1. Introduction
The COVID-19 pandemic has made the world’s population aware of the importance of integrated multidisciplinary approaches to solving global problems, in particular in the field of healthcare. Thus, the pandemic has highlighted the problem of the impossibility of responding effectively to the global epidemic challenge by individual (even influential, global) private companies, putting on the agenda the relevance of combining the efforts of all stakeholders in the fight against the spread of the virus. Such discussions, in particular, refer to vaccine lifecycle management approaches. The current pandemic has highlighted the need to develop comprehensive solutions (organizational and IT) to unify the stakeholders of the entire vaccine lifecycle together in order to perform collaboration processes that are smoother and to provide faster and more effective responses to virus challenges.
Launching a new vaccine is not an easy task, since it involves not only research and development of the substance, but also production, distribution, feedback and performance analysis. Thus, a certain organizational solution, which will unite all the vaccine lifecycle stakeholders into a single system is needed. In the meantime, such a knowledge- and innovation-intensive area as vaccine development requires a free circulation of knowledge and an environment that promotes innovation, technology sharing, and know-how. In order to provide the excellence-oriented governance of such a complicated system, which should be capable of responding to growing challenges over time, it is essential to develop a strong innovation ecosystem [
1]. With the active development of digital technologies and platform solutions, it seems to be possible to provide the appropriate IT support to such an innovation ecosystem.
The World Health Organization (WHO) has repeatedly stated that a new, more severe pandemic awaits humanity in the short term. At the same time, “usual” epidemics also continue to have a serious impact on the social and economic life of the population. A high-tech response, including appropriate business models and information support models, is a panacea in the fight against epidemiological threats. Approaches to the development of an integrated management model for vaccine development, production and delivery, based on the vaccine lifecycle model are the focus of the research presented in this article. Such ka management solution, supported by an appropriate digital platform, is supposed to create a prerequisite for establishing a solid foundation for the national vaccine lifecycle management system, uniting all participants of this cycle into a single ecosystem.
Analysis of the current state showed that the research and practice-oriented community lacks comprehensive approaches to vaccine lifecycle management, which would offer a state-wide organizational form of interaction between the participants of this cycle and an IT solution model to support such interaction. In the meantime, it is evident that such an approach will be in demand both during a pandemic and during periods of a stable seasonal viral load [
2]. The relevance of such a study is determined by the following factors, revealed during the research:
The COVID-19 pandemic. The new coronavirus infection has caused significant damage to public health, living standards, and economies across the world. In addition, it indicated the importance of systemic regulation of vaccine handling at all stages of their lifecycle—from the emergence of a new pathogen to vaccination of the population and monitoring the effectiveness of this vaccination [
3,
4].
The trend toward value-based medicine. In recent decades, the so-called value-based medicine—the ideology of monitoring and evaluation of the effect of treatment (vaccination) after the provision of medical services in order to identify the true value of the treatment provided—has become one of the factors in the development of health care [
5,
6]. From a vaccine point of view, a value-based approach in healthcare encourages the involvement of after-vaccination stages into the vaccine life-cycle as an essential part of the whole process, which creates an input for a new vaccine cycle.
Patient-centered approach to vaccination. The peculiarities of this approach to vaccination are that on the basis of client-orientation it is necessary to create an innovative approach to planning, conducting, evaluating and monitoring the vaccination process, which is based on partnership and mutually beneficial principles of cooperation between the patient and his immediate environment (family) with a medical organization represented by administrative, medical, nursing and support personnel. It is represented as a three-tier structure of patient-oriented healthcare. At the micro level, the key is the model of the relationship “doctor–patient”, at the middle level “medical organization–patient”, and at the macro level “national health care system–patient”.
Platform economy. A shared economy means active roles of industrial ecosystems and IT platforms as a service-oriented environment of ecosystem participants’ communication. It enhances innovation and technology diffusion, involves all the participants in the value creation process, lowers transaction costs, reduce time for new services to enter the market due to the high level of competition, enables rapid development of infrastructures [
7,
8] This way of industrial cooperation completely meets the requirements of a vaccine lifecycle support system.
Open innovation (including open source IT solutions). According to [
9,
10,
11], the obvious advantages of open innovations are lower costs, greater security, greater speed of diffusion, free knowledge circulation, continuous improvement, and customer orientation. The aforementioned advantages are essential components of the vaccine lifecycle management as it is a very innovation-intensive area which has to react fast to the population demand for certain virus protection.
The factors mentioned above determine the need to develop an integrated approach to working with vaccines. This approach should take into account the technological and business processes (supply chain) of development, production and delivery of vaccines; propose a model for IT support of processes at all stages; provide opportunities for the collaboration of participants, including in terms of the use of data; and offer an open solution that involves new entrants in order to stimulate innovation in the sector.
This article is devoted to the description of the methodological basis for the vaccine innovation ecosystem development based on the vaccine lifecycle. The purpose of the study is to develop the architecture model (a set of models) of the development, production, distribution of vaccines and vaccination of the population, supported at every stage by a virtual platform, which would provide the systematic management of the vaccine lifecycle at the state level. The article consistently solves the following tasks:
analysis of the state of the art in the vaccine lifecycle management approaches and IT solutions applied to support this cycle;
creating the supply-chain-based innovative ecosystem model of the vaccine lifecycle management;
development of the virtual IT platform model for innovative ecosystem model of the vaccine lifecycle management.
The article presents the results of the original study, based on both research literature analysis and existing practices of ecosystems’ organization and their IT support, which is still really scarce. The key results of the study are represented as a set of models representing the supply chain-based vaccine lifecycle, the vaccine ecosystem stakeholders view model, the vaccine ecosystem service model, and the IT platform model supporting the vaccine ecosystem.
The following sections of the article address consistently: the theoretical basis and methodology of research, analysis of research literature, the authors’ vision of vaccine lifecycle management in terms of models of the business and IT components of the innovation ecosystem, the topics of future research areas.
2. Materials and Methods
For development the target vaccine lifecycle innovative ecosystem model (described in the
Section 4) the following approaches were chosen as a methodological foundation:
An enterprise architecture approach to the development and analysis of models of interaction of heterogeneous elements (business and IT logic) of socio-economic systems;
Process approach to the analysis of socio-economic systems;
CALS-technologies as models supporting the lifecycle of products;
Supply chain management principles.
Enterprise Architecture. Deservedly accepted by the international scientific and professional community the concept of architecture of the enterprise proclaims consideration of any business system as a set of the interconnected and interdependent diverse elements. Business management as a system includes such elements as goalsetting, strategy, motivation, functional structure, business-processes system, organizational structure, architecture of information systems, applications and data, information exchange models, IT-infrastructure. [
12,
13] Presentation of the above elements in a single complex allows you to understand and analyze the essence of the system and the nature of the interaction of its elements. This creates the prerequisites for the effective development of business systems, because it provides traceability of any changes in the entire system when changing any of its elements.
Enterprise architecture emerged during the period of rapid development of IT for business as a response to the challenge: how to effectively align business and IT elements within a single set of business management. Therefore, special attention is paid to the relationship between business requirements and information system services and applications in enterprise architecture. The functional structure of information systems must take into account the functional structure of the business, while ensuring integration between these functions to support end-to-end enterprise processes. The function-oriented approach to the design of information systems is to ensure effective information exchange within each function at all levels of the IT architecture hierarchy. Architectural approach is applicable to the analysis of both individual enterprises and their complexes, industries, economic systems at the state level.
This article aims to develop an integrated solution for the management of vaccine development, production and delivery processes, and proposes to consider the interaction of such elements as stakeholders, business processes, IT support and data. This is possible in the framework of architectural modeling. [
14] As a result of the study we will offer models of different architectural perspectives of vaccine ecosystem, reflecting the features of interaction of stakeholders, data exchange, IT-support, service architecture of the virtual digital platform.
