Ultra-Responses of Asphodelus tenuifolius L. (Wild Onion) and Convolvulus arvensis L. (Field Bindweed) against Shoot Extract of Trianthema portulacastrum L. (Horse Purslane)
Abstract
1. Introduction
2. Results
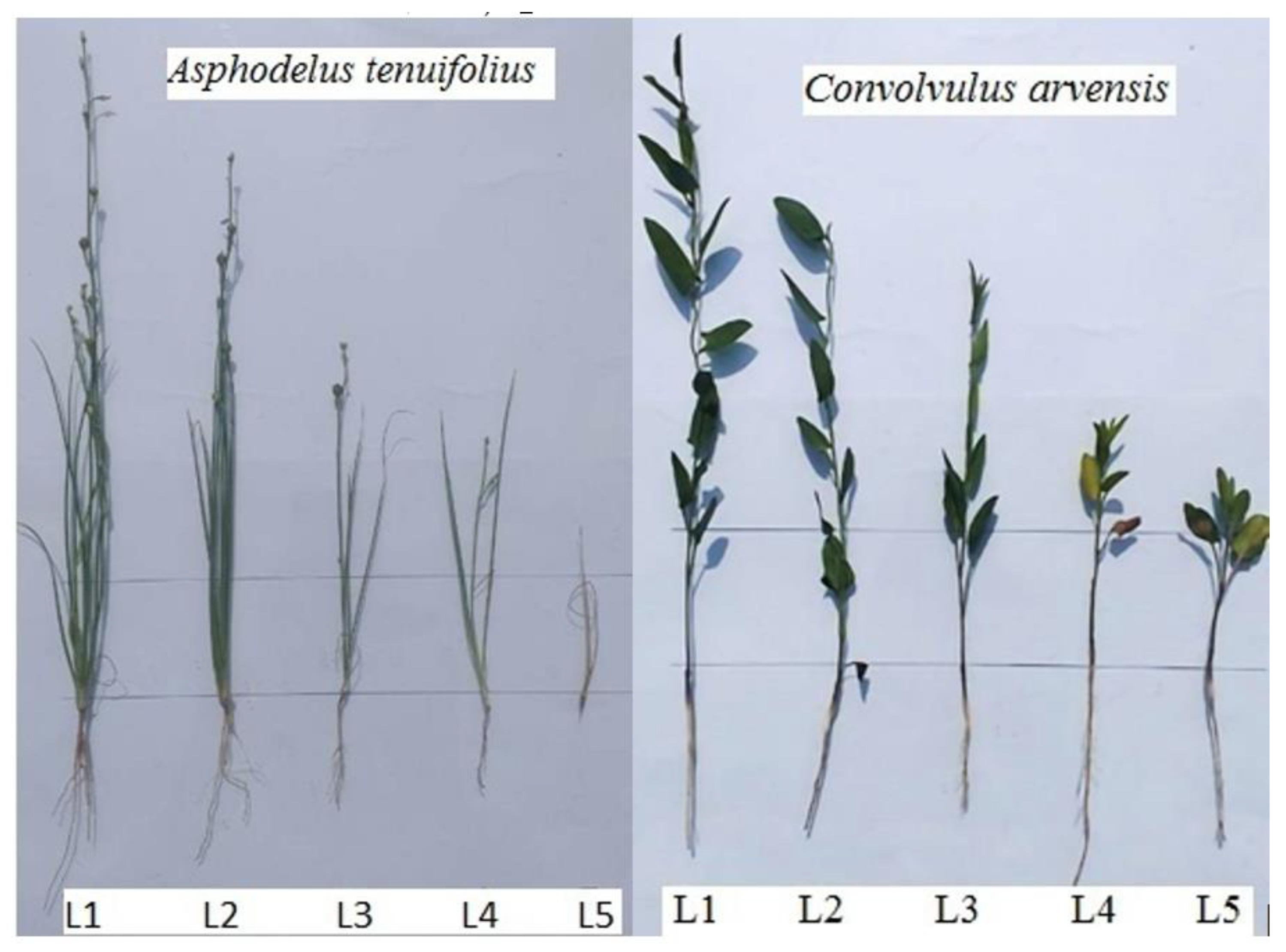
2.1. Morphological Characteristics
2.2. Physiological Characteristics
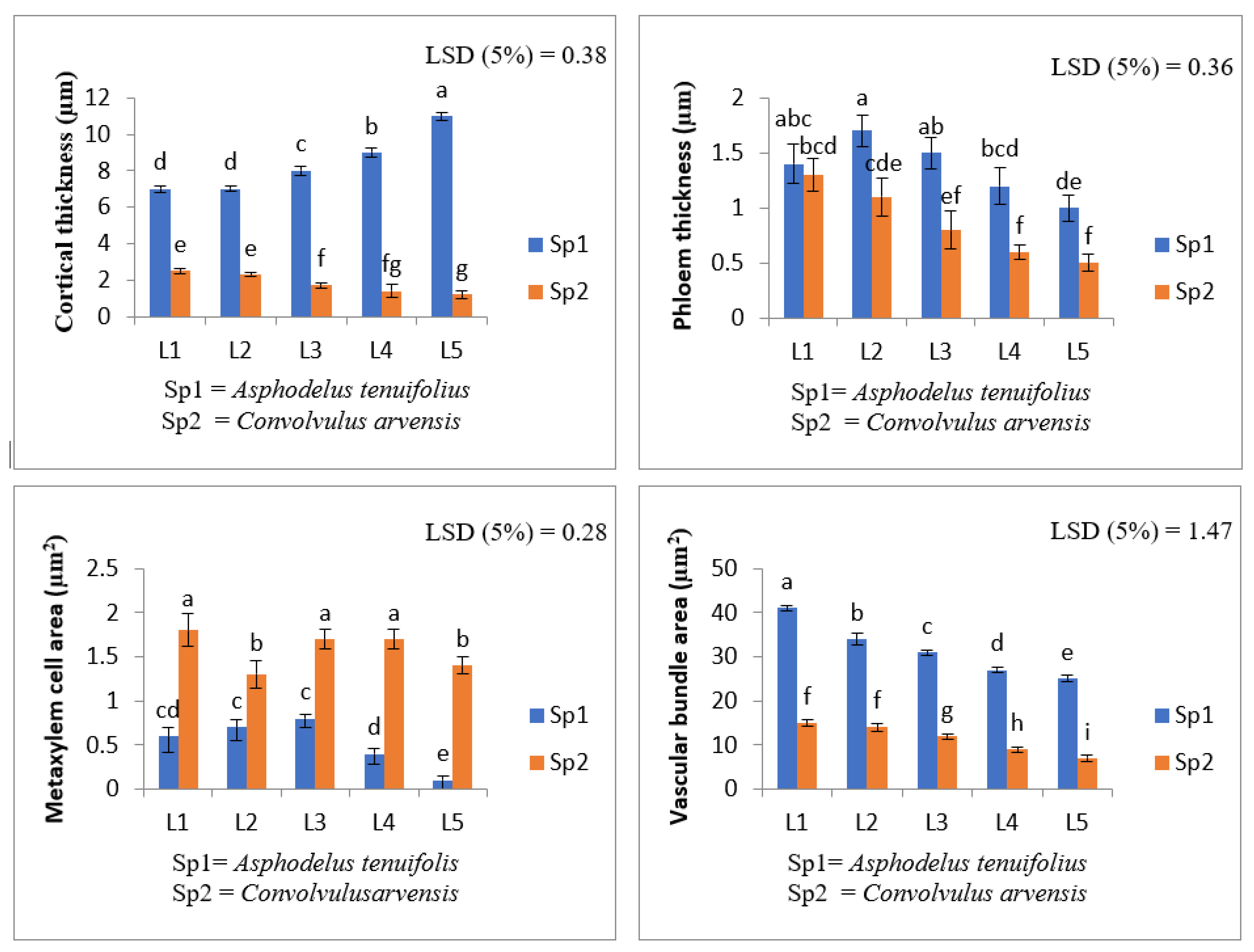
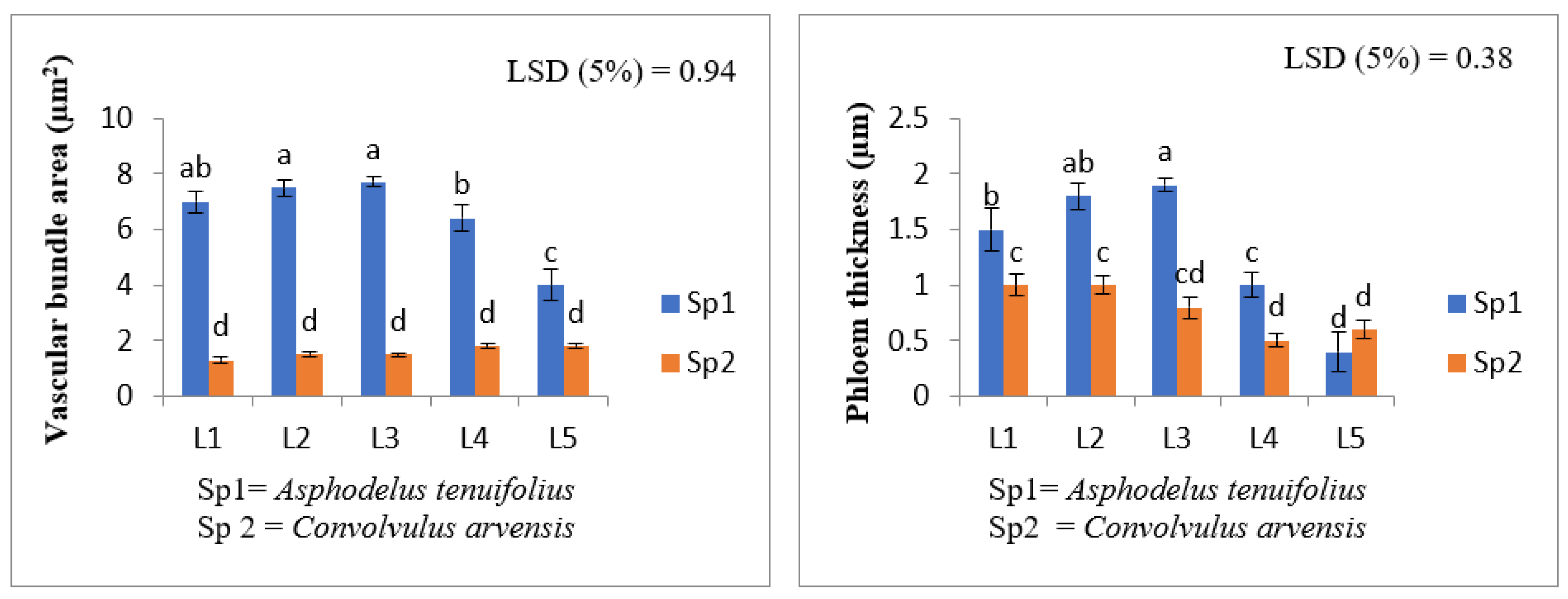
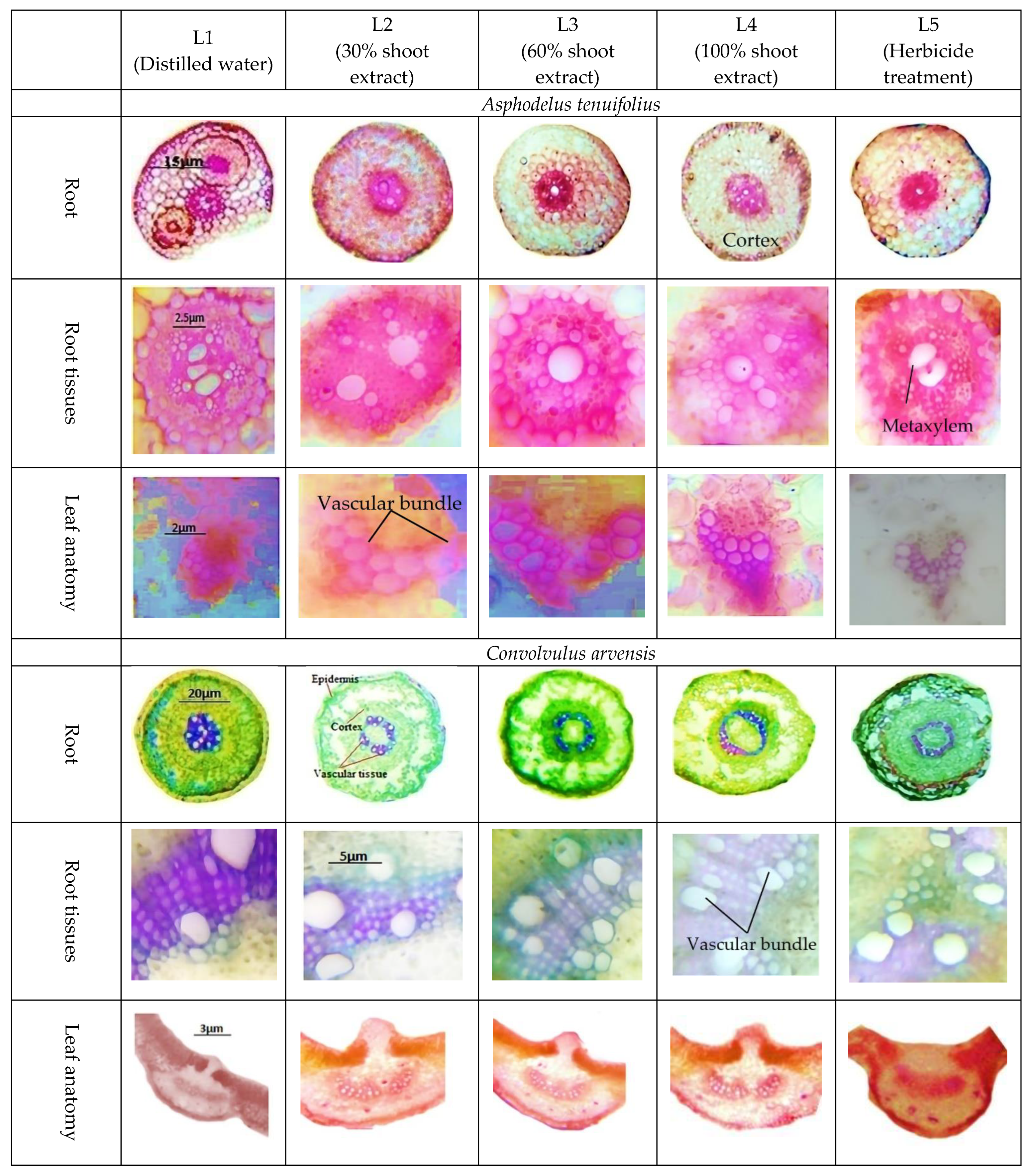
2.3. Anatomical Characteristics
- (i)
- Root anatomy.
- (ii)
- Leaf anatomy.
3. Discussion
4. Materials and Methods
4.1. Anthology of Allelopathic Plant and Preparation of Extract
4.2. Shoot Extract Levels Applied to Weeds
- L1 = Control treatment (Distilled water)
- L2 = 30% allelopathic aqueous treatment of Shoot
- L3 = 60% allelopathic aqueous treatment of Shoot
- L4 = 100% allelopathic aqueous treatment of Shoot
- L5 = Herbicide treatment
4.3. Soil Analysis
4.4. Collection of Seeds of Weeds and Conduction of Pot Experiment
4.5. Morphological Parameters
4.6. Physiological Parameters
- (i)
- Free amino acids (mg/g d.wt.).
- (ii)
- Determination of Ca2+ (mg/g d.wt.).
4.7. Anatomical Parameters
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Babar, M.A.; Khan, A.; Azam, S.; Arif, M.; Hussain, S. Weeds in wheat crop: Weed whole plants nutrient concentration and uptake under fertilizer application and irrigation frequencies. Pure Appl. Biol. 2019, 8, 1724–1735. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Matloob, A.; Khan, F.A.; Khaliq, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.; Ullah, N.; et al. Phytohormones and plant responses to salinity stress: A review. Plant Growth Regul. 2015, 75, 391–404. [Google Scholar] [CrossRef]
- Peterson, M.A.; Collavo, A.; Ovejero, R.; Shivrain, V.; Walsh, M.J. The challenge of herbicide resistance around the world: A current summary. Pest Manag. Sci. 2018, 74, 2246–2259. [Google Scholar] [CrossRef]
