Assessment of “Sameness” and/or Differences between Marketed Creams Containing Miconazole Nitrate Using a Discriminatory in vitro Release Testing (IVRT) Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals and Formulations
2.1.2. HPLC Instrumentation
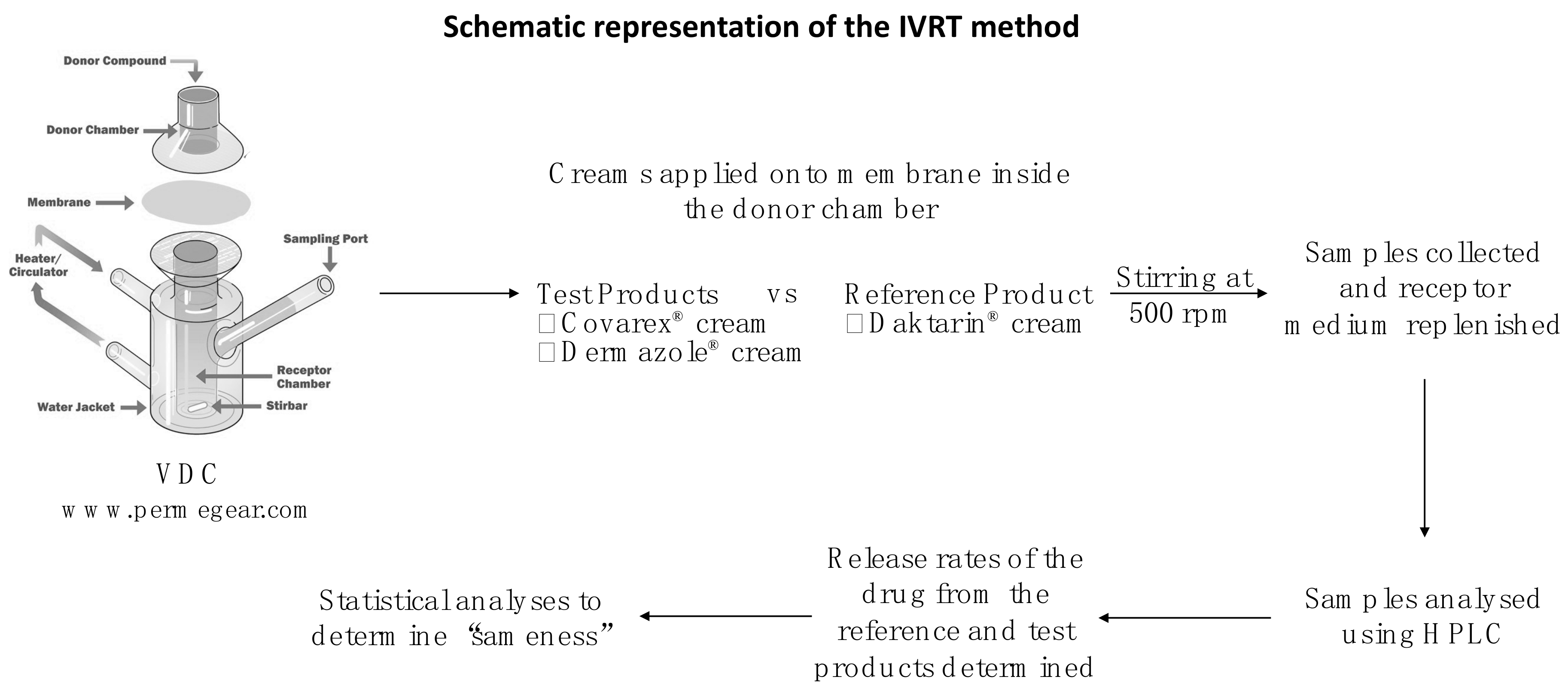
2.1.3. IVRT System
2.2. Methods
2.2.1. HPLC Method Validation
2.2.2. Mechanical Validation
2.2.3. Performance Verification Test (PVT) [15,16]
2.2.4. MCZ Solubility in the Receptor Medium
2.2.5. Membrane Screening
2.2.6. IVRT Method
2.2.7. Calculation of release rates
- Qn = Amount released at time (n) per unit area in µg/cm2
- Cn = Concentration of drug in receptor medium at different sampling times (n) in µg/cm3
- Vs = Volume of the sample in cm3
- Vc = Volume of the cell in cm3
- Ac = Area of the orifice of the cell in cm2
2.2.8. Validation of the IVRT method
