Impacts of the Residual Trace Metals of Aquaculture in Net Cages on the Quality of Sediment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Fish Farming in the Study Area
2.3. Collection, Preparation and Analysis of Sediment
2.4. Geostatistical Assessment
2.5. Geochemical and Ecotoxicological Indices
3. Results and Discussion
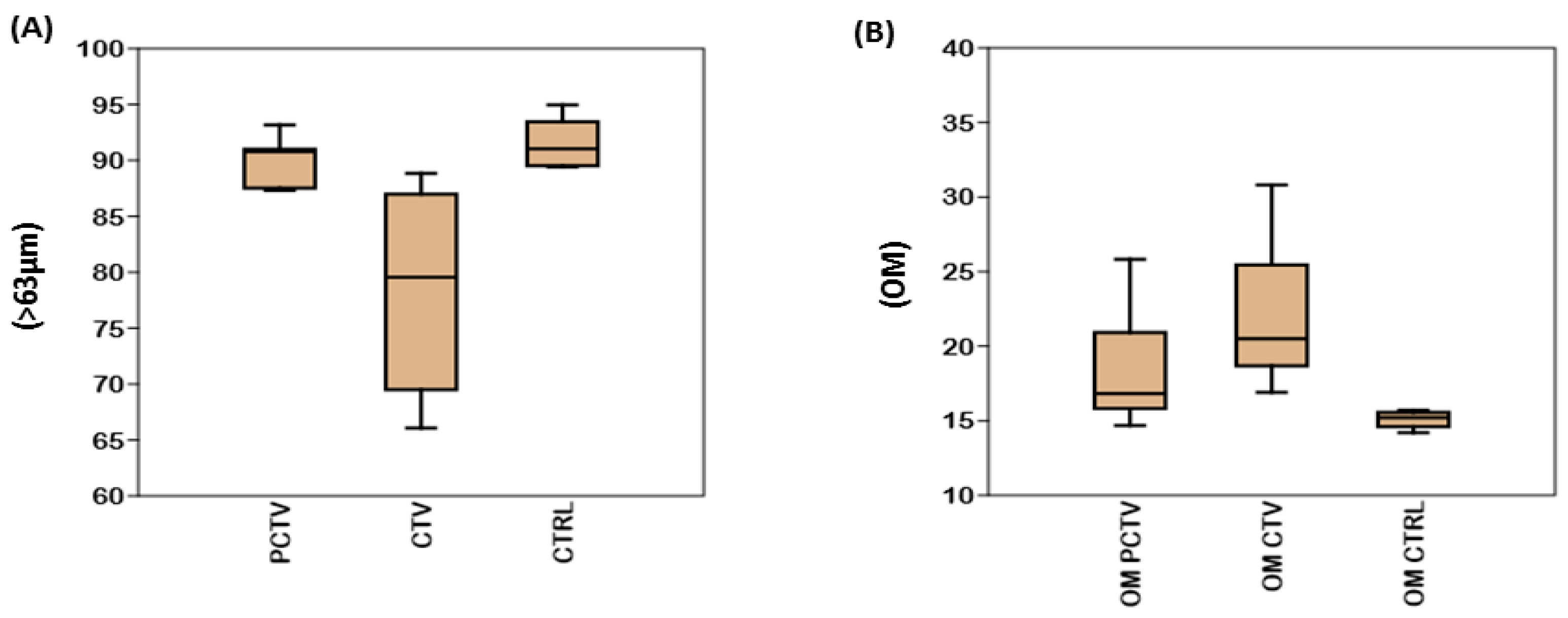
3.1. Sediment Constitution
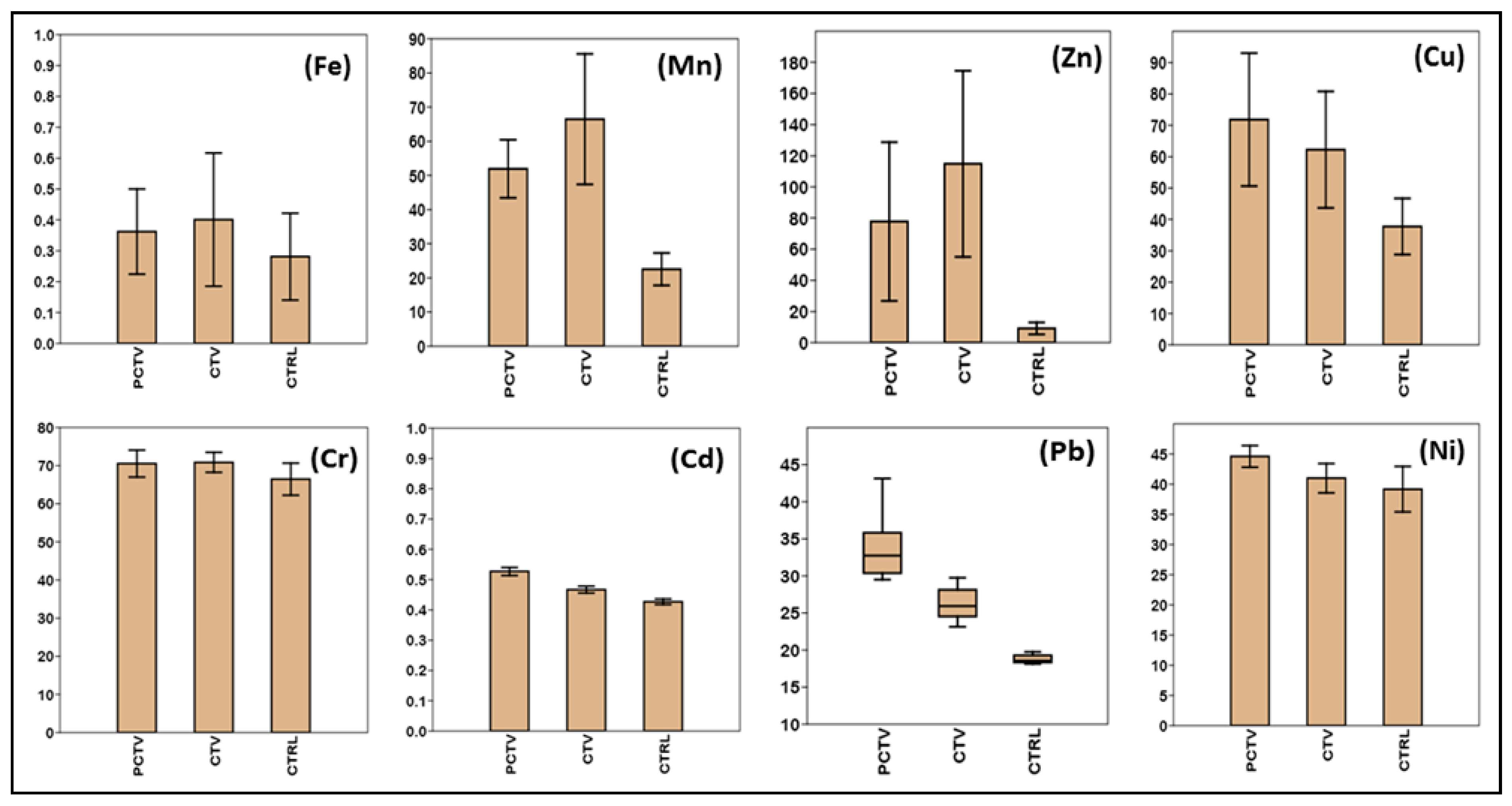
3.2. Concentration and Distribution of Trace Metals in the Sediment
3.3. Percentage Increase in Trace Metal Concentrations in the Sediment
3.4. Sediment Quality Guidelines (SQGs)
3.5. Increase in Metals Trace of Ctv and Pctv Areas in Relation to CTRL
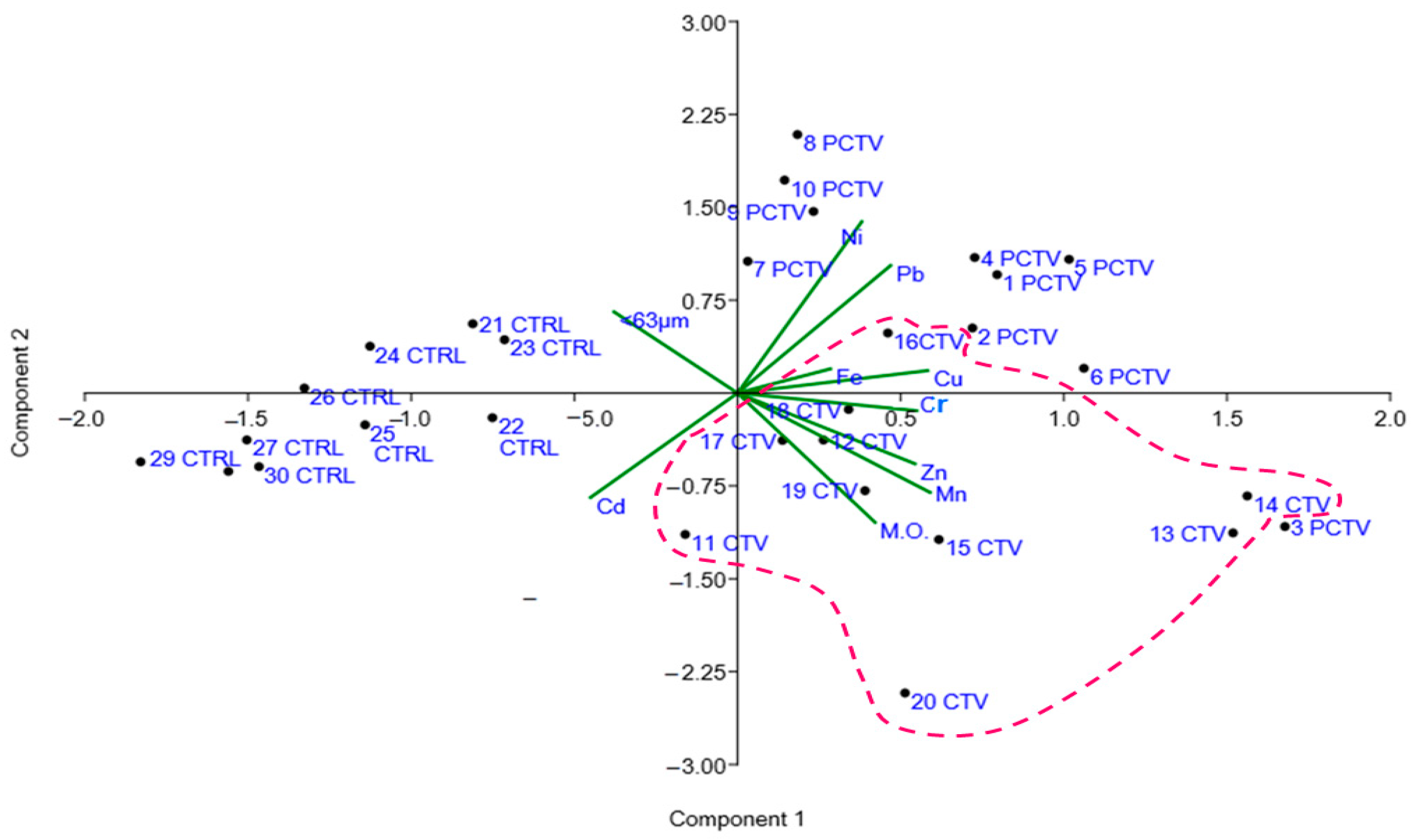
3.6. Patterns of Inter-relationships between Organic Matter and Metal Residual Traces of Pisicultutra
3.7. Geostatistical Analysis
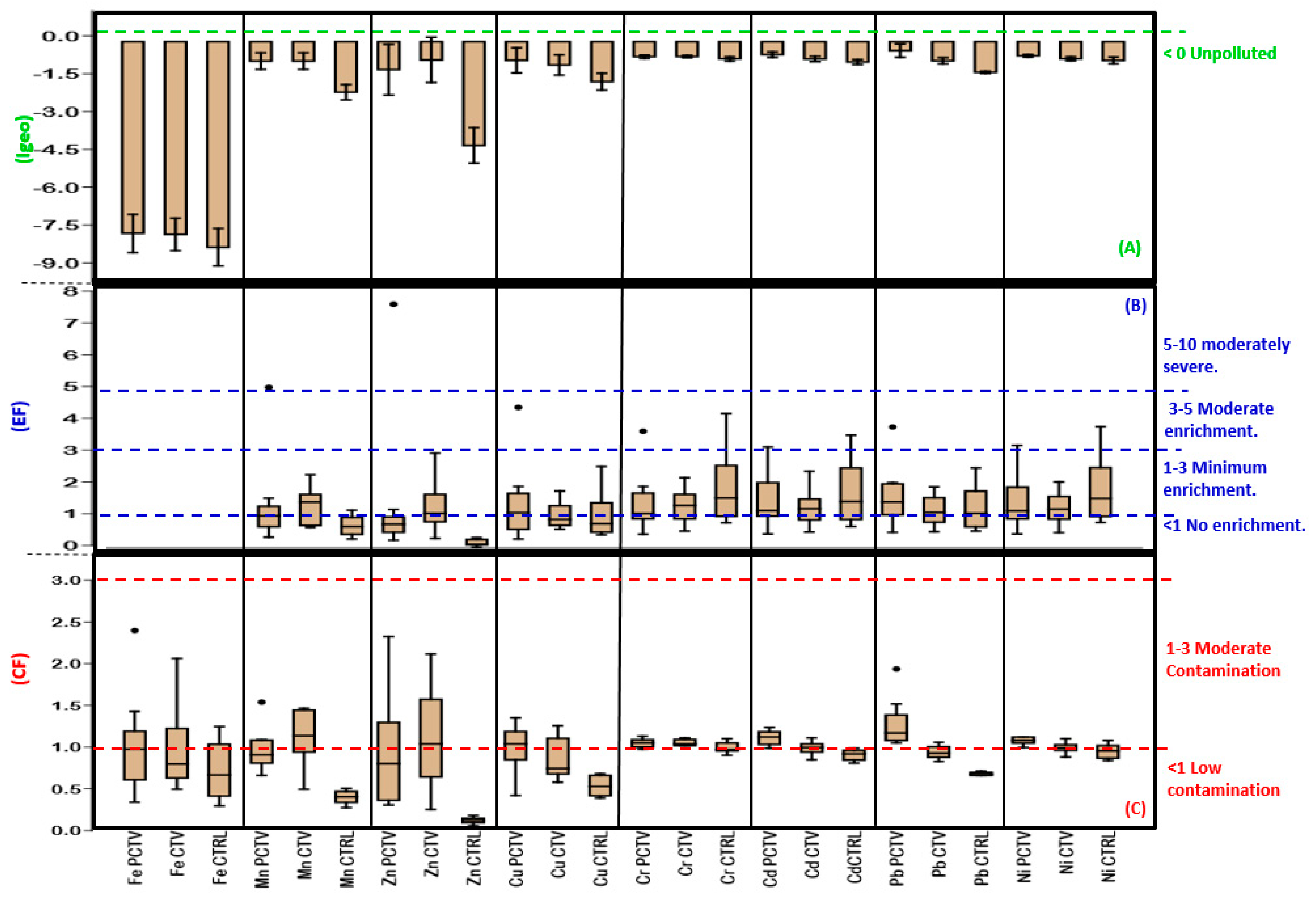
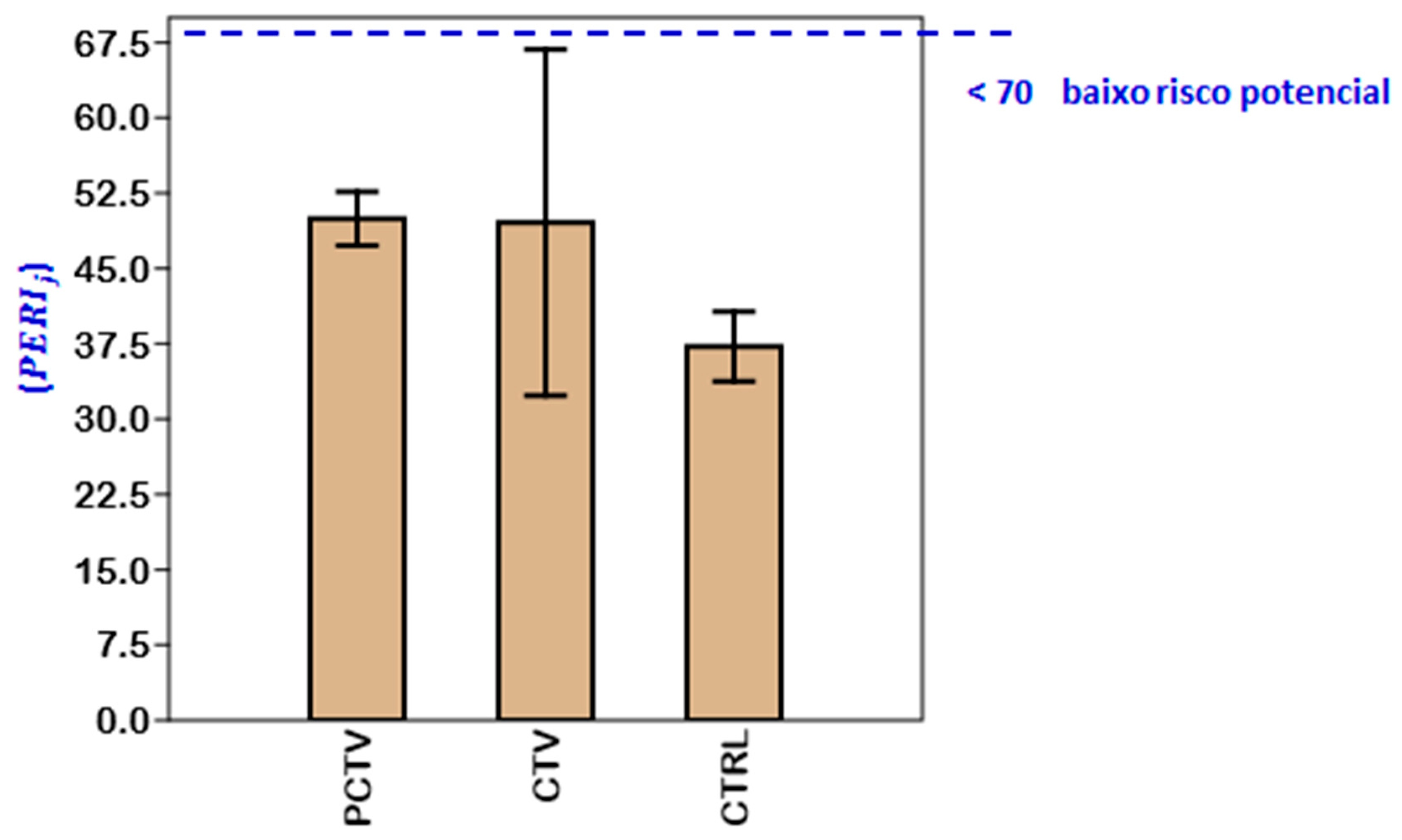
3.8. Geochemical and Ecotoxicological Indexes
3.8.1. Geochemical Indexes
