Abstract
Melatonin functions as a central regulator of cell and organismal function as well as a neurohormone involved in several processes, e.g., the regulation of the circadian rhythm, sleep, aging, oxidative response, and more. As such, it holds immense pharmacological potential. Receptor-mediated melatonin function mainly occurs through MT1 and MT2, conserved amongst mammals. Other melatonin-binding proteins exist. Non-receptor-mediated activities involve regulating the mitochondrial function and antioxidant cascade, which are frequently affected by normal aging as well as disease. Several pathologies display diseased or dysfunctional mitochondria, suggesting melatonin may be used therapeutically. Drosophila models have extensively been employed to study disease pathogenesis and discover new drugs. Here, we review the multiple functions of melatonin through the lens of functional conservation and model organism research to empower potential melatonin therapeutics to treat neurodegenerative and renal diseases.
1. Introduction
Melatonin (5-methoxy N-acetyltryptamine), nicknamed the “hormone of darkness”, was first discovered in the cow pineal gland, and has, since, been found in several mammals and other organisms, including plants (reviewed in [1]). Compared to mammalians, much less is known about how melatonin functions in invertebrates and plants [2]. Responsible for maintaining a steady circadian rhythm, melatonin is primarily synthesized and secreted into the blood by the mammalian pineal gland of the brain, peaking at night and reaching its lowest during the day [2,3,4,5,6,7,8,9,10,11,12]. Melatonin is also secreted by the retina, the lacrimal and Harderian glands, gut, bone marrow, platelets, and thyroid [13,14,15,16,17]. Finally, several (and potentially all) cells produce melatonin in the mitochondria [18,19], and, likely, the cytoplasm [20] with a distinct, non-circadian rhythmicity. Melatonin has been found to influence many cellular pathways and organismal functions [14,17]. Its effects are prominent on the circadian cycle, as well as endocrine function, immunity, and fertility [21]. Moreover, melatonin protects from arterial vasoconstriction and neurodegeneration via at least two types of receptors found in many cell types [22]. Melatonin effectively scavenges reactive oxygen species (ROS) and functions as a mitochondrial and cyto-protector [23,24]. The observed reduction in melatonin levels in several diseases, including neurodegeneration and cancer, constitutes the foundation for pursuing melatonin as a therapeutic. Melatonin is amphipathic and can easily cross cell membranes [25]. Therefore, it is expected to work through binding to transmembrane receptors, intracellular proteins, possibly to nuclear receptors, as well as display antioxidant activity [26].
Melatonin appears to have originated from photosynthetic bacteria, primitive cyanobacteria and alpha-proteobacteria before the endosymbiotic events believed to have originated eukaryotic mitochondria and chloroplasts [27]. Melatonin can donate electrons easily [28,29] and may have initially functioned as a free radical scavenger to reduce oxidative stress from photosynthesis and metabolism [2,27,30,31]. Organismal evolutionary diversification prompted an increased range of melatonin functions beyond its fundamental antioxidant capacity, to include the regulation of circadian rhythm, sleep, ciliary swimming behavior, vision, immunity, and more [2,32].
In contrast to the long evolutionary history of melatonin, its transmembrane receptors appeared relatively late in evolution [2]. Specifically in mammals, melatonin receptor 1 (MT1) and melatonin receptor 2 (MT2) display high affinity for melatonin and belong to the G-protein-coupled receptors (GPCR) superfamily [2,33,34,35,36,37,38]. A third receptor, MT3, identified as a binding site on the cytosolic detoxification enzyme quinone reductase 2 (QR2), is also found in various tissues, including liver, heart, lungs, kidney, intestine, and muscle [26,39,40,41].
Highly conserved functions and ubiquitous effects within the body make melatonin a primary research interest with potential pharmacological application. However, its precise molecular mechanism(s) of action, its reception and signal transduction have yet to be fully elucidated. Model organisms such as the fruit fly Drosophila melanogaster can recapitulate fundamental biological mechanisms, provide mechanistic insight, and contribute to the evolving understanding of melatonin function and its possible use as a therapeutic.
2. Melatonin Receptors
Encoded by melatonin receptor genes found, respectively, on human chromosome 4 and 11, the MT1 (alias MTNR1A, Mel1a) and MT2 (alias MTNR1B, Mel1b) proteins bind melatonin and share a high degree of sequence homology [37,42]. MT1 and MT2, respectively consisting of 350 and 362 amino acids (aa), share a 55% overall aa homology and 70% homology within the transmembrane domains [32]. Both have seven transmembrane α-helices connected by alternating loops with the amino-terminus on the extracellular side and the carboxy-terminus on the intracellular side [41]. The recently reported crystal structures of human MT1 and MT2, unexpectedly revealed the presence of highly specific orthosteric binding sites for melatonin buried within the membrane that, due to melatonin amphipathic properties, may contribute to ligand specificity [43].
While both MT1 and MT2 are high affinity melatonin receptors, the human MT2 exhibits a lower affinity for 125I-melatonin than MT1 [34]. Human MT1 and MT2 can form homo- and heterodimers with each other and heteromers with other GPCRs, consistent with them displaying several cellular functions (reviewed in [44]). MT1 and MT2 heterodimers are common even in the absence of a ligand and conformational changes have been shown to occur upon ligand binding [45]. Oligo- and heteromerization greatly impact the functional diversity of these receptors [46]. MT1 and MT2 primarily remain coupled to Gi/o proteins and display a high affinity for melatonin due to the formation of the ligand–receptor–G-protein (L–R–G) (or β-arrestin) complex [46]. Several other signaling molecules and G proteins have been reported to interact with MT1 and MT2 in specific cases [46]. Dissimilar to MT1 and MT2, the MT3 receptor exhibits a low binding affinity for melatonin [47,48]; however, its inhibition is theorized to play a role in the antioxidant effects of melatonin [49]. An analysis of MTNR genes encoding the melatonin receptors from 45 vertebrate species provided insight into their origin and evolution. In particular, the mtnr1c gene found in lower mammals contains a C-terminal expansion, and is thought to have evolved after the evolutionary branching from Monotremata and Marsupialia [50]. Interestingly, the orphan receptor GPR50 appears to be a mammalian mtnr1c ortholog. Considering the two rounds of whole genome duplications occurred since the common vertebrate ancestor [51], the loss of the ancestral mtnr1c function, and the analysis of the regions adjacent to the MTNR genes, it seems likely that during the recent tetrapod evolution several melatonin receptor-related genes may have been lost [50]. Numerous sequences in GenBank have been automatically annotated as encoding “melatonin-receptor-like” proteins, including entries from invertebrates and insect species. However, their divergence and current lack of functional evidence cautions against considering them as bona fide melatonin receptors. Despite structural similarities in the MT1, MT2, and GPR50 transmembrane motifs, GPR50 in humans does not bind melatonin directly, but inhibits MT1 receptor functions via heteromerization [46,52]. Transcription factors belonging to the retinoic acid-related orphan nuclear hormone receptor (ROR) family are functionally linked to melatonin pathways; however, their capacity to bind melatonin directly is debated [53].
2.1. Melatonin Receptor Functions
Sleep/wake homeostasis and circadian rhythm regulate sleep (reviewed in [54]). In mammalians, the master circadian clock is located within the suprachiasmatic nucleus (SCN) in the hypothalamus, where melatonin is released and controls SCN activity through the MT1 and MT2 receptors [55,56]. Melatonin receptors seem to be involved in a plethora of physiological activities and cellular processes (reviewed in [22,34]) as diverse as phototransduction and ocular growth [57,58], blood pressure regulation [59], immunomodulation [60,61], the hair cycle [62], and may exhibit oncostatic effects in several cancers [34]. Additionally, MTNR1A was found to have regulatory effects on reproductive seasonality that appears tightly linked to the light/dark cycle [63]. Tissue-specific expression of receptors relates to several melatonin physiological effects, yet it has only been partly defined. MT1 and MT2 are expressed in the cell membranes of a variety of tissues throughout the body, including the brain, retina, cardiovascular system, liver, gallbladder, colon, skin, kidneys, among others [64], (reviewed in [22]). To date, the mammalian melatonin receptors have been studied extensively. Some well-described melatonin receptor functions are listed in Table 1. In contrast, melatonin signaling and receptor homologs in other species, including invertebrates, remain largely unknown.

Table 1.
Melatonin receptor functions.
In the central nervous system (CNS), MT1 and/or its mRNA are found in the SCN, pars tuberalis (PT) [89,90,91], and the retina [57]. MT2 is expressed in the olfactory bulb, forebrain, hippocampus, amygdala, and superior colliculus [92], as well as in the septum, within specialized cells of the hippocampus, the pars reticulata of the substantia nigra, ventral tegmental nucleus and other regions associated with non-rapid eye movement (NREM) sleep [90]. MT2 mRNA is found in the retina, sclera, lens, and retinal pigment epithelial cells [93]. Although both MT1 and MT2 receptors were detected in the retina and are essential for retinal function, the MT2 mRNA was, surprisingly, not detected within the photosensitive retinal ganglion cells in mice [93]. Of note, mice are nocturnal. Melatonin synthesis is similar in both nocturnal and diurnal animals, with peak release being recorded during the middle of the night (12–3 am) [94]. It has been shown that melatonin induces typical nighttime behavior, i.e., activity for nocturnal animals, and sleep for diurnal species such as humans [95,96,97] (reviewed in [98]).
The simultaneous activation of MT1 and MT2 can elicit diverse responses with additive, cooperative or opposing effects [38]. In vertebrates, the light/dark cycle affects the retina, which signals to the master circadian clock in the SCN via the retino-hypothalamic tract [99]. Responding to the SCN, the pineal gland synthesizes and releases melatonin that is captured by the target cells through MT1 and MT2 receptors (reviewed in [100]). Creating a regulatory feedback loop, the SCN cells, which express MT1 and MT2, also respond to melatonin (reviewed in [56]). MT1 and MT2 are cyclically expressed daily during the light/dark cycle [101]. Melatonin promotes the expression of clock genes, which is expected to contribute to its circadian functions [102] and may also operate in receptor-independent ways [103], or through interaction with other receptors [87,104,105]. Several GPCRs become desensitized upon ligand binding, which helps to regulate the intensity of cell response. While there is suggestive evidence of such properties for MT1 and MT2 (e.g., [106,107]), both mechanism and physiological roles remain unknown. Genetic studies with murine knockout animals have indicated that MT1 and MT2 may regulate REM sleep and NREM sleep phases, respectively [87]. The relationship between melatonin, MT1, MT2, and sleep appears complex. The genetic and pharmacologic manipulation of the MT1 and MT2 pathways through single and double MT1/MT2 knockout and chemical probing employing agonists, as well as the study of the effects of the surgical removal of the pineal gland, collectively suggest that melatonin contributes to the sleep wake cycle, yet, may not be obligatory [87,105]. The double MT1/MT2 knockout mice displayed an almost normal sleep phase duration but may have altered cycling between NREM and REM sleep [87]. Because both receptors are differentially expressed in the brain areas controlling REM and NREM sleep, one intriguing possibility is that MT1- and MT2-dependent responses to melatonin may be linked. This, together with potentially differential receptor deactivation cycles during melatonin peak time, may yield the REM/NREM cycling [55,87,108]. The mechanism remains, however, to be fully demonstrated experimentally.
In the cardiovascular system, melatonin regulates smooth muscle and endothelial cells [75,109]. Melatonin receptors may increase coronary blood flow and improve cardiac function [109]. Depending on the tissue, melatonin may also induce vasoconstriction or vasodilation. In smooth muscle and coronary arteries, MT1 appeared to mediate vasoconstriction while MT2 activation induced vasodilation [75,77,110], by reducing cyclic adenosine monophosphate (cAMP) levels and phosphatidylinositol 4,5-bisphosphate hydrolysis [111,112]. In animal studies, specific arteries responded differentially to melatonin exposure. Rat and pig coronaries constricted [113], while the rabbit pulmonary, aorta, iliac, and renal arteries dilated [75,77,110,114,115,116,117,118,119]. In humans, while renal blood flow and conductance decreased in response to melatonin, forearm vascular beds exhibited the opposite response, and cerebral circulation was unaffected [120]. Pharmacological studies suggest that some of these effects may be independent of MT1 and MT2 receptors [121]. Studies in pigs also imply that in endothelial cells, MT2 activation may increase nitric oxide (NO) production, which in turn causes vasodilation [109]. However, melatonin antioxidant properties are largely responsible for suppressing NO production in addition to significantly increasing superoxide dismutase (SOD) activity [122,123,124]. While this prompts melatonin’s potential use in treating diseases such as Alzheimer’s disease, further studies will be needed to decipher its effects on endothelial cells.
Melatonin may also impact immunity [60,61]. High levels of melatonin have been shown to promote immune system functions, while, conversely, low levels are associated with the suppression of numerous immune parameters. The discovery of melatonin receptors in multiple lymphoid organs and lymphocytes indicates that there may be multiple mechanisms of action [21]. Mice lacking a functional MT1 gene were used to show that MT2 receptors are responsible for the melatonin enhancement of splenocyte proliferation and the regulation of anti-keyhole limpet hemocyanin (KLH) IgG concentrations [81,125]. Note, that the T-cell-dependent KLH antigen from mollusks is widely employed in immunotoxicology to evaluate immune function in varying conditions (reviewed in [126]). Additionally, the activation of MT2 receptors has been shown to inhibit melanoma cell growth [127].
Due to its high cell permeability, free melatonin in humans can bind to intracellular MT3 and nuclear ROR proteins [128]. MT3 (alias ML2, NQO2) is a quinone reductase expressed in several vital organs, including kidney, liver, heart, and lung and in muscle, intestine, and brown fat, that inhibits the quinones electron transport chain and protects from oxidative stress (reviewed in [34,40]).
Melatonin has one possible nuclear receptor type in the ROR protein family. The ROR/RZR proteins are zinc-finger transcription factors with α, β, and γ subgroups. Dissimilar to RORβ, both RORα and RORγ are known to participate in several pathways that are also regulated by melatonin. For example, the transcriptional regulation of the clock gene Bmal1 by RORα correlates with the mammalian circadian rhythm and has been shown to be necessary for normal circadian regulation in mice [129,130]. Melatonin promotes RORα transcriptional activity and can be co-immunoprecipitated with RORα [131], indicating some functional interaction. Physiological studies also point to a large functional overlap between the two; however, their direct binding is controversial (reviewed in [53]). Based on some experimental observations, it has been proposed that RORα-mediated effects may be indirect and exerted through other factors, e.g., MT1, MT2, sirtuins, the redox state, mitochondria, and, possibly, the expression of ROR antagonist REV-ERB [132,133,134,135,136,137,138,139].
The orthologous GPR50 (alias H9, ML1X; found in vertebrates, except birds and fish) and Mel1c (in fish, amphibians, and birds) proteins are also GPCRs. GPR50 exhibits about a 50% sequence identity with MT1 and MT2; however, it does not appear to bind melatonin [44]. Interestingly, GPR50 can form heteromers with both MT1 and MT2 and negatively regulate the melatonin–MT1 interaction, while leaving MT2 binding activity intact [52] (reviewed in [40]).
2.2. Molecular Mechanisms of Melatonin-Receptor Signaling
MT1 and MT2 are activated by distinct physiological concentrations of melatonin released from the pineal gland due to feedback onto the SCN [41]. The pathways downstream of MT1 and MT2 affect intracellular cAMP and cGMP, calcium (Ca2+) levels, and the activation of specific protein kinases [34].
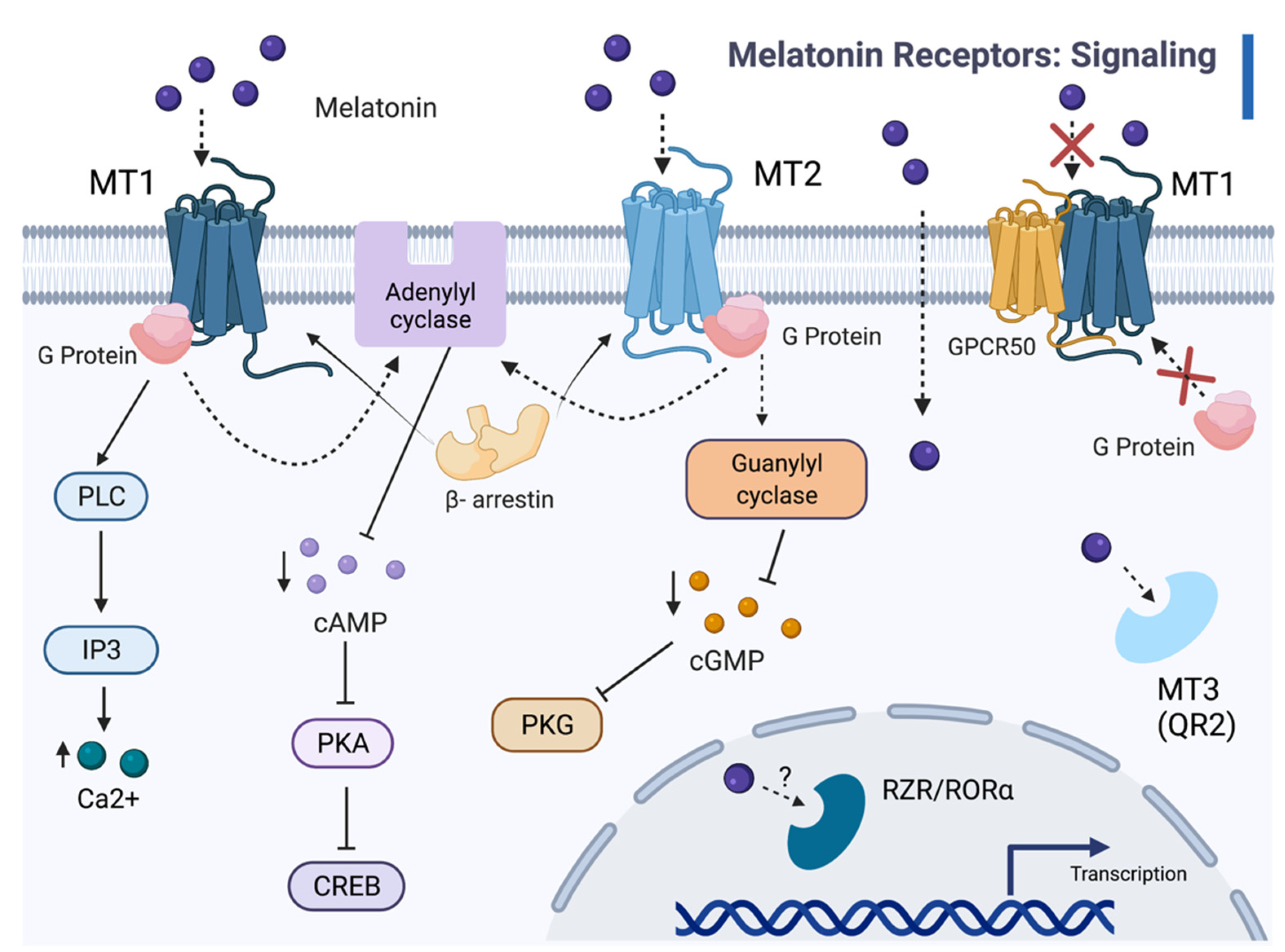
Most notable among MT1 functions, is the inhibition of cAMP accumulation by the pertussis toxin (PTX)-sensitive G proteins in the mammalian pituitary and SCN [26,41,48,86,87]. MT1 engagement by melatonin activates Gi proteins, inhibits adenylyl cyclase activity, and decreases intracellular cAMP (Figure 1) [38,46]. Such a decrease activates protein kinase A and, subsequently, the transcription factor cAMP-responsive element binding (CREB) [38,46]. Additionally, the MT1 receptor augments potassium conductance through Inner Rectifier Potassium (Kir) channels, induces the mitogen-activated protein kinase 1/2 (MAPK), and extracellular signal-regulated kinase (ERK) 1 and 2 [38]. Circadian signaling regulates melatonin binding and the expression of MT1 mRNA [34]. Both responses and the signal transduction pathways themselves variably correlated to the circadian rhythm. Studies with recombinant receptors illustrated the ability of MT1 to activate many types of G proteins [34]. MT2 is closely associated with the inhibition of adenylyl cyclase and guanylyl cyclase, as well as phosphoinositide production [48,88]. MT2 also inhibits forskolin-stimulated cAMP production in addition to cGMP formation and activates PKC in the SCN [38]. In (nocturnal) rodent SCN and PT, varying levels of MT1 mRNA expression and 125I-melatonin binding are exhibited throughout the day, with greater expression during the daytime [140].

