Chrysin Protects Rat Kidney from Paracetamol-Induced Oxidative Stress, Inflammation, Apoptosis, and Autophagy: A Multi-Biomarker Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs and Chemicals
2.2. Animals
2.3. Experimental Design
2.4. Sample Collection
2.5. Renal Function Analysis
2.6. Analysis of Oxidants and Antioxidants
2.7. Tissue Preparation for Autophagy, Inflammation and Apoptosis Markers
2.8. Assay of Inflammation
2.9. Assay of Apoptosis
2.10. Assay of Autophagy
2.11. Statistical Analysis
3. Results
3.1. Serum Biochemical Analysis
3.2. MDA Levels
3.3. Antioxidant Enzymes
3.4. Inflammatory Markers
3.5. Apoptotic Marker
3.6. Autophagic Marker
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Kanno, S.I.; Tomizawa, A.; Yomogida, S. Detecting mRNA predictors of acetaminophen-induced hepatotoxicity in mouse blood using quantitative real-time PCR. Biol. Pharm. Bull. 2016, 39, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.T.; Arjumand, W.; Nafees, S. Hesperidin alleviates acetaminophen induced toxicity in Wistar rats by abrogation of oxidative stress, apoptosis and inflammation. Toxicol. Lett. 2012, 208, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Gamal El-Din, A.M.; Mostafa, A.M.; Othman, A.; Al-Shabanah, O.A.; Al-Bekairi, A.M.; Nagi, M.N. Protective effect of Arabic gum against acetaminophen-induced hepatotoxicity in mice. Pharmacol. Res. 2003, 48, 631–635. [Google Scholar] [CrossRef]
- Abdel-Zaher, A.O.; Abdel-Hady, R.H.; Mahmoud, M.M.; Farrag, M.M. The potential protective role of alpha-lipoic acid against acetaminophen-induced hepatic and renal damage. Toxicology 2008, 243, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Ghosh, J.; Manna, P.; Sil, P.C. Taurine protects rat testes against doxorubicin-induced oxidative stress as well as p53, Fas and caspase 12-mediated apoptosis. Amino Acids 2012, 42, 1839–1855. [Google Scholar] [CrossRef] [PubMed]