3.8.2. Ecotoxicological Indexes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chabukdhara, M.; Nema, A.K. Assessment of Heavy Metal Contamination in Hindon River Sediments: A Chemometric and Geochemical Approach. Chemosphere 2012, 87, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Zahra, A.; Hashmi, M.Z.; Malik, R.N.; Ahmed, Z. Enrichment and Geo-Accumulation of Heavy Metals and Risk Assessment of Sediments of the Kurang Nallah--Feeding Tributary of the Rawal Lake Reservoir, Pakistan. Sci. Total Environ. 2014, 470–471, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Komala, P.S.; Nur, A.; Badriah, N.L.; Harefa, M.; Silvia, S.; Ridwan; Zulkarnaini. An Assessment of Heavy Metals Pollution in the Waters and Sediments of Lake Maninjau, Indonesia. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1041, 012031. [Google Scholar] [CrossRef]
- Förstner, U.; Wittmann, G.T.W. Metal Pollution in the Aquatic Environment; Springer: Berlin/Heidelberg, Germany, 1981. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Teng, Y. Source Apportionment and Source-Oriented Risk Assessment of Heavy Metals in the Sediments of an Urban River-Lake System. Sci. Total Environ. 2020, 737, 140310. [Google Scholar] [CrossRef]
- Esteves, F.D.A. Fundamentals of Limnology, 3rd ed.; Interciencia: Rio de Janeiro, Brazil, 2011. [Google Scholar]
- Varol, M. Impacts of Cage Fish Farms in a Large Reservoir on Water and Sediment Chemistry. Environ. Pollut. 2019, 252, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xiong, X.; Liu, J.; He, C.; Zhao, Q. Carbon and Nitrogen Stable Isotopes Evidence for the Environmental Impact of the Cage Fish Farm in Poyang Lake, China. Bull. Environ. Contam. Toxicol. 2020, 105, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, X.; Cheng, H.; Zeng, E.Y.; Hu, Y. Heavy Metal Pollution in Sediments of a Typical Mariculture Zone in South China. Mar. Pollut. Bull. 2012, 64, 712–720. [Google Scholar] [CrossRef] [PubMed]
- OECD. OECD-FAO Agricultural Outlook 2022–2031. 2022. Available online: https://www.oecd-ilibrary.org/agriculture-and-food/oecd-fao-agricultural-outlook-2022-2031_f1b0b29c-en (accessed on 8 July 2022).
