Experimental Study on Kinetics and Mechanism of Ciprofloxacin Degradation in Aqueous Phase Using Ag-TiO2/rGO/Halloysite Photocatalyst
Abstract
1. Introduction
2. Results and Discussion
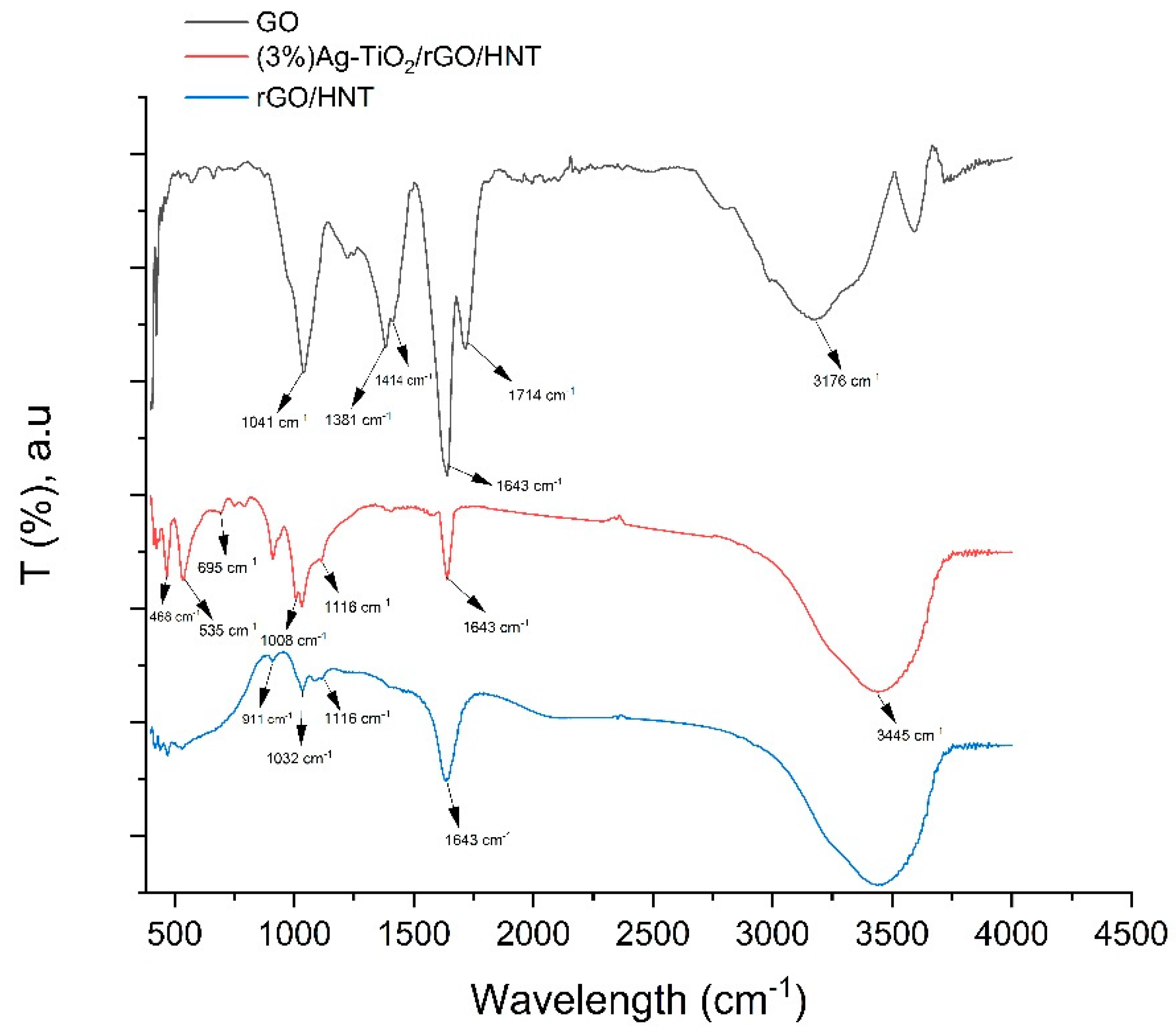
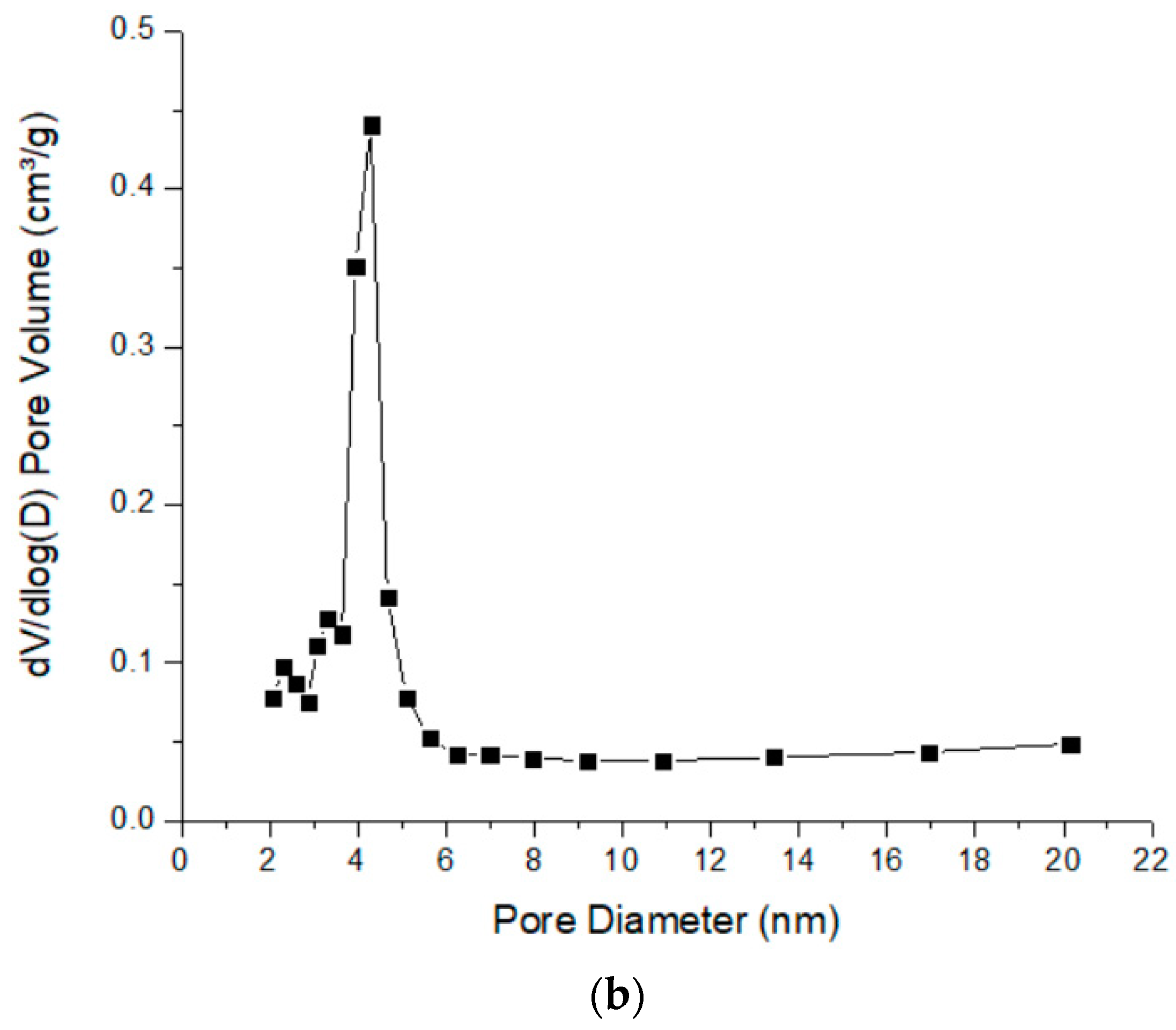
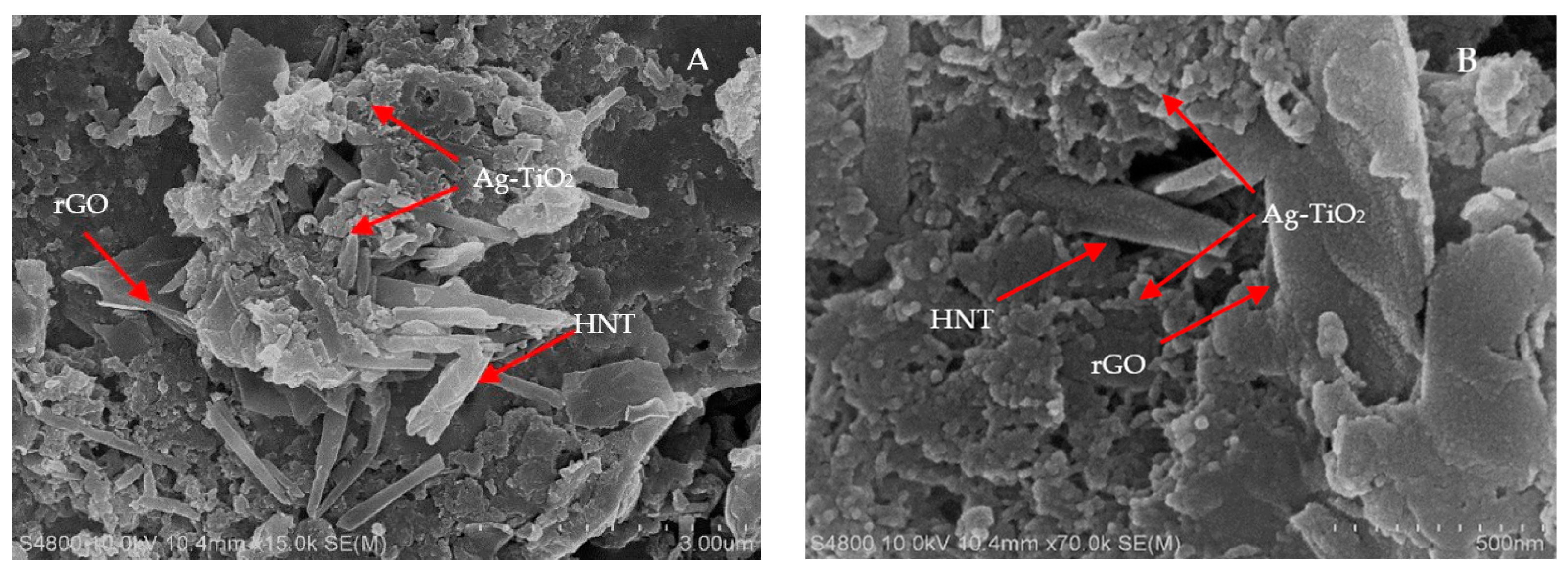
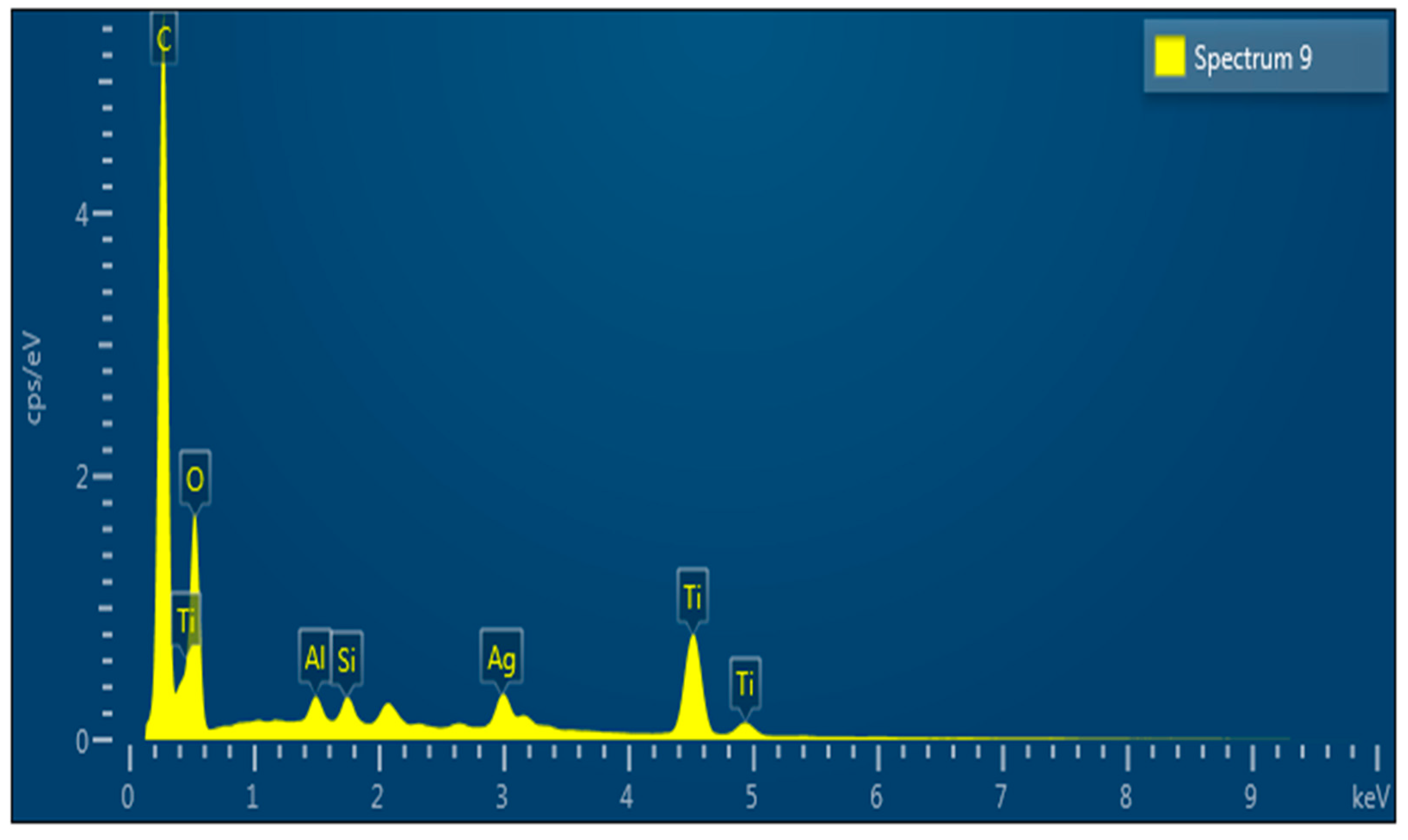
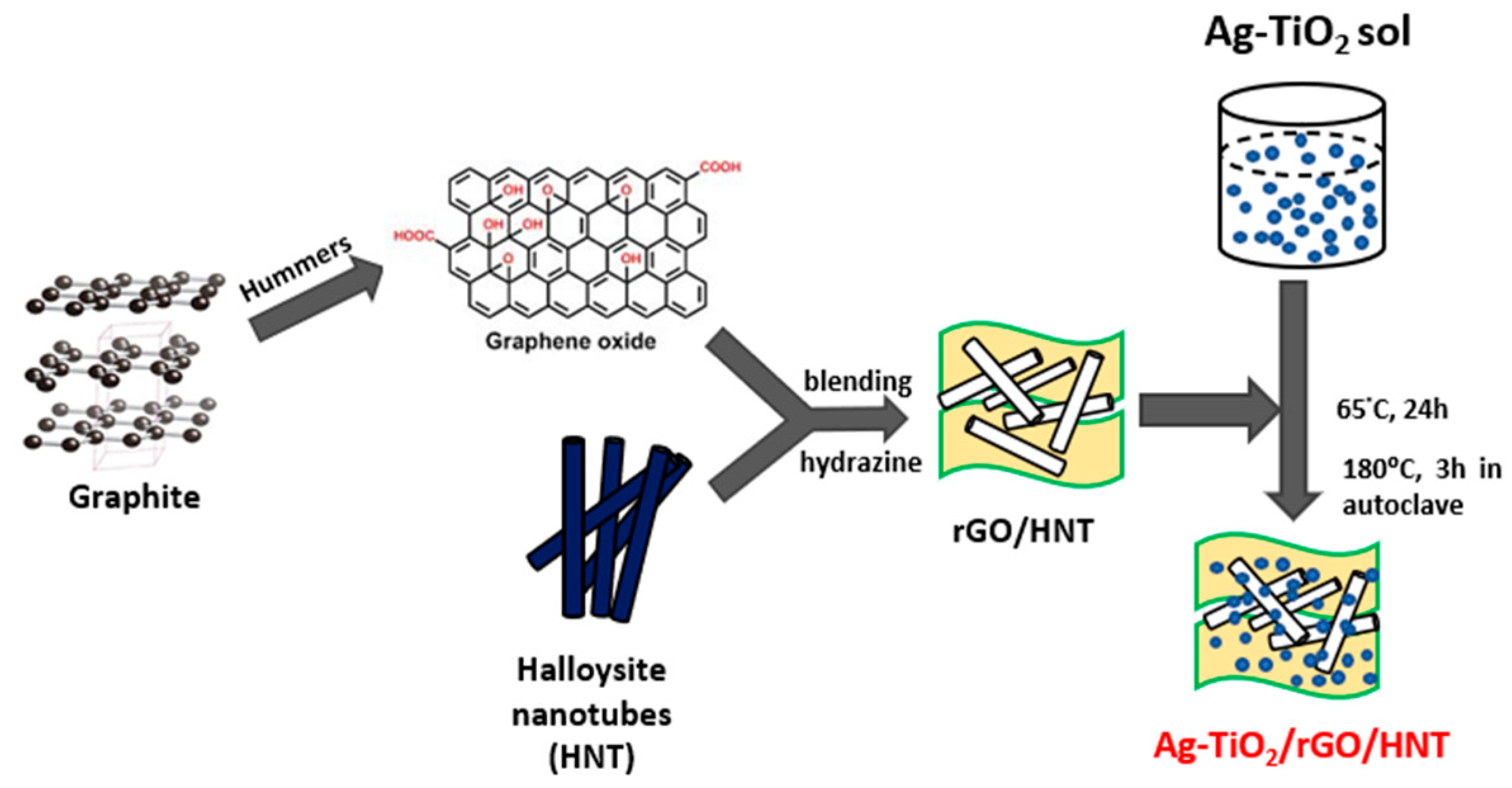
2.1. Material Characterization
2.2. Photocatalytic Activity
2.3. Kinetics of CIP Photodegradation
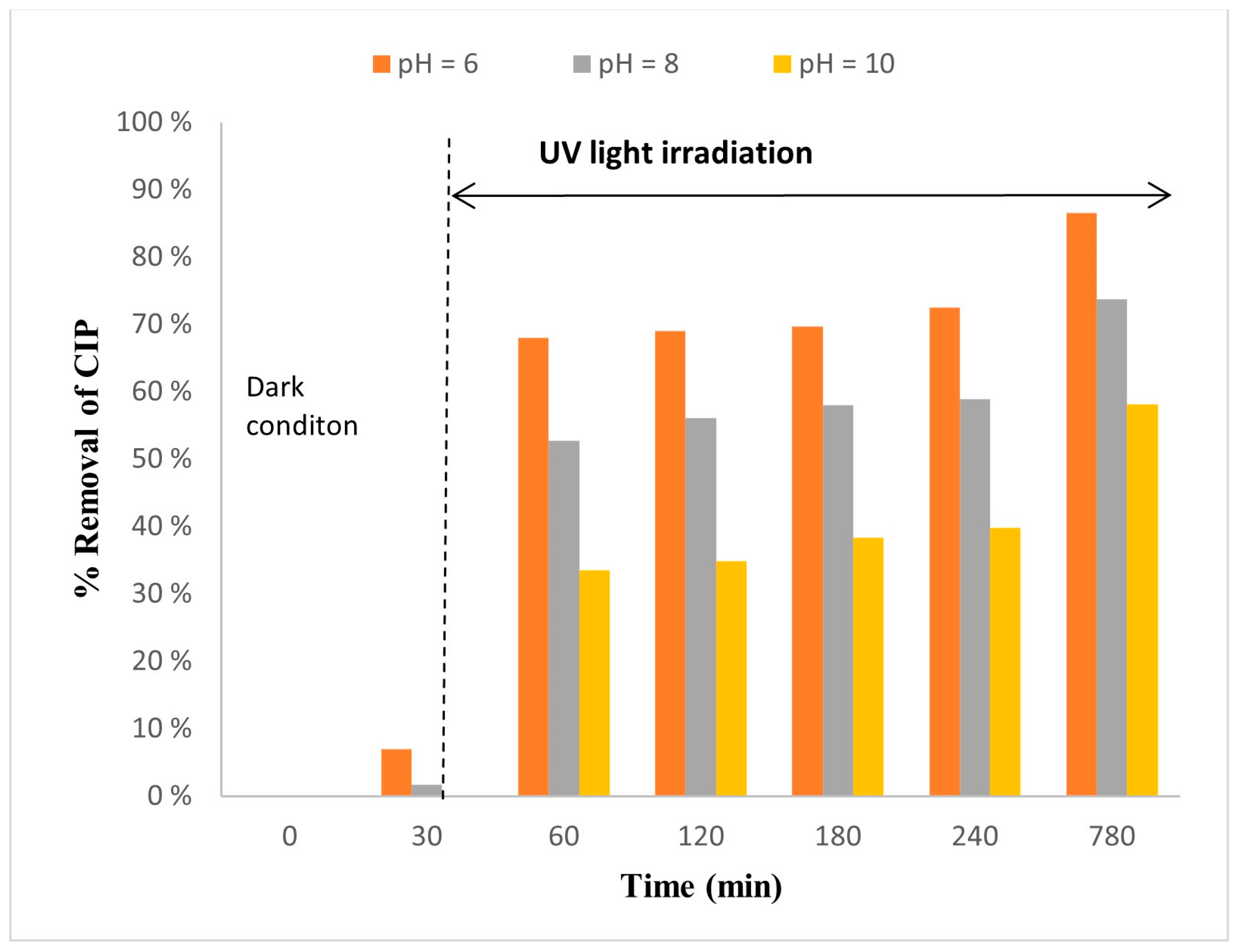
2.4. Effect of pH
2.4.1. Point of Zero Charge (pHPZC) of Ag-TiO2/rGO/HNT
2.4.2. The Influence of pH
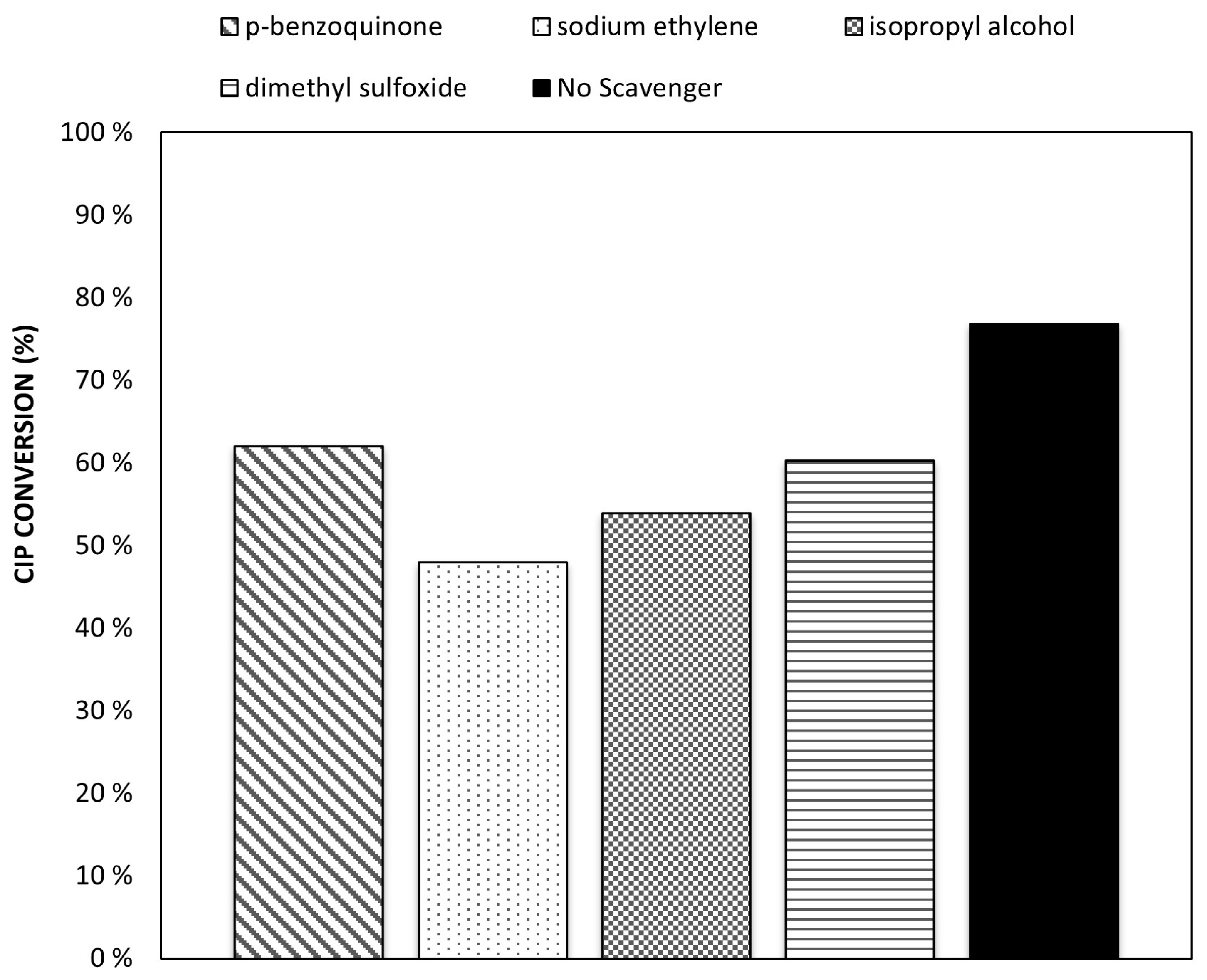
2.5. Scavengers Test
2.6. Mechanism of CIP Photodegradation Reaction Using/rGO/HNT
Conduction Band Energy (ECB) and Valence Band Energy Level (EVB)
2.7. Total Organic Carbon (TOC) Analysis
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of Graphene Oxide
3.3. Halloysite Purification
3.4. Preparation of rGO/HNT
3.5. Preparation of Ag-TiO2/Nanocomposite
3.6. Material Characterizations
3.7. Photocatalytic Activity Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ikehata, K.; Gamal El-Din, M.; Snyder, S.A. Ozonation and Advanced Oxidation Treatment of Emerging Organic Pollutants in Water and Wastewater. Ozone Sci. Eng. 2008, 30, 21–26. [Google Scholar] [CrossRef]
- Watkinson, A.J.; Murby, E.J.; Costanzo, S.D. Removal of Antibiotics in Conventional and Advanced Wastewater Treatment: Implications for Environmental Discharge and Wastewater Recycling. Water Res. 2007, 41, 4164–4176. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, G.; Li, X.; Zou, S.; Li, P.; Hu, Z.; Li, J. Occurrence and Elimination of Antibiotics at Four Sewage Treatment Plants in the Pearl River Delta (PRD), South China. Water Res. 2007, 41, 4526–4534. [Google Scholar] [CrossRef] [PubMed]
