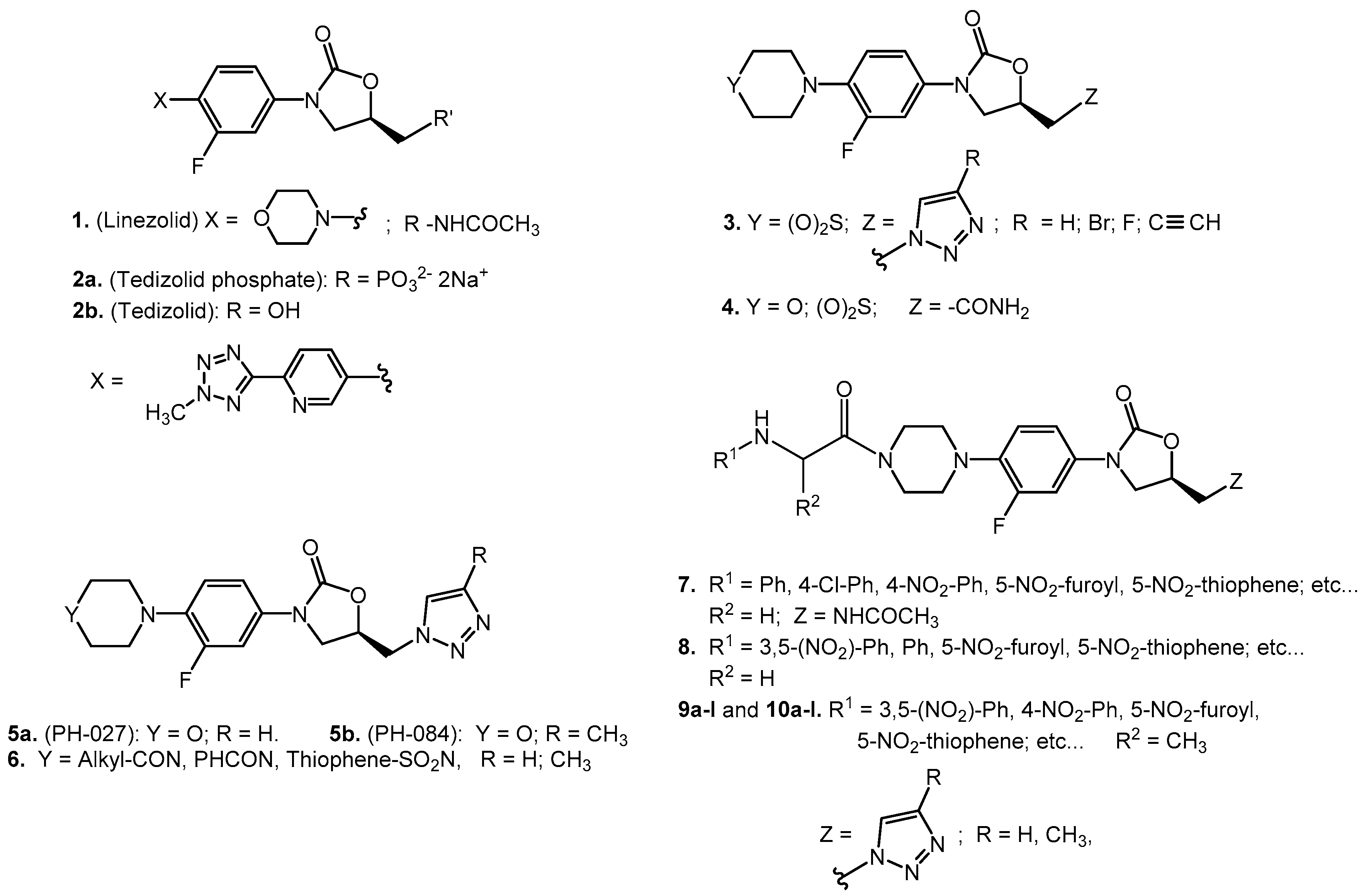
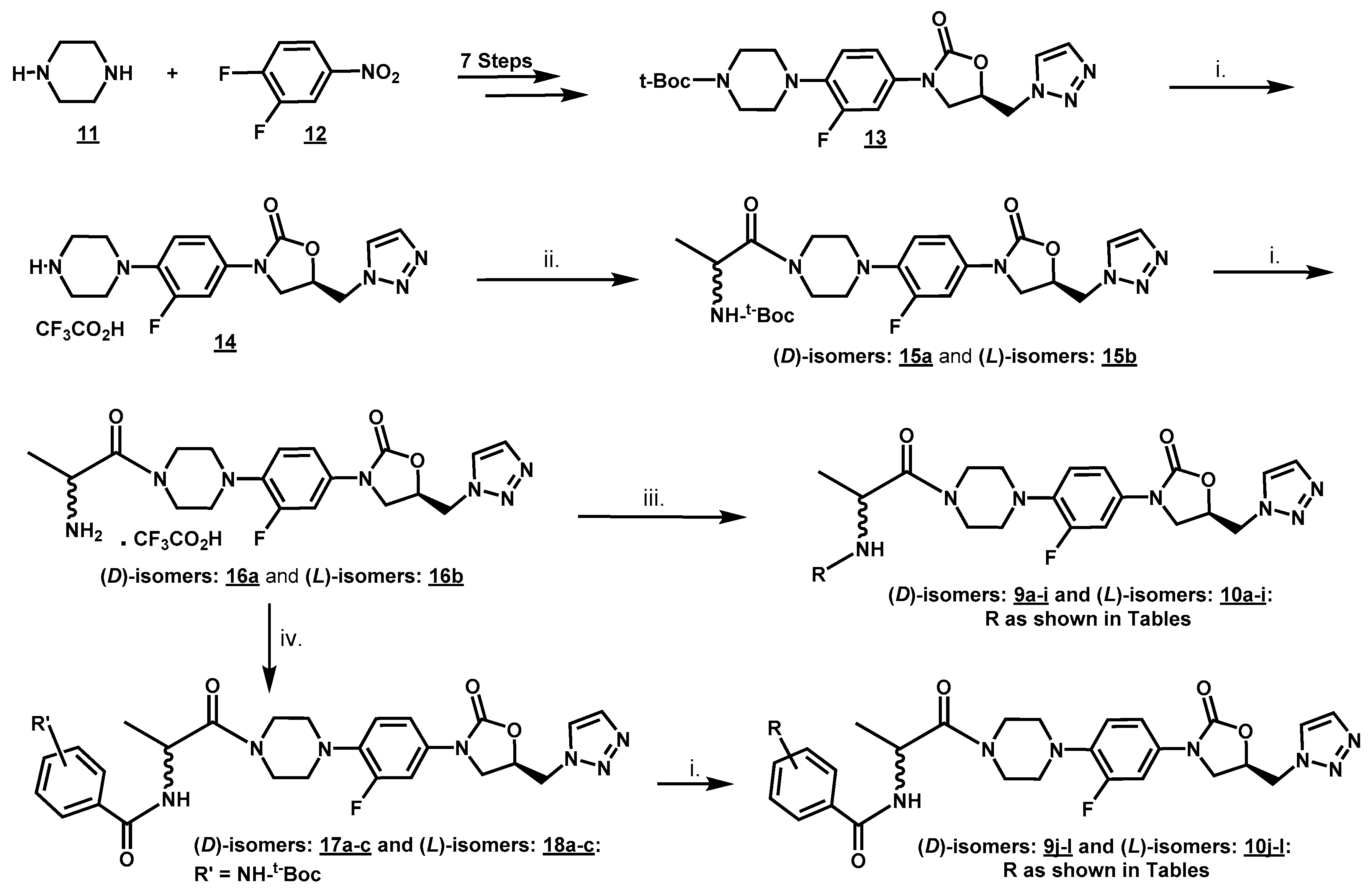
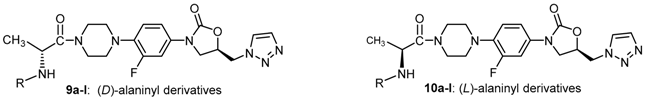
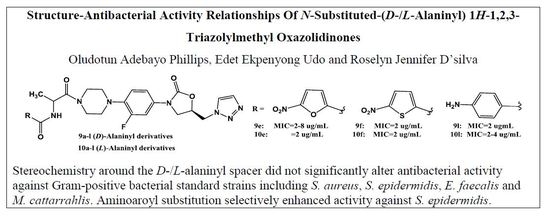
2.2. Syntheses
2.2.1. Preparation of (R)-5-((1H-1,2,3-triazol-1-yl)methyl)-3-(4-(4-(D-alanyl)piperazin-1-yl)-3-fluorophenyl)oxazolidin-2-one 2,2,2-trifluoroacetate 16a
A solution of (tert-butoxycarbonyl)-D-alanine (1.027 gm, 5.430 mmol) in anhydrous DCM (35 mL) was treated with N, N’-dicyclohexylcarbodiimide (1.40 gm, 6.787 mmol) and 1-hydroxybenzotriazole (0.917 gm, 6.787 mmol), and the mixture was stirred under a nitrogen atmosphere for 2 h. The reaction mixture was filtered directly into a solution of the trifluoro acetate salt, 14 (2.50 gm, 5.430 mmol) and TEA (2.19 mL, 15.747 mmol) in anhydrous CH3CN (40 mL) at 0 °C. The reaction mixture was stirred overnight and concentrated on a rotavap under vacuum to give a viscous oil, which was dissolved in DCM (40 mL), and the precipitated urea was filtered off. The DCM layer was washed with water, 10% Na2CO3 solution, brine, dried (Na2SO4), filtered and concentrated under vacuum to give a brown foam, which was triturated with ether to yield a cream-colored crude solid. This solid was recrystallized from ethyl acetate to afford the carbamate 15a as an off-white solid, 2.07 gm, yield: 74%; mp.: 214–216 °C. 1H-NMR (DMSO-d6, 600 MHZ): δ 8.18 (d, 1H, J = 0.7 Hz, triazole H), 7.77 (d, 1H, J = 0.8 Hz, triazole H), 7.43 (dd, 1H J = 2.5 Hz, 14.7 Hz, phenyl H), 7.14 (dd, 1H, J = 2.3 Hz, 8.8 Hz, phenyl H), 7.07 (t, 1H, J = 9.3 Hz, phenyl H), 6.96 (d, 1H, NH, J = 7.9 Hz, exchangeable with D2O), 5.12-5.14 (m, 1H, oxazolidinone H), 4.83 (d, 2H, J = 5.1 Hz, CH2), 4.45–4.50 (m, 1H, d-alanine CH), 4.21 (t, 1H, J = 9.2 Hz, oxazolidione H), 3.87 (q, 1H, J = 5.8 Hz, 9.3 Hz, oxazolidinone H), 3.59–3.66 (br. d, 4H, piperazine H), 2.96 (br. d, 4H, piperazine H), 1.36–1.38 (br., 9H, C(CH3)3), 1.17 (d, 3H, J = 6.9 Hz, CH3)). 13C-NMR (DMSO-d6): δ 171.2, 155.8, 155.3, 154.2, 153.9, 135.8, 133.9, 133.8, 133.7, 126.3, 120.2, 120.2, 114.8, 114.7, 107.3, 107.1, 78.4, 71.3, 52.2, 51.1, 50.8, 47.6, 46.3, 45.2, 42.0, 28.7, 18.2. IR (KBr pellet, cm−1): ν 3328, 3185, 3125, 2929, 2792, 1738, 1628, 1574, 1536, 1386, 1311, 1271, 1462, 1244, 1185, 1088, 1047. LRMS (m/z): 517.3 (M+), HRMS (m/z): calcd. for C24H32FN7O5: 517.2449; found 540.2400 (M+ + Na). Anal. calcd. for C24H32FN7O5: C: 55.70, H: 6.23, N: 18.94; found C: 55.75, H: 6.56, N: 18.41.