Business Process Management. The process approach has long been widely used in business management, as well as in the implementation of IT solutions. If the functional structure of enterprise activity defines “What to do?”, the system of process models answers the question “How is the enterprise activity realized to achieve the required result?”. Ref. [
15,
16] The process approach allows you to describe the sequence of actions to achieve the result, decomposing it into individual steps to the required level of detail. Thanks to the structure of the process approach it became a basis for enterprise activity automation. The process approach enhances smoother and faster innovation implementation as it tracks the whole stages of any prescribed cycle properly.
CALS technology. Ref. [
17,
18] CALS (continuous acquisition and lifecycle support) is a concept that unites the principles and technologies of information support of the product lifecycle at all its stages, based on the use of an integrated information environment (single information space), providing uniform methods of process management and interaction of all participants in this cycle—customers (including government agencies), suppliers and manufacturers, operating and management personnel—in accordance with the requirements of the appropriate standards mainly by means of electronic data exchange.
Key advantages of CALS-technologies are:
The main problems hindering effective management of vaccine information are the enormous amount of information (“information chaos”) and communication barriers between all the participants of the lifecycle. The ways to solve them are laid down in the CALS strategy. CALS strategy is to create a single information space for everyone involved in the vaccine lifecycle, including the consumer. The single information space is based on the use of open architectures, international standards, joint data storage and proven software and hardware tools.
Supply chain management principles. Supply chain management principles were declared in 1997 in [
19]. The principles are still actual over decades. They are intended to provide adopted, service-oriented, smooth path of goods and services through all the participants of the supply chain.
The authors formulated the following methodological and functional requirements for the architectural solution being developed for vaccine lifecycle management. The system of models of the architectural solution should take into account:
the Deming continuous improvement cycle;
ecosystem approach, describing character of interaction of all stakeholders of lifecycle, acting within a single value chain;
requirements of innovations openness, making possible to connect new participants to the cycle (both from organizational and IT points of view), capable;
service-oriented architecture principles for development the IT-support model of the vaccine life-cycle.
3. Literature Review
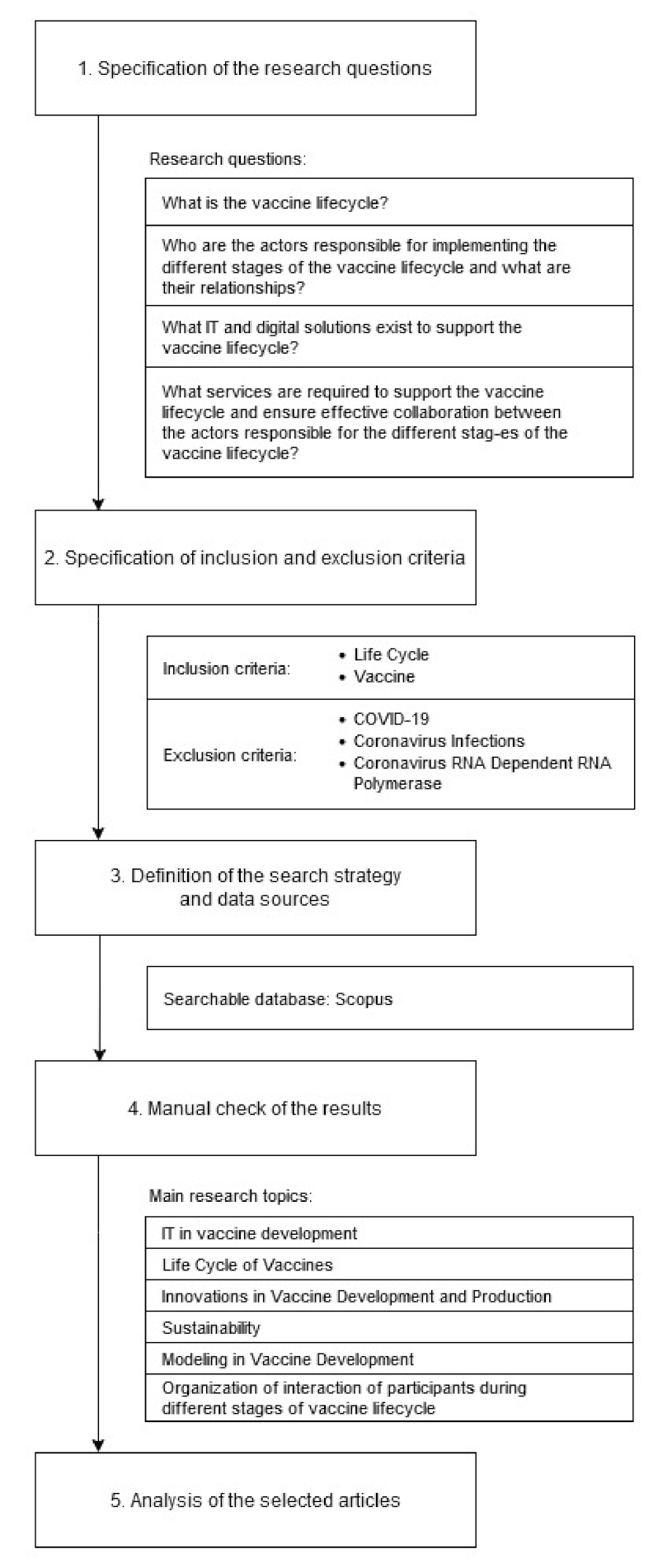
In order to synthesize existing works on a research topic in a fair manner and identify the gap, in
Section 3 a systematic literature review was conducted. The Kitchenham’s [
20] guideline for systematic literature review was used as a reference. According to the proposed literature review algorithm the following steps were implemented (
Figure 1):
Specification of the research questions.
In our study, the following research questions were defined:
What is the vaccine lifecycle?
Who are the actors responsible for implementing the different stages of the vaccine lifecycle and what are their relationships?
What IT and digital solutions exist to support the vaccine lifecycle?
What services are required to support the vaccine lifecycle and ensure effective collaboration between the actors responsible for the different stages of the vaccine lifecycle?
Specification of inclusion and exclusion criteria.
- 2.
Specification of inclusion and exclusion criteria.
The main focus of this research was on the vaccine lifecycle and its information support. The review was made for the period of the last 3 years (2019–2021). In the search it was discovered that quite a number of articles during the mentioned period consider the problem of vaccination through the prism of coronavirus, emphasizing the peculiarities of this virus. The study aims to develop a universal solution for vaccine lifecycle management, so articles examining the specifics of coronavirus vaccine development were excluded from consideration.
It was decided to exclude those studies that could not be accessed without additional investments.
- 3.
Definition of the search strategy and data sources.
In this study, we used an electronic search procedure to identify the set of articles about innovation hubs and a manual search to pick the studies that help to answer the research questions. The electronic search procedure was chosen as it ensures accuracy and completeness of the evidence. The electronic search was applied on the Scopus search engine, because almost 80% of Scopus records include abstract, which makes analysis easier, and a high quality of search outcomes.
In total, 110 open-access articles in English on the target research area were identified.
- 4.
Manual check of the results.
The analysis of articles abstracts resulted in selection of the most relevant ones. The number of analyzed articles decreased to 24. These articles have been categorized into large categories showing the industry of the research (
Table 1).
Moreover, it can be noted that of all the articles that reached the final review, 15 were case studies and referred to a specific region or some kind of industrial example, and nine displayed concepts. Thus, the analyzed articles reflect both the practical and historical states of the art.
- 5.
Analysis of the selected articles.
Stern P.L. reviews the main elements that provide for the development of safe and effective vaccines. The three vaccine development phases (preclinical, clinical, and post-licensure) integrate the requirements to ensure safety, immunogenicity, and efficacy in the final licensed product [
24].
