Fabrication of Anti-HSV-1 Curcumin Stabilized Nanostructured Proniosomal Gel: Molecular Docking Studies on Thymidine Kinase Proteins
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.2.1. Curcumin Isolation
2.2.2. Formulation Study
2.3. Preparation of Curcumin Proniosomes
2.4. Evaluation of Curcumin Proniosomes Formulae
2.4.1. Zeta Potential (ζ) Determination
2.4.2. Vesicle Size and Size Distribution
2.4.3. Determination of Percentage Entrapment Efficiency (% EE)
2.4.4. Optimization of Curcumin Proniosomes
2.4.5. Preparation of Curcumin Proniosomal Gel
2.4.6. Characterization of The Optimal Curcumin Proniosomal Gel Formulae
In-Vitro Release Study
Ex-Vivo Permeation Studies
In-Vitro Anti HSV-1 Assay
Transmission Electron Microscopy (TEM)
Molecular Docking and Targets Preparation
3. Results
3.1. Zeta Potential (ζ), Vesicle Size (VS) and Size Distribution
3.2. Determination of Percentage Entrapment Efficiency (%EE)
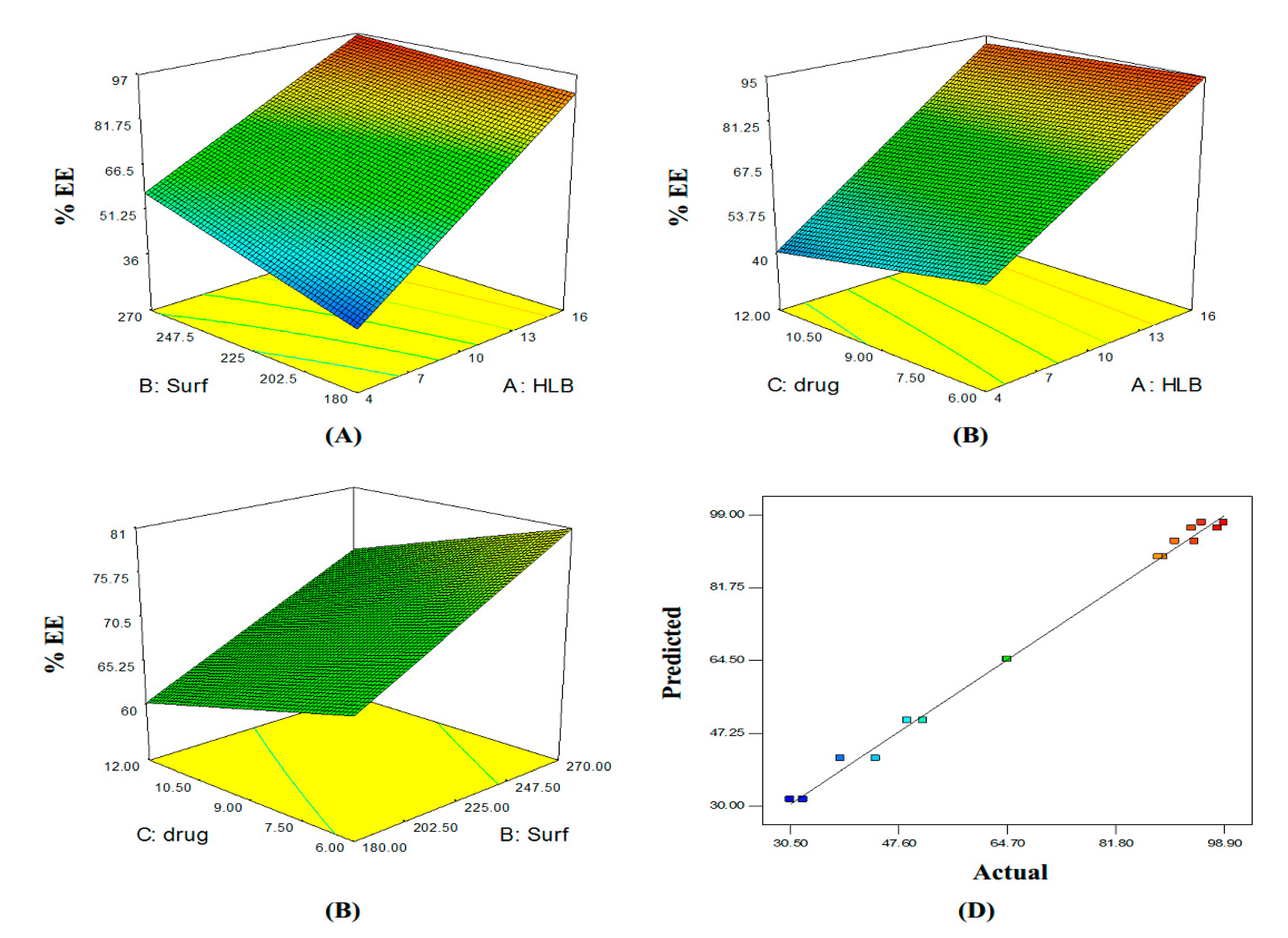
3.3. Optimization of Curcumin Proniosomes
3.4. Characterization of the Selected Curcumin Proniosomal Gel Formulae
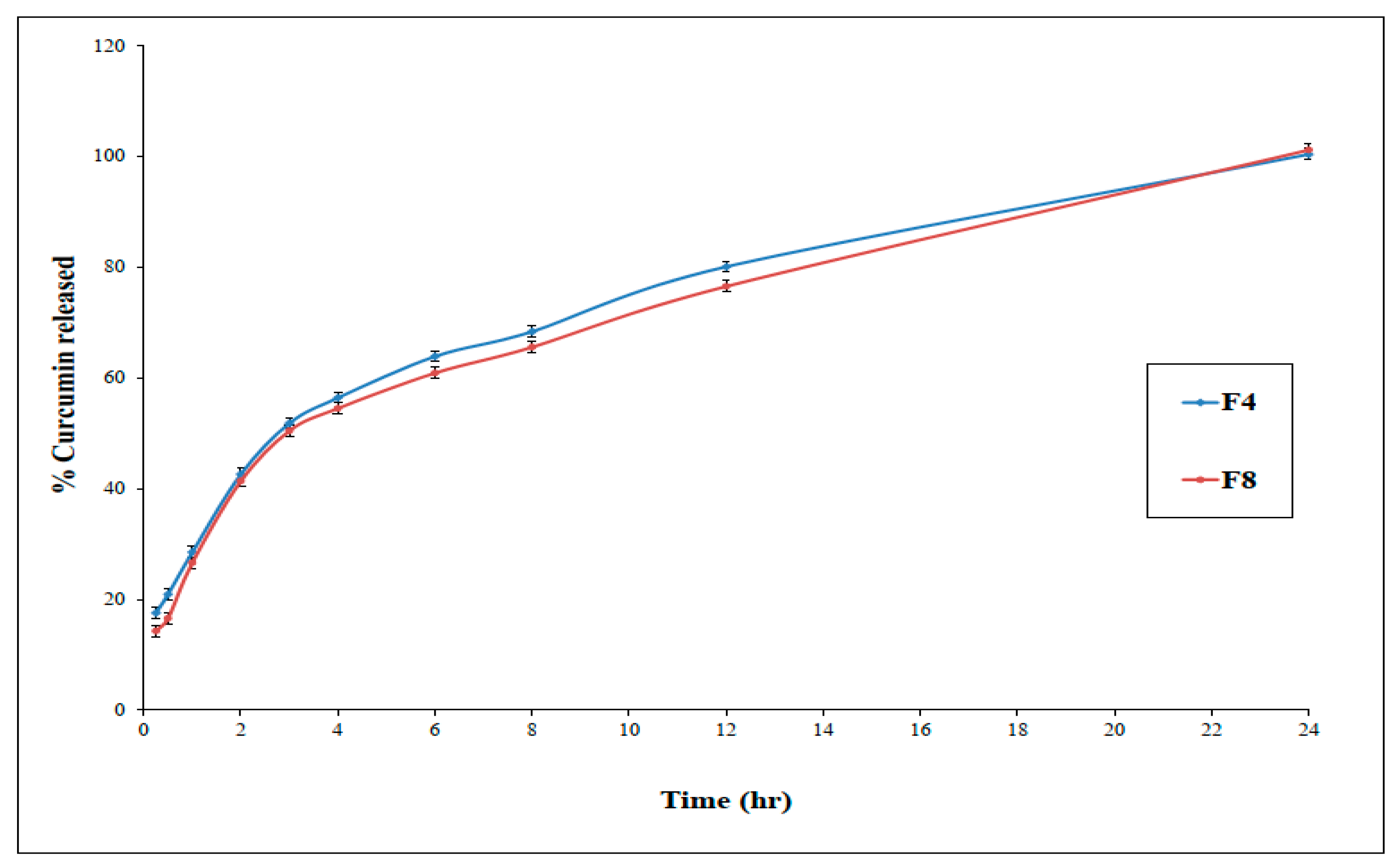
3.4.1. In-Vitro Release Study
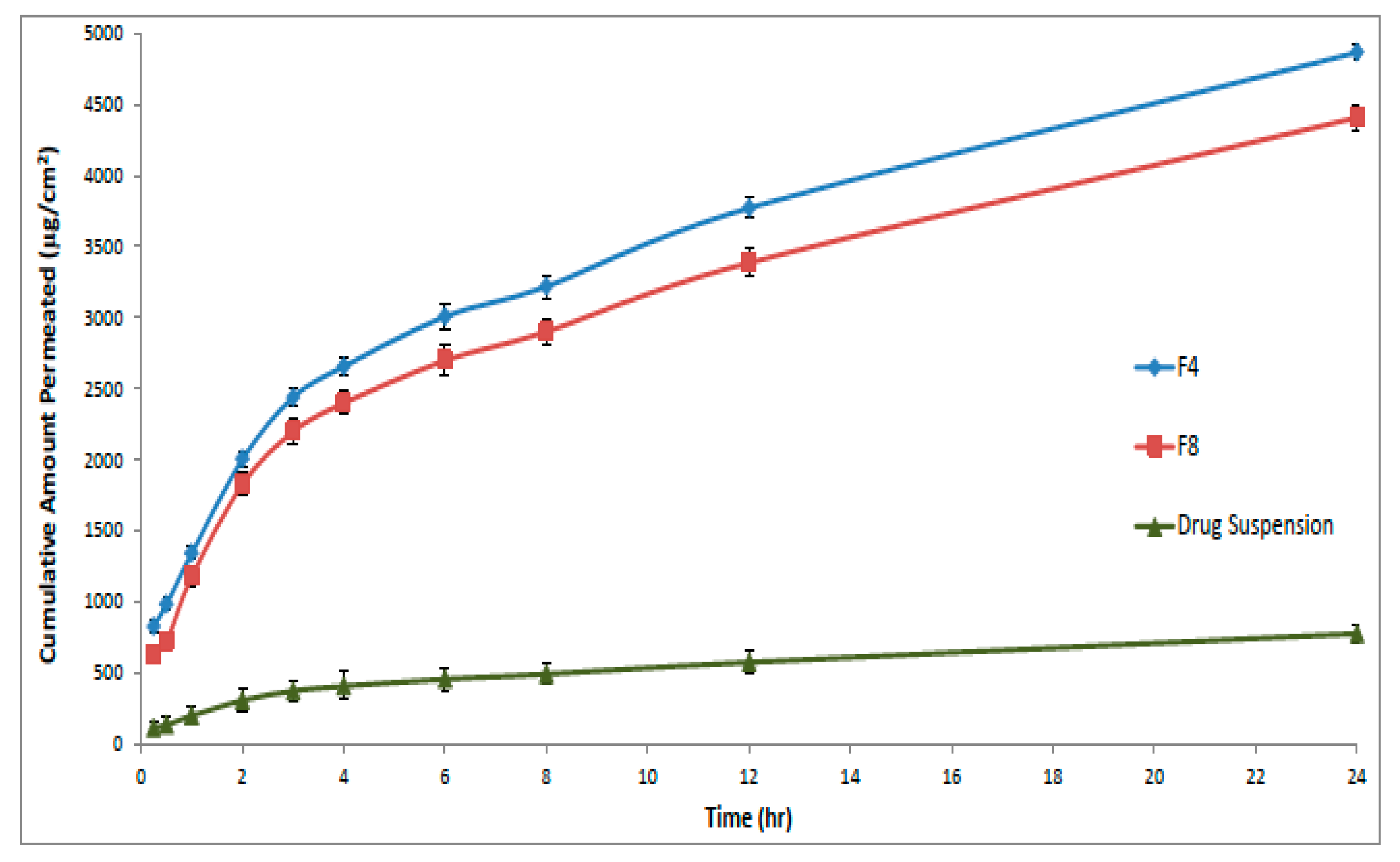
3.4.2. Ex-Vivo Permeation Studies
3.4.3. Cytotoxicity Study of Curcumin on Vero Cells
3.4.4. Transmission Electron Microscopy
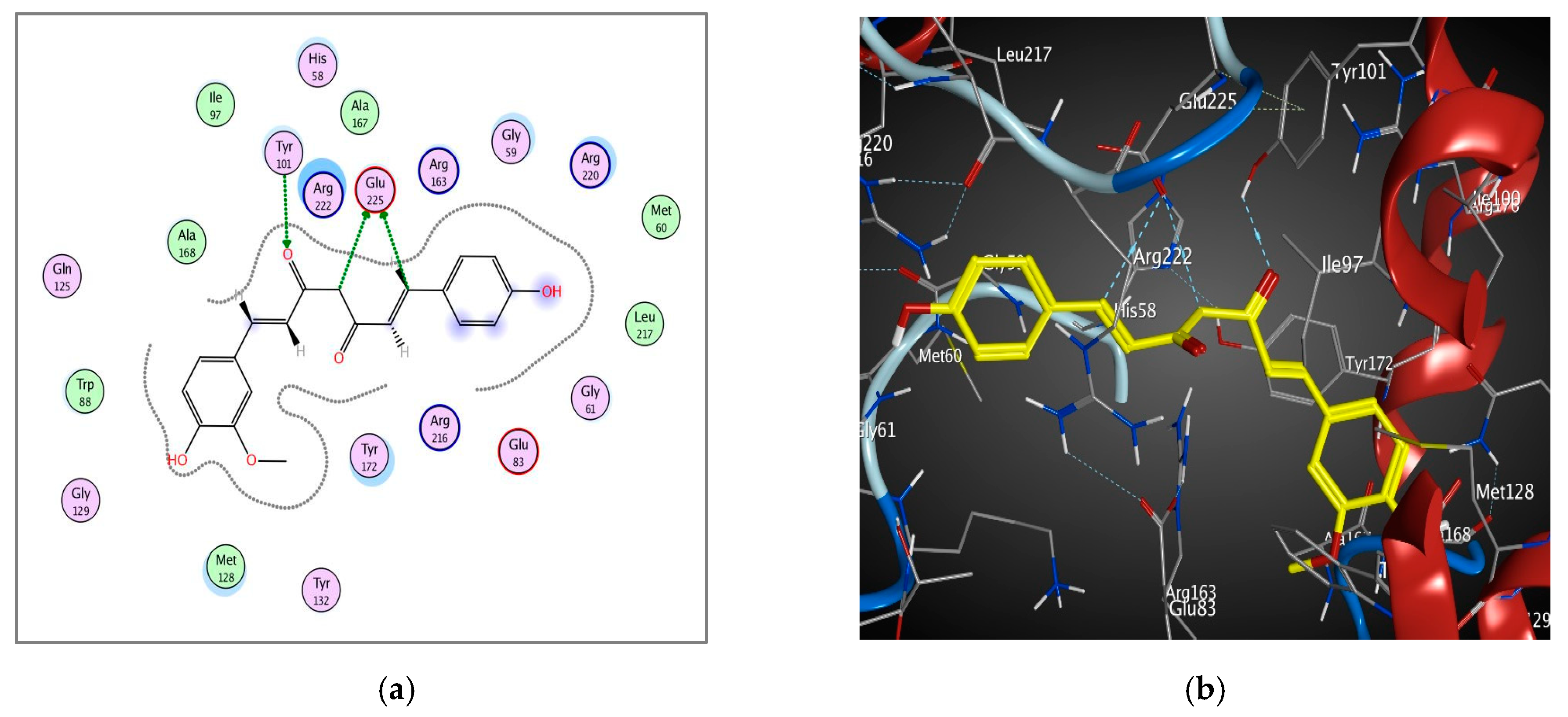
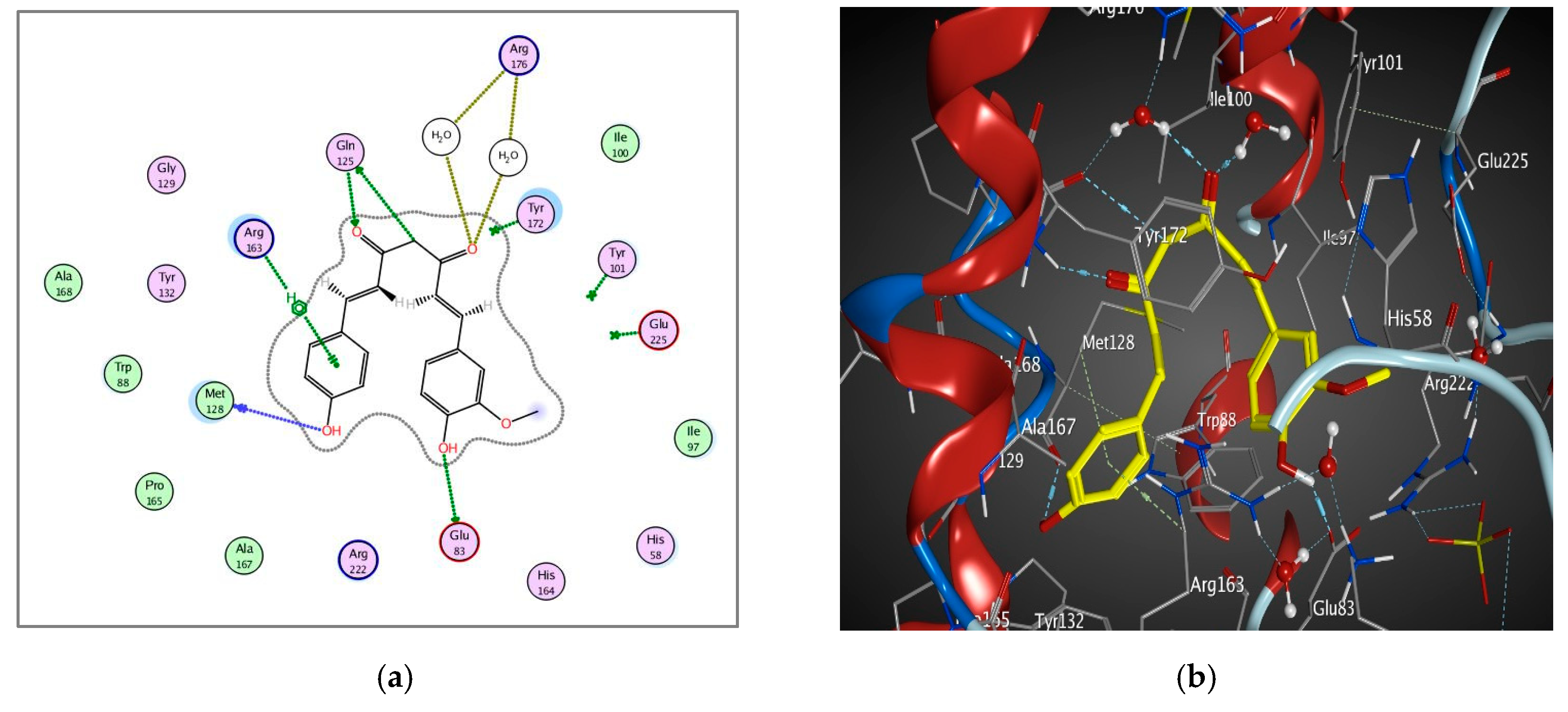
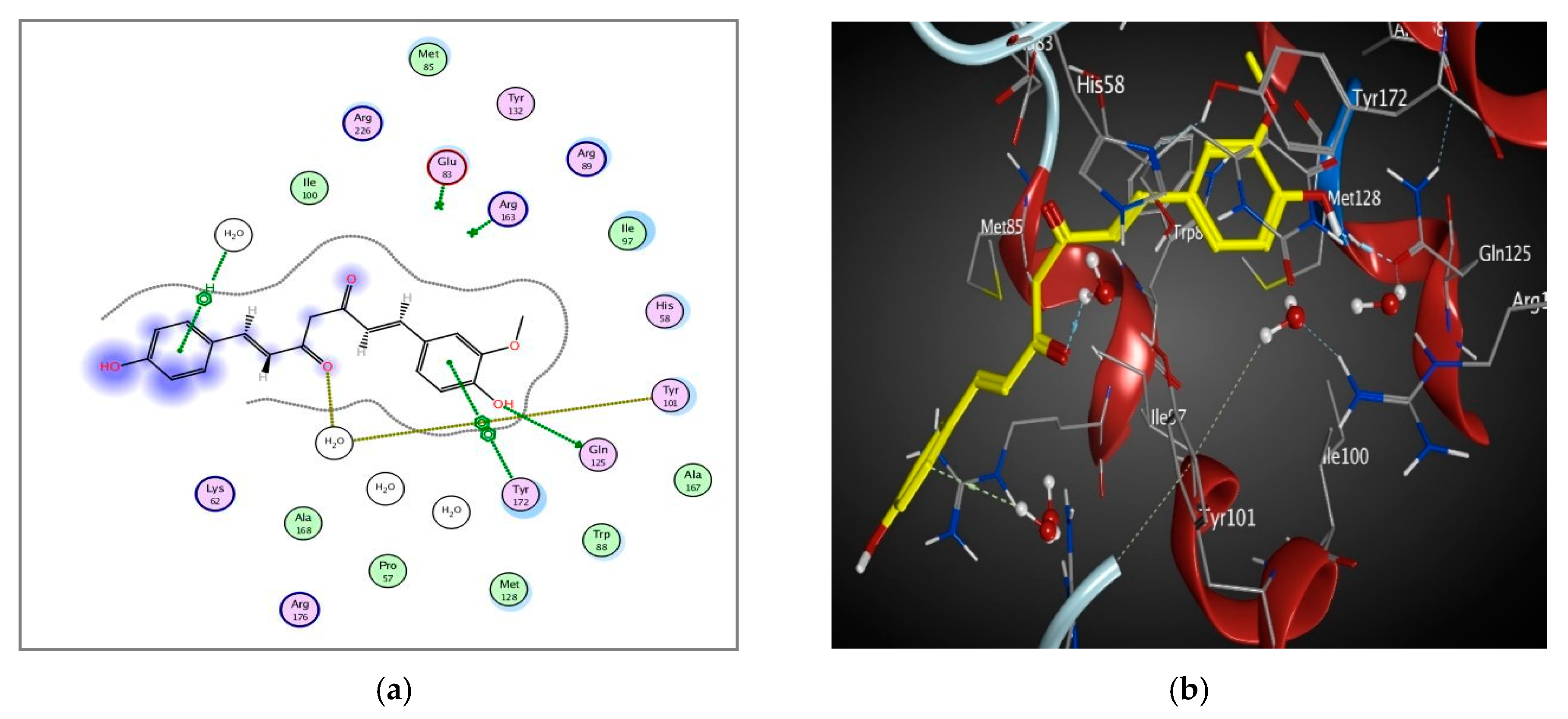
3.4.5. Molecular Docking of Curcumin on HSV-1 Thymidine Kinase Proteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Marchi, S.; Trombetta, C.M.; Gasparini, R.; Temperton, N.; Montomoli, E. Epidemiology of herpes simplex virus type 1 and 2 in Italy: A seroprevalence study from 2000 to 2014. J. Prev. Med. Hyg. 2017, 58, E27–E33. [Google Scholar]
- Cortesi, R.; Ravani, L.; Rinaldi, F.; Marconi, P.; Drechsler, M.; Manservigi, M.; Argnani, R.; Menegatti, E.; Esposito, E.; Manservigi, R. Intranasal immunization in mice with non-ionic surfactants vesicles containing HSV immunogens: A preliminary study as possible vaccine against genital herpes. Int. J. Pharm. 2013, 440, 229–237. [Google Scholar] [CrossRef]
- Arduino, P.G.; Porter, S.R. Oral and perioral herpes simplex virus type 1 (HSV-1) infection: Review of its management. Oral Dis. 2006, 12, 254–270. [Google Scholar] [CrossRef]