Figure 1.
The melatonin signaling cascades. Melatonin binds to transmembrane receptors MT1 and MT2, as well as the MT3 binding site on the cytosolic enzyme QR2, and, possibly, the nuclear receptor RZR/RORα. Melatonin (ligand) binding with MT1 and MT2 receptors recruits β-arrestin and activates G proteins, which inhibit adenylyl cyclase activity and decrease cAMP levels. MT1 coupling to G proteins activates phospholipase C (PLC), which leads to increased intracellular Ca2+. Melatonin-dependent activation of MT2 and associated G proteins prompts interaction with guanylyl cyclase, which reduces cGMP levels; therefore, lowering PKG activity. In vertebrates, except for birds and fish [42,141], heteromerization of GPR50 and MT1 (but not MT2) inhibits both G protein interactions and melatonin binding. Melatonin acts as a ligand for MT3 and, possibly, for RORα, independently of the MT1 and MT2 pathways. With RORα and RORγ, melatonin affects nuclear transcription factor activity and gene expression.
3. Receptor-Independent Melatonin Functions
In addition to its several receptor-mediated functions, melatonin can also function independently. A well-known example of a receptor-independent action is its activity to counter oxidative stress. Melatonin is an effective scavenger for free radicals formed in response to stressors activating the AP1 transcriptional response [142,143,144]. In mitochondria, melatonin functions to neutralize dangerous pro-oxidant metabolic byproducts of oxidative phosphorylation and the electron transport chain, such as radicals and peroxides [145,146]. Reportedly, the mitochondrial melatonin concentration (~100 nM) is approximately one hundred times that of the circulatory melatonin released by the pineal gland (1 nM at peak release) [147]. Such a concentration is achieved from a combination of rapid uptake and transport via the PEPT1/2 transporters [148] and glucose transporter 1 (GLUT1) [149], and endogenous synthesis independent of the circadian pineal release [18]. It has been proposed that mitochondrial melatonin synthesis reflects the capacity of the ancestral bacterial endosymbiont that modern mitochondria originate from (reviewed in [150]). In addition to its ROS scavenging power, melatonin affects several mitochondrial pathways. It improves the function of the electron transport chain [147] and ATP production [151]. It also promotes the synthesis of antioxidant glutathione [152], upregulates mitochondrial sirtuin SIRT3 [153,154,155], which, in turn, increases SOD antioxidant activity and acetyl coenzyme A synthesis. The latter is a necessary cofactor for a limiting melatonin biosynthetic enzyme, arylalkylamine N-acetyl transferase [156]. Altogether, these pathways appeared to reduce mitochondrial ROS damage in rat and mice models [157,158,159] (reviewed in [160]).
Experimental observations suggest that mitochondrial melatonin also exits this organelle [19] and may affect other cell districts and compartments, as well as nearby cells in paracrine fashion [150]. Because the mitochondrial membrane contains abundant MT1 and MT2 [19,161,162], mitochondria can respond to released mitochondrial melatonin in a way reminiscent of cellular autocrine loops.
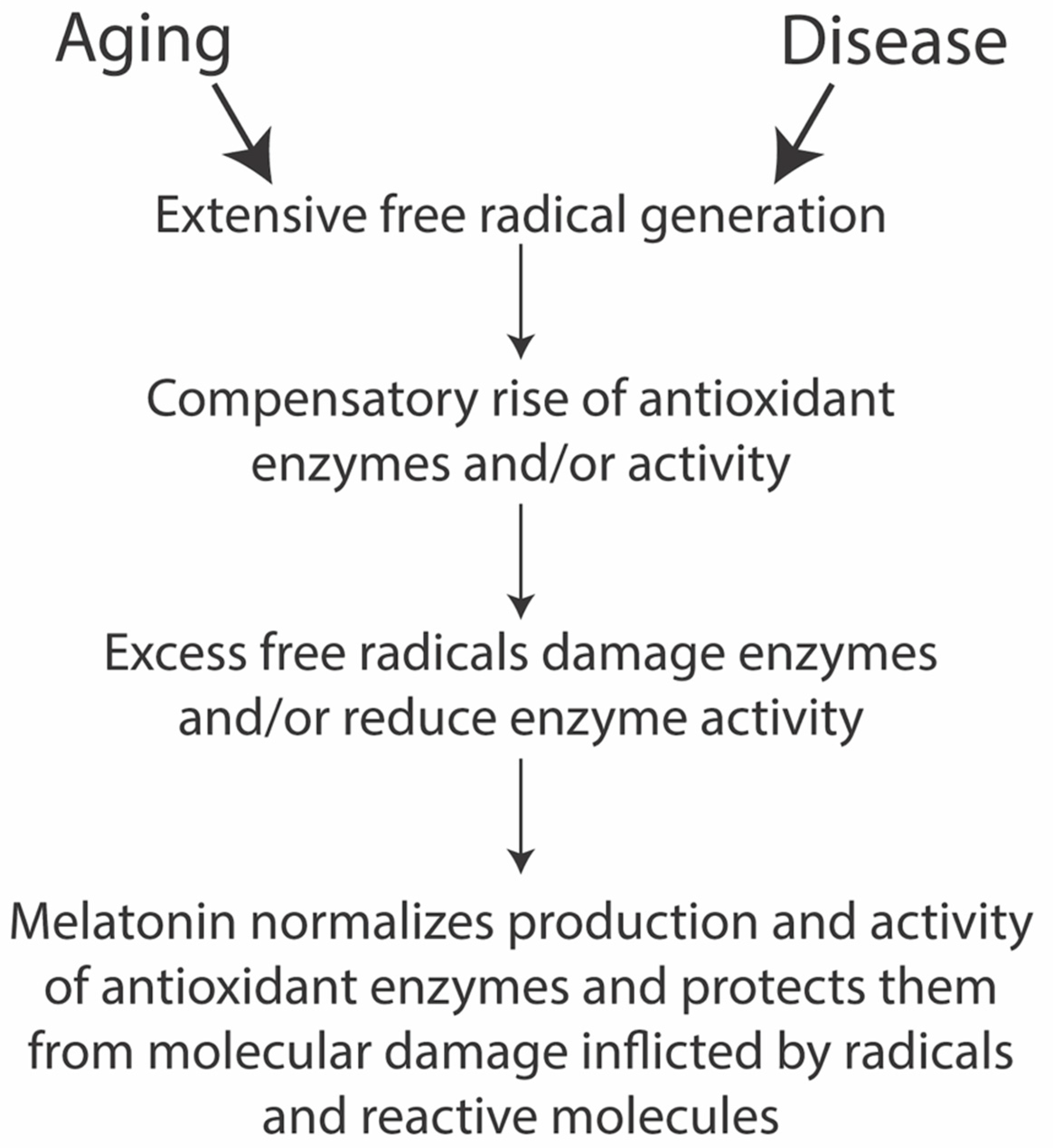
Several diseases and aging feature cellular oxidative damage (Figure 2). Cells appear to have a natural defense against these injuries in their endogenous melatonin production and melatonin signaling. However, the natural buffering capacity may become insufficient and, in such cases, melatonin administration in therapeutical dosages promises to help remedy such degeneration. Additionally, several diseases affect the mitochondria directly or compromise them indirectly (discussed below), in which case, melatonin may become an attractive treatment option.

Figure 2.
Simplified melatonin antioxidant cascade (drawn after [163]).
3.1. Melatonin and Longevity
One of the risk factors for several prevalent diseases, biological aging is the decline in the physiological ability to perform or meet certain demands that occur as time passes [164]. Shared by most organisms and characterized by the accumulation of damage in processes at the molecular, cellular, and, eventually, the organ levels, biological aging increases individual susceptibility to disease, infection, and death. Aging is influenced by genetic, dietary, and environmental factors. Considering the social costs and degraded life quality associated with disease, preventing age-related conditions and prolonging a healthy life span are biomedical topics of great interest.
The genes found to affect survival displayed weak effects on longevity when assayed individually [165]. Consistently, genetic manipulation of the aging process in mice could only increase lifespan modestly [165]. Thus, aging appears to be only partly modulated by genetic factors. A series of observations support such conclusion. Aging may present differently even among individuals of the same species. In fact, in populations of genetically identical yeast or laboratory animals, individual age at death varies. Similarly, monozygotic human twins usually die at different ages [166]. Twin studies estimated that the individual genetic makeup may affect lifespan at most by 30% [167]. Aging is associated with many features, including genomic instability, telomere attrition, and mitochondrial dysfunction. During aging, melatonin production gradually declines [168] (reviewed in [169]). Life-extension protocols and environmental factors such as nutrition appeared to impact longevity at about 70%, more than twice the estimated impact of genetic makeup [170]. Thus, many compounds known to affect aging mechanisms have been tested for life-extending potential. The DrugAge database of aging-related drugs reports that pineal gland extract was found to be the most effective, prolonging mouse lifespan by about 31% [171,172]. Similarly, epithalamin, a tetrapeptide found in pineal gland extract, was also reported to elicit a nearly identical longevity extension [171,173]. Epithalamin was found to be active in both its natural and synthetic form, the latter called epit(h)alon [174,175,176]. Besides lengthening the healthy life span, epithalamin could also reduce carcinogenesis, improve cardiovascular function, and protect retinal and brain function [174,176,177,178,179,180,181,182,183,184,185,186,187]. Epithalamin heightened peroxide chemiluminescence found in the blood of 30-month-old rodent models and humans [173]. Interestingly, epithalamin appeared to induce melatonin production [188,189,190]. Underscoring evolutionary conservation and strongly implying melatonin, the administration of pineal gland extract was found to extend life from humans to Drosophila [177,191,192,193,194,195].
Melatonin administration increased the life span of rodents [191,196,197,198], with an 18% extension reported for mice [171,172]. Melatonin was also found to increase the life span of several invertebrates, including Drosophila [196,199,200]. One study examining the protective effects of melatonin from ionizing radiation in rats observed that melatonin inhibited guanine base oxidation in DNA [201]. In C. elegans, it was found that genes related to mitochondrial function greatly affected life span [202]. RNAi inactivation pertinent to mitochondrial function, surprisingly, extended the life span of the average C. elegans [202].
Oxidative Damage
Oxidative damage is a major contributor to biological aging. The free radical theory of aging states that free radicals induce changes in cellular metabolism, which, eventually, leads to functional decline and organismal death through gradual loss in cellular function and reduced resistance against physiological stress [200,203]. ROS include oxygen-derived molecules and chemical species with one unpaired electron called free radicals [204]. ROS have been linked to oxidative damage of fatty acids, DNA, and proteins, as well as to the production of hydroxyl and peroxyl radicals, hydrogen peroxide, and superoxide radical anions [205]. ROS can also target mitochondria [206], which would induce mitochondrial stress and, eventually, reduce the life span [207]. The cellular capacity to counter oxidative stress is limited and may be overwhelmed when ROS form at an excessive rate [163,205] (Figure 2). Evidence of oxidative stress has been found in several age-related pathologies, including cancer, cardiovascular, inflammatory, and neurodegenerative diseases such as PD and AD [205,208]. Mitochondria are responsible for more than 90% of the oxygen consumption and, thus, produce the greatest amount of ROS [209]. Indeed, ROS can be formed through exogenous and endogenous means. Endogenous promoters of ROS formation include free radical semiquinone anion species (Q−) formed in mitochondria, and cytosolic intracellular enzymes, while extrinsic sources comprise environmental agents (e.g., ultraviolet light, ionizing radiation), including non-DNA reactive carcinogens and chemicals in pollutants such as methyl viologen, also known as herbicide paraquat [205]. Excess oxygen-containing compounds bring about tissue damage, chronic inflammatory processes, and disturb cell function [210]. Antioxidants and free radical scavengers, instead, inhibit the effects of free radicals because of their ability to quench oxidative stress.
Known for being a highly effective antioxidant and free radical scavenger [211], melatonin has been studied and used for anti-aging benefits and longevity extension to prevent oxidative and mitochondrial injury and maintain mitochondrial bioenergetics. Melatonin counters oxidative stress by working alongside other radical scavengers to donate electrons to unstable radicals [212,213,214]. It stimulates antioxidative glutathione peroxidase and glutathione reductase enzymes, as well as upregulates glutathione synthesis (reviewed in [163,215,216]). Melatonin can quench hydrogen peroxide, NO, peroxynitrite anion, superoxide anion, peroxyl, and the more damaging hydroxyl radicals, acting both in lipophilic and hydrophilic manners [215,217] (reviewed in [150,216]). In vitro, melatonin effectively reduced the peroxidation of ox-brain phospholipids [218]. Importantly, the breakdown metabolites formed during the quenching process, such as cyclic 3-hydroxymelatonin, N-acetyl-N-formyl-5-methoxykynuramine, and N-acetyl-5-methoxykynuramine, also donate electrons [219,220,221,222,223], creating an “antioxidant cascade” (reviewed in [163]), where free radicals are progressively eliminated. Resistance to oxidative stress is one of the longevity extending factors [200].
Aging decreases activity of the mitochondrial respiratory chain and slows down ATP production [224]. Steady melatonin administration in the drinking water of one-month-old mice until ten months of age appeared to effectively counteract the age-related decline of lung functionality and increase ATP production, indicating that melatonin administration maintained fully functioning lung mitochondria during aging [225].
3.2. Drosophila in Longevity Studies
Drosophila melanogaster is an ideal model species to specifically study longevity because of its relatively short life span, and conservation of ~75% of the genes and pathways involved in human disease and longevity [226,227]. With a rigorous comparative perspective, core biological mechanisms can be deciphered in the fly and the resulting knowledge may be used to inform complex vertebrate modeling. Fruit fly physiology can be directly (and carefully) compared to that of humans. Relevant for aging studies, Drosophila presents two sexes, sexual dimorphism enabling the examination of sex-specific differences, and highly differentiated tissues [228]. Economic culturing in the laboratory, without the need of expensive containment facilities, and the wealth of accessible genetic resources (e.g., public strain repositories, construct collections available to the research community at minimal cost) make Drosophila particularly amenable to studying the genetics of aging. Moreover, its short life span eases the study of potential life-extending drugs. D. melanogaster, as well as other invertebrate and unicellular model organisms, have first indicated that caloric restriction positively impacts longevity [229]. Adult Drosophila that were administered a mechanistic target of rapamycin (mTOR) inhibitor rapamycin through their food source, displayed the life span extension patterns observed in mTOR mutants [230]. Such effect is also visible in Figure 8 of Gamberi et al., 2017 [231]). Drosophila research confirmed the involvement of the nutrient-sensing insulin/insulin-like growth factor signaling (IIS) pathway in regulating aging. The mutational inactivation of three of the seven insulin-like peptides encoded in the fly genome (dilp2–3,5) yielded a dramatic 30–50% life span increase [232]. Another study provided evidence that circ RNA encoded by sulfateless (circSfl) is downregulated over time, contributing, significantly, to aging; however, circ RNA upregulation only increased the average life span of Drosophila by about 15% [233]. Underscoring mechanistic conservation, dietary restriction positively affected longevity in Drosophila [234]. However, how calorie restriction and altered macronutrient intake balance result in increased longevity, have not yet been identified [235].
4. Melatonin and Neurological Disease
Linked to melatonin function, many neurodegenerative diseases such as Alzheimer’s disease (AD), Huntington’s disease (HD), and Parkinson’s disease (PD) are associated with the disruption of the circadian clock and function [236] (reviewed in [237]). Moreover, oxidative damage is widespread among neurological diseases [238,239,240]. As well, defective mitochondrial function leading to oxidative imbalance is thought to lead to neurological disease pathology directly or indirectly (reviewed in [241]). It has recently been shown that the melatonin synthesized by the mitochondrial matrix can activate MT1 signaling which inhibits cytochrome c release and caspase activation, thereby halting neurodegeneration [19]. Thus, these pathologies may offer opportunities of intervention through melatonin or the modulation of melatonin-responsive pathways.
4.1. Alzheimer’s Disease
The hallmark of AD consists of the accumulation of extracellular senile plaques, composed of amyloid β peptides, and intracellular neurofibrillary tangles, composed of aggregated neuronal cytoskeletal tau protein [241,242,243]. AD is characterized by the disruption of cognitive functions and progressive memory loss [243] (reviewed in [244]). As well, mitochondrial abnormalities have been shown to play an important role in disease pathogenesis, often leading to the inhibition of electron transport in the brain, including reduced activity of cytochrome c oxidase [245] (reviewed in [241]). In AD, inhibited electron transport from the mitochondria is thought to yield oxidative imbalance favoring accumulated mitochondrial oxidants [241]. Melatonin has been shown to play a role in AD because it not only protects against oxidative stress, but also against amyloid β-peptides accumulation, which are typical of AD pathogenesis [243,246,247]. Suggesting a weaker melatonin signal transduction, patients suffering from AD also have reduced melatonin and MT1/MT2 receptor levels; more precisely, in AD patients, the immunoreactivity of both MT1 and MT2 receptors appeared distinctly decreased within the pineal gland cell somata and cellular processes [248].
4.2. Huntington’s Disease
Caused by mutations of the Huntingtin (Htt) gene, HD leads to motor impairment such as involuntary movements, cognitive impairment such as dementia, and psychiatric symptoms among which anxiety and depression are common (reviewed in [249]). From murine models, it has been found that Htt is necessary for mitochondrial metabolism and bioenergetics [250]. Just as AD, HD has also been associated with mitochondrial abnormalities such as a defective electron transport chain and Ca2+ uptake [251,252]. HD has also been associated with a reduction in nightly melatonin levels, accompanied by sleep and circadian function disturbances [253]. Diminished oscillations of core clock genes such as period and timeless have also been observed [236]. In a rat model of HD, melatonin treatment has been proposed to delay the onset of disease symptoms due to its antioxidant properties [254].