- Peixe Brazilian Fish Farming Association (Peixe BR). Brazilian Fish Farming Yearbook. 2022. Available online: https://www.peixebr.com.br/anuario2022/ (accessed on 8 July 2022).
- IBGE. IBGE|Portal Do IBGE|IBGE. Available online: https://www.ibge.gov.br/ (accessed on 16 December 2022).
- Karikari, A.Y.; Asmah, R.; Anku, W.W.; Amisah, S.; Agbo, N.W.; Telfer, T.C.; Ross, L.G. Heavy Metal Concentrations and Sediment Quality of a Cage Farm on Lake Volta, Ghana. Aquac. Res. 2020, 51, 2041–2051. [Google Scholar] [CrossRef]
- Phillips, M.J.; Beveridge, M.C.M.; Ross, L.G. The Environmental Impact of Salmonid Cage Culture on Inland Fisheries: Present Status and Future Trends. J. Fish Biol. 1985, 27, 123–137. [Google Scholar] [CrossRef]
- Camargo, A.F.; Amorim, R.V. Fish farming in cages: A practice to be restricted in Brazil. Acta Limnol. Bras. 2020, 32, 101. [Google Scholar] [CrossRef]
- De Moura, R.S.T.; Lopes, Y.V.D.A.; Henry-Silva, G.G. Sedimentação de Nutrientes e Material Particulado Em Reservatório Sob Influência de Atividades de Piscicultura No Semiárido Do Rio Grande Do Norte. Quim. Nova 2014, 37, 1283–1288. [Google Scholar] [CrossRef]
- Mendiguchía, C.; Moreno, C.; Mánuel-Vez, M.P.; García-Vargas, M. Preliminary Investigation on the Enrichment of Heavy Metals in Marine Sediments Originated from Intensive Aquaculture Effluents. Aquaculture 2006, 254, 317–325. [Google Scholar] [CrossRef]
- Xie, Q.; Qian, L.; Liu, S.; Wang, Y.; Zhang, Y.; Wang, D. Assessment of Long-Term Effects from Cage Culture Practices on Heavy Metal Accumulation in Sediment and Fish. Ecotoxicol. Environ. Saf. 2020, 194, 110433. [Google Scholar] [CrossRef]
- Oliveira, K.F.; Lacerda, L.D.; Peres, T.F.; Marins, R.V.; Santos, J.A. The Fate of Cu, Zn and Mn in an Intensive Fish Aquaculture (Tilapia-Oreochromis niloticus) in an Artificial Reservoir in Northeastern Brazil. Environ. Process. 2017, 4, 107–121. [Google Scholar] [CrossRef]
- Marengoni, N.G.; Possamai, M.; Gonçalves Júnior, A.C.; de Oliveira, A.A.M. Performance e retenção de metais pesados em três linhagens de juvenis de tilápia-do-Nilo em hapas. Acta Sci. Anim. Sci. 2008, 30, 351–358. [Google Scholar] [CrossRef]
- Jiang, H.; Qin, D.; Mou, Z.; Zhao, J.; Tang, S.; Wu, S.; Gao, L. Trace Elements in Farmed Fish (Cyprinus Carpio, Ctenopharyngodon Idella and Oncorhynchus Mykiss) from Beijing: Implication from Feed. Food Addit. Contam. Part B 2016, 9, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Anim-Gyampo, M.; Kumi, M.; Zango, M.S. Heavy Metals Concentrations in Some Selected Fish Species in Tono Irrigation Reservoir in Navrongo, Ghana. J. Environ. Earth Sci. 2013, 3, 109–119. [Google Scholar]
- Weber, P.; Behr, E.R.; Knorr, C.D.L.; Vendruscolo, D.S.; Flores, E.M.M.; Dressler, V.L.; Baldisserotto, B. Metals in the Water, Sediment, and Tissues of Two Fish Species from Different Trophic Levels in a Subtropical Brazilian River. Microchem. J. 2013, 106, 61–66. [Google Scholar] [CrossRef]
- Shang, G.; Wang, X.; Zhu, L.; Liu, S.; Li, H.; Wang, Z.; Wang, B.; Zhang, Z. Heavy Metal Pollution in Xinfengjiang River Sediment and the Response of Fish Species Abundance to Heavy Metal Concentrations. Int. J. Environ. Res. Public 2022, 19, 11087. [Google Scholar] [CrossRef]
- Gao, Y.; Fang, L.; Xiang, Q.Q.; Wang, D.; Ding, L.Y.; Ding, C.Z.; Chen, L.Q. Ecological Risk Assessment of Heavy Metals in Fish from the Dianchi Lake, China Using the Integrated Biomarker Response Approach. Environ. Sci. Pollut. Res. 2020, 27, 45712–45721. [Google Scholar] [CrossRef] [PubMed]
- Berg, H.; Kiibus, M.; Kautsky, N. Heavy Metals in Tropical Lake Kariba, Zimbabwe. Water, Air, Soil Pollut. 1995, 83, 237–252. [Google Scholar] [CrossRef]
- Rashed, M.N. Monitoring of Environmental Heavy Metals in Fish from Nasser Lake. Environ. Int. 2001, 27, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; Wong, P.P.K.; Chu, L.M. Heavy Metal Concentrations in Marine Fishes Collected from Fish Culture Sites in Hong Kong. Arch. Environ. Contam. Toxicol. 2001, 40, 60–69. [Google Scholar] [CrossRef]
- Malik, N.; Biswas, A.K.; Qureshi, T.A.; Borana, K.; Virha, R. Bioaccumulation of Heavy Metals in Fish Tissues of a Freshwater Lake of Bhopal. Environ. Monit. Assess. 2010, 160, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Hamed Abd El Mageed, Y.; Abdelmoneim, T.S.; Elkiki, M.H.; Hassan, M.A.; Berndtsson, R. Assessment of Heavy Metals Pollution and Microbial Contamination in Water, Sediments and Fish of Lake Manzala, Egypt. Life Sci. J. Acta Zhengzhou Univ. Overseas Ed. 2013, 10, 86–99. [Google Scholar]