- El-Shafey, M.M.; Abd-Allah, G.M.; Mohamadin, A.M.; Harisa, G.I.; Mariee, A.D. Quercetin protects against acetaminophen-induced hepatorenal toxicity by reducing reactive oxygen and nitrogen species. Pathophysiology 2015, 22, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Naguib, Y.M.; Azmy, R.M.; Samaka, M.R.; Salem, M.F. Pleurotus ostreatus opposes mitochondrial dysfunction and oxidative stress in acetaminophen-induced hepato-renal injury. BMC Complement. Altern. Med. 2014, 14, 494. [Google Scholar] [CrossRef] [PubMed]
- Winchell, C.G.; Steele, S.; Kawula, T.; Voth, D.E. Dining in: Intracellular bacterial pathogen interplay with autophagy. Curr. Opin. Microbiol. 2016, 29, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Stallons, L.J.; Funk, J.A.; Schnellmann, R.G. Mitochondrial homeostasis in acute organ failure. Curr. Pathobiol. Rep. 2013, 1, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Gul, H.B.; Uysal, E.; Cakir, H. The protective effects of ozone therapy in a rat model of acetaminophen-induced liver injury. Environ. Toxicol. Pharmacol. 2012, 34, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.L.; Yiang, G.T.; Chou, P.L. Dual role of acetaminophen in promoting hepatoma cell apoptosis and kidney fibroblast proliferation. Mol. Med. Rep. 2014, 9, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Ucar, F.; Taslipinar, M.Y.; Alp, B.F. The effects of N-acetylcysteine and ozone therapy on oxidative stress and inflammation in acetaminophen-induced nephrotoxicity model. Ren. Fail. 2013, 35, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Sener, G.; Sehirli, A.O.; Ayanoglu-Dulger, G. Protective effects of melatonin, vitamin E and N-acetylcysteine against acetaminophen toxicity in mice. A comparative study. J. Pineal Res. 2003, 35, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Hamid, Z.A.; Budin, S.B.; Jie, N.W. Nephroprotective effects of Zingiber zerumbet Smith ethyl acetate extract against paracetamol-induced nephrotoxicity and oxidative stress in rats. J. Zhejiang Univ. Sci. B 2012, 13, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Topal, F.; Topal, M.; Gocer, H.; Kalın, P.; Koçyiğit, U.M.; Gülçin, İ.; Alwasel, S.H. Antioxidant activity of taxifolin: An activity-structure relationship. J. Enzym. Inhib. Med. Chem. 