- Elena, S.F.; Sanjuán, R. Adaptive value of high mutation rates of RNA viruses: Separating causes from consequences. J. Virol. 2005, 79, 11555–11558. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef]
- Sanghai, V.D.N.; Kulkarni, S.R.; Sanghai, N.N. Screening of antiviral compounds from plants—A review. J. Pharm. Res. 2014, 8, 1050–1058. [Google Scholar]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Yuan, W.; Li, S.; Gupta, S.C. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: Identification of novel components of turmeric. Mol. Nutr. Food Res. 2013, 57, 1529–1542. [Google Scholar] [CrossRef]
- Flores, D.J.; Lee, L.H.; Adams, S.D. Inhibition of curcumin-treated Herpes Simplex virus 1 and 2 in vero cells. Adv. Microbiol. 2016, 6, 276–287. [Google Scholar] [CrossRef]
- Qin, Y.; Lin, L.; Chen, Y.; Wu, S.; Si, X.; Wu, H.; Zhai, X.; Wang, Y.; Tong, L.; Pan, B.; et al. Curcumin inhibits the replication of enterovirus 71 in vitro. Acta Pharm. Sin. B 2014, 4, 284–294. [Google Scholar] [CrossRef]
- Joe, B.; Vijaykumar, M.; Lokesh, B.R. Biological properties of curcumin-cellular and molecular mechanisms of action. Crit. Rev. Food Sci. Nutr. 2004, 44, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Mathew, D.; Hsu, W.L. Antiviral potential of curcumin. J. Funct. Foods 2018, 40, 692–699. [Google Scholar] [CrossRef]
- Kutluay, S.B.; Doroghazi, J.; Roemer, M.E.; Triezenberg, S.J. Curcumin inhibits herpes simplex virus immediate-early gene expression by a mechanism independent of p300/CBP histone acetyltransferase activity. Virology 2008, 373, 239–247. [Google Scholar] [CrossRef]
- Bourne, K.Z.; Bourne, N.; Reising, S.F.; Stanberry, L.R. Plant products as topical microbicide candidates: Assessment of in vitro and in vivo activity against herpes simplex virus type 2. Antiviral Res. 1999, 42, 219–226. [Google Scholar] [CrossRef]
- Maiti, K.; Mukherjee, K.; Gantait, A.; Saha, B.P.; Mukherjee, P.K. Curcumin-phospholipid complex: Preparation, therapeutic evaluation and pharmacokinetic study in rats. Int. J. Pharm. 2007, 330, 155–163. [Google Scholar] [CrossRef]
- Toden, S.; Goel, A. The holy grail of curcumin and its efficacy in various diseases: Is bioavailability truly a big concern? J. Restor. Med. 2017, 6, 27–36. [Google Scholar] [CrossRef]
- Wahlang, B.; Pawar, Y.B.; Bansal, A.K. Identification of permeability-related hurdles in oral delivery of curcumin using the Caco-2 cell model. Eur. J. Pharm. Biopharm. 2011, 77, 275–282. [Google Scholar] [CrossRef]
