1. Introduction
The blood-brain barrier (BBB) remains an extremely challenging hurdle to therapeutic agents targeted for the central nervous system (CNS) [
1,
2]. Prior attempts to bypass the BBB, and their respective disadvantages, includes: direct injection in to brain tissue (invasive), intra-arterial injection of hyperosmolar solution (toxic, requires ICU stay) [
3,
4], and conjugation of amino acid or other moieties to the desired molecule to use an existing BBB transport system (costly), and focused ultrasound (not widely available, limited indications) [
5].
Sphenopalatine ganglion (SPG) stimulation is a novel approach to modulate BBB permeability in a targeted and transient manner [
6,
7,
8]. The SPG is a parasympathetic ganglion found in the pterygopalatine fossa. It is largely innervated by the greater petrosal nerve (a branch of the facial nerve). Among other functions, it supplies parasympathetic innervation to the brain’s anterior vascular system, covering all the ipsilateral cerebral circulation in humans and bilaterally in rats [
9]. Presently, SPG stimulation is used in humans for the treatment of cluster headaches, with trials showing safety and efficacy of an implantable microstimulator in the pterygopalatine fossa for this purpose [
10,
11,
12].
In previous work from our lab, we have proven the ability of an established SPG stimulation protocol to transport a 70 kDa FITC-dextran molecule across the BBB with statistical significance [
13]. Here, we present work that places our previous endeavors in a clinically relevant context.
Temozolomide (TMZ) is one of the most commonly used chemotherapeutic agents for primary CNS Neoplasms, particularly Glioblastoma Multiforme (GBM). It is an alkylating agent that has a molecular weight of 194 Daltons and BBB permeability of up to 20%. GBM is a grade IV brain tumor, which is the most aggressive form of CNS neoplasm, with an average life expectancy upon diagnosis of 14–18 months [
14,
15,
16].
First-line treatment for the disease includes surgical resection, followed by radiotherapy of the surgical cavity and concurrent chemotherapy. Due to the BBB and blood-brain tumor barrier (BBTB), delivery of chemotherapeutic agents for GBM is a major challenge [
16]. It results in insufficient drug delivery for achieving the necessary anti-neoplastic effect. Despite novel attempts to improve survival in GBM, no evidence-based protocol has out-performed the survival rates from the treatment paradigm presented in the Stupp et al. protocol, which is a course of surgery followed by chemotherapy with TMZ and radiation [
17]. Moreover, re-occurrence rate of GBM is another issue that may be due to insufficient therapy. Many pieces of evidence suggest that current therapies are ineffective towards the prevention of re-occurrence of GBM, and there is an urgent clinical need for developing new technologies to solve this problem. Hence, advanced drug delivery approaches such as the reversible opening of the BBB under functional neurostimulation are essential to impact the survival rates in a scientifically and clinically meaningful way [
2,
18].
2. Materials
TMZ and caffeine reference standards were procured from Sigma-Aldrich Corp. (Round Rock, TX, USA). LC-MS-grade acetonitrile (ACN), LC-MS grade water (with and without 0.1% formic acid (FA), and formic acid were purchased from Fisher Scientific (Fair Lawn, NJ, USA). LC-MS-grade acetonitrile (ACN), water (with and without 0.1% formic acid (FA), formic acid hydrochloric acid (HCl), and sodium hydroxide (NaOH) were purchased from Fisher Scientific (Fair Lawn, NJ, USA).
2.1. Methods
All animal experiments complied with the National Institute of Health Guidelines for the care and use of laboratory animals and were approved by the Thomas Jefferson University Institutional Animal Care and Use Committee (IACUC). All experiments were performed on female Sprague-Dawley rats, 235–245 g in weight. Prior to surgery, a 50 mg/kg solution of TMZ (Sigma Aldrich, Round Rock, TX, USA) in 0.9% normal saline solution with four units of heparin/mL was prepared. Rats were anesthetized with isoflurane and injected with 0.4 mL of subcutaneous anesthetic containing 69 mg/mL ketamine, 3.45 mg/mL xylazine, and 1.38 mg/mL acepromazine. The right internal carotid artery was exposed and cannulated via the standard approach and rats were placed in stereotactic head frame for nerve exposure [
13,
19,
20].
A total of 20 rats were utilized. There were 10 rats in the SPG stimulation group and 10 rats in the control group, without SPG stimulation.
