Convenient Sampling of Xylem Sap from Adult Tree Trunks and Analysis of Its Components
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Trees, Study Site, and Sampling Periods
2.2. Xylem Sap Sampling
2.3. Types of Water-Absorbing Resin and Xylem Sap Components
2.4. Purification of Na-Type Water-Absorbing Resin
2.5. Recovery of Inorganic Anions from the Resin
2.6. Preservative Quality of Inorganic Anions in Xylem Sap Collected in the Resin
2.7. Analysis of Inorganic Anions
2.8. Analysis of Total N, P, and S
2.9. Analysis of Total Free Amino Acids
2.10. Analysis of Ammonium Ions
2.11. Analysis of Total Sugars
2.12. Preparation of Li-Type Water-Absorbing Resin
2.13. Analysis of Metallic Elements
2.14. Statistical Analysis
3. Results and Discussion
3.1. Volume of Xylem Sap Collected
3.2. Characteristics of the Concentrations of Xylem Sap Components
3.2.1. Metallic Elements
3.2.2. Nonmetallic Elements
3.2.3. N compounds
3.2.4. Total Sugars
3.3. Estimation of the Amounts of Specific Components Transported by Xylem Sap
3.4. Seasonal Variation in the Concentrations of K in Xylem Sap
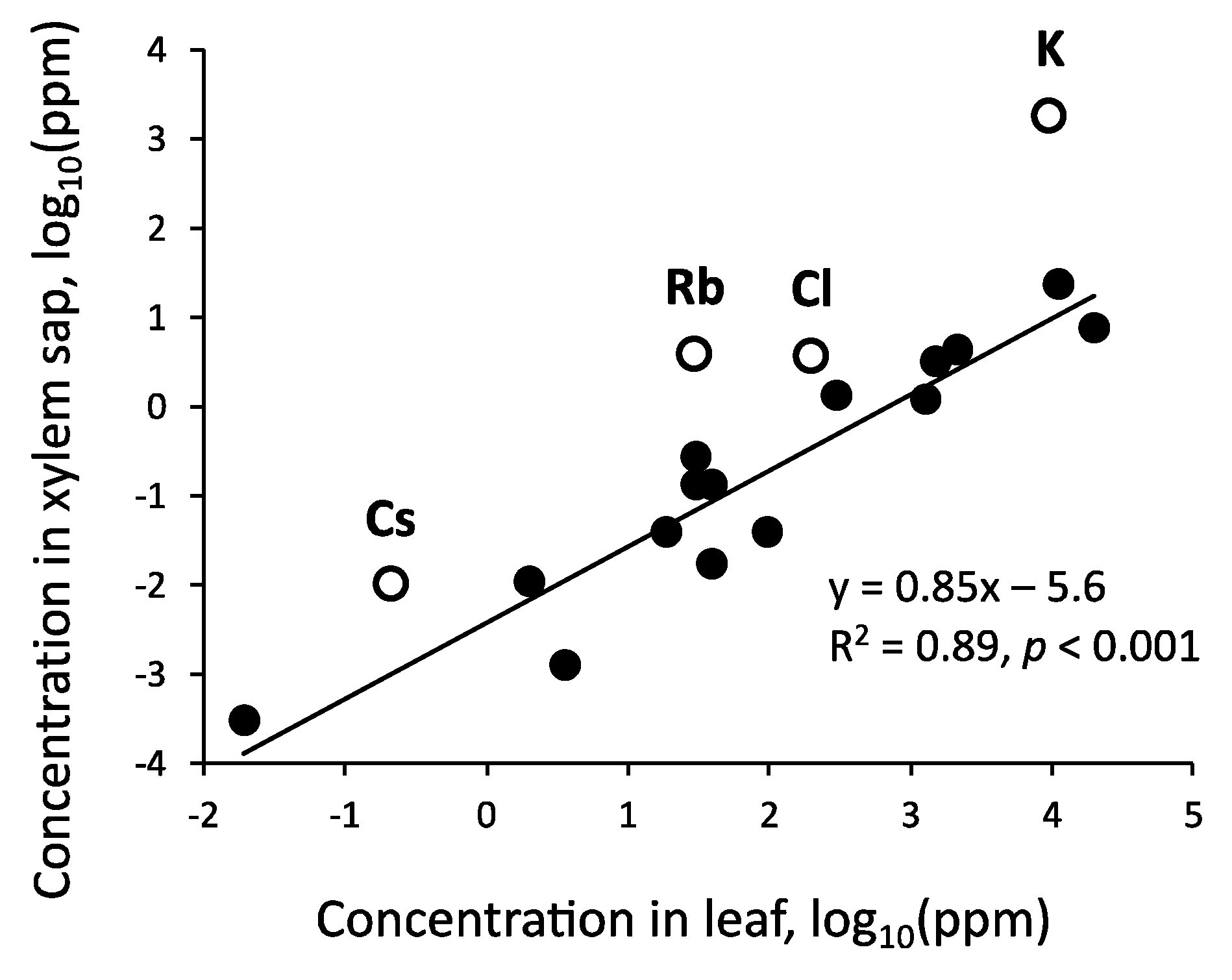
3.5. Comparison of the Elemental Concentrations in the Xylem Sap with Those in Tree Leaves and the Soil Solution
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Element | Conc. Unit | Median (or Mean *) | Q1 (or Min **) | Q3 (or Max ***) | n |
|---|---|---|---|---|---|
| K | ppm | 0.6 | 0.2 | 2.4 | 906 |
| Rb | ppb | 4.3 * | 2.8 ** | 6.6 *** | 5 |
| Cs | ppb | 0.010 * | 0.008 ** | 0.013 *** | 5 |
| Mg | ppm | 1.2 | 0.7 | 1.8 | 906 |
| Ca | ppm | 5.4 | 2.9 | 9.7 | 906 |
| Sr | ppb | 42 | 22 | 66 | 817 |
| Ba | ppb | 30 | 10 | 79 | 701 |
| Al | ppm | 0.34 | 0.02 | 1.0 | 906 |
| Cr | ppb | 0.09 | 0.06 | 0.29 | 63 |
| Mn | ppm | 0.07 | 0.01 | 0.15 | 906 |
| Fe | ppb | 3.0 | 1.3 | 6.9 | 906 |
| Ni | ppb | 4 | 1 | 14 | 63 |
| Cu | ppb | 6 | 3 | 14 | 763 |
| Zn | ppb | 24 | 10 | 54 | 817 |
| Cd | ppb | 0.34 | 0.14 | 0.46 | 63 |
| N (as NO3−) | ppm | 4.8 | 2.4 | 8.6 | 906 |
| P (1) | ppm | – | 0.001 ** | 0.4 *** | – |
| S | ppm | 1.0 | 0.6 | 2.0 | 701 |
| Cl (as Cl−) | ppm | 3.2 | 1.8 | 4.9 | 906 |
| Element | Q. serrata (n = 5) | R * | C. japonica (n = 47) | R ** |
|---|---|---|---|---|
| K | 9610 ± 410 | 185 | 4200 ± 950 | 443 |
| Rb | 29.8 ± 0.7 | 131 | 10.5 ± 2.1 | 343 |
| Cs | 0.21± 0.01 | 47.1 | 0.030 ± 0.005 | 90.0 |
| Mg | 2130 ± 20 | 2.1 | 1180 ±160 | 11.0 |
| Ca | 10,950 ± 130 | 2.2 | 12,290 ± 1840 | 1.1 |
| Sr | 30.3 ± 0.3 | 4.5 | 62.8 ± 13.4 | 0.81 |
| Ba | 30.0 ± 0.4 | 9.3 | 13.2 ± 3.5 | 0.34 |
| Al | 39.1 ± 2.1 | 3.4 | 115 ± 41 | 0.84 |
| Cr | 3.6 ± 0.6 | 0.36 | 0.37 (3) | 3.0 |
| Mn | 301 ± 5 | 4.5 | 28.8 ± 6.5 | 0.12 |
| Fe | 95.0 ± 4.4 | 0.42 | 110 ± 33 | 0.14 |
| Ni | 2.0 ± 0.3 | 5.5 | 0.22 ± 0.06 | 6.8 |
| Cu | 38.4 ± 1.3 | 0.44 | 3.1 ± 0.7 | 9.4 |
| Zn | 18.8 ± 0.2 | 2.1 | 6.8 ± 1.4 | 4.0 |
| Cd | 0.019 ± 0.002 | 15.8 | 0.079 ± 0.016 | 1.3 |
| N | 19,500 (1) | 0.40 | 11,160 ± 1560 | 0.27 |
| P | 1280 ± 20 | 1.0 | 765 ± 157 | 1.7 |
| S | 1510 ± 30 | 2.1 | 890 ± 120 | 1.4 |
| Cl | 200 (2) | 18.4 | 270 (4) | 13.0 |
References
- Alexou, M.; Peuke, A.D. Methods for xylem sap collection. Methods Mol. Biol. 2013, 953, 195–207. [Google Scholar] [PubMed]
- Schurr, U. Xylem sap sampling—New approaches to an old topic. Trends Plant Sci. 1998, 3, 293–298. [Google Scholar] [CrossRef]
- Schurr, U.; Schulze, E.D. The concentration of xylem sap constituents in root exudate, and in sap from intact, transpiring castor bean plants (Ricinus communis L.). Plant Cell Environ. 1995, 18, 409–420. [Google Scholar] [CrossRef]
- Siebrecht, S.; Tischner, R. Changes in the xylem exudate composition of poplar (Populus tremula x P. alba)—Dependent on the nitrogen and potassium supply. J. Exp. Bot. 1999, 50, 1797–1806. [Google Scholar] [CrossRef]
- Anetai, M.; Kojima, H.; Katoh, Y.; Uchino, E.; Izumi, T.; Hori, Y. Time course study of some constituents in sap of Japanese white birch. Rep. Hokkaido Inst. Pub. Health 2000, 50, 41–46, (In Japanese with English Abstract). [Google Scholar]
- Anetai, M.; Kojima, H.; Katoh, Y.; Uchino, E.; Izumi, T.; Hori, Y. A study of constituents in sap of painted maple, Japanese walnut and gloryvine grape. Rep. Hokkaido Inst. Pub. Health 2001, 51, 7–12, (In Japanese with English Abstract). [Google Scholar]
- Jiang, H.; Sakamoto, Y.; Tamai, Y.; Terazawa, M. Proteins in the exudation sap from birch trees, Betula platyphylla Sukatchev var. japonica Hara and Betula verrucosa Her. Eurasian J. For. Res. 2001, 2, 59–64. [Google Scholar]
- Buhtz, A.; Kolasa, A.; Arlt, K.; Walz, C.; Kehr, J. Xylem sap protein composition is conserved among different plant species. Planta 2004, 219, 610–618. [Google Scholar] [CrossRef]
- Bityutskii, N.; Yakkonen, K.; Petrova, A.; Nadporozhskaya, M. Xylem sap mineral analyses as a rapid method for estimation plant-availability of Fe, Zn and Mn in carbonate soils: A case study in cucumber. J. Soil Sci. Plant Nutr. 2017, 17, 279–290. [Google Scholar] [CrossRef]