Article [
25] describes the traditional approach to vaccine R&D and possible improvements, particularly towards being better prepared for emerging viral diseases.
Verbeke R. et al. highlight the challenges in vaccine design, testing and administration, key considerations in the design of mRNA-based vaccines and new opportunities that arise when packaging mRNA in nanoparticulate vaccines [
26].
Hartmann K. et al. in their article say that it is vital that the safety of all vaccines is monitored throughout their lifecycle [
27].
Zawawi A. et al. in research on a vaccine for parasitic helminths say that multiple lifecycle stages exist, each presenting stage-specific antigens [
28].
In [
29] the stages of human papillomavirus (HPV) vaccine development are discussed. Pan-gender vaccination and current clinical trials are also discussed.
This review focuses on the development of CHIKV vaccines that have reached the stage of clinical trials since the late 1960s up until 2018. Also, various stages of vaccine development are considered [
30].
This review outlines the main technological advancements as well as major issues to tackle in the development of vaccines. Possible applications for unmet clinical needs are described [
21].
The research of Zhou, X. et al. summarizes vaccine development paradigms and major types of vaccines [
31].
To better understand the role of innovation in breakthrough drug and vaccine development, the article analyzed recent results for assets developed using different types of innovation [
22].
This report summarizes the major issues and priority areas of research, the roadmap not only encourages research aimed at new solutions, but also provides guidance on the use of innovative tools to drive breakthroughs in the influenza vaccine R&D [
32].
Giersing B. et al. describe the challenges encountered in developing vaccines and a vaccine-product innovation ‘theory of change’, which highlights actions that should be undertaken in parallel to product development to incentivize sustainable investment and prepare the pathway for uptake and impact [
33].
The research was conducted based on a partnership framework which analyzed multiple factors-partnership prerequisites, partnership model, partnership process, and partnership performance, thereby providing a comprehensive insight into the successful utilization of partnership networks for vaccine introduction [
37].
Golan M. et al. analyze current trends in implementing and modeling resilience and recommendations for bridging the gap in the lack of quantitative models, consistent definitions, and trade-off analyses for vaccine supply chains [
35].
Kitney R. I. et al. talk about distributed manufacturing model. The advent of synthetic biology promises much in terms of vaccine design [
34].
The research of Rappuoli R. et al. states that conquering diseases requires considerable investment and a new sustainable model of vaccine development involving close collaborations between public and private sectors [
36].
This study examines the application of systemic vaccinology at various stages of vaccine development. Systems vaccinology is a tool that provides novel and comprehensive understanding if properly used. Data sets retrieved from systems-based studies endorse rational design and effective development of safe and efficacious vaccines [
38].
Mohanty E. et al. review the present scenario of peptide vaccines which are developed using mathematical and computational statistics methods to prevent the spread of disease caused by RNA viruses. We also focus on the importance and current stage of AI and mathematical evolutionary modeling using machine learning tools in the establishment of these new peptide vaccines for the control of viral disease [
39].
The study states that various measures are being taken in India to develop a collaborative ecosystem for vaccine research. The Government of India, and in particular the Department of Biotechnology, is developing mechanisms to support end-to-end vaccine development and testing by strengthening collaboration between industry, academia and government [
42].
Cornwell E. et al. create a susceptible-exposed-infectious-vaccinated hybrid ordinary differential equation and difference equation model [
40].
In research by Sun X. et al. the simulation-based approach combining both route optimization and dynamic simulation to improve the logistics performance for COVID-19 vaccine distribution was developed [
41].
The authors of [
43] have developed a novel deep learning platform—deep docking (DD)—which provides fast prediction of docking scores of Glide (or any other docking program) and, hence, enables structure-based virtual screening of billions of purchasable molecules in a short time.
The article [
44] demonstrates successful pursuit of a platform development approach to manufacture important vaccines. Platform in this article refers to vaccine platform technologies (not digital platforms) that allows the vaccine development cycle to be shortened by means of using the experience of previously developed vaccines.
Jarrett S. et al. tell that the counterfeiting of vaccines is an increasing problem globally with the safety of persons vaccinated, trust in vaccines generally and the associated reputation of vaccine manufacturers and regulatory agencies at risk. This article highlights the efforts of industry and governments on the value of traceability and introduction to 2D barcodes [
23].
The analysis of research papers showed that the most discussed topic concerns development of innovative ways to produce vaccines. Researchers distinguish three phases of vaccine development-preclinical, clinical, and post-licensing. They are united by the requirements for safety, immunogenicity and efficacy of the final licensed product.
In order to understand vaccine handling activities, the authors analyzed the relevant processes through the prism of the Deming cycle (plan-do-check-act) [
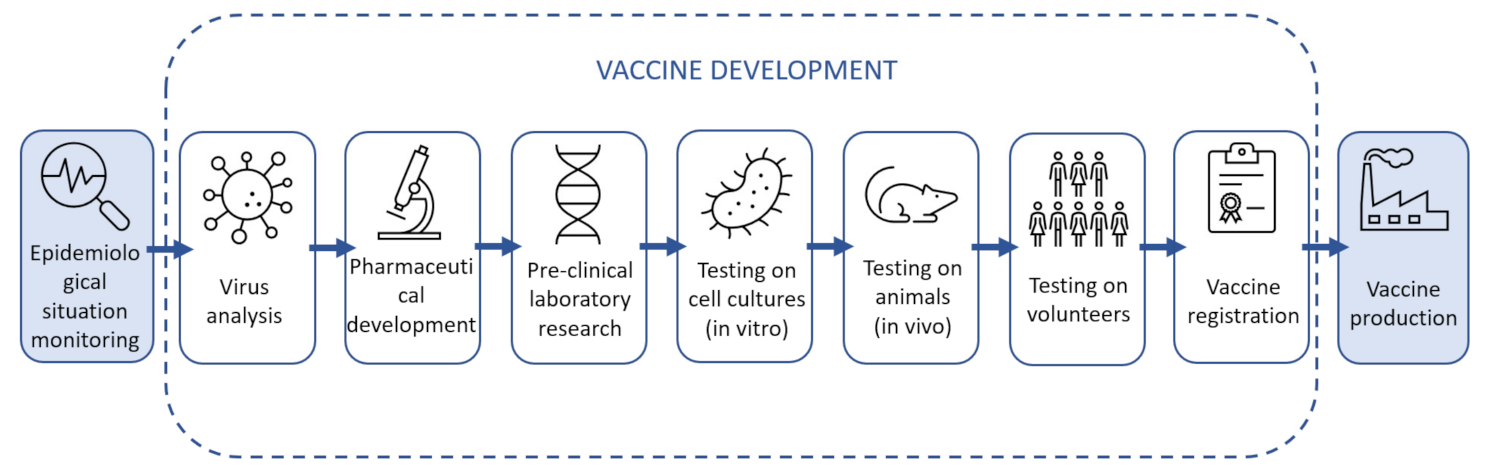
45]. The latter means that any process should be designed in such a way that its implementation is necessarily supported by the possibility of monitoring, feedback gathering and analysis in order to make the necessary adjustments in the next process execution cycle. In this regard, the following top-level Deming cycle model was proposed for vaccination processes: epidemiological situation monitoring–vaccine development–vaccine production–vaccination. It should be noted that the authors have not found any publications that describe the whole proposed cycle or some similar concept. Researchers and specialists often focus on one of the four steps of the proposed cycle without paying attention to the place of the specific step in the overall system and its interfaces with the related processes. At the same time, medical specialists, when describing the processes, often do not pay proper attention to the issues of managing the business process of working with vaccines, in particular to the issues of logistics.
