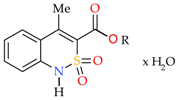
4-Methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic Acid. Peculiarities of Preparation, Structure, and Biological Properties
Abstract
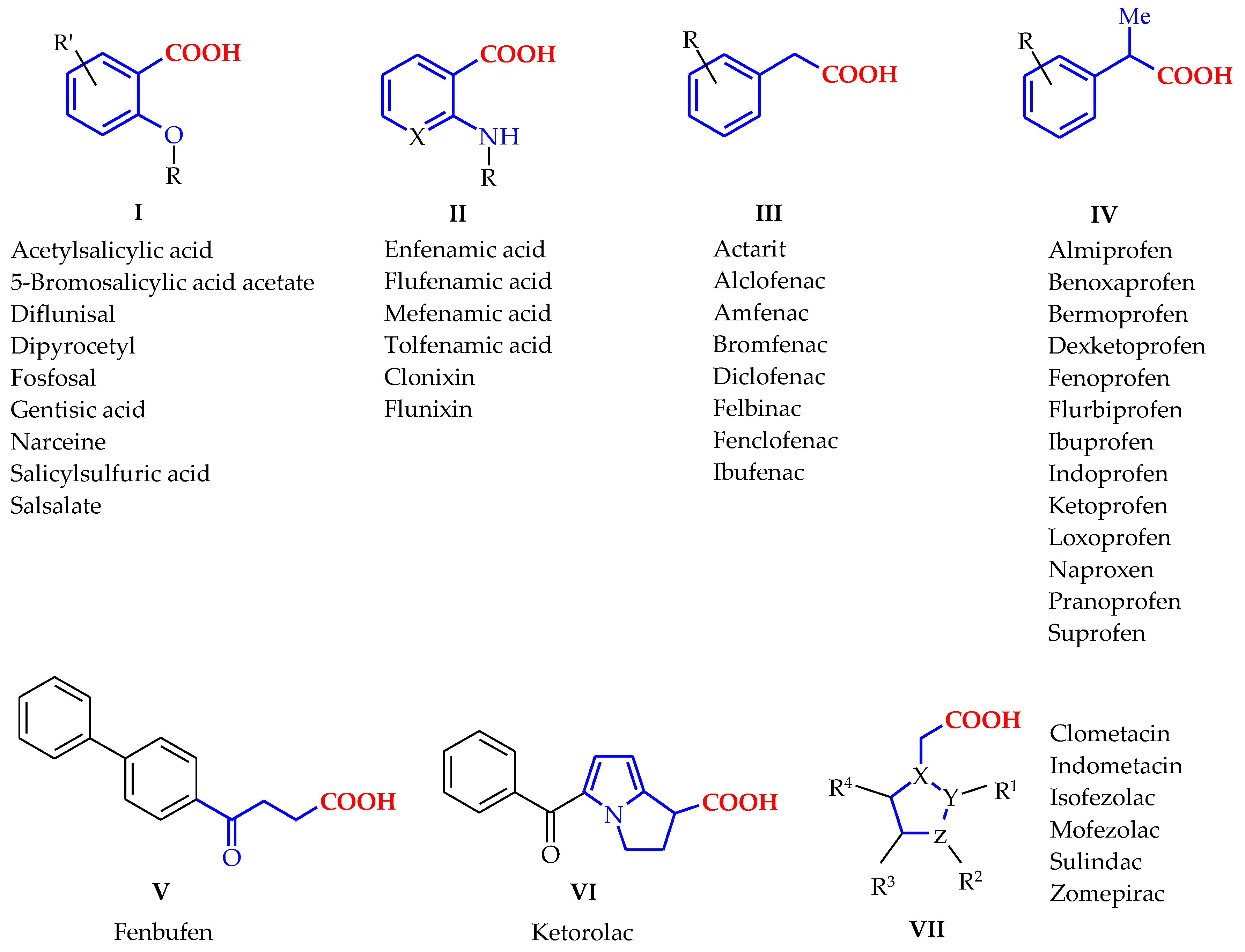
:1. Introduction
2. Materials and Methods
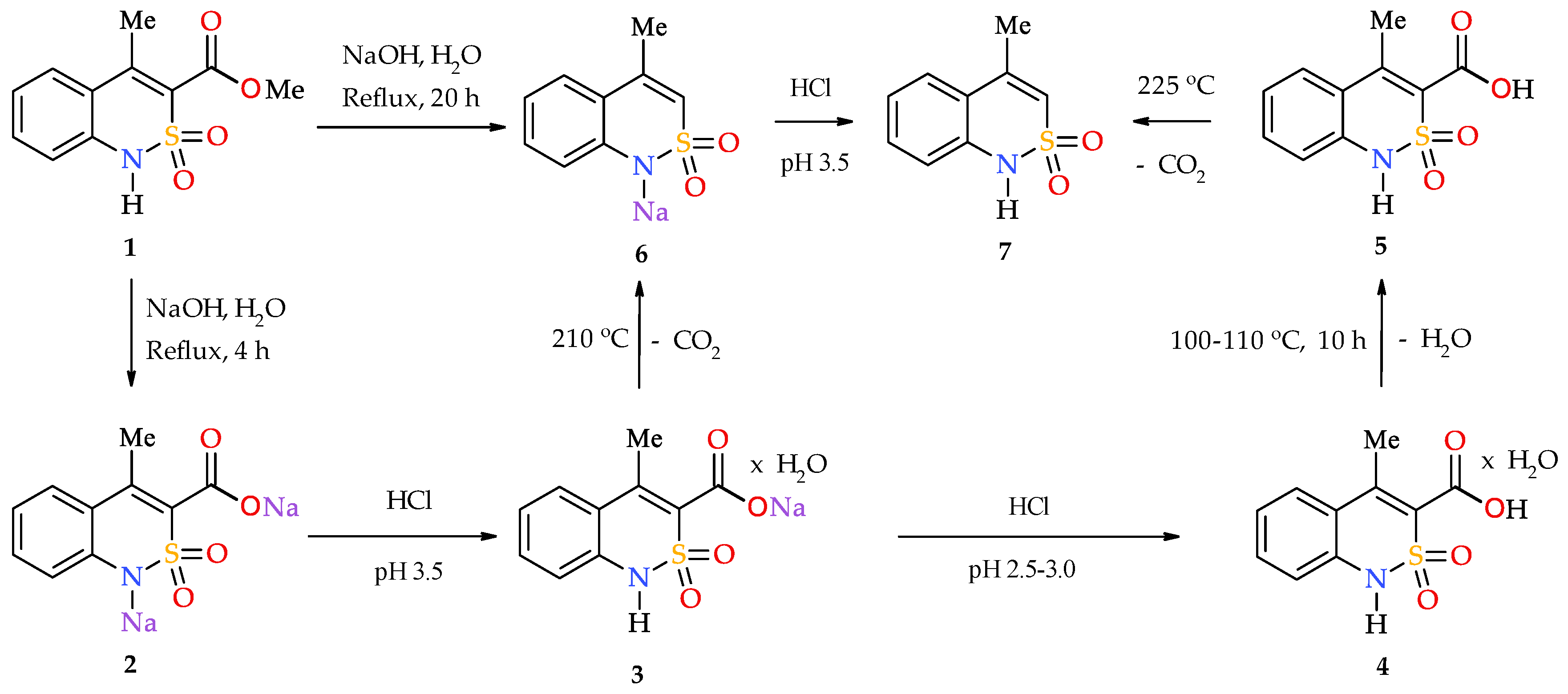
2.1. Chemistry
2.2. Sodium 4-Methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylate Monohydrate (3)
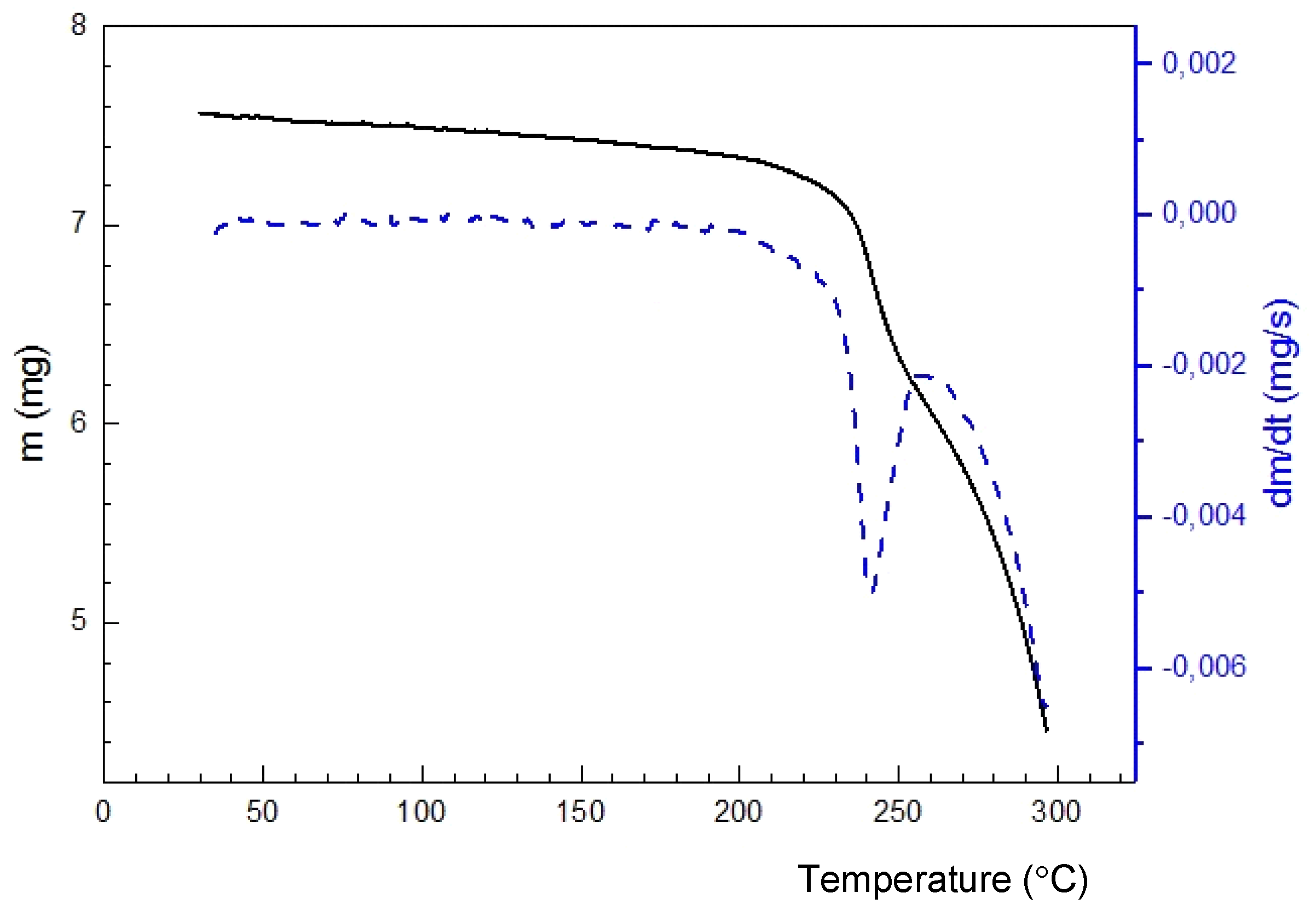
2.3. 4-Methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic Acid Monohydrate (4)
2.4. 4-Methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic Acid (5)