2016, 31, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Polat Köse, L.; Gülçin, İ.; Gören, A.C.; Namiesnik, J.; Martinez-Ayala, A.L.; Gorinstein, S. LC-MS/MS analysis, antioxidant and anticholinergic properties of galanga (Alpinia officinarum Hance) rhizomes. Ind. Crops Prod. 2015, 74, 712–721. [Google Scholar] [CrossRef]
- Ekinci Akdemir, F.N.; Gulcin, İ.; Alwasel, S. A Comparative study on the antioxidant effects of hesperidin and ellagic acid against skeletal muscle ischemia/reperfusion injury. J. Enzym. Inhib. Med. Chem. 2016, 31, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Mehri, S.; Karami, H.V.; Hassani, F.V.; Hosseinzadeh, H. Chrysin reduced acrylamide-induced neurotoxicity in both in vitro and in vivo assessments. Iran. Biomed. J. 2014, 18, 101–106. [Google Scholar] [PubMed]
- Mantawy, E.M.; El-Bakly, W.M.; Esmat, A. Chrysin alleviates acute doxorubicin cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Eur. J. Pharmacol. 2014, 728, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Rashid, S.; Nafees, S.; Hasan, S.K.; Sultana, S. Beneficial effects of Chrysin against Methotrexate-induced hepatotoxicity via attenuation of oxidative stress and apoptosis. Mol. Cell. Biochem. 2014, 385, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Pushpavalli, G.; Veeramani, C.; Pugalendi, K.V. Influence of chrysin on hepatic marker enzymes and lipid profile against d-galactosamine-induced hepatotoxicity rats. Food Chem. Toxicol. 2010, 48, 1654–1659. [Google Scholar] [CrossRef] [PubMed]
- Girish, C.; Koner, B.C.; Jayanthi, S.; Ramachandra, R.K.; Rajesh, B.; Pradhan, S.C. Hepatoprotective activity of picroliv, curcumin and ellagic acid compared to silymarin on paracetamol induced liver toxicity in mice. Fund. Clin. Pharmacol. 2009, 23, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Pushpavalli, G.; Kalaiarasi, P.; Veeramani, C.; Pugalendi, K.V. Effect of chrysin on hepatoprotective and antioxidant status in d-galactosamine-induced hepatitis in rats. Eur. J. Pharmacol. 