An Affordable NIR Spectroscopic System for Fraud Detection in Olive Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Oil Samples
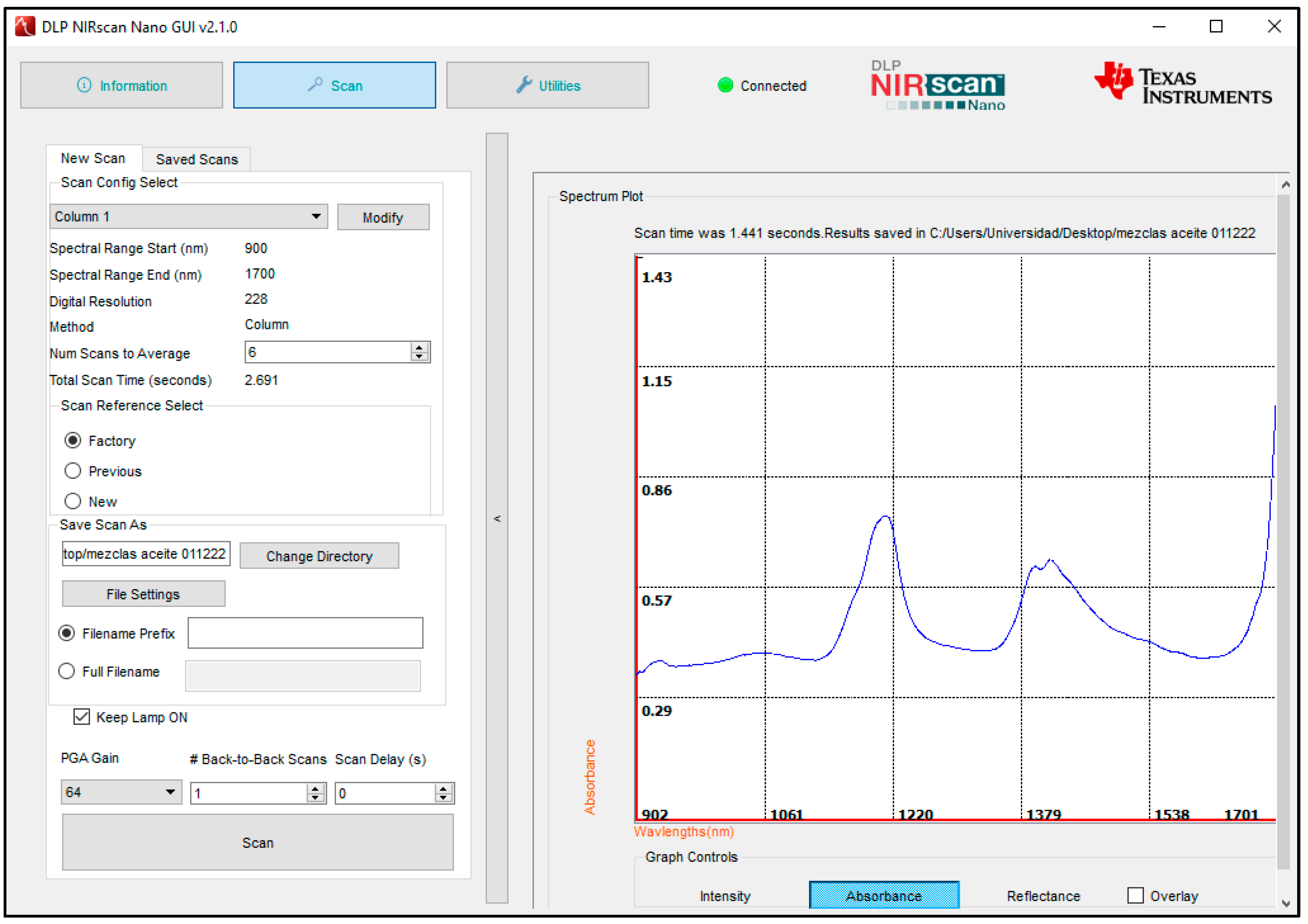
2.2. NIRS Analysis
2.3. Spectra Data Processing
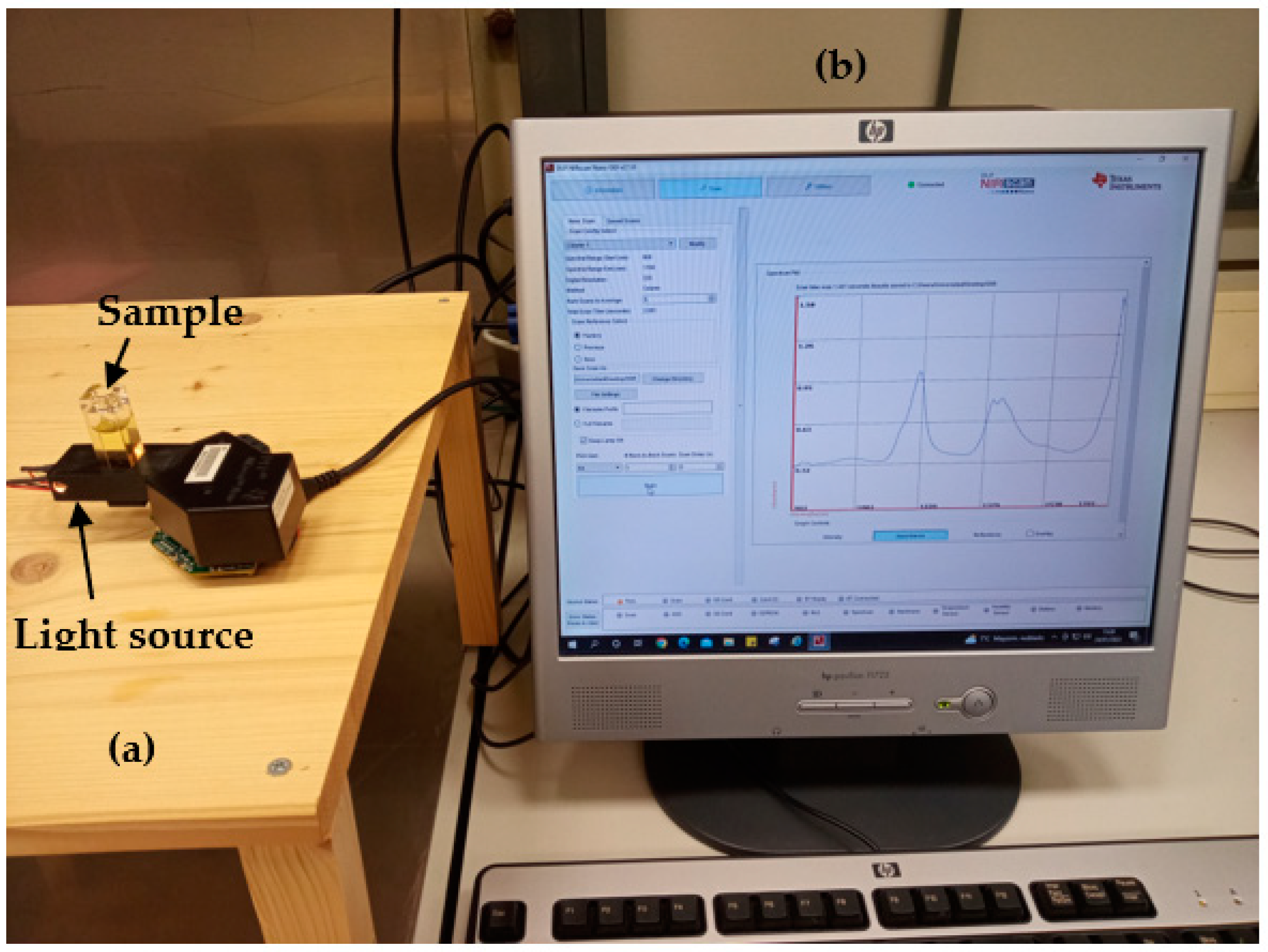
3. Instrumentation
3.1. Spectroscopy Architectures
3.2. Optical Considerations
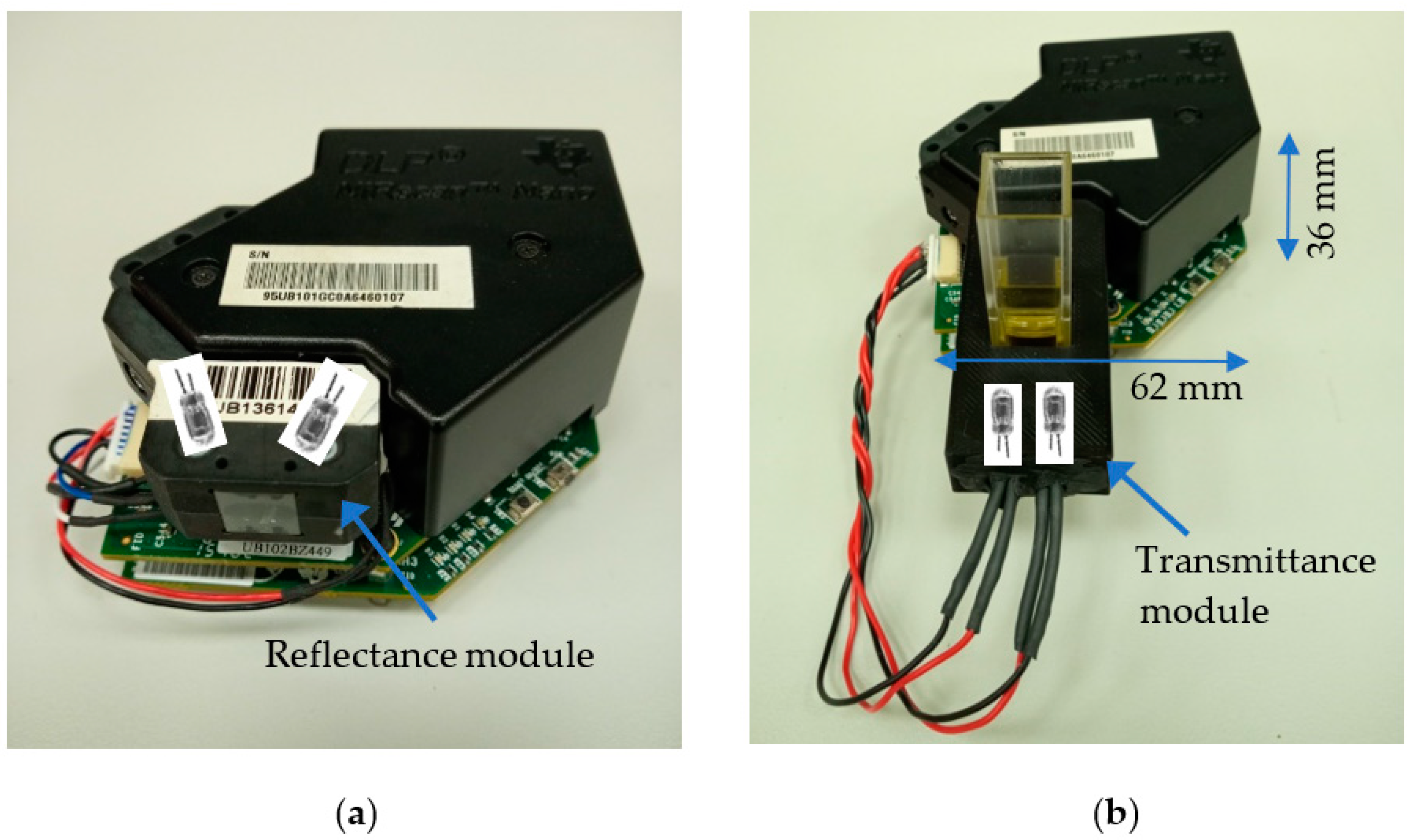
3.3. Hardware Considerations
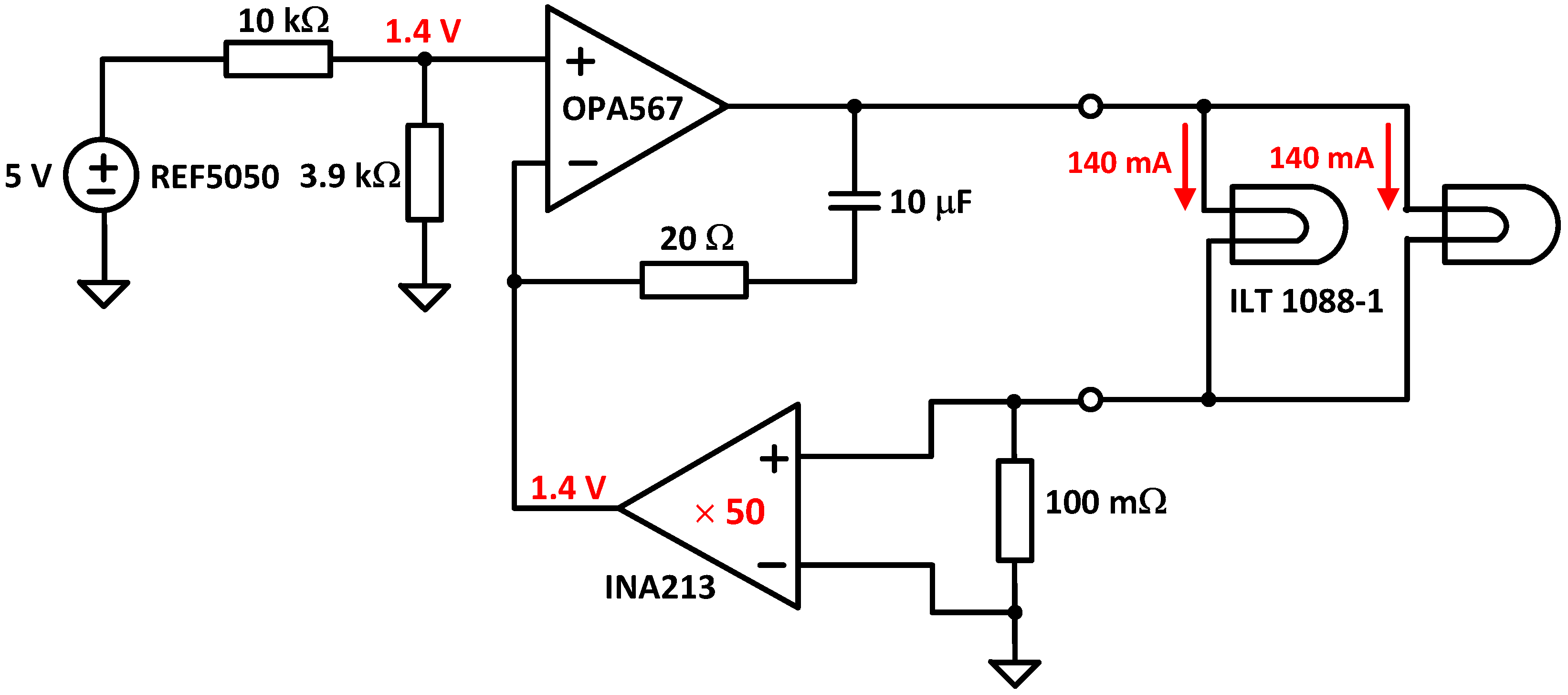
- Light source: It consists of two lens-end Tungsten filaments. They are designated as lens-end lamps because the front end of the glass bulb is formed into a lens to direct more light from the filament to the sample test region. Tungsten halogen lamps are well suited as spectrometer light sources due to their broadband infrared radiation. The transmission module was equipped with two ILT 1088-1 lens-end lamps from International Light Technologies [24]. Figure 7 shows the lamp driver. It provides a constant current of 280 mA at 5 V. The light output of this lamp is sufficient for cuvettes with path lengths of 2–13 mm.