- Scholander, P.F.; Hammel, H.T.; Bradstreet, E.D.; Hemmingsen, E.A. Sap pressure in vascular plants: Negative hydrostatic pressure can be measured in plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef]
- Stark, N.; Spitzner, C.; Essig, D. Xylem sap analysis for determining nutritional status of trees: Pseudotsuga menziesii. Can. J. Forest Res. 1985, 15, 429–437. [Google Scholar] [CrossRef]
- Weber, P.; Stoermer, H.; Gebler, A.; Schneider, S.; von Sengbusch, D.; Hanemann, U.; Rennenberg, H. Metabolic response of Norway spruce (Picea abies) trees to long-term forest management practices and acute (NH4)2SO4 fertilization: Transport of soluble non-protein nitrogen compounds in xylem and phloem. New Phytol. 1998, 140, 461–475. [Google Scholar] [CrossRef]
- Millard, P.; Wendler, R.; Hepburn, A.; Smith, A. Variations in the amino acid composition of xylem sap of Betula pendula Roth. trees due to remobilization of stored N in the spring. Plant Cell Environ. 1998, 21, 715–722. [Google Scholar] [CrossRef]
- Smith, K.T.; Shortle, W.C. Conservation of element concentration in xylem sap of red spruce. Trees 2001, 15, 148–153. [Google Scholar] [CrossRef]
- Guak, S.; Neilsen, D.; Millard, P.; Wendler, R.; Neilsen, G.H. Determining the role of N remobilization for growth of apple (Malus domestica Borkh.) trees by measuring xylem-sap N flux. J. Exp. Bot. 2003, 54, 2121–2131. [Google Scholar] [CrossRef]
- Passioura, J.B. The transport of water from soil to shoot in wheat seedlings. J. Exp. Bot. 1980, 31, 333–345. [Google Scholar] [CrossRef]
- Schurr, U.; Schulze, E.D. Effect of drought on nutrient transport and ABA transport in Ricinus communis. Plant Cell Environ. 1996, 19, 665–674. [Google Scholar] [CrossRef]
- Yong, J.W.H.; Wong, S.C.; Letham, D.S.; Hocart, C.H.; Farquhar, G.D. Effects of elevated [CO2] and nitrogen nutrition on cytokinins in the xylem sap and leaves of cotton. Plant Physiol. 2000, 124, 767–779. [Google Scholar] [CrossRef]
- Bollard, E.G. The use of tracheal sap in the study of apple-tree nutrition. J. Exp. Bot. 1953, 4, 363–368. [Google Scholar] [CrossRef]
- Milburn, J.A.; Ranasinghe, M.S. A comparison of methods for studying pressure and solute potentials in xylem and also in phloem laticifers of Hevea brasiliensis. J. Exp. Bot. 1996, 47, 135–143. [Google Scholar] [CrossRef]
- Nabais, C.; Hagemeyer, J.; Freitas, H. Nitrogen transport in the xylem of Quercus ilex: The role of ornithine. J. Plant Physiol. 2005, 162, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Alves, S.; Gonçalves, M.L.S.; dos Santos, M.M.C. Determination of nickel, calcium and magnesium in xylem sap by flame atomic absorption spectrometry using a microsampling technique. Phytochem. Anal. 2009, 20, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Sugiura, T.; Sakamoto, D.; Moriguchi, T. Effects of dormancy progression and low-temperature response on changes in the sorbitol concentration in xylem sap of Japanese pear during winter season. Tree Physiol. 2013, 33, 398–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Killiny, N.; Hijaz, F. Chemical compositeon of xylem sap from Citrus sinensis L. Osbeck (sweet orange). Proc. Fla. State Hort. Soc. 2015, 128, 114–118. [Google Scholar]
- Newby, R.C. The use of insects for sampling xylem sap. Ann. Bot. 1980, 45, 213–215. [Google Scholar] [CrossRef]
- Malone, M.; Herron, M.; Morales, M.-A. Continuous measurement of macronutrient ions in the transpiration stream of intact plants using the meadow spittlebug coupled with ion chromatography. Plant Physiol. 2002, 130, 1436–1442. [Google Scholar] [CrossRef]
- Miller, S.M.; Hunt, L.C. Collection of xylem sap from apple trees by vacuum. Can. J. Plant Sci. 1971, 51, 438–439. [Google Scholar] [CrossRef]
- Mori, A.; Haibara, K.; Aiba, Y. Seasonal and diurnal variations of mineral concentrations in the branch xylem sap of Cornus contriversa. J. Jpn. For. Soc. 1991, 73, 466–470, (In Japanese with English Abstract). [Google Scholar]
- Furukawa, J.; Abe, Y.; Mizuno, H.; Matsuki, K.; Sagawa, K.; Kojima, M.; Sakakibara, H.; Iwai, H.; Satoh, S. Seasonal fluctuation of organic and inorganic components in xylem sap of Populus nigra. Plant Root 2011, 5, 56–62. [Google Scholar] [CrossRef]
- Glavac, V.; Koenies, H.; Ebben, U. Seasonal variations in mineral concentrations in the trunk xylem sap of beech (Fagus sylvatica L.) in a 42-year-old beech forest stand. New Phytol. 1990, 116, 47–54. [Google Scholar] [CrossRef]
- Rennenberg, H.; Schupp, R.; Glavac, V.; Jochheim, H. Xylem sap composition of beech (Fagus sylvatica L.) trees: Seasonal changes in the axial distribution of sulfur compounds. Tree Physiol. 1994, 14, 541–548. [Google Scholar] [CrossRef]
- Chen, L.; Cao, Y.; Zhang, Z.; Liu, X.; Teramage, M.T.; Zhang, X.; Sun, X. Characteristics of chemical components in the trunk xylem sap of pine trees by means of a centrifugation collection method. Plant Physiol. Biochem. 2019, 142, 482–489. [Google Scholar] [CrossRef]
- Forestry Agency. Types of forest ecosystem. (In Japanese). Available online: https://www.rinya.maff.go.jp/j/keikaku/tayouseichousa/taipu.html (accessed on 1 March 2022).