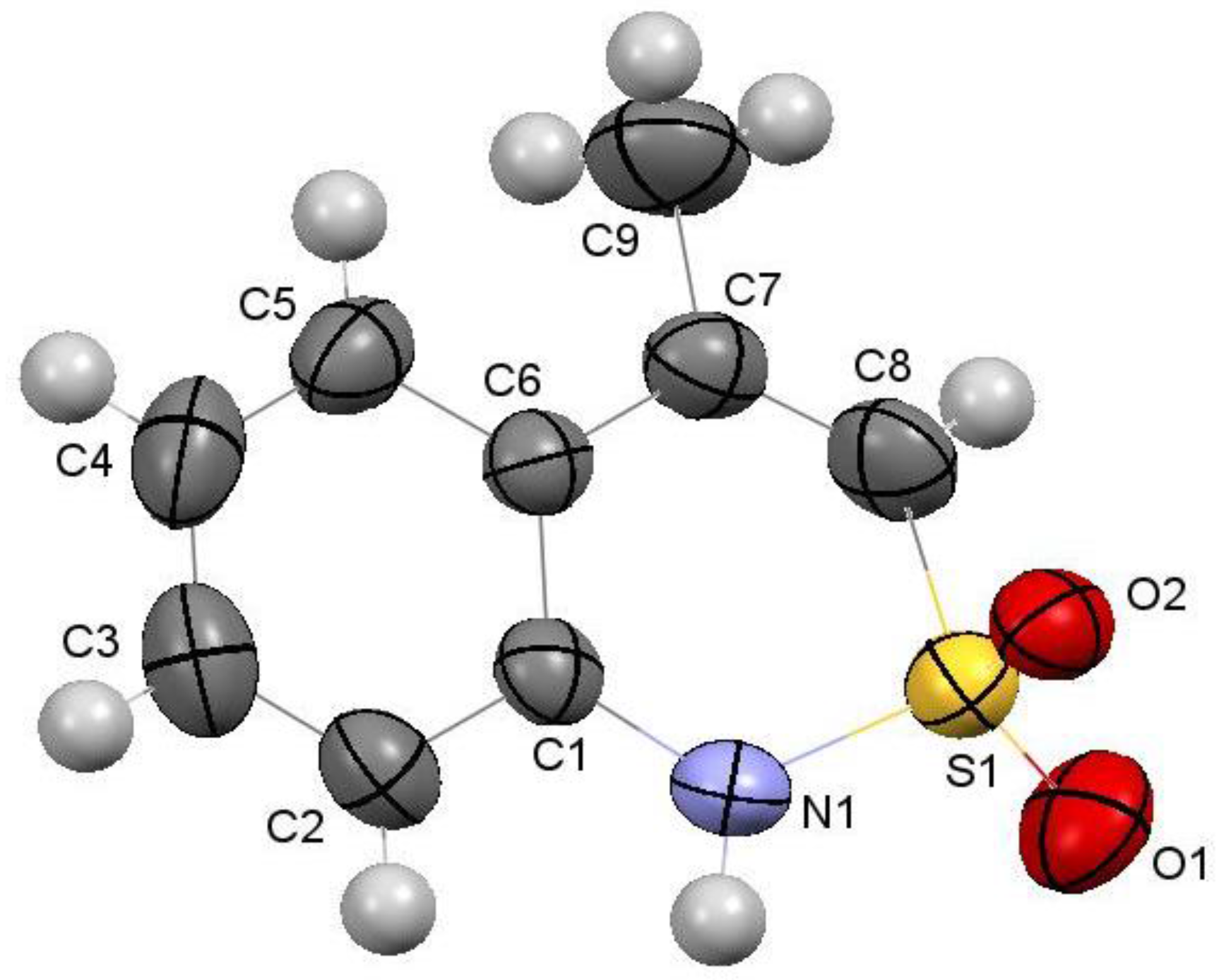
2.5. 4-Methyl-2,2-dioxo-1H-2λ6,1-benzothiazine (7)
- Add sodium hydroxide (1.20 g, 0.03 mol) to the suspension of ester 1 (2.53 g, 0.01 mol) in 15 mL of H2O, then boil under reflux for 20 h. Cool, and acidify the resulting aqueous solution of salt 6 with HCl to рН 3.5. Filter the precipitate of benzothiazine 7 obtained, wash with cold water, and dry in air. Yield: 1.66 g (85%); colorless crystals; mp 179–181 °C (ethanol); 1H-NMR (400 MHz, DMSO-d6): δ 11.31 (br. s, 1H, SO2NH), 7.22 (d, 1Н, J = 7.9 Hz, Н-5), 6.98 (td, 1Н, J = 7.6 and 1.4 Hz, Н-7), 6.61 (d, 1Н, J = 7.6 Hz, Н-8), 6.47 (t, 1Н, J = 7.4 Hz, Н-6), 6.07 (s, 1H, H-3), 2.12 (s, 3Н, 4-CH3). Anal. Calcd. for C9H9NO2S: C, 55.37; H, 4.65; N, 7.17; S 16.42%. Found: C, 55.46; H, 4.73; N, 7.10; S 16.36%.
- Keep 4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic acid (5) (2.39 g, 0.01 mol) at the temperature of 220-225 °C for 10 min. After СО2 evolution is stopped, cool the reaction mixture. Triturate the residue with 10 mL of cold ethanol. Filter the precipitate of benzothiazine 7, and dry in air. Yield: 1.77 g (91%).