- Slit: The input slit specifications affect the ability to couple light into the spectrometer and its spectral resolution. The slit width should be chosen to create an image width at the DMD corresponding to a desired spectral resolution. In addition, the slit length should be large enough to illuminate the full extent of the DMD panel, maximizing the system’s light throughput. Narrowing the slit width increases the spectral resolution of the system but requires a higher-performance optical system to sharply image the smaller slit onto the DMD [25].
- DMD array: The size of this component determines the maximum light collection area and the resolution of the system. The resolution of the system depends on the wavelength spectrum that is spread across one dimension of the DMD (for example, width), the slit width, and the DMD pattern width. The other dimensions of the DMD (for example, height) and the optical transfer function of the system determine the amount of light collected.
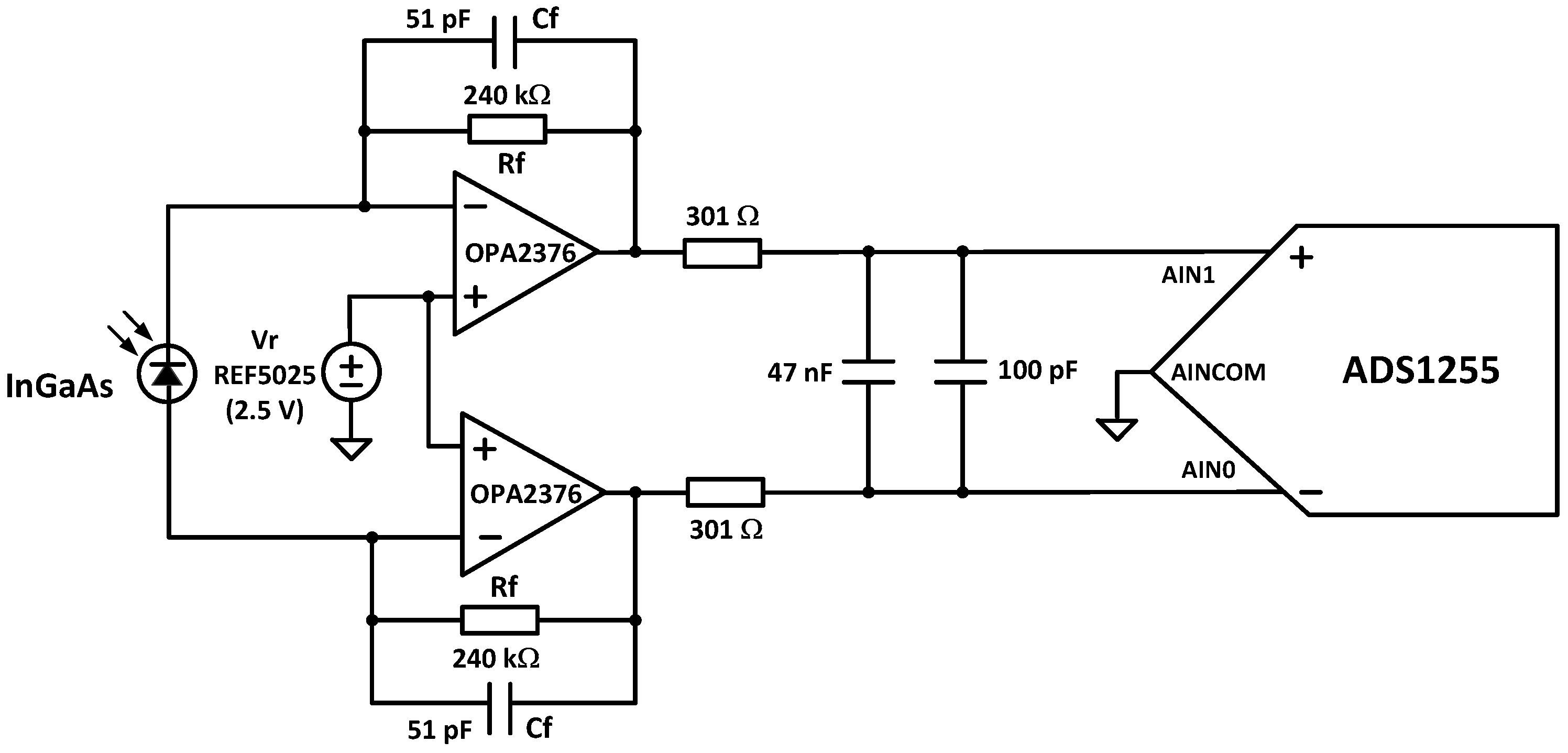
- Photodetector: For NIRS between 900 and 2500 nm, an InGaAs photodiode is the preferred photodetector due to its high quantum efficiency and responsivity. Typically, the photodiode produces a very small current signal proportional to its photosensitive area. A large photosensitive area produces more current in response to light at the expense of higher terminal capacitance. The higher capacitance will result in a slower response to light or lower bandwidth. This capacitance will also affect the capacitive feedback compensation (Cf in Figure 8) of the transimpedance amplifier (TIA). The photodetector also has a dark current when no light is incident. Thus, photodiode selection is a trade-off between cost, wavelength range, photosensitivity, capacitance, and dark current. DLP NIRscan Nano uses the Hamamatsu InGaAs G12183-120K photodiode [26].
- Amplifier: Once the light is converted into a current at the photodetector, a TIA is used to convert the current signal into a voltage. The TIA architecture is best suited for photodiodes that produce higher input currents, achieve wide analog bandwidth, offer high flexibility with simple changes of feedback elements, and handle the high-speed conversion. For noise immunity, the TIA employs a differential signal, with a gain double the feedback resistance, Rf, in Figure 8.
- Analog-to-Digital Converter (ADC): The ADC converts the voltage into a digital signal representation. For best results, an extremely low-noise, high linearity, high-resolution analog-to-digital converter minimizes the noise added by the conversion. To optimize the system’s digital resolution, the ADC’s dynamic range and reference voltages must be matched with the maximum photodetector signal. DLP NIRscan Nano uses the Texas Instruments 24-bit ADS1255.
3.4. Software Considerations
- Method: This controls the scanning process. The DLP NIRscan Nano comes preloaded with two scan configurations from the factory: Column or Hadamard. The Column scan selects one wavelength at a time. The Hadamard scan creates a set with several wavelengths multiplexed at a time and then decodes the individual wavelengths. The Hadamard scan collects much lighter and offers a higher SNR than a column scan [28]. However, this is very dependent on the spectrum being measured and the system used to measure it. To identify adulterations in different olive oils, we consider the Column method to be more effective because the reproducibility study provided better results for the Column method, as stated in this work.
- Wavelength range: Start and End wavelengths (in nm) or spectral range of interest for the scan. The minimum wavelength is 901 nm, and the maximum wavelength is 1700 nm.
- Width in nm: This number selects the width of the groups of pixels in the generated Column or Hadamard patterns. The options displayed correspond to the width of the dispersed spectrum in nm across the quantized pixel width.
- Digital Resolution: This number defines how many wavelength points are captured across the defined spectral range. This corresponds to the number of patterns displayed on the DMD during the scan. By increasing the digital resolution, the spectrum is oversampled. In general, set this resolution to oversample at least twice the desired full-width half maximum desired.