- El-Haddad, A.E.; Sheta, N.M.; Boshra, S.A. Isolation, formulation, and efficacy enhancement of morin emulsified carriers against lung toxicity in rats. AAPS PharmSciTech 2018, 19, 2346–2357. [Google Scholar] [CrossRef]
- Bayindir, Z.; Yuksel, N. Provesicles as novel drug delivery systems. Curr. Pharm. Biotechnol. 2015, 16, 344–364. [Google Scholar] [CrossRef]
- Yasam, V.R.; Jakki, S.L.; Natarajan, J.; Kuppusamy, G. A review on novel vesicular drug delivery: Proniosomes. Drug Deliv. 2014, 21, 243–249. [Google Scholar] [CrossRef]
- Verma, P.; Prajapati, S.K.; Yadav, R.; Senyschyn, D.; Shea, P.R.; Trevaskis, N.L. Single intravenous dose of novel flurbiprofen-loaded proniosome formulations provides prolonged systemic exposure and anti-inflammatory effect. Mol. Pharm. 2016, 13, 3688–3699. [Google Scholar] [CrossRef]
- Taghipour, Y.D.; Bahramsoltani, R.; Marques, A.M.; Naseri, R.; Rahimi, R.; Haratipour, P.; Panah, A.I.; Farzaei, M.H.; Abdollahi, M. A systematic review of nano formulation of natural products for the treatment of inflammatory bowel disease: Drug delivery and pharmacological targets. DARU J. Pharm. Sci. 2018, 26, 229–239. [Google Scholar] [CrossRef]
- M Soliman, S.; M Sheta, N.; M.M. Ibrahim, B.; M El-Shawwa, M.; M Abd El-Halim, S. Novel intranasal drug delivery: Geraniol charged polymeric mixed micelles for targeting cerebral insult as a result of ischaemia/reperfusion. Pharmaceutics 2020, 12, 76. [Google Scholar] [CrossRef]
- Zafar; Quarta; Marradi; Ragusa. Recent developments in the reduction of oxidative stress through antioxidant polymeric formulations. Pharmaceutics 2019, 11, 505. [Google Scholar] [CrossRef]
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between bioavailability and health potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef]
- Jadhav, K.R.; Pawar, A.Y.; Bachhav, A.A.; Ahire, S.A. Proniosome: A novel non-ionic provesicules as potential drug carrier. Asian J. Pharm. 2016, 10, 210–222. [Google Scholar]
- Kumar, G.P.; Rajeshwarrao, P. Nonionic surfactant vesicular systems for effective drug delivery—An overview. Acta Pharm. Sin. B 2011, 1, 208–219. [Google Scholar] [CrossRef]
- Rawat, A.S.; Kumar, M.S.; Khurana, B.; Mahadevan, N. Proniosome gel: A novel topical delivery system. Int. J. Rec. Adv. Pharm. Res. 2011, 3, 1–10. [Google Scholar]