While in cranial fixation, the anterior ethmoidal nerve was exposed via a retroorbital approach, similar to the method described by Yarnitsky [
21] and illustrated in
Figure 1a,b. Orbital contents were subperiosteally dissected from the orbital wall, preserving the ethmoidal nerve and its attachments to the orbital contents and ethmoidal foramen. Next, a platinum-iridium bipolar hook electrode was placed around the nerve proximal to its entry into the ethmoidal foramen, with the cathode on the medial side of the electrode. The electrode was then attached to a pulse generator with stimulation parameters at 5V, with 1 ms duration, and 1 ms delay.
All rats received a uniform low dose (50 mg/kg) of TMZ into the right internal carotid artery and consisted of two groups: one receiving a stimulation protocol and the other serving as a control (no stimulation). SPG stimulation was performed in an intermittent fashion, based on previously published data and work from our lab [
13,
22]. One stimulation cycle consisted of 90 s “on” time followed by a 60 s “off” time. Stimulated rats were subjected to six cycles of priming stimulation, followed by an additional eight cycles of injecting the TMZ as a continuous infusion over 8 min (
Figure 2).
Rats were taken for sacrifice via thoracotomy within 15 min following the stimulation protocol. Following thoracotomy, blood tissue samples were obtained via cardiac puncture and rats were perfused with 1 mL/kg of phosphate buffer saline (PBS). Subsequently, brain specimens were harvested and sectioned to isolate and homogenize each hemisphere.
2.2. Liquid Chromatography Mass Spectrometry (LCMS) Method for Temozolomide
For quantitation of TMZ in the presence of internal standard as caffeine, isocratic elution chromatography was performed using LCMS (Thermo Fisher Scientific, Waltham, MA, USA) for efficient chromatographic elution and separation. A mass spectrometer method for detection of TMZ and caffeine was developed by direct infusion of solution (TMZ and caffeine) mix in a high resolution (Orbitrap) mass spectrometer. The scanning mass range was set as 170 to 196 m/z. This optimized mass spec method (with parameters such as spray ionization voltage: 4.25 kV, capillary voltage 27.50 V, sheath gas flow: 30, auxiliary gas flow rate: 5, capillary temperature as 350 °C) was used for the detection of TMZ and caffeine. Separation of TMZ and caffeine was carried out on C18 reverse phase (2.7 µm particle size, 4.6 × 100mm) Cortecs® HILIC column by Waters (Milford, MA, USA). Both calibration standards and samples were eluted using 30:70 water 0.1% FA and ACN 0.1% FA. LCMS run time was optimized to 6 min (min). Injection volume was set to 5 µL, solvent flow rate of 0.250 mL/min. The column temperature was set to 30 °C. Thermo XCaliber (v. 3.0.63) and Exactive (v. 1.1SP6) software (Thermo Fisher Scientific, Waltham, MA, USA) were used for method development and data acquisition in raw format.
2.3. Calibration Standards and Sample Preparation
A calibration curve was developed in a concentration range of 10 ng/mL to 2000 ng/mL and 5 ng/mL to 2000 ng/mL using TMZ standard spiked in plasma and brain tissue, respectively. In addition, caffeine was also added to both plasma and brain tissue with a final concentration of 1 µg/mL. After spiking standards with an internal standard, plasma and brain tissues were acidified with 10% HCl. Both standards and calibration standards were extracted with acetonitrile (ACN) only. The extraction was performed in a ratio of 1:3 for plasma or brain tissue to ACN only. All the extracted standards and samples vortexed for 40–50 s and were centrifuged at 15,700× g for 15 min. Clear 150 µL supernatant was taken and subjected to LCMS analysis. Calibration standards (from plasma and brain tissue) were subjected to intra and inter-day evaluations with a single cycle of freeze (−20 °C) and thaw (20 °C). This gave n = 2 injections for each day run, total n = 4 injections for standard runs from plasma and brain tissue.
2.4. Statistical Methods and Software
All animal study data were compiled and subsequently analyzed using the GraphPad Prism software (v7.04, GraphPad Software, La Jolla, CA, USA). Analysis of Variance (ANOVA) was used to assess for differences between >2 groups, with multiple comparisons test used to assess for individual differences between groups. Students t-test was used to assess for differences when there were only two groups being analyzed. A p-value of < 0.05 was considered statistically significant.