4.3. Parkinson’s Disease
PD is frequently caused by mutations in the leucine-rich repeat kinase 2 (LRRK2) gene (reviewed in [255,256]) and it leads to severe motor symptoms such as tremors or slow movements and sleep disturbances [257]. Sleep disturbances are found in 60–98% of LRRK2-associated PD patients [258], suggesting the need for therapeutics, potentially targeting the genes and/or symptoms associated with sleep disorders. Melatonin supplements have been widely used to induce sleep and it is hypothesized that treatment in PD patients could normalize melatonin levels, inducing regular sleep patterns [257]. As well, PD patients were found to be defective in the mitochondrial Complex I, leading to abnormal electron transport, neuronal depolarization, impaired mitochondrial Ca2+ uptake, and oxidative imbalance [241,259,260,261,262]. PD pathogenesis also includes the impaired removal of defective mitochondria [241,263] and has been classified as a “mitochondrial disease” (reviewed in [264,265]).
5. Melatonin Life Extension in Drosophila Models of Aging and Disease
Both melatonin and arylalkylamine N-acetyltransferase, a key catalyst for melatonin synthesis, are conserved in flies [200,266]. Moreover, Drosophila extracts can synthesize melatonin from its natural building blocks tryptophan, tryptamine, and serotonin [266]. Melatonin fed to the D. melanogaster Oregon wild strain at a daily concentration of 100 μg/mL within the culture medium increased the maximum life span by 33.2%, compared to vehicle-fed flies [200]. Another study found that exposure of the Drosophila HEM strain to melatonin and pineal peptide epithalamin [173], increased the mean life span by about 17% [196]. Epithalamin administration increased antioxidant activity by 36.6% and SOD by 19.7% [173]. Suggesting mechanistic conservation, epithalamin was found to lengthen the Drosophila lifespan and reduce oxidative stress [267,268,269]. In flies, epithalamin appeared to promote transcription by favoring DNA strand separation at promoter sites while stimulating euchromatin formation, which is instead progressively lost during aging [270].
Melatonin dispensed to the Drosophila Canton-S wild strain at the early stages of development, and a concentration of 0.08% per unit mass in a culture medium, increased male life span by 15% [199]. Melatonin administration (0.43 mM) increased both life span and malondialdehyde levels [271]. Malondialdehyde reduces aging-related free radical damage, and its levels are used as a clinical indicator of antioxidant potential in patients [272]. As that of other insects, the Drosophila genome does not contain clear MT1 and MT2 homologs, although it encodes several orphan GPCRs and the downstream effectors of signal transduction. Worm and fly genomes include quinone reductases of distinct origin from NQO2 [273]. The Drosophila ROR homolog, ultraspiracle, has been studied for its endocrine function related to the hormone ecdysone [274,275], but it is known to be important for retinoid metabolism in eye development and tissue regeneration [276,277] both having functional conservation. Interestingly, retinoids are crucial for retinal development and appear linked to melatonin function [278]. However, functional relatedness and melatonin binding remain to be determined. Due to the complicated melatonin functional overlap in mammalians, Drosophila appears an ideal system in which to distinguish evolutionary recent GPCR receptor-mediated functions from ancient melatonin functional pathways.
5.1. Melatonin Treatments in Drosophila Models of Neurological Disease
With a nervous system similar to humans, yet streamlined [279,280], Drosophila models have recently been key to understanding HD pathogenesis and aspects of other neurodegenerative diseases [226,227]. The Drosophila circadian clock system contains fewer circadian pacemaker neurons than humans, which enabled functional studies and, likely, the most precise definition of the molecular, genetic, physiological, and behavioral aspects of any circadian clock system [281]. In the fly, the setup of the circadian rhythm is regulated largely at the transcriptional level by the daily expression cycle of several genes, including period (per) [282,283,284] and timeless (tim) [285], as well as the pigment dispersing factor (pdf) found in circadian pacemaker neurons [286] (reviewed in [287,288]). While the complex mammalian circadian rhythm relies on multiple clocks (reviewed in [289]), the basic organization of the oscillators is conserved between flies and mammals and several key proteins are also conserved. Therefore, Drosophila is considered a valid model in which to study neurodegenerative disease (reviewed in [290]). Importantly, the high conservation of oxidative response pathways makes Drosophila a useful model to further explore the role of oxidative stress in pathologies, including neurodegenerative and renal diseases [291,292,293,294] (Table 2).

Table 2.
Antioxidant enzymes influenced by melatonin *: Implications in Drosophila disease models.
5.1.1. Huntington’s Disease
Very recently, transgenic HD flies were generated by expressing mHtt in pan neuronal and pdf-specific neurons [236]. The HD fly model displays the progressive loss of motor function and reduced oscillations of core clock genes per and tim [236]. The Drosophila HD model was used to investigate the effects of melatonin and curcumin, a compound found in turmeric plant roots, on eclosion and the characteristic progressive loss of locomotion [236]. Curcumin exhibits several therapeutic properties, e.g., antioxidant and anti-inflammatory, and, notably, it has been suggested to be neuroprotective for PD and AD [309]. This study found that melatonin significantly increased both percent eclosion and motor function measured as the climbing ability of the HD flies, compared to control flies of the same age [236]. Curcumin (10 μM) also improved the climbing ability of the HD flies. Neither melatonin nor curcumin had adverse effects on control flies [236], suggesting they may be non-toxic and suitable to long-term therapeutical administration. Moreover, similar to the aging process which features the progressive loss of circadian functions, HD flies aged 1 through 13 days normally displayed a lower amplitude of per and tim mRNA oscillations compared to the wild-type controls [236,310,311]. However, both melatonin and curcumin rescued the daily (24 h) per and tim mRNA oscillations to normal levels in HD flies [236]. Khyati et al. speculated that melatonin and curcumin supplementation to HD flies may prevent neurodegeneration by inhibiting oxidation and blocking Htt protein aggregation, respectively [236]. Consistently, in a mouse model of HD, Htt protein accumulation and aggregation, as well as transcriptional deficits, were present by six months of age and were both improved by dietary curcumin [312]. Oxidative damage is widespread in neurodegenerative disease [238,239,240] and the antioxidant properties of melatonin make it a promising therapeutic candidate. However, melatonin’s effectiveness appears to be due to more than its antioxidant function. Indeed, while oxidative damage appears important for HD fly pathology, supplementation with antioxidant SOD and dietary antioxidants α-tocopherol and coenzyme Q10 in HD flies was not enough to rescue the lethal HD phenotype [296]. This observation is reminiscent of the Martin and colleagues finding that in rat mitochondria, glutathione levels were responding specifically to melatonin and not to other antioxidants, i.e., ascorbic acid and α-tocopherol [147]. These observations in different models make it tempting to speculate that melatonin may have unique critical properties. A clue about melatonin’s mechanism of action in HD may come from the observation that the skin fibroblasts of HD patients displayed significantly reduced activity of antioxidant catalase [303]; therefore, melatonin may potentially be used to increase catalase activity in HD. Antioxidant enzymes influenced by melatonin are well conserved in Drosophila and can be further studied to gain mechanistic detail (Table 2). As well, the amelioration of HD pathology is likely to involve an improved regulation of core melatonin-responsive clock-gene pathways, that can also be rapidly characterized in Drosophila [17,236]. Overall, melatonin and curcumin both showed promise in ameliorating HD symptoms, and further studies are required to understand their precise action following circadian clock disturbances and the potential for synergistic or additive effects.
5.1.2. Parkinson’s Disease
Recently, transgenic flies were generated by selectively expressing human (h) LRRK2 in mushroom bodies, where sleep is regulated in Drosophila [257]. “Humanized” LRRK2 transgenic flies recapitulate key properties of human PD such as motor impairment and the loss of dopaminergic neurons [257,313]. The hLRRK2-expressing flies displayed sleep fragmentation caused by elevated arousal at night, as well as the disturbance of presynaptic function demonstrated by the decrease in cellular excitability of Kenyon cells in mushroom bodies [257]. Melatonin treatment was found to ameliorate both conditions. The dark phase (night) mean sleep length was increased and the frequency of excitatory postsynaptic potentials (EPSPs) was restored to normal levels upon administering melatonin (4 mM) to the hLRRK2 transgenic flies [257]. The observed rescue was thought to reflect improved synaptic transmission due to a melatonin-dependent reduction in ROS, that would otherwise damage neurotransmitter release [257]. Similarly, SOD and catalase activity were found significantly decreased in PD patients as the disease progressed [295,314]. Melatonin treatment could also improve long-term memory deficits in hLRRK2 flies by regulating the presynaptic membrane Ca2+ activity of Kenyon cells, though exact mechanisms are still unknown [315]. More than 80% of PD patients experience cognitive decline often leading to long-term memory impairment [315,316]. Therefore, melatonin treatment could greatly improve their quality of life. Overall, melatonin seems to be a promising treatment for PD patients expressing LRRK2 mutations with severe sleep-related problems and cognitive decline.
5.1.3. Alzheimer’s Disease
AD is identified by the accumulation of plaques composed of amyloid beta (Aβ) peptides [243]. One dominant form of Aβ peptides, Aβ42, is speculated to play an important role at the start of AD pathogenesis [243]. In AD, Aβ42 oligomers form interactions with mitochondrial proteins, leading to mitochondrial dysfunction and excessive ROS production (reviewed in [317]). Thus, antioxidants such as melatonin have previously been used to inhibit Aβ oligomerization in AD [318]. A recent study has used transgenic flies overexpressing human Aβ42 in the central nervous system to study the effects of melatonin. The treatment was found to have many benefits in the AD Drosophila model. Melatonin (0.43 mM) significantly improved climbing ability and increased the AD fly life span compared to untreated control flies [243]. As well, an immunoblot analysis showed that Aβ42 expression was reduced in flies exposed to melatonin [243]. Moreover, fluorescence assays displayed normalized ROS levels in the mitochondria of treated AD flies, compared to untreated ones that, instead, contained high levels of ROS [243]. Because of these promising results, melatonin seems to be a potential treatment for AD due to its antioxidant properties, although a deeper characterization of these effects is needed.
5.2. Melatonin Treatment in a Novel Drosophila Model of Polycystic Kidney Disease
Recently, we showed that melatonin reduced cysts in a first-in-kind Drosophila model of autosomal-dominant polycystic kidney disease (ADPKD) [319], which raises the novel and intriguing possibility that melatonin may be beneficial in PKD treatment. ADPKD is a genetic disease caused by mutations in genes PKD1 (80% of all cases) or PKD2 (15% of all cases) [320]. ADPKD is characterized by the progressive formation of fluid-filled cysts along the length of the renal tubules (nephrons). Cystic growth disrupts normally regulated proliferation and apoptosis of the epithelial cells forming the renal tubule and it displays some neoplastic characteristics (reviewed in [321]). The first-in-kind Drosophila model of PKD harbors a mutation in the Bicaudal C (BicC) gene [231]. Conserved evolutionarily from flies to humans, the human BicC ortholog is called BICC1 and the murine one Bicc1. Both BICC1 and Bicc1 appear to be genetically downstream of key PKD gene PKD1 [231]. The mutation of any vertebrate BicC genes was sufficient to induce renal cysts (reviewed in [322]), implying that BicC dysregulation may contribute to renal cystic pathogenesis in conditions of PKD1 loss-of-function.
The Drosophila PKD model was developed by crossing flies containing a BicC deletion (Df(2 L)RA5/CyO or Δ) with flies containing a BicC hypomorphic mutation (BicCYC33/CyO), generating BicCΔ/YC33 mutant flies that recapitulate several phenotypic and molecular aspects of human PKD, such as the formation of fluid filled cysts along the renal (Malpighian) tubules, as well as increased activity of the mTOR kinase and myc upregulation, which both control cell proliferation and apoptosis [231,323]. BicC mutants also show conserved pharmacological response to Smac mimics [324,325] and rapamycin [231]. Melatonin (150 μM) was administered at night to the BicCΔ/YC33 mutant flies aged 0–2 days and Malpighian tubules were dissected after 18 days of treatment [319]. Melatonin was found to significantly reduce cysts by over 30% along the entire tubule length of BicCΔ/YC33 flies, compared to vehicle-treated flies [319]. Drosophila features one longer anterior and one shorter posterior Malpighian tubule pair, each with different transcriptomes and functions [326]. As well, each tubule pair contains three functionally distinct regions dubbed proximal, intermediate, and terminal (reviewed in [327]). Melatonin-promoted cyst reduction affected such regions differentially [319]. Compared to vehicle-treated controls, the proximal region of melatonin-treated flies was rescued most effectively with a 59% cyst reduction [319]. The intermediate and terminal regions showed 37 and 31% reduction, respectively [319]. These results highlight the functional differences occurring along the anterior and posterior tubule regions [319,328] (reviewed in [327]) and raise the intriguing possibility that such differences may be conserved to humans. Although not extensively studied, melatonin has been found to support normal kidney function in mammals. Mesenchymal stem cells pre-treated with melatonin and transplanted into the kidneys of a rat model of chronic kidney disease (CKD) displayed less disturbance of the basement membrane and improved renal tubule histology, while reducing overall fibrosis, a common complication [329,330]. As well, the preconditioning of the mesenchymal stem cells with melatonin reduced transforming growth factor (TGF)-β, tumor necrosis factor (TNF)-α, and α-smooth muscle actin expression, while increasing E-cadherin expression, indicating the amelioration of cell-to-cell adhesion in the tubules [329]. It was suggested that TNF-α reduction may be due to melatonin antioxidant activity, which decreases ROS production and inflammation [329,331], but additional investigation is needed to fully characterize the molecular mechanism.
While the potential mechanism of action for melatonin in renal cyst reduction is not known, one important feature of ADPKD is oxidative stress [297,299,332], which could be attenuated by the antioxidant properties of melatonin. Oxidative stress markers such as 8-isoprostane, asymmetric dimethylarginine and prostaglandin PGF2a have been shown to be elevated in ADPKD patients with a preserved estimated glomerular filtration rate (eGFR), as compared to normal individuals [332] (reviewed in [333]). Reduced levels of SOD, as well as glutathione peroxidase were also found in ADPKD patients [297,302]. Consistently, murine models of ADPKD featured decreased antioxidant enzymes glutathione peroxidase, catalase, glutathione S-transferase, and SOD, while displaying aggregates of lipid peroxidation byproducts in plasma and kidneys [299]. Moreover, murine models of ADPKD suggest that mitochondrial dysfunction may also play a role in inducing oxidative stress leading to cyst formation [334]. Mitochondrial function seems indirectly regulated by the PC1–PC2 complex, which promotes mitochondrial Ca2+ uptake and regulates oxidative phosphorylation [335]. Suggesting that renal cystic diseases may share a signature of oxidative stress, rat models for the autosomal recessive (AR) form of PKD also feature increased oxidative stress [336]; however, more markers need to be studied for this condition and to clarify mitochondrial involvement. Because the evolutionary ancient oxidative response pathways are conserved in Drosophila [291,292,293,294] (Table 2), Drosophila seems ideal to model how melatonin affects PKD-type cysts. Melatonin is also known to regulate several pathways implicated in neoplastic growth (e.g., mTOR (reviewed in [337]), MAPK [338,339], JAK/STAT3 [339,340,341], ERK [337,342], and TGF-β/Smad [343]). Several of these pathways are also altered in ADPKD tissue and related neoplastic renal cell carcinoma (reviewed in [321,344]). Thus, the observed improvements in the melatonin treated cystic BicC flies may result from the simultaneous correction of several cellular defects. Another possible contributing mechanism, ADPKD, similar to several other diseases, including cancer, causes a profound metabolic reprogramming of the renal cystic cells and defective glucose metabolism [345] such as or similar to the Warburg effect [346,347]. In these conditions, pyruvate, that is normally transported to the mitochondria, remains in the cytoplasm, and is fermented to lactate in hypoxic conditions. Melatonin appears to counteract the Warburg effect by inhibiting the hypoxia inducible factor (HIF) 1α, which increases pyruvate uptake by the mitochondria and its conversion to acetyl-coenzyme A [348]. As mentioned above, acetyl-coenzyme A is also a cofactor for melatonin synthesis, which consolidates the pathway. These melatonin-mediated effects have been found to rescue cell metabolism in oncology [349] and may also contribute to ameliorating the Warburg-like effects in the renal cyst and ADPKD. Considering that all these pathways are well-conserved in Drosophila (reviewed in [321,327,350]), and corresponding gene knockout and knockdown flies exist, it should be possible to decipher the molecular bases of melatonin efficacy as a cyst-reducing drug candidate. Overall, our Drosophila studies gave insight into a potential novel treatment for ADPKD, that being non-toxic, could likely be administered indefinitely to alleviate symptoms of chronic ADPKD. In oncology, melatonin also exhibited promise as a combination drug that potentiates chemotherapy and, simultaneously, shields normal tissue from its damaging effects [351,352,353,354,355,356,357,358,359,360,361]. Possibly, the melatonin ability to alleviate the side effects of other drugs may be tested in combination with tolvaptan, the only approved ADPKD drug that has displayed signs of potential hepatotoxicity [362,363,364]. While intriguing, further studies will be needed to fully determine the efficacy and applicability of melatonin treatment to ADPKD therapy.
6. Discussion
Melatonin is a universally conserved molecule and major regulator for (virtually) all biological organisms. It displays evolutionary ancient functions as an antioxidant and transcriptional regulator that protects both unicellular and multicellular organisms from endogenous and exogenous stress and oxidative injury. In the latter organisms, melatonin has acquired additional functions to synchronize gene expression with daily and seasonal variations of the light/dark cycle. Overall, melatonin affects key aspects of metabolism, longevity, and biological adaptation. Mostly studied in mammals, the evolutionary recent functions are mediated through multifunctional MT1 and MT2 receptors. Belonging to the GPCR family, MT1 and MT2 can homo- and hetero-dimerize with each other and heteromerize with other receptors, displaying remarkable functional diversity. Through such key roles, melatonin appears to be a master regulator fine-tuned to species-specific biology and conceivably holds immense therapeutic potential. It is, therefore, unsurprising to find disrupted melatonin-related pathways in several diseases. Circadian disruption is known to occur in neurodegenerative diseases such as AD, PD, and HD; however, altered light/dark cycles have been observed in several other conditions that are seemingly unrelated to the CNS, including ADPKD. Increased oxidative stress, and mitochondrial dysfunction, conditions underlying several diseases, could be improved through melatonin-induced renormalizing functions. Being non-toxic, melatonin makes it ideal for the protracted treatment needed in chronic disease. Speculatively, the melatonin capacity to affect and rebalance a multitude of pathways also makes it an ideal drug, especially in conditions causing metabolic reprogramming such as ADPKD and cancer. However, the incomplete knowledge of the myriad of melatonin roles and dosage effects still limits precise manipulation in diseased states. The potential for melatonin treatment is widely recognized in neurological disease and oncology, there is new promise for ADPKD and there appears to be an untapped capacity also in renal cell carcinoma, a malignancy sharing pathological aspects with ADPKD (reviewed in [321,344]). Considering that the complexity and redundancy of the mammalian melatonin pathways substantially complicate mechanistic studies, model organisms may be deployed to probe specific questions and mechanisms. Evolutionary conservation and a vast arsenal of genetic tools make Drosophila a key organism in which to distinguish core conserved ancient roles from those evolutionarily more recent. Due to the lack of bona fide MT1 and MT2 receptors, the powerful Drosophila genetics could potentially be used to generate humanized flies expressing human MT1 or MT2 receptors alone and in combination to test genetic interactions and gene modifiers in the whole organism and in specific tissues. Combined with up-and-coming fly pharmacology (reviewed in [227,350]) and toxicology [365,366], fly research promises to yield new biological knowledge with translational significance.
