Immunological Features in the Process of Blood Platelet-Induced Alloimmunisation, with a Focus on Platelet Component Transfusion
Abstract
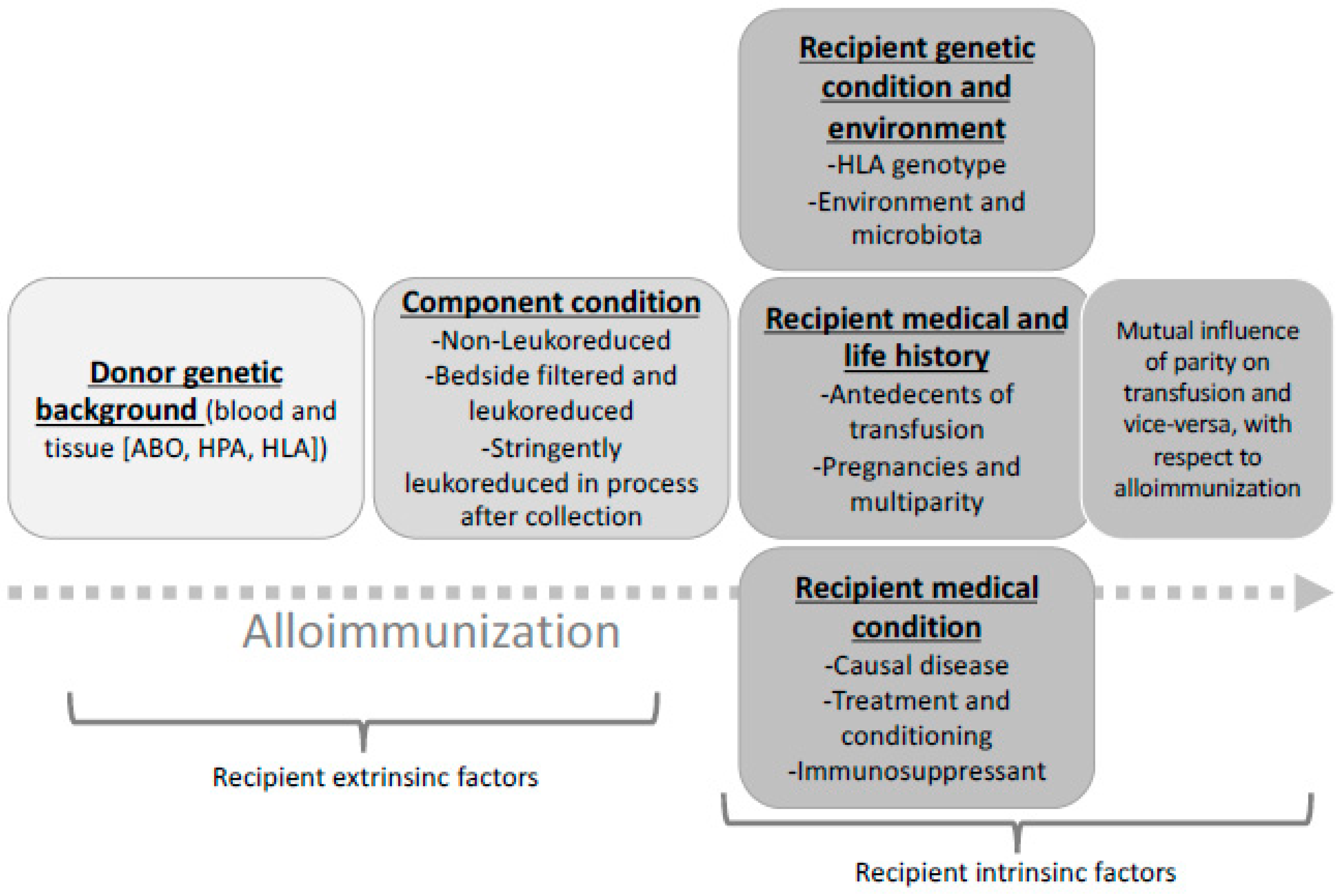