3. Results
3.1. Method Development for Quantitation of TMZ from Plasma and Brain Tissue
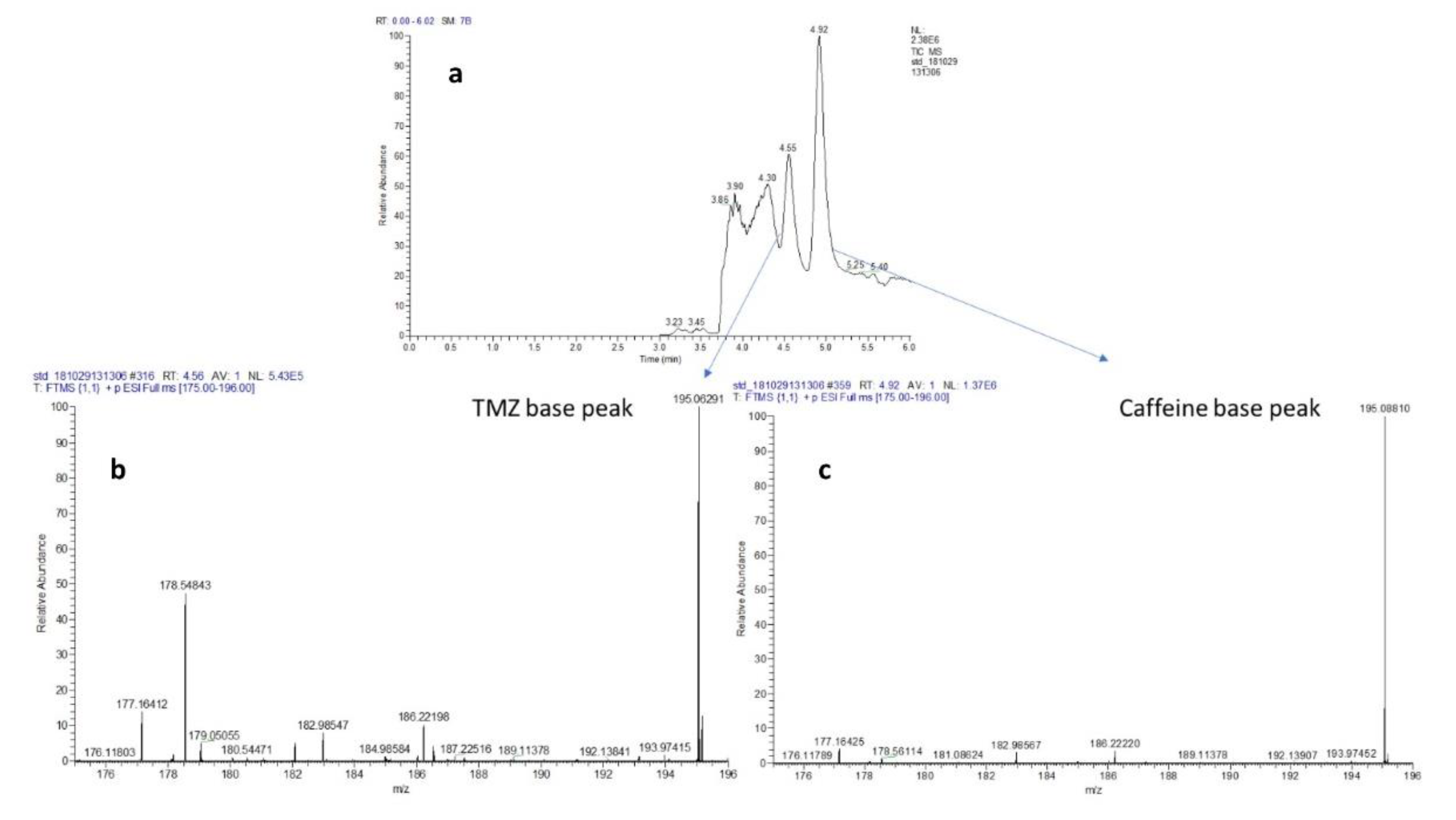
TMZ is a water-insoluble (partition coefficient 22.4) and caffeine is a water-soluble (partition coefficient of −0.07) in nature [
23,
24]. Moreover, caffeine also showed efficient elution profile in ACN with 0.1% FA. Hence, the use of the appropriate balance of aqueous- organic solvent ratio and column chemistries can certainly help in providing an appropriate resolution on HPLC column and their detection in positive ion mode of mass spectrometry. Our preliminary runs show that a ratio of 30:70 water 0.1% FA: ACN 0.1% FA with C18 column provides appropriate chromatography separations for TMZ and caffeine, as shown in
Figure 3a–c.