- Adebileje, T.; Adebileje, S.; Aye, P.O. Ciprofloxacin Hydrochloride Encapsulated into PLGA Nanoparticles for Drug Delivery Application: Fractional Factorial Design. OALib 2018, 05, 1–14. [Google Scholar] [CrossRef]
- Avisar, D.; Lester, Y.; Mamane, H. PH Induced Polychromatic UV Treatment for the Removal of a Mixture of SMX, OTC and CIP from Water. J. Hazard. Mater. 2010, 175, 1068–1074. [Google Scholar] [CrossRef]
- Koo, Y.; Littlejohn, G.; Collins, B.; Yun, Y.; Shanov, V.N.; Schulz, M.; Pai, D.; Sankar, J. Synthesis and Characterization of Ag–TiO2–CNT Nanoparticle Composites with High Photocatalytic Activity under Artificial Light. Compos. Part B Eng. 2014, 57, 105–111. [Google Scholar] [CrossRef]
- Shi, B.; Yin, H.; Gong, J.; Nie, Q. Ag/AgCl Decorated Bi4Ti3O12 Nanosheet with Highly Exposed (001) Facets for Enhanced Photocatalytic Degradation of Rhodamine B, Carbamazepine and Tetracycline. Appl. Surf. Sci. 2017, 419, 614–623. [Google Scholar] [CrossRef]
- Cui, W.; Li, X.; Gao, C.; Dong, F.; Chen, X. Ternary Ag/AgCl-(BiO)2CO3 Composites as High-Performance Visible-Light Plasmonic Photocatalysts. Catal. Today 2017, 284, 67–76. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, E.; Dai, H.; Kang, L.; Wu, H.; Fan, J.; Hu, X.; Liu, H. Photocatalytic Activity of Ag–TiO2-Graphene Ternary Nanocomposites and Application in Hydrogen Evolution by Water Splitting. Int. J. Hydrogen Energy 2014, 39, 7664–7671. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, F.; Wang, F.; Zhou, B.; Chen, H.; Yuan, R.; Liu, Y.; Chen, Y. Combined the Photocatalysis and Fenton-like Reaction to Efficiently Remove Sulfadiazine in Water Using g-C3N4/Ag/γ-FeOOH: Insights Into the Degradation Pathway From Density Functional Theory. Front. Chem. 2021, 9. [Google Scholar] [CrossRef]
- Gholami, P.; Khataee, A.; Soltani, R.D.C.; Dinpazhoh, L.; Bhatnagar, A. Photocatalytic Degradation of Gemifloxacin Antibiotic Using Zn-Co-LDH@biochar Nanocomposite. J. Hazard. Mater. 2020, 382, 121070. [Google Scholar] [CrossRef] [PubMed]