- Linearity, Precision, and Reproducibility
- µ = Estimated mean release rate
- = Inter-run standard deviation
- = Intra-run standard deviation
- Sensitivity, Specificity and Selectivity
- Robustness
- Recovery
2.2.9. Assessment of “Sameness”/Differences between Creams Containing 2% MCZ
2.2.10. Application of IVRT
3. Results
3.1. HPLC Method Validation
- Concentration range - 5, 10, 20, 40, 60, 80 and 100 µg/mL were used as calibrators
- Linearity - R2 ≥ 0.999
- Accuracy - CV < 5% using the following concentrations: 20, 100 and 180 µg/mL
- Repeatability - CV < 5% for 30, 50, 70 µg/mL during 3 separate runs
- Inter- and intra-run precision - CV < 5%
- LLOQ and LOD - 1 and 0.33 µg/mL, respectively
- Sample stability - Bench-top (24 ± 1 °C), HPLC sample tray (21 °C ) and refrigerator (4 ± 1 °C) for 9 days
- Specificity - The placebo extract did not show any interfering peaks. In addition, the specificity was proved when the average MCZ content in three different marketed products was analysed and found to be between 95.4% and 101.3% of the label claim. CV < 5%.
3.2. Mechanical Validation
3.3. Performance Verification Test (PVT)
3.4. MCZ Solubility in the Receptor Medium
3.5. Membrane Screening
3.6. Validation of the IVRT Method
- Linearity, Precision, and Reproducibility
- Sensitivity
- Specificity
- Selectivity
- Robustness
- Recovery
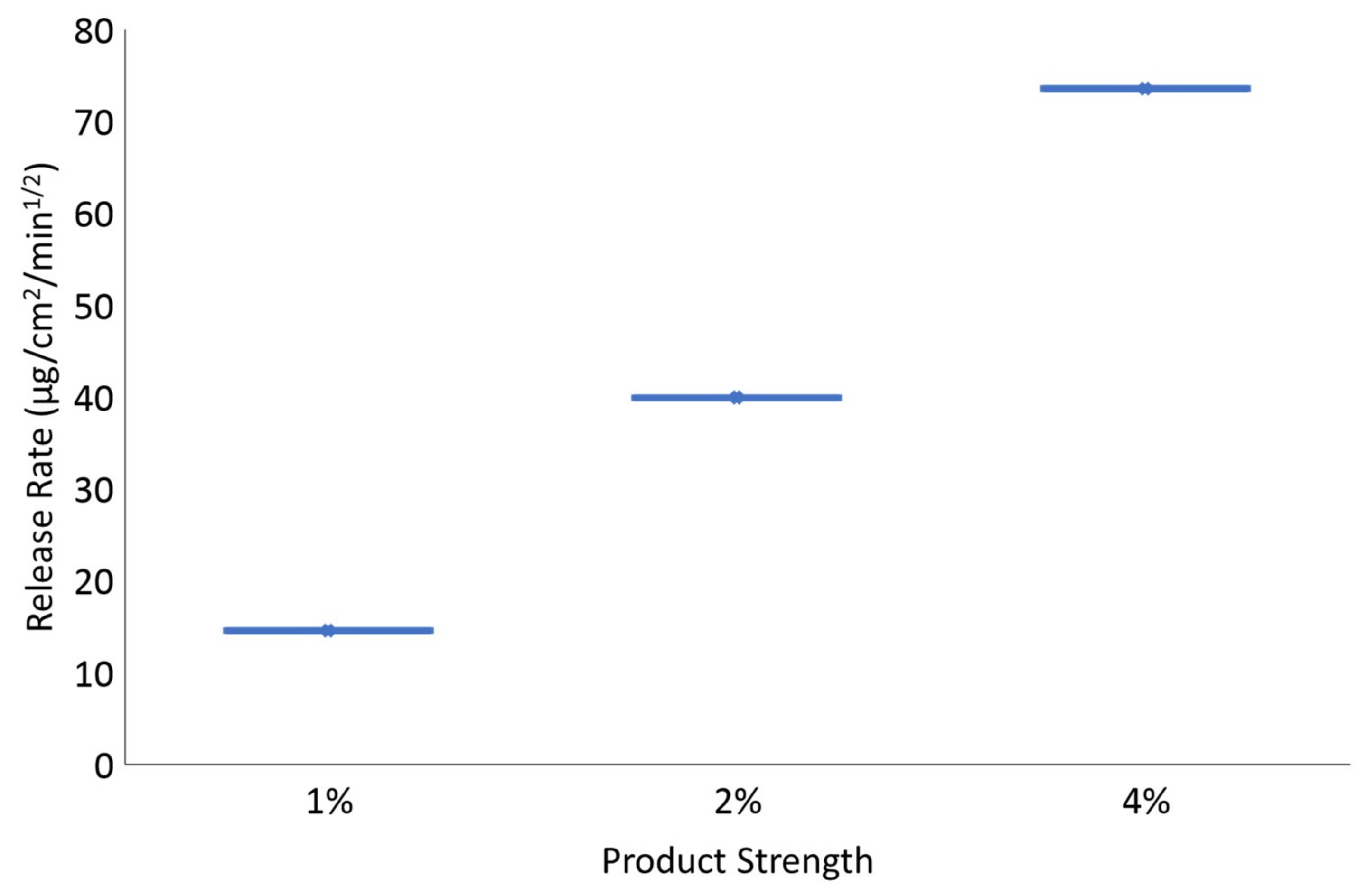
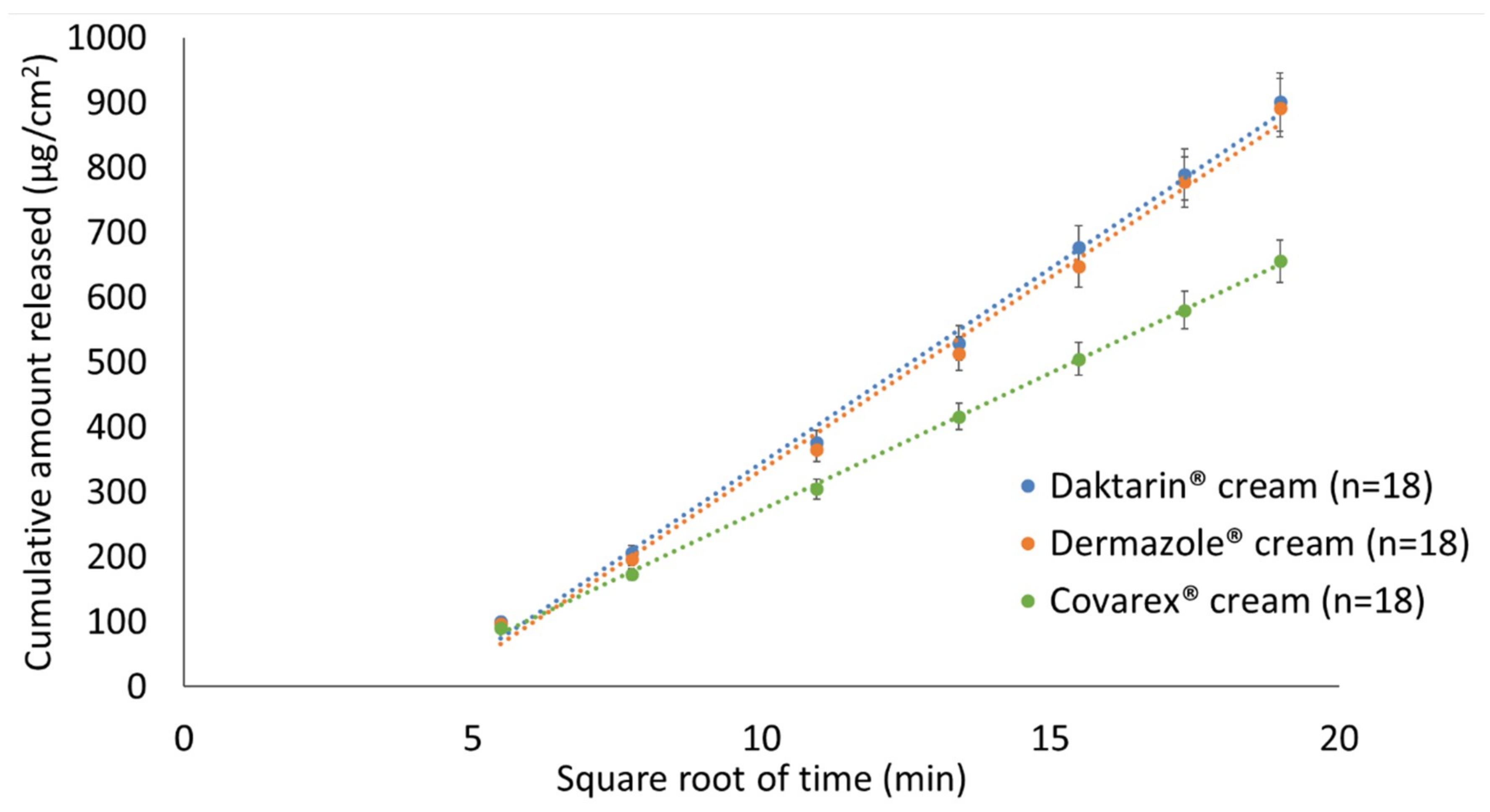
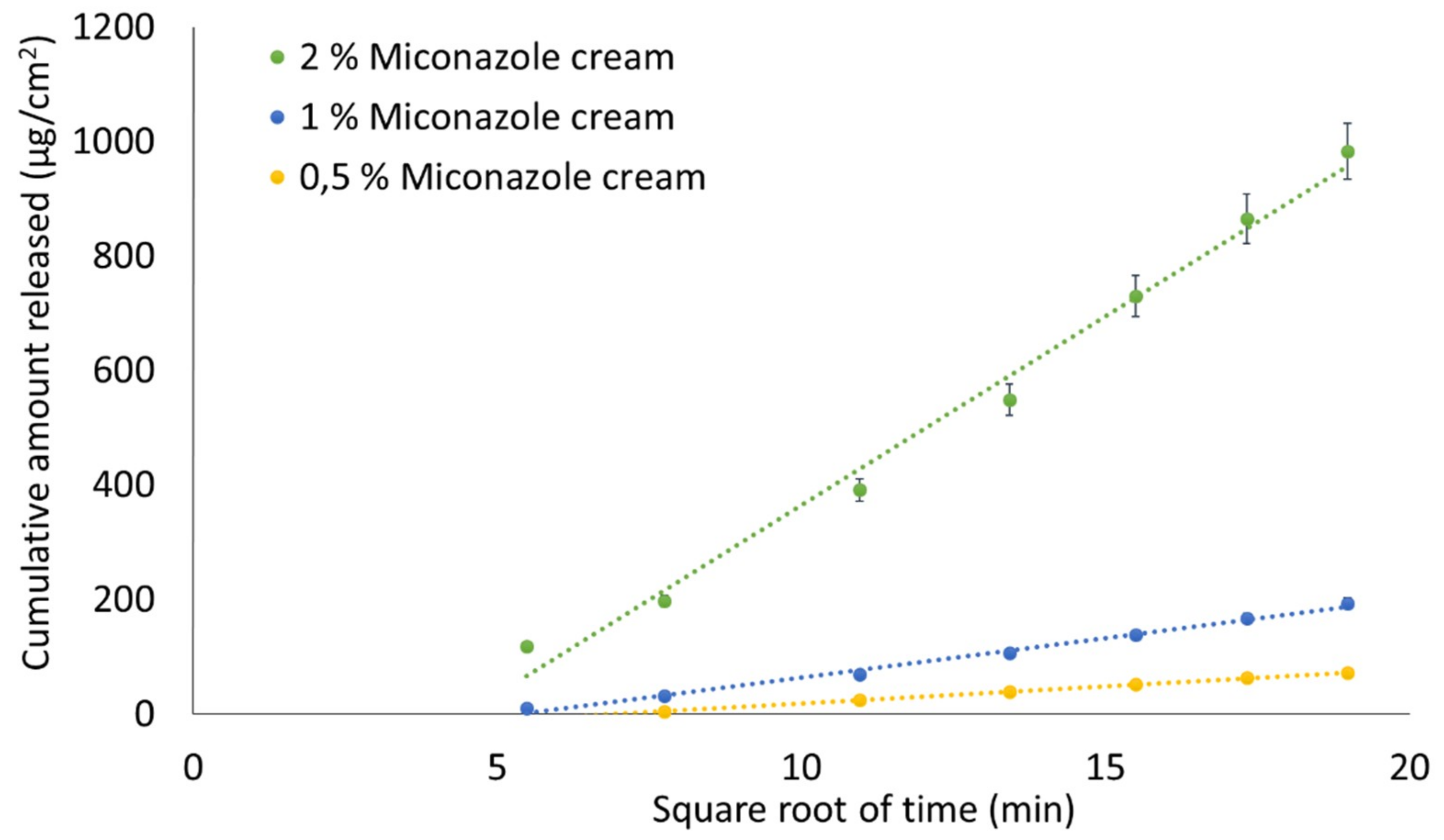
3.7. Assessment of “Sameness”/Differences between Creams Containing 2% MCZ
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nallagundla, S.; Kanfer, I.; Patnala, S. Comparison of in vitro release rates of acyclovir from cream formulations using vertical diffusion