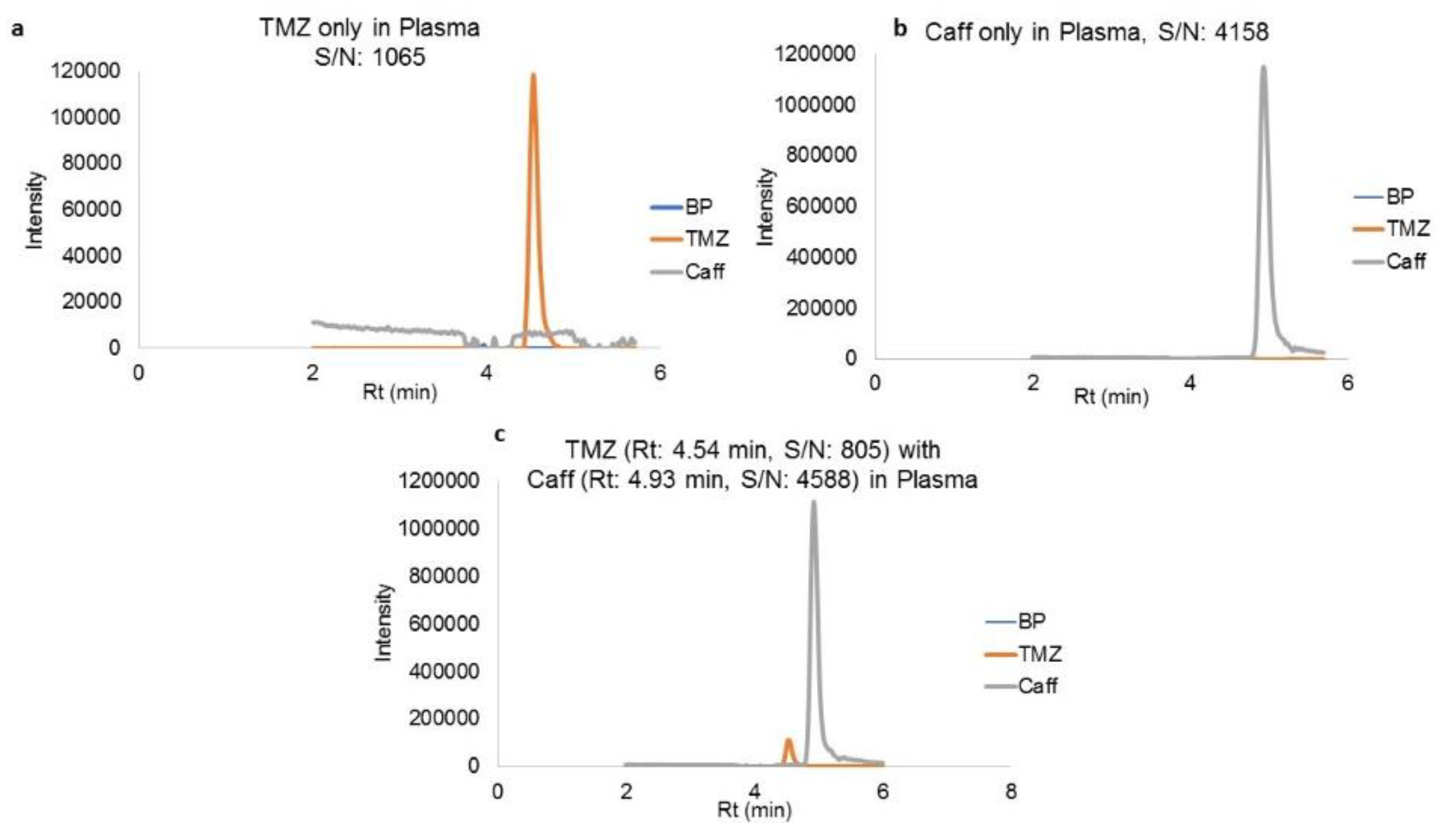
Plasma simultaneously spiked with TMZ and caffeine shows the efficient separations mentioned before, as shown in
Figure 4a–c. Concentration ranges from 10 ng/mL to 2000 ng/mL and 20 ng/mL to 2000 ng/mL for TMZ spiked in plasma and brain tissue, respectively, were used for plotting the calibration curve (concentration. v/s area). This curve resulted in the regression equations of y = 5176.4x (r
2 ≥ 0.99) and y = 5891.8x (r
2 ≥ 0.99) for TMZ in plasma and brain, respectively.