2010, 63, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Ekinci Akdemir, F.N.; Gulcin, İ.; Karagöz, B.; Soslu, R. Quercetin protects rat skeletal muscle from ischemia reperfusion injury. J. Enzym. Inhib. Med. Chem. 2016, 31, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Placer, Z.A.; Cushmanni, L.L.; Johnson, B.C. Estimation of products of lipid peroxidation (as malondialdehyde) in biochemical systems. Anal. Biochem. 1966, 16, 359–364. [Google Scholar] [CrossRef]
- Gülçin, İ.; Beydemir, Ş.; Hisar, O. The effect of α-tocopherol on the antioxidant enzymes activities and lipid peroxidation of rainbow trout (Oncorhynchus mykiss). Acta Vet. Hung. 2005, 53, 425–433. [Google Scholar]
- Aebi, H. Catalase. In Methods in Enzymatic Analysis; Bergmeyer, H.U., Ed.; Academic Press: New York, NY, USA, 1983; pp. 276–286. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Sun, Y.; Larry, W.O.; Ying, L. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [PubMed]
- Sedlak, J.; Lindsay, R.H.C. Estimation of total protein bound and nonprotein sulfhydryl groups in tissue with Ellmann’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Lawrence, R.A.; Burk, R.F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun. 1976, 7, 952–958. [Google Scholar] [CrossRef]
- Srinivasan, V.; Panneerselvam, R.; Gunasekaran, S.; Palani, S. Ethanolic extract of Melia azadirachta against acetaminophen induced nephrotoxicity. Int. J. PharmTech Res. 2014, 6, 70–79. [Google Scholar]
- Wudil, L.A.M.; Sarki, S.I. The effect of aqueous stem bark extract of Erythrina mildbraedii on acetaminophen induced nephrotoxicity in rats. Bayero J. Pure Appl. Sci. 2015, 8, 10–18. [Google Scholar] [CrossRef]
- Singh, R.K.; Gautam, R.K.; Karchuli, M.S. Nephrotoxicity: An overvıew. J. Biomed. Pharm. Res. 2014, 3, 41–47. [Google Scholar]
- Adam, G.O.; Rahman, M.M.; Lee, S.J. Hepatoprotective effects of Nigella sativa seed extract against acetaminophen-induced oxidative stress. Asia Pac. J. Trop. Med. 2016, 9, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.I.; Omar, S.A.; El-Guendi, M.I. Potential protective effects of quercetin and curcumin on paracetamol-induced histological changes, oxidative stress, impaired liver and kidney functions and haematotoxicity in rat. Food Chem. Toxicol. 