cells. AAPS Pharm. Sci. Tech. 2014, 15, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Shargel, L.; Kanfer, I. Drugs and the Pharmaceutical Sciences: Generic Drug Product Development Solid Oral Dosage Forms, 2nd ed.; Taylor & Francis Group: Florida, USA, 2014; Volume 129. [Google Scholar]
- Food and Drugs. Code of Federal Regulations, title 21 § 320.24. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=320.24 (accessed on 30 October 2019).
- Chowhan, Z.T.; Pritchard, R. Release of corticoids from oleaginous ointment bases containing drug in suspension. J. Pharm. Sci. 1975, 64, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, B.J.; Young, E.; Coquilla, V.; Katz, M. Effect of topical vehicle composition on the in vitro release of fluocinolone acetonide and its acetate ester. J. Pharm. Sci. 1968, 57, 928–933. [Google Scholar] [CrossRef] [PubMed]
- The United States Pharmacopeial Convention. USP General Chapter <1724> Semisolid Drug Products—Performance Tests. In United States Pharmacopeia and National Formulary (USP 41-NF 36); The United States Pharmacopeial Convention: North Bethesda, MD, USA, 2018; pp. 7944–7956. [Google Scholar]
- US Food and Drug Administration, Center for Drug Evaluation and Research. Guidance for Industry: Nonsterile Semisolid Dosage Forms, Scale-up and Post Approval Changes, Chemistry, Manufacturing, and Control; In vitro Release Testing and In vivo Bioequivalence Documentation (SUPAC-SS); US Food and Drug Administration, Center for Drug Evaluation and Research: Rockville, MD, USA, 1997. [Google Scholar]
- US Food and Drug Administration. Draft Guidance on Acyclovir. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/psg/Acyclovir_oint_18604_RC03-12.pdf (accessed on 30 October 2019).
