Papillary Meningioma: Case Presentation with Emphasis on Surgical and Medical Therapy of a Rare Variant of Meningioma
Abstract
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Classification of Tumours of the Central Nervous System; World Health Organization: Lyon, France, 2016. [Google Scholar]
- Buerki, R.A.; Horbinski, C.M.; Kruser, T.; Horowitz, P.M.; James, C.D.; Lukas, R.V. An overview of meningiomas. Future Oncol. 2018, 14, 2161–2177. [Google Scholar] [CrossRef] [PubMed]
- Preusser, M.; Brastianos, P.K.; Mawrin, C. Advances in meningioma genetics: Novel therapeutic opportunities. Nat. Rev. Neurol. 2018, 14, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Wiemels, J.; Wrensch, M.; Claus, E.B. Epidemiology and etiology of meningioma. J. Neurooncol. 2010, 99, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Stefanko, S.Z.; Mackay, W.M. Papillary meningioma. Acta Neuropathol. 1981, 7, 126–128. [Google Scholar]
- Ludwin, S.K.; Rubinstein, L.J.; Russell, D.S. Papillary meningioma: A malignant variant of meningioma. Cancer 1975, 36, 1363–1373. [Google Scholar] [CrossRef]
- Wu, Y.T.; Ho, J.T.; Lin, Y.J.; Lin, J.W. Rhabdoid papillary meningioma: A clinicopathologic case series study. Neuropathology 2011, 31, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Kros, J.M.; Cella, F.; Bakker, S.L.; Paz, Y.; Geuze, D.; Egeler, R.M. Papillary meningioma with pleural metastasis: Case report and literature review. Acta Neurol. Scand. 2000, 102, 200–202. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tathe, S.P.; Parate, S.N. Papillary meningioma: Diagnosis by intraoperative crush smear cytology. Diagn. Cytopathol. 