Author Contributions
Conceptualization, C.M.-B. and C.G.; data curation, C.M.-B., C.C.E., J.J., S.M. and A.P.; writing—original draft preparation, C.M.-B., C.C.E., J.J., S.M., A.P. and C.G.; writing—review and editing, C.M.-B., C.C.E., J.J., S.M., A.P. and C.G.; figures, C.C.E. and C.M.-B.; supervision, C.G.; project administration, C.G.; funding acquisition, C.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the NIH SCoRE grant P20GM103499-20 and the APC was funded by the SCoRE grant P20GM103499-20 and startup funds to C.G.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
C.G. is a member of the IDeA Networks of Biomedical Research Excellence (INBRE) and the Center of Excellence in Research on Orphan Diseases—Fondation Courtois.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chowdhury, I.; Sengupta, A.; Maitra, S.K. Melatonin: Fifty years of scientific journey from the discovery in bovine pineal gland to delineation of functions in human. Ind. J. Biochem. Biophys. 2008, 45, 289–304. [Google Scholar]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin synthesis and function: Evolutionary history in animals and plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef]
- Hattori, A.; Migitaka, H.; Iigo, M.; Itoh, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar] [PubMed]
- Manchester, L.C.; Tan, D.X.; Reiter, R.J.; Qi, W.; Karbownik, M.; Calvo, J.R. High levels of melatonin in edible seeds: Possible function in germ cell protection. Life Sci. 2000, 67, 3023–3029. [Google Scholar] [CrossRef]
- Reiter, R.J. Melatonin: Clinical relevance. Best Pract. Res. Clin. Endocrinol. Metab. 2003, 17, 273–285. [Google Scholar] [CrossRef]
- Masters, A.; Pandi-Perumal, S.R.; Seixas, A.; Girardin, J.L.; McFarlane, S.I. Melatonin, the hormone of darkness: From sleep promotion to Ebola treatment. Brain Disord. Ther. 2014, 4, 1000151. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Galano, A. Melatonin: Exceeding expectations. Physiology 2014, 29, 325–333. [Google Scholar] [CrossRef]
- Shi, H.; Chen, K.; Wei, Y.; He, C. Fundamental issues of melatonin-mediated stress signaling in plants. Front. Plant Sci. 2016, 7, 1124. [Google Scholar] [CrossRef]
- Tan, D.X.; Hardeland, R.; Back, K.; Manchester, L.C.; Alatorre-Jimenez, M.A.; Reiter, R.J. On the significance of an alternate pathway of melatonin synthesis via 5-methoxytryptamine: Comparisons across species. J. Pineal Res. 2016, 61, 27–40. [Google Scholar] [CrossRef]
- Cecon, E.; Oishi, A.; Jockers, R. Melatonin receptors: Molecular pharmacology and signalling in the context of system bias. Br. J. Pharmacol. 2018, 175, 3263–3280. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J. Melatonin: The chemical expression of darkness. Mol. Cell. Endocrinol. 1991, 79, C153–C158. [Google Scholar] [CrossRef]
- Reiter, R.J. Pineal melatonin: Cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 1991, 12, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Erren, T.C.; Reiter, R.J.; Piekarski, C. Chronodisruption and melatonin: The need for sensible exposure metrics in epidemiological studies. J. Pineal Res. 2008, 45, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin, hormone of darkness and more: Occurrence, control mechanisms, actions and bioactive metabolites. Cell Mol. Life Sci. 2008, 65, 2001–2018. [Google Scholar] [CrossRef]
- Jenwitheesuk, A.; Nopparat, C.; Mukda, S.; Wongchitrat, P.; Govitrapong, P. Melatonin regulates aging and neurodegeneration through energy metabolism, epigenetics, autophagy and circadian rhythm pathways. Int. J. Mol. Sci. 2014, 15, 16848–16884. [Google Scholar] [CrossRef]
- Venegas, C.; García, J.A.; Escames, G.; Ortiz, F.; López, A.; Doerrier, C.; García-Corzo, L.; López, L.C.; Reiter, R.J.; Acuña-Castroviejo, D. Extrapineal melatonin: Analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 2012, 52, 217–227. [Google Scholar] [CrossRef]
- Suofu, Y.; Li, W.; Jean-Alphonse, F.G.; Jia, J.; Khattar, N.K.; Li, J.; Baranov, S.V.; Leronni, D.; Mihalik, A.C.; He, Y.; et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. USA 2017, 114, E7997–E8006. [Google Scholar] [CrossRef]
- Tan, D.X.; Reiter, R.J. Mitochondria: The birth place, battle ground and the site of melatonin metabolism in cells. Melatonin Res. 2019, 2, 1. [Google Scholar] [CrossRef]
- Macchi, M.M.; Bruce, J.N. Human pineal physiology and functional significance of melatonin. Front. Neuroendocrinol. 2004, 25, 177–195. [Google Scholar] [CrossRef]
- Dubocovich, M.L.; Markowska, M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine 2005, 27, 101–110. [Google Scholar] [CrossRef]
- Reiter, R.J.; Melchiorri, D.; Sewerynek, E.; Poeggeler, B.; Barlow-Walden, L.; Chuang, J.; Ortiz, G.G.; Acuña-Castroviejo, D. A review of the evidence supporting melatonin’s role as an antioxidant. J. Pineal Res. 1995, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Reiter, R.J.; Menéndez-Peláez, A.; Pablos, M.I.; Burgos, A. Characterization of high-affinity melatonin binding sites in purified cell nuclei of rat liver. J. Pineal Res. 1994, 16, 100–112. [Google Scholar] [CrossRef]
- Emet, M.; Ozcan, H.; Ozel, L.; Yayla, M.; Halici, Z.; Hacimuftuoglu, A. A review of melatonin, its receptors and drugs. Eurasian J. Med. 2016, 48, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Zheng, X.; Kong, J.; Manchester, L.C.; Hardeland, R.; Kim, S.J.; Xu, X.; Reiter, R.J. Fundamental issues related to the origin of melatonin and melatonin isomers during evolution: Relation to their biological functions. Int. J. Mol. Sci. 2014, 15, 15858–15890. [Google Scholar] [CrossRef]
- Poeggeler, B.; Saarela, S.; Reiter, R.J.; Tan, D.X.; Chen, L.D.; Manchester, L.C.; Barlow-Walden, L.R. Melatonin—A highly potent endogenous radical scavenger and electron donor: New aspects of the oxidation chemistry of this indole accessed in vitro. Ann. N. Y. Acad. Sci. 1994, 738, 419–420. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Plummer, B.F.; Hardies, L.J.; Weintraub, S.T.; Vijayalaxmi; Shepherd, A.M. A novel melatonin metabolite, cyclic 3-hydroxymelatonin: A biomarker of in vivo hydroxyl radical generation. Biochem. Biophys. Res. Commun. 1998, 253, 614–620. [Google Scholar] [CrossRef]
- Margulis, L. Symbiotic theory of the origin of eukaryotic organelles; criteria for proof. Symp. Soc. Exp. Biol. 1975, 29, 21–38. [Google Scholar]
- Reiter, R.J.; Rosales-Corral, S.; Tan, D.X.; Jou, M.J.; Galano, A.; Xu, B. Melatonin as a mitochondria-targeted antioxidant: One of evolution’s best ideas. Cell. Mol. Life Sci. 2017, 74, 3863–3881. [Google Scholar] [CrossRef]
- Cecon, E.; Liu, L.; Jockers, R. Melatonin receptor structures shed new light on melatonin research. J. Pineal Res. 2019, 67, e12606. [Google Scholar] [CrossRef]
- Barrett, P.; Conway, S.; Morgan, P.J. Digging deep—Structure-function relationships in the melatonin receptor family. J. Pineal Res. 2003, 35, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Pandi-Perumal, S.R.; Trakht, I.; Srinivasan, V.; Spence, D.W.; Maestroni, G.J.M.; Zisapel, N.; Cardinali, D.P. Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 2008, 85, 335–353. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Ursinus, J.; Zhou, J.N.; Scheer, F.A.; Ai-Min, B.; Jockers, R.; van Heerikhuize, J.; Swaab, D.F. Alterations of melatonin receptors MT1 and MT2 in the hypothalamic suprachiasmatic nucleus during depression. J. Affect. Disord. 2013, 148, 357–367. [Google Scholar] [CrossRef]
- Benleulmi-Chaachoua, A.; Chen, L.; Sokolina, K.; Wong, V.; Jurisica, I.; Emerit, M.B.; Darmon, M.; Espin, A.; Stagljar, I.; Tafelmeyer, P.; et al. Protein interactome mining defines melatonin MT1 receptors as integral component of presynaptic protein complexes of neurons. J. Pineal Res. 2016, 60, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Jockers, R.; Delagrange, P.; Dubocovich, M.L.; Markus, R.P.; Renault, N.; Tosini, G.; Cecon, E.; Zlotos, D.P. Update on melatonin receptors: IUPHAR review 20. Br. J. Pharmacol. 2016, 173, 2702–2725. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Clough, S.J.; Hutchinson, A.J.; Adamah-Biassi, E.B.; Popovska-Gorevski, M.; Dubocovich, M.L. MT1 and MT2 melatonin receptors: A therapeutic perspective. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 361–383. [Google Scholar] [CrossRef]
- Nosjean, O.; Ferro, M.; Coge, F.; Beauverger, P.; Henlin, J.M.; Lefoulon, F.; Fauchere, J.L.; Delagrange, P.; Canet, E.; Boutin, J.A. Identification of the melatonin-binding site MT3 as the quinone reductase 2. J. Biol. Chem. 2000, 275, 31311–31317. [Google Scholar] [CrossRef]
- Ekmekcioglu, C. Melatonin receptors in humans: Biological role and clinical relevance. Biomed. Pharmacother. 2006, 60, 97–108. [Google Scholar] [CrossRef]
- Dubocovich, M.L.; Delagrange, P.; Krause, D.N.; Sugden, D.; Cardinali, D.P.; Olcese, J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol. Rev. 2010, 62, 343–380. [Google Scholar] [CrossRef]
- Li, D.Y.; Smith, D.G.; Hardeland, R.; Yang, M.Y.; Xu, H.L.; Zhang, L.; Yin, H.D.; Zhu, Q. Melatonin receptor genes in vertebrates. Int. J. Mol. Sci. 2013, 14, 11208–11223. [Google Scholar] [CrossRef]
- Stauch, B.; Johansson, L.C.; Cherezov, V. Structural insights into melatonin receptors. FEBS J. 2020, 287, 1496–1510. [Google Scholar] [CrossRef]
- Levoye, A.; Jockers, R.; Ayoub, M.A.; Delagrange, P.; Savaskan, E.; Guillaume, J.L. Are G protein-coupled receptor heterodimers of physiological relevance? Focus on melatonin receptors. Chronobiol. Int. 2006, 23, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, M.A.; Couturier, C.; Lucas-Meunier, E.; Angers, S.; Fossier, P.; Bouvier, M.; Jockers, R. Monitoring of ligand-independent dimerization and ligand-induced conformational changes of melatonin receptors in living cells by bioluminescence resonance energy transfer. J. Biol. Chem. 2002, 277, 21522–21528. [Google Scholar] [CrossRef]
- Oishi, A.; Cecon, E.; Jockers, R. Melatonin receptor signaling: Impact of receptor oligomerization on receptor function. Int. Rev. Cell Mol. Biol. 2018, 338, 59–77. [Google Scholar] [CrossRef] [PubMed]
- Luchetti, F.; Canonico, B.; Betti, M.; Arcangeletti, M.; Pilolli, F.; Piroddi, M.; Canesi, L.; Papa, S.; Galli, F. Melatonin signaling and cell protection function. FASEB J. 2010, 24, 3603–3624. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.Y.; Leong, M.K.; Liang, H.; Paxinos, G. Melatonin receptors: Distribution in mammalian brain and their respective putative functions. Brain Struct. Funct. 2017, 222, 2921–2939. [Google Scholar] [CrossRef] [PubMed]
- Jockers, R.; Maurice, P.; Boutin, J.A.; Delagrange, P. Melatonin receptors, heterodimerization, signal transduction and binding sites: What’s new? Br. J. Pharmacol. 2008, 154, 1182–1195. [Google Scholar] [CrossRef]
- Li, Y.; Lv, Y.; Bian, C.; You, X.; Shi, Q. Molecular evolution of melatonin receptor genes (mtnr) in vertebrates and its shedding light on mtnr1c. Gene 2021, 769, 145256. [Google Scholar] [CrossRef]
- Guyomard, R.; Boussaha, M.; Krieg, F.; Hervet, C.; Quillet, E. A synthetic rainbow trout linkage map provides new insights into the salmonid whole genome duplication and the conservation of synteny among teleosts. BMC Genet. 2012, 13, 15. [Google Scholar] [CrossRef]
- Levoye, A.; Dam, J.; Ayoub, M.A.; Guillaume, J.L.; Couturier, C.; Delagrange, P.; Jockers, R. The orphan GPR50 receptor specifically inhibits MT1 melatonin receptor function through heterodimerization. EMBO J. 2006, 25, 3012–3023. [Google Scholar] [CrossRef]
- Ma, H.; Kang, J.; Fan, W.; He, H.; Huang, F. ROR: Nuclear receptor for melatonin or not? Molecules 2021, 26, 2693. [Google Scholar] [CrossRef]
- Pace-Schott, E.F.; Hobson, J.A. The neurobiology of sleep: Genetics, cellular physiology and subcortical networks. Nat. Rev. Neurosci. 2002, 3, 591–605. [Google Scholar] [CrossRef]
- Gobbi, G.; Comai, S. Sleep well. Untangling the role of melatonin MT1 and MT2 receptors in sleep. J. Pineal Res. 2019, 66, e12544. [Google Scholar] [CrossRef]
- Dubocovich, M.L. Melatonin receptors: Role on sleep and circadian rhythm regulation. Sleep Med. 2007, 8, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Scher, J.; Wankiewicz, E.; Brown, G.M.; Fujieda, H. MT1 melatonin receptor in the human retina: Expression and localization. Investig. Ophthalmol. Vis. Sci. 2002, 43, 889–897. [Google Scholar]
- Rada, J.A.; Wiechmann, A.F. Melatonin receptors in chick ocular tissues: Implications for a role of melatonin in ocular growth regulation. Invest. Ophthalmol. Vis. Sci. 2006, 47, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Arangino, S.; Cagnacci, A.; Angiolucci, M.; Vacca, A.M.; Longu, G.; Volpe, A.; Melis, G.B. Effects of melatonin on vascular reactivity, catecholamine levels, and blood pressure in healthy men. Am. J. Cardiol. 1999, 83, 1417–1419. [Google Scholar] [CrossRef]
- Srinivasan, V.; Maestroni, G.J.M.; Cardinali, D.P.; Esquifino, A.I.; Pandi-Perumal, S.R.; Miller, S.C. Melatonin, immune function and aging. Immun. Ageing 2005, 2, 17. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Reiter, R.J.; Lardone, P.J.; Herrera, J.L.; Fernandez-Montesinos, R.; Guerrero, J.M.; Pozo, D. The modulatory role of melatonin on immune responsiveness. Curr. Opin. Investig. Drugs 2006, 7, 423–431. [Google Scholar]
- Slominski, A.; Pisarchik, A.; Wortsman, J. Expression of genes coding melatonin and serotonin receptors in rodent skin. Biochim. Biophys. Acta 2004, 1680, 67–70. [Google Scholar] [CrossRef]
- Weaver, D.R.; Liu, C.; Reppert, S.M. Nature’s knockout: The Mel1b receptor is not necessary for reproductive and circadian responses to melatonin in Siberian hamsters. Mol. Endocrinol. 1996, 10, 1478–1487. [Google Scholar] [CrossRef]
- Adamah-Biassi, E.B.; Zhang, Y.; Jung, H.; Vissapragada, S.; Miller, R.J.; Dubocovich, M.I. Distribution of MT1 melatonin receptor promoter-driven RFP expression in the brains of BAC C3H/HeN transgenic mice. J. Histochem. Cytochem. 2014, 62, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.E.; McKellar, S.; Klein, D.C. Melatonin inhibition of the in vivo pituitary response to luteinizing hormone-releasing hormone in the neonatal rat. Neuroendocrinology 1980, 31, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Vanecek, J.; Vollrath, L. Developmental changes and daily rhythm in melatonin-induced inhibition of 3′,5′-cyclic AMP accumulation in the rat pituitary. Endocrinology 1990, 126, 1509–1513. [Google Scholar] [CrossRef]
- Vanecek, J.; Klein, D.C. Melatonin inhibition of GnRH-induced LH release from neonatal rat gonadotroph: Involvement of Ca2+ not cAMP. Am. J. Physiol. 1995, 269, E85–E90. [Google Scholar] [CrossRef] [PubMed]
- von Gall, C.; Garabette, M.L.; Kell, C.A.; Frenzel, S.; Dehghani, F.; Schumm-Draeger, P.M.; Weaver, D.R.; Korf, H.W.; Hastings, M.H.; Stehle, J.H. Rhythmic gene expression in pituitary depends on heterologous sensitization by the neurohormone melatonin. Nat. Neurosci. 2002, 5, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Frungieri, M.B.; Mayerhofer, A.; Zitta, K.; Pignataro, O.P.; Calandra, R.S.; Gonzalez-Calvar, S.I. Direct effect of melatonin on Syrian hamster testes: Melatonin subtype 1a receptors, inhibition of androgen production, and interaction with the local corticotropin-releasing hormone system. Endocrinology 2005, 146, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Torres-Farfan, C.; Richter, H.G.; Rojas-Garcia, P.; Vergara, M.; Forcelledo, M.L.; Valladares, L.E.; Torrealba, F.; Valenzuela, G.J.; Seron-Ferre, M. MT1 melatonin receptor in the primate adrenal gland: Inhibition of adrenocorticotropin-stimulated cortisol production by melatonin. J. Clin. Endocrinol. Metab. 2003, 88, 450–458. [Google Scholar] [CrossRef]
- Torres-Farfan, C.; Richter, H.G.; Germain, A.M.; Valenzuela, G.J.; Campino, C.; Rojas-Garcia, P.; Forcelledo, M.L.; Torrealba, F.; Seron-Ferre, M. Maternal melatonin selectively inhibits cortisol production in the primate fetal adrenal gland. J. Physiol. 2004, 554, 841–856. [Google Scholar] [CrossRef]
- Alonso-Vale, M.I.C.; Andreotti, S.; Peres, S.B.; Anhe, G.F.; Borges-Silva, C.N.; Neto, J.C.; Lima, F.B. Melatonin enhances leptin expression by rat adipocytes in the presence of insulin. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E805–E812. [Google Scholar] [CrossRef]
- Kemp, D.M.; Ubeda, M.; Habener, J.F. Identification and functional characterization of melatonin Mel 1a receptors in pancreatic beta cells: Potential role in incretin-mediated cell function by sensitization of cAMP signaling. Mol. Cell. Endocrinol. 2002, 191, 157–166. [Google Scholar] [CrossRef]