- Exposure Time: For scan configurations with one section, the exposure time is set to 0.635 ms. For scan configurations with more than one section, the exposure time can be individually set for each section in the range of 0.635 to 60.960 ms.
- Number of scans to average: This determines how many times the pattern set for a scan will be cycled through on the DMD. The microcontroller collects and averages the data, which lowers noise as additional scans are averaged. For scans where noise is a problem, this can be increased.
- Scan Reference Select: This button allows the user to choose the reference for the absorbance or reflectance graph. For transmittance sampling, data are taken with no sample in the cuvette.
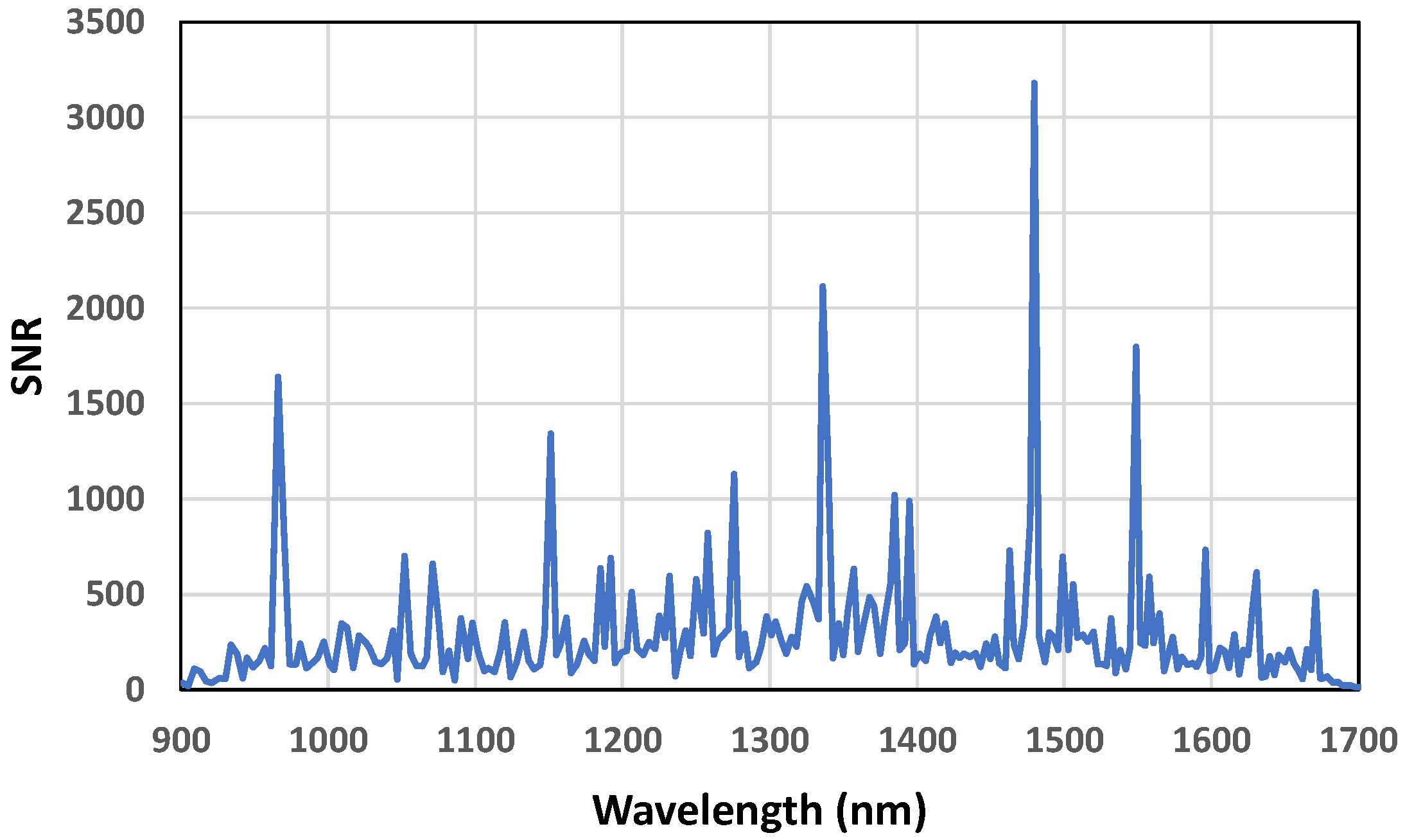
- Execute scan k times (we used k = 4) with scan time t and no time between scans.
- Compute the difference vector of the intensities at wavelength n.
- Compute the average of the intensity measurements at wavelength n.
- Compute the standard deviation of point 2.
- Compute the SNR as the ratio between points 3 and 4.
- Repeat points 1 to 5 at other wavelengths between 900 to 1700 nm.
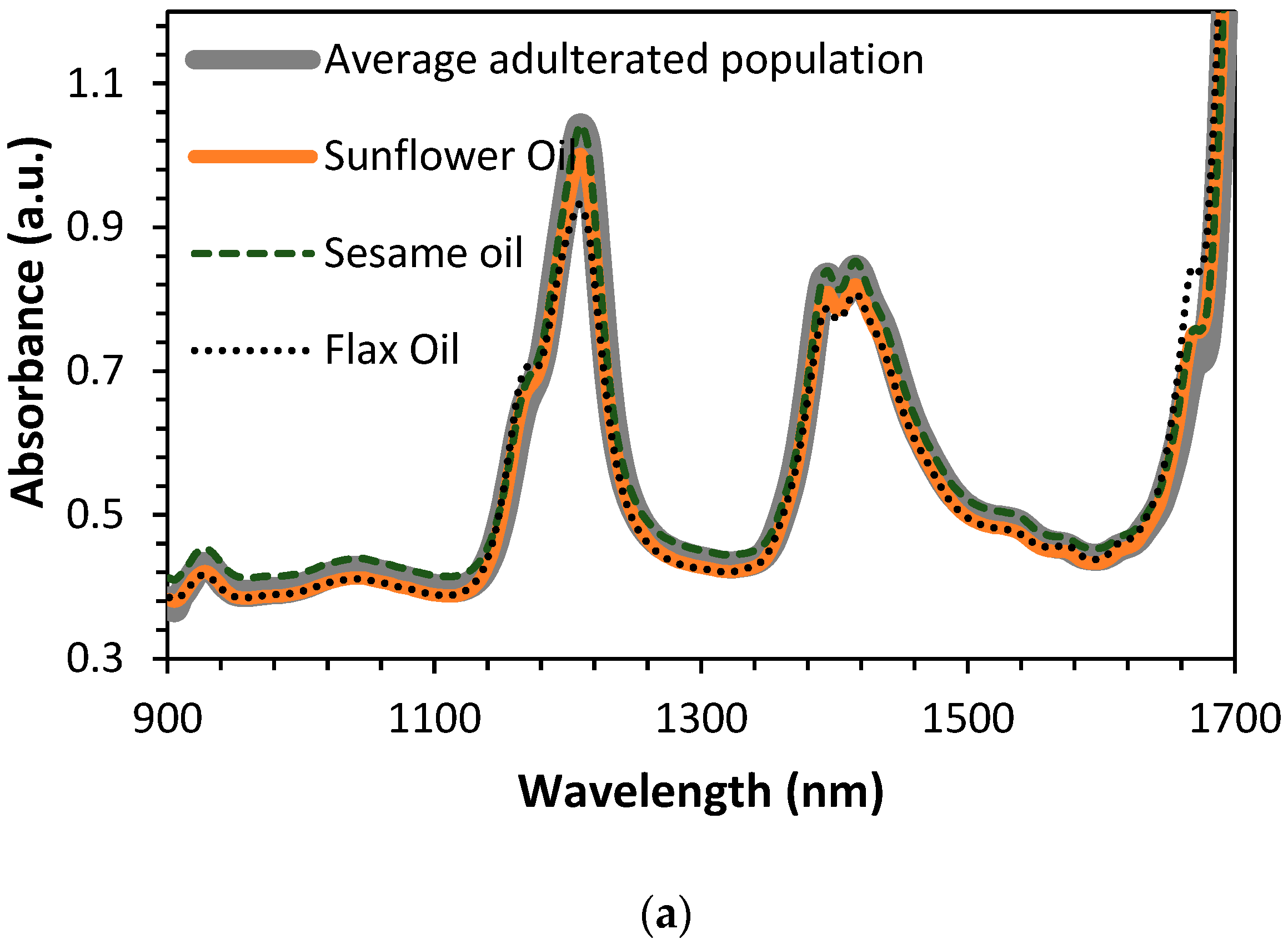
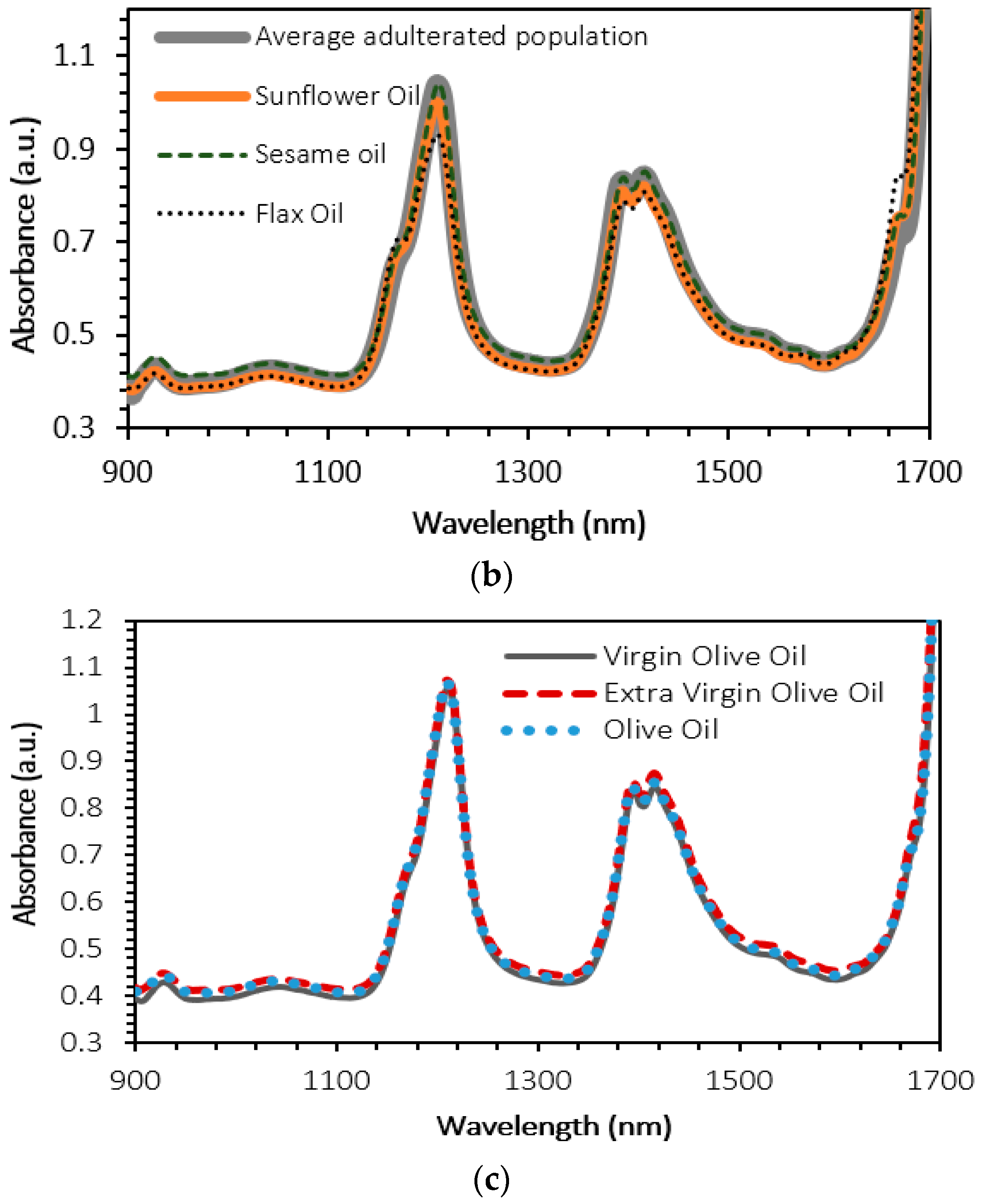
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Toxic Oil Syndrome: Current Knowledge and Future Perspectives. Introduction; World Health Organization. Regional Office for Europe: Geneva, Switzerland, 1992; Volume 42, pp. 1–4.
- De la Paz, M.P.; Philen, R.M.; Borda, I.A. Toxic oil syndrome: The perspective after 20 years. Epidemiol. Rev. 2001, 23, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Andrikopoulos, N.K.; Giannakis, I.G.; Tzamtzis, V. Analysis of olive oil and seed oil triglycerides by capillary gas chromatography as a tool for the detection of the Adulteration of Olive Oil. J. Chromatogr. Sci. 2001, 39, 137–145. [Google Scholar] [PubMed]
- Meenu, M.; Cai, Q.; Xu, B. A critical review on analytical techniques to detect adulteration of extra virgin olive oil. Trends Food Sci. Technol. 2019, 91, 391–408. [Google Scholar]
