Potential Role of Microfibrillar-Associated Protein 4, Fibrotic Indices and Oxidative Stress in Hepatocellular Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Blood Sampling
2.3. Assessment of Biochemical Parameters
2.4. Statistical Analysis
3. Results
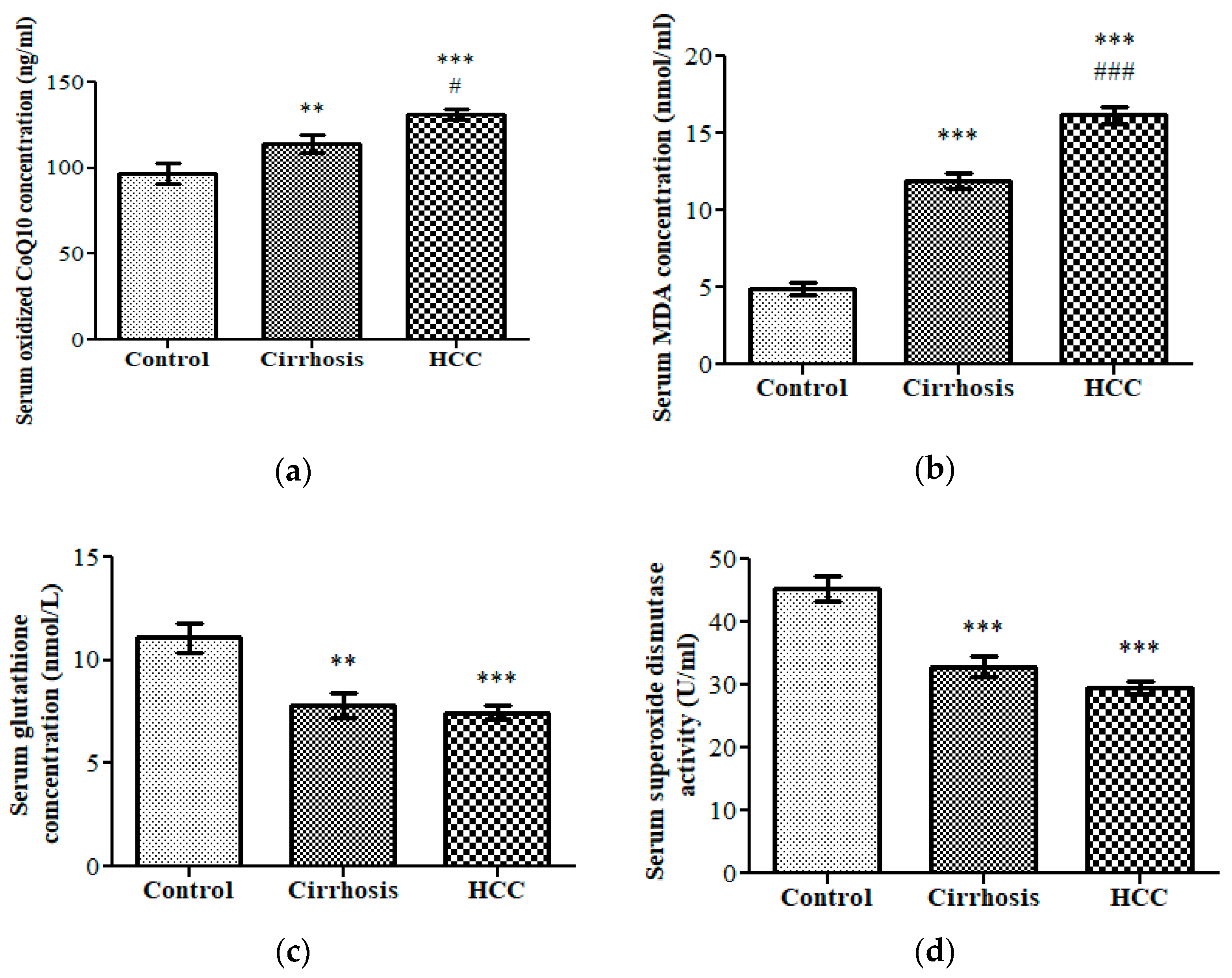
3.1. Oxidative Stress Status
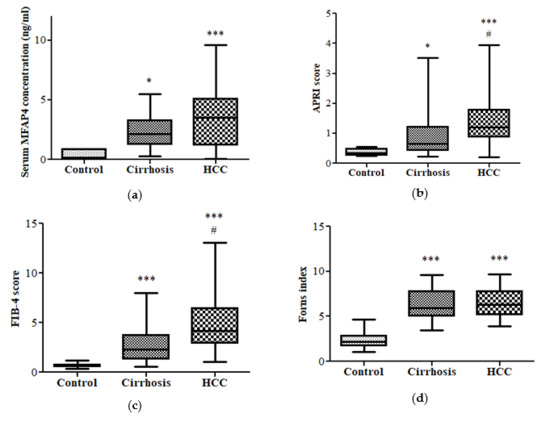
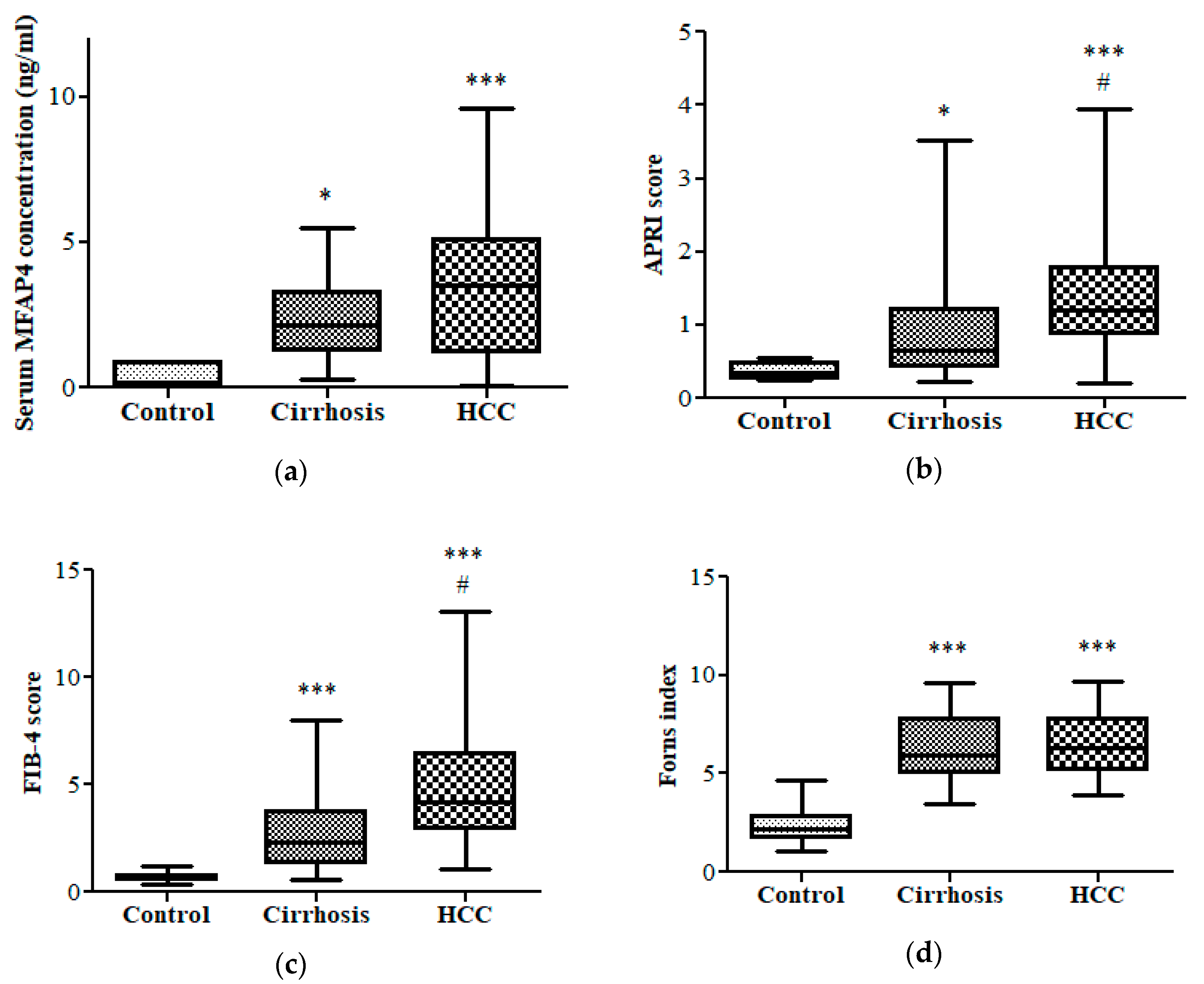
3.2. Fibrotic Biomarkers
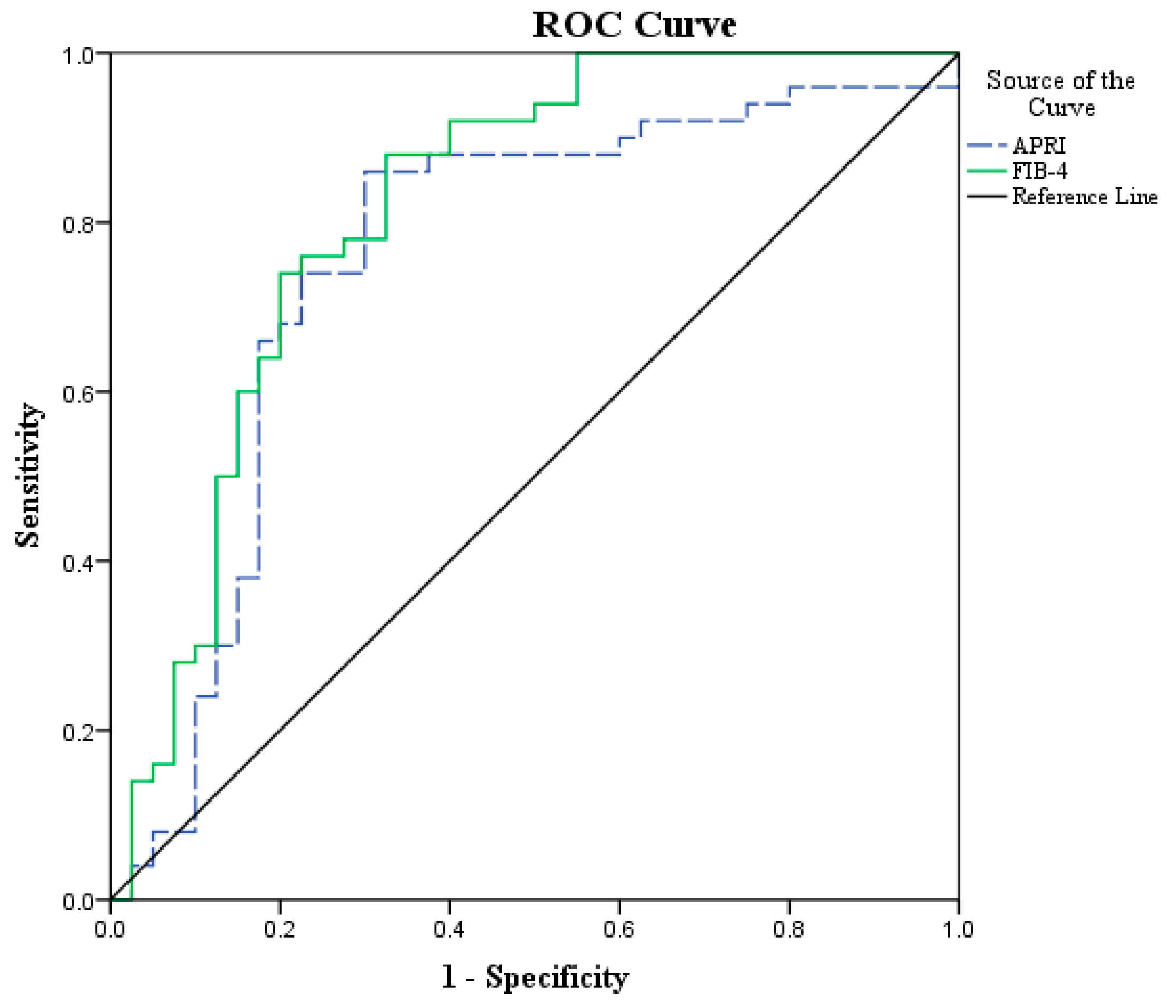
3.3. Diagnostic Performance of APRI and FIB-4 in HCC vs. Cirrhotic Patients and Control Group
3.4. Diagnostic Performance of MFAP4 and Oxidized CoQ10 for the Prediction of Dead HCC Patients
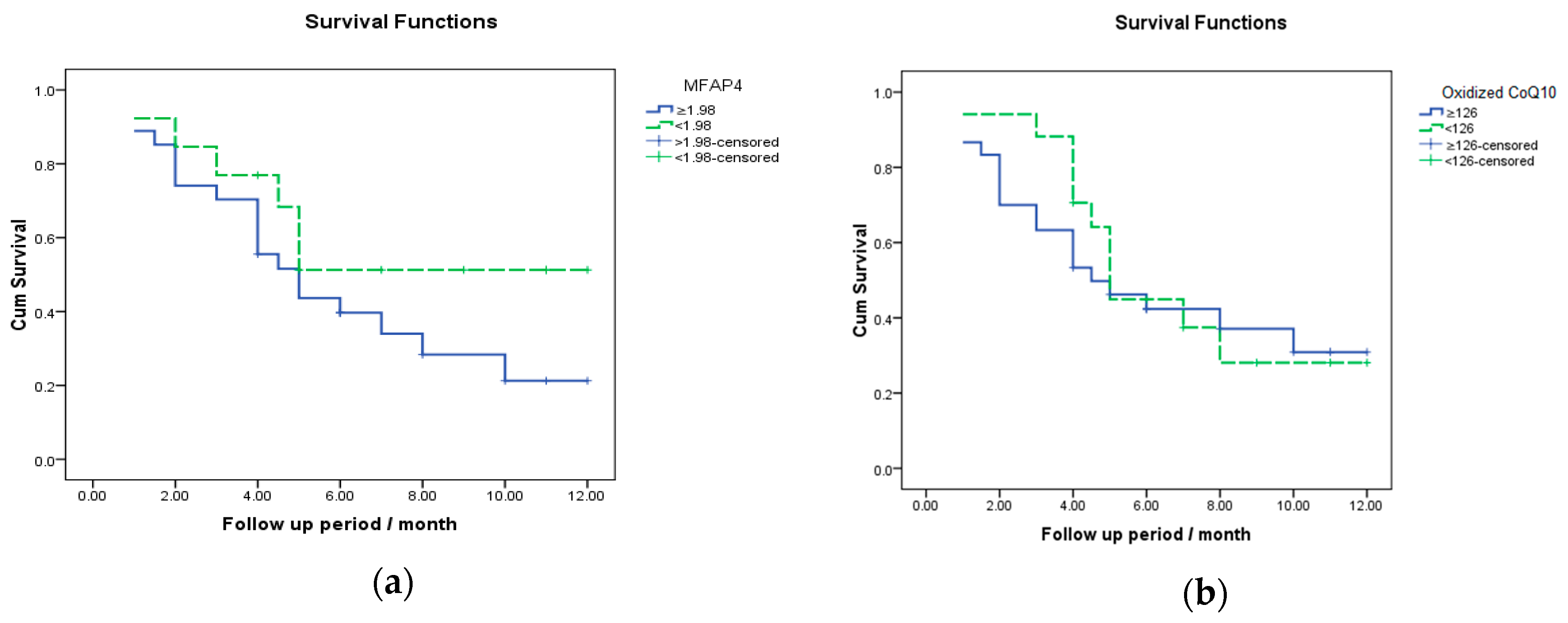
3.5. Survival Analysis for MFAP4 and Oxidized CoQ10 in the Prediction of Death in HCC Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.S.; Khaled, H.M.; Mikhail, N.N.; Baraka, H.; Kamel, H. Cancer incidence in Egypt: Results of the national population-based cancer registry program. J. Cancer Epidemiol. 2014, 2014, 18. [Google Scholar] [CrossRef] [PubMed]
- Goldman, R.; Ressom, H.W.; Abdel-Hamid, M.; Goldman, L.; Wang, A.; Varghese, R.S.; An, Y.; Loffredo, C.A.; Drake, S.K.; Eissa, S.A.; et al. Candidate markers for the detection of hepatocellular carcinoma in low-molecular weight fraction of serum. Carcinogenesis 2007, 28, 2149–2153. [Google Scholar] [CrossRef] [PubMed]
- Nouroz, F.; Shaheen, S.; Mujtaba, G.; Noreen, S. An overview on hepatitis C virus genotypes and its control. Egypt. J. Med. Hum. Genet. 2015, 16, 291–298. [Google Scholar] [CrossRef]
- Hoshida, Y.; Fuchs, B.C.; Bardeesy, N.; Baumert, T.F.; Chung, R.T. Pathogenesis and prevention of hepatitis C virus-induced hepatocellular carcinoma. J. Hepatol. 2014, 61, S79–S90. [Google Scholar] [CrossRef] [PubMed]
