Stem Cell Therapy for Post-Traumatic Stress Disorder: A Novel Therapeutic Approach
Abstract
1. Introduction
2. Stem Cell Therapy: Terminology and Cell Lines
2.1. Overview
2.2. Human Pluripotent Stem Cells (hPSCs)
2.3. Fetal-Derived Neural Progenitor Stem Cells
2.4. Mesenchymal Stem Cells
3. Epilepsy
3.1. Overview
3.2. Mechanism
3.3. Stem Cell Therapy for Epilepsy
4. PTSD
4.1. Overview
4.2. Prevalence of PTSD and Treatment-Resistant PTSD
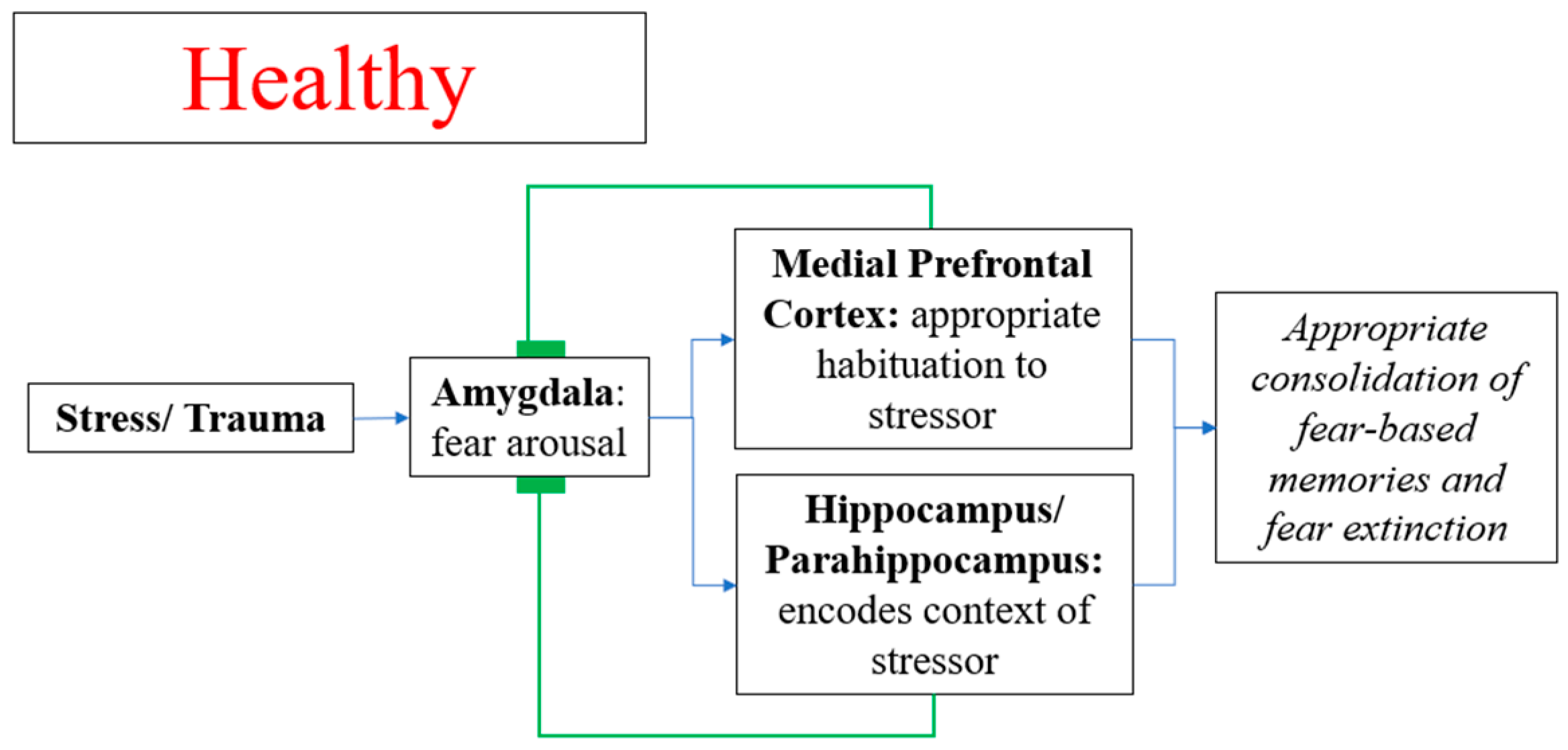
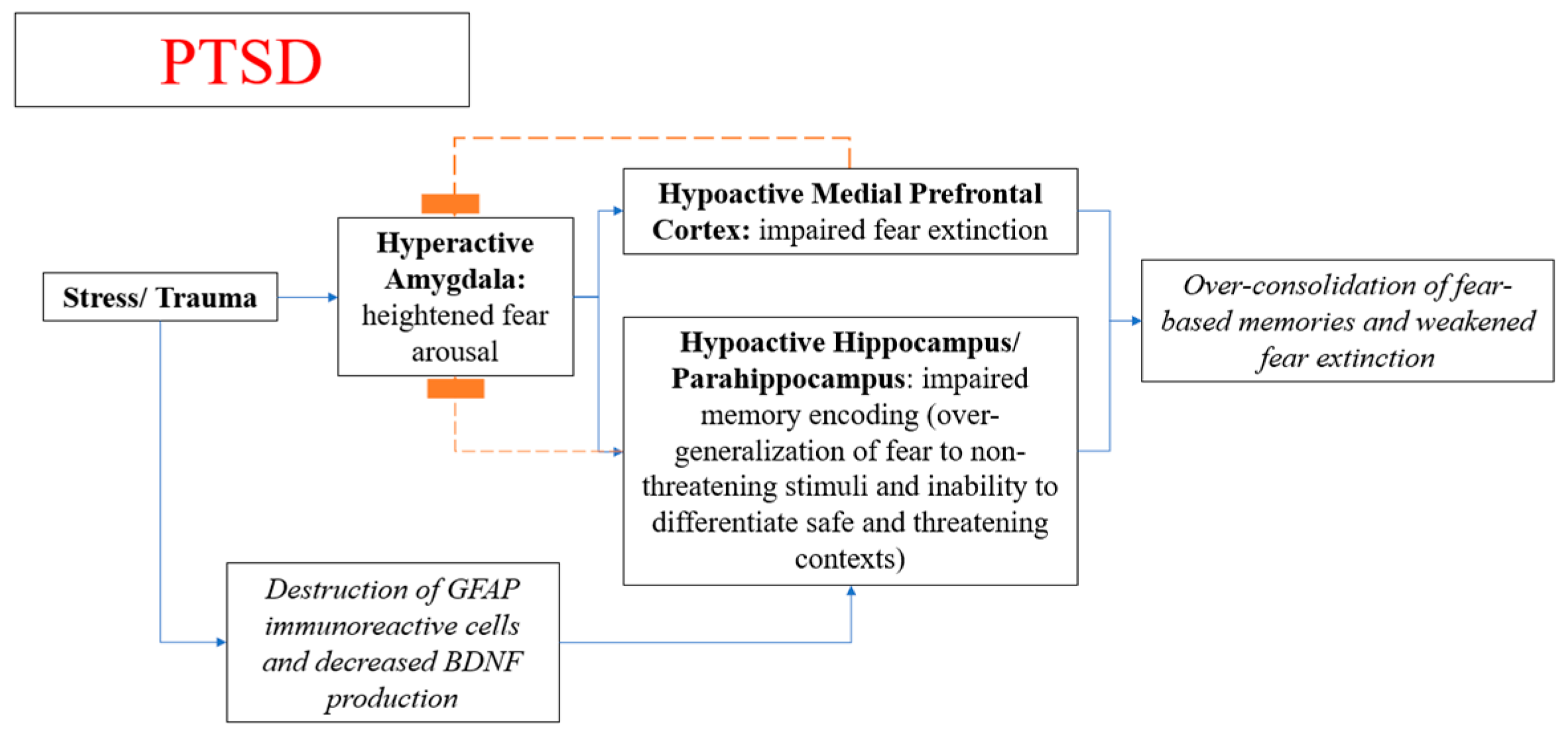
4.3. Neuroanatomy and Pathophysiological Mechanism of PTSD
4.4. Shortcomings of Current Treatments
4.5. Similarities of PTSD to Epilepsy
4.6. Behavioral Sensitization and Electrophysiological Kindling
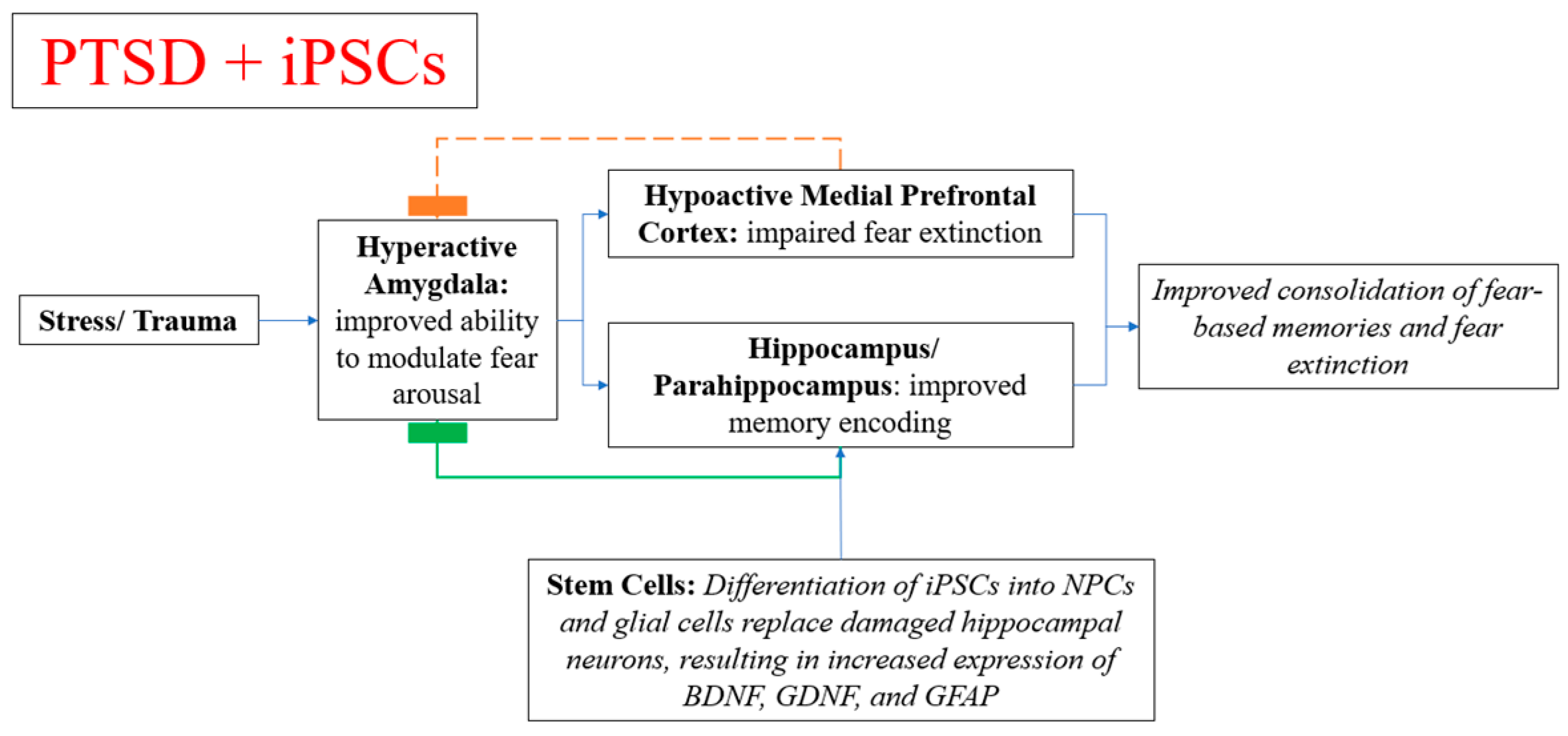
4.7. Stem Cell Therapy in Memory Symptoms, Neuropsychiatric Disorders, and PTSD
5. Conclusions: Stem Cell Therapy for PTSD
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bonanno, G.A. Loss, trauma, and human resilience: Have we underestimated the human capacity to thrive after extremely aversive events? Am. Psychol. 2004, 59, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Dadic-Hero, E.; Toric, I.; Ruzic, K.; Medved, P.; Graovac, M. Comorbidity—A troublesome factor in PTSD treatment. Psychiatr. Danub. 2009, 21, 420–424. [Google Scholar]
- Alonso, J.; Angermeyer, M.C.; Lepine, J.P. European Study of the Epidemiology of Mental Disorders P. The European Study of the Epidemiology of Mental Disorders (ESEMeD) project: An epidemiological basis for informing mental health policies in Europe. Acta Psychiatr. Scand. 2004, 109 (Suppl. 420), 5–7. [Google Scholar] [CrossRef] [PubMed]
- Steel, Z.; Chey, T.; Silove, D.; Marnane, C.; Bryant, R.A.; van Ommeren, M. Association of torture and other potentially traumatic events with mental health outcomes among populations exposed to mass conflict and displacement: A systematic review and meta-analysis. JAMA 2009, 302, 537–549. [Google Scholar] [CrossRef]
- Ferry, F.R.; Brady, S.E.; Bunting, B.P.; Murphy, S.D.; Bolton, D.; O’Neill, S.M. The Economic Burden of PTSD in Northern Ireland. J. Trauma. Stress 2015, 28, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Bisson, J.; Andrew, M. Psychological treatment of post-traumatic stress disorder (PTSD). Cochrane Database Syst. Rev. 2007, CD003388. [Google Scholar]
- Hoskins, M.; Pearce, J.; Bethell, A.; Dankova, L.; Barbui, C.; Tol, W.A. Pharmacotherapy for post-traumatic stress disorder: Systematic review and meta-analysis. Br. J. Psychiatry 2015, 206, 93–100. [Google Scholar] [CrossRef]
- Miller, D.D.; Caroff, S.N.; Davis, S.M.; Rosenheck, R.A.; McEvoy, J.P.; Saltz, B.L. Extrapyramidal side-effects of antipsychotics in a randomised trial. Br. J. Psychiatry 2008, 193, 279–288. [Google Scholar] [CrossRef]
- Maneshi, M.; Vahdat, S.; Fahoum, F.; Grova, C.; Gotman, J. Specific resting-state brain networks in mesial temporal lobe epilepsy. Front. Neurol. 2014, 5, 127. [Google Scholar] [CrossRef] [PubMed]
- Boccia, M.; D’Amico, S.; Bianchini, F.; Marano, A.; Giannini, A.M.; Piccardi, L. Different neural modifications underpin PTSD after different traumatic events: An fMRI meta-analytic study. Brain Imaging Behav. 2016, 10, 226–237. [Google Scholar] [CrossRef]
- Cisler, J.M.; Steele, J.S.; Lenow, J.K.; Smitherman, S.; Everett, B.; Messias, E. Functional reorganization of neural networks during repeated exposure to the traumatic memory in posttraumatic stress disorder: An exploratory fMRI study. J. Psychiatr. Res. 2014, 48, 47–55. [Google Scholar] [CrossRef]
- Fahoum, F.; Lopes, R.; Pittau, F.; Dubeau, F.; Gotman, J. Widespread epileptic networks in focal epilepsies: EEG-fMRI study. Epilepsia 2012, 53, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Fiddick, L. There is more than the amygdala: Potential threat assessment in the cingulate cortex. Neurosci. Biobehav. Rev. 2011, 35, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Thijs, R.D.; Surges, R.; O’Brien, T.J.; Sander, J.W. Epilepsy in adults. Lancet 2019, 393, 689–701. [Google Scholar] [CrossRef]