- Khaledian, H.R.; Zolfaghari, P.; Elhami, V.; Aghbolaghy, M.; Khorram, S.; Karimi, A.; Khataee, A. Modification of Immobilized Titanium Dioxide Nanostructures by Argon Plasma for Photocatalytic Removal of Organic Dyes. Molecules 2019, 24, 383. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.-J.; Niu, C.-G.; Zhang, L.; Liang, C.; Guo, H.; Zeng, G.-M. Photocatalytic Degradation of Ciprofloxacin by a Novel Z-Scheme CeO2–Ag/AgBr Photocatalyst: Influencing Factors, Possible Degradation Pathways, and Mechanism Insight. J. Catal. 2018, 358, 141–154. [Google Scholar] [CrossRef]
- Zhu, L.; Li, H.; Xia, P.; Liu, Z.; Xiong, D. Hierarchical ZnO Decorated with CeO2 Nanoparticles as the Direct Z-Scheme Heterojunction for Enhanced Photocatalytic Activity. ACS Appl. Mater. Interfaces 2018, 10, 39679–39687. [Google Scholar] [CrossRef]
- Syed, A.; Yadav, L.S.R.; Bahkali, A.H.; Elgorban, A.M.; Abdul Hakeem, D.; Ganganagappa, N. Effect of CeO2-ZnO Nanocomposite for Photocatalytic and Antibacterial Activities. Crystals 2020, 10, 817. [Google Scholar] [CrossRef]
- Li, X.; Zhu, W.; Yin, Y.; Lu, X.; Yao, C.; Ni, C. La1−xAgxFeO3/Halloysites Nanocomposite with Enhanced Visible Light Photocatalytic Performance. J. Mater. Sci. Mater. Electron. 2016, 27, 4180–4185. [Google Scholar] [CrossRef]
- Peng, H.; Liu, X.; Tang, W.; Ma, R. Facile Synthesis and Characterization of ZnO Nanoparticles Grown on Halloysite Nanotubes for Enhanced Photocatalytic Properties. Sci. Rep. 2017, 7, 2250. [Google Scholar] [CrossRef]