- Hernandez–Gea, V.; Toffanin, S.; Friedman, S.L.; Llovet, J.M. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology 2013, 144, 512–527. [Google Scholar] [CrossRef] [PubMed]
- Loguercio, C.; Federico, A. Oxidative stress in viral and alcoholic hepatitis. Free Radic. Biol. Med. 2003, 34, 1–10. [Google Scholar] [CrossRef]
- Arauz, J.; Ramos-Tovar, E.; Muriel, P. Redox state and methods to evaluate oxidative stress in liver damage: From bench to bedside. Ann. Hepatol. 2016, 15, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, G.; Gkouvatsos, K.; Pantopoulos, K. Chronic hepatitis C and liver fibrosis. World J. Gastroenterol. 2014, 20, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Sepulveda, S.A.; Bryan-Marrugo, O.L.; Cordova-Fletes, C.; Gutierrez-Ruiz, M.C.; Rivas-Estilla, A.M. Oxidative stress modulation in hepatitis C virus infected cells. World J. Hepatol. 2015, 7, 2880–2889. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Maraver, J.; Cordero, M.D.; Oropesa-Ávila, M.; Fernández Vega, A.; De La Mata, M.; Delgado Pavón, A.; De Miguel, M.; Pérez Calero, C.; Villanueva Paz, M.; Cotán, D. Coenzyme Q10 therapy. Mol. Syndromol. 2014, 5, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Navas, P.; Villalba, J.M.; de Cabo, R. The importance of plasma membrane coenzyme Q in aging and stress responses. Mitochondrion 2007, 7, S34–S40. [Google Scholar] [CrossRef] [PubMed]
- Miles, M.V.; Horn, P.S.; Morrison, J.A.; Tang, P.H.; DeGrauw, T.; Pesce, A.J. Plasma coenzyme Q 10 reference intervals, but not redox status, are affected by gender and race in self-reported healthy adults. Clin. Chim. Acta 2003, 332, 123–132. [Google Scholar] [CrossRef]