2019, 47, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Chao, Q.; Zhang, H.; Wang, D.W.; Shu, H.S. Intraventricular cystic papillary meningioma: A case report and literature review. Medicine 2020, 99, e21514. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.B.; Ke, C.; Han, Z.A.; Lin, S.H.; Mou, Y.G.; Luo, R.Z.; Wu, S.X.; Chen, Z.P. Intraparenchymal papillary meningioma of brainstem: Case report and literature review. World J. Surg. Oncol. 2012, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Lee, Y.S. Molecular characteristics of meningiomas. J. Pathol. Transl. Med. 2020, 54, 45–63. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, C.; Sieberns, J.; Gehlhaar, C.; Simon, M.; Paulus, W.; von Deimling, A. NF2 mutations in secretory and other rare variants of meningiomas. Brain Pathol. 2006, 16, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.A.; Wakimoto, H.; Shankar, G.M.; Barker, F.G., 2nd; Brastianos, P.K.; Santagata, S.; Sokol, E.S.; Pavlick, D.C.; Shah, N.; Reddy, A.; et al. Frequent inactivating mutations of the PBAF complex gene PBRM1 in meningioma with papillary features. Acta Neuropathol. 2020, 140, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Behling, F.; Fodi, C.; Hoffmann, E.; Renovanz, M.; Skardelly, M.; Tabatabai, G.; Schittenhelm, J.; Honegger, J.; Tatagiba, M. The role of Simpson grading in meningiomas after integration of the updated WHO classification and adjuvant radiotherapy. Neurosurg. Rev. 2020, 44, 2329–2336. [Google Scholar] [CrossRef] [PubMed]