A solution of 15a (6.07 g, 11.73 mmol) in DCM (12.0 mL) was treated with trifluoroacetic acid (12.0 mL) and stirred to room temperature overnight. The reaction mixture was concentrated to dryness to give a brown oil, which was digested several times with ether and treated with THF/diethyl ether (1:1 mixture) with stirring to give 16a as a cream-colored solid, 4.92 g, yield: 79%. This solid was utilized for subsequent reactions without further purification. 1H-NMR (DMSO-d6, 600 MHZ): δ 8.17 (d, 1H, J = 0.7 Hz, triazole H), 8.13 (br. d, 3H, J = 3.8 Hz, +NH3, exchangeable with D2O), 7.76 (d, 1H, J = 0.8 Hz, triazole H), 7.42 (dd, 1H, J = 2.5 Hz, 14.7 Hz, phenyl H), 7.12 (dd, 2H, J = 2.3 Hz, 8.8 Hz, phenyl H), 7.07 (t, 1H, J = 9.3 Hz, phenyl H), 5.10–5.14 (m, 1H, oxazolidinone H), 4.83 (d, 1H, J = 5.0 Hz, CH2), 4.42–4.44 (m, 1H, d-alanine CH), 4.22 (t, 1H, J = 9.2 Hz, oxazolidinone H), 3.86 (q, 1H, J = 5.8 Hz, 9.3 Hz, oxazolidinone H), 3.56–3.79 (m, 4H, piperazine H), 2.91–3.03 (m, 4H, piperazine H), 1.33 (d, 3H, J = 7.0 Hz, CH3)). 13C-NMR (DMSO-d6, 600 MHZ): δ 168.0, 158.5, 158.3, 158.1, 157.9, 155.4, 153.8, 153.5, 135.2, 135.2, 135.2, 133.5, 133.4, 125.9, 119.9, 119.8, 117.5, 115.6, 114.6, 113.6, 106.9, 106.7, 70.8, 51.7, 50.5, 50.1, 47.1, 45.9, 44.8, 41.7, 16.4. LRMS (m/z): calcd. for C19H24FN7O3 (M+ − CF3O2H): 417.30. HRMS (m/z): calcd. for C21H25F4N7O5: 531.1853; found 530.2300 (M+ − H).
2.2.2. Preparation of (R)-5-((1H-1,2,3-triazol-1-yl)methyl)-3-(4-(4-(L-alanyl)piperazin-1-yl)-3-fluorophenyl) oxazolidin-2-one 2,2,2-trifluoroacetate 16b
Compound 16b was prepared via a similar procedure to the D-alaninyl isomer 16a from (tert-butoxycarbonyl)-L-alanine (0.410 gm, 2.172 mmol), N,N′-dicyclohexyl carbodiimide (0.560 gm, 2.715 mmol), 1-hydroxy -benzotriazole (0.366 gm, 2.715 mmol), TFA salt (1.00 gm, 2.172 mmol) and TEA (0.877 mL, 6.30 mmol) in a mixture of DCM (40 mL) and acetonitrile (15 mL) and worked up in a similar manner to give the intermediate compound 15b as an off-white solid, 0.913 g, yield: 82%; recrystallized (EtOAc), mp.: 203–207 °C. 1H-NMR (DMSO-d6, 600 MHZ): δ 8.18 (d, 1H, J = 0.7 Hz, triazole H), 7.77 (d, 1H, J = 0.8 Hz, triazole H), 7.43 (dd, 1H, J = 2.5 Hz, 14.4 Hz, phenyl H), 7.28 (dd,1H, J = 2.3 Hz, 8.8 Hz, phenyl H), 7.06 (t, 1H, J = 9.3 Hz, phenyl H), 7.00 (br. d, 1H, J = 7.9 Hz, NH, exchangeable with D2O), 5.09–5.15 (m, 1H, oxazolidinone H), 4.83 (d, 2H, J = 5.1 Hz, CH2), 4.45–4.50 (m, 1H, l-alanine CH), 4.21 (t, 1H, J = 9.2 Hz, oxazolidinone H), 3.87 (q, 1H, J = 5.7 Hz, 9.3 Hz, oxazolidinone H), 3.63–3.65 (br. d, 4H, piperazine H), 2.91–2.98 (br. d, 4H, piperazine H), 1.36 (br d, 9H, (CH3)3), 1.16 (d, 3H, J = 6.9 Hz, CH3). 13C-NMR (DMSO-d6, 600 MHZ): δ 170.7, 155.3, 154.8, 153.7, 153.4, 135.4, 135.3, 133.3, 133.3, 133.2, 125.8, 119.7, 114.2, 106.8, 106.7, 77.9, 70.7, 51.7, 50.6, 50.3, 47.1, 45.8, 44.7, 41.5, 28.2, 17.7. IR (KBr pellet, cm−1): ν 3634, 3500, 3346, 3121, 2980, 2900, 2811, 2722, 1877, 1736, 1653, 1578, 1520, 1447, 1387, 1367, 1280, 1236, 1120, 1061, 1028. LRMS (m/z): 517.3 (M+). Calcd. for C24H32FN7O5: 517.2449; found 540.2500 (M+ + Na). Anal. calcd. for C24H32FN7O5: C: 55.70, H: 6.23, N: 18.94; found C: 55.21, H: 6.31, N: 18.55.
Compound 16b was prepared via a similar procedure to 16a from a solution of 15b (8.0 g, 15.46 mmol) in DCM (16.0 mL) in trifluoroacetic acid (16.0 mL) to afford the title compound as a cream solid, 7.36 g, yield: 90%. This solid was utilized for subsequent reactions without further purification. 1H-NMR (DMSO-d6, 600 MHZ): δ 8.17 (d, 1H, J = 0.7 Hz, triazole H), 8.12 (br. d, 3H, J = 4.0 Hz, +NH3, exchangeable with D2O), 7.76 (d, 1H, J = 0.7 Hz, triazole H), 7.42 (dd, 1H, J = 2.5 Hz, 14.7 Hz, phenyl H), 7.14 (dd, 2H, J = 2.3 Hz, 8.8 Hz, phenyl H), 7.07 (t, 1H, J = 9.3 Hz, phenyl H), 5.11–5.14 (m, 1H, oxazolidinone H), 4.83 (d, 1H, J = 5.0 Hz, CH2), 4.41–4.44 (m, 1H, l-alanine CH), 4.21 (t, 1H, J = 9.1 Hz, oxazolidinone H), 3.86 (q, 1H, J = 5.7 Hz, 9.3 Hz, oxazolidinone H), 3.59–3.67 (m, 4H, piperazine H, overlaps with DOH signal), 2.90–3.08 (m, 4H, piperazine H), 1.33 (d, 3H, J = 7.0 Hz, CH3). 13C-NMR (DMSO-d6, 600 MHZ): δ 167.9, 158.0, 157.8, 155.4, 153.8, 153.5, 135.2, 135.2, 133.4, 133.3, 125.9, 119.8, 119.8, 116.1, 114.3, 114.3, 106.9, 106.7, 70.7, 51.7, 50.4, 50.1, 47.1, 45.9, 44.7, 41.6, 16.4. LRMS (m/z): calcd. for C19H24FN7O3 (M+ − CF3O2H): 417.3. HRMS (m/z): calcd. for C21H25F4N7O5: 531.1853; found 530.2400 (M+ − H).
2.2.3. Preparation of N-((R)-1-(4-(4-((R)-5-((1H-1,2,3-triazol-1-yl)methyl)-2-oxooxazolidin-3-yl)-2-fluorophenyl)piperazin-1-yl)-1-oxopropan-2-yl)-2-nitrobenzamide 9a
To an ice-cooled solution of 16a (0.700 g, 1.317 mmol) in anhyd. CH3CN (20 mL) was added TEA (0.70 mL) and 2-nitrobenzoyl chloride (0.363 g, 1.97 mmol), and the reaction mixture was stirred overnight. The reaction mixture was diluted with DCM (100 mL), washed with water (3 × 80 mL), 10% NaHCO3 solution (80 mL), water (80 mL) and brine (80 mL), dried (Na2SO4), filtered and concentrated to give a yellow solid, which was triturated with ether and filtered to give a yellow solid, 0.580 g, recrystallized (CH3CN) to give the title Compound 9a as a yellow solid, 190 mg, yield: 23%; mp.: 124–126 °C. 1H-NMR (DMSO-d6, 600 MHZ):δ 9.03 (d, 1H, NH, J = 8.0 Hz), 8.17 (d,1H, J = 0.7 Hz, triazole H), 8.03 (dd, 1H, J = 0.9 Hz, 8.1 Hz, nitrobenzene H), 7.79 (t, 1H, J = 7.1 Hz, nitrobenzene H), 7.76 (d, 1H, J = 1.0 Hz, triazole H), 7.66–7.77 (m, 1H, nitrobenzene H), 7.61 (dd, 1H, J = 1.3 Hz, 7.6 Hz, nitrobenzene H), 7.42 (dd,1H, J = 3.4 Hz, 12.1 Hz, phenyl H), 7.27 (dd, 1H, J = 2.3 Hz, 8.8 Hz, phenyl H), 7.06 (t, 1H, J = 7.5 Hz, phenyl H), 5.09–5.14 (m, 1H oxazolidinone H), 4.94–4.95 (m, 1H, d-alanine CH), 4.83 (d, 2H, J = 5.2 Hz, CH2), 4.20 (t,1H, J = 9.0 Hz, oxazolidinone), 3.87 (dd, 1H, J = 5.3 Hz, 8.9 Hz, oxazolidinone CH), 3.74–3.61 (m, 4H, piperazine H), 3.03–2.93 (m, 4H, piperazine H), 1.29 (d, 3H, J = 6.8 Hz, CH3). 13C-NMR (DMSO-d6, 600 MHZ): δ 167.9, 163.1, 153.7, 152.1, 151.8, 145.3, 133.8, 133.7, 131.9, 133.7, 131.7, 131.6, 130.5, 129.1, 127.6, 124.2, 122.4, 118.1, 118.1, 112.6, 112.6, 105.2, 105.0, 69.1, 50.1, 49.1, 48.6, 45.4, 43.3, 43.2, 40.0, 15.7. IR (KBr pellet, cm−1): 3305, 3112, 1753, 1645, 1519, 1282, 1228, 1026, 743. LRMS (m/z) 566.5 (M+). Anal. calcd. for C26H27FN8O6: C: 55.12, H: 4.80, N: 19.78; found C: 55.1, H: 5.21, N: 19.65.
2.2.4. Preparation of N-((S)-1-(4-(4-((R)-(5-((1H-1,2,3-triazol-1-yl)methyl)-2-oxooxazolidin-3-yl)-2-fluorophenyl)piperazin-1-yl)-1-oxopropan-2-yl)-2-nitrobenzamide 10a
Compound 10a was prepared via a similar procedure to 9a from 16b (0.90 g, 1.693 mmol) and 2-nitrobenzoyl chloride (0.471 gm, 2.54 mmol) to give 10a as a yellow solid, 258 mg, yield: 27%; recrystallized (CH3CN), mp.: 185–190 °C. 1H-NMR (DMSO-d6, 600 MHZ): δ 9.05 (d, NH, 1H, J = 8.0 Hz, exchangeable with D2O), 8.17 (s, 1H, triazole H), 8.05 (dd, 1H, J = 0.5 Hz, 8.2 Hz, nitrobenzene H), 7.79–7.82 (m, 1H, nitrobenzene H), 7.77 (s, 1H, triazole H), 7.69–7.72 (m, 1H, nitrobenzene H), 7.62 (dd, 1H, J = 1.1 Hz, 7.6 Hz, nitrobenzene H), 7.44 (dd, 1H, J = 2.4 Hz, 14.7 Hz, phenyl H), 7.14 (dd, 1H, J = 2.3 Hz, 8.8 Hz, phenyl H), 7.07 (t, 1H, J = 9.3 Hz, phenyl H), 5.11–5.15 (m, 1H, oxazolidinone H), 4.96–5.01 (m, 1H, l-alanine CH), 4.84 (d, 2H, J = 5.1, CH2), 4.22 (t, 1H, J = 9.2 Hz, oxazolidinone H), 3.87 (dd, 1H, J = 5.7 Hz, 9.3 Hz, oxazolidinone H), 3.60–3.80 (m, 4H, piperazine H), 2.90–3.06 (m, 4H, piperazine H), 1.30 (d, 3H, J = 6.8 Hz, CH3). 13C-NMR (DMSO-d6, 600 MHZ): δ 175.4, 169.4, 158.9, 158.7, 154.3, 144.8, 140.6, 140.6, 138.6, 134.3, 131.1, 128.7, 125.0, 119.6, 112.1, 111.9, 84.8, 84.5, 84.3, 76.1, 57.0, 55.9, 55.5, 52.4, 50.8, 50.2, 46.9, 22.5. IR (KBr pellet, cm−1): ν 3309, 3122, 2826, 1729, 1635, 1601, 1524, 1442, 1341, 1228, 1160, 1118, 1028. LRMS (m/z) 566.2 (M+). Anal. calcd. for C26H27FN8O6: C: 55.12, H: 4.80, N: 19.78; found C: 54.98, H: 5.22, N: 19.70.