1. Circumstances of Patient Immunisation
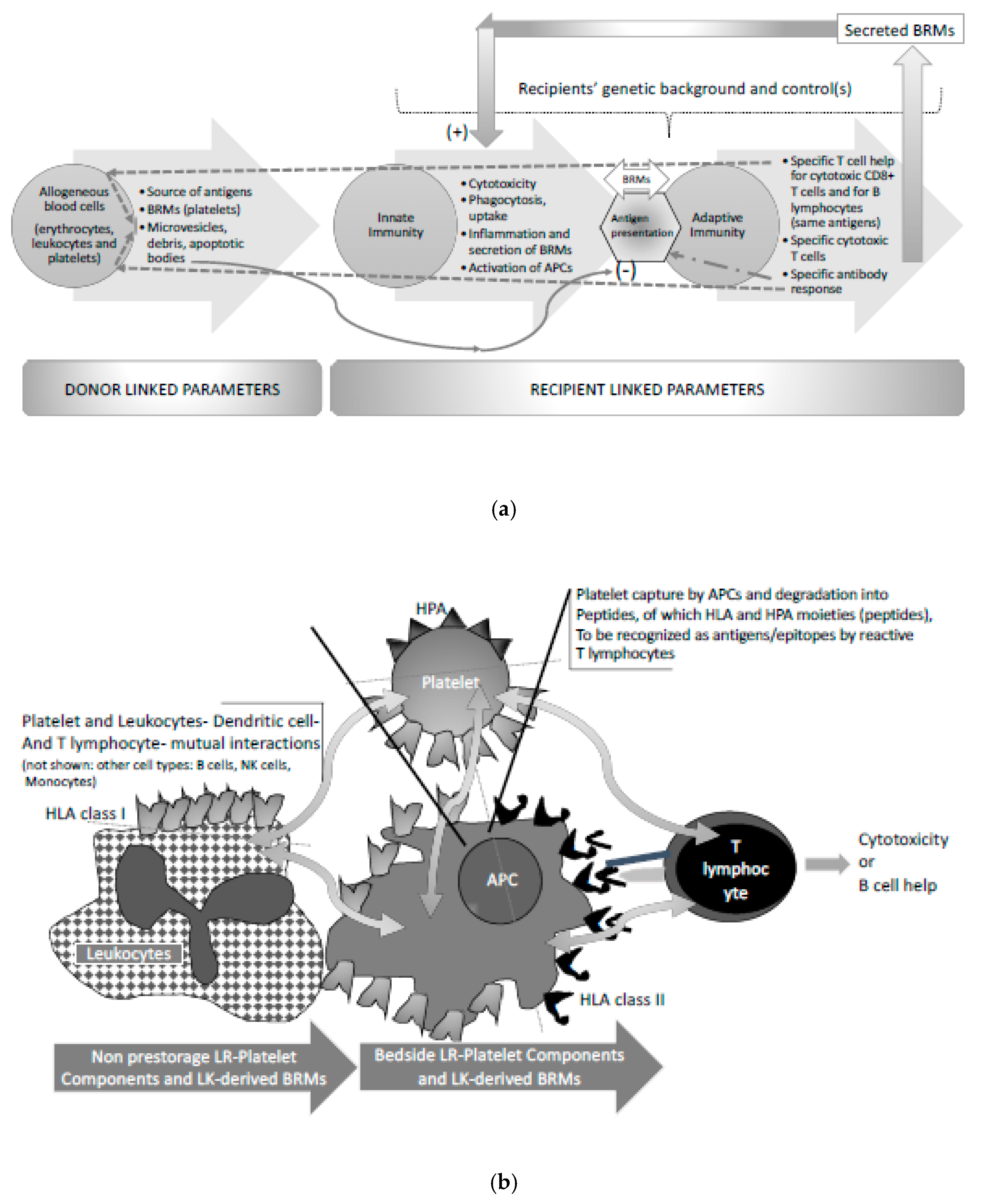
2. Allogenous (Foreign) Blood Cell Related Parameters Involved in Recipient Immunisation
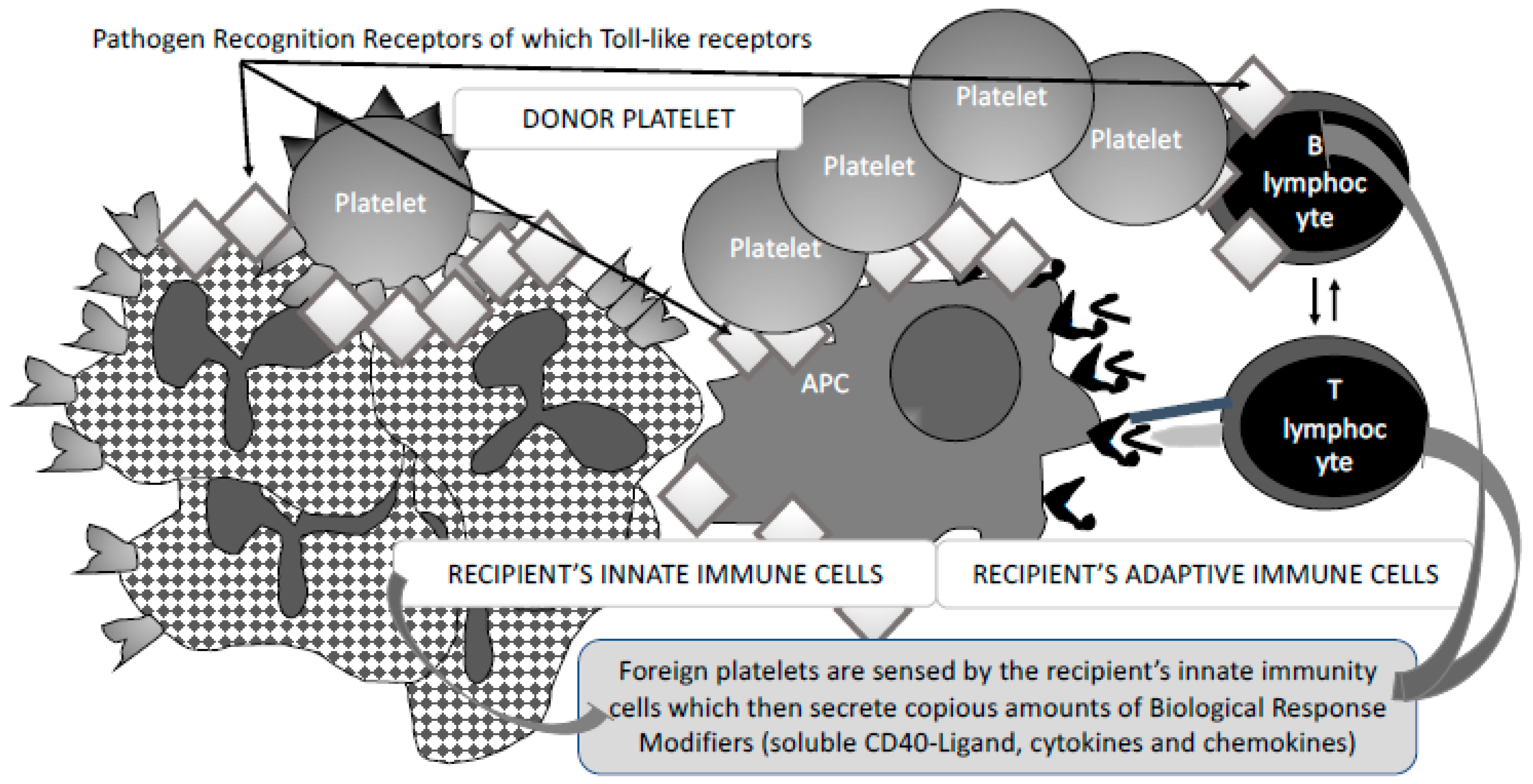