- Mishra, G.; Mukhopadhyay, M. TiO2 Decorated Functionalized Halloysite Nanotubes (TiO2@HNTs) and Photocatalytic PVC Membranes Synthesis, Characterization and Its Application in Water Treatment. Sci. Rep. 2019, 9, 4345. [Google Scholar] [CrossRef]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.T. 5—Graphene Photocatalysts. In Multifunctional Photocatalytic Materials for Energy; Woodhead Publishing in Materials; Lin, Z., Ye, M., Wang, M., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 79–101. ISBN 978-0-08-101977-1. [Google Scholar]
- Lu, K.-Q.; Li, Y.-H.; Tang, Z.-R.; Xu, Y.-J. Roles of Graphene Oxide in Heterogeneous Photocatalysis. ACS Mater. Au 2021, 1, 37–54. [Google Scholar] [CrossRef]
- Graphene in Photocatalysis: A Review—Li-2016-Small-Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1002/smll.201600382 (accessed on 5 November 2022).
- Sher Shah, M.S.A.; Park, A.R.; Zhang, K.; Park, J.H.; Yoo, P.J. Green Synthesis of Biphasic TiO2–Reduced Graphene Oxide Nanocomposites with Highly Enhanced Photocatalytic Activity. ACS Appl. Mater. Interfaces 2012, 4, 3893–3901. [Google Scholar] [CrossRef]
- Nyankson, E.; Olasehinde, O.; John, V.T.; Gupta, R.B. Surfactant-Loaded Halloysite Clay Nanotube Dispersants for Crude Oil Spill Remediation. Ind. Eng. Chem. Res. 2015, 54, 9328–9341. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Kim, G.-S.; Kim, S.J. Graphene Nanosheets: Ultrasound Assisted Synthesis and Characterization. Ultrason. Sonochem. 2013, 20, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Beranek, R.; Kisch, H. Tuning the Optical and Photoelectrochemical Properties of Surface-Modified TiO2. Photochem. Photobiol. Sci. 2008, 7, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Kozuka, H.; Yoko, T. Photoelectrochemical Properties of Dye-Sensitized TiO2 Films Containing Dispersed Gold Metal Particles Prepared by Sol-Gel Method. J. Ceram. Soc. Jpn. 1996, 104, 164–168. [Google Scholar] [CrossRef]