2.2.5. Preparation of N-((R)-1-(4-(4-((R)-5-((1H-1,2,3-triazol-1-yl)methyl)-2-oxooxazolidin-3-yl)-2-fluorophenyl)piperazin-1-yl)-1-oxopropan-2-yl)-3-nitrobenzamide 9b
Compound 9b was prepared via a similar procedure to 9a from 16a (0.700 g, 1.317 mmol) and 3-nitrobenzoyl chloride (0.363 g, 1.97 mmol) to give a yellow solid, 0.580 g, yield: 68%; recrystallized (CH3CN), mp.: 208–210 °C. 1H-NMR (DMSO-d6, 600 MHZ): δ 9.13 (d, NH, 1H, J = 7.4 Hz, exchangeable with D2O), 8.75 (t, 1H, J = 1.9 Hz, nitrobenzene H), 8.38–8.40 (m, 1H, nitrobenzene H), 8.33–8.35 (m, 1H, nitrobenzene H), 8.17 (d, 1H, J = 0.8 Hz, triazole H), 7.79 (t, 1H, J = 8.0 Hz, nitrobenzene H), 7.77 (d, 1H, J = 0.7 Hz, triazole H), 7.42 (dd, 1H, J = 2.5 Hz, 14.7 Hz, phenyl H), 7.12 (dd, 1H, J = 3.8 Hz, 9.4 Hz, phenyl H), 7.06 (t, 1H, J = 9.3 Hz, phenyl H), 5.11–5.14 (m, 1H, oxazolidinone H), 4.98–5.05 (m, 1H, d-alanine CH), 4.83 (d, 2H, J = 5.1 Hz, CH2), 4.21 (t, 1H, J = 9.2 Hz, oxazolidinone H), 3.86 (dd, 1H, J = 5.7 Hz, 9.3 Hz, oxazolidinone H), 3.62–3.70 (m, 4H, piperazine H), 2.95–3.01 (br. d, 4H, piperazine H), 1.35 (d, 3H, J = 7.0 Hz, CH3). 13C-NMR (DMSO-d6, 600 MHZ): δ 170.7, 164.2, 155.8, 154.2, 153.9, 148.2, 135.9, 135.8, 135.8, 134.5, 133.9, 133.8, 133.7, 130.6, 126.5, 126.3, 122.7, 120.3, 120.2, 114.7, 114.7, 107.3, 107.1, 71.3, 52.2, 51.1, 50.8, 47.6, 45.9, 45.4, 42.1, 17.7. IR (KBr pellet, cm−1): 3288, 3117, 2823, 1720, 1637, 1517, 1445, 1349, 1285, 1228. LRMS (m/z): 566.6 (M+). Anal. calcd. for C26H27FN8O6: C: 55.12, H: 4.80, N: 19.78; found: C: 54.91, H: 4.71, N: 19.79.
2.2.6. Preparation of N-((S)-1-(4-(4-((R)-(5-((1H-1,2,3-triazol-1-yl)methyl)-2-oxooxazolidin-3-yl)-2-fluorophenyl)piperazin-1-yl)-1-oxopropan-2-yl)-3-nitrobenzamide 10b
Compound 10b was prepared via a similar procedure to 9a from 16b (0.90 g, 1.693 mmol) and 3-nitrobenzoyl chloride (0.471 g, 2.54 mmol) to give a cream-colored solid, 480 mg, yield: 51%; recrystallized (EtOAc), mp.: 138–145 °C. 1H-NMR (DMSO-d6, 600 MHZ): δ 9.13 (d, NH, 1H, J = 7.4 Hz, exchangeable with D2O), 8.75 (t, 1H, J = 1.9 Hz, nitrobenzene H), 8.38–8.40 (m, 1H, nitrobenzene H), 8.33–8.35 (m, 1H, nitrobenzene H), 8.16 (d, 1H, J = 0.7 Hz, triazole H), 7.78 (t, 1H, J = 8.0 Hz, nitrobenzenene H), 7.76 (d, 1H, J = 0.7 Hz, triazole H), 7.42 (dd, 1H, J = 2.5 Hz, 14.7 Hz, phenyl H), 7.13 (dd, 1H, J = 3.8 Hz, 9.4 Hz, phenyl H), 7.06 (t, 1H, J = 9.3 Hz, phenyl H), 5.10–5.14 (m, 1H, oxazolidinone H), 5.01–5.05 (m, 1H, l-alanine CH), 4.83 (d, 2H, J = 5.1 Hz, CH2), 4.20 (t, 1H, J = 11.8 Hz, oxazolidinone H), 3.86 (dd, 1H, J = 5.7 Hz, 9.3 Hz, oxazolidinone H), 3.62–3.70 (m, 4H, piperazine H), 2.95–3.01 (br. d, 4H, piperazine H), 1.35 (d, 3H, J = 7.0 Hz, CH3). 13C-NMR (DMSO-d6, 600 MHZ): δ 175.4, 168.9, 160.6, 159, 158.7, 153, 140.7, 140.6, 140.5, 139.2, 138.6, 138.6, 138.5, 135.3, 131.3, 131.1, 127.5, 125.0, 119.5, 112.1, 112.9, 76.0, 56.9, 55.9, 55.6, 52.3, 50.7, 50.2, 46.9, 22.5, 19.3. IR (KBr pellet, cm−1): ν 3442, 3309, 3122, 2826, 1729, 1635, 1600, 1523, 1442, 1419, 1340, 1227, 1200, 1160, 1118, 1028. LRMS (m/z): 566.4 (M+). Anal. calcd. for C26H27FN8O6: C: 55.12, H: 4.80, N: 19.78; found C: 55.16, H: 4.88, N: 19.91.
2.2.7. Preparation of N-((R)-1-(4-(4-((R)-5-((1H-1,2,3-triazol-1-yl)methyl)-2-oxooxazolidin-3-yl)-2-fluorophenyl)piperazin-1-yl)-1-oxopropan-2-yl)-4-nitrobenzamide 9c
Compound 9c was prepared via a similar procedure to 9a from 16a (0.700 g, 1.317 mmol) and 4-nitrobenzoyl chloride to give a yellow solid, 300 mg, yield: 38%, recrystallized (CH3CN); mp.: 228–230 °C. 1H-NMR (DMSO-d6, 600 MHZ) δ: 9.04 (d, 1H, J = 9 Hz, NH, exchangeable with D2O), 8.32 (d, 2H, J = 8.9 Hz, phenyl H), 8.16 (d, 1H, J = 0.8 Hz, triazole H), 8.12 (d, 2H, J = 8.9 Hz, phenyl H), 7.76 (d, 1H, J = 0.8 Hz, triazole H), 7.42 (dd, 1H, J = 2.9 Hz, 15.7 Hz, phenyl H), 7.13 (dd, 1H, J = 3.5 Hz, 8.3 Hz, phenyl H), 7.06 (t, 1H, J = 9.6 Hz, phenyl H), 5.10–5.15 (m, 1H, oxazolidinone H), 4.99–5.04 (m, 1H, d-alanine CH), 4.83 (d, 2H, J = 5.1 Hz, CH2), 4.20 (t, 1H, J = 9.2 Hz, oxazolidinone H), 3.86 (dd, 1H, J = 5.5 Hz, 8.3 Hz, oxazolidinone H), 3.58–3.75 (m, 4H, piperazine H), 2.90–3.06 (m, 4H, piperazine H), 1.34 (d, 3H, J = 7.0 Hz, CH3). 13C-NMR (DMSO-d6, 600 MHZ): δ 170.6, 164.7, 155.8, 154.2, 153.9, 149.6, 140.0, 135.9, 135.8, 133.9, 133.8, 133.7, 129.5, 126.3, 123.9, 120.3, 120.2, 114.7, 107.3, 107.1, 71.3, 52.2, 51.1, 50.8, 47.6, 45.9, 45.4, 42.1, 17.7. IR (KBr pellet, cm−1): 3373, 2821, 1742, 1640, 1529, 1444, 1341, 1226, 1162, 1110, 1028. LRMS (m/z): 566.5 (M+). Anal. calcd. for C26H27FN8O6: C: 55.12, H: 4.80, N: 19.78; found: C: 54.73, H: 5.00, N: 20.06.
2.2.8. Preparation of N-((S)-1-(4-(4-((R)-(5-((1H-1,2,3-triazol-1-yl)methyl)-2-oxooxazolidin-3-yl)-2-fluorophenyl)piperazin-1-yl)-1-oxopropan-2-yl)-4-nitrobenzamide 10c
Compound 10c was prepared via a similar procedure to 9a from 16b (0.90 g, 1.693 mmol) and 4-nitrobenzoyl chloride (0.471 g, 2.54 mmol) to afford a yellow solid, 450 mg, yield: 47%, recrystallized (CH3CN), mp.: 245–247 °C. 1H-NMR (DMSO-d6, 600 MHZ): δ 9.06 (d, NH, 1H, J = 7.4, NH, exchangeable with D2O), 8.33 (d, 2H, J = 8.9 Hz, nitrobenzene H), 8.18 (d, 1H, J = 0.7 Hz, triazole H), 8.13 (d, 2H, J = 8.9 Hz, nitrobenzene H), 7.77 (s, 1H, triazole H), 7.43 (dd, 1H, J = 3.0 Hz, 14.2 Hz, phenyl H), 7.13 (dd, 1H, J = 3.5 Hz, 8.3 Hz, phenyl H), 7.07 (t, 1H, J = 8.9, phenyl H), 5.10–5.16 (m, 1H, oxazolidinone H), 4.98–5.18 (m, 1H, l-alanine CH), 4.84 (d, 2H, J = 5.1 Hz, CH2), 4.21 (t, 1H, J = 8.9, oxazolidinone H), 3.87 (dd, 1H, J = 6.2 Hz, 10.1 Hz, oxazolidinone H), 3.58–3.76 (m, 4H, piperazine H), 2.92–3.08 (m, 4H, piperazine H), 1.35 (d, 3H, J = 7.0, CH3). 13C-NMR (DMSO-d6, 600 MHZ): δ 170.6, 164.7, 155.8, 154.2, 154.0, 149.6, 140.1, 135.9, 135.8, 133.9, 133.8, 133.7, 129.5, 126.3, 124.0, 120.3, 120.3, 114.8, 107.3, 107.2, 71.3, 52.2, 51.1, 50.8, 47.6, 45.9, 45.4, 42.1, 17.7. IR (KBr pellet, cm−1): ν 3439, 3309, 3123, 2826, 1728, 1635, 1601, 1525, 1442, 1341, 1282, 1228, 1200, 1161, 1118, 1028. LRMS (m/z) 566.3 (M+). Anal. calcd. for C26H27FN8O6: C: 55.12, H: 4.80, N: 19.78; found C: 54.95, H: 5.20, N: 19.69.