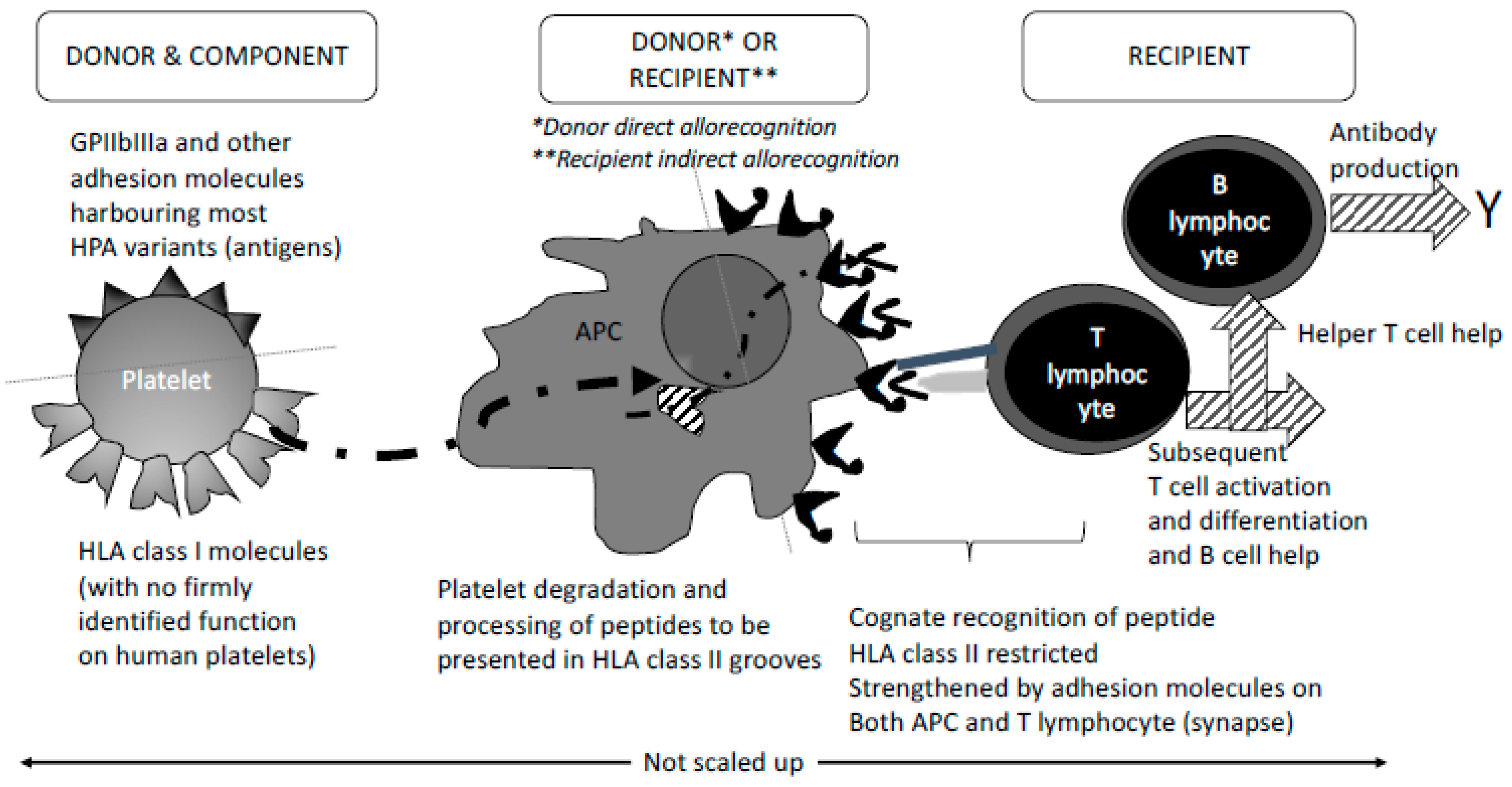
3. Fine-Tuned Mechanisms of Alloimmunisation: As Yet Unexplored
4. Implications for Patient Management
5. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPD—HPA Sequence Database European Bioinformatics Institute All HPA—Alloantigen/Protein Data. Available online: https://www.ebi.ac.uk/ipd/hpa/table1.html (accessed on 5 November 2018).