There are certain paradigms for the development of different types of vaccines. After studying the sources, we have a fairly complete picture of the vaccine development process, which consists of the following steps:
Virus analysis;
Pharmaceutical development;
Pre-clinical laboratory research;
Testing on cell cultures (in vitro);
Testing on animals (in vivo);
Testing on volunteers;
Vaccine registration.
The processes of Epidemiological situation monitoring, Vaccine production and vaccination have received incomparably less attention in the research literature.
In addition to the literature review, the authors analyzed the open information of the world famous vaccine developers and manufacturers (the State Research Center “Vector” (Kolsovo, Russia) the National Research Center for Epidemiology and Microbiology named after N.F. Gamalei (Moscow, Russia), Federal Scientific Center for Research and Development of Immunobiological Preparations named after V.I. M.P. Chumakova (Moscow, Russia) in Russia; the Wuhan Institute of Virology at the Chinese Academy of Sciences (Wuhan, China), the Wuhan Institute of Biologicals (part of the Sinopharm Group, Shanghai, China), Sinovac Research and Development Co. (Beijing, China), CanSino Biologics Inc. (Tianjin, China) in China; The National Institute of Allergy and Infectious Diseases (Rockville, MA, USA), Moderna (Cambridge, MA, USA), Inovio (Plymouth Meeting, PA, USA), Arcturus Therapeutics (San Diego, CA, USA), Johnson and Johnson’s (New Brunswick, NJ, USA) in the USA; Duke-NUS Singapore School of Medicine in Singapore; Petrovax Pharm (Moscow, Russia), Pfizer (New York, NY, USA) and IT vendors in search of the integrated vaccine lifecycle support solution—both business and IT [
46,
47,
48].
All of the above listed companies focus on the full-cycle research and production (up to the finished dosage form) with subsequent registration and promotion in the pharmaceutical markets of medical products, which are the parts of the vaccine supply chain. The companies do not mention the remaining stages of the supply chain in their activities which would include vaccine delivery to the patients and further steps.
Nor do the IT companies describe complex solutions for tracking all stages of the vaccine lifecycle. There are different IT systems for supporting the different stages of the vaccine lifecycle (vaccine development systems, vaccine testing systems, vaccine inventory monitoring systems of a company level, immunization registries of the state level) used by different stakeholders of the cycle and not integrated with each other.
From the business point of view, the solution SAP Vaccine Collaboration Hub (SAP VCH) should be mentioned as the one, supporting a certain part of a vaccine lifecycle and providing the IT support not for the single companies, but to the vaccine ecosystem [
49,
50]. Compared with the research publications mentioned above, in which there was a lack of business focus, the SAP solution is vaccine supply chain oriented, but specifically medical steps of the vaccine lifecycle (vaccine development, epidemiological situation monitoring) are missing.
One important issue is an analysis of current trends in implementation and modeling the sustainability of existing vaccine supply chains. New models of distributed production, sustainable vaccine development involve close collaboration between the public and private sectors.
Additionally, a search for existing organizational forms of cooperation within the vaccine lifecycle was conducted. The search string “vaccine + hub or ecosystem” was formed for the Google search engine. Except the information about SAP Vaccine Collaboration Hub, mentioned above, the following vaccine-related ecosystems were identified.
Various ecosystems such as BioNTech are being set up to organize vaccine research, and mechanisms are being developed to support end-to-end vaccine development and testing by strengthening collaboration between industry, academia, and government. Ref. [
51] Biopharmaceutical New Technologies (BioNTech, Mainz, Germany) is a next-generation immunotherapy company that is developing new treatments for cancer, infectious and rare diseases, as well as developing vaccines. BioNTech has created its own ecosystem for vaccine development.
Modeling contributes immensely to the development of the industry. Various modeling approaches improve the logistics performance of vaccine distribution.
To support vaccine production, various platforms such as The cBio Cancer Genomics Portal and The European Thoracic Oncology Platform are being developed and introduced into production. The cBio Cancer Genomics Portal is a resource for multidimensional cancer genomics and vaccine datasets that currently provides access to data from more than 5000 tumor samples from 20 cancer studies. The cBio Cancer Genomics Portal significantly reduces the barriers between complex genomic data and cancer researchers who want access to molecular profiles and clinical characteristics from large-scale cancer genomics projects, and enables researchers to translate these rich data sets into biological insights and clinical applications. [
52] The European Thoracic Oncology Platform is a platform that promotes academic clinical research and the exchange of ideas in thoracic oncology. It contains research projects and clinical vaccine trials with a focus on knowledge development in the field of thoracic malignancies. ETOP includes more than 50 collaborative groups and institutions from across Europe and beyond. [
53] They simplify the vaccine production process as well as increase its efficiency.
The analysis revealed the following:
No explicit description of the complete vaccine life-cycle, reflecting the Deming continuous improvement cycle, has been found; different researchers consider separate stages of this cycle but not their interconnection; in practice different stakeholders are responsible for implementing a specific stage of the vaccine cycle, which does not provide the synergy effect of the whole ecosystem;
No information has been found on a comprehensive IT solution that supports the entire vaccine cycle.
The current state of the research and the practice in the vaccine lifecycle management make this article relevant.
4. Results and Discussion
4.1. Business Model of a Vaccine Ecosystem
The safe use of vaccines will require proper vaccine management and supply chain management, which will be determined by the characteristics of the finished vaccine product (such as release form, composition, stability, temperature requirements, storage and transport volumes). It is essential to ensure that a national system for tracking and monitoring the use of vaccine products is in place to manage multiple vaccine products, manage the supply of vaccines for subsequent doses of vaccines, participate in the monitoring of vaccine safety, and address potential drug withdrawals, and their series/parties. The following are key points to look out for when managing a vaccine supply chain:
Establish early contact with potential vaccine suppliers to access data on vaccines and plan the necessary infrastructure and procedures for storing, transporting and administering vaccines;
Hold an assessment of vaccine warehouses, transport services, delivery systems and vaccine handling at the national and subnational levels, taking into account the characteristics of vaccine products and filling identified gaps, which may include:
Evaluating peak vaccine storage and transportation options, and developing contingency plans to enhance the current distribution system’s capabilities;
Review, update (as needed), and distribute “standard operating procedures” describing all aspects of vaccine and vaccine supply management.
Planning the management of consumables and auxiliaries used in immunization (syringes, needles, containers for disposal of sharp and stabbing waste, personal protective equipment, thermal containers, cooler bags, cold items, kits for assisting with anaphylactic shock, vaccination card blanks, educational materials).