- Jafarian, M.; Modarres Mousavi, S.M.; Alipour, F.; Aligholi, H.; Noorbakhsh, F.; Ghadipasha, M. Cell injury and receptor expression in the epileptic human amygdala. Neurobiol. Dis. 2019, 124, 416–427. [Google Scholar] [CrossRef]
- Ali, S.O.; Shahin, N.N.; Safar, M.M.; Rizk, S.M. Therapeutic potential of endothelial progenitor cells in a rat model of epilepsy: Role of autophagy. J. Adv. Res. 2019, 18, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Milczarek, O.; Jarocha, D.; Starowicz-Filip, A.; Kwiatkowski, S.; Badyra, B.; Majka, M. Multiple Autologous Bone Marrow-Derived CD271(+) Mesenchymal Stem Cell Transplantation Overcomes Drug-Resistant Epilepsy in Children. Stem Cells Transl. Med. 2018, 7, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Ruschenschmidt, C.; Koch, P.G.; Brustle, O.; Beck, H. Functional properties of ES cell-derived neurons engrafted into the hippocampus of adult normal and chronically epileptic rats. Epilepsia 2005, 46 (Suppl. 5), 174–183. [Google Scholar] [CrossRef]
- Trounson, A.; McDonald, C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 2015, 17, 11–22. [Google Scholar] [CrossRef]
- Huang, H.; Young, W.; Chen, L.; Feng, S.; Zoubi, Z.M.A.; Sharma, H.S. Clinical Cell Therapy Guidelines for Neurorestoration (IANR/CANR 2017). Cell Transplant 2018, 27, 310–324. [Google Scholar] [CrossRef] [PubMed]
- Daley, G.Q.; Hyun, I.; Apperley, J.F.; Barker, R.A.; Benvenisty, N.; Bredenoord, A.L. Setting Global Standards for Stem Cell Research and Clinical Translation: The 2016 ISSCR Guidelines. Stem Cell Rep. 2016, 6, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Salgado, A.J.; Sousa, J.C.; Costa, B.M.; Pires, A.O.; Mateus-Pinheiro, A.; Teixeira, F.G. Mesenchymal stem cells secretome as a modulator of the neurogenic niche: Basic insights and therapeutic opportunities. Front. Cell. Neurosci. 2015, 9, 249. [Google Scholar] [CrossRef] [PubMed]
- Fox, I.J.; Daley, G.Q.; Goldman, S.A.; Huard, J.; Kamp, T.J.; Trucco, M. Stem cell therapy. Use of differentiated pluripotent stem cells as replacement therapy for treating disease. Science 2014, 345, 1247391. [Google Scholar] [CrossRef]
- Huang, L.; Zhou, X.; Tang, S. Optimization of frequency-doubled Er-doped fiber laser for miniature multiphoton endoscopy. J. Biomed. Opt. 2018, 23, 126503. [Google Scholar] [CrossRef]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Simonson, O.E.; Domogatskaya, A.; Volchkov, P.; Rodin, S. The safety of human pluripotent stem cells in clinical treatment. Ann. Med. 2015, 47, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Grealish, S.; Diguet, E.; Kirkeby, A.; Mattsson, B.; Heuer, A.; Bramoulle, Y. Human ESC-derived dopamine neurons show similar preclinical efficacy and potency to fetal neurons when grafted in a rat model of Parkinson’s disease. Cell Stem Cell 2014, 15, 653–665. [Google Scholar] [CrossRef]
- Lukovic, D.; Valdes-Sanchez, L.; Sanchez-Vera, I.; Moreno-Manzano, V.; Stojkovic, M.; Bhattacharya, S.S. Brief report: Astrogliosis promotes functional recovery of completely transected spinal cord following transplantation of hESC-derived oligodendrocyte and motoneuron progenitors. Stem Cells 2014, 32, 594–599. [Google Scholar] [CrossRef]
- Perrier, A.; Peschanski, M. How can human pluripotent stem cells help decipher and cure Huntington’s disease? Cell Stem Cell 2012, 11, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, Z.S.; Talib, S.; Feigal, E.G. The potential for immunogenicity of autologous induced pluripotent stem cell-derived therapies. J. Biol. Chem. 2014, 289, 4571–4577. [Google Scholar] [CrossRef] [PubMed]
- Irion, S.; Zabierowski, S.E.; Tomishima, M.J. Bringing Neural Cell Therapies to the Clinic: Past and Future Strategies. Mol. Ther. Methods Clin. Dev. 2017, 4, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Merkle, F.T.; Maroof, A.; Wataya, T.; Sasai, Y.; Studer, L.; Eggan, K. Generation of neuropeptidergic hypothalamic neurons from human pluripotent stem cells. Development 2015, 142, 633–643. [Google Scholar] [CrossRef]