- Sambhakar, S.; Paliwal, S.; Sharma, S.; Singh, B. Formulation of risperidone loaded proniosomes for effective transdermal delivery: An in-vitro and in-vivo study. Bull. Fac. Pharmacy, Cairo Univ. 2017, 55, 239–247. [Google Scholar] [CrossRef]
- Revathy, S.; Elumalai, S.; Antony, M.B. Isolation, purification and identification of curcuminoids from turmeric (Curcuma longa L.) by column chromatography. J. Exp. Sci. 2011, 2, 21–25. [Google Scholar]
- Khatoon, M.; Shah, K.U.; Din, F.U.; Shah, S.U.; Rehman, A.U.; Dilawar, N.; Khan, A.N. Proniosomes derived niosomes: Recent advancements in drug delivery and targeting. Drug Deliv. 2017, 24, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Nimbalwar, M.; Upadhye, K.; Dixit, G. Fabrication and evaluation of ritonavir proniosomal transdermal gel as a vesicular drug delivery system. Pharmacophore 2016, 7, 82–95. [Google Scholar]
- Loona, S.; Gupta, N.B.; Khan, M.U. Preparation and characterization of metformin proniosomal gel for treatment of diabetes mellitus. Int. J. Pharm. Sci. Rev. Res. 2012, 15, 108–114. [Google Scholar]
- Soliman, S.M.; Abdelmalak, N.S.; El-Gazayerly, O.N.; Abdelaziz, N. Novel non-ionic surfactant proniosomes for transdermal delivery of lacidipine: Optimization using 23factorial design and in vivo evaluation in rabbits. Drug Deliv. 2016, 23, 1608–1622. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Kaur, L. Spectrophotometric method for simultaneous estimation of ornidazole and curcumin in pure form. Pharma Innov. 2014, 3, 1–4. [Google Scholar]
- Sharma, K.; Agrawal, S.S.; Gupta, M. Development and validation of UV spectrophotometric method for the estimation of curcumin in bulk drug and pharmaceutical dosage forms. Int. J. Drug Dev. Res. 2017, 4, 375–380. [Google Scholar]
- Lather, V.; Sharma, D.; Pandita, D. Proniosomal gel-mediated transdermal delivery of bromocriptine: In vitro and ex vivo evaluation. J. Exp. Nanosci. 2016, 11, 1044–1057. [Google Scholar] [CrossRef]
- El-Nabarawi, M.A.; Bendas, E.R.; El Rehem, R.T.A.; Abary, M.Y.S. Transdermal drug delivery of paroxetine through lipid-vesicular formulation to augment its bioavailability. Int. J. Pharm. 2013, 443, 307–317. [Google Scholar] [CrossRef]
- El-Nabarawi, M.A.; Bendas, E.R.; Abd El Rehem, T.R.; Abary, M.Y.S. Formulation and evaluation of dispersed paroxetine liposomes in gel. J. Chem. Pharm. Res. 2012, 4, 2209–2222. [Google Scholar]