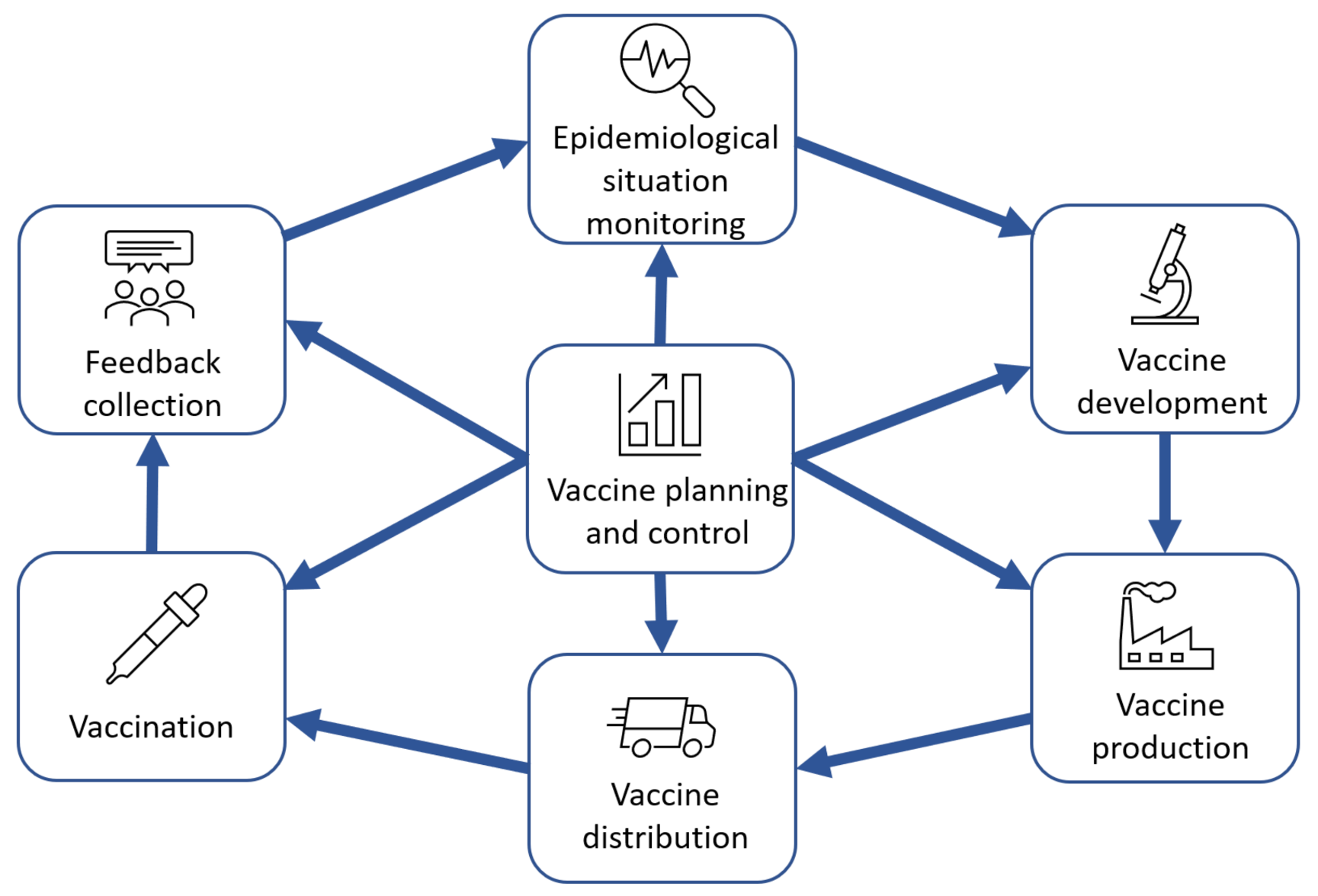
Based on a review of publications, real companies’ experience and IT solutions for vaccine lifecycle support (see
Section 3), the following model of lifecycle stages was proposed as the basis for developing a management model for vaccine development, production, and delivery (
Figure 2).
The cycle proposed in
Figure 2 implies the sequential implementation of the following steps:
The vaccine planning and control processes are implemented continuously throughout the cycle.
The model in
Figure 1 depicts the business processes of vaccine-related activities, while the specific medical, manufacturing and research processes can be described in the decomposition diagrams of the blocks shown in
Figure 2. For example, the decomposition of the vaccine development process is the model in
Figure 3. This view of the process is common among the vaccine development research community. The authors of this article propose to place these specific processes in the context of the overall value chain, which would ensure proper consistency between the inputs and outputs of the vaccine lifecycle stages and provide the individual participants with a common vision of the whole cycle and their place in it.
Vaccine lifecycle management is a multilevel process involving the government, corporate stakeholders (research organizations, manufacturers, distributors, etc.), and the public. The architectural solution for vaccine lifecycle management should provide management of the process at the operational (production), tactical and strategic levels.
Strategic management level. The strategic level is the management at the level of the state, as a key institutional customer, the level of development and implementation of the mission in the field of development, production and delivery of vaccines. Each state has different stages of the vaccine lifecycle under its control. For example, in Uzbekistan there are manufacturers, distributors and vaccinators of almost all global vaccine brands (Sputnik V (with partial production), AstraZeneca, Sinovac, Pfizer, etc.), but no vaccine development here. The situation in Russia is different from that in Uzbekistan: Sputnik V is the main vaccine (developed in the country), but there are others (also domestically developed) that mostly support it.
Most countries are not capable of producing a vaccine on their own. For example, France, having declared its own vaccine ready and of high quality, has not been able to produce it. This leads to the fact that more than 95% of WHO purchasing organizations are focused on importing vaccines. However, looking at the situation in Kazakhstan, we can say that it has export-import capabilities: it produces and partially exports Sputnik V. Belarus has a complete technological cycle of growing vaccine substance for production and sale.
Tactical management level. Tactical level implies management at the level of supply chains—the level of management, compliance with expectations which allows linking the flow of patients allowed for vaccination, vaccines in the appropriate state (temperature storage and transportation, etc.), and specialists providing vaccination services (for vaccination it is necessary to validate the patient, monitor the patient’s condition after vaccination). In addition, this level must take into account the restrictions in force in different countries (e.g., the GMP standard in the EU countries).
Operational management level. The operational level is the level at which the plan from the levels above is executed.