- Hussain, M.I.; El-Sheikh, M.A.; Reigosa, M.J. Allelopathic potential of aqueous extract from Acacia melanoxylon R. Br. on Lactuca sativa. Plants 2020, 9, 1228. [Google Scholar] [CrossRef]
- Khan, M.A.; Afridi, R.A.; Hashim, S.; Khattak, A.M.; Ahmad, Z.; Wahid, F.; Chauhan, B.S. Integrated effect of allelochemicals and herbicides on weed suppression and soil microbial activity in wheat (Triticum aestivum L.). J. Crop Prot. 2016, 90, 34–39. [Google Scholar] [CrossRef]
- Wipf, H.M.L.; Meindl, G.A.; Ashman, T.L. A first test of elemental allelopathy via heterospecific pollen receipt. Am. J. Bot. 2016, 103, 514–521. [Google Scholar] [CrossRef]
- Yang, X.F.; Kong, C.H. Interference of allelopathic rice with paddy weeds at the root level. Plant Biol. 2017, 19, 584–591. [Google Scholar] [CrossRef]
- Cheng, F.; Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef]
- Budzałek, G.; Śliwińska-Wilczewska, S.; Wiśniewska, K.; Wochna, A.; Bubak, I.; Latała, A.; Wiktor, J.M. Macroalgal defense against competitors and herbivores. Int. J. Mol. Sci. 2021, 22, 7865. [Google Scholar] [CrossRef]
- El-Abbassi, A.; Saadaoui, N.; Kiai, H.; Raiti, J.; Hafidi, A. Potential applications of olive mill wastewater as a biopesticide for crop protection. Sci. Total Environ. 2017, 576, 10–21. [Google Scholar] [CrossRef]
- Safdar, M.E.; Hayyat, M.S.; Maajid, M.Z.; Nadeem, M.; Ali, A. Estimation of an economic threshold of Convolvulus arvensis L. weed in wheat (Triticum aestivum L.). Pak. J. Weed Sci. Res. 2019, 25, 17–26. [Google Scholar]
- Pushpa, N.S.S.; Baloch, S.K.; Buriro, M.; Soomro, A.A.; Khan, M.T.; Jogi, Q.U.; Kandhro, M.N.; Soomro, F.D. Allelopathic Effect of Weed Species on Germination and Seedling Traits of Wheat Varieties. J. Innov. Sci. 2020, 5, 100. [Google Scholar] [CrossRef]
- Ara, A.; Akram, A.; Ajmal, M.; Akhund, S.; Nayyar, B.G. Pharmacological, nutritional and allelopathic attributes of noxious weed, Trianthema portulacastrum L. (Horse purslane). Pure Appl. Biol. 2021, 4, 340–352. [Google Scholar] [CrossRef]