- Venkatachalam, N.; Palanichamy, M.; Murugesan, V. Sol–Gel Preparation and Characterization of Alkaline Earth Metal Doped Nano TiO2: Efficient Photocatalytic Degradation of 4-Chlorophenol. J. Mol. Catal. Chem. 2007, 273, 177–185. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, H.; Andino, J.M.; Li, Y. Photocatalytic CO2 Reduction with H2O on TiO2 Nanocrystals: Comparison of Anatase, Rutile, and Brookite Polymorphs and Exploration of Surface Chemistry. ACS Catal. 2012, 2, 1817–1828. [Google Scholar] [CrossRef]
- Batzill, M. Fundamental Aspects of Surface Engineering of Transition Metal Oxide Photocatalysts. Energy Environ. Sci. 2011, 4, 3275–3286. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P.; et al. Band Alignment of Rutile and Anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef]
- Xu, M.; Gao, Y.; Moreno, E.M.; Kunst, M.; Muhler, M.; Wang, Y.; Idriss, H.; Wöll, C. Photocatalytic Activity of Bulk TiO2 Anatase and Rutile Single Crystals Using Infrared Absorption Spectroscopy. Phys. Rev. Lett. 2011, 106, 138302. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why Is Anatase a Better Photocatalyst than Rutile?—Model Studies on Epitaxial TiO2 Films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New Understanding of the Difference of Photocatalytic Activity among Anatase, Rutile and Brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef]
- Prieto-Mahaney, O.-O.; Murakami, N.; Abe, R.; Ohtani, B. Correlation between Photocatalytic Activities and Structural and Physical Properties of Titanium(IV) Oxide Powders. Chem. Lett. 2009, 38, 238–239. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, Y.; Zhou, B.; Wei, Z.; Zhu, Z.; Han, L.; Zhang, P.; Hu, Y. Preparation and Photocatalytic Performance of Silver-Modified and Nitrogen-Doped TiO2 Nanomaterials with Oxygen Vacancies. New J. Chem. 2021, 45, 4694–4704. [Google Scholar] [CrossRef]
