Assessment of Genetic Diversity of Local Coffee Populations in Southwestern Saudi Arabia Using SRAP Markers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. DNA Extraction
2.3. Primer Selection and SRAP-PCR Amplification
2.4. Data Collection
3. Results
3.1. SRAP Analysis
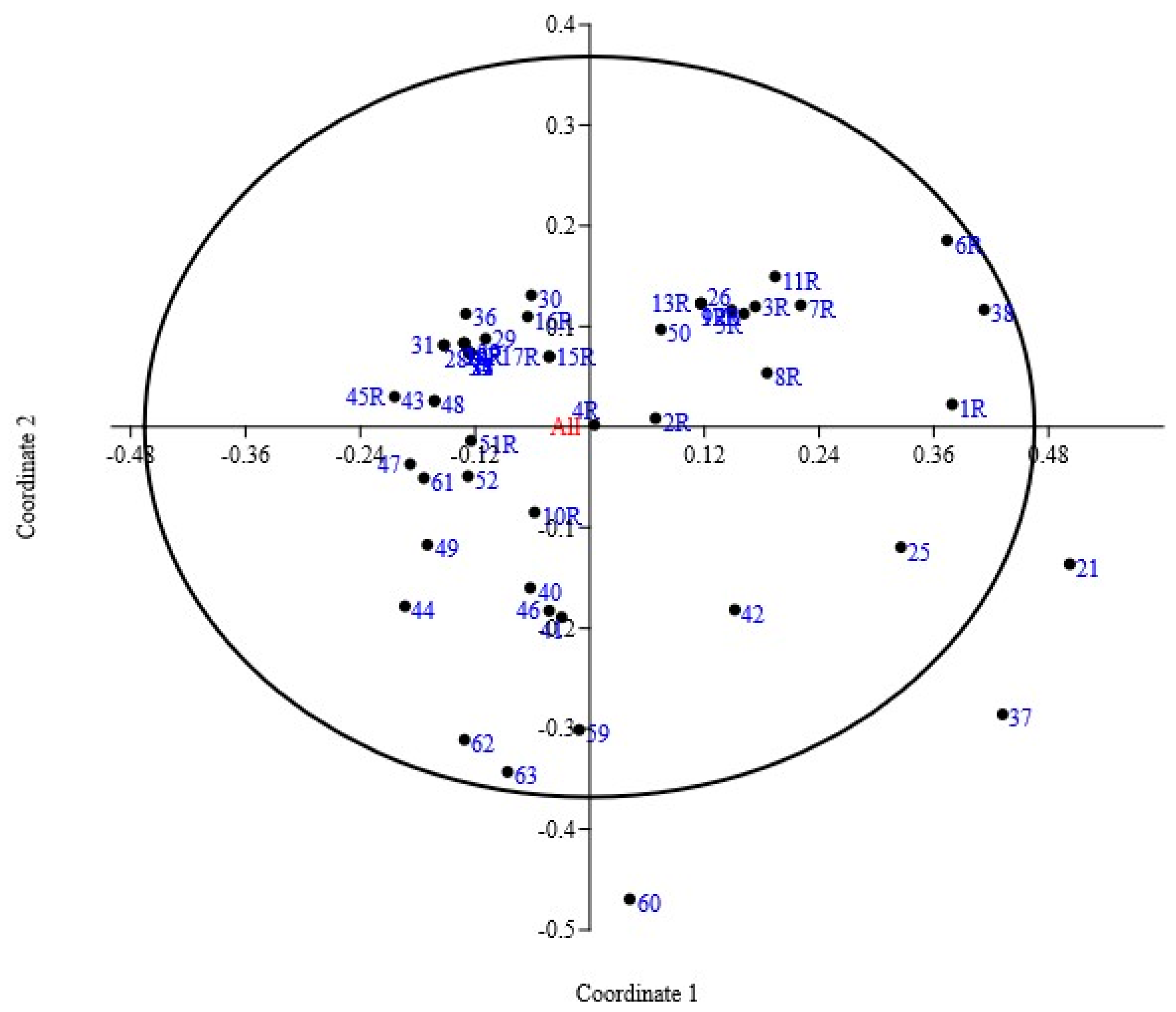
3.2. Principal Coordinate Analysis
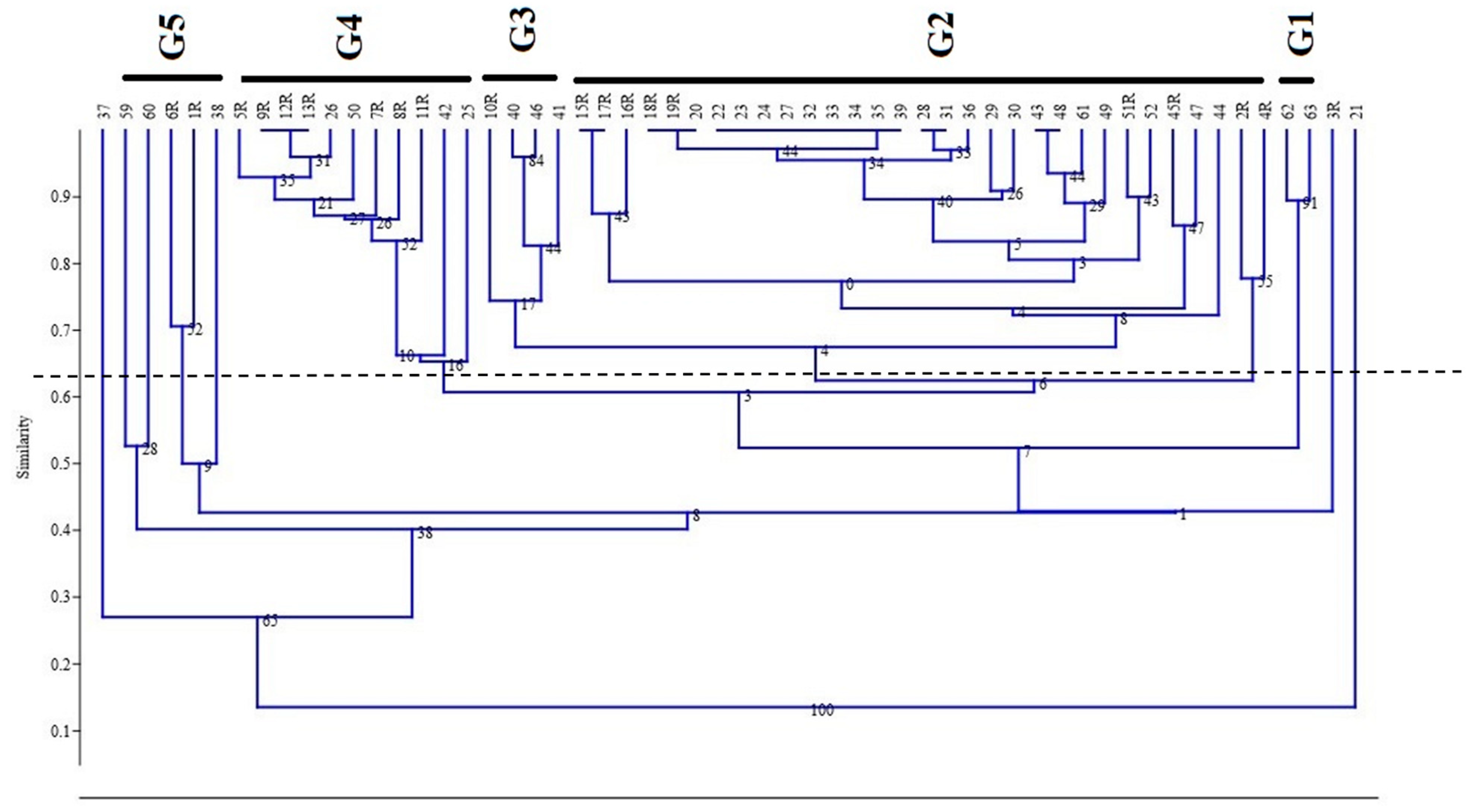
3.3. Hierarchical Cluster Analysis (HCA)
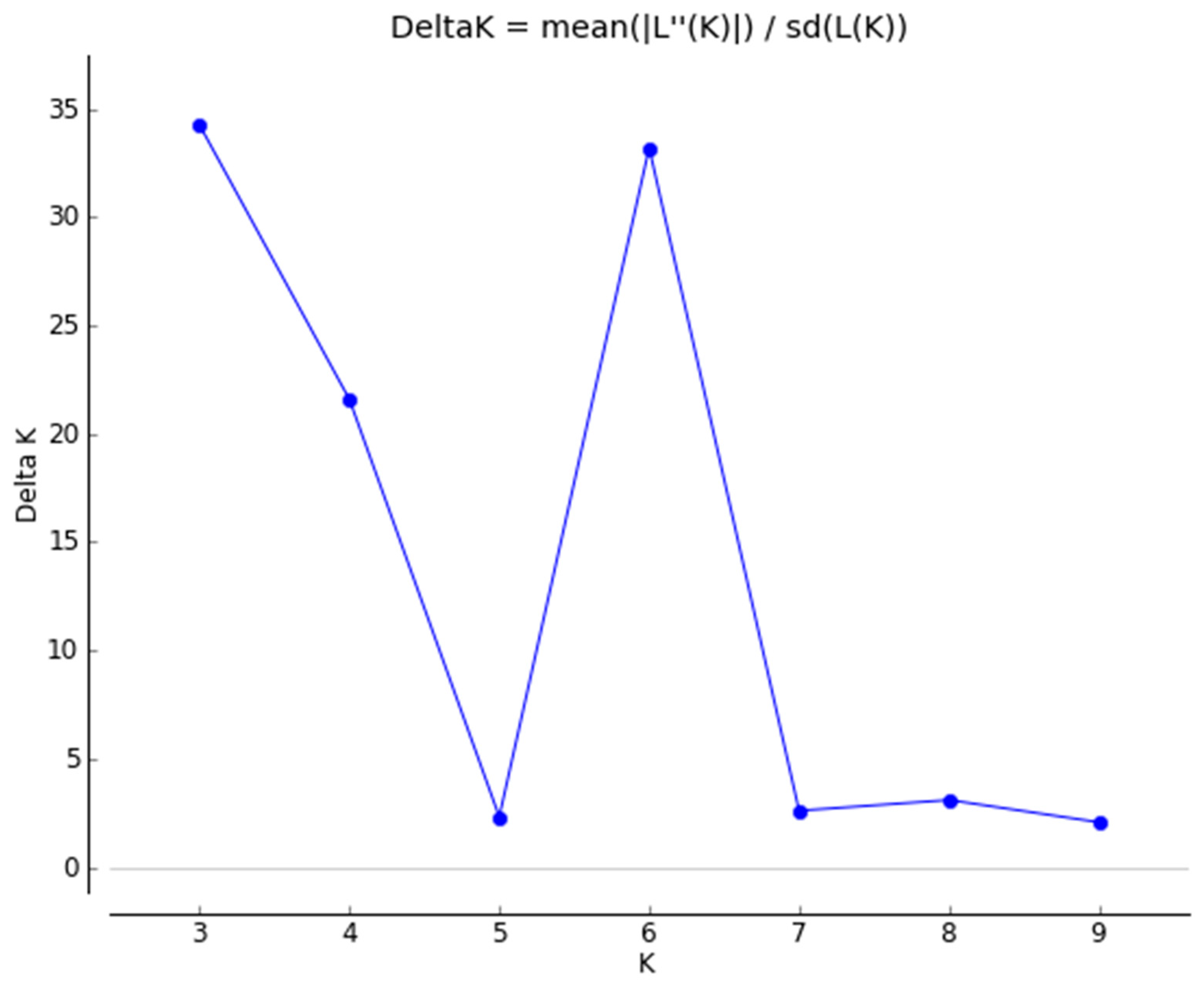
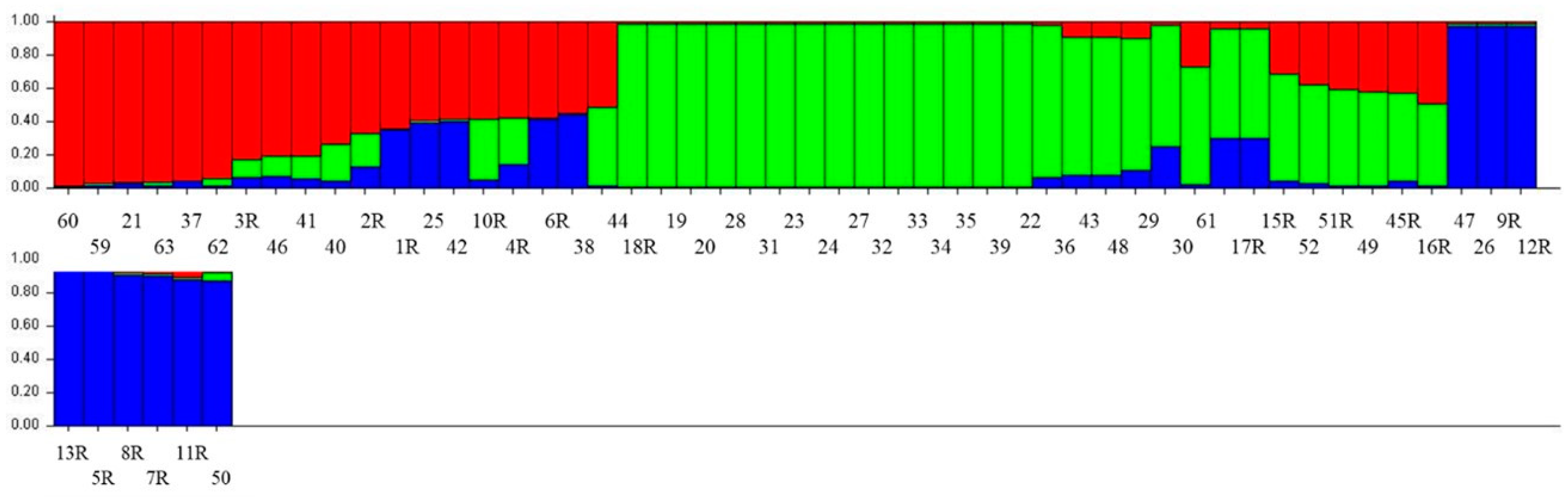
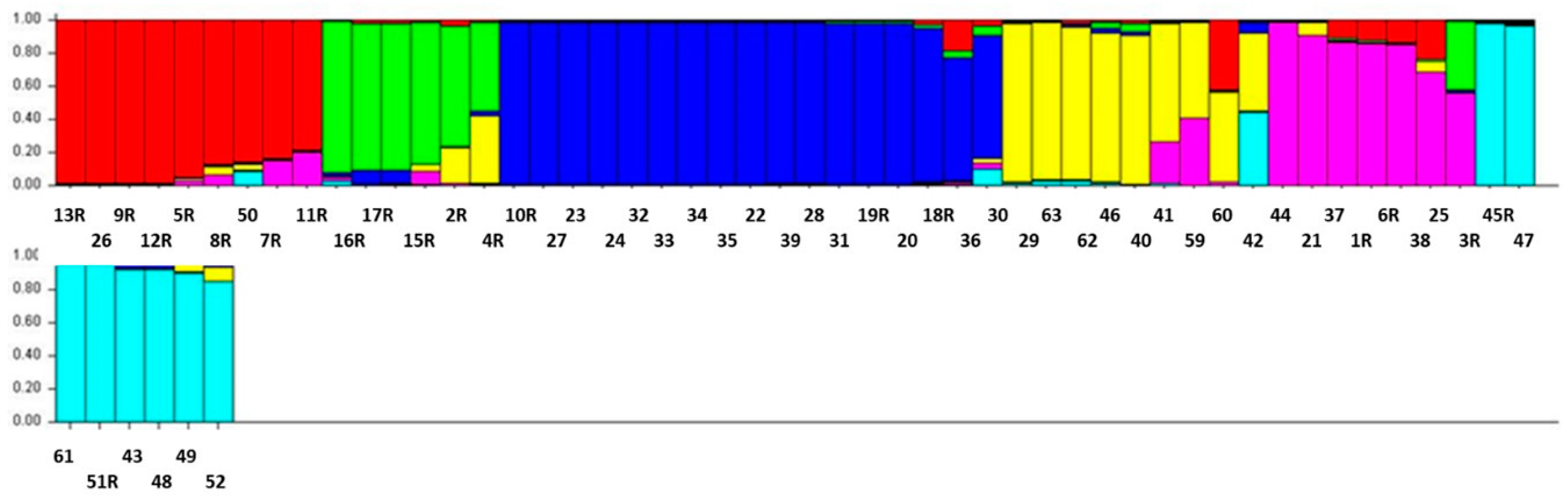
3.4. Structure Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wintgens, J.N. Coffee: Growing, Processing, Sustainable Production. A Guidebook for Growers, Processors, Traders, and Researchers, 1st ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004. [Google Scholar]
- Ferreira, T.; Shuler, J.; Guimarães, R.; Farah, A. Chapter 1 Introduction to coffee plant and genetics. In Coffee: Production, Quality and Chemistry, 1st ed.; Farah, A., Ed.; The Royal Society of Chemistry: London, UK, 2019; pp. 1–25. [Google Scholar]
- Teressa, A.; Crouzillat, D.; Petiard, V.; Brouhan, P. Genetic diversity of arabica coffee (Coffea arabica L.) collections. Ethiop. J. Appl. Sci. Technol. 2010, 1, 63–79. [Google Scholar]
- Anthony, F.; Bertrand, B.; Quiros, O.; Wilches, A.; Lashermes, P.; Berthaud, J.; Charrier, A. Genetic diversity of wild coffee (Coffea arabica L.) using molecular markers. Euphytica 2001, 118, 53–65. [Google Scholar] [CrossRef]
- da Silva, B.S.R.; Sant’Ana, G.C.; Chaves, C.L.; Godoy Androcioli, L.; Ferreira, R.V.; Sera, G.H.; Charmetant, P.; Leroy, T.; Pot, D.; Domingues, D.S. Population structure and genetic relationships between ethiopian and brazilian coffea arabica genotypes revealed by SSR markers. Genetica 2019, 147, 205–216. [Google Scholar] [CrossRef] [PubMed]
- AL-Asmari, K.M.; Zeid, I.M.A.; Al-Attar, A.M. Coffee arabica in Saudi Arabia: An overview. Int. J. Pharm. Phytopharm. Res. (EIJPPR) 2020, 10, 71–78. [Google Scholar]
- Al-Zaidi, A.A.; Baig, M.B.; Shalaby, M.Y.; Hazber, A. Level of knowledge and its application by coffee farmers in the Udeen area, governorate of Ibb--Republic of Yemen. J. Anim. Plant Sci. 2016, 26, 1797–1804. [Google Scholar]