- Chen, H.; Lin, Z.; Tan, C. Fast quantitative detection of sesame oil adulteration by near-infrared spectroscopy and chemometric models. Vib. Spectrosc. 2018, 99, 178–183. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, L.; Wang, D.; Jiang, J.; Harrington, P.D.B.; Mao, J.; Zhang, Q.; Li, P. Detection of flax oil multiple adulteration by near-infrared spectroscopy and nonlinear one class partial least squares discriminant analysis. LWT 2020, 125, 109247. [Google Scholar] [CrossRef]
- Borghi, F.T.; Santos, P.C.; Santos, F.D.; Nascimento, M.H.; Correa, T.; Cesconetto, M.; Pires, A.A.; Ribeiro, A.V.F.N.; Lacerda, V.; Romão, W.; et al. Quantification and classification of vegetable oils in extra virgin olive oil samples using a portable near-infrared spectrometer associated with chemometrics. Microchem. J. 2020, 159, 105544. [Google Scholar] [CrossRef]
- Garrido-Varo, A.; Sánchez, M.T.; De la Haba, M.J.; Torres, I.; Pérez_Marín, D. Fast, Low-Cost and Non-Destructive Physico-Chemical Analysis of Virgin Olive Oils Using Near-Infrared Reflectance Spectroscopy. Sensors. 2017, 17, 2642. [Google Scholar] [CrossRef]
- Pasquini, C. Near infrared spectroscopy: A mature analytical technique with new perspectives–a review. Anal Chim. Acta 2018, 1026, 8–36. [Google Scholar] [CrossRef]
- Crocombe, R.A. Portable Spectroscopy. Appl. Spectrosc. 2018, 72, 1701–1751. [Google Scholar] [CrossRef]
- Yan, H.; Siesler, H.W. Hand-held near-infrared spectrometers: State-of-the-art instrumentation and practical applications. NIR News 2018, 29, 8–12. [Google Scholar] [CrossRef]
- Wolfrum, E.J.; Payne, C.; Schwartz, A.; Jacobs, J.; Kressin, R.W. A Performance Comparison of Low-Cost Near-Infrared (NIR) Spectrometers to a Conventional Laboratory Spectrometer for Rapid Biomass Compositional Analysis. BioEnergy Res. 2020, 13, 1121–1129. [Google Scholar] [CrossRef]
- Texas Instruments. Available online: https://www.ti.com/tool/DLPNIRNANOEVM#tech-docs (accessed on 23 December 2022).