A description of the tasks of the different levels of supply chain management is presented in
Table 2.
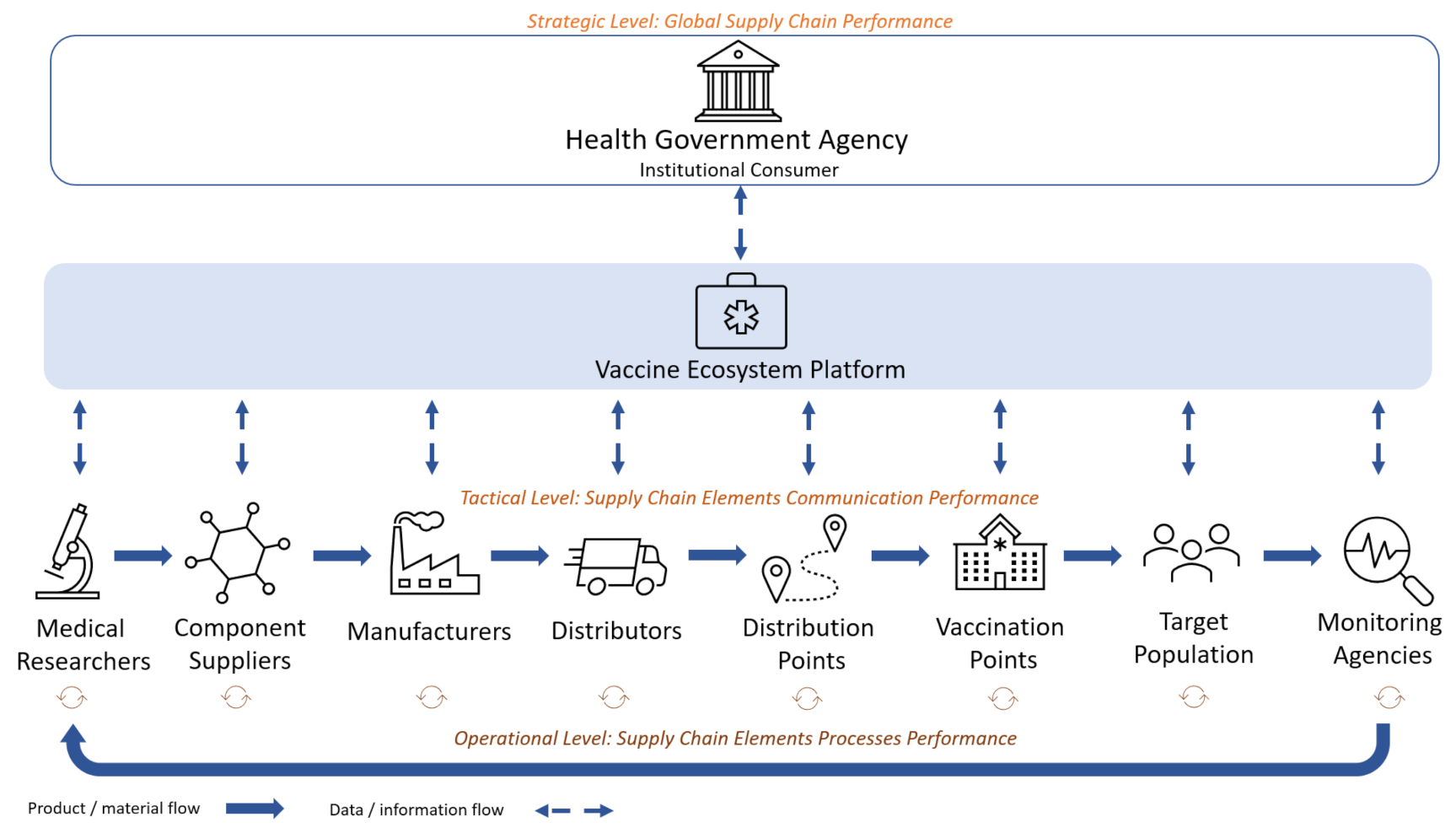
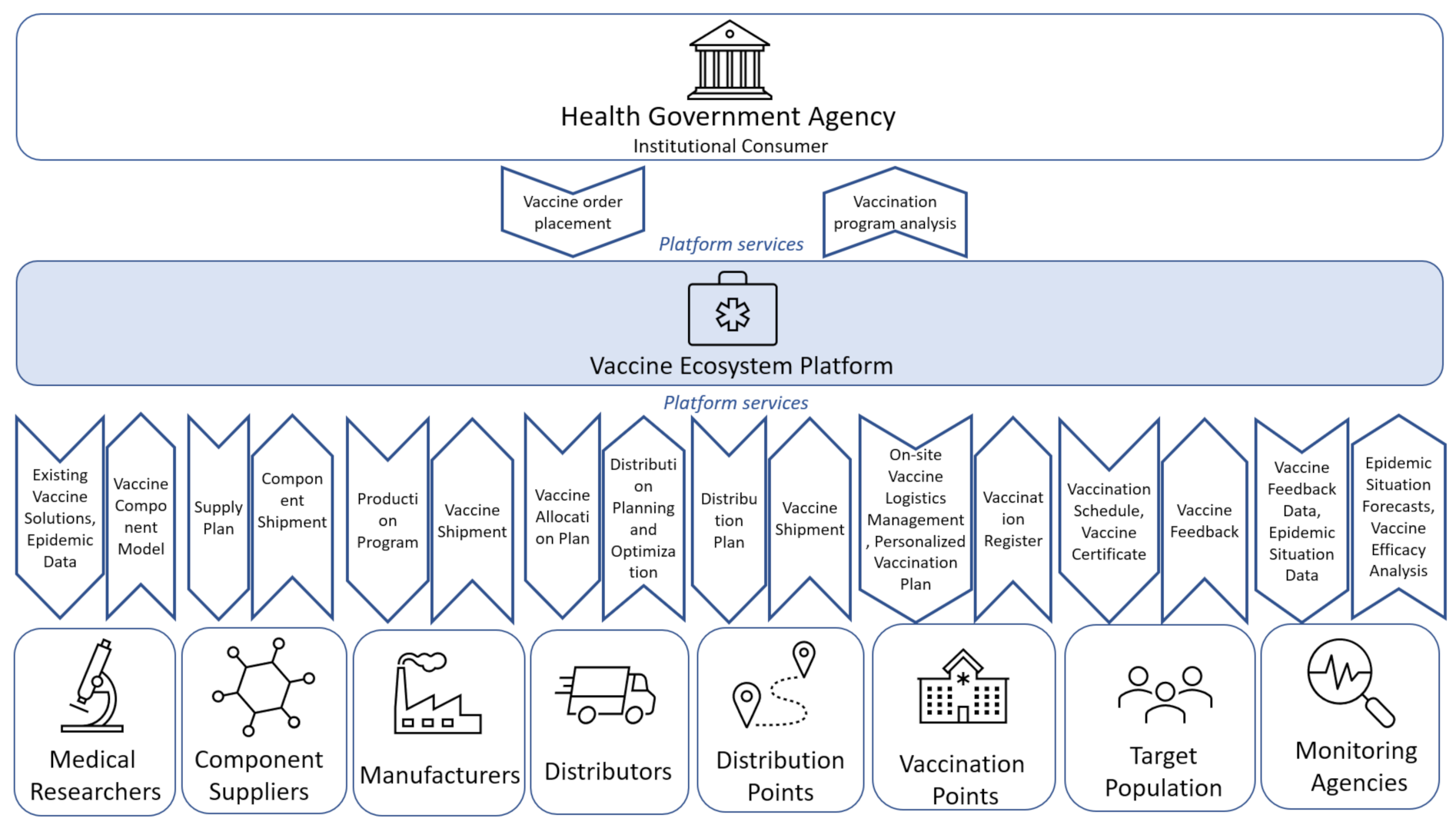
Let us describe the process proposed as the basis for a vaccine lifecycle management system, focusing on the role of stakeholders (
Figure 4).
In the model of interaction between the stakeholders of the vaccine cycle presented in
Figure 3 the following stakeholders are defined:
Institutional consumer (traditionally state government or health ministry)—shapes the mission and strategy for vaccine development, production and delivery, and creates the state order for vaccines;
Medical researchers—develop vaccines for emerging viruses or virus strains. Medical ecosystems bring together many different stakeholders, but the core of such systems should be medical and research organizations as the main centers of expertise accumulation and medical innovations production and the drivers of their dissemination;
Component suppliers—supply vaccine components;
Manufacturers—produce vaccines;
Distributors—transport vaccines between manufacturers and distribution centers;
Distribution points—places where vaccines are stored for further transportation to the next storage sites or places where vaccines are directly administered;
Vaccination points—medical institutions and other facilities where vaccines are administered to patients and recorded;
Target population—groups to be vaccinated;
Monitoring agencies—monitor and analyze the epidemiological situation and the results and consequences of vaccination.
Medical researchers and monitoring agencies in this model are specific entities. They are performing their activities on a permanent manner: development of vaccines and monitoring the epidemiological situation is carried out on the regular basis, not depending on a particular quantitative state order. The trigger of the medical research process is a new virus emergence, and the monitoring of the epidemiological situation is carried out on a regular basis. Whereas the trigger for the entire supply chain shown in
Figure 3 is the placed state order for vaccines, and the trigger for individual blocks of this chain is the completion of activities of the previous block.
The following management tasks are addressed in the supply chain shown in
Figure 3:
- 1.
Public purchasing (institutional consumer).
The state may not be the purchaser of the vaccine, but it will control it in its territory. It is the source of the strategic goal, forms the mission and quantifies the success of the mission. For example, the Ministry of Health sets the target number of vaccinated people in the country as a whole and separately in the regions. The institutional customer carries out planning based on methodologies developed by hygienic health experts.
After the approval of the procurement plan at the state level, based on the percentage of the population in different types of vaccines, the budget is calculated, which is allocated according to the items: the contract price of vaccine production, payment of medical personnel, logistics of delivery, storage, delivery to the place of vaccination (organized groups, the army, power structures, rotational workers, teachers, etc.). Next, a strategic program is formed, which reflects the necessary number of different types of vaccine in the country in a given time, from which follows the planning of the budget.
- 2.
Placement of the requisition with potential suppliers.
The state-approved application is placed with potential suppliers.