- Brokinkel, B.; Spille, D.C.; Brokinkel, C.; Hess, K.; Paulus, W.; Bormann, E.; Stummer, W. The Simpson grading: Defining the optimal threshold for gross total resection in meningioma surgery. Neurosurg. Rev. 2020, 41, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Sumner, W.A.; Amini, A.; Hankinson, T.C.; Foreman, N.K.; Gaspar, L.E.; Kavanagh, B.D.; Karam, S.D.; Rusthoven, C.G.; Liu, A.K. Survival benefit of postoperative radiation in papillarymeningioma: Analysis of the National Cancer Data Base. Rep. Pract. Oncol. Radiother. 2017, 22, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.; Zhu, H.; Li, J.; Tang, H.; Kuang, D.; Wang, Y.; Tang, F.; Chen, X.; Zhou, L.; Xie, Q.; et al. Prognostic value of estrogen receptor in WHO Grade III meningioma: A long-term follow-up study from a single institution. J. Neurosurg. 2018, 128, 1698–1706. [Google Scholar] [CrossRef]
- Pellerino, A.; Bruno, F.; Internò, V.; Rudà, R.; Soffietti, R. Current clinical management of elderly patients with glioma. Expert Rev. Anticancer Ther. 2020, 20, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.C.; Glantz, M.J.; Fadul, C.E. Recurrent meningioma: Salvage therapy with long-acting somatostatin analogue. Neurology 2007, 69, 969–973. [Google Scholar] [CrossRef]
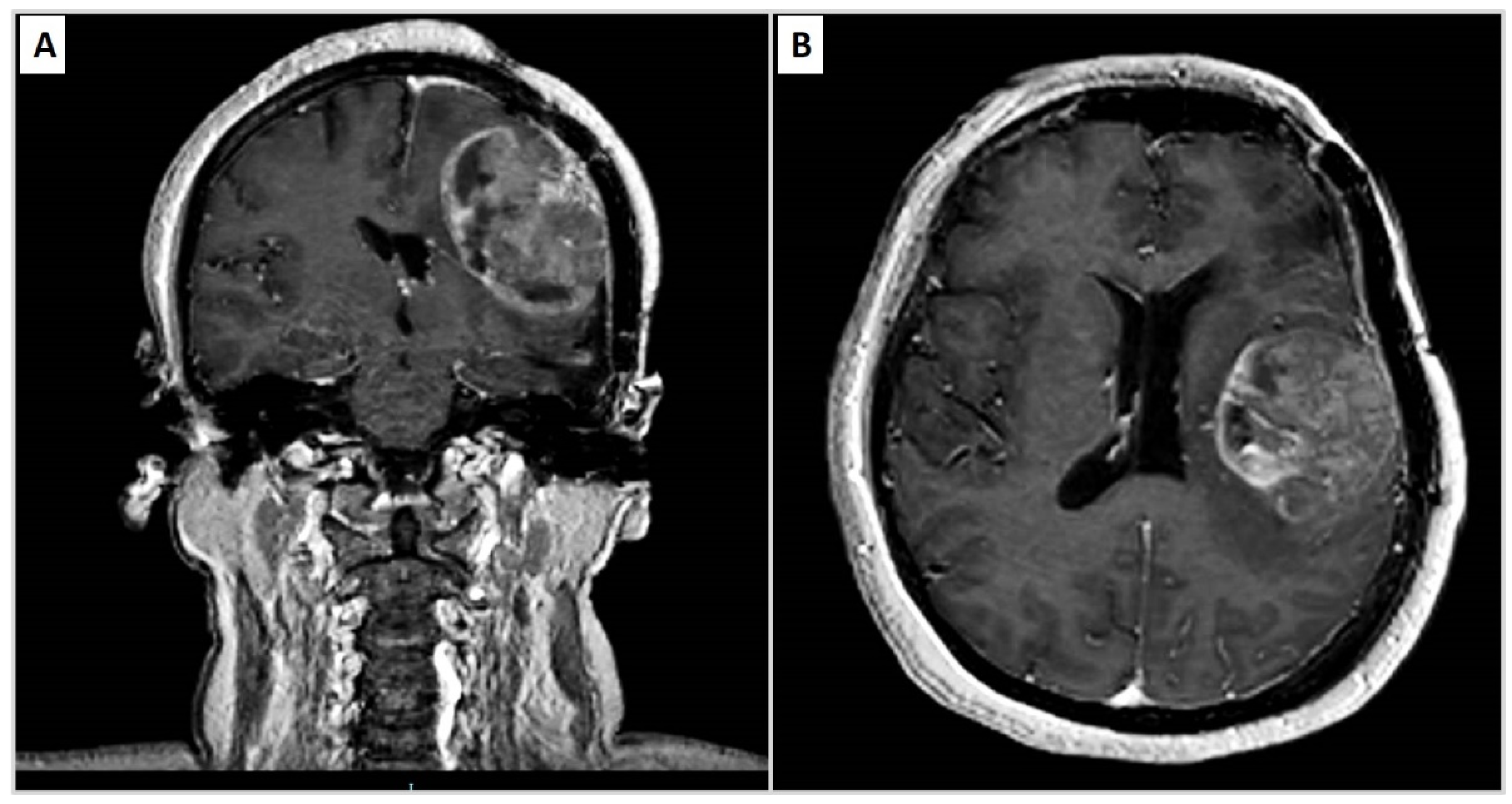
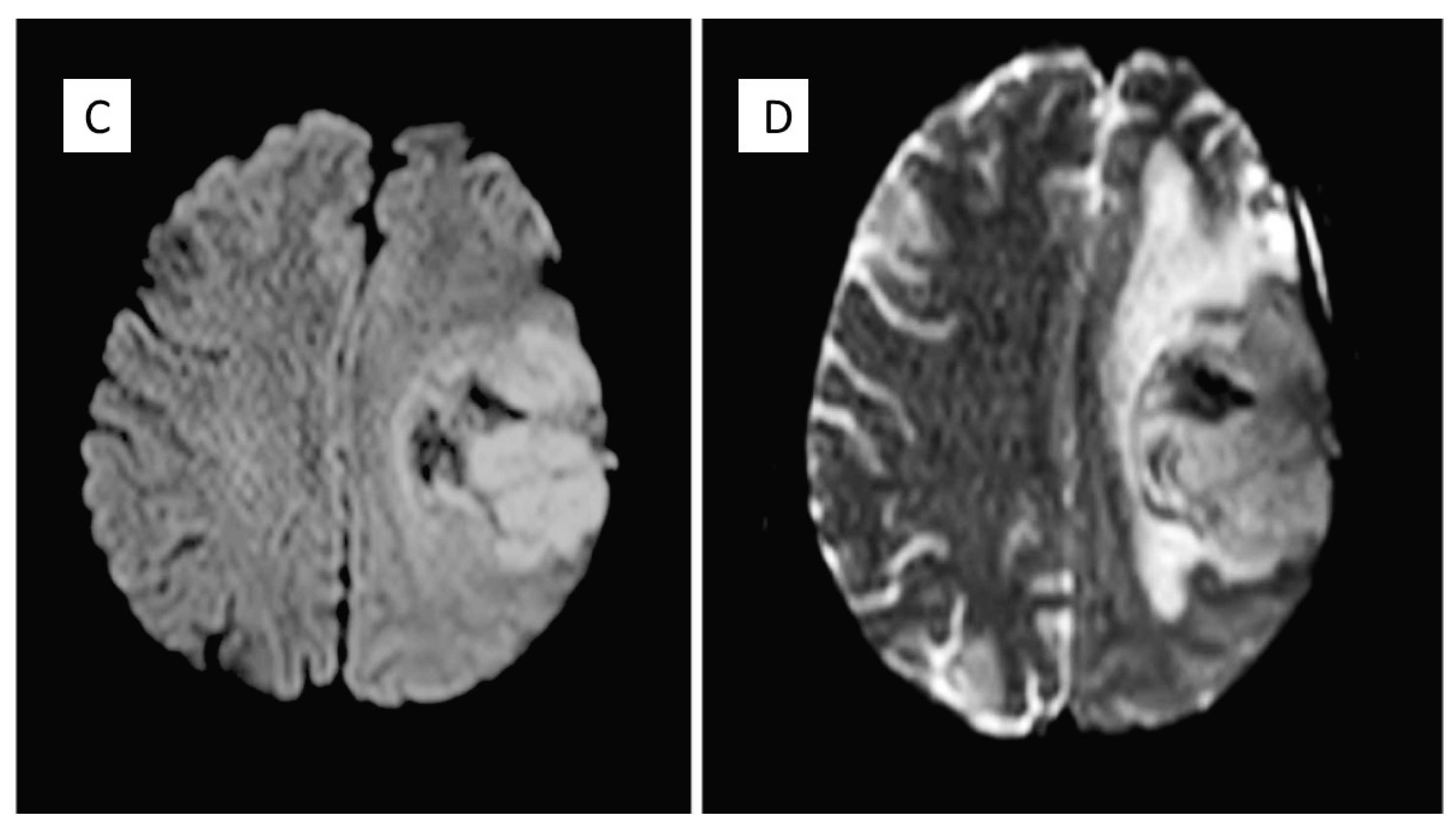
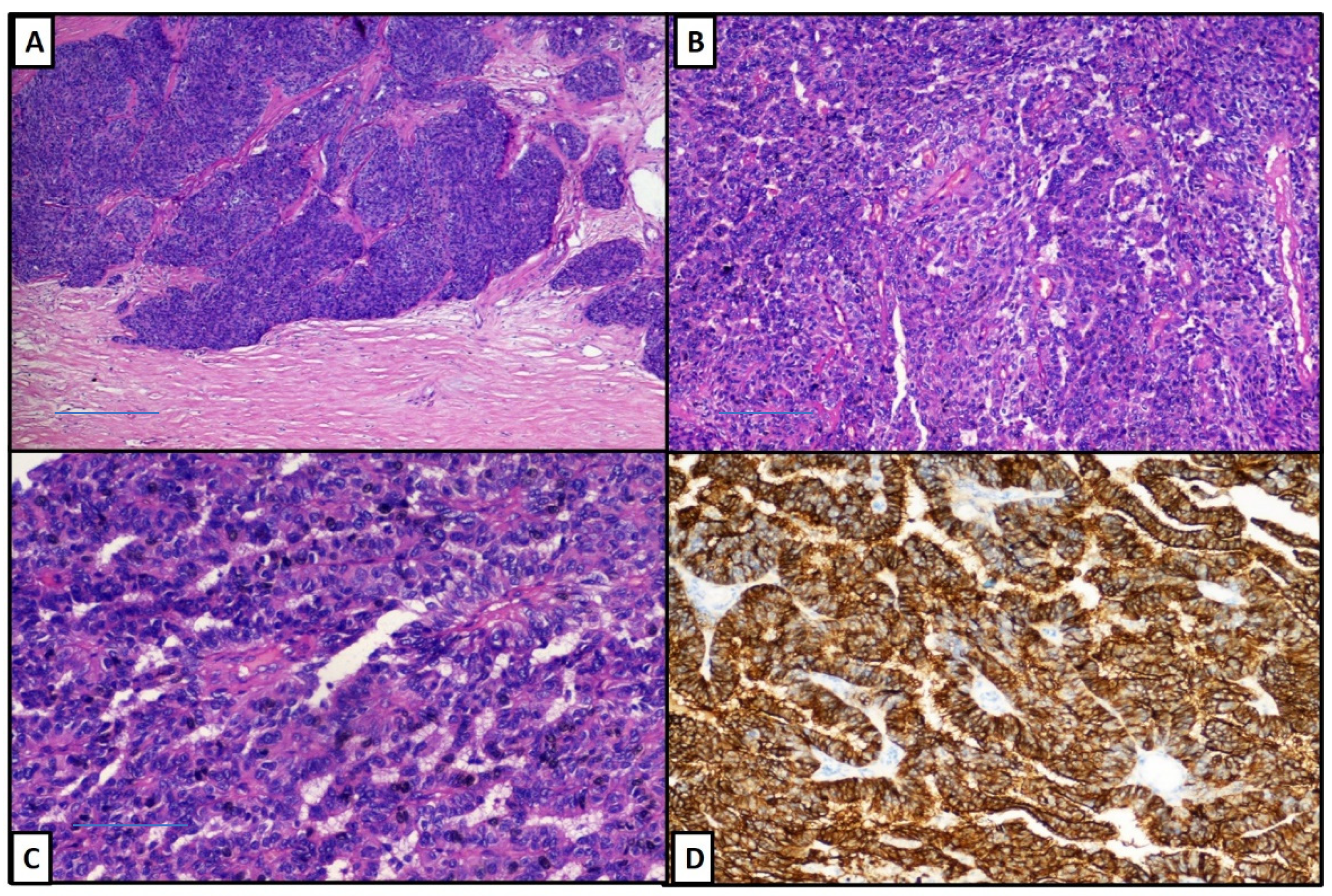
| Years | Symptoms | Diagnosis | Treatment |
|---|---|---|---|