2010, 48, 3246–3261. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ. Antioxidant activity of food constituents-An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ.; Beydemir, S. Phenolic compounds as antioxidants: Carbonic anhydrase isoenzymes inhibitors. Mini Rev. Med. Chem. 2013, 13, 408–430. [Google Scholar] [PubMed]
- Gebaly, Z.M.; Ramadan, B.K.; Hamouda, M.H. The Protective Effect of L-Carnitine on Paracetamol-induced Nephrotoxicity in Adult Male Albino Rats (Microscopic and Biochemical studies). J. Am. Sci. 2012, 8, 906–917. [Google Scholar]
- Ghosh, A.; Sil, P.C. Anti-oxidative Effect of a Protein from Cajanus indicus L against Acetaminophen-induced Hepato-nephro Toxicity. J. Biochem. Mol. Biol. 2007, 40, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Cekmen, M.; Ilbey, Y.O.; Ozbek, E. Curcumin prevents oxidative renal damage induced by acetaminophen in rats. Food Chem. Toxicol. 2009, 47, 1480–1484. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ.; Topal, F.; Çakmakçı, R.; Gören, A.C.; Bilsel, M.; Erdoğan, U. Pomological features, nutritional quality, polyphenol content analysis and antioxidant properties of domesticated and three wild ecotype forms of raspberries (Rubus idaeus L.). J. Food Sci. 2011, 76, C585–C593. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ. Antioxidant Activity of Eugenol: A Structure–Activity Relationship Study. J. Med. Food 2011, 14, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ.; Bursal, E.; Şehitoğlu, H.M.; Bilsel, M.; Gören, A.C. Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum, Turkey. Food Chem. Toxicol. 2010, 48, 2227–2238. [Google Scholar] [CrossRef] [PubMed]
- Anitha, T.A.; Rajadurai, M. Antioxidative potential of chrysin, a flavone in streptozotocin–nicotinamide-induced diabetic rats. Biomed. Prev. Nutr. 2014, 4, 511–517. [Google Scholar] [CrossRef]