- Abd-Elsalam, W.H.; El-Zahaby, S.A.; Al-Mahallawi, A.M. Formulation and in vivo assessment of terconazole-loaded polymeric mixed micelles enriched with Cremophor EL as dual functioning mediator for augmenting physical stability and skin delivery. Drug Deliv. 2018, 25, 484–492. [Google Scholar] [CrossRef]
- Jadhav, B.K.; Mahadik, K.R.; Paradkar, A.R. Development and validation of improved reversed phase-HPLC method for simultaneous determination of curcumin, demethoxycurcumin and bis-demethoxycurcumin. Chromatographia 2007, 65, 483–488. [Google Scholar] [CrossRef]
- Shah, J.; Nair, A.B.; Shah, H.; Jacob, S.; Shehata, T.M.; Morsy, M.A. Enhancement in antinociceptive and anti-inflammatory effects of tramadol by transdermal proniosome gel. Asian J. Pharm. Sci. 2019, in press, e1–e11. [Google Scholar] [CrossRef]
- Üstündaǧ Okur, N.; Apaydin, Ş.; Karabay Yavaşoǧlu, N.Ü.; Yavaşoǧlu, A.; Karasulu, H.Y. Evaluation of skin permeation and anti-inflammatory and analgesic effects of new naproxen microemulsion formulations. Int. J. Pharm. 2011, 416, 136–144. [Google Scholar] [CrossRef]
- Amoros, M.; Sauvager, F.; Girre, L.; Cormier, M. In vitro antiviral activity of propolis. Apidologie 1992, 23, 231–240. [Google Scholar] [CrossRef]
- Simões, C.M.O.; Amoros, M.; Girre, L. Mechanism of antiviral activity of triterpenoid saponins. Phyther. Res. An Int. J. Devoted to Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 1999, 13, 323–328. [Google Scholar] [CrossRef]
- Walum, E.; Stenberg, K.; Jenssen, D. Cell toxicology: Principles and practice. J. Appl. Toxicol. 1990, 11, 389–390. [Google Scholar]
- Langlois, M.; Allard, J.P.; Nugier, F.; Aymard, M. A rapid and automated colorimetric assay for evaluating the sensitivity of herpes simplex strains to antiviral drugs. J. Biol. Stand. 1986, 14, 201–211. [Google Scholar] [CrossRef]
- Kruppenbacher, J.P.; Klass, R.; Eggers, H.J. A rapid and reliable assay for testing acyclovir sensitivity of clinical herpes simplex virus isolates independent of virus dose and reading time. Antiviral Res. 1994, 23, 11–22. [Google Scholar] [CrossRef]
- Sezgin-Bayindir, Z.; Yuksel, N. Investigation of formulation variables and excipient interaction on the production of niosomes. AAPS PharmSciTech 2012, 13, 826–835. [Google Scholar] [CrossRef]