- Shi, Y.; Kirwan, P.; Livesey, F.J. Directed differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. Nat. Protoc. 2012, 7, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, M.W.; Croft, G.F.; Williams, D.J.; O’Keeffe, S.; Carrasco, M.A.; Davis, A.R. Accelerated high-yield generation of limb-innervating motor neurons from human stem cells. J. Neurosci. 2013, 33, 574–586. [Google Scholar] [CrossRef]
- Kefalopoulou, Z.; Politis, M.; Piccini, P.; Mencacci, N.; Bhatia, K.; Jahanshahi, M. Long-term clinical outcome of fetal cell transplantation for Parkinson disease: Two case reports. JAMA Neurol. 2014, 71, 83–87. [Google Scholar] [CrossRef]
- Iwanami, A.; Kaneko, S.; Nakamura, M.; Kanemura, Y.; Mori, H.; Kobayashi, S. Transplantation of human neural stem cells for spinal cord injury in primates. J. Neurosci. Res. 2005, 80, 182–190. [Google Scholar] [CrossRef]
- Deng, X.; Liang, Y.; Lu, H.; Yang, Z.; Liu, R.; Wang, J. Co-transplantation of GDNF-overexpressing neural stem cells and fetal dopaminergic neurons mitigates motor symptoms in a rat model of Parkinson’s disease. PLoS ONE 2013, 8, e80880. [Google Scholar] [CrossRef] [PubMed]
- Bachoud-Levi, A.C.; Perrier, A.L. Regenerative medicine in Huntington’s disease: Current status on fetal grafts and prospects for the use of pluripotent stem cell. Rev. Neurol. (Paris) 2014, 170, 749–762. [Google Scholar] [CrossRef]
- Gao, J.; Prough, D.S.; McAdoo, D.J.; Grady, J.J.; Parsley, M.O.; Ma, L. Transplantation of primed human fetal neural stem cells improves cognitive function in rats after traumatic brain injury. Exp. Neurol. 2006, 201, 281–292. [Google Scholar] [CrossRef]
- Sakata, H.; Niizuma, K.; Yoshioka, H.; Kim, G.S.; Jung, J.E.; Katsu, M. Minocycline-preconditioned neural stem cells enhance neuroprotection after ischemic stroke in rats. J. Neurosci. 2012, 32, 3462–3473. [Google Scholar] [CrossRef]
- Glass, J.D.; Boulis, N.M.; Johe, K.; Rutkove, S.B.; Federici, T.; Polak, M. Lumbar intraspinal injection of neural stem cells in patients with amyotrophic lateral sclerosis: Results of a phase I trial in 12 patients. Stem Cells 2012, 30, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.C.; Kim, K.N.; Yoo, J.; Kim, I.S.; Yun, S.; Lee, H. Clinical Trial of Human Fetal Brain-Derived Neural Stem/Progenitor Cell Transplantation in Patients with Traumatic Cervical Spinal Cord Injury. Neural Plast. 2015, 2015, 630932. [Google Scholar] [CrossRef] [PubMed]
- Kalladka, D.; Sinden, J.; Pollock, K.; Haig, C.; McLean, J.; Smith, W. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): A phase 1, first-in-man study. Lancet 2016, 388, 787–796. [Google Scholar] [CrossRef]
- Neirinckx, V.; Coste, C.; Rogister, B.; Wislet-Gendebien, S. Concise review: Adult mesenchymal stem cells, adult neural crest stem cells, and therapy of neurological pathologies: A state of play. Stem Cells Transl. Med. 2013, 2, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Li, X.L.; Xue, Y.; Zhang, C.X.; Wang, Y.; Hu, X. Bone marrow cells differentiation into organ cells using stem cell therapy. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2899–2907. [Google Scholar] [PubMed]
- Petrou, P.; Gothelf, Y.; Argov, Z.; Gotkine, M.; Levy, Y.S.; Kassis, I. Safety and Clinical Effects of Mesenchymal Stem Cells Secreting Neurotrophic Factor Transplantation in Patients With Amyotrophic Lateral Sclerosis: Results of Phase 1/2 and 2a Clinical Trials. JAMA Neurol. 2016, 73, 337–344. [Google Scholar] [CrossRef]
- Diez-Tejedor, E.; Gutierrez-Fernandez, M.; Martinez-Sanchez, P.; Rodriguez-Frutos, B.; Ruiz-Ares, G.; Lara, M.L. Reparative therapy for acute ischemic stroke with allogeneic mesenchymal stem cells from adipose tissue: A safety assessment: A phase II randomized, double-blind, placebo-controlled, single-center, pilot clinical trial. J. Stroke Cerebrovasc. Dis. 2014, 23, 2694–2700. [Google Scholar] [CrossRef] [PubMed]