- Ramos, A.S.; Correia, A.T.; Antunes, S.C.; Gonçalves, F.; Nunes, B. Effect of acetaminophen exposure in Oncorhynchus mykiss gills and liver: Detoxification mechanisms, oxidative defence system and peroxidative damage. Environ. Toxicol. Pharmacol. 2014, 37, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.U.; Tahir, M.; Khan, A.Q. Chrysin suppresses renal carcinogenesis via amelioration of hyperproliferation, oxidative stress and inflammation: Plausible role of NF-κB. Toxicol. Lett. 2013, 216, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Sathiavelu, J.; Senapathy, G.J.; Devaraj, R.; Namasivayam, N. Hepatoprotective effect of chrysin on prooxidant-antioxidant status during ethanol-induced toxicity in female albino rats. J. Pharm. Pharmacol. 2009, 61, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Bursal, E.; Köksal, E.; Gülçin, İ.; Bilsel, G.; Gören, A.C. Antioxidant activity and polyphenol content of cherry stem (Cerasus avium L.) determined by LC-MS/MS. Food Res. Int. 2013, 51, 66–74. [Google Scholar] [CrossRef]
- Bursal, E.; Gülçin, İ. Polyphenol contents and in vitro antioxidant activities of lyophilized aqueous extract of kiwifruit (Actinidia deliciosa). Food Res. Int. 2011, 44, 1482–1489. [Google Scholar] [CrossRef]
- Basu, S.K.; Rupeshkumar, M.; Kavitha, K. Hepatoprotective and antioxidant effect of Pavonia zeylanica against acetaminophen induced hepatotoxicity in rats. Int. J. Pharm. Biol. Sci. 2012, 3, 407–415. [Google Scholar]
- Bessems, J.G.; Vermeulen, N.P. Paracetamol (acetaminophen)-induced toxicity: Molecular and biochemical mechanisms, analogues and protective approaches. CRC Crit. Rev. Toxicol. 2001, 31, 55–138. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Khan, A.Q.; Qamar, W. Chrysin abrogates cisplatin-induced oxidative stress, p53 expression, goblet cell disintegration and apoptotic responses in the jejunum of Wistar rats. Br. J. Nut. 2012, 108, 1574–1585. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Lee, S.; Kim, S.H. Chrysin suppresses mast cell-mediated allergic inflammation: Involvement of calcium, caspase-1 and nuclear factor-κB. Toxicol. Appl. Pharm. 