- Tounekti, T.; Mahdhi, M.; Al-Turki, T.A.; Khemira, H. Genetic diversity analysis of coffee (Coffea arabica L.)germplasm accessions growing in the southwestern Saudi Arabia using quantitative traits. Nat. Resour. 2017, 8, 321–336. [Google Scholar]
- Montagnon, C.; Sheibani, F.; Benti, T.; Daniel, D.; Bote, A.D. Deciphering early movements and domestication of Coffea arabica through a comprehensive genetic diversity study covering Ethiopia and Yemen. Agronomy 2022, 12, 3203. [Google Scholar] [CrossRef]
- Montagnon, C.; Mahyoub, A.; Solano, W.; Sheibani, F. Unveiling a unique genetic diversity of cultivated Coffea arabica L. In its main domestication center: Yemen. Genet. Resour. Crop Evol. 2021, 68, 2411–2422. [Google Scholar] [CrossRef]
- De Pauw, E. An Agroecological Exploration of the Arabian Peninsula; International Center for Agricultural Research in the Dry Areas (ICARDA): Beirut, Lebanon, 2002. [Google Scholar]
- Ovalle-Rivera, O.; Läderach, P.; Bunn, C.; Obersteiner, M.; Schroth, G. Projected shifts in Coffea arabica suitability among major global producing regions due to climate change. PLoS ONE 2015, 10, e0124155. [Google Scholar] [CrossRef] [Green Version]
- Tounekti, T.; Mahdhi, M.; Al-Turki, T.; Khemira, H. Water relations and photo-protection mechanisms during drought stress in four coffee (Coffea arabica) cultivars from southwestern Saudi Arabia. S. Afr. J. Bot. 2018, 117, 17–25. [Google Scholar] [CrossRef]
- Afzal, M.; Alghamdi, S.S.; Migdadi, H.M.; Khan, M.A.; Farooq, M. Morphological and molecular genetic diversity analysis of chickpea genotypes. Int. J. Agric. Biol. 2018, 20, 1062–1070. [Google Scholar]
- Ahmad, A.; Wang, J.-D.; Pan, Y.-B.; Sharif, R.; Gao, S.-J. Development and use of simple sequence repeats (SSRs) markers for sugarcane breeding and genetic studies. Agronomy 2018, 8, 260. [Google Scholar] [CrossRef] [Green Version]
- Alzahib, R.H.; Migdadi, H.M.; Ghamdi, A.A.A.; Alwahibi, M.S.; Afzal, M.; Elharty, E.H.; Alghamdi, S.S. Exploring genetic variability among and within hail tomato landraces based on sequence-related amplified polymorphism markers. Diversity 2021, 13, 135. [Google Scholar] [CrossRef]
- Benti, T. Progress in arabica coffee breeding in Ethiopia: Achievements, challenges and prospects. Int. J. Sci. Basic Appl. Res. 2017, 33, 15–25. [Google Scholar]