- Satti, H.S.; Waheed, A.; Ahmed, P.; Ahmed, K.; Akram, Z.; Aziz, T. Autologous mesenchymal stromal cell transplantation for spinal cord injury: A Phase I pilot study. Cytotherapy 2016, 18, 518–522. [Google Scholar] [CrossRef]
- Dahbour, S.; Jamali, F.; Alhattab, D.; Al-Radaideh, A.; Ababneh, O.; Al-Ryalat, N. Mesenchymal stem cells and conditioned media in the treatment of multiple sclerosis patients: Clinical, ophthalmological and radiological assessments of safety and efficacy. CNS Neurosci. Ther. 2017, 23, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cheng, H.; Dai, G.; Wang, X.; Hua, R.; Liu, X. Umbilical cord mesenchymal stem cell transplantation significantly improves neurological function in patients with sequelae of traumatic brain injury. Brain Res. 2013, 1532, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Hlebokazov, F.; Dakukina, T.; Ihnatsenko, S.; Kosmacheva, S.; Potapnev, M.; Shakhbazau, A. Treatment of refractory epilepsy patients with autologous mesenchymal stem cells reduces seizure frequency: An open label study. Adv. Med. Sci. 2017, 62, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Lalu, M.M.; McIntyre, L.; Pugliese, C.; Fergusson, D.; Winston, B.W.; Marshall, J.C. Safety of cell therapy with mesenchymal stromal cells (SafeCell): A systematic review and meta-analysis of clinical trials. PLoS ONE 2012, 7, e47559. [Google Scholar] [CrossRef] [PubMed]
- Thurman, D.J.; Begley, C.E.; Carpio, A.; Helmers, S.; Hesdorffer, D.C.; Mu, J. The primary prevention of epilepsy: A report of the Prevention Task Force of the International League Against Epilepsy. Epilepsia 2018, 59, 905–914. [Google Scholar] [CrossRef]
- Sander, J.W.; Hart, Y.M.; Johnson, A.L.; Shorvon, S.D. National General Practice Study of Epilepsy: Newly diagnosed epileptic seizures in a general population. Lancet 1990, 336, 1267–1271. [Google Scholar] [CrossRef]
- Sepkuty, J.P.; Cohen, A.S.; Eccles, C.; Rafiq, A.; Behar, K.; Ganel, R. A neuronal glutamate transporter contributes to neurotransmitter GABA synthesis and epilepsy. J. Neurosci. 2002, 22, 6372–6379. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, W.; Pozzo-Miller, L.D.; Figurov, A.; Lu, B. Presynaptic modulation of synaptic transmission and plasticity by brain-derived neurotrophic factor in the developing hippocampus. J. Neurosci. 1998, 18, 6830–6839. [Google Scholar] [CrossRef] [PubMed]
- Steffens, M.; Huppertz, H.J.; Zentner, J.; Chauzit, E.; Feuerstein, T.J. Unchanged glutamine synthetase activity and increased NMDA receptor density in epileptic human neocortex: Implications for the pathophysiology of epilepsy. Neurochem. Int. 2005, 47, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Norwood, B.A.; Bumanglag, A.V.; Osculati, F.; Sbarbati, A.; Marzola, P.; Nicolato, E. Classic hippocampal sclerosis and hippocampal-onset epilepsy produced by a single “cryptic” episode of focal hippocampal excitation in awake rats. J. Comp. Neurol. 2010, 518, 3381–3407. [Google Scholar] [CrossRef]
- Mathern, G.W.; Babb, T.L.; Vickrey, B.G.; Melendez, M.; Pretorius, J.K. The clinical-pathogenic mechanisms of hippocampal neuron loss and surgical outcomes in temporal lobe epilepsy. Brain 1995, 118, 105–118. [Google Scholar] [CrossRef]
- Cendes, F.; Leproux, F.; Melanson, D.; Ethier, R.; Evans, A.; Peters, T. MRI of amygdala and hippocampus in temporal lobe epilepsy. J. Comput. Assist. Tomogr. 1993, 17, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Kienzler, F.; Norwood, B.A.; Sloviter, R.S. Hippocampal injury, atrophy, synaptic reorganization, and epileptogenesis after perforant pathway stimulation-induced status epilepticus in the mouse. J. Comp. Neurol. 2009, 515, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Bertram, E.H. Temporal lobe epilepsy: Where do the seizures really begin? Epilepsy Behav. 2009, 14 (Suppl. 1), 32–37. [Google Scholar] [CrossRef] [PubMed]