2011, 254, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Zarpelon, A.C.; Rodrigues, F.C.; Lopes, A.H.; Souza, G.R.; Carvalho, T.T.; Pinto, L.G.; Xu, D.; Ferreira, S.H.; Alves-Filho, J.C.; McInnes, I.B.; et al. Spinal cord oligodendrocyte-derived alarmin IL-33 mediates neuropathic pain. FASEB J. 2016, 30, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Pastorelli, L.; De Salvo, C.; Vecchi, M.; Pizarro, T.T. The role of IL-33 in gut mucosal inflammation. Mediat. Inflamm. 2013, 2013, 608187. [Google Scholar] [CrossRef] [PubMed]
- Gujral, J.S.; Knight, T.R.; Farhood, A.; Bajt, M.L.; Jaeschke, H. Mode of cell death after acetaminophen overdose in mice: Apoptosis or oncotic necrosis? Toxicol. Sci. 2002, 67, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Ali, N.; Nafees, S.; Hasan, S.K.; Sultana, S. Mitigation of 5-Fluorouracil induced renal toxicity by chrysin via targeting oxidative stress and apoptosis in wistar rats. Food Chem. Toxol. 2014, 66, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wu, F.; Lin, S. Adiponectin protects against acetaminophen-induced mitochondrial dysfunction and acute liver injury by promoting autophagy in mice. J. Hepatol. 2014, 61, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Koukourakis, M.I.; Kalamida, D.; Giatromanolaki, A. Autophagosome proteins LC3A, LC3B and LC3C have distinct subcellular distribution kinetics and expression in cancer cell lines. PLoS ONE 2015, 10, e0137675. [Google Scholar] [CrossRef] [PubMed]
- Lazova, R.; Camp, R.L.; Klump, V. Punctate LC3B expression is a common feature of solid tumors and associated with proliferation, metastasis, and poor outcome. Clin. Cancer Res. 2012, 18, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.L.; Chen, C.M.; Chang, Y.Z. Pine (Pinus morrisonicola Hayata) Needle Extracts Sensitize GBM8901 Human Glioblastoma Cells to Temozolomide by Downregulating Autophagy and O6-Methylguanine-DNA Methyltransferase Expression. J. Agric. Food Chem. 2014, 62, 10458–10467. [Google Scholar] [CrossRef] [PubMed]

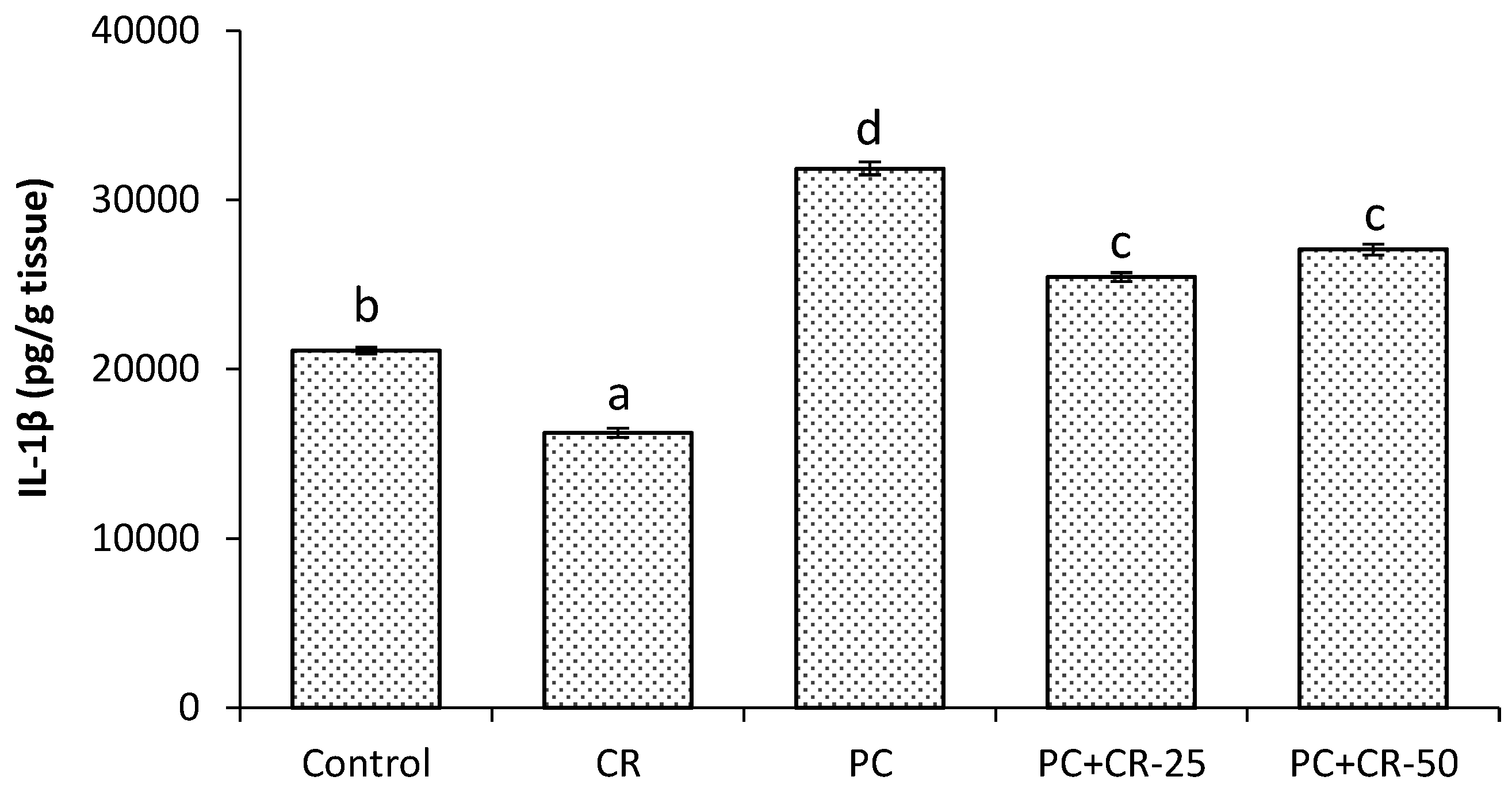
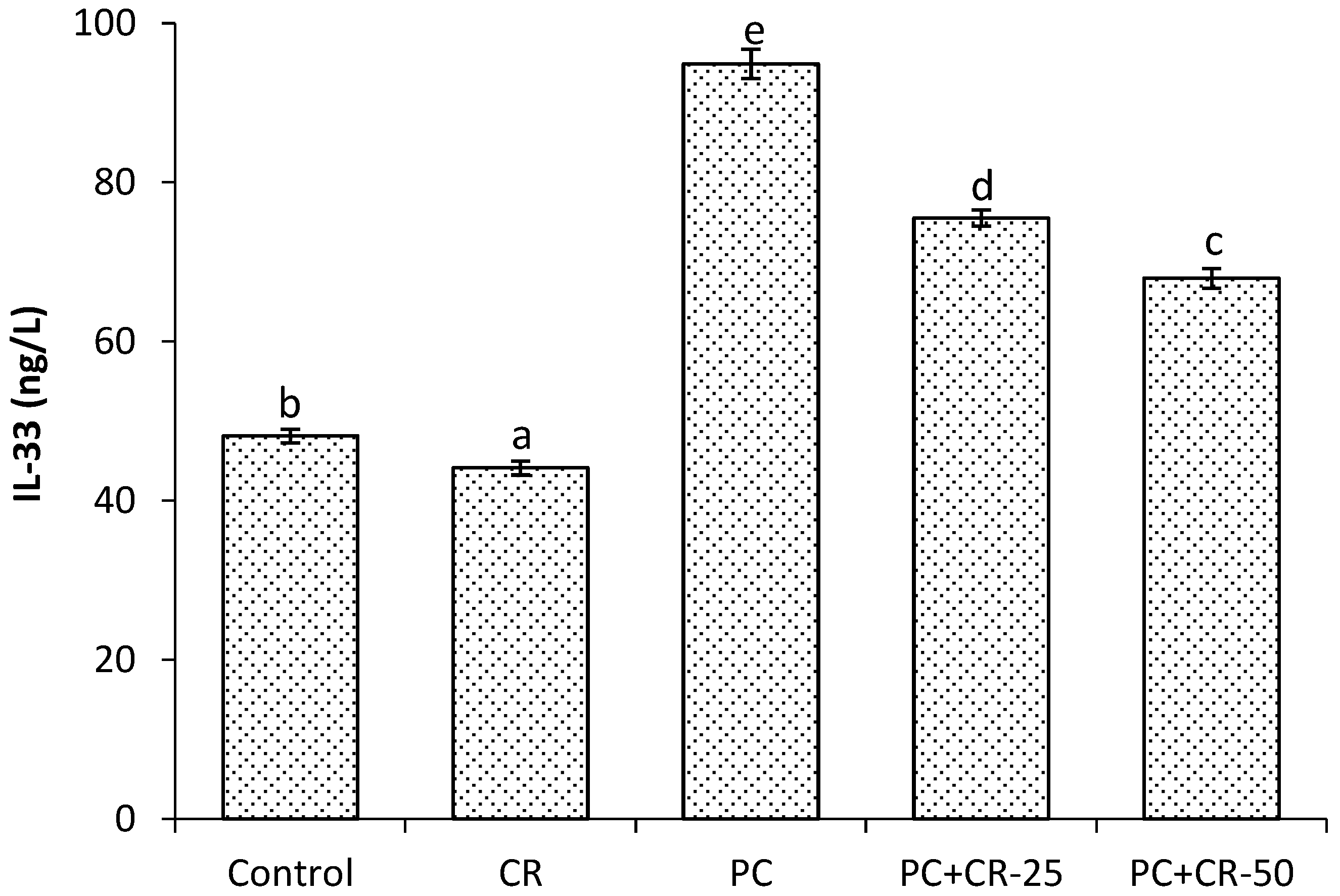