- Al-Murish, T.M.; Elshafei, A.A.; Al-Doss, A.A.; Barakat, M.N. Genetic diversity of coffee (Coffea arabica L.)in Yemen via SRAP, TRAP and SSR markers. J. Food Agric. Environ. 2013, 11, 411–416. [Google Scholar]
- Machado, C.; Pimentel, N.; Golynsk, A.; Ferreira, A.; Vieira, H.; Partelli, F. Genetic diversity among 16 genotypes of Coffea arabica in the Brazilian Cerrado. Genet. Mol. Res. 2017, 16, 16039794. [Google Scholar] [CrossRef]
- Geleta, M.; Herrera, I.; Monzón, A.; Bryngelsson, T. Genetic diversity of arabica coffee (Coffea arabica L.) in Nicaragua as estimated by simple sequence repeat markers. Sci. World J. 2012, 2012, 939820. [Google Scholar] [CrossRef]
- Li, G.; Quiros, C.F. Sequence-related amplified polymorphism (SRAP), a new marker system based on a simple PCR reaction: Its application to mapping and gene tagging in brassica. Theor. Appl. Genet. 2001, 103, 455–461. [Google Scholar] [CrossRef]
- Jaccard, P. Nouvelles recherches sur la distribution florale. Bull. Soc. Vaud. Sci. Nat. 1908, 44, 223–270. [Google Scholar]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Anderson, J.A.; Churchill, G.; Autrique, J.; Tanksley, S.; Sorrells, M. Optimizing parental selection for genetic linkage maps. Genome 1993, 36, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Yeh, F.C.; Yang, R.; Boyle, T.B.; Ye, Z.; Mao, J.X. Popgene Version 1.32, the User-Friendly Shareware for Population Genetic Analysis; Molecular Biology and Biotechnology Centre, University of Alberta: Edmonton, AB, Canada, 1999. [Google Scholar]
- Earl, D.A.; VonHoldt, B.M. Structure harvester: A website and program for visualizing structure output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [Green Version]
- Hopley, T.; Byrne, M. Gene flow and genetic variation explain signatures of selection across a climate gradient in two riparian species. Genes 2019, 10, 579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chown, S.L.; Hodgins, K.A.; Griffin, P.C.; Oakeshott, J.G.; Byrne, M.; Hoffmann, A.A. Biological invasions, climate change, and genomics. In Crop Breeding: Bioinformatics and Preparing for Climate Change; Santosh, K., Ed.; Apple Academic Press: Waretown, NJ, USA, 2016; pp. 37–91. [Google Scholar]