- Nelson, M.T.; Quayle, J.M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am. J. Physiol. 1995, 268, C799–C822. [Google Scholar] [CrossRef] [PubMed]
- Doolen, S.; Krause, D.N.; Dubocovich, M.L.; Duckles, S.P. Melatonin mediates two distinct responses in vascular smooth muscle. Eur. J. Pharmacol. 1998, 345, 67–69. [Google Scholar] [CrossRef]
- Geary, G.G.; Duckles, S.P.; Krause, D.N. Effect of melatonin in the rat tail artery: Role of K+ channels and endothelial factors. Br. J. Pharmacol. 1998, 123, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Masana, M.I.; Doolen, S.; Ersahin, C.; Al-Ghoul, W.M.; Duckles, S.P.; Dubocovich, M.L.; Krause, D.N. MT2 melatonin receptors are present and functional in rat caudal artery. J. Pharmacol. Exp. Ther. 2002, 302, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Zhang, H.; Jin, C.; Qiu, F.; Wu, Y.; Shi, L. Melatonin mediates vasodilation through both direct and indirect activation of BKCa channels. J. Mol. Endocrinol. 2017, 59, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Shiu, S.Y.; Li, L.; Xu, J.N.; Pang, C.S.; Wong, J.T.; Pang, S.F. Melatonin-induced inhibition of proliferation and G1/S cell cycle transition delay of human choriocarcinoma Jar cells: Possible involvement of MT2 (MEL1B) receptor. J. Pineal Res. 1999, 27, 183–192. [Google Scholar] [CrossRef]
- Collins, A.; Yuan, L.; Kiefer, T.L.; Cheng, Q.; Lai, L.; Hill, S.M. Overexpression of the MT1 melatonin receptor in MCF-7 human breast cancer cells inhibits mammary tumor formation in nude mice. Cancer Lett. 2003, 189, 49–57. [Google Scholar] [CrossRef]
- Drazen, D.L.; Nelson, R.J. Melatonin receptor subtype MT2 (Mel 1b) and not MT1 (Mel 1a) is associated with melatonin-induced enhancement of cell-mediated and humoral immunity. Neuroendocrinology 2001, 74, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Lotufo, C.M.; Lopes, C.; Dubocovich, M.L.; Farsky, S.H.; Markus, R.P. Melatonin and N-acetylserotonin inhibit leukocyte rolling and adhesion to rat microcirculation. Eur. J. Pharmacol. 2001, 430, 351–357. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Garcia-Maurino, S.; Calvo, J.R.; Guerrero, J.M. Melatonin counteracts the inhibitory effect of PGE2 on IL-2 production in human lymphocytes via its mt1 membrane receptor. FASEB J. 2003, 17, 755–757. [Google Scholar] [CrossRef]
- Kadekaro, A.L.; Andrade, L.N.; Floeter-Winter, L.M.; Rollag, M.D.; Virador, V.; Vieira, W.; Castrucci, A.M.D.L. MT-1 melatonin receptor expression increases the antiproliferative effect of melatonin on S-91 murine melanoma cells. J. Pineal Res. 2004, 36, 204–211. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Lardone, P.J.; Fernandez-Santos, J.M.; Martin-Lacave, I.; Calvo, J.R.; Karasek, M.; Guerrero, J.M. Human lymphocyte-synthesized melatonin is involved in the regulation of the interleukin-2/interleukin-2 receptor system. J. Clin. Endocrinol. Metab. 2005, 90, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P.J.; Barrett, P.; Howell, H.E.; Helliwell, R. Melatonin receptors: Localization, molecular pharmacology and physiological significance. Neurochem. Int. 1994, 24, 101–146. [Google Scholar] [CrossRef]
- Comai, S.; Ochoa-Sanchez, R.; Gobbi, G. Sleep-wake characterization of double MT1/MT2 receptor knockout mice and comparison with MT1 and MT2 receptor knockout mice. Behav. Brain Res. 2013, 243, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Boutin, J.A.; Audinot, V.; Ferry, G.; Delagrange, P. Molecular tools to study melatonin pathways and actions. Trends Pharmacol. Sci. 2005, 26, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Sugden, D.; McArthur, A.J.; Ajpru, S.; Duniec, K.; Piggins, H.D. Expression of mt1 melatonin receptor subtype mRNA in the entrained rat suprachiasmatic nucleus: A quantitative RT-PCR study across the diurnal cycle. Brain Res. Mol. Brain Res. 1999, 72, 176–182. [Google Scholar] [CrossRef]
- Lacoste, B.; Angeloni, D.; Dominguez-Lopez, S.; Calderoni, S.; Mauro, A.; Faschini, F.; Descarries, L.; Gobbi, G. Anatomical and cellular localization of melatonin MT1 and MT2 receptors in the adult rat brain. J. Pineal Res. 2015, 58, 397–417. [Google Scholar] [CrossRef] [PubMed]
- Waly, N.E.; Hallworth, R. Circadian pattern of melatonin MT1 and MT2 receptor localization in the rat suprachiasmatic nucleus. J. Circadian Rhythms 2015, 13, 1. [Google Scholar] [CrossRef]
- Klosen, P.; Lapmanee, S.; Schuster, C.; Guardiola, B.; Hicks, D.; Pevet, P.; Felder-Schmittbuhl, M.P. MT1 and MT2 melatonin receptors are expressed in nonoverlapping neuronal populations. J. Pineal Res. 2019, 67, e12575. [Google Scholar] [CrossRef]
- Baba, K.; Benleulmi-Chaachoua, A.; Journé, A.S.; Kamal, M.; Guillaume, J.L.; Dussaud, S.; Gbahou, F.; Yettou, K.; Liu, C.; Contreras-Alcantara, S.; et al. Heteromeric MT1/MT2 melatonin receptors modulate photoreceptor function. Sci. Signal. 2013, 6, ra89. [Google Scholar] [CrossRef]
- Perreau-Lenz, S.; Kalsbeek, A.; Garidou, M.L.; Wortel, J.; Van Der Vliet, J.; Van Heijningen, C.; Simonneaux, V.; Pevet, P.; Buijs, R.M. Suprachiasmatic control of melatonin synthesis in rats: Inhibitory and stimulatory mechanisms. Eur. J. Neurosci. 2003, 17, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, W.B.; Gillin, J.C.; Dawson, S.D.; Lewy, A.J.; Wyatt, R.J. Effects of melatonin on propranolol on sleep of the rat. Brain Res. 1980, 201, 240–244. [Google Scholar] [CrossRef]
- Zhdanova, I.V.; Wurtman, R.J.; Morabito, C.; Piotrovska, V.R.; Lynch, H.J. Effects of low oral doses of melatonin, given 2-4 hours before habitual bedtime, on sleep in normal young humans. Sleep 1996, 19, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.; Deboer, T.; Schwierin, B.; Tobler, I. Effect of melatonin on sleep and brain temperature in the Djungarian hamster and the rat. Physiol Behav. 1998, 65, 77–82. [Google Scholar] [CrossRef]
- Cipolla-Neto, J.; do Amaral, F.G. Melatonin as a hormone: New physiological and clinical insights. Endocr. Rev. 2018, 39, 990–1028. [Google Scholar] [CrossRef]
- Kalsbeek, A.; Buijs, R.M. Output pathways of the mammalian suprachiasmatic nucleus: Coding circadian time by transmitter selection and specific targeting. Cell Tissue Res. 2002, 309, 109–118. [Google Scholar] [CrossRef]
- Borjigin, J.; Zhang, L.S.; Calinescu, A.A. Circadian regulation of pineal gland rhythmicity. Mol. Cell Endocrinol. 2012, 349, 13–19. [Google Scholar] [CrossRef]
- Pinato, L.; Ramos, D.; Hataka, A.; Rossignoli, P.S.; Granado, M.D.J.; Mazzetto, M.C. Day/night expression of MT1 and MT2 receptors in hypothalamic nuclei of the primate Sapajus apella. J. Chem. Neuroanat. 2017, 81, 10–17. [Google Scholar] [CrossRef]
- James, F.O.; Cermakian, N.; Boivin, D.B. Circadian rhythms of melatonin, cortisol, and clock gene expression during simulated night shift work. Sleep 2007, 30, 1427–1436. [Google Scholar] [CrossRef]
- Jan, J.E.; Reiter, R.J.; Wong, P.K.H.; Bax, M.C.O.; Ribary, U.; Wasdell, M.B. Melatonin has membrane receptor-independent hypnotic action on neurons: An hypothesis. J. Pineal Res. 2010, 50, 233–240. [Google Scholar] [CrossRef]
- Fisher, S.P.; Sugden, D. Sleep-promoting action of IIK7, a selective MT2 melatonin receptor agonist in the rat. Neurosci. Lett. 2009, 457, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.P.; Sugden, D. Endogenous melatonin is not obligatory for the regulation of the rat sleep-wake cycle. Sleep 2010, 33, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Witt-Enderby, P.A.; Bennett, J.; Jarzynka, M.J.; Firestine, S.; Melan, M.A. Melatonin receptors and their regulation: Biochemical and structural mechanisms. Life Sci. 2003, 72, 2183–2198. [Google Scholar] [CrossRef]
- Gerdin, M.J.; Masana, M.I.; Dubocovich, M.L. Melatonin-mediated regulation of human MT1 melatonin receptors expressed in mammalian cells. Biochem. Pharmacol. 2004, 67, 2023–2030. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, G.; Comai, S. Differential function of melatonin MT1 and MT2 receptors in REM and NREM sleep. Front. Endocrinol. 2019, 10, 87. [Google Scholar] [CrossRef]
- Grossini, E.; Molinari, C.; Uberti, F.; Mary, D.A.S.G.; Vacca, G.; Caimmi, P.P. Intracoronary melatonin increases coronary blood flow and cardiac function through β-adrenoreceptors, MT1/MT2 receptors, and nitric oxide in anesthetized pigs. J. Pineal Res. 2011, 51, 246–257. [Google Scholar] [CrossRef]
- Ersahin, C.; Masana, M.I.; Dubocovich, M.L. Constitutively active melatonin MT1 receptors in male rat caudal arteries. Eur. J. Pharmacol. 2002, 439, 171–172. [Google Scholar] [CrossRef]
- Capsoni, S.; Viswanathan, M.; De Oliveira, A.M.; Saavedra, J.M. Characterization of melatonin receptors and signal transduction system in rat arteries forming the circle of Willis. Endocrinology 1994, 135, 373–378. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Korkmaz, A. The circadian melatonin rhythm and its modulation: Possible impact on hypertension. J. Hypertens. Suppl. 2009, 27, S17–S20. [Google Scholar] [CrossRef]
- Weekley, L.B. Effects of melatonin on pulmonary and coronary vessels are exerted through perivascular nerves. Clin. Auton. Res. 1993, 3, 45–47. [Google Scholar] [CrossRef]
- Satake, N.; Shibata, S.; Takagi, T. The inhibitory action of melatonin on the contractile response to 5-hydroxytryptamine in various isolated vascular smooth muscles. Gen. Pharmacol. 1986, 17, 553–558. [Google Scholar] [CrossRef]
- Shibata, S.; Satake, N.; Takagi, T.; Usui, H. Vasorelaxing action of melatonin in rabbit basilar artery. Gen. Pharmacol. 1989, 20, 677–680. [Google Scholar] [CrossRef]
- Satake, N.; Oe, H.; Sawada, T.; Shibata, S. The mode of vasorelaxing action of melatonin in rabbit aorta. Gen. Pharmacol. 1991, 22, 219–221. [Google Scholar] [CrossRef]
- Satake, N.; Oe, H.; Shibata, S. Vasorelaxing action of melatonin in rat isolated aorta; possible endothelium dependent relaxation. Gen. Pharmacol. 1991, 22, 1127–1133. [Google Scholar] [CrossRef]
- Capsoni, S.; Stankov, B.M.; Fraschini, F. Reduction of regional cerebral blood flow by melatonin in young rats. Neuroreport 1995, 6, 1346–1348. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, C.; Giummelly, P.; Atkinson, J.; Delagrange, P.; Scalbert, E.; Capdeville-Atkinson, C. Melatonin potentiates NE-induced vasoconstriction without augmenting cytosolic calcium concentration. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H420–H425. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.S.; Sauder, C.L.; Ray, C.A. Melatonin differentially affects vascular blood flow in humans. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H670–H674. [Google Scholar] [CrossRef] [PubMed]
- Ting, N.; Thambyraja, A.; Sugden, D.; Scalbert, E.; Delagrange, P.; Wilson, V.G. Pharmacological studies on the inhibitory action of melatonin and putative melatonin analogues on porcine vascular smooth muscle. Naunyn Schmiedebergs Arch. Pharmacol. 2000, 36, 327–333. [Google Scholar] [CrossRef]
- Okatani, Y.; Wakatsuki, A.; Kaneda, C. Melatonin increases activities of glutathione peroxidase and superoxide dismutase in fetal rat brain. J. Pineal Res. 2000, 28, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Terron, M.P.; Flores, L.J.; Czarnocki, Z. Melatonin and its metabolites: New findings regarding their production and their radical scavenging actions. Acta. Biochim. Pol. 2007, 54, 1–9. [Google Scholar] [CrossRef]
- Huang, C.C.; Lai, C.J.; Tsai, M.H.; Wu, Y.C.; Chen, K.T.; Jou, M.J.; Fu, P.I.; Wu, C.H.; Wei, I.H. Effects of melatonin on the nitric oxide system and protein nitration in the hypobaric hypoxic rat hippocampus. BMC Neurosci. 2015, 16, 61. [Google Scholar] [CrossRef]
- Drazen, D.L.; Bilu, D.; Bilbo, S.D.; Nelson, R.J. Melatonin enhancement of splenocyte proliferation is attenuated by luzindole, a melatonin receptor antagonist. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R1476–R1482. [Google Scholar] [CrossRef]
- Swaminathan, A.; Lucas, R.M.; Dear, K.; McMichael, A.J. Keyhole limpet haemocyanin-a model antigen for human immunotoxicological studies. Br. J. Clin. Pharmacol. 2014, 78, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.E.; Wiechmann, A.F.; Hu, D.N. Melatonin receptors in human uveal melanocytes and melanoma cells. J. Pineal Res. 2000, 28, 165–171. [Google Scholar] [CrossRef]
- Tarocco, A.; Caroccia, N.; Morciano, G.; Wieckowski, M.R.; Ancora, G.; Garani, G.; Pinton, P. Melatonin as a master regulator of cell death and inflammation: Molecular mechanisms and clinical implications for newborn care. Cell Death Dis. 2019, 10, 317. [Google Scholar] [CrossRef]
- Ko, C.H.; Takahashi, J.S. Molecular components of the mammalian circadian clock. Hum. Mol. Genet. 2006, 15, R271–R277. [Google Scholar] [CrossRef]
- Jetten, A.M. Retinoid-related orphan receptors (RORs): Critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl. Recept. Signal. 2009, 7, e003. [Google Scholar] [CrossRef] [PubMed]
- Lardone, P.J.; Guerrero, J.M.; Fernández-Santos, J.M.; Rubio, A.; Martín-Lacave, I.; Carrillo-Vico, A. Melatonin synthesized by T lymphocytes as a ligand of the retinoic acid-related orphan receptor. J. Pineal Res. 2011, 51, 454–462. [Google Scholar] [CrossRef]
- Ram, P.T.; Dai, J.; Yuan, L.; Dong, C.; Kiefer, T.L.; Lai, L.; Hill, S.M. Involvement of the mt1 melatonin receptor in human breast cancer. Cancer Lett. 2002, 179, 141–150. [Google Scholar] [CrossRef]
- Chang, H.C.; Guarente, L. SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell 2013, 153, 1448–1460. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Feng, N.; Tang, D.; Feng, J.; Li, Z.; Jia, M.; Liu, Z.; Gu, X.; Wang, Y.; Fu, F.; et al. Melatonin prevents Drp1-mediated mitochondrial fission in diabetic hearts through SIRT1-PGC1a pathway. J. Pineal Res. 2018, 65, e12491. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin and inflammation-Story of a double-edged blade. J. Pineal Res. 2018, 65, e12525. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Jou, M.J.; Acuna-Castroviejo, D. Melatonin mitigates mitochondrial meltdown: Interactions with SIRT3. Int. J. Mol. Sci. 2018, 19, 2439. [Google Scholar] [CrossRef]
- Sánchez, D.I.; González-Fernández, B.; Crespo, I.; San-Miguel, B.; Álvarez, M.; González-Gallego, J.; Tuñón, M.J. Melatonin modulates dysregulated circadian clocks in mice with diethylnitrosamine-induced hepatocellular carcinoma. J. Pineal Res. 2018, 65, e12506. [Google Scholar] [CrossRef]
- Han, D.; Wang, Y.; Chen, J.; Zhang, J.; Yu, P.; Zhang, R.; Li, S.; Tao, B.; Wang, Y.; Qiu, Y.; et al. Activation of melatonin receptor 2 but not melatonin receptor 1 mediates melatonin-conferred cardioprotection against myocardial ischemia/reperfusion injury. J. Pineal Res. 2019, 67, e12571. [Google Scholar] [CrossRef]
- Merlo, S.; Luaces, J.P.; Spampinato, S.F.; Toro-Urrego, N.; Caruso, G.I.; D’Amico, F.; Capani, F.; Sortino, M.A. SIRT1 mediates melatonin’s effects on microglial activation in hypoxia: In vitro and in vivo evidence. Biomolecules 2020, 10, 364. [Google Scholar] [CrossRef]
- Guerrero, H.Y.; Gauer, F.; Pevet, P.; Masson-Pevet, M. Daily and circadian expression patterns of mt1 melatonin receptor mRNA in the rat pars tuberalis. Adv. Exp. Med. Biol. 1999, 460, 175–179. [Google Scholar] [CrossRef]
- Gubitz, A.K.; Reppert, S.M. Assignment of the melatonin-related receptor to human chromosome X (GPR50) and mouse chromosome X (Gpr50). Genomics 1999, 55, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, I.R.; Mazuruk, K.; Schoen, T.J.; Chader, G.J. Structural analysis of the human hydroxyindole-O-methyltransferase gene. Presence of two distinct promoters. J. Biol. Chem. 1994, 269, 31969–31977. [Google Scholar] [CrossRef]
- Estrada-Rodgers, L.; Levy, G.N.; Weber, W.W. Characterization of a hormone response element in the mouse N-acetyltransferase 2 (Nat2*) promoter. Gene Expr. 1998, 7, 13–24. [Google Scholar]
- Muxel, S.M.; Laranjeira-Silva, M.F.; Carvalho-Sousa, C.E.; Floeter-Winter, L.M.; Markus, R.P. The RelA/cRel nuclear factor-κB (NF-κB) dimer, crucial for inflammation resolution, mediates the transcription of the key enzyme in melatonin synthesis in RAW 264.7 macrophages. J. Pineal Res. 2016, 60, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Boveris, A. Mitochondrial production of superoxide radical and hydrogen peroxide. Adv. Exp. Med. Biol. 1977, 78, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Sohal, R.S.; Brunk, U.T. Mitochondrial production of pro-oxidants and cellular senescence. Mutat. Res. 1992, 275, 295–304. [Google Scholar] [CrossRef]