- Ministry of Land, Infrastructure, Transport and Tourism. Fundamental Land Classification Survey—Geomorphology, Subsurface Geology & Soil–, 1:50,000 (Makabe). (In Japanese). Available online: https://nlftp.mlit.go.jp/kokjo/inspect/landclassification/land/5-1/prefecture08.html#prefecture08-04 (accessed on 1 March 2022).
- Japan Meteorological Agency. Search for the Past Meteorological Data. (In Japanese). Available online: http://www.data.jma.go.jp/obd/stats/etrn/index.php (accessed on 1 March 2022).
- Masuda, F.; Tanaka, K. Super water-absorbing polymer and its use. J. Home Econ. Jpn. 1989, 40, 721–724. (In Japanese) [Google Scholar]
- Sakimoto, S.; Hosoda, K. Development of Concentration Technology. (In Japanese). Available online: https://agriknowledge.affrc.go.jp/RN/2039016818.pdf (accessed on 16 January 2023).
- Charlot, G. L’analyse Qualitative et les Réactions en Solution, 4th ed.; Masson et Cie: Paris, France, 1957; 365p. [Google Scholar]
- Martell, A.E. Stability Constants of Metal-Ion Complexes, Section II: Organic Ligands, Special Publication No. 17; Chemical Society: London, UK, 1964; 754p. [Google Scholar]
- Okamoto, K.; Matsushima, A.; Ota, T.; Akasaki, T. A comparative study on the amino nitrogen values determined by the Van Slyke method, Sorensen method, and ninhydrin colorimetric method. Rep. Central Customs Labo. 2013, 53, 11–17, (In Japanese with English Abstract). [Google Scholar]
- Moore, S.; Stein, W.H. Photometric ninhydrin method for use in the chromatography of amino acids. J. Biol. Chem. 1948, 176, 367–388. [Google Scholar] [CrossRef]
- Moore, S.; Stein, W.H. A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J. Biol. Chem. 1954, 211, 907–913. [Google Scholar] [CrossRef]
- Searle, P.L. The berthelot or indophenol reaction and its use in the analytical chemistry of nitrogen. A review. Analyst 1984, 109, 549–568. [Google Scholar] [CrossRef]
- Kempers, A.J.; Kok, C.J. Re-examination of the determination of ammonium as the indophenol blue complex using salicylate. Anal. Chim. Acta 1989, 221, 147–155. [Google Scholar] [CrossRef]
- Rhine, E.D.; Sims, G.K.; Mulvaney, R.L.; Pratt, E.J. Improving the Berthelot reaction for determining ammonium in soil extracts and water. Soil Sci. Soc. Am. J. 1998, 62, 473–480. [Google Scholar] [CrossRef]
- Arakawa, Y.; Akagi, I.; Yamamoto, K. Determination of ammonium nitrogen in KCl extracts of cropland soils by using 2-hydroxybiphenyl sodium salt. Jpn. J. Soil Sci. Plant Nutr. 2003, 74, 657–659. (In Japanese) [Google Scholar]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Kitamura, S.; Nakaya, M. Determination methods of sugars. Seibutu-Kogaku Kaishi 2012, 90, 790–793. (In Japanese) [Google Scholar]
- Hashimoto, K.; Isobe, N.; Takahashi, Z. Studies on the carbohydrates of plants, Fractional quantitative analysis of carbohydrate pattern in agricultural products. Rep. Toyo Inst. Food Tech. 1967, 8, 395–403, (In Japanese with English Abstract). [Google Scholar]
- Fujikawa, T.; Shiomi, M.; Mukai, S.; Wada, M. Investigation of the phenol-sulfuric acid method for the quantitative analysis of sugar, mainly on the influence of impurity. J. Agric. Chem. Soc. Jpn. 1974, 48, 483–491, (In Japanese with English Abstract). [Google Scholar]
- Fiora, A.; Cescatti, A. Vertical foliage distribution determines the radial pattern of sap flux density in Picea abies. Tree Physiol. 2008, 28, 1317–1323. [Google Scholar] [CrossRef]
- Čermák, J.; Nadezhdina, N.; Trcala, M.; Simon, J. Open field-applicable instrumental methods for structural and functional assessment of whole trees and stands. iForest 2015, 8, 226–278. [Google Scholar] [CrossRef]
- Nadezhdina, N.; Čermák, J.; Ceulemans, R. Radial patterns of sap flow in woody stems of dominant and understory species: Scaling errors associated with positioning of sensors. Tree Physiol. 2002, 22, 907–918. [Google Scholar] [CrossRef]
- Kumagai, T.; Aoki, S.; Nagasawa, H.; Mabuchi, T.; Kubota, K.; Inoue, S.; Utsumi, Y.; Otsuki, K. Effects of tree-to-tree and radial variations on sap flow estimates of transpiration in Japanese cedar. Agric. For. Meteorol. 2005, 135, 110–116. [Google Scholar] [CrossRef]
- Matisons, R.; Bardulis, A.; Kanberga-Silina, K.; Krisans, O.; Jansons, A. Sap flow in xylem of mature Norway spruce: A case study in Northwestern Latvia during the season of 2014–2015. Baltic Forestry 2017, 23, 477–481. [Google Scholar]
- Kubota, M.; Tenhunen, J.; Zimmermann, R.; Schmidt, M.; Kakubari, Y. Influence of environmental conditions on radial patterns of sap flux density of a 70-year Fagus crenata trees in the Naeba Mountains. Ann. For. Sci. 2005, 62, 289–296. [Google Scholar] [CrossRef]
- Sato, T.; Oda, T.; Igarashi, Y.; Suzuki, M.; Uchiyama, Y. Circumferential sap flow variation in the trunks of Japanese cedar and cypress trees growing on a steep slope. Hydrol. Res. Lett. 2012, 6, 104–108. [Google Scholar] [CrossRef]
- Annamalainathan, K.; Joseph, J.; Alam, B.; Satheesh, P.R.; Jacob, J. Seasonal changes in xylem sap flow rate in mature rubber plants. J. Plant. Crops 2013, 41, 343–349. [Google Scholar]
- Zhang, J.-G.; He, Q.-Y.; Shi, W.-Y.; Otsuki, K.; Yamanaka, N.; Du, S. Radial variations in xylem sap flow and their effect on whole-tree water use estimates. Hydrol. Process. 2015, 29, 4993–5002. [Google Scholar] [CrossRef]