- US Food and Drug Administration. Draft Guidance on Dapsone Gel. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/psg/Dapsone_Topical%20gel_NDA%20207154_RV%20Nov%202018.pdf (accessed on 30 October 2019).
- US Food and Drug Administration. Draft Guidance on Acyclovir Cream. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/psg/Acyclovir_topical%20cream_RLD%2021478_RV12-16.pdf (accessed on 30 October 2019).
- US Food and Drug Administration. Draft Guidance on Docosanol Topical Cream. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/psg/Docosanol_topical%20cream_NDA%20020941_RC08-17.pdf (accessed on 30 October 2019).
- European Medicines Agency. Committee for Medicinal Products for Human Use. Draft guideline on quality and equivalence of topical products. London, UK. 2018. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-quality-equivalence-topical-products_en.pdf (accessed on 30 October 2019).
- Aiache, J.; Aoyagi, N.; Bashaw, D.; Brown, C.; Brown, W.; Burgess, D.; Siewert, M.; Dressman, J.; Shah, V.P.; Crison, J.; et al. FIP/ AAPS Guidelines to Dissolution/ In Vitro Release Testing of Novel/Special Dosage Forms. AAPS Pharm. Sci. Tech. 2003, 4, 1–10. [Google Scholar]
- US Food and Drug Administration, Center for Drug Evaluation and Research. Guidance for Industry: Q2B Validation of Analytical Procedures: Methodology; US Food and Drug Administration, Center for Drug Evaluation and Research: Rockville, MD, USA, 1996. [Google Scholar]
- Ueda, C.T.; Shah, V.P.; Derdzinski, K.; Ewing, G.; Flynn, G.; Maibach, H.; Marques, M.; Rytting, H.; Shaw, S.; Thakker, K.; et al. Topical and Transdermal Drug Products. Pharmacopeial Forum 2009, 35, 750–764. [Google Scholar] [CrossRef]
- Klein, R.R.; Bechtel, J.L.; Burchett, K.; Thakker, K.D. Hydrocortisone as a Standard for In Vitro Release Testing Hydrocortisone as a Standard for In Vitro Release Testing. Dissolution Technol. 2010, 17, 37–38. [Google Scholar] [CrossRef]
- The United States Pharmacopeial Convention. USP General Chapter <711> Dissolution. In United States Pharmacopeia and National Formulary (USP 41-NF 36); The United States Pharmacopeial Convention: North Bethesda, MD, USA, 2018. [Google Scholar]
- Hauck, W.W.; Shah, V.P.; Shaw, S.W.; Ueda, C.T. Reliability and reproducibility of vertical diffusion cells for determining release rates from semisolid dosage forms. Pharm. Res. 2007, 24, 2018–2024. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.R.; Heckart, J.L.; Thakker, K.D. In vitro release testing methodology and variability with the vertical diffusion cell (VDC). Dissolution Technol. 2018, 25, 52–61. [Google Scholar] [CrossRef]
- Higuchi, W.I. Analysis of data on the medicament release from ointments. J. Pharm. Sci. 1962, 51, 802–804. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T. Rate of Release of Medicaments from Ointment Bases Containing Drugs in Suspension. J. Pharm. Sci. 1961, 50, 874–875. [Google Scholar] [CrossRef] [PubMed]
- Richardson, N.; Ethier, A.; Romanski, F. The effect of excipient selection on the microstructure and performance attributes of Topical Semi-solid emulsion. In Optimizing Topical Semi-Solid Drug Formulation and Performance; Pharm Tech: London, UK, 2016. [Google Scholar]