- Varol, M.; Sünbül, M.R. Environmental Contaminants in Fish Species from a Large Dam Reservoir and Their Potential Risks to Human Health. Ecotoxicol. Environ. Saf. 2019, 169, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, M.; Patimar, R. Ecological Risk Assessment of Heavy Metals in Surface Sediments from the Gorgan Bay, Caspian Sea. Mar. Pollut. Bull. 2018, 137, 662–667. [Google Scholar] [CrossRef]
- Lu, L.; Liu, J.; Li, Z.; Zou, X.; Guo, J.; Liu, Z.; Yang, J.; Zhou, Y. Antibiotic Resistance Gene Abundances Associated with Heavy Metals and Antibiotics in the Sediments of Changshou Lake in the Three Gorges Reservoir Area, China. Ecol. Indic. 2020, 113, 106275. [Google Scholar] [CrossRef]
- Salami, I.R.S.; Rahmawati, S.; Sutarto, R.I.H.; Jaya, P.M. Accumulation of Heavy Metals in Freshwater Fish in Cage Aquaculture at Cirata Reservoir, West Java, Indonesia. Ann. N. Y. Acad. Sci. 2008, 1140, 290–296. [Google Scholar] [CrossRef]
- Hardaway, C.J.; Sneddon, J.; Sneddon, E.J.; Kiran, B.; Lambert, B.J.; McCray, T.C.; Bowser, D.Q.; Douvris, C. Study of Selected Metal Concentrations in Sediments by Inductively Coupled Plasma-Optical Emission Spectrometry from a Metropolitan and More Pristine Bayou in Southwest Louisiana, United States. Microchem. J. 2016, 127, 213–219. [Google Scholar] [CrossRef]
- Dean, R.J.; Shimmield, T.M.; Black, K.D. Copper, Zinc and Cadmium in Marine Cage Fish Farm Sediments: An Extensive Survey. Environ. Pollut. 2007, 145, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Basaran, A.K.; Aksu, M.; Egemen, O. Impacts of the Fish Farms on the Water Column Nutrient Concentrations and Accumulation of Heavy Metals in the Sediments in the Eastern Aegean Sea (Turkey). Environ. Monit. Assess. 2010, 162, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Wu, S.C.; Zhang, J.; Cao, Y.; Yu, S.; Wong, M.H. The Effects of Mariculture on Heavy Metal Distribution in Sediments and Cultured Fish around the Pearl River Delta Region, South China. Chemosphere 2016, 148, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Ammar, R.; Kazpard, V.; Wazne, M.; El Samrani, A.G.; Amacha, N.; Saad, Z.; Chou, L. Reservoir Sediments: A Sink or Source of Chemicals at the Surface Water-Groundwater Interface. Environ. Monit. Assess. 2015, 187, 579. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.G.M.; Tanaka, A.; Stagnitti, F.; Allinson, G.; Maekawa, T. Observations on the Effects of Caged Carp Culture on Water and Sediment Metal Concentrations in Lake Kasumigaura, Japan. Ecotoxicol. Environ. Saf. 2001, 48, 107–115. [Google Scholar] [CrossRef]
- Karaouzas, I.; Kapetanaki, N.; Mentzafou, A.; Kanellopoulos, T.D.; Skoulikidis, N. Heavy Metal Contamination Status in Greek Surface Waters: A Review with Application and Evaluation of Pollution Indices. Chemosphere 2021, 263, 128192. [Google Scholar] [CrossRef]
- Kalantzi, I.; Shimmield, T.M.; Pergantis, S.A.; Papageorgiou, N.; Black, K.D.; Karakassis, I. Heavy Metals, Trace Elements and Sediment Geochemistry at Four Mediterranean Fish Farms. Sci. Total Environ. 2013, 444, 128–137. [Google Scholar] [CrossRef]
- Wenchuan, Q.; Dickman, M.; Sumin, W. Multivariate Analysis of Heavy Metal and Nutrient Concentrations in Sediments of Taihu Lake, China. Hydrobiologia 2001, 450, 83–89. [Google Scholar] [CrossRef]
- de Carvalho Braga, C.; Cabral, J.B.P.; Lopes, S.M.F.; Oliveira, S.F.; da Rocha, I.R. Qualidade Dos Sedimentos Em Relação à Presença de Metais Pesados No Reservatório Da Usina Hidrelétrica de Caçu–GO (Quality of Sediments in Relation to the Presence of Heavy Metals in the Hydroelectric Plant Reservoir in Caçu–Goiás). Rev. Bras. Geogr. Física 2018, 11, 959–972. [Google Scholar] [CrossRef]
- Gu, Y.; Yang, Y.; Lin, Q.; Li, Q.; Wang, Z.; Wang, X. Spatial, Temporal, and Speciation Variations of Heavy Metals in Sediments of Nan’ao Island, a Representative Mariculture Base in Guangdong Coast, China. J. Environ. Monit. 2012, 14, 1943–1950. [Google Scholar] [CrossRef]
- CONAMA Brazilian National Council of the Environment. Resolution No. 454. Publication DOU, of 11/08/2012. Available online: http://www2.mma.gov.br/port/conama/legiabre.cfm?codlegi=693 (accessed on 14 January 2020).
- MacDonald, D.D.; Ingersoll, C.G.; Berger, T.A. Development and Evaluation of Consensus-Based Sediment Quality Guidelines for Freshwater Ecosystems. Arch. Environ. Contam. Toxicol. 2000, 39, 20–31. [Google Scholar] [CrossRef]
- EPA-Taiwan Soil and Groundwater Pollution Remediation Act—Article Content—Laws & Regulations Database of The Republic of China (Taiwan). Available online: https://law.moj.gov.tw/ENG/LawClass/LawAll.aspx?pcode=O0110001 (accessed on 16 December 2022).