- Luo, Y.; Zhang, X.; Xu, J.; Zheng, Y.; Pu, S.; Duan, Z.; Li, Z.; Liu, G.; Chen, J.; Wang, Z. Phenotypic and molecular marker analysis uncovers the genetic diversity of the grass Stenotaphrum secundatum. BMC Genet. 2020, 21, 86. [Google Scholar] [CrossRef]
- Hasan, N.; Choudhary, S.; Naaz, N.; Sharma, N.; Laskar, R.A. Recent advancements in molecular marker-assisted selection and applications in plant breeding programmes. J. Genet. Eng. Biotechnol. 2021, 19, 128. [Google Scholar] [CrossRef]
- Nadeem, M.A.; Nawaz, M.A.; Shahid, M.Q.; Doğan, Y.; Comertpay, G.; Yıldız, M.; Hatipoğlu, R.; Ahmad, F.; Alsaleh, A.; Labhane, N. DNA molecular markers in plant breeding: Current status and recent advancements in genomic selection and genome editing. Biotechnol. Biotechnol. Equip. 2018, 32, 261–285. [Google Scholar] [CrossRef] [Green Version]
- Yunita, R.; Oktavioni, M.; Chaniago, I.; Syukriani, L.; Setiawan, M.A.; Jamsari, J. Analysis of genetic diversity of arabica coffee [Coffea arabica L.] in Solok regency by SRAP molecular markers. IOP Conf. Ser. Earth Environ. Sci. 2020, 497, 012018. [Google Scholar] [CrossRef]
- Kumar, J.; Heikrujam, M.; Sharma, K.; Agrawal, V. SRAP and SSR marker-assisted genetic diversity, population structure analysis and sex identification in jojoba (Simmondsia chinensis). Ind. Crop. Prod. 2019, 133, 118–132. [Google Scholar] [CrossRef]
- Yan, W.; Li, J.; Zheng, D.; Friedman, C.; Wang, H. Analysis of genetic population structure and diversity in Mallotus oblongifolius using ISSR and SRAP markers. PeerJ 2019, 7, e7173. [Google Scholar] [CrossRef] [Green Version]
- Subositi, D.; Mujahid, R. Karakterisasi genetik tempuyung (Sonchus arvensis L.) berdasarkan penanda molekuler sequence-related amplified polymorphism. J. Biol. Indones. 2013, 9, 167–174. [Google Scholar]
- Jingade, P.; Huded, A.K.; Kosaraju, B.; Mishra, M.K. Diversity genotyping of Indian coffee (Coffea arabica L.)germplasm accessions by using SRAP markers. J. Crop Improv. 2019, 33, 327–345. [Google Scholar] [CrossRef]
- Liu, C.C.; Shringarpure, S.; Lange, K.; Novembre, J. Exploring population structure with admixture models and principal component analysis. In Statistical Population Genomics; Dutheil, J.Y., Ed.; Springer US: New York, NY, USA, 2020; pp. 67–86. [Google Scholar]