Table 1 and
Table 2 show the percentage coefficient of variation (%CV) for TMZ in calibration concentration ranges from plasma and brain matrix. Retention time (Rt) for the TMZ and caffeine was 4.56 min and 4.91 min, respectively.
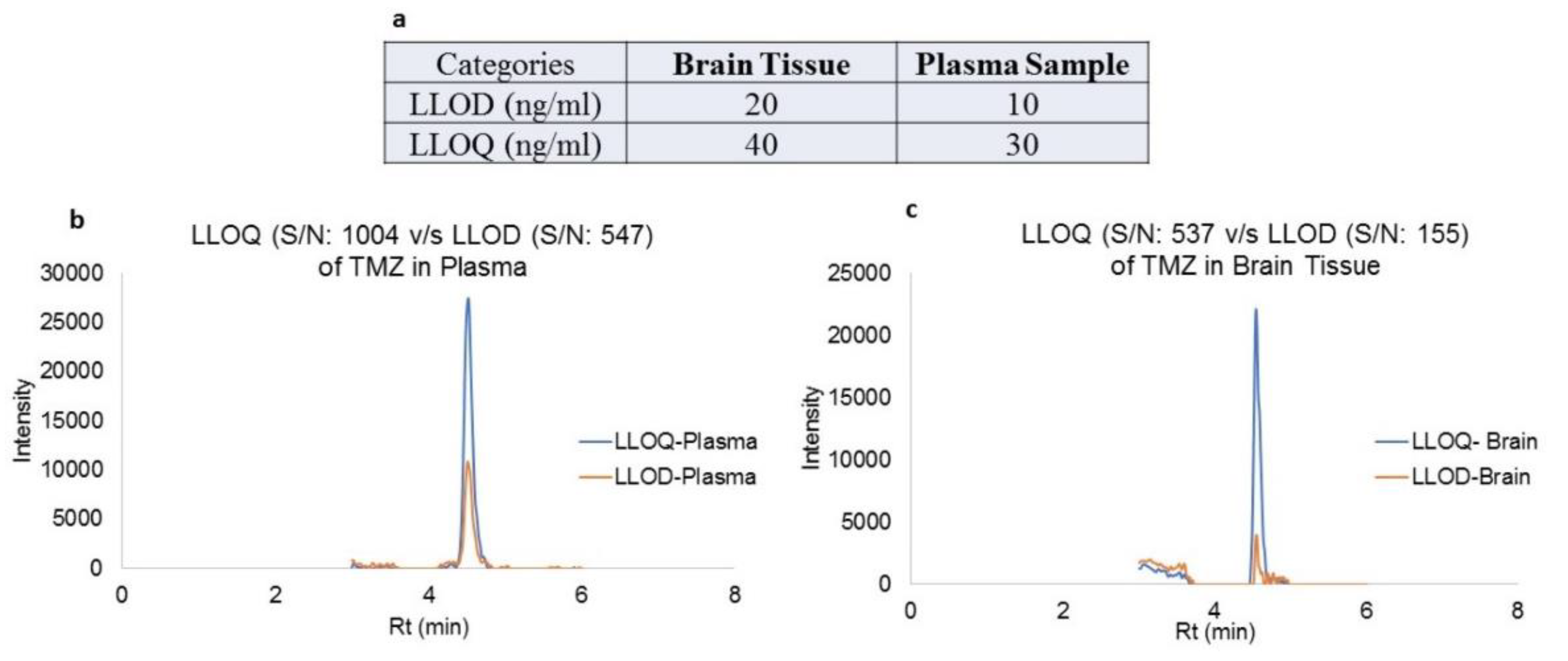
The lower limit of detection (LLOD) and lower limit of quantitation (LLOQ), along with their representative extracted ion chromatograms (XIC) for both plasma and brain tissues, are shown in
Figure 5. This method provided a simplest and effective quantitation TMZ by single ion monitoring (SIM) protocol as per standards set by FDA and ICH guidelines for bioanalytical method development. This simultaneous estimation method was used for the quantitative measurements TMZ in plasma and brain tissue of rats treated with TMZ under SPG stimulation vs. non-stimulation condition.