- Bose, S.K.; Ray, R. Hepatitis C virus infection and insulin resistance. World J. Diabetes 2014, 5, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Wai, C.T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S.F. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Forns, X.; Ampurdanes, S.; Llovet, J.M.; Aponte, J.; Quintó, L.; Martínez-Bauer, E.; Bruguera, M.; Sánchez-Tapias, J.M.; Rodés, J. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology 2002, 36, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Jiang, J.H.; Yang, Y.T.; Guo, Z.; Li, J.J.; Liu, X.H.; Lu, F.; Zeng, F.H.; Ye, J.S.; Zhang, K.L.; et al. Stratified aspartate aminotransferase-to-platelet ratio index accurately predicts survival in hepatocellular carcinoma patients undergoing curative liver resection. Tumour Biol. 2017, 39. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Li, C.; Wen, T.F.; Yan, L.N.; Li, B.; Wang, W.T.; Yang, J.Y.; Xu, M.Q. Postoperative aspartate aminotransferase to platelet ratio index change predicts prognosis for hepatocellular carcinoma. Medicine (Baltim.) 2016, 95, e4160. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Nishijima, N.; Enomoto, H.; Sakamoto, A.; Nasu, A.; Komekado, H.; Nishimura, T.; Kita, R.; Kimura, T.; Iijima, H.; et al. Comparison of FIB-4 index and aspartate aminotransferase to platelet ratio index on carcinogenesis in chronic hepatitis B treated with entecavir. J. Cancer 2017, 8, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.J.; Tseng, C.W.; Chang, T.T.; Tzeng, S.J.; Hsieh, Y.H.; Hung, T.H.; Huang, H.T.; Wu, S.F.; Tseng, K.C. Aspartate aminotransferase to platelet ratio index and sustained virologic response are associated with progression from hepatitis C associated liver cirrhosis to hepatocellular carcinoma after treatment with pegylated interferon plus ribavirin. Clin. Interv. Aging 