- Al Sherif, E.A.; Gharieb, H.R. Allelochemical effect of Trianthema portulacastrum L. on Amaranthus viridis L. supports the ecological importance of allelopathy. Afr. J. Agric. Res. 2011, 6, 6690–6697. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Singh Sidhu, G.P.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic response of plants under different abiotic stresses: A review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Lal, M.K.; Naga, K.C.; Kumar, R.; Chourasia, K.N.; Subhash, S.; Kumar, D.; Sharma, S. Emerging roles of melatonin in mitigating abiotic and biotic stresses of horticultural crops. Sci. Hortic. 2020, 272, 109592. [Google Scholar] [CrossRef]
- Shahzad, A.; Ullah, S.; Dar, A.A.; Sardar, M.F.; Mehmood, T.; Tufail, M.A.; Shakoor, A.; Haris, M. Nexus on climate change: Agriculture and possible solution to cope future climate change stresses. Environ. Sci. Pollut. Res. 2021, 28, 14211–14232. [Google Scholar] [CrossRef]
- Palaniappan, S.P.; Annadurai, K. Organic Farming Theory and Practice; Scientific Publishers: Hackensack, NJ, USA, 2018. [Google Scholar]
- Islam, A.M.; Yeasmin, S.; Qasem, J.R.S.; Juraimi, A.S.; Anwar, M.P. Allelopathy of medicinal plants: Current status and future prospects in weed management. Agric. Sci. 2018, 9, 1569–1588. [Google Scholar] [CrossRef]
- Głąb, L.; Sowiński, J.; Bough, R.; Dayan, F.E. Allelopathic potential of sorghum in weed control: A comprehensive review. Adv. Agron. 2017, 145, 43–95. [Google Scholar]
- Gomaa, N.H.; AbdElgawad, H.R. Phytotoxic effects of Echinochloa colona (L.) Link.(Poaceae) extracts on the germination and seedling growth of weeds. Span. J. Agric. Res. 2012, 10, 492–501. [Google Scholar] [CrossRef]
- Kandhro, M.N.; Tunio, S.D.; Rajpar, I.; Chachar, Q.D.; Gandahi, A.W. Allelopathic impact of sorghum and sunflower on germination and seedling growth of summer broadleaf weeds. Pak. J. Agric. Sci. 2015, 31, 229–239. [Google Scholar]