- Shenk, J.S.; Westerhaus, M.O. Routine Operation, Calibration, Development and Network System Management Manual; NIRSystems Inc.: Silver Spring, MD, USA, 1995. [Google Scholar]
- Shenk, J.S.; Westerhaus, M.O. Calibration the ISI Way. Near Infrared Spectroscopy: The Future Waves; Davies, A.M.C., Williams, P.C., Eds.; NIR Publications: Chichester, UK, 1996; pp. 198–202. [Google Scholar]
- ISO 5725e1; Accuracy (Trueness and Precision) of Measurement Methods and Results. Part 1: General Principles and Definitions. The International Organization for Standardization (ISO): Geneva, Switzerland, 1994.
- ISO 5725e2; Accuracy (Trueness and Precision) of Measurement Methods and Results. Part 2: Basic Method for the Determination of Repeatability and Reproducibility of a Standard Measurement Method. The International Organization for Standardization (ISO): Geneva, Switzerland, 1994.
- Mark, H.; Workman, J. Statistics in Spectroscopy; Academic Press: Cambridge, MA, USA, 2003. [Google Scholar]
- Thompson, M.; Wood, R. Using uncertainty functions to predict and specify the performance of analytical methods. Accred. Qual. Assur. 2006, 10, 471–478. [Google Scholar]
- Burns, D.A.; Ciurczak, E.W. Handbook of Near-Infrared Analysis, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Nelson, P. DLP Technology for Spectroscopy; DLPA048; Texas Instruments: Dallas, TX, USA, 2014; pp. 1–7. [Google Scholar]
- Perrella, G. Texas Instruments DLP® NIRscan™ Nano Evaluation Module (EVM) Optical Design Considerations; DLPA062; Texas Instruments: Dallas, TX, USA, 2016; pp. 1–8. [Google Scholar]
- Texas Instruments. DLP Spectrometer Design Considerations. DLPA049. 2014. Available online: https://www.ti.com/lit/pdf/dlpa049 (accessed on 23 December 2022).
- International Light Technologies. Available online: https://www.intl-lighttech.com/node/730 (accessed on 25 December 2022).
- Gelabert, P. Signal Chain Performance Optimizations in the TI DLP Technology-Based Spectrometers; DLPA072; Texas Instruments: Dallas, TX, USA, 2016; pp. 1–16. [Google Scholar]
- Hamamatsu Photonics. Available online: https://www.hamamatsu.com/eu/en/resources/library.html (accessed on 23 December 2022).
- Texas Instruments. DLP© NIRscanTM Nano EVM User´s Guide. DLPU030G. 2017. Available online: https://www.ti.com/lit/pdf/dlpu030 (accessed on 2 January 2022).
- Dyer, S.A. Hadamard transform spectrometry. Chemom. Intell. Lab. Syst. 1991, 12, 101–115. [Google Scholar] [CrossRef]
- Pruett, E. Flexible Trade-Offs in Maximizing SNR and Resolution in TI DLP® Technology-Based Spectrometer Systems; DLPA066; Texas Instruments: Dallas, TX, USA, 2016. [Google Scholar]
- Panero, J.S.; da Silva, H.E.; Panero, P.S.; Smiderle, O.J.; Panero, F.S.; Faria, F.S.E.D.V.; Rodríguez, A.F. Separation of Cultivars of Soybeans by Chemometric Methods Using Near Infrared Spectroscopy. J. Agric. Sci. 2018, 10, 351–360. [Google Scholar] [CrossRef]
- Ali, H.; Saleem, M.; Anser, M.R.; Khan, S.; Ullah, R.; Bilal, M. Validation of Fluorescence Spectroscopy to Detect Adulteration of Edible Oil in Extra Virgin Olive Oil (EVOO) by Applying Chemometrics. Appl. Spectrosc. 2018, 72, 1371–1379. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, Y.; Min, S. Data fusion strategy in quantitative analysis of spectroscopy relevant to olive oil adulteration. Vib. Spectrosc. 2019, 101, 20–27. [Google Scholar] [CrossRef]
- Gurdeniz, G.; Ozen, B. Detection of adulteration of extra-virgin olive oil by chemometric analysis of mid-infrared spectral data. Food Chem. 2009, 116, 519–525. [Google Scholar] [CrossRef]
- Downey, G.; McIntyre, P.; Davies, A.N. Detecting and Quantifying Sunflower Oil Adulteration in Extra Virgin Olive Oils from the Eastern Mediterranean by Visible and Near- Infrared Spectroscopy. J. Agric. Food Chem. 2002, 50, 5520–5525. [Google Scholar]
- Torrecilla, J.S.; Cancilla, J.C.; Matute, G.; Díaz-Rodríguez, P.; Flores, A.I. Self-organizing maps based on chaotic parameters to detect adulterations of extra virgin olive oil with inferior edible oils. J. Food Eng. 2013, 118, 400–405. [Google Scholar]