- Sato, T.; Tanaka, N.; Inoue, M.; Sawada, H.; Watanabe, S.; Suzuki, M. Report on an experiment of dye injection into xylem sap of a Quercus serrata tree in University Forest in Aichi. Miscellaneous Info. Univ. Tokyo For. 2010, 49, 29–41, (In Japanese with English Abstract). [Google Scholar]
- Takeuchi, N. Measurement of evapotranspiration rate using a lysimeter method. Water Sci. 1989, 33, 70–84. (In Japanese) [Google Scholar]
- Kondo, T.; Ohga, S. Bud break and water movement of deciduous broad-leaved trees (review). Bull. Kyushu Univ. For. 2000, 81, 63–76, (In Japanese with English Abstract). [Google Scholar]
- Komiyama, A. Relationships between stem-diameter growth periods and leaf growth periods of deciduous broadleaved tree species with reference to environmental factors. J. Jpn. For. Soc. 1991, 73, 409–418, (In Japanese with English abs.tract). [Google Scholar]
- Yamashita, K.; Okada, N.; Kamo, K. Application of the wire dendrometer for monitoring the radial growth of trees, A comparison with the conventional band dendrometer and the pinning method. J. Japan Wood Res. Soc. 2006, 52, 8–18, (In Japanese with English Abstract). [Google Scholar] [CrossRef]
- Motoyanagi, Y.; Chiba, Y.; Kawasaki, T.; Migita, C.; Sato, A. Relationship between climate conditions and tree growths and canopy foliage dynamics in a Quercus serrata forest in Tsukuba, Japan. Kanto J. For. Res. 2014, 65, 319–322, (In Japanese with English Abstract). [Google Scholar]
- Sánchez-Costa, E.; Poyatos, R.; Sabaté, S. Contrasting growth and water use strategies in four co-occurring Mediterranean tree species revealed by concurrent measurements of sap flow and stem diameter variations. Agric. For. Meteorol. 2015, 207, 24–37. [Google Scholar] [CrossRef]
- Moore, D.B. Winter-dormant-season sap flow dynamics and the environmental conditions that drive them. Acta Hortic. 2020, 1300, 97–104. [Google Scholar] [CrossRef]
- Shibata, S.; Kobashi, S.; Hanayama, H. Influence of soil moisture and air temperature to photosynthetic activity of some ever-green broad-leaved trees. J. Jpn. Soc. Reveget. Tech. 1991, 17, 1–8, (In Japanese with English Abstract). [Google Scholar] [CrossRef]
- Kosugi, Y.; Shibata, S.; Matsui, K.; Kobashi, S. Differences between deciduous and evergreen broad-leaved trees in the pattern of seasonal change of leaf-scale photosynthetic net assimilation rate and transpiration rate. J. Jpn. Soc. Reveget. Tech. 1997, 22, 205–215. [Google Scholar] [CrossRef]
- Catovsky, S.; Holbrook, N.M.; Bazzaz, F.A. Coupling whole-tree transpiration and canopy photosynthesis in coniferous and broad-leaved tree species. Can. J. For. Res. 2002, 32, 295–309. [Google Scholar] [CrossRef]
- Komatsu, H.; Kume, T.; Yoshifuji, N.; Hotta, N.; Suzuki, M. Transpiration of a Cryptomeria japonica plantation in winter: Analysis based on one-year sap flow measurements. Bull. Tokyo Univ. For. 2007, 117, 1–9. [Google Scholar]
- Liu, X.; Zhang, B.; Zhuang, J.-Y.; Han, C.; Zhai, L.; Zhao, W.-R.; Zhang, J.-C. The relationship between sap flow density and environmental factors in the Yangtze River delta region of China. Forests 2017, 8, 74. [Google Scholar] [CrossRef]
- Zhao, X.-w.; Ouyang, L.; Zhao, P.; Zhang, C.-f. Effects of size and microclimate on whole-tree water use and hydraulic regulation in Schima superba trees. PeerJ 2018, 6, e5164. [Google Scholar] [CrossRef]
- Xu, S.; Yu, Z. Environmental control on transpiration: A case study of a desert ecosystem in northwest China. Water 2020, 12, 1211. [Google Scholar] [CrossRef]
- Morikawa, Y. Seasonal variation in transpiration of Chamaecyparis obtusa on clear days. J. Jpn. For. Soc. 1970, 52, 259–262. [Google Scholar]
- Ortuño, M.F.; Garcia-Orellana, Y.; Conejero, W.; Ruiz-Sánchez, M.C.; Mounzer, O.; Alarcón, J.J.; Torrecillas, A. Relationship between climatic variables and sap flow, stem water potential and maximum daily trunk shrinkage in lemon trees. Plant Soil 2006, 279, 229–242. [Google Scholar] [CrossRef]
- Pfautsch, S.; Bleby, T.M.; Rennenberg, H.; Adams, M.A. Phloem as capacitor: Radial transfer of water into xylem of tree stems occurs via symplastic transport in ray parenchyma. For. Ecol. Manag. 2010, 259, 1190–1199. [Google Scholar] [CrossRef]
- Ma, C.; Luo, Y.; Shao, M.; Li, X.; Sun, L.; Jia, X. Environmental controls on sap flow in black locust forest in Loess Plateau, China. Sci. Rep. 2017, 7, 13160. [Google Scholar] [CrossRef]
- Gupta, S.K.; Ram, J.; Singh, H. Comparative study of transpiration in cooling effect of tree species in the atmosphere. J. Geosci. Environ. Protect. 2018, 6, 151–166. [Google Scholar] [CrossRef]
- Laiju, N.; Otieno, D.; Jung, E.-Y.; Lee, B.; Tenhunen, J.; Lim, J.-H.; Sung, J.-H.; Kang, S. Environmental controls on growing-season sap flow density of Quercus serrata Thunb in a temperate deciduous forest of Korea. J. Ecol. Field Biol. 2012, 35, 213–225. [Google Scholar] [CrossRef]
- Nagakura, J.; Shigenaga, H.; Akama, A.; Takahashi, M. Growth and transpiration of Japanese cedar (Cryptomeria japonica) and Hinoki cypress (Chamaecyparis obtusa) seedlings in response to soil water content. Tree Physiol. 2004, 24, 1203–1208. [Google Scholar] [CrossRef]
- Chiu, C.-W.; Tateishi, M.; Komatsu, H.; Otsuki, K. Comparison of transpiration for Quercus serrata estimated based on the sap-flux method and measured based on the cutting-tree experiment. Bull. Kyushu Univ. For. 2014, 95, 1–4. [Google Scholar]
- Utsumi, Y. Analytical methods for water distribution and water movement in secondary xylem of trees. J. Japan Wood Res. Soc. 2013, 59, 1–12, (In Japanese with English abstract). [Google Scholar] [CrossRef]