- Champness, J.N.; Bennett, M.S.; Wien, F.; Visse, R.; Summers, W.C.; Herdewijn, P.; de Clerq, E.; Ostrowski, T.; Jarvest, R.L.; Sanderson, M.R. Exploring the active site of herpes simplex virus type-1 thymidine kinase by X-ray crystallography of complexes with aciclovir and other ligands. Proteins 1998, 32, 350–361. [Google Scholar] [CrossRef]
- Wurth, C.; Kessler, U.; Vogt, J.; Schulz, G.E.; Folkers, G.; Scapozza, L. The effect of substrate binding on the conformation and structural stability of Herpes Simplex virus type 1 thymidine kinase. Protein Sci. 2001, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Khalil, R.M.; Abdelbary, G.A.; Basha, M.; Awad, G.E.A.; El-Hashemy, H.A. Design and evaluation of proniosomes as a carrier for ocular delivery of lomefloxacin HCl. J. Liposome Res. 2017, 27, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.M.; Farid, R.M.; Kassem, A.A. Nano-proniosomes enhancing the transdermal delivery of mefenamic acid. J. Liposome Res. 2014, 24, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Shamma, R.N.; Basalious, E.B.; Shoukri, R.A. Development and optimization of a multiple-unit controlled release formulation of a freely water soluble drug for once-daily administration. Int. J. Pharm. 2011, 405, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Bayindir, Z.S.; Yuksel, N. Characterization of niosomes prepared with various nonionic surfactants for paclitaxel oral delivery. J. Pharm. Sci. 2010, 99, 2049–2060. [Google Scholar] [CrossRef]
- Ismail, S.; Khattab, A. Optimization of proniosomal itraconazole formulation using Box Behken design to enhance oral bioavailability. J. Drug Deliv. Sci. Technol. 2018, 45, 142–150. [Google Scholar] [CrossRef]
- Abdelbary, G.A.; Amin, M.M.; Zakaria, M.Y. Ocular ketoconazole-loaded proniosomal gels: Formulation, ex vivo corneal permeation and in vivo studies. Drug Deliv. 2017, 24, 309–319. [Google Scholar] [CrossRef]
- Hathout, R.M.; Mansour, S.; Mortada, N.D.; Guinedi, A.S. Liposomes as an ocular delivery system for acetazolamide: In vitro and in vivo studies. AAPS PharmSciTech 2007, 8, E1–E12. [Google Scholar] [CrossRef]
- Ramezani, V.; Honarvar, M.; Seyedabadi, M.; Karimollah, A.; Ranjbar, A.M.; Hashemi, M. Formulation and optimization of transfersome containing minoxidil and caffeine. J. Drug Deliv. Sci. Technol. 2018, 44, 129–135. [Google Scholar] [CrossRef]
- Alsarra, I.A. Evaluation of proniosomes as an alternative strategy to optimize piroxicam transdermal delivery. J. Microencapsul. 2009, 26, 272–278. [Google Scholar] [CrossRef]