- Parrent, A.G.; Blume, W.T. Stereotactic amygdalohippocampotomy for the treatment of medial temporal lobe epilepsy. Epilepsia 1999, 40, 1408–1416. [Google Scholar] [CrossRef]
- Robbins, R.J.; Brines, M.L.; Kim, J.H.; Adrian, T.; de Lanerolle, N.; Welsh, S. A selective loss of somatostatin in the hippocampus of patients with temporal lobe epilepsy. Ann. Neurol. 1991, 29, 325–332. [Google Scholar] [CrossRef]
- Levesque, M.; Avoli, M. The kainic acid model of temporal lobe epilepsy. Neurosci. Biobehav. Rev. 2013, 37, 2887–2899. [Google Scholar] [CrossRef]
- Kucker, S.; Tollner, K.; Piechotta, M.; Gernert, M. Kindling as a model of temporal lobe epilepsy induces bilateral changes in spontaneous striatal activity. Neurobiol. Dis. 2010, 37, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Ryu, B.; Nagappan, S.; Santos-Valencia, F.; Lee, P.; Rodriguez, E.; Lackie, M. Chronic loss of inhibition in piriform cortex following brief, daily optogenetic stimulation. Cell Rep. 2021, 35, 109001. [Google Scholar] [CrossRef]
- Van Dycke, A.; Raedt, R.; Verstraete, A.; Theofilas, P.; Wadman, W.; Vonck, K. Astrocytes derived from fetal neural progenitor cells as a novel source for therapeutic adenosine delivery. Seizure 2010, 19, 390–396. [Google Scholar] [CrossRef] [PubMed][Green Version]
- de Gois da Silva, M.L.; da Silva Oliveira, G.L.; de Oliveira Bezerra, D.; da Rocha Neto, H.J.; Feitosa, M.L.T.; Argolo Neto, N.M. Neurochemical properties of neurospheres infusion in experimental-induced seizures. Tissue Cell 2018, 54, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Papazian, I.; Kyrargyri, V.; Evangelidou, M.; Voulgari-Kokota, A.; Probert, L. Mesenchymal Stem Cell Protection of Neurons against Glutamate Excitotoxicity Involves Reduction of NMDA-Triggered Calcium Responses and Surface GluR1, and Is Partly Mediated by TNF. Int. J. Mol. Sci. 2018, 19, 651. [Google Scholar] [CrossRef]
- Acharya, M.M.; Christie, L.A.; Lan, M.L.; Donovan, P.J.; Cotman, C.W.; Fike, J.R. Rescue of radiation-induced cognitive impairment through cranial transplantation of human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2009, 106, 19150–19155. [Google Scholar] [CrossRef]
- Xian, P.; Hei, Y.; Wang, R.; Wang, T.; Yang, J.; Li, J. Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics 2019, 9, 5956–5975. [Google Scholar] [CrossRef] [PubMed]
- Backofen-Wehrhahn, B.; Gey, L.; Broer, S.; Petersen, B.; Schiff, M.; Handreck, A. Anticonvulsant effects after grafting of rat, porcine, and human mesencephalic neural progenitor cells into the rat subthalamic nucleus. Exp. Neurol. 2018, 310, 70–83. [Google Scholar] [CrossRef]
- Shetty, A.K.; Upadhya, D. GABA-ergic cell therapy for epilepsy: Advances, limitations and challenges. Neurosci. Biobehav. Rev. 2016, 62, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Karstoft, K.I.; Galatzer-Levy, I.R.; Statnikov, A.; Li, Z.; Shalev, A.Y.; members of Jerusalem Trauma Outreach. Bridging a translational gap: Using machine learning to improve the prediction of PTSD. BMC Psychiatry 2015, 15, 30. [Google Scholar] [CrossRef]
- Knipscheer, J.; Sleijpen, M.; Frank, L.; de Graaf, R.; Kleber, R.; Ten Have, M. Prevalence of Potentially Traumatic Events, Other Life Events and Subsequent Reactions Indicative for Posttraumatic Stress Disorder in the Netherlands: A General Population Study Based on the Trauma Screening Questionnaire. Int. J. Environ. Res. Public Health 2020, 17, 1725. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, P.S.; Hertzberg, J.S.; Kirby, A.C.; Dennis, M.F.; Hair, L.P.; Dedert, E.A. The effect of draft DSM-V criteria on posttraumatic stress disorder prevalence. Depress. Anxiety 2012, 29, 1032–1042. [Google Scholar] [CrossRef]
- Qassem, T.; Aly-ElGabry, D.; Alzarouni, A.; Abdel-Aziz, K.; Arnone, D. Psychiatric Co-Morbidities in Post-Traumatic Stress Disorder: Detailed Findings from the Adult Psychiatric Morbidity Survey in the English Population. Psychiatr. Q. 2021, 92, 321–330. [Google Scholar] [CrossRef]