- Kuroda, K. Tree tissue and structures and its hydraulic conductance. Green Age 2008, 417, 36–39. (In Japanese) [Google Scholar]
- Van Bel, A.J.E. Xylem-phloem exchange via the rays: The undervalued route of transport. J. Exp. Bot. 1990, 41, 631–644. [Google Scholar] [CrossRef]
- Kitin, P.; Fujii, T.; Abe, H.; Takata, K. Anatomical features that facilitate radial flow across growth rings and from xylem to cambium in Cryptomeria japonica. Ann. Bot. 2009, 103, 1145–1157. [Google Scholar] [CrossRef]
- Sane, S.P.; Singh, A.K. Water movement in vascular plants: A primer. J. Indian Inst. Sci. 2011, 9, 233–242. [Google Scholar]
- Barnard, D.M.; Lachenbruch, B.; McCulloh, K.A.; Kitin, P.; Meinzer, F.C. Do ray cells provide a pathway for radial water movement in the stems of conifer trees. Amer. J. Bot. 2013, 100, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K. Functions of parenchyma cells in wood; material transport and heartwood formation. J. Jpn. Wood Res. Soc. 2015, 61, 131–135, (In Japanese with English abstract). [Google Scholar] [CrossRef] [Green Version]
- Kuroda, K.; Yamane, K.; Itoh, Y. Radial movement of minerals in the trunks of standing Japanese cedar (Cryptomeria japonica D. Don) trees in summer by tracer analysis. Forests 2020, 11, 562. [Google Scholar] [CrossRef]
- Mahara, Y.; Ohta, T.; Ohshima, J.; Iizuka, K. Origin and hydrodynamics of xylem sap in tree stems, and relationship to root uptake of soil water. Sci. Rep. 2021, 11, 8404. [Google Scholar] [CrossRef]
- Dambrine, E.; Martin, F.; Carisey, N.; Granier, A.; Hällgren, J.-E.; Bishop, K. Xylem sap composition: A tool for investigating mineral uptake and cycling in adult spruce. Plant Soil 1995, 168/169, 233–241. [Google Scholar] [CrossRef]
- Minato, K.; Kojima, N.; Ishimaru, Y.; Katayama, Y. Mobility of trace elements in stem of Sugi (Cryptomeria japonica D. Don) by some simple extraction methods. Bull. Kyoto Univ. For. 1990, 62, 326–337, (In Japanese with English abstract). [Google Scholar]
- Bundt, M.; Kretzschmar, S.; Zech, W.; Wilcke, W. Seasonal dynamics of nutrients in leaves and xylem sap of coffee plants as related to different soil compartments. Plant Soil 1997, 197, 157–166. [Google Scholar] [CrossRef]
- Tunca, R.I.; Ozgul, O.; Yabanli, M.; Sener, I. Evaluation of heavy metal concentrations in the xylem sap of Turkish pine (Pinus brutia Ten.) and honeydew of Marchalina hellenica, Gennadius (Hemiptera: Marchalinidae) collected in western Anatolia, Turkey. Fresenius Environ. Bull. 2018, 27, 9812–9815. [Google Scholar]
- Bilek, M.; Szwerc, W.; Kużniar, P.; Stawarczyk, K.; Kocjan, R. Time-related variability of the mineral content in birch tree sap. J. Elem. 2017, 22, 497–515. [Google Scholar] [CrossRef]
- Katakura, M. Growth and thickness of heartwood, sapwood, and bark of Quercus serrata and Quercus acutissima in Nagano Prefecture. Rep. Nagano Pref. For. Res. Ctr. 1987, 3, 7–12. (In Japanese) [Google Scholar]
- Iida, S.; Noguchi, S.; Shimizu, T.; Kaneko, T. Relationships of sapwood area and width of bark to diameter at breast height of Japanese cedar planted in the Tohoku district, Japan. J. Jpn. Assoc. Hydrol. Sci. 2017, 47, 3–9, (in Japanese with English abstract). [Google Scholar]
- Uchida, E.; Osada, K.; Nakao, K. Major and trace element chemical compositional signatures of some granitic tocks related to metal mineralization in Japan. Open J. Geol. 2017, 7, 559–576. [Google Scholar] [CrossRef]
- Uozumi, N. Ion transporters. seibutsu-kogaku kaishi 2018, 96, 589–592. (In Japanese) [Google Scholar]
- Rout, G.; Samantaray, S.; Das, P. Aluminum toxicity in plants: A review. Agronomie 2001, 21, 3–21. [Google Scholar] [CrossRef]
- Raveh, E.; Levy, Y. Analysis of xylem water as an indicator of current chloride uptake status in citrus trees. Sci. Hortic. 2005, 103, 317–327. [Google Scholar] [CrossRef]
- Pate, J.S.; Jeschke, W.D. Mineral uptake and transport in xylem and phloem of the proteaceous tree, Banksia prionotes. Plant Soil 1993, 155/156, 273–276. [Google Scholar] [CrossRef]
- Anderssen, F.G. Some seasonal changes in the tracheal sap of pear and apricot trees. Plant Physiol. 1929, 4, 459–476. [Google Scholar] [CrossRef] [Green Version]
- Köstner, B.; Schupp, R.; Schulze, E.-D.; Rennenberg, H. Organic and inorganic sulfur transport in the xylem sap and the sulfur budget of Picea abies trees. Tree Physiol. 1998, 18, 1–9. [Google Scholar] [CrossRef]
- Schupp, R.; Glavac, V.; Rennenberg, H. Thiol composition of xylem sap of beech trees. Phytochemistry 1991, 30, 1415–1418. [Google Scholar] [CrossRef]
- Schneider, A.; Kreuzwieser, J.; Schupp, R.; Sauter, J.J.; Rennenberg, H. Thiol and amino acid composition of the xylem sap of poplar trees (Populus × canadensis ‘robusta’). Can. J. Bot. 1994, 72, 347–351. [Google Scholar] [CrossRef]
- Saito, K. Sulfur assimilatory metabolism. The long and smelling road. Plant Physiol. 2004, 136, 2443–2450. [Google Scholar] [CrossRef]
- Gigolashvili, T.; Kopriva, S. Transporters in plant sulfur metabolism. Front. Plant Sci. 2014, 5, 442. [Google Scholar] [CrossRef]
- Schachtman, D.P.; Reid, R.J.; Ayling, S.M. Phosphorous uptake by plants: From soil to cell. Plant Physiol. 1998, 116, 447–453. [Google Scholar] [CrossRef]
- Escher, P.; Eiblmeier, M.; Hetzger, I.; Rennenberg, H. Spatial and seasonal variation in amino compounds in the xylem sap of a mistletoe (Viscum album) and its hosts (Populus spp. and Abies alba). Tree Physiol. 2004, 24, 639–650. [Google Scholar] [CrossRef]