2.2.9. Preparation of N-((R)-1-(4-(4-((R)-5-((1H-1,2,3-triazol-1-yl)methyl)-2-oxooxazolidin-3-yl)-2-fluorophenyl)piperazin-1-yl)-1-oxopropan-2-yl)-3,5-dinitrobenzamide 9d
Compound 9d was prepared via a similar procedure to 9a from 16a (1.00 g, 1.881 mmol) and 3,5-dinitrobenzoyl chloride (0.65 g, 2.82 mmol) to give a yellow solid, 390 mg, yield: 32%, purified by silica gel column chromatography (EtOAc-Hex 10-1, EtOAc and EtOAc-MeOH 10:1)], mp.: 223–225 °C. 1H-NMR (DMSO-d6, 600 MHZ): δ 9.52 (d, 1H, J = 8.8 Hz, NH, exchangeable with D2O), δ 9.13 (d, 2H, J = 2.1 Hz, nitrobenzene H), 8.96 (t, 1H, J = 2.1 Hz, nitrobenzene H), 8.16 (d, 1H, J = 1.0 Hz, triazole H), 7.76 (d, 1H, J = 0.8 Hz, triazole H), 7.42 (d, 1H, J = 3.0 Hz, 14.2 Hz, phenyl H), 7.13 (d, 1H, J = 3.5 Hz, 8.3 Hz, phenyl H), 7.07 (t, 1H, J = 11.1Hz, phenyl H), 5.02–5.14 (m, 2H, oxazolidinone H and d-alanine CH), 4.83 (d, 2H, J = 5.0 Hz, CH2), 4.2 (t, 1H, J = 10.7 Hz, oxazolidinone H), 3.87 (dd, 1H, J = 6.2 Hz, 10.1 Hz, oxazolidinone H), 3.60–3.78 (m, 4H, piperazine H), 2.96–3.08 (m, 4H, piperazine H), 1.38 (d, 3H, J = 7.0 Hz, CH3). 13C-NMR (DMSO-d6, 600 MHZ): δ 170.4, 162.2, 155.8, 154.2, 153.9, 148.7, 136.9, 135.9, 135.8, 133.9, 133.8, 133.7, 128.3, 126.3, 121.5, 120.3, 120.3, 114.8, 114.7, 107.3, 107.1, 71.3, 67.5, 65.4, 60.2, 52.2, 51.1, 50.8, 47.6, 46.2, 45.6, 42.1, 17.7. IR (KBr pellet, cm−1): ν 3481, 3276, 3094.23, 1756, 1633, 1542, 1517, 1445, 1346, 1230, 1028. HRMS (m/z): calcd. for C26H26FN9O8: 611.1888, found 612.20 (M+ + H), LRMS (m/z) 611.5 (M+). Anal. CHN, calcd.: C: 51.06, H: 4.29, N: 20.61; found: C: 49.95, H: 4.70, N: 20.12.
2.2.10. Preparation of N-((S)-1-(4-(4-((R)-5-((1H-1,2,3-triazol-1-yl)methyl)-2-oxooxazolidin-3-yl)-2-fluoro phenyl)piperazin-1-yl)-1-oxopropan-2-yl)-3,5-dinitrobenzamide 10d
Compound 10d was prepared via a similar procedure to 9a from 16b (0.700 g, 1.317 mmol) and 3,5-dinitro benzoyl chloride (0.455 g, 1.976 mmol) to give a yellow solid, 382 mg, yield: 45%, recrystallization (CH3CN), mp.: 191–194 °C. 1H-NMR (DMSO-d6, 400 MHZ): δ 9.53 (d, 1H, J = 7.2 Hz, NH, exchangeable with D2O), 9.12 (d, 2H, J = 2.0 Hz, nitrobenzene H), 8.96 (t, 1H, J = 2.0 Hz, nitrobenzene H), 8.16 (s, 1H, triazole H), 7.76 (s, 1H, triazole H), 7.42 (dd, 1H, J = 2.2 Hz, 14.7 Hz, phenyl H), 7.06–7.14 (m, 2H, phenyl H), 5.05–5.12 (m, 2H, oxazolidinone H and l-alanine CH), 4.82 (d, 2H, J = 5.0 Hz, CH2), 4.20 (t, 1H, J = 9.1 Hz, oxazolidinone H), 3.85 (dd, 1H, J = 5.7 Hz, 9.3 Hz, oxazolidinone H), 3.64–3.72 (m, 4H, piperazine H), 2.95–3.01 (m, 4H, piperazine H), 1.37 (d, 3H, J = 6.9 Hz, CH3). 13C-NMR (DMSO-d6, 400 Hz): δ 170, 161.8, 153.5, 148.2, 136.4, 135.4, 135.3, 133.4, 133.2, 127.8, 125.9, 121.0, 119.8, 114.3, 107.3, 107.1, 70.8, 51.7, 50.7, 50.3, 47.1, 45.7, 45.0, 42.4, 17.2. IR (KBr pellet, cm−1): ν 3425, 3278, 3109, 1752, 1668, 1631, 1541, 1518, 1484, 1446, 1345, 1230, 1028. HRMS (m/z): calcd. for C26H26FN9O8: 611.1888, found 612.2090 (M+ + H), LRMS (m/z) 611.4 (M+). Anal. CHN, expected: C: 51.06, H: 4.29, N: 20.61, found C: 50.72, H: 4.25, N: 19.61.
2.2.11. Preparation of N-(R)-1-(4-(4-((R)-(5-((1H-1,2,3-triazol-1-yl)methyl)-2-oxooxazolidin-3-yl)-2-fluorophenyl)piperazin-1-yl)-1-oxopropan-2-yl)-5-nitrofuran-2-carboxamide 9e
To a stirred solution of 5-nitrofuran-2-carboxylic acid (1.77 gm, 11.288 mmol) in anhyd. DCM (30 mL) cooled in an ice bath was added oxalyl chloride (2.46 mL, 28.22 mmol) under nitrogen followed by 2 drops of dry DMF, and effervescence ensued. The ice bath was removed, and the reaction mixture was stirred 2 h. The mixture was evaporated to dryness on a rotavap to give the acid chloride as a yellow semisolid, which was dried under vacuum. The resulting acid chloride was dissolved in anhyd. DCM (30 mL) and added in rapid drops to a solution of the TFA salt 16a (1.5 g, 2.822 mmol) and TEA (2.14 mL, 11.288 mmol) in CH3CN (32 mL) with cooling in an ice bath. The reaction mixture was left to stir to room temperature overnight under nitrogen and concentrated to dryness. The residue was dissolved in DCM and washed with water, 10% Na2CO3 solution, water, brine and dried with Na2SO4, filtered and concentrated to give a brown foam, which was triturated with ether and hexane (1:1) to afford 9e as a brown solid, 624 mg, yield: 40%, recrystallized (EtOAc), mp.: 180–185 °C. 1H-NMR (DMSO-d6, 600 MHZ): δ 9.08 (d, 1H, J = 8.7 Hz, NH, exchangeable with D2O), 8.18 (d, 1H, J = 0.7 Hz, triazole H), 7.77 (d, 1H, J = 0.7 Hz, triazole H), 7.76 (d, J = 3.9 Hz, nitrofuran H), 7.55 (d, 1H, J = 5.3 Hz, nitrofuran H), 7.43 (dd, 1H, J = 3.7 Hz, 15.0 Hz, phenyl H), 7.14 (dd, 1H, J = 3.7 Hz, 8.0 Hz, phenyl H), 7.08 (t, 1H, J = 7.7 Hz, phenyl H), 5.14–5.12 (m, 1H, oxazolidinone H), 4.97–5.01 (m, 1H, d-alanine CH), 4.83 (d, 2H, J = 6.7 Hz, CH2), 4.21 (t, 1H, J = 9.2 Hz, oxazolidinone H), 3.85–3.88 (q, 1H, J = 4.0 Hz, 8.7 Hz, oxazolidinone H), 3.62–3.71 (m, 4H, piperazine H), 3.00 (m, 4H, piperazine H), 1.34 (d, 3H, J = 6.9 Hz, CH3). IR (KBr pellet, cm−1): ν 3403, 3131, 2929, 2811, 1753, 1642, 1590, 1548, 1521, 1444, 1386, 1350, 1233, 1157, 1113, 1023. LRMS (m/z): 556.5 (M+). Anal. calcd. for C24H25FN8O7: C: 51.80, H: 4.53, N: 20.14; found: C: 51.97, H: 4.26, N: 20.36.
2.2.12. Preparation of N-((S)-1-(4-(4-((R)-(5-((1H-1,2,3-triazol-1-yl)methyl)-2-oxo oxazolidin-3-yl)-2-fluorophenyl)piperazin-1-yl)-1-oxopropan-2-yl)-5-nitrofuran-2-carboxamide 10e
Compound 10e was prepared via a similar procedure to 9e from 16b (0.700 g, 1.317 mmol) and 5-nitrofuran-2-carboxylic acid (0.827 g, 5.268mmol) to give a yellowish-brown solid, 356 mg, yield 46%, recrystallized (CH3CN/MeOH), mp.: 218–222 °C. 1H-NMR (DMSO-d6, 600 MHZ): δ 9.09 (d, 1H, J = 7.5 Hz, NH, exchangeable with D2O), 8.18 (d, 1H, J = 0.7 Hz, triazole H), 7.78 (d, 1H, J = 0.7 Hz, triazole H), 7.77 (d, 1H, J = 3.8 Hz, nitrofuran H), 7.44 (d, 1H, J = 3.8 Hz, nitrofuran H), 7.42 (d, 1H, J = 2.5 Hz, phenyl H), 7.13 (d, 1H, J = 2.3 Hz, phenyl H), 7.08 (t, 1H, J = 9.4 Hz, phenyl H), 5.12–5.14 (m, 1H, oxazolidinone H), 4.98–5.02 (m, 1H, l-alanine CH), 4.84 (d, 1H, J = 5.0, CH2), 4.21 (t, 1H, J = 9.3 Hz, oxazolidinone H), 3.85–3.88 (q, 1H, J = 4.0 Hz, 8.7 Hz, oxazolidinone H), 3.60–3.74 (m, 4H, piperazine H), 2.93–3.06 (m, 4H, piperazine H), 1.34 (d, 3H, J = 7.0 Hz, CH3). 13C-NMR (DMSO-d6, 400 Hz): δ 169.7, 155.5, 155.3, 153.7, 153.4, 147.7, 135.3, 135.3, 133.3, 133.3, 133.2, 125.8, 119.8, 119.8, 115.9, 114.3, 113.3, 106.8, 106.7, 70.7, 51.7, 50.6, 50.2, 47.1, 45, 44.9, 41.7, 17.3. IR (KBr pellet, cm−1): ν 3396, 3351, 3107, 2917, 2823, 1748, 1637, 1542, 1488, 1445, 1421, 1381, 1342, 1276, 11648, 1105, 1074, 1029. LRMS (m/z): 556.5 (M+). HRMS (m/z): calcd. for C24H25FN8O7: 556.1830, found 579.1700 (M+ + Na). Anal. calcd. for C24H25FN8O7: C: 51.80, H: 4.53, N: 20.14, found: C: 51.41, H: 4.78, N: 20.44.
2.2.13. Preparation of N-((R)-1-(4-(4-((R)-(5-((1H-1,2,3-triazol-1-yl)methyl)-2-oxooxazolidin-3-yl)-2-fluorophenyl) piperazin-1-yl)-1-oxopropan-2-yl)-5-nitrothiophene-2-carboxamide 9f
Compound 9f was prepared via a similar procedure to 9e from 16a (1.00 g, 1.881 mmol) and 5-nitrothiophene-2-carboxylic acid (1.302 g (7.524 mmol) to give an off-white solid, 262 mg, yield: 24%, recrystallized (CH3CN), mp.: 223–227 °C. 1H-NMR (DMSO-d6, 600 MHZ): 9.27 (d, NH,1 H, J = 7.4 Hz, exchangeable with D2O), 8.17 (d, 1H, J = 0.5 Hz, triazole H), 8.15 (d, nitrothiophene, J = 4.3 Hz, 1H, nitrothiophene H), 7.95 (d, 1H, J = 4.4 Hz, nitrothiophene H), 7.77 (s, 1H, triazole H), 7.43 (dd, 1H, J = 2.4 Hz, 14.7 Hz, phenyl H), 7.27 (dd, 1H, J = 8.8 Hz, 2.3 Hz, phenyl H), 7.07 (t, 1H, J = 9.4 Hz, phenyl H), 5.12–5.14 (m, 1H, oxazolidinone H), 4.97–5.01 (m, 1H, d-alanine CH), 4.83 (d, 1H, J = 5.0 Hz, CH2), 4.21 (t, 1H, J = 9.1 Hz, oxazolidinone H), 3.87 (dd, 1H, J = 5.7 Hz, 9.3 Hz, CH), δ 3.58–3.70 (m, 4H, piperazine H), 2.90–3.05 (m, 4H, piperazine H), 1.34 (d, 3H, J = 7.0 Hz, CH3). 13C-NMR (DMSO-d6): δ 159.5, 155.8, 154.2, 154.0, 153.5, 146.3, 135.8, 133.9, 133.8, 133.7, 130.7, 128.4, 126.3, 120.3, 114.8, 107.3, 107.2, 71.3, 52.2, 51.1, 50.8, 47.6, 46, 45.4, 42.1, 17.7. IR (KBr pellet, cm−1): ν 3396, 3351, 3107, 2917, 2823, 1748, 1637, 1542, 1488, 1445, 1421, 1381, 1342, 1276, 1164, 1105, 1074, 1029. MS: 572.5 (M+). HRMS (m/z): calcd. for C24H25FN8O7S: 572.1602, found 573.1700 (M+ + H), LRMS (m/z): 572.5 (M+). Anal. calcd. for C24H25FN8O6S: C: 49.91, H: 4.57, N: 19.54.; found C: 50.35, H: 4.40, N: 19.57.