- Mokhtar, M.; Sammour, O.A.; Hammad, M.A.; Megrab, N.A. Effect of some formulation parameters on flurbiprofen encapsulation and release rates of niosomes prepared from proniosomes. Int. J. Pharm. 2008, 361, 104–111. [Google Scholar] [CrossRef]
- Marwa, A.; Omaima, S.; Hanaa, E.-G.; Mohammed, A.-S. Preparation and in-vitro evaluation of diclofenac sodium niosomal formulations. Int. J. Pharm. Sci. Res. 2013, 4, 1757–1765. [Google Scholar]
- Pardakhty, A.; Varshosaz, J.; Rouholamini, A. In vitro study of polyoxyethylene alkyl ether niosomes for delivery of insulin. Int. J. Pharm. 2007, 328, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Aboelwafa, A.A.; El-Setouhy, D.A.; Elmeshad, A.N. Comparative study on the effects of some polyoxyethylene alkyl ether and sorbitan fatty acid ester surfactants on the performance of transdermal carvedilol proniosomal gel using experimental design. AAPS PharmSciTech 2010, 11, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Yu, S.Y.; Wu, P.C.; Huang, Y.B.; Tsai, Y.H. In vitro skin permeation of estradiol from various proniosome formulations. Int. J. Pharm. 2001, 215, 91–99. [Google Scholar] [CrossRef]
- Zandi, K.; Ramedani, E.; Mohammadi, K.; Tajbakhsh, S.; Deilami, I.; Rastian, Z.; Fouladvand, M.; Yousefi, F.; Farshadpour, F. Evaluation of antiviral activities of curcumin derivatives against HSV-1 in Vero cell line. Nat. Prod. Commun. 2010, 5, 1935–1938. [Google Scholar] [CrossRef] [PubMed]


| Code | Composition (mg) * | Mean Zeta Potential (mV) | PDI | Mean Vesicle Size (nm), (Y1) | Entrapment Efficiency (%), (Y2) | ||
|---|---|---|---|---|---|---|---|
| Drug | Kolliphor RH40 | Brij 93 | |||||
| F1 | 6 | - | 180 | −56.57 ± 1.29 | 0.49 | 171.40 ± 3.96 | 41.27 ± 3.91 |
| F2 | 6 | 180 | - | −39.50 ± 2.10 | 0.32 | 161.55 ± 1.06 | 92.75 ± 2.19 |
| F3 | 6 | - | 270 | −63.27 ± 0.91 | 0.48 | 209.30 ± 0.57 | 64.75 ± 0.07 |
| F4 | 6 | 270 | - | −35.20 ± 0.52 | 0.34 | 173.70 ± 2.26 | 97.15 ± 2.47 |
| F5 | 12 | - | 180 | −66.27 ± 0.59 | 0.41 | 250.60 ± 1.27 | 31.55 ± 1.48 |
| F6 | 12 | 180 | - | −45.90 ± 1.23 | 0.52 | 179.30 ± 0.42 | 89.00 ± 0.57 |
| F7 | 12 | - | 270 | −56.97 ± 0.40 | 0.42 | 218.05 ± 5.66 | 50.25 ± 1.77 |
| F8 | 12 | 270 | - | −45.13 ± 1.32 | 0.59 | 206.15 ± 4.17 | 95.85 ± 2.90 |
| Code | Flux (JSS) (µg/cm2/h) | Kp (cm/h) | Er |
|---|---|---|---|
| F4 | 160.43 | 0.225 | 6.14 |
| F8 | 148.66 | 0.208 | 5.69 |
| Drug suspension | 26.12 | 0.037 | - |