- Sutradhar, T.; Lokho, A.; Das, A.P. Effects of extracts and leachates of Trianthema portulacastrum L. (Aizoaceae) on the seed germination and performance of young jute seedlings (Corchorus olitorius L. of Malvaceae) in Bardhaman district of West Bengal, India. Int. J. Agric. Sci. 2018, 8, 279–282. [Google Scholar]
- Khaliq, A.; Matloob, A.; Khan, M.B.; Tanveer, A. Differential suppression of rice weeds by allelopathic plant aqueous extracts. Planta Daninha 2013, 31, 21–28. [Google Scholar] [CrossRef]
- Natarajan, A.; Elavazhagan, P.; Prabhakaran, J. Allelopathic potential of billy goat weed Ageratum conyzoides L. and Cleome viscosa L. on germination and growth of Sesamum indicum L. Int. J. Curr. Biotechnol. 2014, 2, 21–24. [Google Scholar]
- Ghimire, B.K.; Hwang, M.H.; Sacks, E.J.; Yu, C.Y.; Kim, S.H.; Chung, I.M. Screening of allelochemicals in Miscanthus sacchariflorus extracts and assessment of their effects on germination and seedling growth of common weeds. Plants 2020, 9, 1313. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Cheema, Z.A.; Ihsan, M.Z.; Hussain, Y.; Mazari, A.; Abbas, H.T. Allelopathic effects of different plant water extract on yield and weeds of wheat. Planta Daninha 2018, 36. [Google Scholar] [CrossRef]
- Zohaib, A.; Abbas, T.; Tabassum, T. Weeds cause losses in field crops through allelopathy. Not. Sci. Biol. 2016, 8, 47–56. [Google Scholar] [CrossRef]
- Al-Johani, N.S.; Aytah, A.A.; Boutraa, T. Allelopathic impact of two weeds, Chenopodium murale and Malva parviflora on growth and photosynthesis of barley (Hordeum vulgare L.). Pak. J. Bot. 2012, 44, 1865–1872. [Google Scholar]
- Sarabi, V.; Mahallati, M.N.; Nezami, A.; Rashed Mohassel, M.H. Effects of common lambsquarters (‘Chenopodium album’ L.) Emergence time and density on growth and competition of maize (‘Zea mays’ L.). Aust. J. Crop Sci. 2013, 7, 532–537. [Google Scholar]