- Anthony, F.; Combes, M.; Astorga, C.; Bertrand, B.; Graziosi, G.; Lashermes, P. The origin of cultivated Coffea arabica L. varieties revealed by AFLP and SSR markers. Theor. Appl. Genet. 2002, 104, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Berecha, G.; Aerts, R.; Vandepitte, K.; Van Glabeke, S.; Muys, B.; Roldán-Ruiz, I.; Honnay, O. Effects of forest management on mating patterns, pollen flow and intergenerational transfer of genetic diversity in wild arabica coffee (Coffea arabica L.) from Afromontane rainforests. Biol. J. Linn. Soc. 2014, 112, 76–88. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, R.; Lenoir, J.; Piedallu, C.; Riofrío-Dillon, G.; De Ruffray, P.; Vidal, C.; Pierrat, J.-C.; Gégout, J.-C. Changes in plant community composition lag behind climate warming in lowland forests. Nature 2011, 479, 517–520. [Google Scholar] [CrossRef]
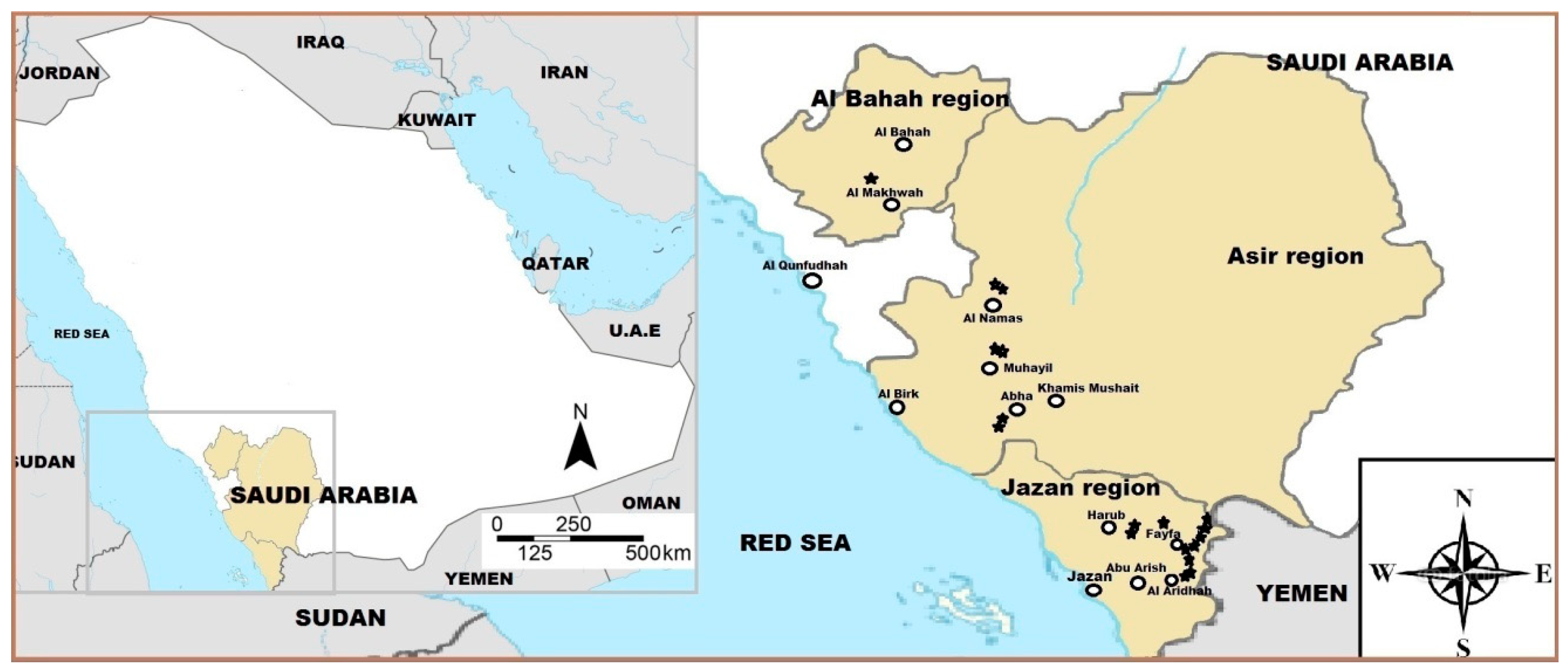
| # | Accession No. | Region | District | Altitude (m a.s.l.) | Latitude |
|---|---|---|---|---|---|
| 1 | KSA1R | Jazan | Khacher/Al-Zoughli | 1254 | 17°18′03″ N |
| 2 | KSA2R | Jazan | Khacher/Al-Guatil | 1484 | 17°19′01″ N |
| 3 | KSA3R | Jazan | Khacher/Al-Guatil | 1484 | 17°19′01″ N |
| 4 | KSA4R | Jazan | Jebel Fayfa | 1541 | 17°15′21″ N |
| 5 | KSA5R | Jazan | Wadi Dafa | 1254 | 17°25′41″ N |
| 6 | KSA6R | Jazan | Tallan | 1672 | 17°23′12″ N |
| 7 | KSA7R | Jazan | Tallan | 1672 | 17°23′12″ N |
| 8 | KSA8R | Jazan | Tallan | 1546 | 17°23′01″ N |
| 9 | KSA9R | Jazan | Tallan | 1672 | 17°23′12″ N |
| 10 | KSA10R | Al-Baha | Shada Al-ala | 1548 | 19°50′54″ N |
| 11 | KSA11R | Jazan | Khacher/Al-Zoughli | 1254 | 17°18′03″ N |
| 12 | KSA12R | Jazan | Maaddi | 1287 | 17°29′29″ N |
| 13 | KSA13R | Jazan | Maaddi | 1344 | 17°29′29″ N |
| 14 | KSA15R | Assir | Rayda | 1594 | 18°11′37″ N |
| 15 | KSA16R | Assir | Rayda | 1594 | 18°11′37″ N |
| 16 | KSA17R | Assir | Rayda | 1519 | 18°11′37″ N |
| 17 | KSA18R | Jazan | Jebel Fayfa | 1260 | 17°15′20″ N |
| 18 | KSA19R | Jazan | Jebel Fayfa | 1660 | 17°15′55″ N |
| 19 | KSA20 | Jazan | Jebel Fayfa | 1260 | 17°15′20″ N |
| 20 | KSA21 | Jazan | Jebel Fayfa | 1260 | 17°15′20″ N |
| 21 | KSA22 | Jazan | Jebel Fayfa | 1260 | 17°15′20″ N |
| 22 | KSA23 | Jazan | Jebel Fayfa | 1260 | 17°15′20″ N |
| 23 | KSA24 | Jazan | Jebel Fayfa | 1260 | 17°15′20″ N |
| 24 | KSA25 | Jazan | Jebel Fayfa | 1260 | 17°15′20″ N |
| 25 | KSA26 | Jazan | Jebel Fayfa | 1550 | 17°15′24″ N |