- Grabowska, E.; Zaleska, A.; Sorgues, S.; Kunst, M.; Etcheberry, A.; Colbeau-Justin, C.; Remita, H. Modification of Titanium(IV) Dioxide with Small Silver Nanoparticles: Application in Photocatalysis. J. Phys. Chem. C 2013, 117, 1955–1962. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Oba, S.N.; Aniagor, C.O.; Adeniyi, A.G.; Ighalo, J.O. Adsorption of Ciprofloxacin from Water: A Comprehensive Review. J. Ind. Eng. Chem. 2021, 93, 57–77. [Google Scholar] [CrossRef]
- Guo, J.; Ma, D.; Sun, F.; Zhuang, G.; Wang, Q.; Al-Enizi, A.M.; Nafady, A.; Ma, S. Substituent Engineering in G-C3N4/COF Heterojunctions for Rapid Charge Separation and High Photo-Redox Activity. Sci. China Chem. 2022, 65, 1704–1709. [Google Scholar] [CrossRef]
- Song, S.; Wu, K.; Wu, H.; Guo, J.; Zhang, L. Synthesis of Z-Scheme Multi-Shelled ZnO/AgVO3 Spheres as Photocatalysts for the Degradation of Ciprofloxacin and Reduction of Chromium(VI). J. Mater. Sci. 2020, 55, 4987–5007. [Google Scholar] [CrossRef]
- Blondel, C.; Delsart, C.; Valli, C.; Yiou, S.; Godefroid, M.R.; Van Eck, S. Electron Affinities of 16 O, 17 O, 18 O, the fine structure of 16O−, and the hyperfine structure of 17O−. Phys. Rev. A 2001, 64, 052504. [Google Scholar] [CrossRef]
- Tang, R.; Fu, X.; Ning, C. Accurate Electron Affinity of Ti and Fine Structures of Its Anions. J. Chem. Phys. 2018, 149, 134304. [Google Scholar] [CrossRef]
- High-Precision Electron Affinity of Oxygen/Nature Communications. Available online: https://www.nature.com/articles/s41467-022-33438-y (accessed on 10 January 2023).
- Lide, D.R. CRC Handbook of Chemistry and Physics, 84th Edition Edited by David R. Lide (National Institute of Standards and Technology). J. Am. Chem. Soc. 2004, 126, 1586. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Yao, H.; Dang, L.; Li, Z. Efficient Decomposition of Organic Compounds and Reaction Mechanism with BiOI Photocatalyst under Visible Light Irradiation. J. Mol. Catal. Chem. 2011, 334, 116–122. [Google Scholar] [CrossRef]