- Vaishali, R.; Chaturvedi, A. Allelopathic effect of some plants on morphological attributes of invasive alien weed: Malachra capitata (L.) L. GSC Biol. Pharm. Sci. 2019, 6, 108–114. [Google Scholar]
- Sidhu, G.P.S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Alterations in photosynthetic pigments, protein, and carbohydrate metabolism in a wild plant Coronopus didymus L. (Brassicaceae) under lead stress. Acta Physiol. Plant 2017, 39, 176. [Google Scholar] [CrossRef]
- Maqbool, N.; Wahid, A.; Farooq, M.; Cheema, Z.A.; Siddique, K.H.M. Allelopathy and abiotic stress interaction in crop plants. In Allelopathy; Springer: Berlin/Heidelberg, Germany, 2013; pp. 451–468. [Google Scholar]
- Obata, T.; Witt, S.; Lisec, J.; Palacios-Rojas, N.; Florez-Sarasa, I.; Yousfi, S.J.; Araus, L.; Cairns, J.E.; Fernie, A.R. Metabolite profiles of maize leaves in drought, heat, and combined stress field trials reveal the relationship between metabolism and grain yield. Plant Physiol. 2015, 169, 2665–2683. [Google Scholar] [CrossRef] [PubMed]
- Hadi, M.R.; Karimi, N. The role of calcium in plants salt tolerance. J. Plant Nutr. 2012, 35, 2037–2054. [Google Scholar] [CrossRef]
- Xiong, T.C.; Ronzier, E.; Sanchez, F.; Corratgé-Faillie, C.; Mazars, C.; Thibaud, J.B. Imaging long distance propagating calcium signals in intact plant leaves with the BRET-based GFP-aequorin reporter. Front. Plant Sci. 2014, 5, 43. [Google Scholar] [CrossRef]
- Rewald, B.; Shelef, O.; Ephrath, J.E.; Rachmilevitch, S. Adaptive plasticity of salt-stressed root systems. In Ecophysiology and Responses of Plants under Salt Stress; Springer: New York, NY, USA, 2013; pp. 169–201. [Google Scholar]
- Lamalakshmi Devi, E.; Kumar, S.; Basanta Singh, T.; Sharma, S.K.; Beemrote, A.; Devi, C.P.; Chongtham, S.K.; Singh, C.H.; Yumlembam, R.A.; Haribhushan, A.; et al. Adaptation strategies and defense mechanisms of plants during environmental stress. In Medicinal Plants and Environmental Challenges; Springer: Cham, Switzerland, 2017; pp. 359–413. [Google Scholar]