- Prima-Putra, D.; Botton, B. Organic and inorganic compounds of xylem exudates from five woody plants at the stage of bud breaking. J. Plant Physiol. 1998, 153, 670–676. [Google Scholar] [CrossRef]
- Suzuki, T.; Kohno, K. Changes in the nitrogen compounds of xylem sap of mulberry (Morus alba L.) during regrowth after pruning. Ann. Bot. 1983, 51, 441–448. [Google Scholar] [CrossRef]
- Grassi, G.; Millard, P.; Wendler, R.; Minotta, G.; Tagliavini, M. Measurement of xylem sap amino acid concentrations in conjugation with whole tree transpiration estimates spring N remobilization by cherry (Prunus avium L.) trees. Plant Cell Environ. 2002, 25, 1689–1699. [Google Scholar] [CrossRef]
- Biles, C.L.; Abeles, F.B. Xylem sap proteins. Plant Physiol. 1991, 96, 597–601. [Google Scholar] [CrossRef]
- Soper, F.M.; Richards, A.E.; Siddique, I.; Aidar, M.P.M.; Cook, G.D.; Hutley, L.B.; Robinson, N.; Schmidt, S. Natural abundance (δ15N) indicates shifts in nitrogen relations of woody taxa along a savanna-woodland continental rainfall gradient. Oecologia 2015, 178, 297–308. [Google Scholar] [CrossRef]
- Stewart, G.R.; Pearson, J.; Kershaw, J.L.; Clough, E.C.M. Biochemical aspects of inorganic nitrogen assimilation by woody plants. Ann. Sci. For. 1989, 46, 648s–653s. [Google Scholar] [CrossRef]
- Suzuki, T. Physiology and ecology of forest trees, focusing on nutrition and metabolism (assimilation) of nitrogen. Kagaku To Seibutsu 1993, 31, 621–626. (In Japanese) [Google Scholar] [CrossRef]
- Miller, A.J.; Cramer, M.D. Root nitrogen acquisition and assimilation. Plant Soil 2004, 274, 1–36. [Google Scholar] [CrossRef]
- Fukasawa, Y.; Kuishi, T.; Seiwa, K. Underground of forest edges–role of mycorrhizal communities on seedling establishment. Japanese J. Ecol. 2013, 63, 239–249. (In Japanese) [Google Scholar]
- Toju, H.; Yamamoto, S.; Sato, H.; Tanabe, A.S.; Gilbert, G.S.; Kadowaki, K. Community composition of root-associated fungi in a Quercus-dominated temperate forest: “codominance” of mycorrhizal and root-endophytic fungi. Ecol. Evol. 2013, 3, 1281–1293. [Google Scholar] [CrossRef]
- Kimoto, R.; Sone, K.; Hata, K. The infection of Japanese cedar by arbuscular mycorrhizal fungi in Takakuma Experimental Forest of Kagoshima University. Kyushu J. For. Res. 2013, 66, 17–20. (In Japanese) [Google Scholar]
- Govindarajulu, M.; Pfeffer, P.E.; Jin, H.; Abubaker, J.; Douds, D.D.; Allen, J.W.; Bücking, H.; Lammers, P.J.; Shachar-Hill, Y. Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 2005, 435, 819–823. [Google Scholar] [CrossRef]
- Castro-Rodriguez, V.; Cañas, R.A.; de la Torre, F.N.; Pascual, M.B.; Avila, C.; Cánovas, F.M. Molecular fundamentals of nitrogen uptake and transport in trees. J. Exp. Bot. 2017, 68, 2489–2500. [Google Scholar] [CrossRef]
- Makarov, M.I. The role of mycorrhiza in transformation of nitrogen compounds in soil and nitrogen nutrition of plants: A review. Eurasian Soil Sci. 2019, 52, 193–205. [Google Scholar] [CrossRef]
- Kato, T.; Yamagata, M.; Tsukahara, S. Seasonal variations in nitrogenous compounds in xylem sap of Satsuma mandarin trees. J. Japan. Soc. Hort. Sci. 1984, 53, 13–16. [Google Scholar] [CrossRef] [Green Version]
- Loescher, W.H.; McCamant, T.; Keller, J.D. Carbohydrate reserves, translocation, and storage in woody plant roots. HortScience 1990, 25, 274–281. [Google Scholar] [CrossRef]
- Ketchie, D.O.; Lopushinsky, W. Composition of Root Pressure Exudate from Conifers; Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station: Portland, OR, USA, 1981; pp. 1–7. [Google Scholar]
- Kumagai, T.; Aoki, S.; Shimizu, T.; Otsuki, K. Sap flow estimates of stand transpiration at two slope positions in a Japanese ceder forest watershed. Tree Physiol. 2007, 27, 161–168. [Google Scholar] [CrossRef]
- Wind, C.; Arend, A.; Fromm, J. Potassium-dependent cambial growth in poplar. Plant Biol. 2004, 6, 30–37. [Google Scholar]
- Fromm, J. Wood formation of trees in relation of potassium and calcium nutrition. Tree Physiol. 2010, 30, 1140–1147. [Google Scholar] [CrossRef]
- Hamada, S.; Kumagai, T.; Kochi, K.; Kobayashi, N.; Hiyama, T.; Miyazawa, Y. Spatial and temporal variations in photosynthetic capacity of a temperate deciduous-evergreen forest. Trees 2016, 30, 1083–1093. [Google Scholar] [CrossRef]
- Yasue, K. Clarification of the mechanisms of variations in radial growth and wood properties of Cryptomeria japonica and Chamaecyparis obtusa due to climate change. In Research Report on Grants-in-Aid for Scientific Research (B); Ministry of Education, Culture, Sports, Science and Technology: Tokyo, Japan, 2018; (In Japanese with English abstract); Available online: https://kaken.nii.ac.jp/file/KAKENHI-PROJECT-26292092/26292092seika.pdf (accessed on 1 March 2022).
- Tighe-Neira, R.; Alberdi, M.; Arce-Johnson, P.; Romero, J.; Reyes, M.; Rengel, Z.; Inostroza-Blancheteau, C. Role of potassium in governing photosynthetic processes and plant yield. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore, 2018; pp. 191–203. [Google Scholar]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef]
- Yamori, W.; Hikosaka, K.; Way, D.A. Temperature response of photosynthesis in C3, C4, and CAM plants: Temperature acclimation and temperature adaptation. Photosynth. Res. 2014, 119, 101–117. [Google Scholar] [CrossRef]