- Martin, M.; Macias, M.; Escames, G.; Leon, J.; Acuna-Castroviejo, D. Melatonin but not vitamins C and E maintains glutathione homeostasis in t-butyl hydroperoxide-induced mitochondrial oxidative stress. FASEB J. 2000, 14, 1677–1679. [Google Scholar] [CrossRef]
- Huo, X.; Wang, C.; Yu, Z.; Peng, Y.; Wang, S.; Feng, S.; Zhang, S.; Tian, X.; Sun, C.; Liu, K.; et al. Human transporters, PEPT1/2, facilitate melatonin transportation into mitochondria of cancer cells: An implication of the therapeutic potential. J. Pineal Res. 2017, 62, e12390. [Google Scholar] [CrossRef]
- Hevia, D.; González-Menéndez, P.; Quiros-González, I.; Miar, A.; Rodríguez-García, A.; Tan, D.X.; Reiter, R.J.; Mayo, J.C.; Sainz, R.M. Melatonin uptake through glucose transporters: A new target for melatonin inhibition of cancer. J. Pineal Res. 2015, 58, 234–250. [Google Scholar] [CrossRef]
- Reiter, R.J.; Ma, Q.; Sharma, R. Melatonin in mitochondria: Mitigating clear and present dangers. Physiology 2020, 35, 86–95. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; López, L.C.; Escames, G.; López, A.; García, J.A.; Reiter, R.J. Melatonin-mitochondria interplay in health and disease. Curr. Top. Med. Chem. 2011, 11, 221–240. [Google Scholar] [CrossRef]
- Fridovich, I. Oxygen: How do we stand it? Med. Princ. Pract. 2013, 22, 131–137. [Google Scholar] [CrossRef]
- Han, L.; Wang, H.; Li, L.; Li, X.; Ge, J.; Reiter, R.J.; Wang, Q. Melatonin protects against maternal obesity-associated oxidative stress and meiotic defects in oocytes via the SIRT3-SOD2-dependent pathway. J. Pineal Res. 2017, 63, e12431. [Google Scholar] [CrossRef]
- Zhai, M.; Li, B.; Duan, W.; Jing, L.; Zhang, B.; Zhang, M.; Yu, L.; Liu, Z.; Yu, B.; Ren, K.; et al. Melatonin ameliorates myocardial ischemia reperfusion injury through SIRT3-dependent regulation of oxidative stress and apoptosis. J. Pineal Res. 2017, 63, e12419. [Google Scholar] [CrossRef]
- Naaz, S.; Mishra, S.; Pal, P.K.; Chattopadhyay, A.; Das, A.R.; Bandyopadhyay, D. Activation of SIRT1/PGC 1α/SIRT3 pathway by melatonin provides protection against mitochondrial dysfunction in isoproterenol induced myocardial injury. Heliyon 2020, 6, e05159. [Google Scholar] [CrossRef]
- Klein, D.C. Arylalkylamine N-acetyltransferase: “The Timezyme”. J. Biol. Chem. 2007, 282, 4233–4237. [Google Scholar] [CrossRef] [PubMed]
- Okatani, Y.; Wakatsuki, A.; Reiter, R.J. Melatonin protects hepatic mitochondrial respiratory chain activity in senescence-accelerated mice. J. Pineal Res. 2002, 32, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Okatani, Y.; Wakatsuki, A.; Reiter, R.J.; Enzan, H.; Miyahara, Y. Protective effect of melatonin against mitochondrial injury induced by ischemia and reperfusion of rat liver. Eur. J. Pharmacol. 2003, 469, 145–152. [Google Scholar] [CrossRef]
- Jou, M.J.; Peng, T.I.; Reiter, R.J.; Jou, S.B.; Wu, H.Y.; Wen, S.T. Visualization of the antioxidative effects of melatonin at the mitochondrial level during oxidative stress-induced apoptosis of rat brain astrocytes. J. Pineal Res. 2004, 37, 55–70. [Google Scholar] [CrossRef]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Protective role of melatonin in mitochondrial dysfunction and related disorders. Arch. Toxicol. 2015, 89, 923–939. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sirianni, A.; Pei, Z.; Cormier, K.; Smith, K.; Jiang, J.; Zhou, S.; Wang, H.; Zhao, R.; Yano, H.; et al. The melatonin MT1 receptor axis modulates mutant Huntingtin-mediated toxicity. J. Neurosci. 2011, 31, 14496–14507. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, A.; Brzozowska, I.M.; Hoa, N.; Jones, M.K.; Tarnawski, A.S. Melatonin signaling in mitochondria extends beyond neurons and neuroprotection: Implications for angiogenesis and cardio/gastroprotection. Proc. Natl. Acad. Sci. USA 2018, 115, E1942–E1943. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Koppisepi, S. Medical implications of melatonin: Receptor-mediated and receptor-independent actions. Adv. Med. Sci. 2007, 52, 11–28. [Google Scholar] [PubMed]
- Adams, J.M.; White, M. Biological ageing: A fundamental, biological link between socio-economic status and health? Eur. J. Public Health 2004, 14, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C.J. The genetics of ageing. Nature 2010, 464, 504–512. [Google Scholar] [CrossRef]
- Talens, R.P.; Christensen, K.; Putter, H.; Willemsen, G.; Christiansen, L.; Kremer, D.; Suchiman, H.E.D.; Slagboom, P.E.; Boomsma, D.I.; Heijmans, B.T. Epigenetic variation during the adult lifespan: Cross-sectional and longitudinal data on monozygotic twin pairs. Aging Cell 2012, 11, 694–703. [Google Scholar] [CrossRef]
- Gurland, B.J.; Page, W.F.; Plassman, B.L. A twin study of the genetic contribution to age-related functional impairment. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 859–863. [Google Scholar] [CrossRef][Green Version]
- Reiter, R.J.; Craft, C.M.; Johnson, J.E., Jr.; King, T.S.; Richardson, B.A.; Vaughan, G.M.; Vaughan, M.K. Age-associated reduction in nocturnal pineal melatonin levels in female rats. Endocrinology 1981, 109, 1295–1297. [Google Scholar] [CrossRef]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Barardo, D.; Thornton, D.; Thoppil, H.; Walsh, M.; Sharifi, S.; Ferreira, S.; Anzic, A.; Fernandes, M.; Monteiro, P.; Grum, T.; et al. The DrugAge database of aging-related drugs. Aging Cell 2017, 16, 594–597. [Google Scholar] [CrossRef]
- DrugAge Database of Aging-Related Drugs. Available online: http://genomics.senescence.info/drugs/index.php (accessed on 24 June 2021).
- Anisimov, V.N.; Arutjunyan, A.V.; Khavinson, V.K. Effects of pineal peptide preparation epithalamin on free-radical processes in humans and animals. Neuro. Endocrinol. Lett. 2001, 22, 9–18. [Google Scholar]
- Khavinson, V.; Goncharova, N.; Lapin, B. Synthetic tetrapeptide epitalon restores disturbed neuroendocrine regulation in senescent monkeys. Neuro. Endocrinol. Lett. 2001, 22, 251–254. [Google Scholar]
- Kossoy, G.; Anisimov, V.N.; Ben-Hur, H.; Kossoy, N.; Zusman, I. Effect of the synthetic pineal peptide epitalon on spontaneous carcinogenesis in female C3H/He mice. In Vivo 2006, 20, 253–257. [Google Scholar] [PubMed]
- Vinogradova, I.A. Comparative study of the effects of melatonin and epitalon on the protracted memory under the shuttle labyrinth test conditions in rats in the course of aging. Eksp. Klin. Farmakol. 2006, 69, 13–16. [Google Scholar] [PubMed]
- Anisimov, V.N.; Bondarenko, L.A.; Khavinson, V. Effect of pineal peptide preparation (epithalamin) on life span and pineal and serum melatonin level in old rats. Ann. N. Y. Acad. Sci. 1992, 673, 53–57. [Google Scholar] [CrossRef]
- Anisimov, V.N.; Khavinson, V.K.; Popovich, I.G.; Zabezhinski, M.A. Inhibitory effect of peptide Epitalon on colon carcinogenesis induced by 1,2-dimethylhydrazine in rats. Cancer Lett. 2002, 183, 1–8. [Google Scholar] [CrossRef]
- Anisimov, V.N.; Khavinson, V.K.; Provinciali, M.; Alimova, I.N.; Baturin, D.A.; Popovich, I.G.; Zabezhinski, M.A.; Imyanitov, E.N.; Mancini, R.; Franceschi, C. Inhibitory effect of the peptide epitalon on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Int. J. Cancer 2002, 101, 7–10. [Google Scholar] [CrossRef]
- Khavinson, V.; Razumovsky, M.; Trofimova, S.; Grigorian, R.; Razumovskaya, A. Pineal-regulating tetrapeptide epitalon improves eye retina condition in retinitis pigmentosa. Neuroendocrinol. Lett. 2002, 23, 365–368. [Google Scholar]
- Khavinson, V.K.; Korneva, E.A.; Malinin, V.V.; Rybakina, E.G.; Pivanovich, I.Y.; Shanin, S.N. Effect of epitalon on interleukin-1beta signal transduction and the reaction of thymocyte blast transformation under stress. Neuro. Endocrinol. Lett. 2002, 23, 411–416. [Google Scholar]
- Anisimov, V.N.; Khavinson, V.K.; Popovich, I.G.; Zabezhinski, M.A.; Alimova, I.N.; Rosenfeld, S.V.; Zavarzina, N.Y.; Semenchenko, A.V.; Yashin, A.I. Effect of Epitalon on biomarkers of aging, life span and spontaneous tumor incidence in female Swiss-derived SHR mice. Biogerontology 2003, 4, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Sibarov, D.A.; Kovalenko, R.I.; Malinin, V.V.; Khavinson, V. Epitalon influences pineal secretion in stress-exposed rats in the daytime. Neuro Endocrinol. Lett. 2002, 23, 452–454. [Google Scholar] [PubMed]
- Kossoy, G.; Zandbank, J.; Tendler, E.; Anisimov, V.; Khavinson, V.; Popovich, I.; Zabezhinski, M.; Zusman, I.; Ben-Hur, H. Epitalon and colon carcinogenesis in rats: Proliferative activity and apoptosis in colon tumors and mucosa. Int. J. Mol. Med. 2003, 12, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Anisimov, V.N.; Popovich, I.G.; Zabezhinski, M.A.; Rosenfeld, S.V.; Khavinson, V.K.; Semenchenko, A.V.; Yashin, A.I. Effect of epitalon and melatonin on life span and spontaneous carcinogenesis in senescence accelerated mice (SAM). Vopr. Onkol. 2005, 51, 93–98. [Google Scholar] [PubMed]
- Svechkina, E.B.; Tiutiunnik, N.N.; Vinogradova, I.A. Influence of light regimens, melatonin, and epitalon on amylase activity in the pancreas and small intestine in rats of different age. Adv. Gerontol. 2006, 19, 66–71. [Google Scholar] [PubMed]
- Vinogradova, I.A. Effect of preparations melatonin and epitalon on the age-related dynamics of thyrotrophic activity of the hypophysis and thyroid gland function in different light regimes. Adv. Gerontol. 2009, 22, 631–638. [Google Scholar]
- Goncharova, N.D.; Khavinson, B.K.; Lapin, B.A. Regulatory effect of Epithalon on production of melatonin and cortisol in old monkeys. Bull. Exp. Biol. Med. 2001, 131, 394–396. [Google Scholar] [CrossRef]
- Djeridane, Y.; Khavinson, V.K.; Anisimov, V.N.; Touitou, Y. Effect of a synthetic pineal tetrapeptide (Ala-Glu-Asp-GLy) on melatonin secretion by the pineal gland of young and old rats. J. Endocrinol. Invest. 2003, 26, 211–215. [Google Scholar] [CrossRef]
- Korkushko, O.V.; Lapin, B.A.; Goncharova, N.D.; Khavinson, V.K.; Shatilo, V.B.; Vengerin, A.A.; Antoniuk-Shcheglova, I.A.; Magdich, L.V. Normalizing effect of the pineal gland peptides on the daily melatonin rhythm in old monkeys and elderly people. Adv. Gerontol. 2007, 20, 74–85. [Google Scholar]
- Dilman, V.M.; Anisimov, V.N.; Ostroumova, M.N.; Khavinson, V.K.; Morozov, V.G. Increase in lifespan of rats following polypeptide pineal extract treatment. Exp. Pathol. 1979, 17, 539–545. [Google Scholar] [CrossRef]
- Dilman, V.M.; Anisimov, V.N.; Ostroumova, M.N.; Morosov, V.G.; Khavinson, V.K.; Azarova, M.A. Study of the anti-tumor effect of polypeptide pineal extract. Oncology 1979, 36, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Anisimov, V.N.; Khavinson, V.; Morozov, V.G. Twenty years of study on effects of pineal peptide preparation: Epithalamin in experimental gerontology and oncology. Ann. N. Y. Acad. Sci. 1994, 719, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S.I.S. “I think that the small peptides are the best for healthy ageing...”, an interview with Vladimir Khavinson. Biogerontology 2013, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Khavinson, V.K.; Pronyaeva, V.E.; Linkova, N.S.; Trofimova, S.V. Peptidegic regulation of differentiation of embryonic retinal cells. Bull. Exp. Biol. Med. 2013, 155, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Anisimov, V.N.; Mylnikov, S.V.; Oparina, T.I.; Khavinson, V.K. Effect of melatonin and pineal peptide preparation epithalamin on life span and free radical oxidation in Drosophila melanogaster. Mech. Ageing Dev. 1997, 97, 81–91. [Google Scholar] [CrossRef]
- Anisimov, V.N.; Zavarzina, N.Y.; Zabezhinski, M.A.; Popovich, I.G.; Zimina, O.A.; Shtylick, A.V.; Arutjunyan, A.V.; Oparina, T.I.; Prokopenko, V.M.; Mikhalski, A.I.; et al. Melatonin increases both life span and tumor incidence in female CBA mice. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, B311–B323. [Google Scholar] [CrossRef]
- Anisimov, V.N. Effect of melatonin on life span and longevity. In Melatonin: Biological Basis of Its Function in Health and Disease, 1st ed.; Pandi-Perumal, S.R., Cardinali, D.P., Eds.; Eurekah.com/Landes Biosciences: Georgetown, TX, USA, 2006; pp. 45–59. [Google Scholar]
- Izmaylov, D.M.; Obukhova, L.K. Geroprotector effectiveness of melatonin: Investigation of lifespan of Drosophila melanogaster. Mech. Ageing Dev. 1999, 106, 233–240. [Google Scholar] [CrossRef]
- Bonilla, E.; Medina-Leendertz, S.; Díaz, S. Extension of life span and stress resistance of Drosophila melanogaster by long-term supplementation with melatonin. Exp. Gerontol. 2002, 37, 629–638. [Google Scholar] [CrossRef]
- Karbownik, M.; Reiter, R.J.; Qi, W.; Garcia, J.J.; Tan, D.X.; Manchester, L.C. Protective effects of melatonin against oxidation of guanine bases in DNA and decreased microsomal membrane fluidity in rat liver induced by whole body ionizing radiation. Mol. Cell. Biochem. 2000, 211, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Lee, R.Y.N.; Fraser, A.G.; Kamath, R.S.; Ahringer, J.; Ruvkun, G. A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat. Genet. 2003, 33, 40–48. [Google Scholar] [CrossRef]
- Squier, T.C. Oxidative stress and protein aggregation during biological aging. Exp. Gerontol. 2001, 36, 1539–1550. [Google Scholar] [CrossRef]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Krumova, K.; Cosa, G. Chapter 1: Overview of reactive oxygen species. In Singlet Oxygen: Applications in Biosciences and Nanosciences; Nonell, S., Flors, C., Eds.; Royal Society of Chemistry: London, UK, 2016; Volume 1, pp. 1–21. [Google Scholar] [CrossRef]
- Salvioli, S.; Bonafe, M.; Capri, M.; Monti, D.; Franceschi, C. Mitochondria, aging and longevity—A new perspective. FEBS Lett. 2001, 492, 9–13. [Google Scholar] [CrossRef]
- Schroeder, E.A.; Raimundo, N.; Shadel, G.S. Epigenetic silencing mediates mitochondria stress-induced longevity. Cell Metab. 2013, 17, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, R.; Gonzalez, B.; Johnson, S.C. Mitochondrial pathways in human health and aging. Mitochondrion 2020, 54, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. The free radical theory of aging. Antioxid. Redox Signal 2003, 5, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Pavelescu, L.A. On reactive oxygen species measurement in living systems. J. Med. Life 2015, 8, 38–42. [Google Scholar]
- Acuña-Castroviejo, D.; Escames, G.L.C.; Lopez, L.C.; Ortiz, F.; Lopez, A.; García, J.A. Evidencias de la utilidad de la melatonina frente al envejecimiento y los procesos neurodegenerativos. Psicogeriatría 2009, 1, 3–21. [Google Scholar]
- Tan, D.X.; Chen, L.D.; Poeggeler, B.; Manchester, L.C.; Reiter, R.J. Melatonin: A potent endogenous hydroxyl radical scavenger. Endocrine J. 1993, 1, 57–60. [Google Scholar]
- Reiter, R.J. Lowering the high price of free radicals. News Physiol. Sci. 2000, 15, 246–250. [Google Scholar] [CrossRef]
- Hardeland, R. Antioxidative protection by melatonin: Multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine 2005, 27, 119–130. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Manchester, L.C.; Qi, W. Biochemical reactivity of melatonin with reactive oxygen and nitrogen species. Cell. Biochem. Biophys. 2001, 34, 237–256. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Karasek, M. Does melatonin play a role in aging processes? J. Physiol. Pharmacol. 2007, 58, 105–113. [Google Scholar] [PubMed]
- Marshall, K.A.; Reiter, R.J.; Poeggeler, B.; Aruoma, O.I.; Halliwell, B. Evaluation of the antioxidant activity of melatonin in vitro. Free Radic. Biol. Med. 1996, 21, 307–315. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Burkhardt, S.; Sainz, R.M.; Mayo, J.C.; Kohen, R.; Shohami, E.; Huo, Y.S.; Hardeland, R.; Reiter, R.J. N1-acetyl-N2-formyl-5-methoxykynuramine, a biogenic amine and melatonin metabolite, functions as a potent antioxidant. FASEB J. 2001, 15, 2294–2296. [Google Scholar] [CrossRef]