2016, 11, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Mölleken, C.; Sitek, B.; Henkel, C.; Poschmann, G.; Sipos, B.; Wiese, S.; Warscheid, B.; Broelsch, C.; Reiser, M.; Friedman, S.L. Detection of novel biomarkers of liver cirrhosis by proteomic analysis. Hepatology 2009, 49, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Pilecki, B.; Holm, A.T.; Schlosser, A.; Moeller, J.B.; Wohl, A.P.; Zuk, A.V.; Heumüller, S.E.; Wallis, R.; Moestrup, S.K.; Sengle, G. Characterization of microfibrillar-associated protein 4 (MFAP4) as a tropoelastin-and fibrillin-binding protein involved in elastic fiber formation. J. Biol. Chem. 2016, 291, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Yasui, Y.; Abe, T.; Kurosaki, M.; Higuchi, M.; Komiyama, Y.; Yoshida, T.; Hayashi, T.; Kuwabara, K.; Takaura, K.; Nakakuki, N.; et al. Elastin Fiber Accumulation in Liver Correlates with the Development of Hepatocellular Carcinoma. PLoS ONE 2016, 11, e0154558. [Google Scholar] [CrossRef]
- Saekmose, S.G.; Mossner, B.; Christensen, P.B.; Lindvig, K.; Schlosser, A.; Holst, R.; Barington, T.; Holmskov, U.; Sorensen, G.L. Microfibrillar-Associated Protein 4: A Potential Biomarker for Screening for Liver Fibrosis in a Mixed Patient Cohort. PLoS ONE 2015, 10, e0140418. [Google Scholar] [CrossRef] [PubMed]
- Bracht, T.; Mölleken, C.; Ahrens, M.; Poschmann, G.; Schlosser, A.; Eisenacher, M.; Stühler, K.; Meyer, H.E.; Schmiegel, W.H.; Holmskov, U. Evaluation of the biomarker candidate MFAP4 for non-invasive assessment of hepatic fibrosis in hepatitis C patients. J. Transl. Med. 2016, 14, 201. [Google Scholar] [CrossRef] [PubMed]