- 3.
Logistics management.
After confirming the application, logistics (logistics service for delivery of vaccines to vaccination sites through the elements of the logistics chain) is built. At this level the following tasks are solved:
If raw materials are purchased abroad, they must be validated to the GMP quality standard;
Within the framework of export-import operations, the strategic requirement calculated at the state level is verified against the production and delivery capacities;
Determination of technical feasibility of storage and delivery of vaccines to the central storage facility;
Once the release and delivery calendar is approved, the question of the technical feasibility of storing vaccines purchased domestically or through import operations and delivering them to a central customer audited storage facility to meet existing state-approved standards is addressed. This step aims to ensure the quality of the vaccine.
- 4.
Internal logistics.
Internal logistics is harmonized with the logistics flow to the central storage facility. The delivery is organized from the central storage sites (production warehouse of finished products, etc.) to the vaccination sites. For example, in a geographically distributed country like Russia, the main flow for vaccinations is brought to a regional distribution center, and then distributed to vaccination centers. In some regions, however, the vaccine is delivered directly to vaccination sites due to low population densities. An example of such an experience is the Trans-Baikal Territory of Russia. In such regions, the problems of organized collection of a population and their organized delivery to vaccination sites should be solved, while taking into account the factor of lack of vaccine storage capacity.
- 5.
Providing transportation capacities.
Transport capacities ensure the movement of vaccines to vaccination sites. The task is technologically complex because of the transportation requirements.
- 6.
Performing vaccinations.
The execution of a vaccination is an internal production process of the vaccination point, from the questionnaire and control examination of the patient, to the administration of the vaccine to the patient.
- 7.
Feedback collection.
Collecting feedback involves monitoring post-vaccination phenomena in vaccinated patients (temperature increase, etc.).
Thus, the process management process consists of three levels: strategic, tactical and operational, and it looks like a simple linear process chain.
4.2. IT Support Model of a Vaccine Ecosystem
The spread and development of ecosystem approaches in recent years has been made possible in large part by digital technology. It is they that provide the proper level of information exchange between participants and data processing that is necessary to organize effective interaction and obtain the necessary synergies. The role of IT companies in healthcare ecosystems is really significant: they are responsible for creating platforms, providing appropriate services and developing other IT solutions for doctors and patients on the basis of healthcare ecosystem data [
17].
The process described in
Section 4.1 implies efficient data exchange between subjects of a vaccine lifecycle and information processing. In the authors’ opinion, the form of implementation of such IT support is to become an ecosystem platform that integrates the levels of ecosystem interaction necessary for decision-making and for data exchange between the participants.
A virtual platform that allows tracking support for the development and production of vaccines and further vaccination of the population, taking into account all the requirements of a patient-centered and open innovation philosophy, should meet a number of requirements:
- 1.
The solution must provide control of the process at the operational (value-chain visibility), strategic (supply chain planning) and tactical (mission control) levels.
- 2.
Meeting expectations at the strategic level will link patient flows to vaccines and physical vaccines themselves in the respective states. For example, some vaccines need to be transported under stringent conditions according to the temperatures of the containers in which they are stored. It is necessary to consider not only the delivery of the substance, but also to take into account the requirements of the developer and operator of the vaccine, such as temperature. It is also necessary to monitor the vaccination process in a timely way, because this is required:
Apart from physical and medical requirements, there are a number of legal requirements. For example, it is necessary to follow the GMP (international quality standard for medicinal products) certificate protocol.
- 3.
At the operational level, it is necessary to ensure the execution of the plan developed from the tactical level.
- 4.
For the vaccine development stage the openness of the solution for new participants and new data is crucially important as this stage is very sensitive for innovations. The ability to access new (including external) data and ideas will allow for faster creation of new vaccines and more effective feedback on existing vaccines.
The top-level services model of such a platform is shown in
Figure 5.
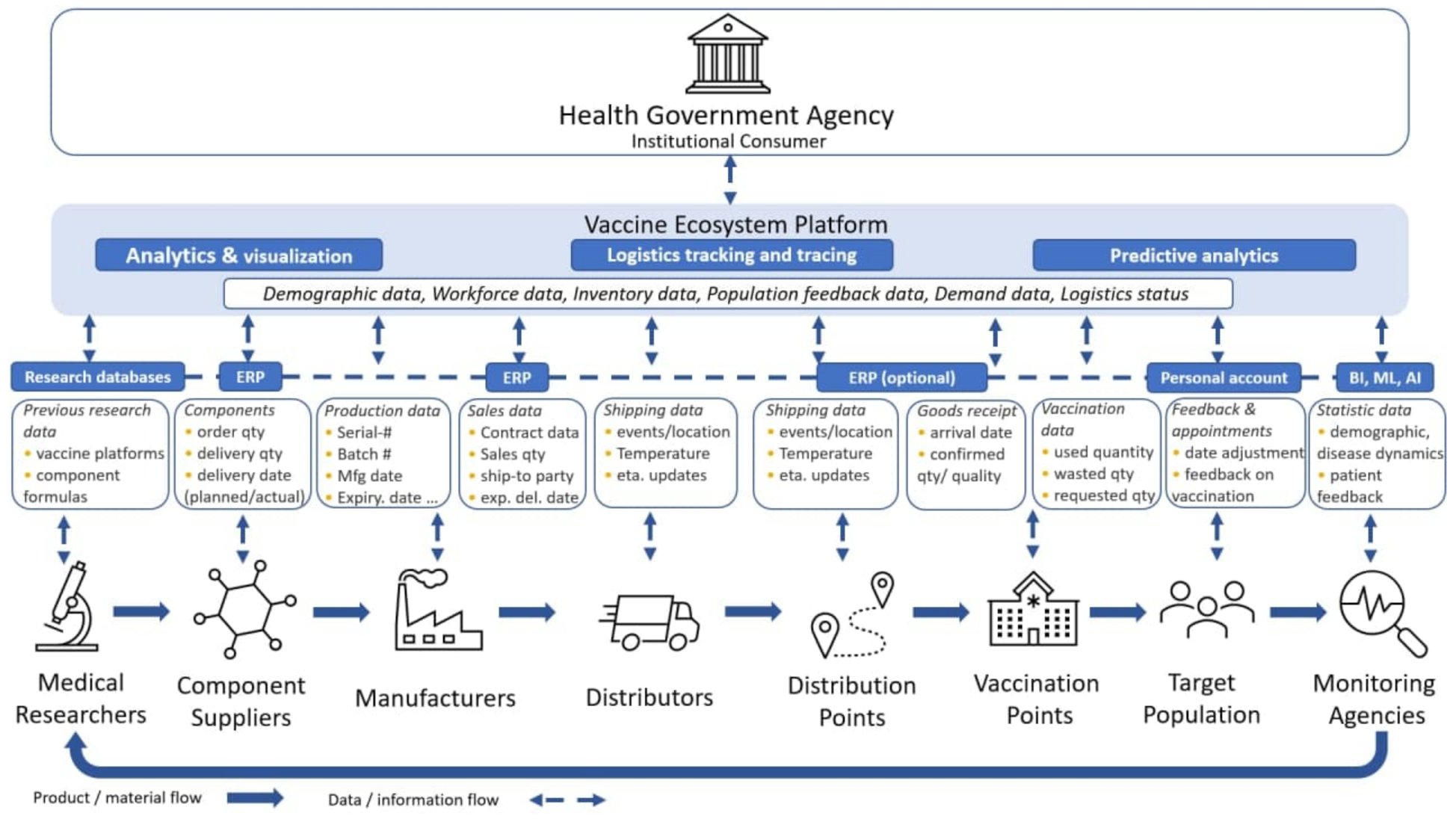
In terms of IT support, the following tasks must be accomplished:
ERP accounting task—implementation of calculations underlying the strategy.