- Fu, Y.; Tan, M.; Guo, Z.; Hao, D.; Xu, Y.; Du, H.; Zhang, C.; Guo, J.; Li, Q.; Wang, Q. Fabrication of Wide-Spectra-Responsive NA/NH2-MIL-125(Ti) with Boosted Activity for Cr(VI) Reduction and Antibacterial Effects. Chem. Eng. J. 2023, 452, 139417. [Google Scholar] [CrossRef]
- Borgarello, E.; Harris, R.; Serpone, N. Photochemical Deposition and Photorecovery of Gold Using Semiconductor Dispersions. A Practical Application of Photocatalysis. Photochem. Depos. Photorecovery Gold Using Semicond. Dispers. Pract. Appl. Photocatal. 1985, 9, 743–747. [Google Scholar]
- Kraeutler, B.; Bard, A.J. Heterogeneous Photocatalytic Preparation of Supported Catalysts. Photodeposition of Platinum on Titanium Dioxide Powder and Other Substrates. J. Am. Chem. Soc. 1978, 100, 4317–4318. [Google Scholar] [CrossRef]
- Herrmann, J.-M.; Disdier, J.; Pichat, P. Photocatalytic Deposition of Silver on Powder Titania: Consequences for the Recovery of Silver. J. Catal. 1988, 113, 72–81. [Google Scholar] [CrossRef]
- Choi, W.; Termin, A.; Hoffmann, M.R. The Role of Metal Ion Dopants in Quantum-Sized TiO2: Correlation between Photoreactivity and Charge Carrier Recombination Dynamics. J. Phys. Chem. 1994, 98, 13669–13679. [Google Scholar] [CrossRef]
- Subramanian, V.; Wolf, E.; Kamat, P.V. Semiconductor−Metal Composite Nanostructures. to What Extent Do Metal Nanoparticles Improve the Photocatalytic Activity of TiO2 Films? J. Phys. Chem. B 2001, 105, 11439–11446. [Google Scholar] [CrossRef]
- Li, X.Z.; Li, F.B. Study of Au/Au3+-TiO2 Photocatalysts toward Visible Photooxidation for Water and Wastewater Treatment. Environ. Sci. Technol. 2001, 35, 2381–2387. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Nasiruddin Khan, M.; Sarwar, A. Determination of Points of Zero Charge of Natural and Treated Adsorbents. Surf. Rev. Lett. 2007, 14, 461–469. [Google Scholar] [CrossRef]




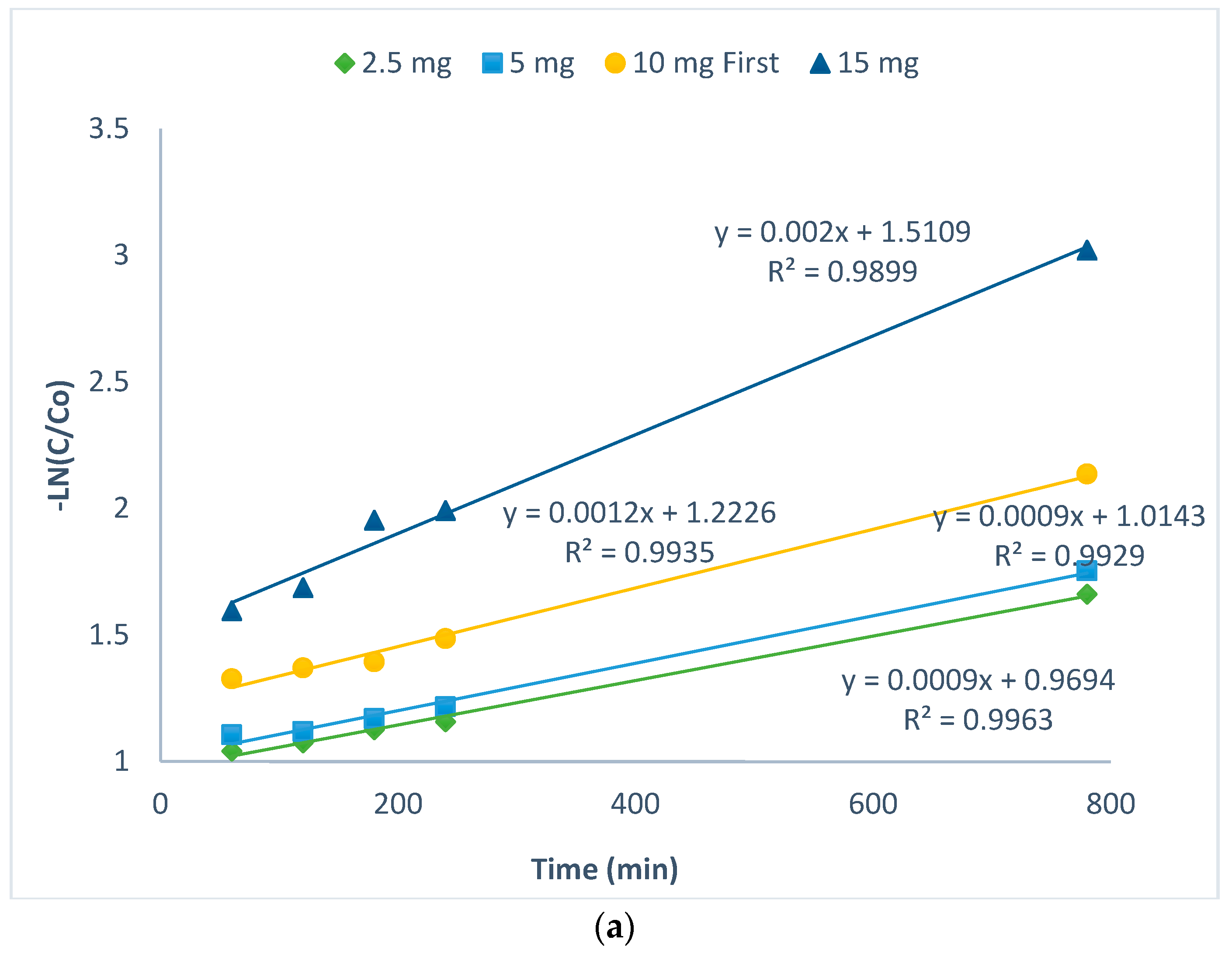



| Pseudo First Order | Pseudo Second Order | |||||