- Dalmolin, A.C.; Dalmagro, H.J.; De A. Lobo, F.; Antunes Jr., M.Z.; Ortiz, C.E.R.; Vourlitís, G.L. Photosynthetic light and carbon dioxide response of the invasive tree, Vochysia divergens Pohl, to experimental flooding and shading. Photosynthetica 2013, 51, 379–386. [Google Scholar] [CrossRef]
- Pereira, S.; Frosi, G.; de Oliveira, M.T.; Lustosa, B.M.; Arruda, E.P.; Santos, M.G. Changes in phenotypic variability of two tropical woody species due to short and long-term exposure to different irradiances. Bragantia 2018, 77, 429–439. [Google Scholar] [CrossRef]
- Tohyama, T.; Ishii, Y.; Kamuro, Y.; Okabe, K. Seasonal light quality for plant growth. Reg. Plant Growth Develop. 2001, 36, 202–207. (In Japanese) [Google Scholar]
- Schaberg, P.G.; Snyder, M.C.; Shane, J.B.; Donnelly, J.R. Seasonal patterns of carbohydrate reserves in red spruce seedlings. Tree Physiol. 2000, 20, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Trifilò, P.; Lo Gullo, M.A.; Salleo, S.; Callea, K.; Nardini, A. Xylem embolism alleviated by ion-mediated increase in hydraulic conductivity of functional xylem: Insights from field measurements. Tree Physiol. 2008, 28, 1505–1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Losso, A.; Nardini, A.; Dämon, B.; Mayr, S. Xylem sap chemistry: Seasonal changes in timberline conifers Pinus cembra, Picea abies, and Larix decidua. Biol. Plant. 2018, 62, 157–165. [Google Scholar] [CrossRef]
- Reid, R.; Hayes, J. Mechanisms and control of nutrient uptake in plants. Int. Rev. Cytol. 2003, 229, 73–114. [Google Scholar] [PubMed]
- Weatherall, A.; Proe, M.F.; Craig, J.; Cameron, A.D.; Midwood, A.J. Internal cycling of nitrogen, potassium and magnesium in young Sitka spruce. Tree Physiol. 2006, 26, 673–680. [Google Scholar] [CrossRef]
- Yoshida, S.; Ichikuni, M. Migration of elements through forests with special emphasis on the role of canopy. Environ. Sci. 1988, 1, 31–38, (In Japanese with English abstract). [Google Scholar]
- Hudgins, J.W.; Krekling, T.; Franceschi, V.R. Distribution of calcium oxalate crystals in the secondary phloem of conifers: A constitutive defense mechanisms? New Phytol. 2003, 159, 677–690. [Google Scholar] [CrossRef]
- Lautner, S.; Ehlting, B.; Windeisen, E.; Rennenberg, H.; Matyssek, R.; Fromm, J. Calcium nutrition has a significant influence on wood formation in poplar. New Phytol. 2007, 173, 743–752. [Google Scholar] [CrossRef]
- He, H.; Bleby, T.M.; Veneklaas, E.J.; Lambers, H.; Kuo, J. Precipitation of calcium, magnesium, strontium and barium in tissues of four Acacia species (Leguminosae: Mimosoideae). PLoS ONE 2012, 7, e41563. [Google Scholar] [CrossRef]
- Nozaki, T.; Kurihara, H. Metal coprecipitation on calcium oxalate precipitated from homogeneous solution. Japan Analyst 1960, 9, 930–933, (in Japanese with English abstract). [Google Scholar] [CrossRef]
- Pongrac, P.; Serra, T.S.; Castillo-Michel, H.; Vogel-Mikuš, K.; Arčon, I.; Kelemen, M.; Jenčič, B.; Kavčič, A.; Carvalhoc, M.T.V.; Aarts, M.G.M. Cadmium associates with oxalate in calcium oxalate crystals and competes with calcium for translocation to stems in the cadmium bioindicator Gomphrena claussenii. Metallomics 2018, 10, 1576–1584. [Google Scholar] [CrossRef]
- Paiva, E.A.S. Are calcium oxalate crystals a dynamic calcium store in plants? New Phytol. 2019, 223, 1707–1711. [Google Scholar] [CrossRef]
- Cline, G.R.; Powell, P.E.; Szaniszlo, P.J.; Reid, C.P.P. Comparison of the abilities of hydroxamic and other natural organic acids to chelate iron and other ions in soil. Soil Sci. 1983, 136, 145–157. [Google Scholar] [CrossRef]
- Holmen, B.A.; Casey, W.H. Hydroxamate ligands, surface chemistry, and the mechanism of ligand-promoted dissolution of goethite [α-FeOOH(s)]. Geochim. Cosmochim. Acta 1996, 60, 4403–4416. [Google Scholar] [CrossRef]
- Duckworth, O.W.; Akafia, M.M.; Andrews, M.Y.; Bargar, J.R. Siderophore-promoted dissolution of chromium from hydroxide minerals. Environ. Sci. Process Impacts 2014, 16, 1348–1359. [Google Scholar] [CrossRef]
- Godo, G.H.; Reisenauer, H.M. Plant effects on soil manganese availability. Soil Sci. Soc. Am. J. 1980, 44, 993–995. [Google Scholar] [CrossRef]
- Gupta, N.; Gaurav, S.S.; Kumar, A. Molecular basis of aluminium toxicity in plants: A review. Am. J. Plant Sci. 2013, 4, 21–37. [Google Scholar] [CrossRef]
- De-Jesús-García, R.; Rosas, U.; Dubrovsky, J.G. The barrier function of plant roots: Biological bases for selective uptake and avoidance of soil compounds. Funct. Plant Biol. 2020, 47, 383–397. [Google Scholar] [CrossRef]
- Bol, R.; Julich, D.; Brödlin, D.; Siemens, J.; Kaiser, K.; Dippold, M.A.; Spielvogel, S.; Zilla, T.; Mewes, D.; von Blanckenburg, F.; et al. Dissolved and colloidal phosphorus fluxes in forest ecosystems–an almost blind spot in ecosystem research. J. Plant Nutr. Soil Sci. 2016, 179, 425–438. [Google Scholar] [CrossRef]
- Katagiri, S.; Miyake, N.; Sakagoshi, H. Studies on mineral cycling in a deciduous broad-leaved forest at Sanbe forest of Shimane University (XIII) The concentration and amount of nutrient elements in dominant trees. Bull. Fa. Agr. Shimane Univ. 1986, 20, 67–74, (In Japanese with English abstract). [Google Scholar]
- Sera, K.; Goto, S.; Takahashi, C.; Saitoh, Y. Movement of light elements in living plants measured by means of a standard-free method in in-air PIXE. NMCC Annual Repot 2013, 20, 52–63, (In Japanese with English abstract). [Google Scholar] [CrossRef]