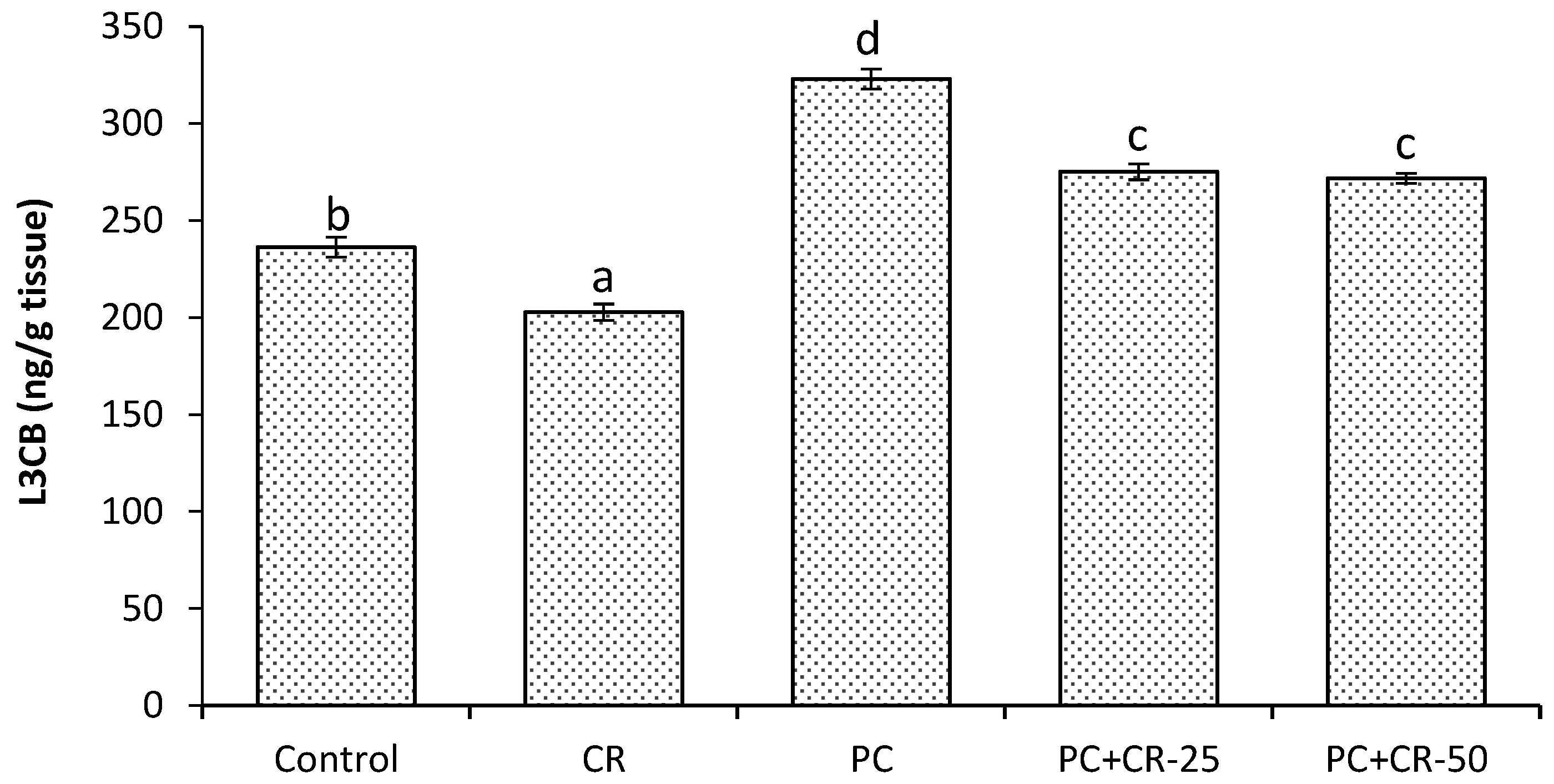
| Parameter | Control | CR | PC | PC+CR-25 | PC+CR-50 |
|---|---|---|---|---|---|
| Urea (mg/dL) | 6.26 ± 0.09 a | 5.74 ± 0.12 a | 23.37 ± 0.75 d | 15.53 ± 0.3 c | 11.50 ± 0.35 b |
| Creatinine (mg/dL) | 0.72 ± 0.01 a | 0.67 ± 0.01 a | 2.74 ± 0.04 d | 1.57 ± 0.02 c | 1.27 ± 0.04 b |
| MDA (nmol/g tissue) | 63.26 ± 0.98 a | 59.81 ± 0.57 a | 120.39 ± 3.3 d | 89.35 ± 1.14 c | 76.94 ± 1.04 b |
| SOD (U/g protein) | 30.32 ± 0.41 c | 31.40 ± 0.25 c | 22.30 ± 0.58 a | 25.47 ± 0.29 b | 24.78 ± 1.53 b |
| GPx (U/g protein) | 36.26 ± 0.40 d | 37.64 ± 0.38 d | 24.80 ± 0.54 a | 30.32 ± 0.59 b | 33.68 ± 0.55 c |
| CAT (katal/g protein) | 65.95 ± 1.40 d | 68.68 ± 0.87 d | 45.36 ± 0.78 a | 51.18 ± 0.66 b | 56.22 ± 0.92 c |
| GSH (nmol/g tissue) | 4.37 ± 0.05 d | 4.58 ± 0.04 d | 1.70 ± 0.03 a | 2.28 ± 0.04 b | 2.98 ± 0.06 c |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kandemir, F.M.; Kucukler, S.; Eldutar, E.; Caglayan, C.; Gülçin, İ. Chrysin Protects Rat Kidney from Paracetamol-Induced Oxidative Stress, Inflammation, Apoptosis, and Autophagy: A Multi-Biomarker Approach. Sci. Pharm. 2017, 85, 4. https://doi.org/10.3390/scipharm85010004
Kandemir FM, Kucukler S, Eldutar E, Caglayan C, Gülçin İ. Chrysin Protects Rat Kidney from Paracetamol-Induced Oxidative Stress, Inflammation, Apoptosis, and Autophagy: A Multi-Biomarker Approach. Scientia Pharmaceutica. 2017; 85(1):4. https://doi.org/10.3390/scipharm85010004
Chicago/Turabian StyleKandemir, Fatih Mehmet, Sefa Kucukler, Eyup Eldutar, Cuneyt Caglayan, and İlhami Gülçin. 2017. "Chrysin Protects Rat Kidney from Paracetamol-Induced Oxidative Stress, Inflammation, Apoptosis, and Autophagy: A Multi-Biomarker Approach" Scientia Pharmaceutica 85, no. 1: 4. https://doi.org/10.3390/scipharm85010004
APA StyleKandemir, F. M., Kucukler, S., Eldutar, E., Caglayan, C., & Gülçin, İ. (2017). Chrysin Protects Rat Kidney from Paracetamol-Induced Oxidative Stress, Inflammation, Apoptosis, and Autophagy: A Multi-Biomarker Approach. Scientia Pharmaceutica, 85(1), 4. https://doi.org/10.3390/scipharm85010004