- Keep sodium 4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylate monohydrate (3) (2.79 g, 0.01 mol) at the temperature of 200–210 °C for 15 min. The initially colorless salt 3 turns into a yellow powder without melting. Dissolve the resulting sodium salt 6 in 15 mL of H2O and acidify with HCl to рН 3.5. Filter the precipitate of 7, wash with cold water, and dry in air. Yield: 1.81 g (93%).
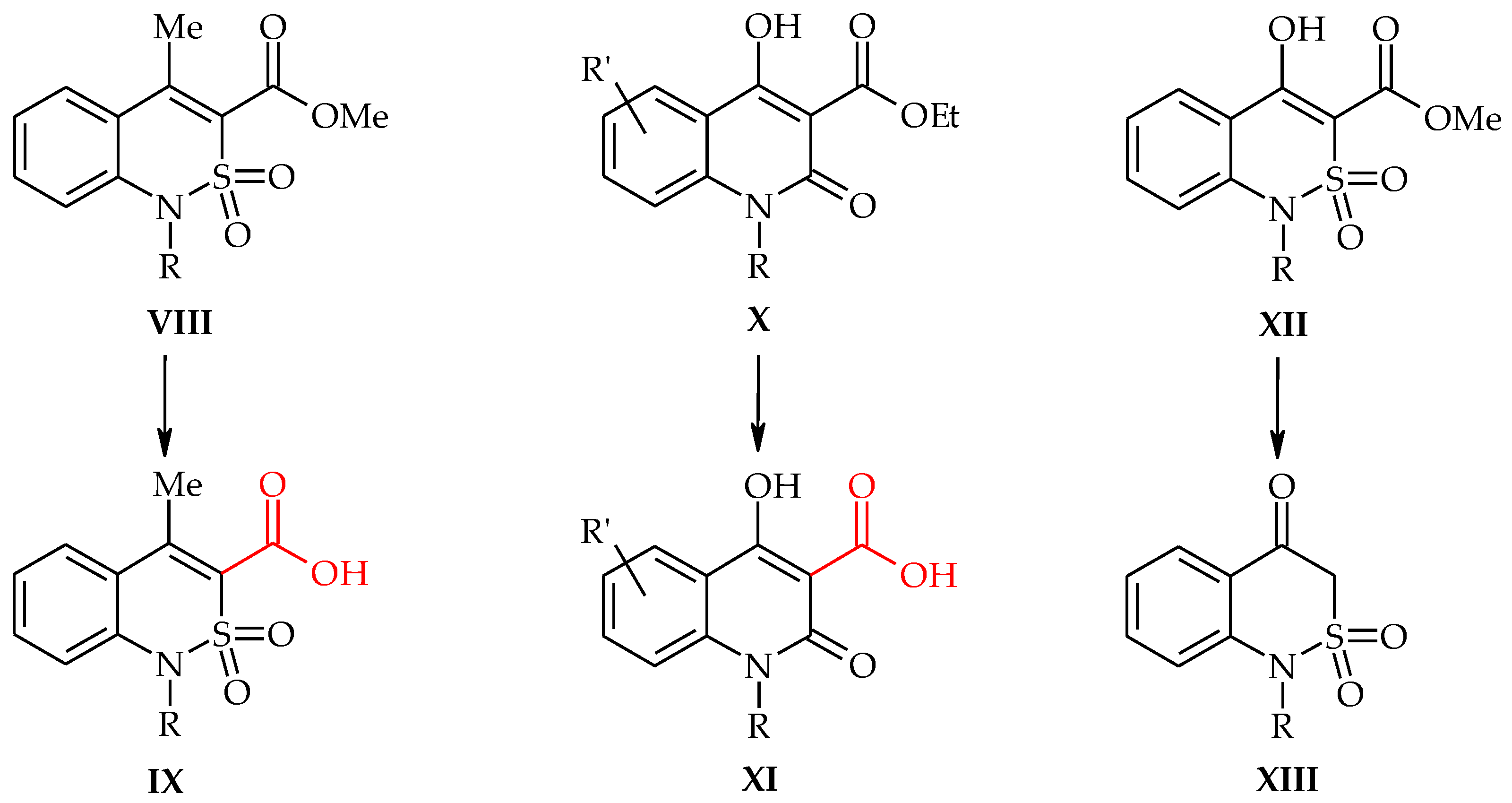
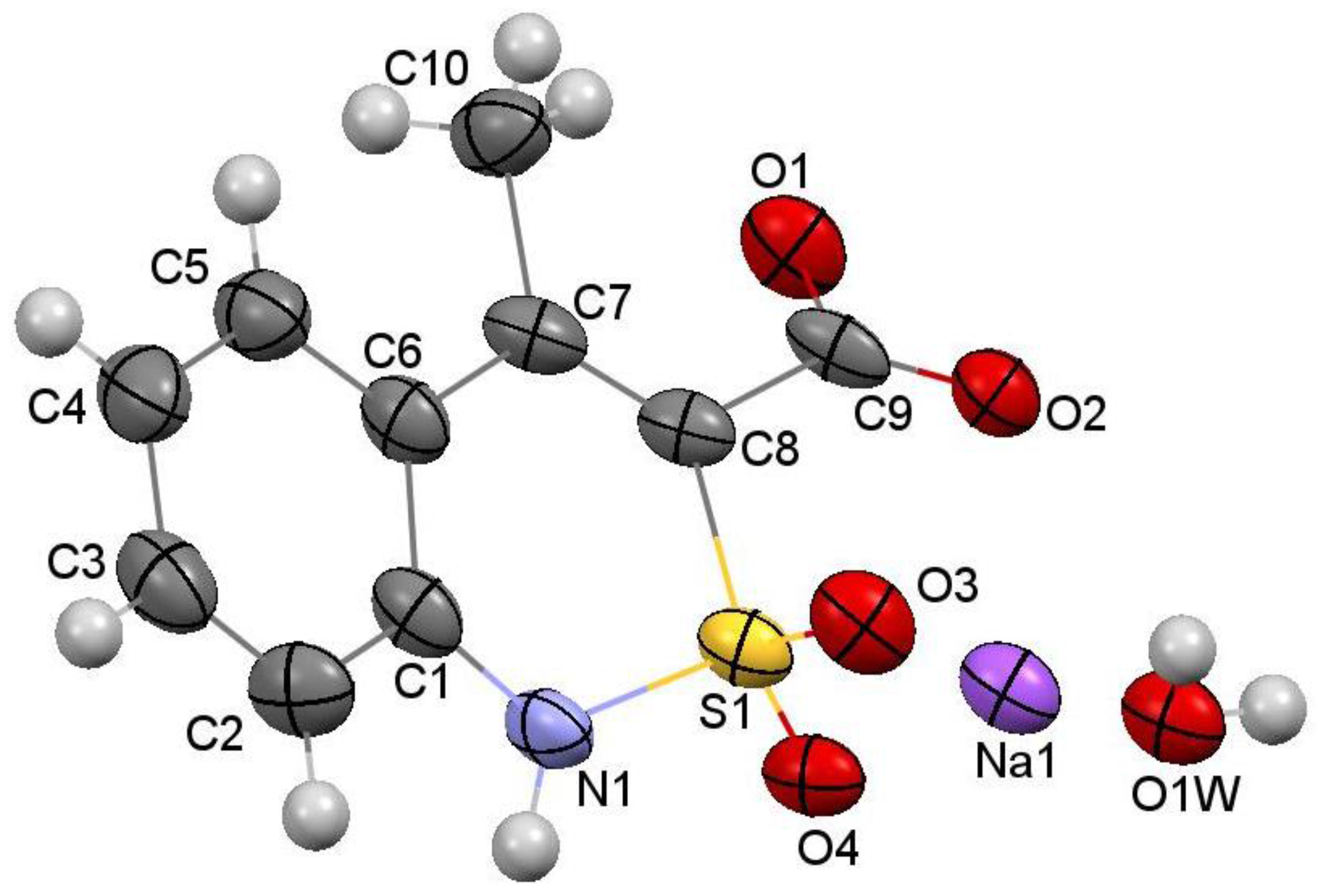
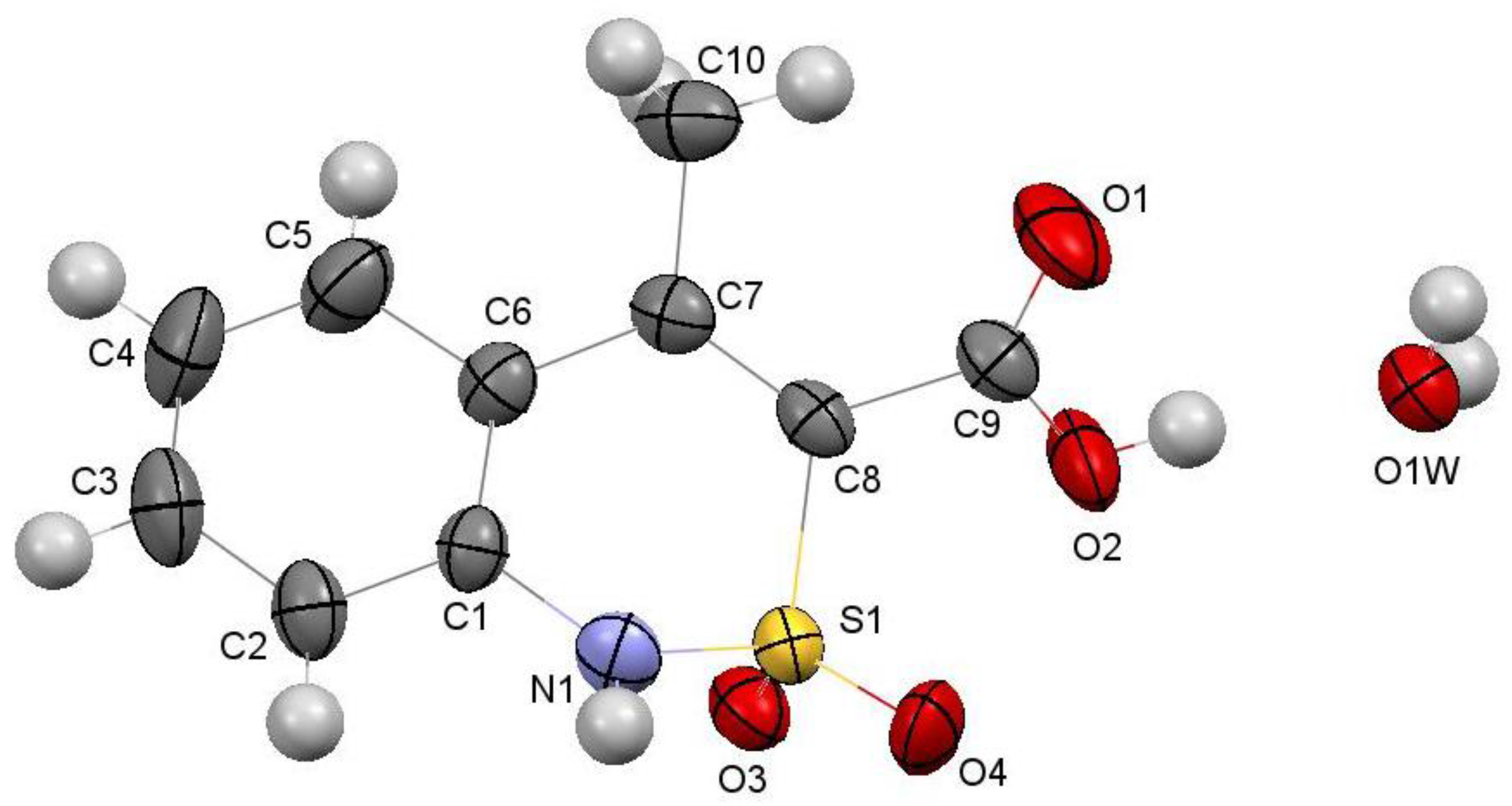
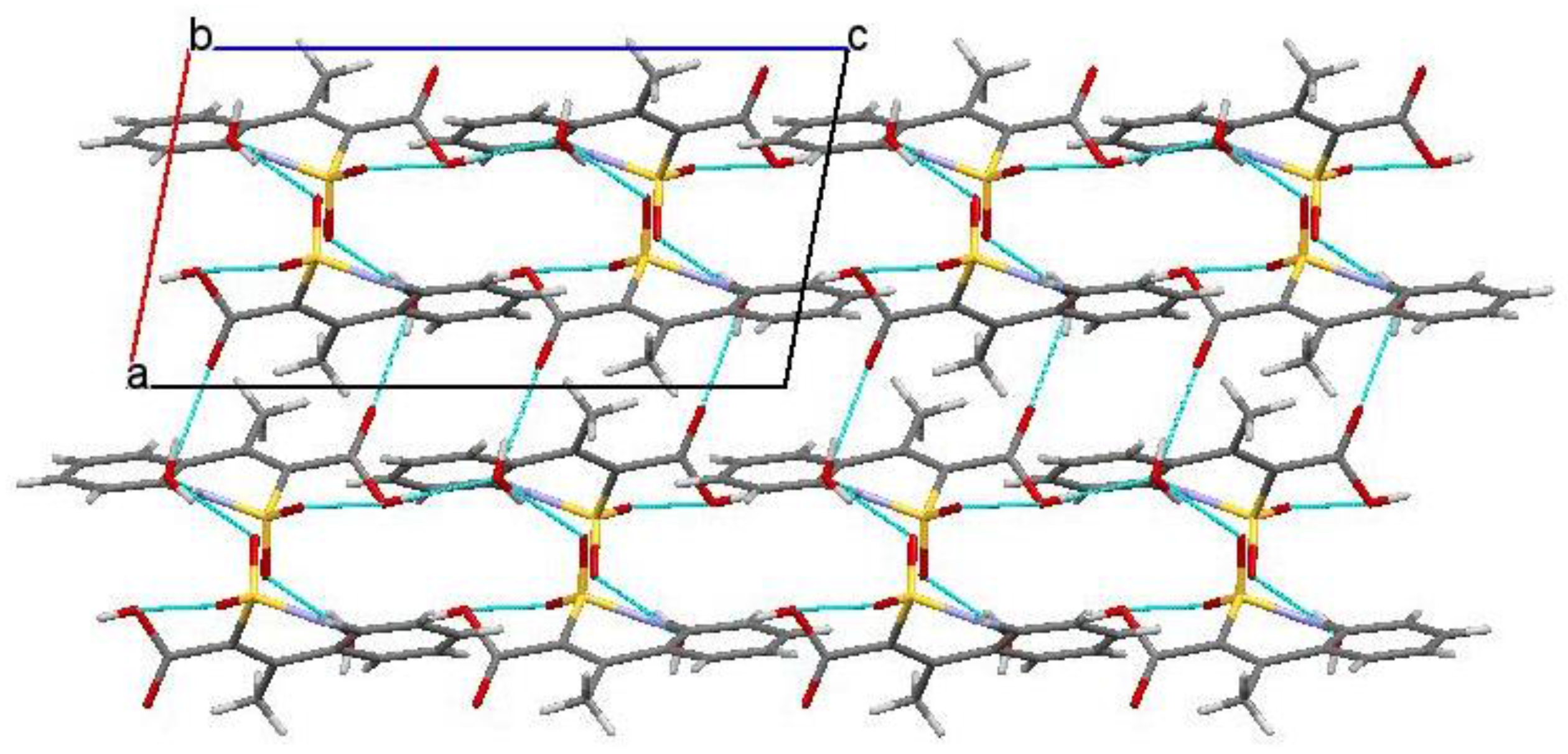
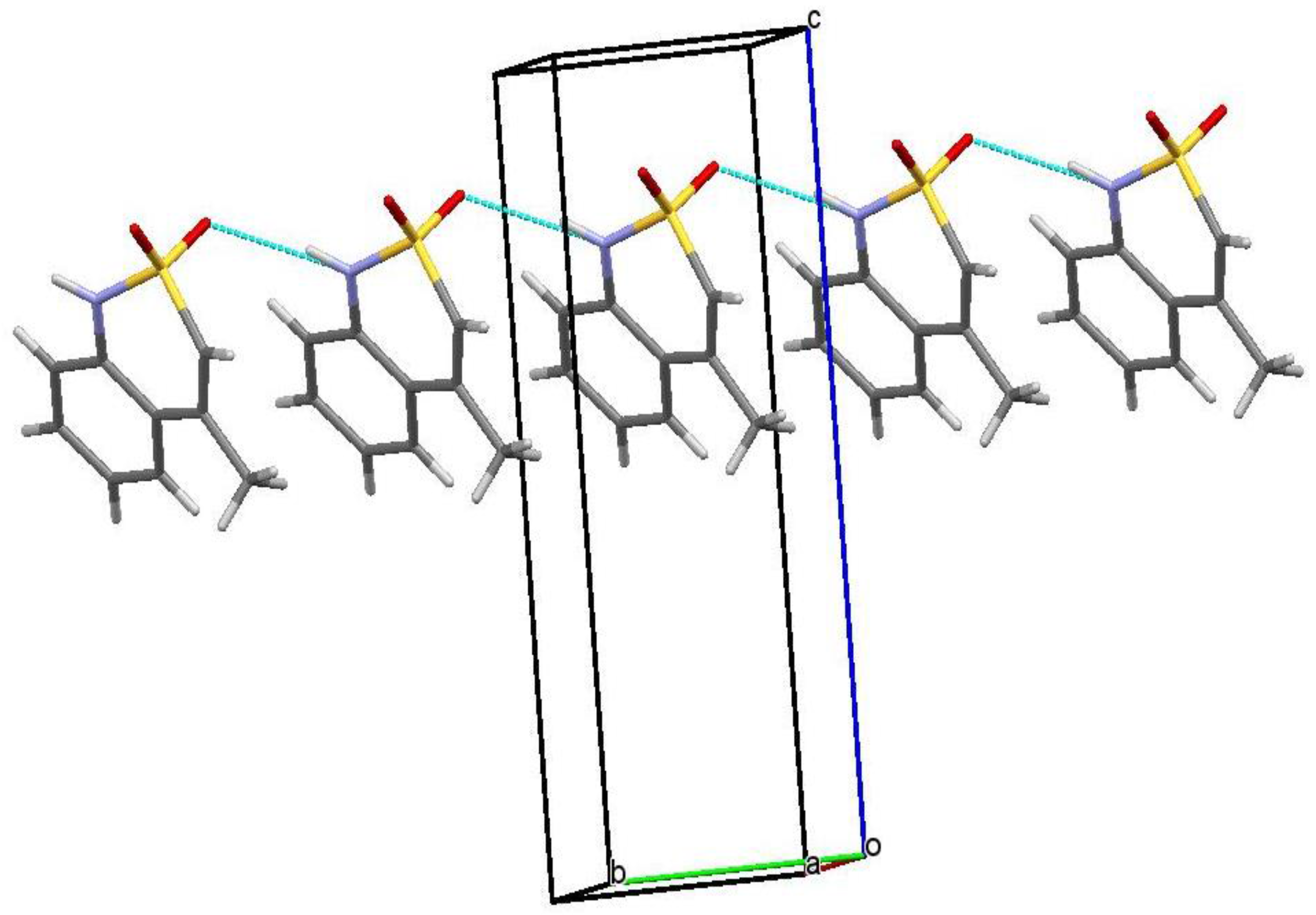
2.6. X-ray Structural Analysis of Sodium 4-Methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylate Monohydrate (3)
2.7. X-ray Structural Analysis of 4-Methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic Acid Monohydrate (4)
2.8. X-ray Structural Analysis of 4-Methyl-2,2-dioxo-1H-2λ6,1-benzothiazine (7)