- Rahman, M.M.; Rahman, M.A.; Miah, M.G.; Saha, S.R.; Karim, M.A.; Mostofa, M.G. Mechanistic insight into salt tolerance of Acacia auriculiformis: The importance of ion selectivity, osmoprotectant, tissue tolerance, and Na+ exclusion. Front. Plant Sci. 2017, 8, 155. [Google Scholar] [CrossRef]
- Hameed, M.; Fatima, S.; Shah, S.M.R.; Ahmad, F.; Ashraf, M.; Ghulam-Nabi, M.A.R.Y.A.M.; Ahmad, M.S.A.; Ahmad, I.; Iqbal, U. Ultrastructural response of wheat (Triticum aestivum L.) lines to potential allelopathy of Alstonia scholaris (L.) R. Br. leaf extract. Turk. J. Bot. 2020, 44, 509–525. [Google Scholar] [CrossRef]
- Chen, T.; White, J.F.; Li, C. Fungal endophyte Epichloë bromicola infection regulates anatomical changes to account for salt stress tolerance in wild barley (Hordeum brevisubulatum). Plant Soil 2020, 461, 533–546. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Diaz-Vivancos, P.; Alvarez, S.; Fernandez-García, N.; Sanchez-Blanco, M.J.; Hernández, J.A. Physiological and biochemical mechanisms of the ornamental Eugenia myrtifolia L. plants for coping with NaCl stress and recovery. Planta 2015, 242, 829–846. [Google Scholar] [CrossRef]
- Naz, N.; Batool, R.; Fatima, S.; Hameed, M.; Ashraf, M.; Ahmad, F.; Ahmad, M.S.A. Adaptive components of tolerance to salinity in a saline desert grass Lasiurus scindicus Henrard. Ecol. Res. 2015, 30, 429–438. [Google Scholar] [CrossRef]
- Ruiz, K.B.; Biondi, S.; Martínez, E.A.; Orsini, F.; Antognoni, F.; Jacobsen, S.E. Quinoa–a model crop for understanding salt-tolerance mechanisms in halophytes. Plant Biosyst. 2016, 150, 357–371. [Google Scholar] [CrossRef]
- Nassar, R.M.; Kamel, H.A.; Ghoniem, A.E.; Alarcón, J.J.; Sekara, A.; Ulrichs, C.; Abdelhamid, M.T. Physiological and anatomical mechanisms in wheat to cope with salt stress induced by seawater. Plants 2020, 9, 237. [Google Scholar] [CrossRef]