- ANZECC; ARMCANZ. Australian and New Zealand Guidelines for Fresh and Marine Water Quality; The Guidelines Australian and New Zealand Environment and Conservation Council Agriculture and Resource Management Council of Australia and New Zealand; ARMCANZ: Sydney, Australia, 2000. [Google Scholar]
- Kanda, A.; Ncube, F.; Mabote, R.R.; Mudzamiri, T.; Kunaka, K.; Dhliwayo, M. Trace Elements in Water, Sediment and Commonly Consumed Fish from a Fish Farm (NE Zimbabwe) and Risk Assessments. SN Appl. Sci. 2020, 2, 1502. [Google Scholar] [CrossRef]
- Custodio, M.; Fow, A.; Peñaloza, R.; Chanamé, F.; Cano, D. Evaluation of Surface Sediment Quality in Rivers with Fish Farming Potential (Peru) Using Indicators of Contamination, Accumulation and Ecological Risk of Heavy Metals and Arsenic. J. Ecol. Eng. 2021, 22, 78–87. [Google Scholar] [CrossRef]
- Muller, G. Index of Geoaccumulation in Sediments of the Rhine River. GeoJournal 1969, 2, 108–118. [Google Scholar]
- Taylor, S.R. Abundance of Chemical Elements in the Continental Crust: A New Table. Geochim. Cosmochim. Acta 1964, 28, 1273–1285. [Google Scholar] [CrossRef]
- Hakanson, L. An Ecological Risk Index for Aquatic Pollution Control: A Sedimentological Approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Kowalska, J.B.; Mazurek, R.; Gąsiorek, M.; Zaleski, T. Pollution Indices as Useful Tools for the Comprehensive Evaluation of the Degree of Soil Contamination-A Review. Environ. Geochem. Health 2018, 40, 2395–2420. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Jiang, X.; Wang, Y.; Zhuang, D. Spatial Characteristics of Heavy Metal Pollution and the Potential Ecological Risk of a Typical Mining Area: A Case Study in China. Process Saf. Environ. Prot. 2018, 113, 204–219. [Google Scholar] [CrossRef]
- Caeiro, S.; Costa, M.H.; Ramos, T.B.; Fernandes, F.; Silveira, N.; Coimbra, A.; Medeiros, G.; Painho, M. Assessing Heavy Metal Contamination in Sado Estuary Sediment: An Index Analysis Approach. Ecol. Indic. 2005, 5, 151–169. [Google Scholar] [CrossRef]
- Zhao, H.; Li, X. Risk Assessment of Metals in Road-Deposited Sediment along an Urban-Rural Gradient. Environ. Pollut. 2013, 174, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Cheng, C.; Feng, H.; Hong, Z.; Zhu, Q.; Kolenčík, M.; Chang, X. Assessment of Metal Mobility in Sediment, Commercial Fish Accumulation and Impact on Human Health Risk in a Large Shallow Plateau Lake in Southwest of China. Ecotoxicol. Environ. Saf. 2020, 194, 110346. [Google Scholar] [CrossRef] [PubMed]
- Ajani, G.E.; Popoola, S.O.; Oyatola, O.O. Evaluation of the Pollution Status of Lagos Coastal Waters and Sediments, Using Physicochemical Characteristics, Contamination Factor, Nemerow Pollution Index, Ecological Risk and Potential Ecological Risk Index. Int. J. Environ. Clim. Chang. 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Segura, R.; Arancibia, V.; Zúñiga, M.C.; Pastén, P. Distribution of Copper, Zinc, Lead and Cadmium Concentrations in Stream Sediments from the Mapocho River in Santiago, Chile. J. Geochem. Explor. 2006, 91, 71–80. [Google Scholar] [CrossRef]
- Yari, A.A.; Varvani, J.; Zare, R. Assessment and Zoning of Environmental Hazard of Heavy Metals Using the Nemerow Integrated Pollution Index in the Vineyards of Malayer City. Acta Geophys. 2021, 69, 149–159. [Google Scholar] [CrossRef]
- Jung, M.C.; Thornton, I. Heavy Metal Contamination of Soils and Plants in the Vicinity of a Lead-Zinc Mine, Korea. Appl. Geochem. 1996, 11, 53–59. [Google Scholar] [CrossRef]
- Lee, C.G.; Chon, H.T.; Jung, M.C. Heavy Metal Contamination in the Vicinity of the Daduk Au-Ag-Pb-Zn Mine in Korea. Appl. Geochem. 2001, 16, 1377–1386. [Google Scholar] [CrossRef]
- Prasad, B.; Sangita, K. Heavy Metal Pollution Index of Ground Water of an Abandoned Open Cast Mine Filled with Fly Ash: A Case Study. Mine Water Environ. 2008, 27, 265–267. [Google Scholar] [CrossRef]
- COGERH Water Resources Management Company. Secretary of Water Resources; Rosário Dam Environmental Inventory: Fortaleza, Brazil, 2021; Available online: https://portal.cogerh.com.br/wpcontent/uploads/pdf/Inventarios/2011/InventarioAmbientaldoAcudeRosario2011.pdf (accessed on 22 February 2020).
- SRH Secretary of Water Resources. Secretary of Water Resources. Water Resources Management Company. Environmental Diagnosis Report for the Salgado Sub-Basin. Available online: https://portal.cogerh.com.br/wpcontent/uploads/2018/10/RelatorioDiagnosticoAmbientaldaSub-BaciadoSalgado.pdf (accessed on 22 February 2020).
- CPMR Repositório Institucional de Geociências: Projeto Rede Integrada de Monitoramento Das Águas Subterrâneas: Relatório Diagnóstico Aquífero Missão Velha Bacia Sedimentar Do Araripe. Available online: https://rigeo.cprm.gov.br/jspui/handle/doc/22231 (accessed on 16 December 2022).
- Nascimento, H.; Melo-Junior; Campeche, D.F.B. Fish Farming in Semi-Arid Reservoir and Weirs: Climatological and Limnogical Aspetcs. 2019. Available online: https://www.aquaculturebrasil.com/revista/21/21-edicao (accessed on 3 January 2022).
- Cortez, H.S.; Lima, G.P.; Sakamoto, M.S. A Seca de 2010-2016 e as Medidas Do Estado Do Ceará Para Mitigar Seus Efeitos. Parcer. Estratégicas 2017, 22, 83–118. [Google Scholar]
- CCME Canadian Council of Ministers of the Environment. Canadian Sediment Quality Guidelines for the Protection of Aquatic Life: Introduction, Updated in Canadian Environmental Quality Guidelines. 1999. Available online: https://www.pla.co.uk/Environment/Canadian-Sediment-Quality-Guidelines-forthe-Protection-of-Aquatic-Life (accessed on 20 December 2021).
- Dantas Martins, L.; Otaviano Praça de Souza, J. Caracterização Morfológica e Granulométrica de Depósitos Aluviais Semiáridos. Rev. Geociências Nord. 2016, 2, 63–72. [Google Scholar] [CrossRef]