|---|---|---|---|---|---|---|
| Sample | Linear equation | k | R2 | Linear equation | k | R2 |
| 2.5 mg | y = 0.0004x + 1.0018 | 0.0004 | 0.989 | y = 7 × 10−5x + 0.0849 | 0.00007 | 0.9933 |
| 5 mg | y = 0.0007x + 0.9848 | 0.0007 | 0.9991 | y = 0.0001x + 0.0802 | 0.0001 | 0.9967 |
| 10 mg | y = 0.0013x + 1.4992 | 0.0013 | 0.9665 | y = 0.0003x + 0.1088 | 0.0003 | 0.9908 |
| 15 mg | y = 0.0011x + 1.3574 | 0.0011 | 0.9945 | y = 0.0004x + 0.1279 | 0.0004 | 0.9972 |
| Initial pH | 0 | 5.07 | 7.08 | 8.99 | 10.97 |
| Final pH | 0 | 7.01 | 7.84 | 8.02 | 10.66 |
| Ag (eV) | Ti (eV) | O (eV) | |
|---|---|---|---|
| EIE | 7.6 | 6.82 | 13.62 |
| EEA | 1.3 | 0.075 | 1.461 |
| (1%)Ag-TiO2 | (3%)Ag-TiO2 | TiO2 | |
|---|---|---|---|
| ECB | −0.445 | −0.43 | −0.3 |
| EVB | 2.165 | 2.32 | 2.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngo, H.-S.; Nguyen, T.-L.; Tran, N.-T.; Le, H.-C. Experimental Study on Kinetics and Mechanism of Ciprofloxacin Degradation in Aqueous Phase Using Ag-TiO2/rGO/Halloysite Photocatalyst. Catalysts 2023, 13, 225. https://doi.org/10.3390/catal13020225
Ngo H-S, Nguyen T-L, Tran N-T, Le H-C. Experimental Study on Kinetics and Mechanism of Ciprofloxacin Degradation in Aqueous Phase Using Ag-TiO2/rGO/Halloysite Photocatalyst. Catalysts. 2023; 13(2):225. https://doi.org/10.3390/catal13020225
Chicago/Turabian StyleNgo, Ha-Son, Thi-Linh Nguyen, Ngoc-Tuan Tran, and Hanh-Chi Le. 2023. "Experimental Study on Kinetics and Mechanism of Ciprofloxacin Degradation in Aqueous Phase Using Ag-TiO2/rGO/Halloysite Photocatalyst" Catalysts 13, no. 2: 225. https://doi.org/10.3390/catal13020225
APA StyleNgo, H.-S., Nguyen, T.-L., Tran, N.-T., & Le, H.-C. (2023). Experimental Study on Kinetics and Mechanism of Ciprofloxacin Degradation in Aqueous Phase Using Ag-TiO2/rGO/Halloysite Photocatalyst. Catalysts, 13(2), 225. https://doi.org/10.3390/catal13020225