2.2.14. Preparation of N-((S)-1-(4-(4-((R)-(5-((1H-1,2,3-triazol-1-yl) methyl)-2-oxooxazolidin-3-yl)-2-fluorophenyl) piperazin-1-yl)-1-oxopropan-2-yl)-5-nitrothiophene-2-carboxamide 10f
Compound 10f was prepared via a similar procedure to 9e from 16b (0.700 g, 1.317 mmol) and 5-nitrothiophene-2-carboxylic acid (0.912 g, 5.268 mmol) to give a yellowish-brown solid, 560 mg, yield: 76%, recrystallized (EtOAc), mp.: 211–214 °C. 1H-NMR (DMSO-d6, 600 MHZ): δ 9.27 (d, 1H, J = 7.4 Hz, NH, exchangeable with D2O), 8.18 (d, 1H, J = 0.6 Hz, triazole H), 8.16 (d, 1H, J = 4.4 Hz, nitrothiophene H), 7.98 (d, 1H, J = 4.4 Hz, nitrothiophene H), 7.78 (s, 1H, triazole H), 7.43 (dd, 1H, J = 2.4 Hz, 14.7 Hz, phenyl H), 7.27 (dd, 1H, J = 2.3 Hz, 8.8 Hz, phenyl H), 7.07 (t, 1H, J = 9.4 Hz, phenyl H), 5.11–5.15 (m, 1H, oxazolidinone H), 4.96–5.01 (m, 1H, l-alanine CH), 4.83 (d, 1H, J = 5.0 Hz, CH2), 4.21 (t, 1H, J = 9.4 Hz, oxazolidinone H), 3.87 (dd, 1H, J = 5.3 Hz, 8.9 Hz, oxazolidinone H), 3.56–3.76 (m, 4H, piperazine H), 2.90–3.06 (m, 4H, piperazine H), 1.34 (d, 3H, J = 7.0 Hz, CH3). 13C-NMR (DMSO-d6): δ 169.8, 159.1, 155.4, 153.8, 153.5, 153.1, 145.8, 135.4 135.3, 133.4, 133.3, 133.3, 130.2, 128, 125.9, 119.8, 114.3, 106.8, 106.7, 70.8, 51.7, 50.6, 50.3, 47.1, 45.5, 45.0, 41.7, 17.2. IR (KBr pellet, cm−1): 3425, 3296, 3079, 2918, 2843, 1742, 1643, 1553, 1517, 1424, 1336, 1276, 1228, 1030. LRMS (m/z) 572.5 (M+). HRMS (m/z): calcd. for C24H25FN8O6S (M+): 572.1602, found 573.1800 (M+ + H). Anal. calcd. for C24H25FN8O6S C: 50.35, H: 4.40, N: 19.57, found C: 50.36, H: 4.52, N: 19.20.
2.2.15. Preparation of N-(R)-1-(4-(4-((R)-5-((1H-1,2,3-triazol-1-yl)methyl)-2-oxooxazolidin-3-yl)-2-fluorophenyl)piperazin-1-yl)-1-oxopropan-2-yl)-2-nitrobenzenesulfonamide 9g
Compound 9g was prepared via a similar procedure to 9a from 16a (650 mg, 1.222 mmol), TEA (0.650 mL) and 2-nitrobenzenesulfonyl chloride (406 mg, 1.834 mmol) in anhyd. CH3CN (15 mL) to give a yellow solid, 200 mg, yield: 20%, recrystallized (CH3CN), mp.: 128–130 °C. 1H-NMR (DMSO-d6, 600 MHZ): δ 8.33 (br. s,1H, NH exchangeable with D2O), 8.17 (d, 1H, J = 0.7 Hz, triazole H), 8.01–8.03 (m, 1H, nitrobenzene H), 7.94–7.96 (m, 1H, nitrobenzene H), 7.83–7.86 (m, 2H, nitrobenzene H), 7.77 (d, 1H, J = 0.7 Hz, triazole H), 7.41 (dd, 1H, J = 2.6 Hz, 14.7 Hz, phenyl H), 7.13 (dd, 1H, J = 2.4 Hz, J = 8.4 Hz, phenyl H), 7.02 (t, 1H, J = 9.5 Hz, phenyl H), 5.10–5.14 (m, 1H oxazolidinone H), 4.83 (d, 2H, J = 5.1 Hz, CH2), 4.48 (q, 1H, J = 6.8 Hz, d-alanine CH), 4.20 (t, 1H, J = 8.9 Hz, oxazolidinone H), 3.86 (q, 1H, J = 5.7 Hz, 9.3 Hz, oxazolidinone H), 3.60–3.37 (m, 4H, piperazine H), 2.67–2.97 (m, 4H, piperazine H), 1.22 (d, 3H, J = 7.0 Hz, CH3). 13C-NMR (DMSO-d6, 600 MHZ): δ 169.4, 155.8, 154.2, 153.9, 147.8, 135.8, 135.7, 134.5, 133.9, 133.8, 133.6, 133.0, 130.4, 126.3, 124.7, 120.2, 120.2, 114.8, 114.7, 107.3, 107.1, 71.3, 52.2, 50.9, 50.5, 49.1, 47.6, 45.3, 42.0, 19.4. IR (KBr pellet, cm−1): ν 3446, 3303, 3100, 2829, 1752, 1650, 1541, 1518, 1483, 1444, 1417, 1356, 1330, 1233, 1170, 1123, 1028. LRMS (m/z): 602.5 (M+). Anal. calcd. for C25H27FN8O7S: C: 49.83, H: 4.52, N: 18.60; found: C: 49.48, H: 4.73, N: 18.80.
2.2.16. Preparation of N-((S)-1-(4-(4-((R)-(5-((1H-1,2,3-triazol-1-yl)methyl)-2-oxooxazolidin-3-yl)-2-fluorophenyl) piperazin-1-yl)-1-oxopropan-2-yl)-2-nitrobenzene sulfonamide 10g
Compound 10g was prepared via a similar procedure to 9a from 16b (700 mg, 1.317 mmol) and 2-nitrobenzenesulfonyl chloride (4–6 mg, 1.834 mmol), to give a yellow solid, 265 mg, yield 30%, recrystallized (CH3CN), mp.: 78–80 °C. 1H-NMR (DMSO-d6, 400 MHZ): δ 8.30 (br., 1H, NH, exchangeable with D2O), 8.16 (s, 1H, triazole H), 8.00–8.03 (m, 1H, nitrobenzene H), 7.93–7.96 (m, 1H, nitrobenzene H), 7.82–7.85 (m, 2H, nitrobenzene H), 7.76 (s, 1H, triazole H), 7.41 (dd, 1H, J = 2.4 Hz, 14.7 Hz, phenyl H), 7.13 (dd, 1H, J = 2.0 Hz, 8.7 Hz, phenyl H), 7.02 (t, 1H, J = 9.3 Hz, phenyl H), 5.09–5.15 (m, 1H, oxazolidinone H), 4.82 (d, 2H, J = 5.0 Hz, CH2), 4.48 (q, 1H, J = 6.8 Hz, l-alanine CH), 4.20 (t, 1H, J = 9.2 Hz, oxazolidinone H), 3.85 (dd, 1H, J = 5.7 Hz, 9.3 Hz, oxazolidinone H), 3.45–3.63 (m, 4H, piperazine H), 2.80–2.96 (m, 4H, piperazine H), 1.21 (m, 3H, J = 6.9 Hz, CH3). IR (KBr pellet, cm−1): ν 3446, 3287, 3130, 2984, 2903, 2827, 1756, 1647, 1542, 1517, 1481, 1442, 1417, 1371, 1230, 1169, 1121, 1028. HRMS (m/z): calcd. for C25H27FN8O7S: 602.1707, found 603.1812 (M+ + H), LRMS (m/z) 602.4 (M+). Anal. calcd. for C25H27FN9O7S: C: 49.83, H: 4.52, N: 18.6; found C: 49.36, H: 4.72, N: 18.34
2.2.17. Preparation of N-((R)-1-(4-(4-((R)-5-((1H-1,2,3-triazol-1-yl)methyl)-2-oxooxazolidin-3-yl)-2-fluorophenyl) piperazin-1-yl)-1-oxopropan-2-yl)-3-nitrobenzenesulfonamide 9h
Compound 9h was prepared via a similar procedure to 9a from 16a (660 mg, 1.241 mmol) and 3-nitrobenzenesulfonyl chloride (412 mg, 1.862 mmol) to give a yellow solid, which was triturated with ether and filtered to give a yellow solid, 300 mg, 40% yield, recrystallized (CH3CN); mp.: 115–118 °C. 1H-NMR (DMSO-d6, 600 MHZ): δ 8.45–8.52 (m, 3H, nitrobenzene H and NH, exchangeable with D2O), 8.18–8.20 (m, 1H, nitrobenzene H), 8.17 (s, 1H, triazole H), 7.88 (t, 1H, J = 8.01 Hz, phenyl H) 7.76 (s, 1H, triazole H), 7.42 (dd, 1H, J = 2.5 Hz, 14.7 Hz, phenyl H), 7.14 (dd, 1H, J = 2.3 Hz, 8.8 Hz, phenyl H), 7.00 (t, 1H, J = 9.3 Hz, phenyl H), 5.11–5.14 (m, 1H oxazolidinone H), 4.83 (d, 2H, CH2), 4.44–4.50 (br., 1H, d-alanine CH), 4.20 (t, 1H, J = 9.2 Hz, oxazolidinone H), 3.86 (dd, 1H, J = 5.7 Hz, 9.3 Hz, oxazolidinone H), 3.36–3.64 (m, 2H, piperazine H), 3.28–3.38 (m, 2H, piperazine H, overlaps with DOH signal), 2.58–2.96 (m, 4H, piperazine H), 1.12 (d, 3H, J = 6.9 Hz, CH3). 13C-NMR (DMSO-d6): δ 169.3, 155.8, 154.2, 153.9, 148.1, 143.3, 135.7, 135.7, 133.9, 133.8, 133.3, 131.5, 127.4, 126.3, 122.0, 120.2, 120.2, 114.7, 107.3, 107.1, 71.3, 52.2, 51.1, 50.4, 48.7, 47.6, 45.2, 41.8, 19.2. IR (KBr pellet, cm−1): ν 3149, 3102, 2828, 1756, 1645, 1524, 1422, 1351, 1230, 1167, 1121, 1079, 1027. LRMS (m/z): 602.5. Anal. calcd. for C25H27FN9O7S: C: 49.83, H: 4.52, N: 18.6; found C: 49.55, H: 4.85, N: 18.68.
2.2.18. Preparation of N-((S)-1-(4-(4-((R)-5-((1H-1,2,3-triazol-1-yl)methyl)-2-oxooxazolidin-3-yl)-2-fluorophenyl) piperazin-1-yl)-1-oxopropan-2-yl)-3-nitrobenzamide 10h
Compound 10h was prepared via a similar procedure to 9a from 16b (700 mg, 1.317 mmol) and 3-nitrobenzenesulfonyl chloride (437 mg, 1.975 mmol) to give a yellow solid, 180 mg, yield 23%, recrystallized (EtOAc); mp.: 108–110 °C. 1H-NMR (DMSO-d6, 600 MHZ): δ 8.52 (t, 1H, J = 2.0 Hz, nitrobenzene H), 8.46-–8.48 (m, 2H, nitrobenzene H and of NH; the NH broad peak is exchangeable with D2O), 8.20–8.21 (m, 1H, nitrobenzene H), 8.18 (d, 1H, J = 0.8 Hz, triazole H), 7.89 (t, 1H, J = 8.0 Hz, nitrobenzene H), 7.78 (d, 1H, J = 0.8 Hz, triazole H), 7.43 (dd, 1H, J = 2.6 Hz, 14.7 Hz, phenyl H), 7.15 (dd, 1H, J = 2.6 Hz, 8.7 Hz, phenyl H), 7.02 (t, 1H, J = 9.3 Hz, phenyl H), 5.12–5.15 (m, 1H, oxazolidinone H), 4.84 (d, 2H, J = 5.2 Hz, CH2), 4.47–4.50 (m, 1H, l-alanine CH), 4.22 (t, 1H, J = 9.2 Hz, oxazolidinone H), 3.87 (dd, 1H, J = 5.7 Hz, 9.3 Hz, oxazolidinone H), 3.55–3.67 (m, 2H, piperazine H), 3.33–3.403 (m, 2H, piperazine H, overlaps with DOH signal), 2.80–2.98 (m, 3H, piperazine H), 2.62 (br., 1H, piperazine H), 1.13 (d, 3H, J = 6.9 Hz, CH3). 13C-NMR (DMSO-d6, 600 MHZ): δ 155.8, 154.0, 148.1, 133.9, 133.3, 131.5, 126.3, 122.0, 120.2, 114.7, 107.3, 107.1, 71.3, 65.4, 52.2, 51.1, 47.6, 45.2, 41.8, 19.2. IR (KBr pellet, cm−1): ν 3449, 3271, 3150, 2895, 1754, 1645, 1518, 1442, 1421, 1352, 1229, 1169, 1124, 1028. LRMS (m/z) 602.5 (M+). Anal. calcd. for C25H27FN9O7S: C: 49.83, H: 4.52, N: 18.60; found: C: 49.38, H: 4.85, N: 18.74.