2.9. Pharmacology
Anti-Inflammatory and Analgesic Test
3. Results and Discussion
3.1. Chemistry
3.2. Evaluation of the Anti-Inflammatory and Analgesic Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kleemann, A.; Engel, J.; Kutscher, B.; Reichert, D. Pharmaceutical Substances: Syntheses, Patents, Applications of the Most Relevant APIs, 5th ed.; Thieme: Stuttgart, Germany, 2008. [Google Scholar]
- O’Neil, M.J.; Heckelman, P.E.; Koch, C.B.; Roman, K.J. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 14th ed.; Merck and Co., Inc.: Whitehouse Station, NJ, USA, 2006. [Google Scholar]
- Proschak, E.; Heitel, P.; Kalinowsky, L.; Merk, D. Opportunities and challenges for fatty acid mimetics in drug discovery. J. Med. Chem. 2017, 60, 5235–5266. [Google Scholar] [CrossRef] [PubMed]
- Ukrainets, I.V.; Mospanova, E.V.; Savchenkova, L.V.; Yankovich, S.I. 4-Hydroxy-2-quinolones. 195. Synthesis of novel, potential analgesics based on 4-(hetarylmethyl)amino-2-oxo-1,2-dihydroquinoline-3-carboxylic acids. Chem. Heterocycl. Compd. 2011, 47, 67–73. [Google Scholar] [CrossRef]
- Kuznetsov, S.G.; Chigareva, S.M.; Ramsh, S.M. Prodrugs. Chemical aspect. Results Sci. Technol. VINITI. Ser. Org. Chem. 1981, 19, 1–176. [Google Scholar]
- Azotla-Cruz, L.; Lijanova, I.V.; Ukrainets, I.V.; Likhanova, N.V.; Olivares-Xometl, O.; Bereznyakova, N.L. New synthesis, structure and analgesic properties of methyl 1-R-4-Methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylates. Sci. Pharm. 2017, 85, 2. [Google Scholar] [CrossRef] [PubMed]
- Ukrainets, I.V.; Davidenko, A.A.; Mospanova, E.V.; Sidorenko, L.V.; Svechnikova, E.N. 4-Hydroxy-2-quinolones. 176. 4-R-2-oxo-1,2-dihydroquinoline-3-carboxylic acids. Synthesis, physicochemical and biological properties. Chem. Heterocycl. Compd. 2010, 46, 559–568. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Petrushova, L.A.; Dzyubenko, S.P.; Liu, Y.Y. 2,1-Benzothiazine 2,2-dioxides. 5. Hydrolysis of alkyl 1-R-4-hydroxy-2,2-dioxo-1Н-2λ6,1-benzothiazine-3-carboxylate. Chem. Heterocycl. Compd. 2014, 50, 1047–1052. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Cambridge Crystallographic Data Center. Request Quoting via: CCDC1826303. Available online: www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 27 February 2018).
- Cambridge Crystallographic Data Center. Request Quoting via: CCDC1826302. Available online: www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 27 February 2018).
- Cambridge Crystallographic Data Center. Request Quoting via: CCDC1826304. Available online: www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 27 February 2018).