- Tan, D.X.; Reiter, R.J.; Manchester, L.C.; Yan, M.T.; El-Sawi, M.; Sainz, R.M.; Mayo, J.C.; Kohen, R.; Allegra, M.; Hardeland, R. Chemical and physical properties and potential mechanisms: Melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top Med. Chem. 2002, 2, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Ressmeyer, A.R.; Mayo, J.C.; Zelosko, V.; Sáinz, R.M.; Tan, D.X.; Poeggeler, B.; Antolín, I.; Zsizsik, B.K.; Reiter, R.J.; Hardeland, R. Antioxidant properties of the melatonin metabolite N1-acetyl-5-methoxykynuramine (AMK): Scavenging of free radicals and prevention of protein destruction. Redox Rep. 2003, 8, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Guenther, A.L.; Schmidt, S.I.; Laatsch, H.; Fotso, S.; Ness, H.; Ressmeyer, A.R.; Poeggeler, B.; Hardeland, R. Reactions of the melatonin metabolite AMK (N1-acetyl-5-methoxykynuramine) with reactive nitrogen species: Formation of novel compounds, 3-acetamidomethyl-6-methoxycinnolinone and 3-nitro-AMK. J. Pineal Res. 2005, 39, 251–260. [Google Scholar] [CrossRef]
- Onuki, J.; Almeida, E.A.; Medeiros, M.H.; Di Mascio, P. Inhibition of 5-aminolevulinic acid-induced DNA damage by melatonin, N1-acetyl-N2-formyl-5-methoxykynuramine, quercetin or resveratrol. J. Pineal Res. 2005, 38, 107–115. [Google Scholar] [CrossRef]
- López-Lluch, G.; Irusta, P.M.; Navas, P.; de Cabo, R. Mitochondrial biogenesis and healthy aging. Exp. Gerontol. 2008, 43, 813–819. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Carretero, M.; Doerrier, C.; López, L.C.; García-Corzo, L.; Tresguerres, J.A.; Escames, G. Melatonin protects lung mitochondria from aging. AGE 2012, 34, 681–692. [Google Scholar] [CrossRef]
- Reiter, L.T.; Potocki, L.; Chien, S.; Gribskov, M.; Bier, E. A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster. Genome Res. 2001, 11, 1114–1125. [Google Scholar] [CrossRef]
- Pandey, U.B.; Nichols, C.D. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol. Rev. 2011, 63, 411–436. [Google Scholar] [CrossRef] [PubMed]
- Toivonen, J.M.; Partridge, L. Endocrine regulation of aging and reproduction in Drosophila. Mol. Cell. Endocrinol. 2009, 299, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Partridge, L. Promoting health and longevity through diet: From model organisms to humans. Cell 2015, 161, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Bjedov, I.; Toivonen, J.M.; Kerr, F.; Slack, C.; Jacobson, J.; Foley, A.; Partridge, L. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 2010, 11, 35–46. [Google Scholar] [CrossRef]
- Gamberi, C.; Hipfner, D.R.; Trudel, M.; Lubell, W.D. Bicaudal C mutation causes myc and TOR pathway up-regulation and polycystic kidney disease-like phenotypes in Drosophila. PLoS Genet. 2017, 13, e1006694. [Google Scholar] [CrossRef]
- Grönke, S.; Clarke, D.F.; Broughton, S.; Andrews, T.D.; Partridge, L. Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genet. 2010, 6, e1000857. [Google Scholar] [CrossRef]
- Weigelt, C.M.; Sehgal, R.; Tain, L.S.; Cheng, J.; Eßer, J.; Pahl, A.; Dieterich, C.; Grönke, S.; Partridge, L. An insulin-sensitive circular RNA that regulates lifespan in Drosophila. Mol. Cell. 2020, 79, 268–279.e5. [Google Scholar] [CrossRef]
- Mair, W.; Piper, M.D.; Partridge, L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 2005, 3, e223. [Google Scholar] [CrossRef] [PubMed]
- Piper, M.D.W.; Partridge, L. Drosophila as a model for ageing. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2707–2717. [Google Scholar] [CrossRef] [PubMed]
- Khyati; Malik, I.; Agrawal, N.; Kumar, V. Melatonin and curcumin reestablish disturbed circadian gene expressions and restore locomotion ability and eclosion behavior in Drosophila model of Huntington’s disease. Chronobiol. Int. 2021, 38, 61–78. [Google Scholar] [CrossRef]
- Musiek, E.S.; Holtzman, D.M. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science 2016, 354, 1004–1008. [Google Scholar] [CrossRef]
- Dabbeni-Sala, F.; Di Santo, S.; Franceschini, D.; Skaper, S.D.; Giusti, P. Melatonin protects against 6-OHDA-induced neurotoxicity in rats: A role for mitochondrial complex I activity. FASEB J. 2001, 15, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Antolín, I.; Mayo, J.C.; Sainz, R.M.; del Brío, M.A.; Herrera, F.; Martín, V.; Rodríguez, C. Protective effect of melatonin in a chronic experimental model of Parkinson’s disease. Brain Res. 2002, 943, 163–173. [Google Scholar] [CrossRef]
- Polimeni, G.; Esposito, E.; Bevelacqua, V.; Guarneri, C.; Cuzzocrea, S. Role of melatonin supplementation in neurodegenerative disorders. Front. Biosci. 2014, 19, 429–446. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Costa, J.V.; Kowaltowski, A.J. Neurological disorders and mitochondria. Mol. Aspects Med. 2020, 71, 100826. [Google Scholar] [CrossRef] [PubMed]
- Sarantseva, S.; Timoshenko, S.; Bolshakova, O.; Karaseva, E.; Rodin, D.; Schwarzman, A.L.; Vitek, M.P. Apolipoprotein E-mimetics inhibit neurodegeneration and restore cognitive functions in a transgenic Drosophila model of Alzheimer’s disease. PLoS ONE 2009, 4, e8191. [Google Scholar] [CrossRef]
- Khatoon, R.; Rasheed, M.Z.; Rawat, M.; Alam, M.M.; Tabassum, H.; Parvez, S. Effect of melatonin on Aβ42 induced changes in the mitochondrial function related to Alzheimer’s disease in Drosophila melanogaster. Neurosci. Lett. 2019, 711, 134376. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Parker, W.D., Jr.; Parks, J.; Filley, C.M.; Kleinschmidt-DeMasters, B.K. Electron transport chain defects in Alzheimer’s disease brain. Neurology 1994, 44, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Pappolla, M.; Chyan, Y.J.; Poeggeler, B.; Frangione, B.; Wilson, G.; Ghiso, J.; Reiter, R.J. An assessment of the antioxidant and the antiamyloidogenic properties of melatonin: Implications for Alzheimer’s disease. J. Neural Transm. 2000, 107, 203–231. [Google Scholar] [CrossRef]
- Savaskan, E.; Wirz-Justice, A.; Olivieri, G.; Pache, M.; Kräuchi, K.; Brydon, L.; Jockers, R.; Müller-Spahn, F.; Meyer, P. Distribution of melatonin MT1 receptor immunoreactivity in human retina. J. Histochem. Cytochem. 2002, 50, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Brunner, P.; Sözer-Topcular, N.; Jockers, R.; Ravid, R.; Angeloni, D.; Fraschini, F.; Eckert, A.; Müller-Spahn, F.; Savaskan, E. Pineal and cortical melatonin receptors MT1 and MT2 are decreased in Alzheimer’s disease. Eur. J. Histochem. 2006, 50, 311–316. [Google Scholar]
- Roos, R.A. Huntington’s disease: A clinical review. Orphanet. J. Rare Dis. 2010, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Ismailoglu, I.; Chen, Q.; Popowski, M.; Yang, L.; Gross, S.S.; Brivanlou, A.H. Huntingtin protein is essential for mitochondrial metabolism, bioenergetics and structure in murine embryonic stem cells. Dev. Biol. 2014, 391, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Panov, A.V.; Gutekunst, C.A.; Leavitt, B.R.; Hayden, M.R.; Burke, J.R.; Strittmatter, W.J.; Greenamyre, J.T. Early mitochondrial calcium defects in Huntington’s disease are a direct effect of polyglutamines. Nat. Neurosci. 2002, 5, 731–736. [Google Scholar] [CrossRef]
- Panchal, K.; Tiwari, A.K. Mitochondrial dynamics, a key executioner in neurodegenerative diseases. Mitochondrion 2019, 47, 151–173. [Google Scholar] [CrossRef]
- Kalliolia, E.; Silajdžić, E.; Nambron, R.; Hill, N.R.; Doshi, A.; Frost, C.; Watt, H.; Hindmarsh, P.; Bjorkqvist, M.; Warner, T.T. Plasma melatonin is reduced in Huntington’s disease. Mov. Disord. 2014, 29, 1511–1515. [Google Scholar] [CrossRef]
- Túnez, I.; del Carmen Muñoz, M.; Feijóo, M.; Muñoz-Castañeda, J.R.; Bujalance, I.; Valdelvira, M.E.; López, P.M. Protective melatonin effect on oxidative stress induced by okadaic acid into rat brain. J. Pineal Res. 2003, 34, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Greggio, E.; Cookson, M.R. Leucine-rich repeat kinase 2 mutations and Parkinson’s disease: Three questions. ASN Neuro 2009, 1, e00002. [Google Scholar] [CrossRef]
- Cookson, M.R. The role of leucine-rich repeat kinase 2 (LRRK2) in Parkinson’s disease. Nat. Rev. Neurosci. 2010, 11, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ran, D.; Zhao, X.; Huang, Y.; Long, S.; Liang, F.; Guo, W.; Nucifora, F.C., Jr.; Gu, H.; Lu, X.; et al. Melatonin attenuates hLRRK2-induced sleep disturbances and synaptic dysfunction in a Drosophila model of Parkinson’s disease. Mol. Med. Rep. 2016, 13, 3936–3944. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.; Schweitzer, K.J.; Leitner, P.; Zimprich, A.; Lichtner, P.; Belcredi, P.; Brussel, T.; Schulte, C.; Maass, S.; Nagele, T.; et al. Type and frequency of mutations in the LRRK2 gene in familial and sporadic Parkinson’s disease. Brain 2005, 128, 3000–3011. [Google Scholar] [CrossRef]
- Parker, W.D., Jr.; Boyson, S.J.; Parks, J.K. Abnormalities of the electron transport chain in idiopathic Parkinson’s disease. Ann. Neurol. 1989, 26, 719–723. [Google Scholar] [CrossRef]
- Alam, Z.I.; Jenner, A.; Daniel, S.E.; Lees, A.J.; Cairns, N.; Marsden, C.D.; Jenner, P.; Halliwell, B. Oxidative DNA damage in the parkinsonian brain: An apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J. Neurochem. 1997, 69, 1196–1203. [Google Scholar] [CrossRef]
- Sheehan, J.P.; Swerdlow, R.H.; Parker, W.D.; Miller, S.W.; Davis, R.E.; Tuttle, J.B. Altered calcium homeostasis in cells transformed by mitochondria from individuals with Parkinson’s disease. J. Neurochem. 1997, 68, 1221–1233. [Google Scholar] [CrossRef]
- Chinopoulos, C.; Adam-Vizi, V. Mitochondria deficient in complex I activity are depolarized by hydrogen peroxide in nerve terminals: Relevance to Parkinson’s disease. J. Neurochem. 2001, 76, 302–306. [Google Scholar] [CrossRef]
- Kim, I.; Rodriguez-Enriquez, S.; Lemasters, J.J. Selective degradation of mitochondria by mitophagy. Arch. Biochem. Biophys. 2007, 462, 245–253. [Google Scholar] [CrossRef]
- Gorman, G.S.; Chinnery, P.F.; DiMauro, S.; Hirano, M.; Koga, Y.; McFarland, R.; Suomalainen, A.; Thorburn, D.R.; Zeviani, M.; Turnbull, D.M. Mitochondrial diseases. Nat. Rev. Dis. Primers 2016, 2, 16080. [Google Scholar] [CrossRef]
- Craven, L.; Alston, C.L.; Taylor, R.W.; Turnbull, D.M. Recent advances in mitochondrial disease. Annu. Rev. Genomics Hum. Genet. 2017, 18, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, L.; Callebert, J.; Launay, J.M.; Jallon, J.M. Melatonin biosynthesis in Drosophila: Its nature and its effects. J. Neurochem. 1988, 50, 382–387. [Google Scholar] [CrossRef]
- Khavinson, V.K.; Izmaylov, D.M.; Obukhova, L.K.; Malinin, V.V. Effect of epitalon on the lifespan increase in Drosophila melanogaster. Mech. Ageing Dev. 2000, 120, 141–149. [Google Scholar] [CrossRef]
- Khavinson, V.K.; Myl’nikov, S.V. Effect of epithalone on the age-specific changes in the time course of lipid peroxidation in Drosophila melanogaster. Bull. Exp. Biol. Med. 2000, 130, 1116–1119. [Google Scholar] [CrossRef]
- Khavinson, V.K.; Myl’nikov, S.V.; Oparina, T.I.; Arutyunyan, A.V. Effects of peptides on generation of reactive oxygen species in subcellular fractions of Drosophila melanogaster. Bull. Exp. Biol. Med. 2001, 132, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Khavinson, V.K.; Lezhava, T.A.; Monaselidze, J.R.; Jokhadze, T.A.; Dvalishvili, N.A.; Bablishvili, N.K.; Trofimova, S.V. Peptide Epitalon activates chromatin at the old age. Neuro. Endocrinol. Lett. 2003, 24, 329–333. [Google Scholar]
- Terán, R.; Bonilla, E.; Medina-Leendertz, S.; Mora, M.; Villalobos, V.; Paz, M.; Arcaya, J.L. The life span of Drosophila melanogaster is affected by melatonin and thioctic acid. Investig. Clín. 2012, 53, 250–261. [Google Scholar]
- Gaweł, S.; Wardas, M.; Niedworok, E.; Wardas, P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad. Lek. 2004, 57, 453–455. [Google Scholar]
- Vasiliou, V.; Ross, D.; Nebert, D.W. Update of the NAD(P)H:quinone oxidoreductase (NQO) gene family. Hum. Genom. 2006, 2, 329–335. [Google Scholar] [CrossRef]
- Hall, B.L.; Thummel, C.S. The RXR homolog ultraspiracle is an essential component of the Drosophila ecdysone receptor. Development 1998, 125, 4709–4717. [Google Scholar] [CrossRef]
- Ghbeish, N.; Tsai, C.C.; Schubiger, M.; Zhou, J.Y.; Evans, R.M.; McKeown, M. The dual role of ultraspiracle, the Drosophila retinoid X receptor, in the ecdysone response. Proc. Natl. Acad. Sci. USA 2001, 98, 3867–3872. [Google Scholar] [CrossRef]
- Picking, W.L.; Chen, D.M.; Lee, R.D.; Vogt, M.E.; Polizzi, J.L.; Marietta, R.G.; Stark, W.S. Control of Drosophila opsin gene expression by carotenoids and retinoic acid: Northern and western analyses. Exp. Eye. Res. 1996, 63, 493–500. [Google Scholar] [CrossRef]
- Arshavsky, V.Y. Vision: The retinoid cycle in Drosophila. Curr. Biol. 2010, 20, R96–R98. [Google Scholar] [CrossRef][Green Version]
- Ashton, A.; Stoney, P.N.; Ransom, J.; McCaffery, P. Rhythmic diurnal synthesis and signaling of retinoic acid in the rat pineal gland and its action to rapidly downregulate ERK phosphorylation. Mol. Neurobiol. 2018, 55, 8219–8235. [Google Scholar] [CrossRef]
- Mackay, T.F.; Anholt, R.R. Of flies and man: Drosophila as a model for human complex traits. Annu. Rev. Genomics Hum. Genet. 2006, 7, 339–367. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.; Evans, J.M.; Hendricks, J.C.; Sehgal, A. A Drosophila model for age-associated changes in sleep: Wake cycles. Proc. Natl. Acad. Sci. USA 2006, 103, 13843–13847. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sharma, A. Common features of circadian timekeeping in diverse organisms. Curr. Opin. Physiol. 2018, 5, 58–67. [Google Scholar] [CrossRef]
- Konopka, R.J.; Benzer, S. Clock mutants of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 1971, 68, 2112–2116. [Google Scholar] [CrossRef] [PubMed]
- Bargiello, T.A.; Young, M.W. Molecular genetics of a biological clock in Drosophila. Proc. Natl. Acad. Sci. USA 1984, 81, 2142–2146. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Zehring, W.A.; Wheeler, D.A.; Pirrotta, V.; Hadfield, C.; Hall, J.C.; Rosbash, M. Molecular analysis of the period locus in Drosophila melanogaster and identification of a transcript involved in biological rhythms. Cell 1984, 38, 701–710. [Google Scholar] [CrossRef]
- Sehgal, A.; Price, J.L.; Man, B.; Young, M.W. Loss of circadian behavioral rhythms and per RNA oscillations in the Drosophila mutant timeless. Science 1994, 263, 1603–1606. [Google Scholar] [CrossRef]
- Kaneko, M.; Park, J.H.; Cheng, Y.; Hardin, P.E.; Hall, J.C. Disruption of synaptic transmission or clock-gene-product oscillations in circadian pacemaker cells of Drosophila cause abnormal behavioral rhythms. J. Neurobiol. 2000, 43, 207–233. [Google Scholar] [CrossRef]
- Rosbash, M.; Bradley, S.; Kadener, S.; Li, Y.; Luo, W.; Menet, J.S.; Nagoshi, E.; Palm, K.; Schoer, R.; Shang, Y.; et al. Transcriptional feedback and definition of the circadian pacemaker in Drosophila and animals. Cold Spring Harb. Symp. Quant. Biol. 2007, 72, 75–83. [Google Scholar] [CrossRef]
- Rosbash, M. Circadian rhythms and the transcriptional feedback loop (Nobel lecture). Angew. Chem. 2021, 60, 8650–8666. [Google Scholar] [CrossRef]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Zordan, M.A.; Sandrelli, F. Circadian clock dysfunction and psychiatric disease: Could fruit flies have a say? Front. Neurol. 2015, 6, 80. [Google Scholar] [CrossRef]
- Ferrandon, D.; Imler, J.L.; Hetru, C.; Ho_mann, J.A. The Drosophila systemic immune response: Sensing and signaling during bacterial and fungal infections. Nat. Rev. Immunol. 2007, 7, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Buchon, N.; Broderick, N.A.; Chakrabarti, S.; Lemaitre, B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 2009, 23, 2333–2344. [Google Scholar] [CrossRef]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.C.; Liao, C.W.; Pan, R.L.; Juang, J.L. Infection-induced intestinal oxidative stress triggers organ-to-organ immunological communication in Drosophila. Cell Host Microbe 2012, 11, 410–417. [Google Scholar] [CrossRef]
- Bostantjopoulou, S.; Kyriazis, G.; Katsarou, Z.; Kiosseoglou, G.; Kazis, A.; Mentenopoulos, G. Superoxide dismutase activity in early and advanced Parkinson’s disease. Funct. Neurol. 1997, 12, 63–68. [Google Scholar] [PubMed]
- Bahadorani, S.; Hilliker, A.J. Antioxidants cannot suppress the lethal phenotype of a Drosophila melanogaster model of Huntington’s disease. Genome 2008, 51, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Rudym, D.; Chandra, P.; Miskulin, D.; Perrone, R.; Sarnak, M. Inflammation, oxidative stress, and insulin resistance in polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 7–13. [Google Scholar] [CrossRef]
- Kish, S.J.; Morito, C.; Hornykiewicz, O. Glutathione peroxidase activity in Parkinson’s disease brain. Neurosci. Lett. 1985, 58, 343–346. [Google Scholar] [CrossRef]
- Maser, R.L.; Vassmer, D.; Magenheimer, B.S.; Clavet, J.P. Oxidant stress and reduced antioxidant enzyme protection in polycystic kidney disease. J. Am. Soc. Nephrol. 2002, 13, 991–999. [Google Scholar] [CrossRef]