2.2.19. Preparation of N-((R)-1-(4-(4-((R)-5-((1H-1,2,3-triazol-1-yl)methyl)-2-oxooxazolidin-3-yl)-2-fluorophenyl) piperazin-1-yl)-1-oxopropan-2-yl)-4-nitrobenzenesulfonamide 9i
Compound 10h was prepared via a similar procedure to 9a from 16a (600 mg, 1.128 mmol) and 4-nitrobenzenesulfonyl chloride (374 mg, 1.862 mmol) to give a yellow solid, 200 mg, yield: 29%, recrystallized (CH3CN); mp.: 190–192 °C. 1H-NMR (DMSO-d6, 600 MHZ):δ 8.49 (br., 1H, NH, exchangeable with D2O), 8.38–8.40 (m, 2H, nitrobenzene H), 8.17 (d, 1H, J = 0.7 Hz, triazole H), 8.00–8.04 (m, 2H, nitrobenzene H), 7.77 (s, 1H, J = 0.7 Hz, triazole H), 7.42 (dd, 1H, J = 2.5 Hz, 14.7 Hz, phenyl H), 7.13 (dd, 1H, J = 2.6 Hz, 8.7 Hz, phenyl H), 7.02 (t, 1H, J = 9.5 Hz, phenyl H), 5.11–5.14 (m, 1H, oxazolidinone H), 4.83 (d, 2H, J = 5.0 Hz, CH2), 4.45–4.48 (m, 1H, d-alanine CH), 4.21 (t, 1H, J = 9.2 Hz, oxazolidinone H), 3.86 (dd, 1H, J = 5.5 Hz, 9.1 Hz, oxazolidinone H), 3.57–3.65 (m, 2H, piperazine H), 3.38–3.41 (m, 2H, piperazine H, overlaps with the DOH signal, 2.83–2.94 (m, 3H, piperazine H), 2.67–2.71 (br., 1H, piperazine H), 1.11(d, 3H, J = 6.8 Hz, CH3). 13C-NMR (DMSO-d6, 600 MHZ): δ 169.2, 153.7, 152.1, 151.8, 147.8, 145.1, 133.6, 133.6, 131.7, 131.7, 126.6, 124.2, 122.6, 118.1, 118.1, 112.6, 112.6, 105.2, 105.0, 69.1, 50.1, 48.9, 48.3, 46.5, 45.4, 43.1, 39.7, 17.1. IR (KBr pellet, cm−1): ν 3268, 3102, 2833, 1747, 1643, 1525, 1426, 1347, 1317, 1228, 1165, 1027. LRMS (m/z) 602.4 (M+). Anal. calcd. for C25H27FN9O7S: C: 49.83, H: 4.52, N: 18.6; found: C: 49.47, H: 4.46, N: 18.4
2.2.20. Preparation of N-((S)-1-(4-(4-((R)-(5-((1H-1,2,3-triazol-1-yl) methyl)-2-oxooxazolidin-3-yl)-2-fluorophenyl) piperazin-1-yl)-1-oxopropan-2-yl)-4-nitrobenzene sulfonamide 10i
Compound 10l was prepared via a similar procedure to 9a from 16b (700 mg, 1.317 mmol) and 4-nitrobenzenesulfonyl chloride (437 mg, 1.975 mmol) to give a yellow solid, 411 mg, yield 52%, recrystallized (EtOAc); mp.: 203–205 °C. 1H-NMR (DMSO-d6, 400 MHZ): δ 8.48 (br., 1H, NH, exchangeable with D2O), 8.39 (d, 2H, J = 8.8 Hz, nitrobenzene H), 8.16 (s, 1H, triazole H), 8.02 (d, 2H, J = 8.8 Hz, nitrobenzene H), 7.76 (s, 1H, triazole H), 7.41 (dd, 1H, J = 2.4 Hz, 14.8 Hz, phenyl H), 7.14 (dd, 1H, J = 2.1 Hz, 8.5 Hz, phenyl H), 7.02 (t, 1H, J = 9.3 Hz, phenyl H), 5.10–5.14 (m, 1H, oxazolidinone H), 4.82 (d, 2H, J = 5.1 Hz, CH2), 4.40–4.50 (m, 1H, l-alanine CH), 4.20 (t, 1H, J = 9.1 Hz, oxazolidinone H), 3.86 (d, 1H, J = 5.7 Hz, 9.3 Hz, oxazolidinone H), 3.54–3.60 (m, 2H, piperazine H), 3.33 (m, 2H, piperazine H overlaps with DOH signal), 2.83–3.04 (br., 4H, piperazine H), 1.10 (d, 3H, J = 6.9 Hz, CH3). 13C-NMR (DMSO-d6, 600 MHZ): δ 169.9, 155.3, 153.7, 153.4, 149.4, 146.7, 135.2, 135.2, 133.3, 133.3, 128.2, 125.8, 124.2, 119.7, 119.7, 114.2, 106.8, 106.6, 70.7, 51.7, 50.5, 49.9, 48.1, 47.1, 44.7, 41.3, 18.7. HRMS (m/z): calcd. for C25H27FN8O7S: 602.1707, found 603.1912 (M+ + H), LRMS (m/z) 602.4 (M+). IR (KBr pellet, cm−1): ν 3452, 3279, 3102, 1732, 1649, 1520, 1424, 1352, 1310, 1228, 1196, 1168, 1078, 1042, 1026. Anal. calcd. for C25H27FN9O7S: C: 49.83, H: 4.52, N: 18.6; found C: 49.7, H: 4.704, N: 18.4.
2.2.21. Preparation of N-((R)-1-(4-(4-((R)-5-((1H-1,2,3-triazol-1-yl)methyl)-2-oxooxazolidin-3-yl)-2-fluorophenyl)piperazin-1-yl)-1-oxopropan-2-yl)-2-aminobenzamide 2,2,2-trifluoroacetate 9j
An ice cooled solution of 2-((tert-butoxycarbonyl) amino-benzoic acid (0.446 gm, 1.881 mmol) in anhyd. DCM (40 mL) was treated with N, N′-dicyclohexylcarbodiimide (485 mg, 2.351 mmol) and 1-hydroxybenzotriazole (318 mg, 6.787 mmol), respectively, and the reaction mixture was stirred for 2 h under nitrogen. The reaction mixture was then filtered into a solution of the TFA salt 16a (1.00 g, 1.881 mmol) and TEA (0.760 mL, 5.45 mmol) in anhyd. CH3CN (15 mL) and left stirring at room temperature overnight. The reaction mixture was concentrated to give a crude brown oil, which was dissolved in DCM, washed with water, 10% Na2CO3 solution, brine, dried Na2SO4, filtered and concentrated to yield a cream-colored solid. The solid was triturated with ether, collected by filtration and recrystallized from ethyl acetate to give the intermediate compound carbamate 17a as a solid, 507 mg, yield 43%; mp.: 188–191 °C. This product was utilized for subsequent reactions. 1H-NMR (DMSO-d6, 600 MHZ): δ 10.45 (s, 1H, NH, exchangeable with D2O), 8.89 (d, 1H, J = 7.3 Hz, NH, exchangeable with D2O), 8.19 (d, 1H, J = 10.1 Hz, phenyl H), 8.17 (d, 1H, J = 0.9 Hz, triazole H), 7.81 (dd, 1H, J = 1.4 Hz, 7.9 Hz, phenyl H), 7.77 (d, 1H, J = 0.8 Hz, triazole H), 7.48 (t, 1H, J = 7.1 Hz, phenyl H), 7.43 (dd, 1H, J = 2.4 Hz, 15.4 Hz, phenyl H), 7.04–7.15 (m, 3H, phenyl H), 5.11–5.15 (m, 1H, oxazolidinone H), 4.95–5.00 (m, 1H, d-alanine CH), 4.83 (d, 2H, J = 5.0 Hz, CH2), 4.21 (t, 1H, J = 8.2 Hz, oxazolidinone H), 3.86 (dd, 1H, J = 5.5 Hz, 9.1 Hz, oxazolidinone H), 3.60–3.78 (m, 4H, piperazine H), 2.94–3.04 (m, 4H, piperazine H), 1.45 (s, 9H, (CH3)3), 1.34 (d, 3H, J = 7.0 Hz, CH3). MS 636.8 (M+). 13C-NMR (DMSO-d6, 400 MHZ): δ 170.2, 167.8, 155.8, 153.5, 153.4, 152.1, 139.4, 135.4, 135.4, 133.4, 133.3, 132.2, 128.6, 125.9, 121.4, 119.8, 119.3, 118.5, 114.3, 106.9, 82.8, 79.8, 70.8, 51.7, 50.3, 47.1, 45.3, 44.9, 41.7, 27.9. IR (KBr pellet, cm−1): ν 3350, 3116, 2978, 2931, 1758, 1718, 1647, 1587, 1520, 1442, 1227, 1159, 1089, 1026. LRMS (m/z): 536.4 (M+ − (CH3)2C=CH2 + CO2). Anal. calcd. for C31H37FN8O6: C: 58.48, H: 5.86, N: 17.18, found C: 58.36, H: 6.19, N: 17.18.
Compound 9j was prepared via a similar procedure to 16a from the intermediate 17a (372 mg, 0.70 mmol) to give a white solid, 157 mg, yield 28%; recrystallized (EtOAc); mp.: 106–109 °C. 1H-NMR (DMSO-d6, 600 MHZ): δ 8.40 (d, 1H, J = 7.4 Hz, NH, exchangeable with D2O), 8.18 (d, 1H, J = 0.7 Hz, triazole H), 7.78 (s, 1H, J = 0.6 Hz, triazole H), 7.60 (d, 1H, J = 7.0 Hz, aminobenzene H), 7.43 (dd, 1H, J = 2.5 Hz, 14.7 Hz, phenyl H), 7.18 (t, 1H, J = 14.7 Hz, aminobenzene H), 7.14 (dd, 1H, J = 2.3 Hz, 8.8 Hz, phenyl H), 7.07 (t, 1H, J = 9.3 Hz, phenyl H), 6.75 (d, 1H, J = 8.2 Hz, aminobenzene H), 6.59 (t, 1H, J = 7.5 Hz, aminobenzene H), 5.11–5.14 (m, 1H, oxazolidinone H), 4.90–4.97 (m, 1H, d-alanine CH), 4.84 (d, 2H, J = 5.0 Hz, CH2), 4.21 (t, 1H, J = 9.2 Hz, oxazolidinone H), 3.87 (dd, 1H, J = 5.7 Hz, 9.3 Hz, oxazolidinone H, overlapping with +NH3 signal), 3.60–3.71 (br. m, 7H, piperazine H, overlapping with +NH3 signal, which is exchangeable with D2O), 2.95–3.01 (br. d, 4H, J = 7.0 Hz, piperazine H), 1.31 (s, 3H, J = Hz, CH3). 13C-NMR (DMSO-d6, 600 MHZ): δ 170.6, 168.1, 158.3, 158.0, 155.4, 153.7, 153.5, 135.4, 135.4, 133.4, 133.3, 133.2, 131.8, 128.5, 125.9, 119.8, 119.7, 116.8, 115.4, 115.1, 114.3, 114.3, 106.8, 106.7, 70.8, 64.9, 51.7, 50.7, 50.3, 47.1, 44.9, 44.7, 41.6, 17.4, 15.2. IR (KBr pellet, cm−1): ν 3447, 3354, 2981, 2829, 1755, 1635, 1517, 1446, 1326, 1230, 1200, 1162, 1027. HRMS (m/z): for C28H30F4N8O6: 650.2224, found 650.0167.