- Kessler, R.C.; Sonnega, A.; Bromet, E.; Hughes, M.; Nelson, C.B. Posttraumatic stress disorder in the National Comorbidity Survey. Arch. Gen. Psychiatry 1995, 52, 1048–1060. [Google Scholar] [CrossRef] [PubMed]
- Kar, N. Cognitive behavioral therapy for the treatment of post-traumatic stress disorder: A review. Neuropsychiatr. Dis. Treat. 2011, 7, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Perez Benitez, C.I.; Zlotnick, C.; Stout, R.I.; Lou, F.; Dyck, I.; Weisberg, R. A 5-year longitudinal study of posttraumatic stress disorder in primary care patients. Psychopathology 2012, 45, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Shin, L.M.; Liberzon, I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 2010, 35, 169–191. [Google Scholar] [CrossRef] [PubMed]
- Rauch, S.L.; Shin, L.M.; Wright, C.I. Neuroimaging studies of amygdala function in anxiety disorders. Ann. N. Y. Acad. Sci. 2003, 985, 389–410. [Google Scholar] [CrossRef]
- Lanius, R.A.; Williamson, P.C.; Boksman, K.; Densmore, M.; Gupta, M.; Neufeld, R.W. Brain activation during script-driven imagery induced dissociative responses in PTSD: A functional magnetic resonance imaging investigation. Biol. Psychiatry 2002, 52, 305–311. [Google Scholar] [CrossRef]
- Milad, M.R.; Wright, C.I.; Orr, S.P.; Pitman, R.K.; Quirk, G.J.; Rauch, S.L. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol. Psychiatry 2007, 62, 446–454. [Google Scholar] [CrossRef]
- Alvarez, R.P.; Biggs, A.; Chen, G.; Pine, D.S.; Grillon, C. Contextual fear conditioning in humans: Cortical-hippocampal and amygdala contributions. J. Neurosci. 2008, 28, 6211–6219. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.; Kroll, A.; Lipinski, S.J.; Wessa, M.; Ridder, S.; Christmann, C. Context conditioning and extinction in humans: Differential contribution of the hippocampus, amygdala and prefrontal cortex. Eur. J. Neurosci. 2009, 29, 823–832. [Google Scholar] [CrossRef]
- Lonsdorf, T.B.; Haaker, J.; Kalisch, R. Long-term expression of human contextual fear and extinction memories involves amygdala, hippocampus and ventromedial prefrontal cortex: A reinstatement study in two independent samples. Soc. Cogn. Affect. Neurosci. 2014, 9, 1973–1983. [Google Scholar] [CrossRef]
- Rougemont-Bucking, A.; Linnman, C.; Zeffiro, T.A.; Zeidan, M.A.; Lebron-Milad, K.; Rodriguez-Romaguera, J. Altered processing of contextual information during fear extinction in PTSD: An fMRI study. CNS Neurosci. Ther. 2011, 17, 227–236. [Google Scholar] [CrossRef]
- Garfinkel, S.N.; Abelson, J.L.; King, A.P.; Sripada, R.K.; Wang, X.; Gaines, L.M. Impaired contextual modulation of memories in PTSD: An fMRI and psychophysiological study of extinction retention and fear renewal. J. Neurosci. 2014, 34, 13435–13443. [Google Scholar] [CrossRef]
- Bouton, M.E. Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biol. Psychiatry 2002, 52, 976–986. [Google Scholar] [CrossRef]
- Coventry, P.A.; Meader, N.; Melton, H.; Temple, M.; Dale, H.; Wright, K. Psychological and pharmacological interventions for posttraumatic stress disorder and comorbid mental health problems following complex traumatic events: Systematic review and component network meta-analysis. PLoS Med. 2020, 17, e1003262. [Google Scholar] [CrossRef] [PubMed]
- Steinert, C.; Hofmann, M.; Leichsenring, F.; Kruse, J. The course of PTSD in naturalistic long-term studies: High variability of outcomes. A systematic review. Nord. J. Psychiatry 2015, 69, 483–496. [Google Scholar] [CrossRef]
- Ursano, R.J.; Bell, C.; Eth, S.; Friedman, M.; Norwood, A.; Pfefferbaum, B. Practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. Am. J. Psychiatry 2004, 161, 3–31. [Google Scholar] [PubMed]
- Hu, X.H.; Bull, S.A.; Hunkeler, E.M.; Ming, E.; Lee, J.Y.; Fireman, B. Incidence and duration of side effects and those rated as bothersome with selective serotonin reuptake inhibitor treatment for depression: Patient report versus physician estimate. J. Clin. Psychiatry 2004, 65, 959–965. [Google Scholar] [CrossRef]