| Test | Min | Max | V | Acceptance Criteria | Pass |
|---|---|---|---|---|---|
| Bench top levelness | - | - | - | not more than 1° | Yes |
| Capacity of the cells | 11.98 mL | 12.04mL | 0.06 mL | Yes | |
| Temperature of the receptor medium | 32.0 °C | 32.3 °C | 0.3 °C | Yes | |
| Diameter of the orifice of the cell | 14.90 mm | 15. 32 mm | 0.42 mm | Yes | |
| Dispensed sampling volume | 200 µL | 200 µL | - | Yes | |
| Stirring speed | 499.6 rpm | 500.2 rpm | 0.6 | Yes |
| Parameter | Acceptance Criteria | Results |
|---|---|---|
| Intra-run variability | CV for the first run (n = 6 VDCs) <15% | 3.01% |
| CV for the second run (n = 6 VDCs) <15% | 11.90% | |
| Inter-run variability | CV for both runs (n = 12 VDCs) <15% | 8.87% |
| Product “sameness” testing | The 90% CI must fall within the limits of 75–133.33% | Lower limit: 88.41% Upper limit: 113.37% |
| Pairwise Comparison | Computed 90% CI | “Sameness” Confirmed | |
|---|---|---|---|
| Lower Limit | Upper Limit | ||
| MCZ cream 1% vs MCZ cream 2% | 25.25 | 51.65 | No |
| MCZ cream 4% vs MCZ cream 2% | 165.43 | 210.76 | No |
| Daktarin® cream (2%) (Run 1) vs Daktarin® cream (2%) (Run 2) | 83.39 | 104.20 | Yes |
| Daktarin® cream (2%) (Run 1) vs Daktarin® cream (2%) (Run 3) | 88.88 | 112.88 | Yes |
| Daktarin® cream (2%) (Run 2) vs Daktarin® cream (2%) (Run 3) | 93.29 | 122.03 | Yes |
| Pairwise Comparison | Computed 90% CI | “Sameness” Confirmed | |
|---|---|---|---|
| Lower Limit | Upper Limit | ||
| Daktarin® Cream (2%) vs Dermazole® cream (2%) | 93.93 | 103.74 | Yes |
| Daktarin® cream (2%) vs Covarex® cream (2%) | 61.93 | 72.98 | No |
| Dermazole® cream (2%) vs Covarex® Cream (2%) | 68.26 | 74.50 | No |
| Daktarin® cream (2%) vs Daktarin® cream (2%) | 97.10 | 113.39 | Yes |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purazi, P.; Rath, S.; Ramanah, A.; Kanfer, I. Assessment of “Sameness” and/or Differences between Marketed Creams Containing Miconazole Nitrate Using a Discriminatory in vitro Release Testing (IVRT) Method. Sci. Pharm. 2020, 88, 6. https://doi.org/10.3390/scipharm88010006
Purazi P, Rath S, Ramanah A, Kanfer I. Assessment of “Sameness” and/or Differences between Marketed Creams Containing Miconazole Nitrate Using a Discriminatory in vitro Release Testing (IVRT) Method. Scientia Pharmaceutica. 2020; 88(1):6. https://doi.org/10.3390/scipharm88010006
Chicago/Turabian StylePurazi, Potiwa, Seeprarani Rath, Ashmita Ramanah, and Isadore Kanfer. 2020. "Assessment of “Sameness” and/or Differences between Marketed Creams Containing Miconazole Nitrate Using a Discriminatory in vitro Release Testing (IVRT) Method" Scientia Pharmaceutica 88, no. 1: 6. https://doi.org/10.3390/scipharm88010006
APA StylePurazi, P., Rath, S., Ramanah, A., & Kanfer, I. (2020). Assessment of “Sameness” and/or Differences between Marketed Creams Containing Miconazole Nitrate Using a Discriminatory in vitro Release Testing (IVRT) Method. Scientia Pharmaceutica, 88(1), 6. https://doi.org/10.3390/scipharm88010006