| 26 | KSA27 | Jazan | Jebel Fayfa | 1550 | 17°15′24″ N |
| 27 | KSA28 | Jazan | Jebel Fayfa | 1550 | 17°15′24″ N |
| 28 | KSA29 | Jazan | Al-Gahr | 1846 | 17°38′08″ N |
| 29 | KSA30 | Jazan | Al-Gahr | 1846 | 17°38′08″ N |
| 30 | KSA31 | Jazan | Al-Gahr | 1846 | 17°38′08″ N |
| 31 | KSA32 | Jazan | Al-Gahr | 1846 | 17°38′08″ N |
| 32 | KSA33 | Jazan | Al-Gahr | 1846 | 17°38′08″ N |
| 33 | KSA34 | Jazan | Jebel Fayfa | 1660 | 17°15′55″ N |
| 34 | KSA35 | Jazan | Jebel Fayfa | 1660 | 17°15′55″ N |
| 35 | KSA36 | Jazan | Jebel Fayfa | 1450 | 17°15′59″ N |
| 36 | KSA37 | Jazan | Eddayer | 1100 | 17°22′10″ N |
| 37 | KSA38 | Jazan | Eddayer | 1228 | 17°22′10″ N |
| 38 | KSA39 | Jazan | Eddayer | 1228 | 17°22′10″ N |
| 39 | KSA40 | Jazan | Haroub | 1100 | 17°29′29″ N |
| 40 | KSA41 | Assir | Rayda | 1450 | 18°11′37″ N |
| 41 | KSA42 | Assir | Rayda | 1450 | 18°11′37″ N |
| 42 | KSA43 | Assir | Rayda | 1400 | 18°11′37″ N |
| 43 | KSA44 | Jazan | Jebel Fayfa | 1524 | 17°15′48″ N |
| 44 | KSA45R | Jazan | Jebel Fayfa | 1524 | 17°15′48″ N |
| 45 | KSA46 | Jazan | Al-Gahr | 1750 | 17°39′01″ N |
| 46 | KSA47 | Jazan | Al-Gahr | 1750 | 17°39′01″ N |
| 47 | KSA48 | Jazan | Jebel Fayfa | 1260 | 17°15′20″ N |
| 48 | KSA49 | Jazan | Jebel Fayfa | 1260 | 17°15′20″ N |
| 49 | KSA50 | Jazan | Jebel Fayfa | 1260 | 17°15′20″ N |
| 50 | KSA51R | Jazan | Jebel Fayfa | 1524 | 17°17′13″ N |
| 51 | KSA52 | Jazan | Jebel Fayfa | 1550 | 17°15′24″ N |
| 52 | KSA59 | Assir | Al-Majarda | 1329 | 19°09′35″ N |
| 53 | KSA60 | Assir | Al-Majarda | 1300 | 19°09′35″ N |
| 54 | KSA61 | Al-Baha | Shada Al-ala | 1548 | 19°50′54″ N |
| 55 | KSA62 | Al-Baha | Shada Al-ala | 1548 | 19°50′54″ N |
| 56 | KSA63 | Al-Baha | Shada Al-ala | 1548 | 19°50′54″ N |
| Primer Pair | Forward Sequence | Reverse Sequence |
|---|---|---|
| F1 × R11 | 5‘-TGAGTCCAAACCGGATA-3’ | 5‘-GACTGCGTACGAATTCAG-3’ |
| F3 × R3 | 5‘-TGAGTCCAAACCGGAT-3’ | 5‘-GACTGCGTACGAATTGAC-3’ |
| F4 × R10 | 5‘-TGAGTCCAAACCGGAGA-3’ | |
| F4 × R11 | 5‘-TGAGTCCAAACCGGAGA-3’ | 5‘-GACTGCGTACGAATTCAG-3’ |
| F6 × R2 | 5‘-TGAGTCCAAACCGGACC-3’ | 5‘-GACTGCGTACGAATTTGC-3’ |
| F9 × R10 | 5‘-TGAGTCCAAACCGGTCC-3’ |
| Primer Combination | Total Number of Fragments | Total Number of Bands | Number of Polymorphic Loci | Fraction Polymorphism (FP) | % Polymorphism | PIC Value | Marker Index (MI) | Resolving Power (RP) |