- Hermes, E.; Sernyak, M.; Rosenheck, R. The use of second generation antipsychotics for post-traumatic stress disorder in a US Veterans Health Administration Medical Center. Epidemiol. Psychiatr. Sci. 2014, 23, 281–288. [Google Scholar] [CrossRef]
- Jinno, S.; Klausberger, T.; Marton, L.F.; Dalezios, Y.; Roberts, J.D.; Fuentealba, P. Neuronal diversity in GABAergic long-range projections from the hippocampus. J. Neurosci. 2007, 27, 8790–8804. [Google Scholar] [CrossRef]
- Zijlmans, M.; van Campen, J.S.; de Weerd, A. Post traumatic stress-sensitive epilepsy. Seizure 2017, 52, 20–21. [Google Scholar] [CrossRef]
- Giotakos, O. Neurobiology of emotional trauma. Psychiatriki 2020, 31, 162–171. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, L.; Zhang, J. Induced pluripotent stem cell-derived neural progenitor cell transplantation promotes regeneration and functional recovery after post-traumatic stress disorder in rats. Biomed. Pharmacother. 2021, 133, 110981. [Google Scholar] [CrossRef]
- Saur, L.; Baptista, P.P.; Bagatini, P.B.; Neves, L.T.; de Oliveira, R.M.; Vaz, S.P. Experimental Post-traumatic Stress Disorder Decreases Astrocyte Density and Changes Astrocytic Polarity in the CA1 Hippocampus of Male Rats. Neurochem. Res. 2016, 41, 892–904. [Google Scholar] [CrossRef]
- Andero, R.; Ressler, K.J. Fear extinction and BDNF: Translating animal models of PTSD to the clinic. Genes Brain Behav. 2012, 11, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Tynan, R.J.; Beynon, S.B.; Hinwood, M.; Johnson, S.J.; Nilsson, M.; Woods, J.J. Chronic stress-induced disruption of the astrocyte network is driven by structural atrophy and not loss of astrocytes. Acta Neuropathol. 2013, 126, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Thelin, E.P.; Hall, C.E.; Tyzack, G.E.; Frostell, A.; Giorgi-Coll, S.; Alam, A. Delineating Astrocytic Cytokine Responses in a Human Stem Cell Model of Neural Trauma. J. Neurotrauma 2020, 37, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Neigh, G.N.; Ali, F.F. Co-morbidity of PTSD and immune system dysfunction: Opportunities for treatment. Curr. Opin. Pharmacol. 2016, 29, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Lorente, I.L.; Xie, Y.; Mazzitelli, J.A.; Hatanaka, E.A.; Cinkornpumin, J.; Miller, D.R. Patient-derived glial enriched progenitors repair functional deficits due to white matter stroke and vascular dementia in rodents. Sci. Transl. Med. 2021, 13, 102458. [Google Scholar]
- Lee, H.J.; Lim, I.J.; Park, S.W.; Kim, Y.B.; Ko, Y.; Kim, S.U. Human neural stem cells genetically modified to express human nerve growth factor (NGF) gene restore cognition in the mouse with ibotenic acid-induced cognitive dysfunction. Cell Transplant. 2012, 21, 2487–2496. [Google Scholar] [CrossRef]
- Yang, H.; Xie, Z.; Wei, L.; Yang, H.; Yang, S.; Zhu, Z. Human umbilical cord mesenchymal stem cell-derived neuron-like cells rescue memory deficits and reduce amyloid-beta deposition in an AbetaPP/PS1 transgenic mouse model. Stem Cell Res. Ther. 2013, 4, 76. [Google Scholar] [CrossRef] [PubMed]
- Xuan, A.G.; Long, D.H.; Gu, H.G.; Yang, D.D.; Hong, L.P.; Leng, S.L. BDNF improves the effects of neural stem cells on the rat model of Alzheimer’s disease with unilateral lesion of fimbria-fornix. Neurosci. Lett. 2008, 440, 331–335. [Google Scholar] [CrossRef]
- Koh, S.H.; Kim, K.S.; Choi, M.R.; Jung, K.H.; Park, K.S.; Chai, Y.G. Implantation of human umbilical cord-derived mesenchymal stem cells as a neuroprotective therapy for ischemic stroke in rats. Brain Res. 2008, 1229, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Ajami, B.; Bennett, J.L.; Krieger, C.; Tetzlaff, W.; Rossi, F.M. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 2007, 10, 1538–1543. [Google Scholar] [CrossRef] [PubMed]
- Riordan, N.H.; Hincapie, M.L.; Morales, I.; Fernandez, G.; Allen, N.; Leu, C. Allogeneic Human Umbilical Cord Mesenchymal Stem Cells for the Treatment of Autism Spectrum Disorder in Children: Safety Profile and Effect on Cytokine Levels. Stem Cells Transl. Med. 2019, 8, 1008–1016. [Google Scholar] [CrossRef]