- Pugh, R.; Murray-Lyon, I.; Dawson, J.; Pietroni, M.; Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y. Coenzyme Q10 as a front-line antioxidant against oxidative stress. J. Clin. Biochem. Nutr. 2005, 36, 29–35. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Yamashita, S.; Fujisawa, A.; Kokura, S.; Yoshikawa, T. Oxidative stress in patients with hepatitis, cirrhosis, and hepatoma evaluated by plasma antioxidants. Biochem. Biophys. Res. Commun. 1998, 247, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Ngoka, L.C. Dramatic down-regulation of oxidoreductases in human hepatocellular carcinoma hepG2 cells: Proteomics and gene ontology unveiling new frontiers in cancer enzymology. Proteome Sci. 2008, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Nishitsuji, H.; Funami, K.; Shimizu, Y.; Ujino, S.; Sugiyama, K.; Seya, T.; Takaku, H.; Shimotohno, K. Hepatitis C virus infection induces inflammatory cytokines and chemokines mediated by the cross talk between hepatocytes and stellate cells. J. Virol. 2013, 87, 8169–8178. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.H.K.; Omrani, M.-D.; Kheradmand, F. Oxidative stress response in patients infected by diverse hepatitis C virus genotypes. Hepat. Mon. 2015, 15, e22069. [Google Scholar] [CrossRef]
- Yahya, R.S.; Ghanem, O.H.; Foyouh, A.; Atwa, M.; Enany, S.A. Role of interleukin-8 and oxidative stress in patients with hepatocellular carcinoma. Clin. Lab. 2013, 59, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Elewa, M.A.; Al-Gayyar, M.M.; Schaalan, M.F.; El Galil, K.H.A.; Ebrahim, M.A.; El-Shishtawy, M.M. Hepatoprotective and anti-tumor effects of targeting MMP-9 in hepatocellular carcinoma and its relation to vascular invasion markers. Clin. Exp. Metastasis 2015, 32, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Levent, G.; Ali, A.; Ahmet, A.; Polat, E.C.; Aytac, C.; Ayse, E.; Ahmet, S. Oxidative stress