ERP as an accounting system connected with external production—in the situation when production is an external entity in relation to the customer, but it is possible to obtain data on the readiness to produce a vaccine (a confirmed application). ERP 4.0 is an entity that connects various unaffiliated players within a single landscape that quickly adapts to the needs of companies that are part of a vertically integrated chain of unaffiliated companies. Today, there are ERP and vendor systems that are linked to the ERP in use through a production bus.
Coordination of logistics—as a “web”, which connects the players of different types: transport logisticians, warehouse logisticians, production managers, coordinators, calculators, controllers of the budget process, the treasury center. When building logistics there is coordination between the various players, which leads to achieving a common strategic goal through the work of a coherent single mechanism.
Coordination planning—it connects three logistic flows: patients–doctors–vaccines, taking into account the technological limitations of production, delivery and storage at different stages in case of any contradictions. To smoothly enter the solution of main medical tasks and avoid failures after vaccination, balancing strategic tasks is necessary to eliminate contradictions on finances, terms, target benchmarks on the vaccinated population, preservation of economic efficiency of enterprises, which are involved in the production of medical products, specialization of doctors.
The model of the platform solving the above tasks is presented in
Figure 6. The described platform is primarily focused on efficient and prompt distribution and implementation of innovations, and therefore should be open to potential access by admitted players. At the same time, it is not a homogeneous, but a heterogeneous solution, i.e., there can be several platforms, but they are integrated through a data bus and become a virtual single platform. For example, in Uzbekistan, the state itself implements the task of controlling the logistics flows, because a ready-made solution was already created. Thus, three platforms were combined into a single platform with access for authorized players. Within a single landscape of similar platform solutions several components from different vendors can co-exist, and the presence of a single virtual platform solves the problem of access to data related to the vaccination process for interested players. Through the bus (portal), it is possible to gain the necessary access and operational or synthetic analytics, which allows the process to be managed.
The proposed vaccine ecosystem model solution is relevant not only during the current pandemic, but also during periods of stable viral load. There are effective vaccines with short lifecycles that do not allow long storage, and there are those with long lifecycles with expensive storage. These contradictions lead to a decision on the timing of production and delivery, storage, etc. Thus there is a return to the Deming cycle, which is interpreted not only on the operational, but also on the tactical circuit. We can say that the population will experience a standard viral load: corona virus, influenza, acute respiratory viral infections, etc. The viral load is stationary and equal to the constant. The volume of diseased people will have a stable value due to the principles of immunity realization. After the end of one disease, it is necessary to go to the planning stage relying on statistics and expert data. In order to collect statistical data, it is necessary to have a tool for receiving feedback from the population.
The article proposes the reference solutions for the vaccine lifecycle management in a form of the innovative ecosystem and its virtual platform. Implementing the proposed model to the real situation will require tailoring it to the particular conditions. While adopting the solution, it is necessary to consider the following input factors:
Cyclical planning requirements;
Specificity of the presence of supervisory bodies at different levels;
Multi-level;
Openness of the architecture;
Compliance with the general methodology of the enterprise architecture;
Cloud/non-cloud deployment;
Methodological and methodological business requirements.
The organizational issues of vaccine ecosystem development are beyond the scope of this article. In other words, the symbiosis of medical institutions with IT and telecom companies into medical ecosystems is a clearly formed trend. The authors have already analyzed the features of innovative ecosystems as a form of cooperation activity within the supply chain [
54,
55]. The further research in this direction could be a study of the peculiarities of organizing innovative ecosystems in health care and, specifically, in vaccine development, production, and delivery. The growing activity in the sphere of ecosystem cooperation in health care and vaccination can be undoubtedly considered as one of the consequences of the COVID-19 pandemic.
The proposed IT solution models (
Figure 5 and
Figure 6) are largely based on and develop the SAP VCH solution. The emergence of a SAP VCH solution is the result of an architectural composition of ready-made components for the actual business task, which appeared quite quickly as a huge challenge. In this context, the concept proposed in the article allows us to approach the task of designing, creating, analyzing, and developing a solution in vaccine management in the most holistic and, as a consequence, effective way.
The architecture model of the development, production, distribution of vaccines and vaccination of the population, supported at every stage by a virtual platform, proposed in the article, provides the following benefits:
Single information space, effective data management at all stages;
Effective domain knowledge management;
Effective innovation management and implementation throughout the whole vaccine supply chain;
Life-cycle management, providing the key benefits of the CALS approach;
A virtual platform without being tied to a specific IT solution allows new members of the system to be connected;
The ability to scale the platform for its further use in tracking the stages of drug development.
5. Conclusions
The COVID-19 pandemic was a serious test for humanity, revealing the need to develop and improve the medical, economic, managerial, and IT components of vaccine development, production, and delivery systems. New and re-emerging infections with pandemic potential will undoubtedly arise. The problem is how best to prepare for such an event. One of the key lessons learned from the current pandemic should be to understand how to avoid such global socio-biological disasters.
Developing a reliable and effective vaccine is no easy task, since it is not only important to create the vaccine itself in the laboratory, but also to produce, distribute and administer it to the population. In this regard, it is important to have a systematic view of the lifecycle of vaccine development, production and delivery, including a model of this lifecycle, a model of interaction between the participants in this cycle, as well as a model of its IT support within a single information space. The article describes the set of architecture models for state-level management of the vaccine lifecycle, including the model of the supply chain-based lifecycle model, the model of the vaccine lifecycle ecosystem and its virtual platform model.
The proposed complex architecture solution provides a theoretical foundation for the organization of the vaccine management and control activities at the national or regional levels. It can be considered as the upper-level reference model for creating a real vaccine lifecycle ecosystem. The ecosystem solution proposed in the article, ensuring the lifecycle of vaccines, is relevant not only in periods of global pandemics, but also during stable seasonal viral load. With a hypothetical victory over coronavirus in the near future, we will still face other epidemics (seasonal influenza). The creation of a vaccine ecosystem model on the basis of this complex will provide society with an effective organizational and management mechanism for continuous improvement and development of the vaccine system both during the pandemic and the acceptable epidemiological situation.
The results presented in this article are essentially the fruit of the open innovation philosophy [
56,
57]. The article was prepared by the staff of Peter the Great Saint Petersburg Polytechnic University, a university that is a member of the SAP University Alliance. SAP brought to market the SAP VCH solution which, despite a certain integrity in the sense of IT support of the set of tasks in the specific area, did not contain a proper scientific justification and did not offer a common framework for the formation of this kind of IT platform. The IT landscape of the solution was in fact heterogeneous in each case. How do we correctly, quickly, cost-effectively, in accordance with future projections, develop such an architecture, taking into account epidemiological, immunological, country, economic, logical, production peculiarities? The trial and error method or long discussions are hardly applicable in this case: people’s lives and well-being are at stake. The scientific approach proposed by the authors of this article is the result of the interaction of organizations external to each other and their knowledge, which they openly share with each other and with professionals in society. The approach, in its most open presentation and interpretation, is the very tool that allows us to find a solution that takes into account the multifaceted specifics of a particular case, the constraints and dynamically changing goals.
The article does not address the issues of evaluating the effectiveness of the proposed ecosystem and platform solutions—this is a topic for a separate study. The authors have a strong track record on approaches to assessing the effectiveness of IT solutions. The study of the effectiveness of ecosystem interactions and platform solutions used in ecosystems includes several significant aspects:
Assessing changes in supply chain efficiency through the creation of an innovative industry ecosystem;
Assessing the effectiveness of platform solutions that support information exchange in an industry innovation ecosystem;
Assessing the value and the feasible cost of participation in the industry innovation ecosystem for its participants.
All the listed issues are supposed to be topics for further research.