- Datta, D.; Subburaju, S.; Kaye, S.; Baruah, J.; Choi, Y.K.; Nian, Y. Human forebrain endothelial cell therapy for psychiatric disorders. Mol. Psychiatry 2020. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kipper, F.C.; Angolano, C.; Vissapragada, R.; Contreras, M.A.; Moore, J.; Bhasin, M. Embryonic periventricular endothelial cells demonstrate a unique pro-neurodevelopment and anti-inflammatory gene signature. Sci. Rep. 2020, 10, 20393. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, A.; Long, J.E.; Crandall, J.E.; Rubenstein, J.L.; Bhide, P.G. Compartment-specific transcription factors orchestrate angiogenesis gradients in the embryonic brain. Nat. Neurosci. 2008, 11, 429–439. [Google Scholar] [CrossRef]
- Li, S.; Kumar, T.P.; Joshee, S.; Kirschstein, T.; Subburaju, S.; Khalili, J.S. Endothelial cell-derived GABA signaling modulates neuronal migration and postnatal behavior. Cell Res. 2018, 28, 221–248. [Google Scholar] [CrossRef]
| Variables Tested | Control | PTSD | PTSD + PBS | PTSD + iPSC Day 7 | PTSD + iPSC Day 14 | PTSD + iPSC Day 21 |
|---|---|---|---|---|---|---|
| Total distance in Open Field Test | No change | Significantly decreased relative to control | Significantly decreased relative to control | Increased relative to PTSD | Increased relative to PTSD | Increased relative to PTSD |
| Interest area stay time in Open Field Test | No change | Significantly decreased relative to control | Significantly decreased relative to control | No significant change from PTSD | Significantly increased relative to PTSD | Significantly increased relative to PTSD |
| Behavior modification in Open Field Test | No change | Significantly decreased relative to control | Significantly decreased relative to control | No significant change from PTSD | Significantly increased relative to PTSD | Significantly increased relative to PTSD |
| Freezing time in Fear Conditioning Test | No change | Significantly increased relative to control | Significantly increased relative to control | Significantly decreased relative to PTSD | Significantly decreased relative to PTSD | Significantly decreased relative to PTSD |
| Astrogliosis | No change | Increased GFAP (+) astrocytes compared to control | Significantly increased GFAP (+) astrocytes compared to control and PBS | |||
| NeuN neuronal maturation marker expression | No change | Significantly decreased expression relative to control | Significantly decreased expression relative to control | Increased relative to PTSD+PBS | Increased relative to PTSD+PBS | Increased relative to PTSD+PBS |
| BDNF expression | No change | Significantly decreased expression relative to control | Significantly decreased expression relative to control | Slightly increased expression relative to PTSD | Significantly increased expression relative to PTSD | Significantly increased expression relative to PTSD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gala, D.; Gurusamy, V.; Patel, K.; Damodar, S.; Swaminath, G.; Ullal, G. Stem Cell Therapy for Post-Traumatic Stress Disorder: A Novel Therapeutic Approach. Diseases 2021, 9, 77. https://doi.org/10.3390/diseases9040077
Gala D, Gurusamy V, Patel K, Damodar S, Swaminath G, Ullal G. Stem Cell Therapy for Post-Traumatic Stress Disorder: A Novel Therapeutic Approach. Diseases. 2021; 9(4):77. https://doi.org/10.3390/diseases9040077
Chicago/Turabian StyleGala, Dhir, Vikram Gurusamy, Krishna Patel, Sreedevi Damodar, Girish Swaminath, and Gautam Ullal. 2021. "Stem Cell Therapy for Post-Traumatic Stress Disorder: A Novel Therapeutic Approach" Diseases 9, no. 4: 77. https://doi.org/10.3390/diseases9040077
APA StyleGala, D., Gurusamy, V., Patel, K., Damodar, S., Swaminath, G., & Ullal, G. (2021). Stem Cell Therapy for Post-Traumatic Stress Disorder: A Novel Therapeutic Approach. Diseases, 9(4), 77. https://doi.org/10.3390/diseases9040077