- Ukrainian Law No. 3447-IV. On protection of animals from severe treatment. Available online: http://zakon2.rada.gov.ua/laws/show/3447-15 (accessed on 04 August 2017).
- Vogel, H.G. Drug Discovery and Evaluation: Pharmacological Assays, 2nd ed.; Springer: Berlin, Germany, 2008; pp. 1103–1106. [Google Scholar]
- Gregory, N.S.; Harris, A.L.; Robinson, C.R.; Dougherty, P.M.; Fuchs, P.N.; Sluka, K.A. An overview of animal models of pain: Disease models and outcome measures. J. Pain. 2013, 14, 1255–1269. [Google Scholar] [CrossRef] [PubMed]
- Ukrainets, I.V.; Shishkina, S.V.; Baumer, V.N.; Gorokhova, O.V.; Petrushova, L.A.; Sim, G. The structure of two pseudo-enantiomeric forms of N-benzyl-4-hydroxy-1-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide and their analgesic properties. Acta Crystallogr. Sect. C Struct. Chem. 2016, 72, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Ukrainets, I.V.; Petrushova, L.A.; Shishkina, S.V.; Grinevich, L.A.; Sim, G. Synthesis, spatial structure and analgesic activity of sodium 3-benzylaminocarbonyl-1-methyl-2,2-dioxo-1H-2λ6,1-benzothiazin-4-olate solvates. Sci. Pharm. 2016, 84, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Orpen, A.G.; Brammer, L.; Allen, F.H.; Kennard, O.; Watson, D.G.; Taylor, R. Typical interatomic distances in organic compounds and organometallic compounds and coordination complexes of the d- and f-block metals. In Structure Correlation; Burgi, H.-B., Dunitz, J.D., Eds.; Wiley-VCH: Weinheim, Germany, 1994. [Google Scholar]
- Zefirov, N.S.; Palyulin, V.A.; Dashevskaya, E.E. Stereochemical studies. XXXIV. Quantitative description of ring puckering via torsional angles. The case of six-membered rings. J. Phys. Org. Chem. 1990, 3, 147–158. [Google Scholar] [CrossRef]
- Zefirov, Y.V. Reduced intermolecular contacts and specific interactions in molecular crystals. Crystallogr. Rep. 1997, 42, 865–886. [Google Scholar]

| Entry | Product | R | Volume of Damaged Extremity (mm3) | Volume of Non-Damaged Extremity (mm3) | ΔVolume (Volume Increase) | Anti- Inflammatory Activity, Compared to Control (%) |
|---|---|---|---|---|---|---|
| 1 | 3 | Na | 516.3 ± 15.8 | 304.9 ± 14.0 | 211.4 ± 16.5 1,2,3 | 48.9 |
| 2 | 4 | H | 568.6 ± 16.5 | 268.7 ± 7.59 | 299.9 ± 20.1 1,2,3 | 27.5 |
| 3 | Lornoxicam | – | 360.5 ± 26.4 | 263.9 ± 19.8 | 96.58 ± 7.62 1 | 76.7 |
| 4 | Diclofenac | – | 397.6 ± 11.9 | 306.6 ± 9.36 | 91.05 ± 5.52 1 | 78.0 |
| 5 | Control | – | 768.7 ± 27.3 | 354.9 ± 11.6 | 413.7 ± 32.2 | 0 |
| Entry | Product | R | Pain Threshold on Damaged Extremity (g/mm2) | Pain Threshold on Non-Damaged Extremity (g/mm2) | ΔPain Threshold | Analgesic Activity, Compared to Control (%) |
|---|---|---|---|---|---|---|
| 1 | 3 | Na | 350.0 ± 15.8 | 306.0 ± 19.6 | 44.0 ± 8.12 1,3 | 86.2 |
| 2 | 4 | H | 294.0 ± 32.3 | 208.0 ± 17.7 | 86.0 ± 18.6 1 | 73.0 |
| 3 | Lornoxicam | – | 441.0 ± 25.6 | 346.0 ± 23.4 | 95.0 ± 4.47 1,2 | 70.1 |
| 4 | Diclofenac | – | 738.0 ± 18.3 | 679.0 ± 25.4 | 59.0 ± 9.27 1 | 81.4 |
| 5 | Control | – | 593.0 ± 56.3 | 275.0 ± 32.1 | 318.0 ± 34.9 | 0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ukrainets, I.V.; Hamza, G.M.; Burian, A.A.; Shishkina, S.V.; Voloshchuk, N.I.; Malchenko, O.V. 4-Methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic Acid. Peculiarities of Preparation, Structure, and Biological Properties. Sci. Pharm. 2018, 86, 9. https://doi.org/10.3390/scipharm86010009
Ukrainets IV, Hamza GM, Burian AA, Shishkina SV, Voloshchuk NI, Malchenko OV. 4-Methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic Acid. Peculiarities of Preparation, Structure, and Biological Properties. Scientia Pharmaceutica. 2018; 86(1):9. https://doi.org/10.3390/scipharm86010009
Chicago/Turabian StyleUkrainets, Igor V., Ganna M. Hamza, Anna A. Burian, Svitlana V. Shishkina, Natali I. Voloshchuk, and Oxana V. Malchenko. 2018. "4-Methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic Acid. Peculiarities of Preparation, Structure, and Biological Properties" Scientia Pharmaceutica 86, no. 1: 9. https://doi.org/10.3390/scipharm86010009
APA StyleUkrainets, I. V., Hamza, G. M., Burian, A. A., Shishkina, S. V., Voloshchuk, N. I., & Malchenko, O. V. (2018). 4-Methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic Acid. Peculiarities of Preparation, Structure, and Biological Properties. Scientia Pharmaceutica, 86(1), 9. https://doi.org/10.3390/scipharm86010009