2.2.22. Preparation of N-(R)-1-(4-(4-((R)-5-((1H-1,2,3-triazol-1-yl)methyl)-2-oxooxazolidin-3-yl)-2-fluorophenyl)piperazin-1-yl)-1-oxopropan-2-yl)-3-aminobenzamide 2,2,2-trifluoroacetate 9k
Compound 9k was prepared via a similar procedure to 9j from 3-((tert-butoxycarbonyl) amino benzoic acid and 16a (1.00 g, 1.881 mmol) to give the intermediate compound 17b as a white solid, 314 mg, yield 26%, recrystallized (EtOAc). mp.: 194–197 °C. 1H-NMR (DMSO-d6, 600 MHZ): δ 9.48 (s, 1H, NH, exchangeable with D2O), 8.55 (d, 1H, J = 10.0 Hz, NH, exchangeable with D2O), 8.17 (d, 1H, J = 0.5 Hz, triazole H), 7.90 (s, 1H, aminobenzene H), 7.77 (s, 1H, triazole H), 7.54 (d, 1H, J = 19.9 Hz, aminobenzene H), 7.47 (d, 1H, J = 14.9 Hz, aminobenzene H), 7.41 (dd, 1H, J = 2.5 Hz, 14.7 Hz, phenyl H), 7.33 (t, 1H, J = 14.9 Hz, aminobenzene H), 7.05–7.15 (m, 2H, phenyl H), 5.10–5.14 (m, 1H, oxazolidinone H), 4.94–4.08 (m, 1H, d-alanine CH), 4.83 (d, 2H, J = 5.10, CH2), 4.21 (t, 1H, J = 9.0 Hz, oxazolidinone H), 3.86 (dd, 1H, J = 5.5 Hz, 9.1 Hz, oxazolidinone H), 3.58–3.70 (m, 4H, piperazine H), 2.92–3.05 (m, 4H, piperazine H), 1.48 (s, 9H, (CH3)3), 1.31(d, 3H, J = 7.0 Hz, CH3). IR (KBr pellet, cm−1): ν 3409, 3255, 2979, 2934, 1763, 1639, 1517, 1480, 1443, 1322, 1233, 1159, 1028. HRMS (m/z): for C31H37FN8O6: 636.2820, found 637.2976 (M+ + H).
The intermediate compound 17b was deprotected via a similar procedure to 16a to give the title Compound 9k as a solid, 180 mg, yield 75%, mp.: 129–132 °C. 1H-NMR (DMSO-d6, 600 MHZ): δ 8.54 (d, 1H, J = 7.4 Hz, NH, exchangeable with D2O), 8.18 (s,1H, triazole H), 7.78 (d, 1H, J = 0.5 Hz, triazole H), 7.35–7.45 (m, 3H, phenyl H), 7.28 (t, 1H, J = 7.8 Hz, phenyl H), 7.12–7.46 (m, 1H, phenyl H), 7.07 (t, 1H, J = 9.3 Hz, phenyl H), 7.01 (d, 1H, J = 7.5 Hz, phenyl H), 5.11–5.15 (m, 1H, oxazolidinone H), 4.94–4.99 (m, 1H, d-alanine CH), 4.84 (d, 2H, J = 5.0 Hz, CH2), 4.20–4.23 (m, 2H, oxazolidinone H), 3.87 (dd, 1H, J = 5.7 Hz, 9.3 Hz, oxazolidinone H, overlapping with +NH3 signal), 3.50–3.88 (br. m, 7H, piperazine H, overlapping with +NH3 signal, which is exchangeable with D2O), 2.95–3.27 (br. d, 4H, piperazine H), 1.31 (d, 3H, J = 7.0 Hz, CH3). 13C-NMR (DMSO-d6, 600 MHZ): δ 170.4, 165.7, 158.3, 158.0, 155.4, 153.7, 153.5, 135.4, 135.1, 133.4, 133.3, 133.2, 129.1, 125.9, 125.9, 119.9, 119.8, 117.1, 116.4, 115.1, 114.3, 106.9, 106.7, 70.8, 51.7, 50.6, 50.3, 47.3, 47.1, 45.1, 44.9, 43.0, 41.6, 17.5. IR (KBr pellet, cm−1): ν 3423, 2921, 1753, 1674, 1638, 1518, 1447, 1326, 1231, 1201, 1135, 1028. HRMS (m/z): for C28H30F4N8O6: 650.2224, found 650.0204 (M+).
2.2.23. Preparation of N-(R)-1-(4-(4-((R)-5-((1H-1,2,3-triazol-1-yl)methyl)-2-oxooxazolidin-3-yl)-2-fluorophenyl)piperazin-1-yl)-1-oxopropan-2-yl)-4-aminobenzamide 2,2,2-trifluoroacetate 9l
Compound 9l was prepared via a similar procedure to 9j from 3-((tert-butoxycarbonyl) amino benzoic acid and 16a (1.00 g, 1.881 mmol) to give the intermediate compound 17c as a white solid, 352 mg, yield 32%, recrystallized (EtOAc), mp.: 142–145 °C. 1H-NMR (DMSO-d6, 600 MHZ): δ 9.62 (s, 1H, NH, exchangeable with D2O), 8.47 (d, 1H, J = 7.6 Hz, NH, exchangeable with D2O), 8.17 (d, 1H, J = 0.9 Hz, triazole H), 7.82 (d, 2H, J = 8.8 Hz, aminobenzene H), 7.77 (d, 1H, J = 0.7 Hz, triazole H), 7.52 (d, 2H, J = 8.8 Hz, aminobenzene H), 7.43 (dd, 1H, J = 2.5 Hz, 14.7 Hz, phenyl H), 7.13 (dd, 1H, J = 2.3 Hz, 8.8 Hz, phenyl H), 7.06 (t, 1H, J = 8.8 Hz, phenyl H), 5.11–5.14 (m, 1H, oxazolidinone H), 4.95–5.00 (m, 1H, d-alanine CH), 4.83 (d, 2H, J = 5.1 Hz, CH2), 4.21 (t, 1H, J = 9.2 Hz, oxazolidinone H), 3.86 (dd, 1H, J = 5.7 Hz, 9.3 Hz, oxazolidinone H), 3.61–3.70 (m, 4H, piperazine H), 2.95–2.98 (m, 4H, piperazine H), 1.49 (s, 9H, (CH3)3), 1.31 (d, 3H, J = 7.0 Hz, CH3). IR (KBr pellet, cm−1): ν 3409, 2979, 2932, 1758, 1730, 1637, 1517, 1446, 1320, 1232, 1159, 1027. HRMS (m/z): for C31H37FN8O6: 636.2820, found 637.3028 (M+ + H).
The intermediate compound 17c was deprotected via a similar procedure to 16a to give the title Compound 9l as a solid, 261 mg, yield 71%, recrystallized (EtOAc); mp.: 139–142 °C. 1H-NMR (DMSO-d6, 600 MHZ): δ 8.20 (d, 1H, J = 7.6 Hz, NH, exchangeable with D2O), 8.18 (d,1H, J = 0.5 Hz, triazole H), 7.78 (s, 1H, triazole H), 7.67 (d, 1H, J = 8.6 Hz, aminobenzene H), 7.43 (dd, 1H, J = 2.4 Hz, 14.6 Hz, phenyl H), 7.14 (dd, 1H, J = 2.6 Hz, 8.9 Hz, phenyl H), 7.06 (t, 1H, J = 9.3 Hz, phenyl H), 6.63 (d, 2H, J = 8.5 Hz, phenyl H), 5.11–5.16 (m, 1H, oxazolidinone H), 4.92–4.98 (m, 1H, d-alanine CH), 4.83 (d, 2H, J = 5.0 Hz, CH2), 4.21 (t, 2H, oxazolidinone H overlaps with +NH3 signal), 3.90–4.50 (br. m, 4H, oxazolidinone H overlaps with +NH3 signal), 3.68 (dd, 1H, J = Hz, oxazolidinone H), 3.60–3.69 (m, 4H, piperazine H), 2.94–3.30 (br. d, 4H, piperazine H), 1.29 (d, 3H, J = 6.9 Hz, CH3). 13C-NMR (DMSO-d6, 600 MHZ): δ 170.8, 165.5, 158.3, 158.1, 155.4, 153.7, 153.5, 135.4, 135.4, 133.8, 133.7, 133.4, 133.3, 133.2, 129.2, 129.0, 128.3, 125.9, 125.9, 119.9, 119.7, 114.8, 114.3, 106.8, 106.7, 70.8, 51.7, 51.7, 50.6, 50.3, 47.3, 47.1, 47.1, 44.9, 44.7, 43.0, 41.6, 17.6. IR (KBr pellet, cm−1): ν 3364, 2985, 2835, 1750, 1674, 1635, 1518, 1448, 1231, 1199, 1134, 1027. HRMS (m/z): for C28H30F4N8O6: 650.2224, found 650.0215 (M+).
2.2.24. Preparation of N-((S)-1-(4-(4-((R)-5-((1H-1,2,3-triazol-1-yl)methyl)-2-oxooxazolidin-3-yl)-2-fluorophenyl)piperazin-1-yl)-1-oxopropan-2-yl)-2-aminobenzamide 2,2,2-trifluoroacetate 10j
Compound 10j was prepared via a similar procedure to 9j starting from 3-((tert-butoxycarbonyl) amino benzoic acid and 16b (1.00 g, 1.881 mmol) to give the intermediate compound 18a as a white solid, 377 mg, yield 32%, recrystallized (EtOAc), mp.: 129–131 °C. 1H-NMR (DMSO-d6, 600 MHZ): δ 10.46 (s, 1H, NH, exchangeable with D2O), 8.91 (d, 1H, J = 7.3 Hz, NH, exchangeable with D2O), 8.20 (d, 1H, J = 8.5 Hz, phenyl H), 8.18 (d, 1H, J = 0.5 Hz, triazole H), 7.81 (d,1H, J = 7.9 Hz, phenyl H), 7.78 (s, 1H, triazole H), 7.47–7.50 (m, 1H, phenyl H), 7.43 (dd, 1H, J = 2.4 Hz, 14.7 Hz, phenyl H), 7.08–7.14 (m, 3H, phenyl H), 5.12–5.14 (m, 1H, oxazolidinone H), 4.95–5.00 (m, 1H, l-alanine CH), 4.84 (d, 2H, J = 5.0 Hz, CH2), 4.21 (t, 1H, J = 9.2 Hz, oxazolidinone H), 3.87 (dd, 1H, J = 5.7 Hz, 9.3 Hz, oxazolidinone H), 3.60–3.78 (m, 4H, piperazine H), 2.90–3.06 (m, 4H, piperazine H), 1.45 (s, 9H, (CH3)3), 1.34 (d, 3H, J = 7.0 Hz, CH3). 13C-NMR (DMSO-d6, 600 MHZ): δ 170.2, 167.8, 155.4, 153.8, 153.5, 152.1, 139.4, 135.4, 135.4, 133.4, 133.3, 133.3, 132.2, 128.6, 125.9, 121.4, 119.8, 119.3, 118.5, 114.3, 106.8, 106.7, 79.8, 70.8, 64.9, 51.7, 50.7, 50.2, 47.1, 45.3, 44.9, 41.7, 30.7, 27.9, 17.1. IR (KBr pellet, cm−1): ν 3350, 3114, 2978, 2931, 1757, 1718, 1647, 1589, 1442, 1227, 1160, 1110, 1050, 1026. HRMS (m/z): for C31H37FN8O6: 636.2820, found 637.0000 (M+ + H). LRMS (m/z): 536.3 (M+ − (CH3)2C=CH2 + CO2). Anal. calcd. for CHN: C: 58.48, H: 5.86, N: 17.6, found C: 58.50, H: 6.38, N: 18.04.