|---|---|---|---|---|---|---|---|---|
| F01 × R11 | 240 | 6 | 6 | 1 | 100 | 0.22 | 1.30 | 6.85 |
| F03 × R03 | 171 | 4 | 4 | 1 | 100 | 0.49 | 1.96 | 5.95 |
| F04 × R10 | 103 | 2 | 2 | 1 | 50 | 0.13 | 0.26 | 3.75 |
| F04 × R11 | 293 | 6 | 6 | 1 | 100 | 0.37 | 2.22 | 6.92 |
| F06 × R02 | 108 | 4 | 4 | 1 | 100 | 0.33 | 1.32 | 4.98 |
| F09 × R10 | 210 | 7 | 7 | 1 | 100 | 0.42 | 2.94 | 7.95 |
| Total | 1125 | 29 | 1.98 | 10 | 36.40 | |||
| Average number of bands | 187.5 | 4.83 | 4.83 | 1 | 91.66 | 0.32 | 1.66 | 6.06 |
| Axis | Eigenvalue | Cumulative Eigenvalue | Percent % | Cumulative (%) |
|---|---|---|---|---|
| 1 | 1.84 | 1.84 | 26.84 | 26.84 |
| 2 | 1.16 | 3.00 | 16.82 | 43.66 |
| 3 | 0.70 | 3.70 | 10.18 | 53.84 |
| 4 | 0.58 | 4.28 | 8.48 | 62.32 |
| 5 | 0.43 | 4.72 | 6.30 | 68.62 |
| 6 | 0.34 | 5.06 | 4.99 | 73.61 |
| 7 | 0.29 | 5.35 | 4.17 | 77.78 |
| 8 | 0.22 | 5.56 | 3.17 | 80.95 |
| 9 | 0.13 | 5.69 | 1.88 | 82.82 |
| 10 | 0.11 | 5.80 | 1.58 | 84.40 |
| 11 | 0.08 | 5.88 | 1.21 | 85.61 |
| 12 | 0.07 | 5.95 | 1.02 | 86.63 |
| 13 | 0.05 | 6.00 | 0.73 | 87.36 |
| Accession No. | Region | District | Structure Status | Accession No. | Region | District | Structure Status |
|---|---|---|---|---|---|---|---|
| KSA1R | Jazan | Khacher/Al-Zoughli | AM | KSA30 | Jazan | Al-Gahr | SPII |
| KSA2R | Jazan | Khacher/Al-Guatil | AM | KSA31 | Jazan | Al-Gahr | SPII |
| KSA3R | Jazan | Khacher/Al-Guatil | SPI | KSA32 | Jazan | Al-Gahr | SPII |
| KSA4R | Jazan | Jebel Fayfa | AM | KSA33 | Jazan | Al-Gahr | SPII |
| KSA5R | Jazan | Wadi Dafa | SPIII | KSA34 | Jazan | Jebel Fayfa | SPII |
| KSA6R | Jazan | Tallan | AM | KSA35 | Jazan | Jebel Fayfa | SPII |
| KSA7R | Jazan | Tallan | SPIII | KSA36 | Jazan | Jebel Fayfa | SPII |
| KSA8R | Jazan | Tallan | SPIII | KSA37 | Jazan | Eddayer | SPI |
| KSA9R | Jazan | Tallan | SPIII | KSA38 | Jazan | Eddayer | AM |
| KSA10R | Al-Baha | Shada Al-ala | AM | KSA39 | Jazan | Eddayer | SPII |
| KSA11R | Jazan | Khacher/Al-Zoughli | SPIII | KSA40 | Jazan | Haroub | SPI |
| KSA12R | Jazan | Maadi | SPIII | KSA41 | Assir | Rayda | SPI |
| KSA13R | Jazan | Maadi | SPIII | KSA42 | Assir | Rayda | AM |
| KSA15R | Assir | Rayda | SPII | KSA43 | Assir | Rayda | SPII |
| KSA16R | Assir | Rayda | AM | KSA44 | Jazan | Jebel Fayfa | AM |