- Christy, A.A.; Kasensumran, S.; Du, Y.; Uzaki, Y. The detection and quantification of adulteration in olive oil by NIR and chemometrics. Anal. Sci. 2004, 20, 935–940. [Google Scholar] [PubMed]
- Zou, M.Q.; Zhang, X.F.; Xiao-Hua, Q.I.; Han-Lu, M.; Dong, Y.; Chun-Wei LI, U.; Guo XU, N.; Wang, H. Rapid authentication of olive oil adulteration by Raman spectrometry. J. Agric. Food Chem. 2009, 57, 6001–6006. [Google Scholar] [PubMed]
- Aparicio, R.; Morales, M.T.; Aparicio-Ruiz, R.; Tena, N.; García-González, D.L. Authenticity of olive oil: Mapping and comparing official methods and promising alternatives. Food Res. Int. 2013, 54, 2025–2038. [Google Scholar]
- Mildner-Szkudlarz, S.; Jelen´, H.H. The potential of different techniques for volatile compounds analysis coupled with PCA for the detection of the adulteration of olive oil with hazelnut oil. Food Chem. 2008, 110, 751–761. [Google Scholar]
- Mildner-Szkudlarz, S.; Jelen´, H.H. Detection of olive oil adulteration with rapeseed and sunflower oils using mos electronic nose and SMPE-MS. J. Food Qual. 2010, 33, 21–41. [Google Scholar] [CrossRef]
- Torrecilla, J.S.; García, J.; García, S.; Rodríguez, F. Quantification of adulterant agents in extra virgin olive oil by models based on its thermophysical properties. J. Food Eng. 2011, 103, 211–218. [Google Scholar]
- Wetten IA, V.; Herwaarden AW, V.; Splinter, R.; Boerrigter-Eenling, R.; Ruth SM, V. Detection of sunflower oil in extra virgin olive oil by fast differential scanning calorimetry. Thermochim. Acta 2015, 603, 237–243. [Google Scholar] [CrossRef]
- Aparicio-Ruiz, R.; Romero, I.; García-González, D.L.; Oliver-Pozo, C.; Aparicio, R. Soft-deodorization of virgin olive oil: Study of the changes of quality and chemical composition. Food Chem. 2017, 220, 42–50. [Google Scholar] [CrossRef]
- Mariani, C.; Bellan, G.; Lestini, E.; Aparicio, R. The detection of the presence of hazelnut oil in olive oil by free and esterified sterols. Eur. Food Res. Technol. 2006, 223, 655–661. [Google Scholar]
- Azadmard-Damirchi, S. Review of the use of phytosterols as a detection tool for adulteration of olive oil with hazelnut oil. Food Aditives Contam. Part A 2010, 27, 1–10. [Google Scholar]
- García-González, D.L.; Viera-Macías, M.; Aparicio-Ruiz, R.; Morales, M.T.; Aparicio, R. Validation of a method based on triglycerides for the detection of low percentages of hazelnut oil in olive oil by column liquid chromatography. J. AOAC Int. 2007, 90, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- International Olive Council (IOC). COI/T.20/Doc. No. 25 Global Method for the Detection of Extraneous Oils in Olive Oils; International Olive Council: Madrid, Spain, 2006. [Google Scholar]
- Dais, P.; Hatzakis, E. Quality assessment and authentication of virgin olive oil by NMR spectroscopy: A critical review. Anal. Chim. Acta 2013, 765, 1–27. [Google Scholar] [PubMed]






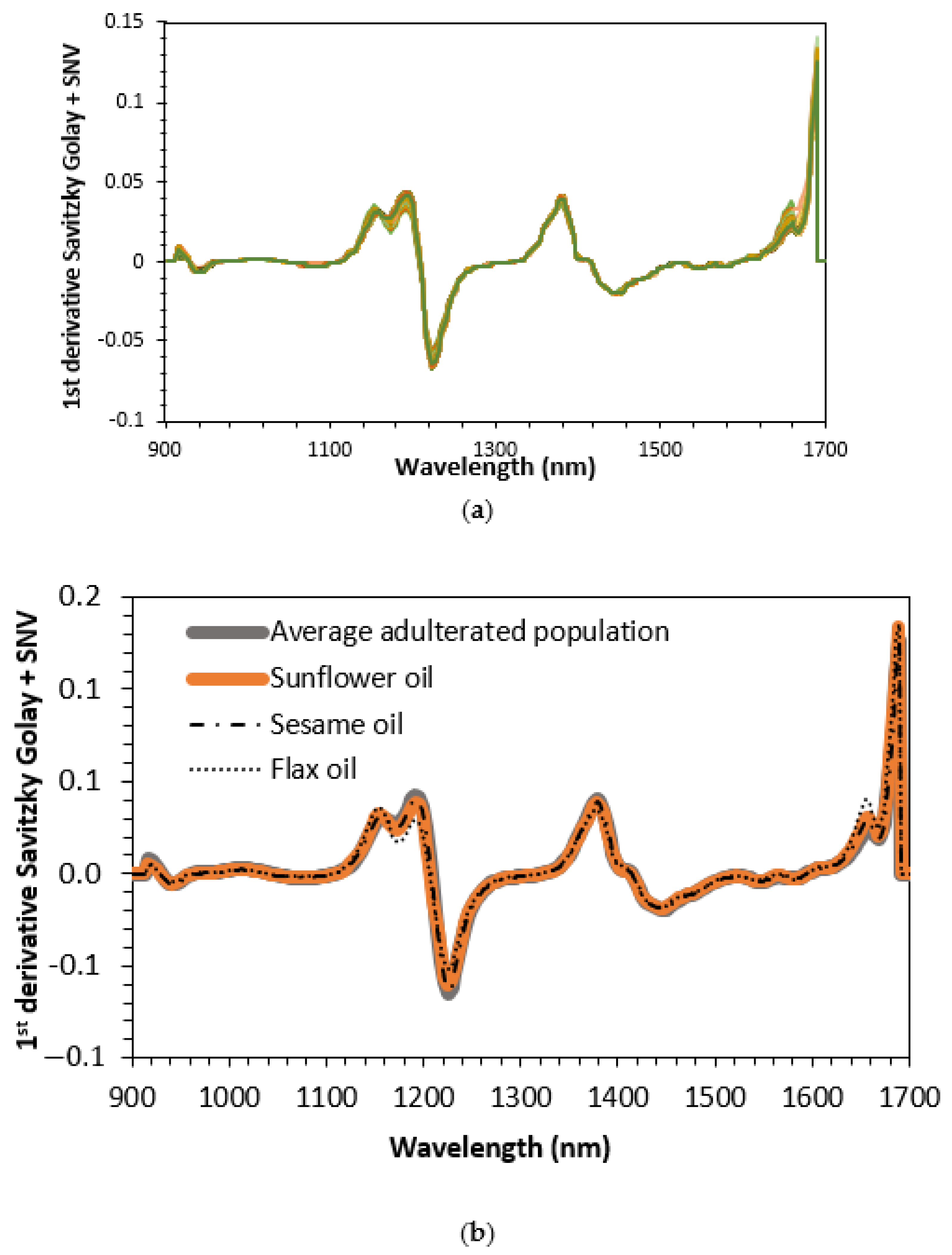
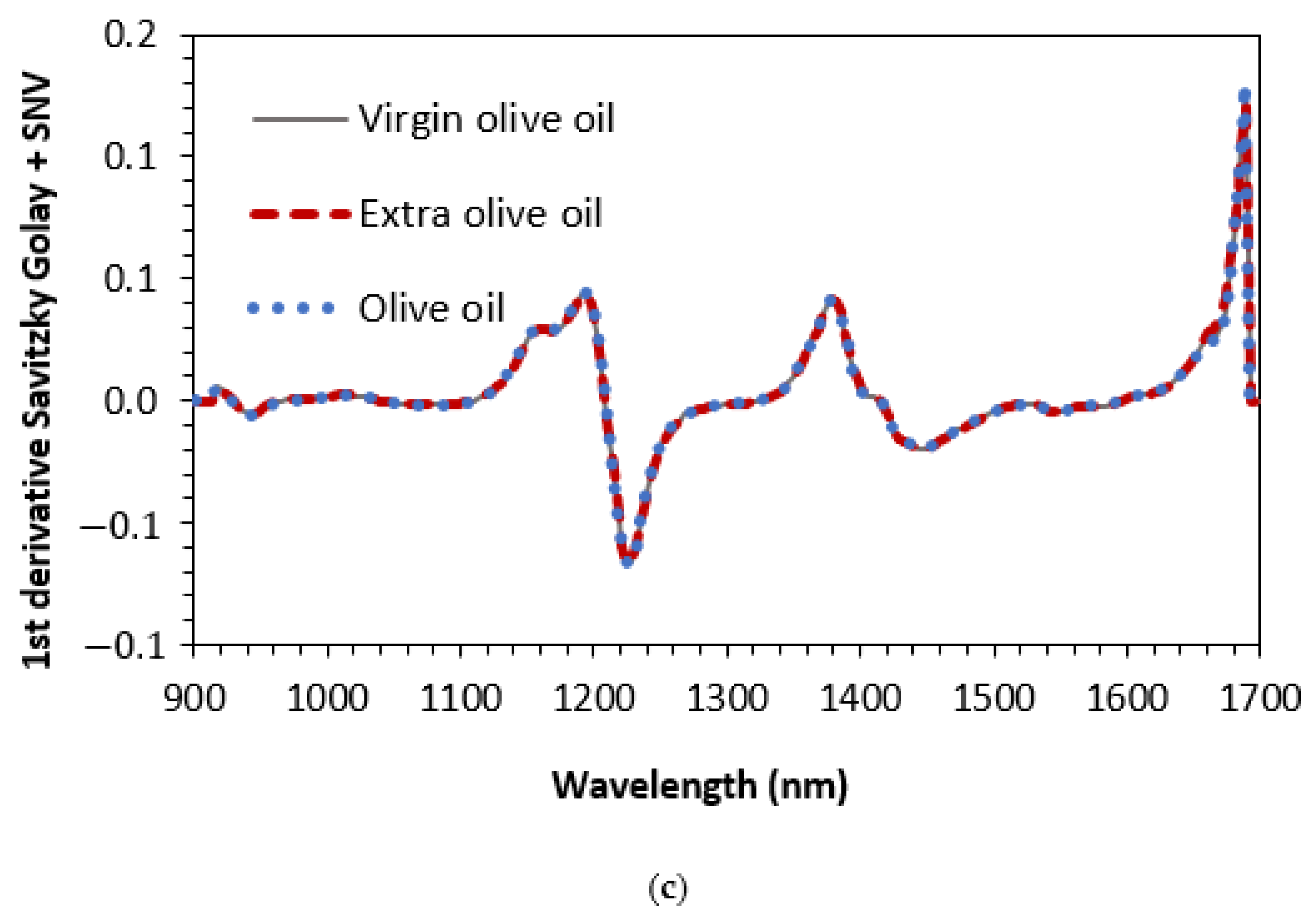

| Binary Mixtures | Ternary Mixtures | |||
|---|---|---|---|---|
| % Olive Oil * | % Adulterant Oil | % Olive Oil * | % Adulterant Oil | % Adulterant Oil |
| 98 | 2 | 90 | 5 | 5 |
| 90 | 10 | 80 | 10 | 10 |
| 80 | 20 | 70 | 15 | 15 |
| 70 | 30 | 88 | 9 | 3 |
| 76 | 8 | 16 | ||
| 92 | 5 | 3 | ||
| 86 | 11 | 3 | ||
| 82 | 10 | 8 | ||
| Parameter | Design Value |
|---|---|
| Slit ƒ-number | 2.5 |
| Slit dimensions | 1.8 × 0.025 mm |
| DMD | DLP2010NIR |
| DMD ƒ-number | 3.8 |
| Spectral range | 900 to 1700 nm |
| Spectral resolution | 10 nm |
| Detector diameter | 1 mm |
| Detector type | Uncooled InGaAs |
| Optical engine dimensions | 33 × 29 × 10 mm |
| Math Pre-Treatment | Parameter | N | R2 | SEC | r2 | SECV | RER |
|---|---|---|---|---|---|---|---|
| SG 1 2 4 4 | Sesame oil | 57 | 0.815 | 3.065 | 0.672 | 3.938 | 7.1 |
| Sunflower oil | 39 | 0.886 | 2.718 | 0.653 | 4.958 | 5.6 | |
| Flax oil | 42 | 0.776 | 2.940 | 0.771 | 3.249 | 8.6 | |
| SG 2 2 4 4 | Sesame oil | 53 | 0.805 | 3.179 | 0.615 | 4.326 | 6.5 |
| Sunflower oil | 38 | 0.852 | 3.014 | 0.608 | 5.041 | 5.6 | |
| Flax oil | 44 | 0.772 | 2.975 | 0.689 | 3.266 | 8.6 | |