The intermediate compound 18a was deprotected via a similar procedure to 16b to give 10j as a white solid, 240 mg, yield 57%; recrystallized (EtOAc), mp.: 168–173 °C. 1H-NMR (DMSO-d6, 600 MHZ): δ 8.40 (d, 1H, J = 7.4 Hz, NH, exchangeable with D2O), 8.18 (d, 1H, J = 0.9 Hz, triazole H), 7.78 (d, 1H, J = 0.7 Hz, triazole H), 7.60 (dd, 1H, J = 1.3 Hz, 7.9 Hz, phenyl H), 7.43 (dd, 1H, J = 2.5 Hz, 14.7 Hz, phenyl H), 7.17–7.20 (m, 1H, phenyl H), 7.14 (dd, 1H, J = 2.3 Hz, 8.8 Hz, phenyl H), 7.07 (t, 1H, J = 9.3 Hz, phenyl H), 6.75 (d, 1H, J = 7.9 Hz, phenyl H), 6.60 (t, 1H, J = 7.5 Hz, phenyl H), 5.10–5.15 (m, 1H, oxazolidinone H), 4.92–4.96 (m, 1H, l-alanine CH), 4.83 (d, 2H, J = 5.1 Hz, CH2), 4.21 (t, 1H, J = 9.2 Hz, oxazolidinone H), 3.87 (dd, 1H, J = 5.7 Hz, 9.3 Hz, oxazolidinone H), 3.40–3.80 (br. m, 7H, piperazine H, overlapping with +NH3 signal, which is exchangeable with D2O), 2.95–3.01 (br. d, 4H, piperazine H), 1.31 (s, 3H, J = 7.0 Hz, CH3). 13C-NMR (DMSO-d6, 600 MHZ): δ 170.6, 168.0, 158.3, 158.0, 155.4, 153.7, 153.5, 135.4, 135.4, 133.4, 133.3, 133.2, 131.9, 128.5, 125.9, 119.7, 116.8, 115.5, 115.2, 114.3, 106.8, 106.7, 70.8, 51.7, 50.7, 50.3, 47.1, 44.9, 44.7, 41.6, 30.7, 17.4. IR (KBr pellet, cm−1): ν 3427, 2984, 2922, 1755, 1635, 1587, 1517, 1447, 1327, 1230, 1200, 1138, 1028. HRMS (m/z): for C28H30F4N8O6: 650.2224, found 650.0234 (M+).
2.2.25. Preparation of N-((S)-1-(4-(4-((R)-5-((1H-1,2,3-triazol-1-yl)methyl)-2-oxooxazolidin-3-yl)-2-fluorophenyl)piperazin-1-yl)-1-oxopropan-2-yl)-3-aminobenzamide 2,2,2-trifluoroacetate 10k
Compound 10k was prepared via a similar procedure to 9j starting from 3-((tert-butoxycarbonyl) amino benzoic acid and 16b (1.00 g, 1.881 mmol) to give the intermediate compound 18b as a white solid, 480 mg, yield 43%, recrystallized (EtOAc); mp.: 171–174 °C. 1H-NMR (DMSO-d6, 600 MHZ): δ 9.50 (s, 1H, NH, exchangeable with D2O), 8.57 (d, 1H, J = 7.6 Hz, NH, exchangeable with D2O), 8.18 (d, 1H, J = 0.9 Hz, triazole H), 7.98 (s, 1H, phenyl H), 7.78 (d,1H, J = 0.9 Hz, triazole H), 7.55 (d, 1H, J = 7.9 Hz, phenyl H), 7.48 (d, 1H, J = 7.9 Hz, phenyl H), 7.44 (dd, 1H, J = 2.5 Hz, 14.7 Hz, phenyl H), 7.34 (t, 1H, J = 9.2 Hz, phenyl H), 7.13 (dd, 1H, J = 2.3 Hz, 9.3 Hz, phenyl H), 7.07 (t, 1H, J = 9.3 Hz, phenyl H), 5.12–5.13 (m, 1H, oxazolidinone H), 4.95–4.97 (m, 1H, l-alanine CH), 4.84 (d, 2H, J = 5.1 Hz, CH2), 4.21 (t, 1H, J = 9.2 Hz, oxazolidinone H), 3.87 (dd, 1H, J = 5.7 Hz, 9.3 Hz, oxazolidinone H), 3.61–3.70 (br. m, 4H, piperazine H), 2.96–3.00 (br. d, 4H, piperazine H), 1.49 (s, 9H, (CH3)3), 1.32 (d, 3H, J = 7.0 Hz, CH3). 13C-NMR (DMSO-d6, 600 MHZ): δ 170.4, 165.9, 155.4, 153.7, 153.5, 152.8, 139.6, 135.4, 135.4, 134.8, 133.4, 133.3, 133.2, 128.4, 125.8, 120.9, 120.8, 119.8, 119.7, 117.5, 114.3, 106.8, 106.6, 79.2, 70.8, 51.7, 50.6, 50.3, 47.1, 45.1, 44.9, 41.6, 28.1, 17.4. IR (KBr pellet, cm−1): ν 3414, 3256, 2978, 1760, 1730, 1637, 1556, 1519, 1441, 1414, 1237, 1158, 1029. HRMS (m/z): for C31H37FN8O6: 636.2820, found 637.0000 (M+ + H). CHN Anal. calcd.: C: 58.48, H: 5.86, N: 17.60, found C: 58.78, H: 6.29, N: 17.60.
The intermediate compound 18b was deprotected via a similar procedure to 16b to give 10k as a white solid, 323 mg, yield 71%; recrystallized (EtOAc), mp.: 203–208 °C. 1H-NMR (DMSO-d6, 600 MHZ): δ 8.80 (d, 1H, J = 7.4 Hz, NH, exchangeable with D2O), 8.17 (d, 1H, J = 0.6 Hz, triazole H), 7.87 (d, 1H, J = 7.8 Hz, phenyl H), 7.77 (d, 1H, J = 6.1 Hz, triazole H and phenyl H), 7.56 (t, 1H, J = 7.9 Hz, phenyl H), 7.47 (d, 1H, J = 8.0 Hz, phenyl H), 7.43 (dd, 1H, J = 2.4 Hz, 14.7 Hz, phenyl H), 7.13 (dd, 1H, J = 2.2 Hz, 8.9 Hz, phenyl H), 7.06 (t, 1H, J = 9.3 Hz, phenyl H), 5.10–5.14 (m, 1H, oxazolidinone H), 4.96–5.00 (m, 1H, l-alanine CH), 4.82 (d, 2H, J = 5.0 Hz, CH2), 4.20 (t, 1H, J = 9.2 Hz, oxazolidinone H), 3.86 (dd, 1H, J = 5.7 Hz, 9.3 Hz, oxazolidinone H, overlapping with +NH3 signal), 3.50–3.86 (br. m, 7H, piperazine H, overlapping with +NH3 signal, exchangeable with D2O), 2.95–3.00 (m, 4H, piperazine H), 1.32 (d, 3H, J = 6.9 Hz, CH3). 13C-NMR (DMSO-d6, 600 MHZ): δ 170.4, 165.7, 158.3, 158.0, 155.4, 153.7, 153.5, 135.4, 135.1, 133.4, 133.3, 133.2, 129.1, 125.9, 125.9, 119.9, 119.8, 117.1, 116.4, 115.1, 114.3, 106.9, 106.7, 70.9, 51.7, 50.6, 50.3, 47.3, 47.1, 45.1, 44.9, 43.0, 41.6, 17.5. IR (KBR pellet, cm−1): 3406, 3061, 2921, 2854, 2585, 1758, 1641, 1519, 1482, 1443, 1417, 1336, 1280, 1216, 1167, 1147, 1023. HRMS (m/z): for C28H30F4N8O6: 650.2224, found 650.0000 (M+).
2.2.26. Preparation of N-((S)-1-(4-(4-((R)-5-((1H-1,2,3-triazol-1-yl)methyl)-2-oxooxazolidin-3-yl)-2-fluorophenyl)piperazin-1-yl)-1-oxopropan-2-yl)-4-aminobenzamide 2,2,2-trifluoroacetate 10l
Compound 10l was prepared via a similar procedure to 9j starting from 3-((tert-butoxycarbonyl) amino benzoic acid and 16b (1.00 g, 1.881 mmol) to give the intermediate compound 18c as a white solid, 485 mg, yield 43%, recrystallized (EtOAc); mp.: 139–142 °C. 1H-NMR (DMSO-d6, 600 MHZ): δ 9.64 (s, 1H, NH, exchangeable with D2O), 8.49 (d, 1H, J = 7.6 Hz, NH, exchangeable with D2O), 8.18 (d, 1H, J = 0.9 Hz, triazole H), 7.83 (d, 2H, J = 8.8 Hz, phenyl H), 7.78 (d, 1H, J = 0.8 Hz, triazole H), 7.52 (d, 2H, J = 8.7 Hz, phenyl H), 7.43 (dd, 1H, J = 2.5 Hz, 14.7 Hz, phenyl H), 7.13 (dd, 1H, J = 2.3 Hz, 8.8 Hz, phenyl H), 7.05 (t, 1H, J = 9.3 Hz, phenyl H), 5.11–5.15 (m, 1H, oxazolidinone H), 4.95–5.00 (m, 1H, d-alanine CH), 4.84 (d, 2H, J = 5.1 Hz, CH2), 4.21 (t, 1H, J = 9.2 Hz, oxazolidinone H), 3.87 (dd, 1H, J = 5.7 Hz, 9.3 Hz, oxazolidinone H), 3.61–3.70 (br. m, 4H, piperazine H), 2.95-2.98 (br. m, 4H, piperazine H), 1.49 (s, 9H, (CH3)3), 1.31 (d, 3H, J = 7.0 Hz, CH3). 13C-NMR (DMSO-d6, 600 MHZ): δ 170.6, 165.2, 155.3, 153.7, 153.5, 152.9, 142.4, 135.4, 135.4, 133.4, 133.3, 133.2, 128.3, 127.2, 125.8, 119.8, 119.7, 117.0, 114.3, 114.3, 106.8, 106.7, 79.5, 70.8, 64.9, 51.7, 50.6, 50.3, 47.1, 44.9, 41.6, 28.1, 17.5. IR (KBr pellet, cm−1): ν 3409, 2979, 2932, 1757, 1727, 1637, 1446, 1320, 1232, 1159, 1027. HRMS (m/z): for C31H37FN8O6: 636.2820, found 637.0000 (M+ + H).
The intermediate compound 18c was deprotected via a similar procedure to 16a to give 10l as a white solid, 229 mg, yield 63%; recrystallized (EtOAc), mp.: 239–242 °C. 1H-NMR (DMSO-d6, 600 MHZ): δ 8.60 (br. d, 1H, J = 6.5 Hz, NH, exchangeable with D2O), 8.17 (d, 1H, J = 0.8 Hz, triazole H), 7.89 (d, 2H, J = 8.5 Hz, phenyl H), 7.76 (s, 1H, J = 0.7 Hz, triazole H), 7.42 (dd, 1H, J = 2.5 Hz, 14.7 Hz, phenyl H), 7.21 (d, 2H, J = 8.0 Hz, phenyl H), 7.12 (dd, 1H, J = 2.3 Hz, 8.8 Hz, phenyl H), 7.06 (t, 1H, J = 9.3 Hz, phenyl H), 5.10–5.13 (m, 1H, oxazolidinone H), 4.94–4.99 (m, 1H, l-alanine CH), 4.82 (d, 2H, J = 5.1 Hz, CH2), 4.20 (t, 1H, J = 9.2 Hz, oxazolidinone H, partially overlaps with the +NH3 signal), 3.74–4.30 (br., 3H, +NH3, exchangeable with D2O, partially overlaps with oxazolidinone H) 3.86 (dd, 1H, J = 5.7 Hz, 9.3 Hz, oxazolidinone H, partially overlaps with the +NH3 signal), 3.59–3.72 (br. m, 4H, piperazine H), 2.95-2.99 (br. d, 4H, piperazine H), 1.31 (s, 3H, J = 7.0 Hz, CH3). 13C-NMR (DMSO-d6, 600 MHZ): δ 170.5, 165.0, 155.3, 153.7, 153.4, 135.3, 135.3, 133.3, 133.2, 129.1, 128.9, 125.8, 119.7, 119.4, 114.3, 106.9, 106.7, 70.7, 51.7, 50.6, 50.2, 47.1, 47.1, 45.0, 44.9, 41.9, 17.4. IR (KBr pellet, cm−1): ν 3407, 2833, 2575, 1758, 1640, 1515, 1417, 1234, 1217, 1020. HRMS (m/z): for C28H30F4N8O6: 650.2224, found 649.0000 (M+ − H) and 537.000 (M+ − CF3CO2H).