- Ola, H.A.E.; Reham, E.F.; Eisa, S.S.; Habib, S.A. Morpho-anatomical changes in salt-stressed kallar grass (Leptochloa fusca L. Kunth). Am. J. Agric. Biol. Sci. 2020, 8, 158–166. [Google Scholar]
- Hassan, M.S.; Naz, N.; Ali, H. Evaluation of the allelopathic potential of Trianthema portulacastrum L. on Convolvulus arvensis L. Biochem. Syst. Ecol. 2022, 104, 104491. [Google Scholar] [CrossRef]
- Hamilton, P.B.; Van Slyke, D.D. Amino acid determination with ninhydrin. J. Biol. Chem. 1943, 150, 231–250. [Google Scholar] [CrossRef]
- Kacar, B. Practice Guide of Plant Nutrition; Ankara University: Ankara, Turkey, 1984; 900p. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics; McGraw-Hill Book Company, Inc.: New York, NY, USA, 1960. [Google Scholar]
| Treatment Levels | L1 | L2 | L3 | L4 | L5 | LSD | CV | GM | F Value |
|---|---|---|---|---|---|---|---|---|---|
| Morphological parameters | Asphodelus tenuifolius | ||||||||
| RL (cm) | 6.3 a | 6 a | 5 b | 4 c | 2 d | 0.42 | 4.99 | 4.6 | 168 ** |
| SL (cm) | 21 a | 20 a | 20 a | 18 b | 10 c | 1.19 | 3.68 | 17.8 | 142 ** |
| RDW (g) | 0.12 a | 0.08 b | 0.05 c | 0.05 c | 0.04 c | 0.03 | 24.25 | 0.07 | 12.4 ** |
| SDW (g) | 2.2 a | 1.7 b | 1.5 b | 1.0 c | 0.6 d | 0.32 | 12.78 | 1.4 | 36.1 ** |
| NL (per plant) | 13 a | 12 a | 8 b | 4 c | 3 c | 3.82 | 26.22 | 8.0 | 14.0 ** |
| LA (cm2) | 10 a | 7 b | 4 c | 4 c | 3 c | 1.15 | 11.27 | 5.6 | 62.6 ** |
| Morphological parameters | Convolvulus arvensis | ||||||||
| RL (cm) | 14 a | 12 b | 11 c | 7 d | 6 e | 0.77 | 4.22 | 10 | 193 ** |
| SL (cm) | 19 a | 15 b | 12 c | 9 d | 7 e | 1.18 | 5.25 | 12.4 | 161 ** |
| RDW (g) | 0.49 a | 0.41 a | 0.29 b | 0.21 b | 0.1 c | 0.10 | 18.52 | 0.30 | 22.9 ** |
| SDW (g) | 1.0 a | 0.7 b | 0.4 c | 0.3 c | 0.2 c | 0.27 | 29.16 | 0.52 | 14 ** |
| NL (per plant) | 14 a | 10 b | 7 bc | 7 bc | 5 c | 3.04 | 19.46 | 8.6 | 13.2 ** |
| LA (cm2) | 4.2 a | 3.0 b | 2.5 bc | 2.3 bc | 1.5 c | 1.04 | 21.15 | 2.7 | 9.16 ** |
| Physiological parameters | Asphodelus tenuifolius | ||||||||