3.2. Concentrations of TMZ in Brain and Plasma and the Percentage of TMZ Crossing the BBB
A total of 20 rats underwent injection of low-dose TMZ (50 mg/kg) for this analysis. There were 10 rats in each group, which consisted of SPG stimulation and no SPG stimulation. TMZ has been established in the literature [
14,
25] to have a baseline BBB permeability ranging from 0–20%, thus serving as an internal control and standard. All stimulation occurred at 10 Hz, based on prior literature and work from our lab establishing this as an optimal low-frequency stimulation parameter for modulating the BBB [
13,
21,
26]. Each hemisphere was sectioned into right and left, to study for regional differences in TMZ concentration.
Table 3 shows the raw numerical concentrations (ng/mL) of TMZ in brain and plasma for each group, as detected by the LCMS method; it also shows the relative percentage of TMZ crossing the BBB and entering brain parenchyma vs. the amount in the plasma. TMZ was administered via an intra-arterial route at a dose of 50 mg/kg, as a slow bolus over 5 min. Rats were harvested 30 min after drug administration. SPG stimulation showed a statistically significant (
p = 0.0006), a five-fold increase in comparing the percent permeability of the right hemispheres of each group (stimulation vs. no stimulation, shown in
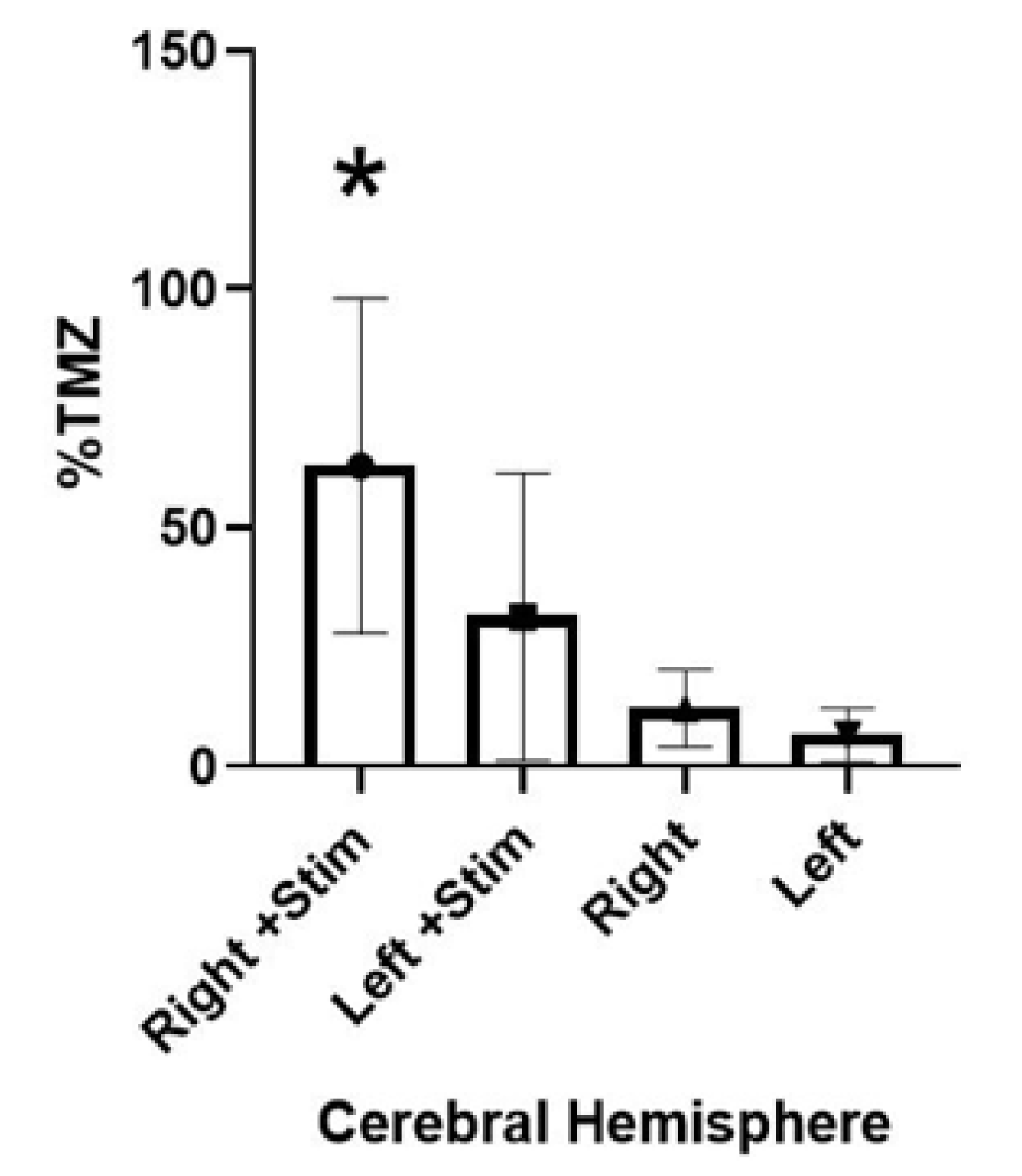
Figure 6). The right hemispheres of the stimulation group showed an average of 62.94% (range 25.61–137.66%) BBB permeability relative to that of plasma, while the non-stimulation group showed an average of 12.12% (range 2.12–25.20%) BBB permeability.