| KSA17R | Assir | Rayda | SPII | KSA45R | Jazan | Jebel Fayfa | AM |
| KSA18R | Jazan | Jebel Fayfa | SPII | KSA46 | Jazan | Al-Gahr | SPI |
| KSA19R | Jazan | Jebel Fayfa | SPII | KSA47 | Jazan | Al-Gahr | AM |
| KSA20 | Jazan | Jebel Fayfa | SPII | KSA48 | Jazan | Jebel Fayfa | SPII |
| KSA21 | Jazan | Jebel Fayfa | SPI | KSA49 | Jazan | Jebel Fayfa | AM |
| KSA22 | Jazan | Jebel Fayfa | SPII | KSA50 | Jazan | Jebel Fayfa | SPIII |
| KSA23 | Jazan | Jebel Fayfa | SPII | KSA51R | Jazan | Jebel Fayfa | AM |
| KSA24 | Jazan | Jebel Fayfa | SPII | KSA52 | Jazan | Jebel Fayfa | AM |
| KSA25 | Jazan | Jebel Fayfa | AM | KSA59 | Assir | Al-Majarda | SPI |
| KSA26 | Jazan | Jebel Fayfa | SPIII | KSA60 | Assir | Al-Majarda | SPI |
| KSA27 | Jazan | Jebel Fayfa | SPII | KSA61 | Al-Baha | Shada Al-ala | SPII |
| KSA28 | Jazan | Jebel Fayfa | SPII | KSA62 | Al-Baha | Shada Al-ala | SPI |
| KSA29 | Jazan | Al-Gahr | SPII | KSA63 | Al-Baha | Shada Al-ala | SPI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ghamedi, K.; Alaraidh, I.; Afzal, M.; Mahdhi, M.; Al-Faifi, Z.; Oteef, M.D.Y.; Tounekti, T.; Alghamdi, S.S.; Khemira, H. Assessment of Genetic Diversity of Local Coffee Populations in Southwestern Saudi Arabia Using SRAP Markers. Agronomy 2023, 13, 302. https://doi.org/10.3390/agronomy13020302
Al-Ghamedi K, Alaraidh I, Afzal M, Mahdhi M, Al-Faifi Z, Oteef MDY, Tounekti T, Alghamdi SS, Khemira H. Assessment of Genetic Diversity of Local Coffee Populations in Southwestern Saudi Arabia Using SRAP Markers. Agronomy. 2023; 13(2):302. https://doi.org/10.3390/agronomy13020302
Chicago/Turabian StyleAl-Ghamedi, Khalef, Ibrahim Alaraidh, Muhammad Afzal, Mosbah Mahdhi, Zarraq Al-Faifi, Mohammed D. Y. Oteef, Taieb Tounekti, Salem S. Alghamdi, and Habib Khemira. 2023. "Assessment of Genetic Diversity of Local Coffee Populations in Southwestern Saudi Arabia Using SRAP Markers" Agronomy 13, no. 2: 302. https://doi.org/10.3390/agronomy13020302
APA StyleAl-Ghamedi, K., Alaraidh, I., Afzal, M., Mahdhi, M., Al-Faifi, Z., Oteef, M. D. Y., Tounekti, T., Alghamdi, S. S., & Khemira, H. (2023). Assessment of Genetic Diversity of Local Coffee Populations in Southwestern Saudi Arabia Using SRAP Markers. Agronomy, 13(2), 302. https://doi.org/10.3390/agronomy13020302