- Alpaslan, A.; Pulatsü, S. The Effect of Rainbow Trout (Oncorhynchus Mykiss Walbaum, 1792) Cage Culture on Sediment Quality in Kesikköprü Reservoir, Turkey. Turkish J. Fish. Aquat. Sci. 2008, 70, 65–70. [Google Scholar]
- De la Lanza, G. Materia Orgánica En Los Sedimentos Del Sistema Lagunar Huizache y Caimanero. Importancia, Comportamiento y Significado En Modelos de Predicción. Cienc. del Mar y Limnol. UNAM 1986, 13, 251–286. [Google Scholar]
- López-Jamar, E. Spatial Distribution of the Infaunal Benthic Communities of the Ría de Muros, North-West Spain. Mar. Biol. 1981 631 1981, 63, 29–37. [Google Scholar] [CrossRef]
- Méndez, N. Annelid Assemblages in Soft Bottoms Subjected to Human Impact in the Urías Estuary (Sinaloa, Mexico). Oceanol. Acta 2002, 25, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Cornel, G.E.; Whoriskey, F.G. The Effects of Rainbow Trout (Oncorhynchus Mykiss) Cage Culture on the Water Quality, Zooplankton, Benthos and Sediments of Lac Du Passage, Quebec. Aquaculture 1993, 109, 101–117. [Google Scholar] [CrossRef]
- Lao, Q.; Cai, S.; Huang, P.; Chen, F.; Su, Q.; Lei, X.; Zhou, X.; Chen, C.; Zhu, Q.; Lu, X.; et al. Contaminant Characteristics and Influencing Factors of Heavy Metals in Seawater and Sediments in a Typical Mariculture Bay in South China. Front. Mar. Sci. 2022, 9, 915. [Google Scholar] [CrossRef]
- Magna, E.K.; Koranteng, S.S.; Donkor, A.; Gordon, C. Health Risk Assessment and Levels of Heavy Metals in Farmed Nile Tilapia (Oreochromis niloticus) from the Volta Basin of Ghana. J. Chem. 2021, 2021, 2273327. [Google Scholar] [CrossRef]
- da Silva Cacho, J.C.; Teixeira de Moura, R.S.; Henry-Silva, G.G. Influence of Nile Tilapia (Oreochromis niloticus) Fish Farming in Net Cages on the Nutrient and Particulate Matter Sedimentation Rates in Umari Reservoir, Brazilian Semi-Arid. Aquac. Reports 2020, 17, 100358. [Google Scholar] [CrossRef]
- Kashem, M.A. Metal Availability in Contaminated Soils: I. Effects of Floodingand Organic Matter on Changes in Eh, PH and Solubility of Cd, Ni AndZn. Nutr. Cycl. Agroecosyst. 2001, 61, 247–255. [Google Scholar] [CrossRef]
- Henry-Silva, G.G.; Melo-Junior, H.D.N.; Attayde, J.L. Extreme Drought Events and the Sustainability of Fish Farming in Net Cages in Reservoirs of the Semi-Arid Northeastern Region in Brazil. Acta Limnol. Bras. 2019, 31, 112. [Google Scholar] [CrossRef]
- Melo Junior, H.N.; Sampaio, F.G. Circulação Vertical Na Piscicultura Em Tanque-Rede de Ambientes Tropicais e Semiárido. Aquac. Bras. 2021, 22, 10–16. [Google Scholar]
- Karakoca, S.; Topcu, A.; Karakoca, S.; Topcu, A. Rainbow Trout (Oncorhynchus Mykiss) Cage Culture: Preliminary Observations of Surface Sediment’s Chemical Parameters and Phosphorus Release in Gokcekaya Reservoir, Turkey. J. Geosci. Environ. Prot. 2017, 5, 12–23. [Google Scholar] [CrossRef]
- Montanhini Neto, R.; Ostrensky, A. Evaluation of Commercial Feeds Intended for the Brazilian Production of Nile Tilapia (Oreochromis niloticus L.): Nutritional and Environmental Implications. Aquac. Nutr. 2015, 21, 311–320. [Google Scholar] [CrossRef]
- de Paula Filho, F.J.; Marins, R.V.; Santos, D.V.; Pereira Junio, R.F.; Menezes, J.M.C.; da Gastão, F.G.C.; Guzzi, A.; Teixeira, R.N.P. Assessment of Heavy Metals in Sediments of the Parnaíba River Delta in the Semi-Arid Coast of Brazil. Environ. Earth Sci. 2021, 80, 167. [Google Scholar] [CrossRef]
- Yang, Y.M.; Chae, M.; Cheon, S.; Shim, M.J. Geochemical Properties and Pollution Assessment of Heavy Metals in the Sediments of Daechung Lake, Korea. Environ. Qual. Manag. 2018, 27, 163–171. [Google Scholar] [CrossRef]
- Chou, C.L.; Haya, K.; Paon, L.A.; Burridge, L.; Moffatt, J.D. Aquaculture-Related Trace Metals in Sediments and Lobsters and Relevance to Environmental Monitoring Program Ratings for near-Field Effects. Mar. Pollut. Bull. 2002, 44, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Zhang, Y.; Zhang, H.; Huang, C.; Pei, Y. Fraction Spatial Distributions and Ecological Risk Assessment of Heavy Metals in the Sediments of Baiyangdian Lake. Ecotoxicol. Environ. Saf. 2019, 174, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, N.; Wojciechowska, E.; Mohsin, M.; Kuittinen, S.; Pappinen, A.; Rezania, S. Trace Metal Contamination of Bottom Sediments: A Review of Assessment Measures and Geochemical Background Determination Methods. Minerals 2021, 11, 872. [Google Scholar] [CrossRef]




| Heavy Metal | Certified Values a | Detection Limits | Observed Concentration | Recovery (%) |
|---|---|---|---|---|
| Fe b | 2.008 ± 0.039 | 0.12 | 1.98 ± 0.06 | 99 |
| Mn c | 234.5 ± 2.8 | 0.10 | 215.70 ± 4.90 | 92 |
| Zn c | 48.9 ± 1.6 | 0.26 | 46.90 ± 2.70 | 96 |
| Cu c | 10.01 ± 0.34 | 0.08 | 9.41 ± 0.25 | 94 |
| Cd | 0.148± 0.007 | 0.03 | 0.14± 0.01 | 95 |
| Cr | 40.9 ± 1.9 | 0.10 | 36.40± 2.50 | 89 |
| Pb | 11.7 ± 1.2 | 0.10 | 10.40± 0.90 | 89 |
| Ni d | 23 | 0.03 | 21.20± 1.10 | 92 |
| Individual Indices | Classification |
|---|---|
| Geoaccumulation index [52]. Where: = Metal concentration. ) = Background value. 1.5 = Minimizing factor of possible background value variation. | Value/category |
| <0: Unpolluted; 0–1: Unpolluted to moderately polluted; 1–2: Moderately polluted; 2–3: Moderately to heavily polluted; 3–4: Heavily polluted; 4–5: Heavily to extremely polluted; >5: Extremely polluted. | |
| Enrichment factor [53]. Where: = Metal concentration. = Background concentration. = Metal concentration. = Background concentration. | Value/category |
| <1: No enrichment; 1–3: Minimum enrichment; 3–5: Moderate enrichment; 5–10: Moderately severe enrichment; 10–25: Severe enrichment; 25–50: Very severe enrichment; >50: Extremely severe enrichment. | |
| Contamination factor [54]. ( Where: individual by metal (i) = Metal concentration. = Background concentration. | Value/category |