and antioxidant defense in patients with chronic hepatitis C patients before and after pegylated interferon alfa-2b plus ribavirin therapy. J. Transl. Med. 2006, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, H.; Koruk, M.; Akcay, F. The Relationship Between Serum Malondialdehyde and Ceruloplasmin in Chronic Liver Disease. Turk. J. Biochem. 2003, 28, 32–34. [Google Scholar]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef] [PubMed]
- De Nardo, D.; Labzin, L.I.; Kono, H.; Seki, R.; Schmidt, S.V.; Beyer, M.; Xu, D.; Zimmer, S.; Lahrmann, C.; Schildberg, F.A. High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat. Immunol. 2014, 15, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Hanigan, M.H. Gamma-Glutamyl Transpeptidase: Redox Regulation and Drug Resistance. Adv. Cancer Res. 2014, 122, 103–141. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Tsai, W.-L.; Shao, R.-X.; Wu, G.; Peng, L.F.; Barlow, L.L.; Chung, W.J.; Zhang, L.; Zhao, H.; Jang, J.-Y.; et al. HCV regulates TGF-β1 production through the generation of reactive oxygen species in an NFκB-dependent manner. Gastroenterology 2010, 138, 2509–2518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-Y.; Yuan, W.-G.; He, P.; Lei, J.-H.; Wang, C.-X. Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets. World J. Gastroenterol. 2016, 22, 10512–10522. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Genda, T.; Ichida, T.; Amano, N.; Sato, S.; Murata, A.; Tsuzura, H.; Narita, Y.; Kanemitsu, Y.; Hirano, K. Prediction of Hepatocellular Carcinoma Development after Hepatitis C Virus Eradication Using Serum Wisteria floribunda Agglutinin-Positive Mac-2-Binding Protein. Int. J. Mol. Sci. 2016, 17, 2143. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Zhou, R.; Wang, W.; Bai, D.; He, C.; Cai, Z.; Shen, Y.; Wang, S.; Deng, H.; Li, Z. High Post-treatment alpha-Fetoprotein Levels and Aspartate Aminotransferase-to-Platelet Ratio Index Predict Hepatocellular Carcinoma in Hepatitis C Virus Decompensated Cirrhotic Patients with Sustained Virological Response After Antiviral Therapy. J. Interferon Cytokine Res. 2017, 37, 362–368. [Google Scholar] [CrossRef] [PubMed]
| Variable | No. of Patients (%) | Variable | No. of Patients (%) |
|---|---|---|---|
| Age | Size of lesion | ||
| ≤60 years | 21 (42%) | ≤5 cm | 21 (42%) |
| >60 years | 29 (58%) | >5 cm | 29 (58%) |
| Gender | Portal vein | ||
| Male | 39 (78%) | Thrombosed | 22 (44%) |
| Female | 11 (22%) | Patent | 28 (56%) |
| Number of lesions | Lymph node involvement | ||
| Single | 15 (30%) | N0 | 31 (62%) |
| Multiple | 35 (70%) | N1 | 19 (38%) |
| Site of lesion | Distal metastasis | ||
| Unilobar | 16 (32%) | M0 | 43 (86%) |
| Bilobar | 34 (68%) | M1 | 7 (14%) |
| Parameters | Control (n = 15) | Cirrhosis (n = 25) | HCC (n = 50) |
|---|---|---|---|
| ALT activity (U/L) | 37.0 (20.0–55.0) | 40.22 (28.44–78.5) | 50.25 (36.59–75.66) * |
| AST activity (U/L) | 28.0 (21.0–38.0) | 48.0 (28.15–69.5) * | 70.5 (54.7–92.5) *** ## |
| γ-GT activity (U/L) | 14.0 (11.0–19.7) | 33.6 (17.2–62.5) ** | 33.2 (18.28–53.85) ** |
| Total bilirubin (mg/dL) | 0.61 ± 0.04 | 0.94 ± 0.07 | 1.28 ± 0.07 *** ## |
| Albumin (mg/dL) | 4.48 ± 0.07 | 4.04 ± 0.1 | 3.8 ± 0.09 *** |
| Prothrombin time (s) | 13.05 ± 0.21 | 13.46 ± 0.33 | 14.62 ± 0.28 ** # |
| α-Fetoprotein (ng/mL) | 0.86 ± 0.085 | 9.2 ± 3.35 | 636.3 ± 92.57 *** ### |
| Cholesterol (mg/dL) | 170.7 ± 9.79 | 170.3 ± 7.42 | 177.7 ± 7.72 |
| TAG (mg/dL) | 96.6 ± 15.6 | 115.3 ± 9.46 | 130.4 ± 6.77 |
| LDL (mg/dL) | 103.7 ± 6.81 | 90.8 ± 4.4 | 114.31 ± 7.35 |