| TFA (mg/g) | 7 c | 8.6 b | 8.6 b | 9.9 a | 8.3 b | 0.41 | 2.65 | 8.5 | 63.4 ** |
| Ca ions (mg/g) | 5 c | 7.4 a | 8 a | 8 a | 6 b | 0.77 | 6.17 | 6.9 | 29.5 ** |
| Physiological parameters | Convolvulus arvensis | ||||||||
| TFA (mg/g) | 6 b | 7.3 a | 7.8 a | 7 ab | 6.8 ab | 1.23 | 9.7 | 7.0 | 2.9 NS |
| Ca ions (mg/g) | 6.5 b | 7.5 a | 7.5 a | 6.5 b | 6.3 b | 0.64 | 5.17 | 6.8 | 8.2 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, M.S.; Naz, N.; Ali, H.; Ali, B.; Akram, M.; Iqbal, R.; Ajmal, S.; Ali, B.; Ercisli, S.; Golokhvast, K.S.; et al. Ultra-Responses of Asphodelus tenuifolius L. (Wild Onion) and Convolvulus arvensis L. (Field Bindweed) against Shoot Extract of Trianthema portulacastrum L. (Horse Purslane). Plants 2023, 12, 458. https://doi.org/10.3390/plants12030458
Hassan MS, Naz N, Ali H, Ali B, Akram M, Iqbal R, Ajmal S, Ali B, Ercisli S, Golokhvast KS, et al. Ultra-Responses of Asphodelus tenuifolius L. (Wild Onion) and Convolvulus arvensis L. (Field Bindweed) against Shoot Extract of Trianthema portulacastrum L. (Horse Purslane). Plants. 2023; 12(3):458. https://doi.org/10.3390/plants12030458
Chicago/Turabian StyleHassan, Muhammad Shahid, Nargis Naz, Habib Ali, Basharat Ali, Muhammad Akram, Rashid Iqbal, Sidra Ajmal, Baber Ali, Sezai Ercisli, Kirill S. Golokhvast, and et al. 2023. "Ultra-Responses of Asphodelus tenuifolius L. (Wild Onion) and Convolvulus arvensis L. (Field Bindweed) against Shoot Extract of Trianthema portulacastrum L. (Horse Purslane)" Plants 12, no. 3: 458. https://doi.org/10.3390/plants12030458
APA StyleHassan, M. S., Naz, N., Ali, H., Ali, B., Akram, M., Iqbal, R., Ajmal, S., Ali, B., Ercisli, S., Golokhvast, K. S., & Hassan, Z. (2023). Ultra-Responses of Asphodelus tenuifolius L. (Wild Onion) and Convolvulus arvensis L. (Field Bindweed) against Shoot Extract of Trianthema portulacastrum L. (Horse Purslane). Plants, 12(3), 458. https://doi.org/10.3390/plants12030458