The pattern of permeability in the left hemispheres from each group followed a similar pattern to that of the upregulation in the right hemispheres. In the stimulation group, the left hemispheres showed an average BBB permeability of 31.29% (range 2.26–105.10%), while the non-stimulation group of left hemispheres showed an average of 6.54% uptake (range 1.26–20.59%). In most specimens, the right hemisphere contained almost twice the amount of TMZ versus that of the left hemisphere.
3.3. Hemispheric Concentrations of TMZ
There appeared to be a regionality of effect, approaching but not definitively reaching statistical significance. Comparing the right and left hemispheres of the stimulation group revealed an average of 62.94% vs. 31.29% permeability (p = 0.028). Similarly, comparing the right and left hemispheres of the group with no stimulation revealed an average of 12.12% vs. 6.54% permeability (p = 0.06).
3.4. Gradients of TMZ Concentration—Difference in Response to SPG Stimulation
Within the stimulation group, there appeared to be several tiers of responses to the stimulation. Generally, the rats seem to fall into one of the following types of responders to SPG stimulation: low (up to 30%), medium (up to 60%), or high response (>60%). Two rats had a lower-end response to stimulation (25.61% and 28.41% permeability). Four rats had an intermediate response to the stimulation (43.89%, 43.45%, 45.34%, and 60.15%). Finally, the remaining the rats had an extremely robust response to the SPG stimulation (65.06%, 103.77%, and 137.664%).
It is also important to note that all the rats in the stimulation group exceeded the threshold of 20%, which is a well-established number in the literature of maximum permeability of TMZ in the absence of interventions to upregulate its permeability. Furthermore, most of the rats (all but two) exhibited at least double this amount. All permeabilities of the stimulation group in the right hemispheres also exceeded the values exhibited in their counterparts in the non-stimulation group.
4. Discussion
In this study, we show the therapeutic potential for SPG stimulation in a clinical context. There was a statistically significant (
p = 0.0006) upregulation of TMZ (as shown in
Figure 6) in the brain parenchyma of healthy rats versus those without stimulation. The rationale for performing these experiments in healthy rats was to test our theory and application of SPG stimulation to deliver a drug in a totally intact, otherwise uncompromised BBB. In GBM, there can be some compromise and alterations of the BBB [
16]. Our goal was to show that the permeability of a relevant CNS drug, with TMZ as a representative prototype, is superior with SPG stimulation and avoids any confounding variables secondary to pathological states that may alter BBB permeability.
Furthermore, in a prior publication from our group [
13], we studied the integrity of the tight junctions of the BBB via electron microscopy. We showed that 4 h following the SPG stimulation, the integrity of the BBB returned to baseline and in an intact state. SPG stimulation provides a means of both targeted therapy and reversible modulation of the BBB. Additionally, in the “real world,” one of the main explanations for why patients with systemic cancers (breast, lung) progress to CNS metastatic disease because of the inefficiency of chemotherapeutic agents to penetrate the BBB during initial treatments [
18]. Therefore, by showing a therapy is effective on an intact BBB, it holds potential not only in patients with CNS disease, but also in those who have the high potential to develop it.