- Takada, J.; Takamatsu, T.; Satake, K.; Sase, H. Data on Elemental Concentration in Land Plants by Neutron Activation Analysis (No. 1); National Institute for Environmental Studies: Tsukuba-City, Japan, 1993; 260p. (In Japanese) [Google Scholar]
- Sasaki, Y.; Nishihagi, K.; Kuroki, Y. Elemental analysis of leaves on polyploids in Japanese cedar (Cryptomeria japonica) and Japanese cypress (Chamaecyparis obtusa). In Research Report of the Oita Prefectural Forest Experiment Station; Oita Prefectural Forest Experiment Station: Oita, Japan, 1996; Volume 22, pp. 1–7, (In Japanese with English Abstract). [Google Scholar]
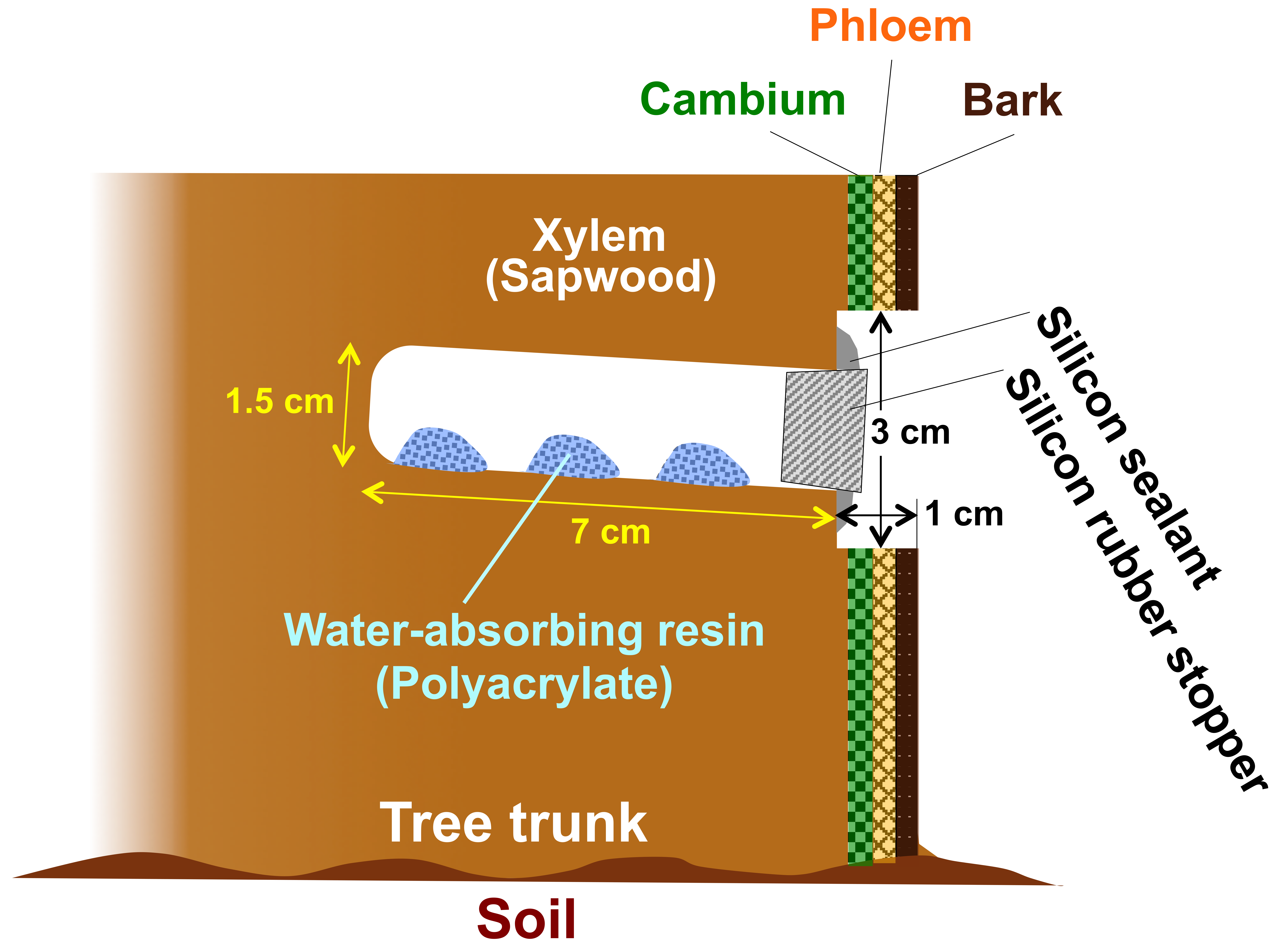






| Tree Species | Experiment 1 | Experiment 2 | Experiment 3 | |
|---|---|---|---|---|
| Q. serrata | Mean | 9.6 | 8.9 | 8.9 |
| SD | 2.8 | 2.7 | 1.9 | |
| Min | 5.4 | 4.9 | 6.5 | |
| Max | 14.0 | 12.6 | 11.9 | |
| MCV | 0.18 | 0.14 | 0.08 | |
| C. japonica | Mean | 10.4 | 11.5 | 10.4 |
| SD | 2.3 | 2.4 | 2.6 | |
| Min | 6.9 | 7.8 | 6.2 | |
| Max | 14.0 | 13.6 | 15.5 | |
| MCV | 0.52 | 0.52 | 0.50 |
| Element /Conc. Unit | Q. serrata | C. japonica | Literature | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean/SD | Min–Max | MCV | Mean/SD | Min–Max | MCV | Mean | Median | Min–Max | n | |
| K/ppm | 1780/620 | 917–2590 | 0.26 | 1860/430 | 1080–2360 | 0.36 | 155 | 80 1,2 | 8.3 3–1500 4 | 30 |
| Rb/ppm | 3.9/1.3 | 2.0–5.6 | 0.36 | 3.6/0.9 | 2.0–4.7 | 0.44 | – | – | – | – |
| Cs/ppb | 9.9/3.7 | 4.5–15 | 0.58 | 2.7/0.8 | 1.4–3.8 | 0.44 | – | – | – | – |
| Mg/ppm | 4.4/6.0 | 0.5–21 | 0.46 | 13/5 | 5.7–20 | 0.26 | 11 | 13 1,5 | 0.6 2–53 6 | 24 |
| Ca/ppm | 24/25 | 3.9–83 | 0.29 | 14/6 | 6.0–24 | 0.30 | 38 | 30 4,7 | 2.1 2–270 5 | 24 |
| Sr/ppb | 136/140 | 22–460 | 0.34 | 51/24 | 22–94 | 0.36 | – | – | – | – |
| Ba/ppb | 280/296 | 40–959 | 0.42 | 4.5/3.1 | 0.8–9.9 | 0.54 | – | – | – | – |
| Al/ppb | 134/45 | 60–226 | 0.26 | 97/53 | 23–206 | 0.45 | 140 | 73 8,9 | 12 8–900 9 | 6 |
| Cr/ppb | 1.3/0.8 | 0.4–3.3 | 0.65 | 1.1/1.0 | <LQ–3.3 | 1.0 | 48 | 48 | 48 10 | 1 |
| Mn/ppb | 1340/1520 | 212–5290 | 0.38 | 3.4/1.1 | 1.6–5.6 | 0.58 | 3300 | 1000 9 | 20 3–21,400 11 | 11 |
| Fe/ppb | 40/39 | 4.2–143 | 0.31 | 15/6 | 4.5–24 | 0.40 | 150 | 90 9,12 | 2.5 13–600 7 | 8 |
| Ni/ppb | 11/8 | 2.3–27 | 0.27 | 1.5/0.9 | 0.4–3.1 | 0.66 | 51 | 51 | 21 14–110 3 | 2 |
| Cu/ppb | 17/8 | 2.2–29 | 1.0 | 29/16 | <LQ–56 | 0.92 | 150 | 37 8 | 0.39 15–2050 13 | 7 |
| Zn/ppb | 39/16 | 14–62 | 0.44 | 27/13 | 10–47 | 0.66 | 740 | 500 9 | 10 13–5800 8 | 8 |
| Cd/ppb | 0.3/0.3 | <LQ–0.9 | 0.42 | 0.1/0.1 | <LQ–0.3 | 0.46 | 1 | 1 | 1 10 | 1 |
| Element /Conc. Unit | Q. serrata | C. japonica | Literature | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean/SD | Min–Max | MCV | Mean/SD | Min–Max | MCV | Mean | Median | Min–Max | n | ||
| Cl/ppm | 4.5/2.8 | 1.7–11 | 0.43 | 2.4/0.5 | 1.4–3.1 | 1.1 | 100 | 26 1 | 0.08 2–1200 3 | 7 | |
| PO4-P/ppm | 0.69/0.56 | 0.15–1.7 | 0.84 | 0.42/0.27 | 0.08–0.96 | 0.83 | 12 | 4.8 4,5 | 0.02 2–62 6 | 6 | |
| Org. P/ppm | 0.17/0.15 | <LQ–0.41 | 1.2 | 0.23/0.15 | 0.03–0.50 | 1.2 | – | – | – | – | |
| Total P/ppm | 0.86/0.60 | 0.15–1.9 | 0.71 | 0.65/0.30 | 0.26–1.3 | 0.84 | 9.6 | 6.4 7 | 2.1 7–29 8 | 4 | |
| SO4-S/ppm | 0.07/0.10 | <LQ–0.31 | 1.4 | 0.05/0.01 | 0.03–0.08 | 1.3 | 13 | 9.3 4,9 | 0.25 2–90 6 | 8 | |
| Org. S/ppm | 3.2/1.8 | 1.2–6.0 | 0.40 | 1.2/0.1 | 1.1–1.5 | 0.38 | – | – | – | – | |
| Total S/ppm | 3.2/1.8 | 1.3–6.2 | 0.38 | 1.3/0.1 | 1.1–1.6 | 0.33 | 2.8 | – | 2.1 10–3.6 10 | 1 | |
| Cys-S/ppb | – | – | – | – | – | – | 33 | 28 11,12 | 0.20 11–130 13 | 3 | |
| GSH-S/ppb | – | – | – | – | – | – | 70 | 34 10,12 | 0.06 12–417 13 | 3 | |
| Q. serrata | C. japonica | Literature | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N-Forms | Mean/SD | Min–Max | MCV | Mean/SD | Min–Max | MCV | Mean | Median | Min–Max | n |
| Conc./ppm NH4-N | 0.55/0.60 | <LQ–1.9 | 0.69 | 0.07/0.02 | <LQ–0.13 | 0.63 | 2.0 | 1.3 1,2 | 0.01 3–7.7 4 | 9 |
| Amino N | 1.8/1.3 | 0.68–4.9 | 0.50 | 0.33/0.09 | 0.18–0.49 | 0.20 | 300 | 106 5 | 2.9 6–2150 5 | 15 |
| Others N | 5.5/3.1 | 1.8–11 | 0.53 | 2.5/0.4 | 1.9–3.1 | 0.34 | – | – | – | – |
| Protein N | – | – | – | – | – | – | 9.3 | 3.7 7,8 | 0.12 9–45 10 | 8 |
| Total N | 7.8/4.7 | 2.5–17 | 0.53 | 2.9/0.4 | 2.2–3.6 | 0.34 | 48 | 36 11 | 3.5 12–280 13 | 18 |
| R/% NH4-N | 5.4/3.5 | 2.0–12.3 | – | 2.5/1.0 | 1.7–5.0 | – | ||||
| Amino N | 23.6/5.1 | 15.5–29.4 | – | 11.5/2.2 | 8.2–15.9 | – | ||||
| Others N | 71.0/6.5 | 58.4–79.3 | – | 86.0/2.3 | 81.5–89.3 | – | ||||
| Q. serrata | C. japonica | Literature | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean/SD | Min–Max | MCV | Mean/SD | Min–Max | MCV | Mean | Median | Min–Max | n |
| 379/206 | 137–708 | 0.28 | 198/63 | 125–310 | 0.53 | 12,680 | 3850 1 | 800 1–50,000 2 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takamatsu, T.; Watanabe, M.; Koshikawa, M.K. Convenient Sampling of Xylem Sap from Adult Tree Trunks and Analysis of Its Components. Forests 2023, 14, 389. https://doi.org/10.3390/f14020389
Takamatsu T, Watanabe M, Koshikawa MK. Convenient Sampling of Xylem Sap from Adult Tree Trunks and Analysis of Its Components. Forests. 2023; 14(2):389. https://doi.org/10.3390/f14020389
Chicago/Turabian StyleTakamatsu, Takejiro, Mirai Watanabe, and Masami Kanao Koshikawa. 2023. "Convenient Sampling of Xylem Sap from Adult Tree Trunks and Analysis of Its Components" Forests 14, no. 2: 389. https://doi.org/10.3390/f14020389