| 2010 | Headache, speech difficulties and hypersomnia | Neoplastic lesion in the left frontparietal area compatible with papillary meningioma | Surgical removal and radiotherapy adjuvant treatment |
| 2015 | Headache | Recurrence of PM | Neurosurgical removal |
| 2020 | Headache, sleep disturbances and functional symptoms | Recurrence of PM | Tumour excision |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazzato, G.; Internò, V.; Cimmino, A.; Messina, R.; Tucci, M.; Lettini, T.; Resta, L.; Ingravallo, G. Papillary Meningioma: Case Presentation with Emphasis on Surgical and Medical Therapy of a Rare Variant of Meningioma. Diseases 2021, 9, 63. https://doi.org/10.3390/diseases9030063
Cazzato G, Internò V, Cimmino A, Messina R, Tucci M, Lettini T, Resta L, Ingravallo G. Papillary Meningioma: Case Presentation with Emphasis on Surgical and Medical Therapy of a Rare Variant of Meningioma. Diseases. 2021; 9(3):63. https://doi.org/10.3390/diseases9030063
Chicago/Turabian StyleCazzato, Gerardo, Valeria Internò, Antonietta Cimmino, Raffaella Messina, Marco Tucci, Teresa Lettini, Leonardo Resta, and Giuseppe Ingravallo. 2021. "Papillary Meningioma: Case Presentation with Emphasis on Surgical and Medical Therapy of a Rare Variant of Meningioma" Diseases 9, no. 3: 63. https://doi.org/10.3390/diseases9030063
APA StyleCazzato, G., Internò, V., Cimmino, A., Messina, R., Tucci, M., Lettini, T., Resta, L., & Ingravallo, G. (2021). Papillary Meningioma: Case Presentation with Emphasis on Surgical and Medical Therapy of a Rare Variant of Meningioma. Diseases, 9(3), 63. https://doi.org/10.3390/diseases9030063