| SG 1 2 4 4 + SNV | Sesame oil | 52 | 0.839 | 2.821 | 0.681 | 4.030 | 6.9 |
| Sunflower oil | 37 | 0.921 | 2.256 | 0.681 | 4.817 | 5.8 | |
| Flax oil | 42 | 0.778 | 2.921 | 0.747 | 3.097 | 9.0 | |
| SG 2 2 4 4 + SNV | Sesame oil | 56 | 0.747 | 3.576 | 0.685 | 4.584 | 6.1 |
| Sunflower oil | 35 | 0.313 | 6.554 | 0.276 | 6.792 | 4.1 | |
| Flax oil | 41 | 0.832 | 2.620 | 0.699 | 3.458 | 8.1 | |
| SNV + SG 1 2 4 4 | Sesame oil | 54 | 0.771 | 3.458 | 0.635 | 4.204 | 6.7 |
| Sunflower oil | 38 | 0.852 | 3.014 | 0.608 | 5.041 | 5.6 | |
| Flax oil | 44 | 0.746 | 3.139 | 0.746 | 3.359 | 8.3 | |
| SNV + SG 2 2 4 4 | Sesame oil | 56 | 0.769 | 3.319 | 0.627 | 4.325 | 6.5 |
| Sunflower oil | 39 | 0.848 | 3.008 | 0.695 | 5.051 | 5.5 | |
| Flax oil | 43 | 0.788 | 2.873 | 0.717 | 3.197 | 8.8 |
| Technique | Discrimination/ Classification Procedures | Advantages | Disadvantages | Ref |
|---|---|---|---|---|
| Fluorescence | PCA and PLS | No sample pretreatment, Robustness to detect sunflower adulteration | Laboratory instrumentation, different models for each adulteration type | [31] |
| MIR | SIMCA-PLS-A | Rapid and easy-to-implement method | Lab expensive instrumentation, sometimes unstable mathematical equations | [32,33] |
| UV-VIS and VIS-NIR | SIMCA, SOM | Rapid analysis, easy to implement at lab level | Sample pretreatment with organic solvents for UV-VIS, Expensive instrumentation for VIS-NIR | [34,35] |
| NIR | PCA | No sample pretreatment, non-destructive analysis | Time-consuming for calibration development, expensive laboratory instrumentation | [36] |
| Raman | PCA | Non-destructive, minimum sample preparation required, rapid method | Interferences due to fluorescence properties of sample or adulterants, Expensive instrumentation, Unstable mathematical equations | [37,38] |
| E-nose (Voltammetric) | SPME –GC-FID or SPME-GC/MS plus PCA or PLS | High sensitivity, reproducibility, and accuracy. | Tedious sample pretreatment, expensive instrumentation, expert personnel required | [39,40] |
| Fast DSC | Temperature controller system, Nitrogen supply | Rapid analysis and easy-to-use technique, | Tedious and time-consuming sample pretreatment required | [41,42] |
| GC | Highly polar capillary column, Flame ionization or Mass Spectrometer detectors | Traditional method, accurate and precise, high reproducibility | Time-consuming sample pretreatment. expensive instrumentation, well-trained analyst | [43,44,45] |
| HPLC | C18 Column, Diode array, photometric of refractive index detector, high-pressure pumps | Accurate and precise, high reproducibility | Tedious and time-consuming sample pretreatment, expertise personnel required | [46,47] |
| NMR | NMR tube, Superconducting magnet, deuterated solvent | Short analysis time, easy sample preparation, good reproducibility | Expensive instrumentation, low sensitivity, expert personnel required | [48] |
| Portable NIR prototype | PCA and PLS | No sample pretreatment, on-site and real-time analysis, easy-to-use, inexpensive instrumentation | Large database for calibration development models | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melendreras, C.; Soldado, A.; Costa-Fernández, J.M.; López, A.; Valledor, M.; Campo, J.C.; Ferrero, F. An Affordable NIR Spectroscopic System for Fraud Detection in Olive Oil. Sensors 2023, 23, 1728. https://doi.org/10.3390/s23031728
Melendreras C, Soldado A, Costa-Fernández JM, López A, Valledor M, Campo JC, Ferrero F. An Affordable NIR Spectroscopic System for Fraud Detection in Olive Oil. Sensors. 2023; 23(3):1728. https://doi.org/10.3390/s23031728
Chicago/Turabian StyleMelendreras, Candela, Ana Soldado, José M. Costa-Fernández, Alberto López, Marta Valledor, Juan Carlos Campo, and Francisco Ferrero. 2023. "An Affordable NIR Spectroscopic System for Fraud Detection in Olive Oil" Sensors 23, no. 3: 1728. https://doi.org/10.3390/s23031728
APA StyleMelendreras, C., Soldado, A., Costa-Fernández, J. M., López, A., Valledor, M., Campo, J. C., & Ferrero, F. (2023). An Affordable NIR Spectroscopic System for Fraud Detection in Olive Oil. Sensors, 23(3), 1728. https://doi.org/10.3390/s23031728