- Nikam, S.; Nikam, P.; Ahaley, S.K.; Sontakke, A.V. Oxidative stress in Parkinson’s disease. Indian J. Clin. Biochem. 2009, 24, 98–101. [Google Scholar] [CrossRef]
- Mason, R.P.; Casu, M.; Butler, N.; Breda, C.; Campesan, S.; Clapp, J.; Green, E.W.; Dhulkhed, D.; Kyriacou, C.P.; Giorgini, F. Glutathione peroxidase activity is neuroprotective in models of Huntington’s disease. Nat. Genet. 2013, 45, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Zima, T.; Crkovska, J.; Merta, M.; Stípek, S.; Nĕmecek, K.; Tesar, V. Activity of the antioxidant enzyme, glutathione peroxidase, on autosomal dominant polycystic kidney disease patients. Biochem. Mol. Biol. Int. 1995, 35, 699–704. [Google Scholar]
- del Hoyo, P.; García-Redondo, A.; de Bustos, F.; Molina, J.A.; Sayed, Y.; Alonso-Navarro, H.; Caballero, L.; Arenas, J.; Jiménez-Jiménez, F.J. Oxidative stress in skin fibroblasts cultures of patients with Huntington’s disease. Neurochem. Res. 2006, 31, 1103–1109. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of catalase in oxidative stress-and age-associated degenerative diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef]
- Torres, V.E.; Bengal, R.J.; Litwiller, R.D.; Wilson, D.M. Aggravation of polycystic kidney disease in Han: SPRD rats by buthionine sulfoximine. J. Am. Soc. Nephrol. 1997, 8, 1283–1291. [Google Scholar] [CrossRef]
- Ilic, T.V.; Jovanovic, M.; Jovicic, A.; Tomovic, M. Oxidative stress indicators are elevated in de novo Parkinson’s disease patients. Funct. Neurol. 1999, 14, 141–147. [Google Scholar]
- Martin, H.L.; Teismann, P. Glutathione—A review on its role and significance in Parkinson’s disease. FASEB J. 2009, 23, 3263–3272. [Google Scholar] [CrossRef] [PubMed]
- Peña-Sánchez, M.; Riverón-Forment, G.; Zaldívar-Vaillant, T.; Soto-Lavastida, A.; Borrero-Sánchez, J.; Lara-Fernández, G.; Esteban-Hernandez, E.M.; Hernandez-Diaz, Z.; Gonzalez-Quevedo, A.; Fernandez-Almirall, I.; et al. Association of status redox with demographic, clinical and imaging parameters in patients with Huntington’s disease. Clin. Biochem. 2015, 48, 1258–1263. [Google Scholar] [CrossRef] [PubMed]
- Darvesh, A.S.; Carroll, R.T.; Bishayee, A.; Novotny, N.A.; Geldenhuys, W.J.; Van der Schyf, C.J. Curcumin and neurodegenerative diseases: A perspective. Expert Opin. Investig. Drugs. 2012, 21, 1123–1140. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Rakshit, K.; Chow, E.S.; Wentzell, J.S.; Kretzschmar, D.; Giebultowicz, J.M. Loss of circadian clock accelerates aging in neurodegeneration-prone mutants. Neurobiol. Dis. 2012, 45, 1129–1135. [Google Scholar] [CrossRef]
- Mattis, J.; Sehgal, A. Circadian rhythms, sleep, and disorders of aging. Trends Endocrinol. Metab. 2016, 27, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Hickey, M.A.; Zhu, C.; Medvedeva, V.; Lerner, R.P.; Patassini, S.; Franich, N.R.; Maiti, P.; Frautschy, S.A.; Zeitlin, S.; Levine, M.S.; et al. Improvement of neuropathology and transcriptional deficits in CAG 140 knock-in mice supports a beneficial effect of dietary curcumin in Huntington’s disease. Mol. Neurodegener. 2012, 7, 12. [Google Scholar] [CrossRef]
- Lee, S.B.; Kim, W.; Lee, S.; Chung, J. Loss of LRRK2/PARK8 induces degeneration of dopaminergic neurons in Drosophila. Biochem. Biophys. Res. Commun. 2007, 358, 534–539. [Google Scholar] [CrossRef]
- Ambani, L.M.; Van Woert, M.H.; Murphy, S. Brain peroxidase and catalase in Parkinson disease. Arch. Neurol. 1975, 32, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Ran, D.; Xie, B.; Gan, Z.; Sun, X.; Gu, H.; Yang, J. Melatonin attenuates hLRRK2-induced long-term memory deficit in a Drosophila model of Parkinson’s disease. Biomed. Rep. 2018, 9, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Hely, M.A.; Morris, J.G.; Reid, W.G.; Trafficante, R. Sydney multicenter study of Parkinson’s disease: Non-L-dopa–responsive problems dominate at 15 years. Mov. Disord. 2005, 20, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Spuch, C.; Ortolano, S.; Navarro, C. New insights in the amyloid-Beta interaction with mitochondria. J. Aging Res. 2012, 2012, 324968. [Google Scholar] [CrossRef]
- He, H.; Dong, W.; Huang, F. Anti-amyloidogenic and anti-apoptotic role of melatonin in Alzheimer disease. Curr. Neuropharmacol. 2010, 8, 211–217. [Google Scholar] [CrossRef]
- Millet-Boureima, C.; Rozencwaig, R.; Polyak, F.; Gamberi, C. Cyst reduction by melatonin in a novel Drosophila model of polycystic kidney disease. Molecules 2020, 25, 5477. [Google Scholar] [CrossRef]
- Bergmann, C.; Guay-Woodford, L.M.; Harris, P.C.; Horie, S.; Peters, D.J.M.; Torres, V.E. Polycystic kidney disease. Nat. Rev. Dis. Primers 2018, 4, 50. [Google Scholar] [CrossRef]
- Millet-Boureima, C.; He, S.; Le, T.B.U.; Gamberi, C. Modeling neoplastic growth in renal cell carcinoma and polycystic kidney disease. Int. J. Mol. Sci. 2021, 22, 3918. [Google Scholar] [CrossRef]
- Gamberi, C.; Lasko, P. The Bic-C family of developmental translational regulators. Comp. Funct. Genom. 2012, 2012, 141386. [Google Scholar] [CrossRef]
- Trudel, M.; Lanoix, J.; Barisoni, L.; Blouin, M.J.; Desforges, M.; L’Italien, C.; D’Agati, V. C-myc–induced apoptosis in polycystic kidney disease is Bcl-2 and p53 independent. J. Exp. Med. 1997, 186, 1873–1884. [Google Scholar] [CrossRef]
- Fan, L.X.; Zhou, X.; Sweeney, W.E.; Wallace, D.P.; Avner, E.D.; Grantham, J.J.; Li, X. Smac-mimetic-induced epithelial cell death reduces the growth of renal cysts. J. Am. Soc. Nephrol. 2013, 24, 2010–2022. [Google Scholar] [CrossRef]
- Millet-Boureima, C.; Chingle, R.; Lubell, W.D.; Gamberi, C. Cyst reduction in a polycystic kidney disease Drosophila model using Smac mimics. Biomedicines 2019, 7, 82. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kean, L.; Yang, J.; Allan, A.K.; Davies, S.A.; Herzyk, P.; Dow, J.A.T. Function-informed transcriptome analysis of Drosophila renal tubule. Genome Biol. 2004, 5, R69. [Google Scholar] [CrossRef] [PubMed]
- Millet-Boureima, C.; Porras, J.M.; Gamberi, C. Modeling renal disease “on the fly”. BioMed Res. Int. 2018, 2018, 5697436. [Google Scholar] [CrossRef]
- Sozen, M.A.; Armstrong, J.D.; Yang, M.; Kaiser, K.; Dow, J.A.T. Functional domains are specified to single-cell resolution in a Drosophila epithelium. Proc. Natl. Acad. Sci. USA 1997, 94, 5207–5212. [Google Scholar] [CrossRef] [PubMed]
- Saberi, K.; Pasbakhsh, P.; Omidi, A.; Borhani-Haghighi, M.; Nekoonam, S.; Omidi, N.; Ghasemi, S.; Kashani, I.R. Melatonin preconditioning of bone-marrow derived mesenchymal stem cells promotes their engraftment and improves renal regeneration in a rat model of chronic kidney disease. J. Mol. Hist. 2019, 50, 129–140. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, C.; Zhang, P.; Jiang, H.; Chen, J. Melatonin preconditioning is an effective strategy for mesenchymal stem cell-based therapy for kidney disease. J. Cell. Mol. Med. 2019, 24, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gong, Y.; Xiong, K.; Ye, Y.; Xiong, Y.; Zhuang, Z.; Luo, Y.; Jiang, Q.; He, F. Melatonin mediates protective effects on inflammatory response induced by interleukin-1 beta in human mesenchymal stem cells. J. Pineal Res. 2013, 55, 14–25. [Google Scholar] [CrossRef]
- Klawitter, J.; Reed-Gitomer, B.Y.; McFann, K.; Pennington, A.; Klawitter, J.; Abebe, K.Z.; Klepacki, J.; Cadnapaphornchai, M.A.; Brosnahan, G.; Chonchoi, M.; et al. Endothelial dysfunction and oxidative stress in polycystic kidney disease. Am. J. Physiol. Renal Physiol. 2014, 307, F1198–F1206. [Google Scholar] [CrossRef]
- Andries, A.; Daenen, K.; Jouret, F.; Bammens, B.; Mekahli, D.; Van Schepdael, A. Oxidative stress in autosomal dominant polycystic kidney disease: Player and/or early predictor for disease progression? Pediatr. Nephrol. 2019, 34, 993–1008. [Google Scholar] [CrossRef]
- Ishimoto, Y.; Inagi, R.; Yoshihara, D.; Kugita, M.; Nagao, S.; Shimizu, A.; Takeda, N.; Wake, M.; Honda, K.; Zhou, J.; et al. Mitochondrial abnormality facilitates cyst formation in autosomal dominant polycystic kidney disease. Mol. Cell. Biol. 2017, 37, e00337-17. [Google Scholar] [CrossRef]
- Padovano, V.; Podrini, C.; Boletta, A.; Caplan, M.J. Metabolism and mitochondria in polycystic kidney disease research and therapy. Nat. Rev. Nephrol. 2018, 14, 678–687. [Google Scholar] [CrossRef]
- Peterson, K.M.; Franchi, F.; Loeffler, D.L.; Psaltis, P.J.; Harris, P.C.; Lerman, L.O.; Lerman, A.; Rodriguez-Porcel, M. Endothelial dysfunction occurs prior to clinical evidence of polycystic kidney disease. Am. J. Nephrol. 2013, 38, 233–240. [Google Scholar] [CrossRef]
- Mehrzadi, M.H.; Hosseinzadeh, A.; Juybari, K.B.; Mehrzadi, S. Melatonin and urological cancers: A new therapeutic approach. Cancer Cell Int. 2020, 20, 444. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.L.; Zhang, B.L.; Reiter, R.J.; Liu, Y.X. Melatonin ameliorates inflammation and oxidative stress by suppressing the p38MAPK signaling pathway in LPS-induced sheep orchitis. Antioxidants 2020, 9, 1277. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, Z.; Sun, H.; Song, J.; Chen, X.; Huang, J.; Lin, X.; Zhou, R. Effect of melatonin on T/B cell activation and immune regulation in pinealectomy mice. Life Sci. 2020, 242, 117191. [Google Scholar] [CrossRef] [PubMed]
- Zhen, G.; Liang, W.; Jia, H.; Zheng, X. Melatonin relieves sepsis-induced myocardial injury via regulating JAK2/STAT3 signaling pathway. Minerva Med. 2020. [Google Scholar] [CrossRef]
- Ma, S.; Zhu, L.; Fan, X.; Luo, T.; Liu, D.; Liang, Z.; Hu, X.; Shi, T.; Tan, W.; Wang, Z. Melatonin derivatives combat with inflammation-related cancer by targeting the Main Culprit STAT3. Eur. J. Med. Chem. 2021, 211, 113027. [Google Scholar] [CrossRef]
- Lu, K.H.; Su, S.C.; Lin, C.W.; Hsieh, Y.H.; Lin, Y.C.; Chien, M.H.; Reiter, R.J.; Yang, S.F. Melatonin attenuates osteosarcoma cell invasion by suppression of C-C motif chemokine ligand 24 through inhibition of the c-Jun N-terminal kinase pathway. J. Pineal Res. 2018, 65, e12507. [Google Scholar] [CrossRef]
- Wei, J.; Wang, Y.; Qi, X.; Fan, Z.; Wu, Y. Melatonin ameliorates hyperglycaemia-induced renal inflammation by inhibiting the activation of TLR4 and TGF-b1/Smad3 signalling pathway. Am. J. Transl. Res. 2020, 12, 1584–1599. [Google Scholar]
- Seeger-Nukpezah, T.; Geynisman, D.M.; Nikonova, A.S.; Benzing, T.; Golemis, E.A. The hallmarks of cancer: Relevance to the pathogenesis of polycystic kidney disease. Nat. Rev. Nephrol. 2015, 11, 515–534. [Google Scholar] [CrossRef]
- Rowe, I.; Chiaravalli, M.; Mannella, V.; Ulisse, V.; Quilici, G.; Pema, M.; Song, X.W.; Xu, H.; Mari, S.; Qian, F.; et al. Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat. Med. 2013, 19, 488–493. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, M.; Li, L.; Chen, L. Involvement of the Warburg effect in non-tumor diseases processes. J. Cell Physiol. 2018, 233, 2839–2849. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. We need to talk about the Warburg effect. Nat. Metab. 2020, 2, 127–129. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R.; Ma, Q. Switching diseased cells from cytosolic aerobic glycolysis to mitochondrial oxidative phosphorylation: A metabolic rhythm regulated by melatonin? J. Pineal Res. 2021, 70, e12677. [Google Scholar] [CrossRef]
- de Lima Mota, A.; Jardim-Perassi, B.V.; de Castro, T.B.; Colombo, J.; Sonehara, N.M.; Nishiyama, V.K.G.; Pierri, V.A.G.; de Campos Zuccari, D.A.P. Melatonin modifies tumor hypoxia and metabolism by inhibiting HIF-1a and energy metabolic pathway in the in vitro and in vivo models of breast cancer. Melatonin Res. 2019, 2, 83–98. [Google Scholar] [CrossRef]
- Millet-Boureima, C.; Selber-Hnatiw, S.; Gamberi, C. Drug discovery and chemical probing in Drosophila. Genome 2021, 64, 147–159. [Google Scholar] [CrossRef]
- Wilson, S.T.; Blask, D.E.; Lemus-Wilson, A.M. Melatonin augments the sensitivity of MCF-7 human breast cancer cells to tamoxifen in vitro. J. Clin. Endocrinol. Metab. 1992, 75, 669–670. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Sainz, R.M.; Mayo, J.C.; Lopez-Burillo, S. Melatonin: Reducing the toxicity and increasing the efficacy of drugs. J. Pharm. Pharmacol. 2002, 54, 1299–1321. [Google Scholar] [CrossRef] [PubMed]
- Lissoni, P.; Brivio, F.; Fumagalli, L.; Messina, G.; Vigore, L.; Parolini, D.; Colciago, M.; Rovelli, F. Neuroimmunomodulation in medical oncology: Application of psychoneuroimmunology with subcutaneous low-dose IL-2 and the pineal hormone melatonin in patients with untreatable metastatic solid tumors. Anticancer Res. 2008, 28, 1377–1381. [Google Scholar] [PubMed]
- Ruiz-Rabelo, J.; Vazquez, R.; Arjona, A.; Perea, D.; Montilla, P.; Tunez, I.; Muntane, J.; Padillo, J. Improvement of capecitabine antitumoral activity by melatonin in pancreatic cancer. Pancreas 2011, 40, 410–414. [Google Scholar] [CrossRef]
- Kim, J.H.; Jeong, S.J.; Kim, B.; Yun, S.M.; Choi, D.Y.; Kim, S.H. Melatonin synergistically enhances cisplatin-induced apoptosis via the dephosphorylation of ERK/p90 ribosomal S6 kinase/heat shock protein 27 in SK-OV-3 cells. J. Pineal Res. 2012, 52, 244–252. [Google Scholar] [CrossRef]
- Margheri, M.; Pacini, N.; Tani, A.; Nosi, D.; Squecco, R.; Dama, A.; Masala, E.; Francini, F.; Zecchi-Orlandini, S.; Formigli, L. Combined effects of melatonin and all-trans retinoic acid and somatostatin on breast cancer cell proliferation and death: Molecular basis for the anticancer effect of these molecules. Eur. J. Pharmacol. 2012, 681, 34–43. [Google Scholar] [CrossRef]
- Uguz, A.C.; Cig, B.; Espino, J.; Bejarano, I.; Naziroglu, M.; Rodriguez, A.B.; Pariente, J.A. Melatonin potentiates chemotherapy-induced cytotoxicity and apoptosis in rat pancreatic tumor cells. J. Pineal Res. 2012, 53, 91–98. [Google Scholar] [CrossRef]
- Jung, J.H.; Sohn, E.J.; Shin, E.A.; Lee, D.; Kim, B.; Jung, D.B.; Kim, J.H.; Yun, M.; Lee, H.J.; Park, Y.K.; et al. Melatonin suppresses the expression of 45S preribosomal RNA and upstream binding factor and enhances the antitumor activity of puromycin in MDA-MB-231 breast cancer cells. Evid.-Based Complement. Altern. Med. 2013, 2013, 879746. [Google Scholar] [CrossRef] [PubMed]
- Kosar, P.A.; Naziroglu, M.; Ovey, I.S.; Cig, B. Synergic e_ects of doxorubicin and melatonin on apoptosis and mitochondrial oxidative stress in MCF-7 breast cancer cells: Involvement of TRPV1 channels. J. Membr. Biol. 2016, 249, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.M.; Woo, S.H.; Oh, S.T.; Hong, S.E.; Choe, T.B.; Ye, S.K.; Kim, E.K.; Seong, M.K.; Kim, H.A.; Noh, W.C.; et al. Melatonin enhances arsenic trioxide-induced cell death via sustained upregulation of Redd1 expression in breast cancer cells. Mol. Cell. Endocrinol. 2016, 422, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Ho_man, K.; Gao, C.; Petrulionis, M.; Herr, I.; Schemmer, P. Melatonin promotes sorafenib-induced apoptosis through synergistic activation of JNK/c-jun pathway in human hepatocellular carcinoma. J. Pineal Res. 2017, 62, e12398. [Google Scholar] [CrossRef]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Grantham, J.J.; Higashihara, E.; Perrone, R.D.; Krasa, H.B.; Ouyang, J.; Czerwiec, F.S. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2012, 367, 2407–2418. [Google Scholar] [CrossRef]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Perrone, R.D.; Koch, G.; Ouyang, J.; McQuade, R.D.; Blais, J.D.; Czerwiec, F.S.; et al. Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2017, 377, 1930–1942. [Google Scholar] [CrossRef]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Perrone, R.D.; Dandurand, A.; Ouyang, J.; Czerwiec, F.S.; Blais, J.D. Multicenter, open-label, extension trial to evaluate the long-term efficacy and safety of early versus delayed treatment with tolvaptan in autosomal dominant polycystic kidney disease: The TEMPO 4:4 Trial. Nephrol. Dial. Transplant 2018, 33, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Luoma, S.E.; St Armour, G.E.; Thakkar, E.; Mackay, T.F.C.; Anholt, R.R.H. A Drosophila model for toxicogenomics: Genetic variation in susceptibility to heavy metal exposure. PLoS Genet. 2017, 13, e1006907. [Google Scholar] [CrossRef] [PubMed]
- Affleck, J.G.; Walker, V.K. Drosophila as a model for developmental toxicology: Using and extending the Drosophotoxicology model. Methods Mol. Biol. 2019, 1965, 139–153. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).