| <1: Low contamination; 1 ≤ FC < 3: Moderate contamination; 3 ≤ FC < 6: Considerable contamination; CF > 6: Very high contamination. | |
| Complex index. | Classification |
| degree of contamination [54]. CDj = Total metals in a sampling area (j). = Contamination factor. | Value/category |
| CD < 6: Low degree of contamination; 6 ≤ CD < 12: Moderate degree of contamination; 12≤ CD < 24: Considerable degree of contamination; CD > 24: High degree of contamination. |
| Complex Index | Classification | ||
|---|---|---|---|
| Ecological risk [54]. = = Index of potential ecological risk of a single heavy metal (i) at the sampling site (j). TC = Heavy metal toxic response coefficient (i) = (Zn = 1, Cu = 5, Cr = 2, Cd = 30, Pb and Ni = 5). | Value/Category | ||
| 1- < 40: low ecological risk; 2–40 < < 80: moderate; 3–80 < < 160: strong; 4–160 < < 320: very strong; 5-> 320: extremely strong. | |||
| Potential ecological risk [56]. = Comprehensive potential ecological risk at the sampling site (j). | Ecological risks | ||
| 1- < 70: low; 2–70 < 140: moderate; 3–140 < 280: strong; 4–280 < 560: very strong; 5- > 560: extremely strong. | |||
| Toxic risk index [9]. = = toxic risk index = metal concentration in the sediment TEL *: threshold effect level ***; PEL **: likely effect level ***; | Classification | ||
| Value/category | |||
| 1-TRI ≤ 5: no toxic risk; 2–5 < TRI ≤ 10: low toxic risk; 3–10< TRI ≤ 15: moderate toxic risk; 4–15 < TRI ≤ 20: considerable toxic risk; 5-TRI > 20: very high toxic risk. | |||
| Sediment Quality Guidelines [46] | Metal | Concentration (mg/kg−1) | |
| Level I | Level II | ||
| Zn | 123 | 315 | |
| Cu | 35.7 | 197 | |
| Cr | 37.3 | 90 | |
| Cd | 0.6 | 3.5 | |
| Pb | 35 | 91.3 | |
| Ni | 18 | 35.9 | |
| Area | Sand | Clay | Silt |
|---|---|---|---|
| PCTV | 12.70 ± 6.00 | 35.49 ± 22.35 | 51.81 ± 26.48 |
| CTV | 21.32 ± 8.35 | 30.04 ± 22.66 | 51.33 ± 23.10 |
| CTRL | 9.41 ± 8.35 | 40.11 ± 20.60 | 48.47 ± 22.68 |
| PCTV | CTV | CTRL | SQGs * | |||||
|---|---|---|---|---|---|---|---|---|
| (Non-Fish-Farming Area) | (Fish Farming) | (Control Area) | ||||||
| Min/Max | Average/σ | Min/Max | Average/σ | Min/Max | Average/σ | Level N1 | Level N2 | |
| Fe a | 0.13 | 0.36 ± 0.14 | 0.19 | 0.40 ± 0.22 | 0.11 | 0.28 ± 0.14 | - | - |
| 0.59 | 0.85 | 0.51 | ||||||
| Mn b | 38.3 | 51.93 ± 8.49 | 27.88 | 66.48 ± 19.11 | 14.46 | 22.55 ± 4.75 | - | - |
| 64.51 | 86.91 | 28.56 | ||||||
| Zn b | 29.3 | 77.77 ± 50.98 | 22.79 | 114.83 ± 59.75 | 2.6 | 9.13 ± 3.80 | 123 | 315 |
| 187.09 | 220.56 | 15.33 | ||||||
| Cu b | 29.1 | 71.83 ± 21.20 | 40.46 | 62.23 ± 18.57 | 26.89 | 37.74 ± 8.96 | 35.7 | 197 |
| 98.75 | 91.3 | 48.3 | ||||||
| Cr b | 65.06 | 70.53 ± 3.54 | 68.21 | 70.85 ± 2.62 | 60.18 | 66.45 ± 4.21 | 37.3 | 90 |
| 76.06 | 74.69 | 74.13 | ||||||
| Cd b | 0.46 | 0.53 ± 0.04 | 0.4 | 0.47 ± 0.04 | 0.38 | 0.43 ± 0.03 | 0.6 | 3.5 |
| 0.59 | 0.53 | 0.46 | ||||||
| Pb b | 29.5 | 33.71 ± 4.34 | 23.13 | 26.29 ± 2.32 | 18.13 | 18.77 ± 0.59 | 35 | 91.3 |
| 43.13 | 29.75 | 19.75 | ||||||
| Ni b | 41.26 | 44.60 ± 1.79 | 36.45 | 40.99 ± 2.43 | 34.4 | 39.17 ± 3.76 | 18 | 39 |
| 46.75 | 45.8 | 44.81 | ||||||
| Concentrations | Percentage Increases | ||
|---|---|---|---|
| Metals | CTRL | CTV | PCTV |
| Fe (%) | 0.28 ± 0.14 | 30% | 34.88% |
| Mn (mg/kg−1) | 22.55 ± 4.75 | 66.08% | 59.66% |
| Zn (mg/kg−1) | 9.13 ± 3.80 | 92.05% | 90.32% |
| Cu (mg/kg−1) | 37.74 ± 8.96 | 39.35% | 47.46% |
| Cr (mg/kg−1) | 66.45 ± 4.21 | 6.21% | 5.78% |
| Pb (mg/kg−1) | 18.77 ± 0.59 | 28.60% | 47.69% |
| Ni (mg/kg−1) | 39.17 ± 3.76 | 4.44% | 12.17% |
| Fe | Mn | Zn | Cu | Cr | Cd | Ni | <63 μm | M.O. | |
|---|---|---|---|---|---|---|---|---|---|
| Fe | |||||||||
| Mn | 0.29 ns | ||||||||
| Zn | 0.41 ** | 0.76 * | |||||||
| Cu | 0.21 ns | 0.62 * | 0.56 * | ||||||
| Cr | 0.20 ** | 0.58 * | 0.51 * | 0.68 * | |||||
| Cd | 0.18 ns | −0.33 ns | −0.34 ns | −0.51 * | −0.34 ns | ||||
| Ni | 0.18 ns | 0.13 ns | 0.07 ns | 0.48 * | 0.47 ** | −0.50 * | |||
| <63 μm | 0.01 ns | −0.02 ns | 0.09 ns | −0.07 ns | 0.04 ns | 0.08 ns | 0.01 ns | ||
| M.O. | 0.04 ns | 0.65 ns | 0.46 ** | 0.46 * | 0.40 ** | −0.41 ** | 0.13 ns | 0.36 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melo Júnior, H.d.N.; de Paula Filho, F.J.; Menezes, J.M.C.; de Araújo, J.A.S.; Gonçalves Santana, J.E.; Melo, H.V.S.; Vieira, R.d.S.; de Morais Oliveira-Tintino, C.D.; Tintino, S.R.; Coutinho, H.D.M.; et al. Impacts of the Residual Trace Metals of Aquaculture in Net Cages on the Quality of Sediment. Life 2023, 13, 338. https://doi.org/10.3390/life13020338
Melo Júnior HdN, de Paula Filho FJ, Menezes JMC, de Araújo JAS, Gonçalves Santana JE, Melo HVS, Vieira RdS, de Morais Oliveira-Tintino CD, Tintino SR, Coutinho HDM, et al. Impacts of the Residual Trace Metals of Aquaculture in Net Cages on the Quality of Sediment. Life. 2023; 13(2):338. https://doi.org/10.3390/life13020338
Chicago/Turabian StyleMelo Júnior, Hênio do Nascimento, Francisco José de Paula Filho, Jorge Marcel Coelho Menezes, José Augusto Soares de Araújo, Jorge Ederson Gonçalves Santana, Hênio Vitor Sobral Melo, Rosimara de Sales Vieira, Cícera Datiane de Morais Oliveira-Tintino, Saulo Relison Tintino, Henrique Douglas Melo Coutinho, and et al. 2023. "Impacts of the Residual Trace Metals of Aquaculture in Net Cages on the Quality of Sediment" Life 13, no. 2: 338. https://doi.org/10.3390/life13020338
APA StyleMelo Júnior, H. d. N., de Paula Filho, F. J., Menezes, J. M. C., de Araújo, J. A. S., Gonçalves Santana, J. E., Melo, H. V. S., Vieira, R. d. S., de Morais Oliveira-Tintino, C. D., Tintino, S. R., Coutinho, H. D. M., & Teixeira, R. N. P. (2023). Impacts of the Residual Trace Metals of Aquaculture in Net Cages on the Quality of Sediment. Life, 13(2), 338. https://doi.org/10.3390/life13020338