Penetration of biomolecule into cerebrospinal fluid (CSF) by crossing the blood-CSF barrier has also been a key area of CNS targeted delivery. The CSF compartment is separated from the systemic central compartment by the blood–CSF barrier, which is formed by the tight junctions of adjacent epithelial cells known in the choroid plexus. Blood flow to the CSF is not a rate-limiting step for drug penetration because the vascular capillaries are fenestrated, permitting drugs to have rapid access to the blood-CSF barrier. The influx into the CSF is controlled by the permeability-area product (PAcsf) that is associated with drug diffusion. The efflux of temozolomide from the CSF consists of three distinct processes: the equivalent PAcsf as used for influx, the bulk flow or convective transport of temozolomide (Qcsf), and a CSF clearance (CLcsf).
In our prior publication, we used a 70 kDa dextran molecule, which is impermeable across the blood-brain CSF barrier. We were able to show that, indeed, SPG stimulation can facilitate transport of a molecule of such large caliber across into the brain tissue of rats. In our present study, we chose temozolomide because it is both clinically relevant for one of the most common primary CNS brain tumors and we can readily measure and compare it against a control. While we know it can cross into the CSF space, it reaches only a fraction of peak plasma concentrations, one source quoting 12–18% of what is peak in the plasma [
27]. Hence, the focus of this work was to study the possibility of the overall increase of drug penetration to CNS. SPG stimulation provides a potential means by which we can modulate the permeability of BBB to get more temozolomide into the CNS and therefore less in the plasma. In this regard, we may be able to decrease systemic toxicity and increase drug delivery to its target (a brain tumor). The proposed mechanism by which this is achieved could to be through an alteration of tight junctions, stated previously [
13].
Furthermore, our study shows that SPG stimulation does not appear to have any effect on the pharmacological structure of the TMZ molecule. Our method developed for measuring TMZ detects the molecule in the state in which it is administered. This molecule is a biological precursor, which at a physiological pH will rapidly break down to an active molecule with alkylating activity. Therefore, our data demonstrate that not only is SPG stimulation safe (no rats experienced any adverse side effects prior to sacrifice), but little is being lost systemically (due to the direct carotid injection) and that a significantly greater percentage of the drug is reaching the brain parenchyma in its native state—where it can break down and exhibit its anti-neoplastic effects. The clinical implications of this fact are tremendous, where it could result in decreased systemic morbidity that is very commonly experienced from chemotherapeutic agents or other drugs designed to specifically target the CNS.
The most common and clinically relevant side effects of temozolomide are systemic in nature: bone marrow suppression, nausea/vomiting, and very rarely acute respiratory failure or liver damage. This drug can cross the BBB to an extent, and no direct CNS side effects have been described. Administering the drug directly to the CNS via an intra-arterial route is in the best interest of the patient, because it would significantly decrease all these systemic risks, which are often reasons why a patient may need to terminate a life-saving therapy.
Furthermore, when a drug is delivered intra-arterially to the brain, it would be delivered to the exact hemisphere and vascular territory where the tumor is located. This is out of the scope of the paper—but endovascular microcatheters are available that can reach distal vessels that would be feeding specifically the vascular territory and brain tissue associated with the brain tumor. Therefore, in this scenario, the benefits of delivering an extremely tailored and targeted therapy outweigh the risks in a clinical context. However, clinicians would certainly monitor patients closely for the development of any therapy related side effects to the CNS. The use of SPG stimulation along with intra-arterial administration of TMZ could significantly help in providing targeted drug delivery and likely reduce systemic toxicity.
The clinical impact that this therapy (SPG stimulation) could have is both timely and necessary. Pardridge et al. noted that a mere 1% of U.S. academic neuroscience programs emphasize BBB transport biology [
1,
2]. His paper was published over a decade ago, and there have been few breakthroughs in this area of research that have had any true impact or relevance.
Safe delivery of therapeutics across the BBB is certainly a real-world challenge for clinicians. In this manuscript, we show a technique that is already used in humans for cluster headaches (SPG stimulation). Despite mechanistic insight, we show how SPG stimulation can potently modulate the BBB and the powerful therapeutic implications of this novel approach.
Limitations
The biggest limitation of this study is the fact that this is a healthy rat model. Additionally, the observed variability in permeability with SPG stimulation may present some limitations from patient to patient. Next logical steps would be to evaluate in a pathological state in rats with GBM. Furthermore, much remains to be learned about the exact mechanistic underpinnings of SPG stimulation.