| HDL (mg/dL) | 50.39 ± 3.97 | 55.6 ± 3.7 | 37.27 ± 2.0 * ### |
| Variable | AUC | p-Value | Cut Off Point | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|---|
| APRI | 0.76 | <0.001 | ≥0.7 | 86.0 | 70.0 | 78.18 | 80.0 | 78.89 |
| FIB-4 | 0.82 | <0.001 | ≥1.57 | 92.0 | 60.0 | 74.19 | 85.71 | 77.78 |
| Variable | AUC | p-Value | Cut Off Point | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|---|
| MFAP4 | 0.533 | 0.727 | ≥1.98 | 76.0 | 46.7 | 70.4 | 53.8 | 65.0 |
| CoQ10 | 0.525 | 0.791 | ≥126 | 64.0 | 40.0 | 63.3 | 35.3 | 53.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salama, M.M.; Nomir, M.M.; Fahmi, M.W.; El-Gayar, A.M.; El-Shishtawy, M.M. Potential Role of Microfibrillar-Associated Protein 4, Fibrotic Indices and Oxidative Stress in Hepatocellular Carcinoma. Sci. Pharm. 2018, 86, 44. https://doi.org/10.3390/scipharm86040044
Salama MM, Nomir MM, Fahmi MW, El-Gayar AM, El-Shishtawy MM. Potential Role of Microfibrillar-Associated Protein 4, Fibrotic Indices and Oxidative Stress in Hepatocellular Carcinoma. Scientia Pharmaceutica. 2018; 86(4):44. https://doi.org/10.3390/scipharm86040044
Chicago/Turabian StyleSalama, Mohamed M., Manal M. Nomir, Maryan W. Fahmi, Amal M. El-Gayar, and Mamdouh M. El-Shishtawy. 2018. "Potential Role of Microfibrillar-Associated Protein 4, Fibrotic Indices and Oxidative Stress in Hepatocellular Carcinoma" Scientia Pharmaceutica 86, no. 4: 44. https://doi.org/10.3390/scipharm86040044
APA StyleSalama, M. M., Nomir, M. M., Fahmi, M. W., El-Gayar, A. M., & El-Shishtawy, M. M. (2018). Potential Role of Microfibrillar-Associated Protein 4, Fibrotic Indices and Oxidative Stress in Hepatocellular Carcinoma. Scientia Pharmaceutica, 86(4), 44. https://doi.org/10.3390/scipharm86040044