| Tested Materials | Non-Toxic Dose on Vero Cell | Initial Viral Titre | Final Viral Titre | Mean % of Reduction |
|---|---|---|---|---|
| curcumin suspension | 1/100 | 1 × 105 | 4 × 104 | 50 ± 2.2 |
| 1 × 106 | 4 × 105 | |||
| 1 × 107 | 4 × 106 | |||
| F4 (1%) | 1/70 | 1 × 105 | 5 × 104 | 85.4 ± 1.05 |
| 1 × 106 | 5 × 105 | |||
| 1 × 107 | 5 × 106 | |||
| F8 (2%) | 1/80 | 1 × 105 | 5 × 105 | 87.9 ± 1.65 |
| 1 × 106 | 4 × 106 | |||
| 1 × 107 | 4 × 107 | |||
| Control | 1/50 | 1 × 105 | 1 × 105 | 0 |
| 1 × 106 | 1 × 106 | |||
| 1 × 107 | 1 × 107 |
| HSV-1 Thymidine Kinase Proteins | Energy Score (kcal/mol) | No. of Interactions | H Bonding Residues | Distance/E (kcal/mol) |
|---|---|---|---|---|
| 1KI7 | −5.4 | 3 | GLU 225 | 3.44/−0.7 |
| GLU 225 | 3.34/−0.8 | |||
| TYR 101 | 2.94/−0.9 | |||
| 1KI4 | −3.8 | 6 | GLN 125 | 3.14/−1.1 |
| MET 128 | 2.91/−1.8 | |||
| GLU 83 | 2.48/−1.3 | |||
| HOH 568 | 2.45/1.3 | |||
| HOH 572 | 2.65/−1.0 | |||
| GLN 125 | 2.86/−1.6 | |||
| ARG 163 (pi-H) | 3.51/−0.6 | |||
| 1E2P | −7.318 | 4 | GLN 125 | 2.73/−2.4 |
| HOH 2009 | 2.96/−1.0 | |||
| HOH 2036 (pi-H) | 4.22/−1.4 | |||
| TYR 172 (pi-pi) | 3.98/0.0 | |||
| 1KIM | Not docked | - | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Halim, S.M.A.; Mamdouh, M.A.; El-Haddad, A.E.; Soliman, S.M. Fabrication of Anti-HSV-1 Curcumin Stabilized Nanostructured Proniosomal Gel: Molecular Docking Studies on Thymidine Kinase Proteins. Sci. Pharm. 2020, 88, 9. https://doi.org/10.3390/scipharm88010009
El-Halim SMA, Mamdouh MA, El-Haddad AE, Soliman SM. Fabrication of Anti-HSV-1 Curcumin Stabilized Nanostructured Proniosomal Gel: Molecular Docking Studies on Thymidine Kinase Proteins. Scientia Pharmaceutica. 2020; 88(1):9. https://doi.org/10.3390/scipharm88010009
Chicago/Turabian StyleEl-Halim, Shady M. Abd, Mohamed A. Mamdouh, Alaadin E. El-Haddad, and Sara M. Soliman. 2020. "Fabrication of Anti-HSV-1 Curcumin Stabilized Nanostructured Proniosomal Gel: Molecular Docking Studies on Thymidine Kinase Proteins" Scientia Pharmaceutica 88, no. 1: 9. https://doi.org/10.3390/scipharm88010009
APA StyleEl-Halim, S. M. A., Mamdouh, M. A., El-Haddad, A. E., & Soliman, S. M. (2020). Fabrication of Anti-HSV-1 Curcumin Stabilized Nanostructured Proniosomal Gel: Molecular Docking Studies on Thymidine Kinase Proteins. Scientia Pharmaceutica, 88(1), 9. https://doi.org/10.3390/scipharm88010009