- Curtis, B.R.; McFarland, J.G. Human platelet antigens—2013. Vox Sang. 2014, 106, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Ohto, H.; Miura, S.; Ariga, H.; Ishii, T.; Fujimori, K.; Morita, S. The natural history of maternal immunization against fetal platelet alloantigens. Transfus. Med. 2004, 14, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Kovanlikaya, A.; Tiwari, P.; Bussel, J.B. Imaging and management of fetuses and neonates with alloimmune thrombocytopenia. Pediatr. Blood Cancer 2017, 64. [Google Scholar] [CrossRef] [PubMed]
- Petermann, R. Thirty years of platelet immunology in fetal and neonatal alloimmune thrombocytopeniamanagement, current situation. Transfus. Clin. Biol. 2017, 24, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Winkelhorst, D.; Oepkes, D.; Lopriore, E. Fetal and neonatal alloimmune thrombocytopenia: Evidence based antenatal and postnatal management strategies. Expert Rev. Hematol. 2017, 10, 729–737. [Google Scholar] [CrossRef]
- Tiller, H.; Husebekk, A.; Ahlen, M.T.; Stuge, T.B.; Skogen, B. Current perspectives on fetal and neonatal alloimmune thrombocytopenia—Increasing clinical concerns and new treatment opportunities. Int. J. Womens Health 2017, 9, 223–234. [Google Scholar] [CrossRef]
- Bonstein, L.; Haddad, N. Taking a wider view on fetal/neonatal alloimmune thrombocytopenia. Thromb. Res. 2017, 151 (Suppl. 1), 100S–102S. [Google Scholar] [CrossRef]
- Winkelhorst, D.; Murphy, M.F.; Greinacher, A.; Shehata, N.; Bakchoul, T.; Massey, E.; Baker, J.; Lieberman, L.; Tanael, S.; Hume, H.; et al. Antenatal management in fetal and neonatal alloimmune thrombocytopenia: A systematic review. Blood 2017, 129, 1538–1547. [Google Scholar] [CrossRef]
- Yougbaré, I.; Lang, S.; Yang, H.; Chen, P.; Zhao, X.; Tai, W.S.; Zdravic, D.; Vadasz, B.; Li, C.; Piran, S.; et al. Maternal anti-platelet β3 integrins impair angiogenesis and cause intracranial hemorrhage. J. Clin. Investig. 2015, 125, 1545–1556. [Google Scholar] [CrossRef]
- Quach, M.E.; Chen, W.; Li, R. Mechanisms of platelet clearance and translation to improve platelet storage. Blood 2018, 131, 1512–1521. [Google Scholar] [CrossRef]
- Chen, W.; Druzak, S.A.; Wang, Y.; Josephson, C.D.; Hoffmeister, K.M.; Ware, J.; Li, R. Refrigeration-Induced Binding of von Willebrand Factor Facilitates Fast Clearance of Refrigerated Platelets. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2271–2279. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liang, X.; Syed, A.K.; Jessup, P.; Church, W.R.; Ware, J.; Josephson, C.D.; Li, R. Inhibiting GPIbα Shedding Preserves Post-Transfusion Recovery and Hemostatic Function of Platelets after Prolonged Storage. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Quach, M.E.; Dragovich, M.A.; Chen, W.; Syed, A.K.; Cao, W.; Liang, X.; Deng, W.; De Meyer, S.F.; Zhu, G.; Peng, J.; et al. Fc-independent immune thrombocytopenia via mechanomolecular signaling in platelets. Blood 2018, 131, 787–796. [Google Scholar] [CrossRef]
- Dahl, J.; Refsum, E.; Ahlen, M.T.; Egeland, T.; Jensen, T.; Viken, M.K.; Stuge, T.B.; Acharya, G.; Husebekk, A.; Skogen, B.; et al. Unraveling the role of maternal anti-HLA class I antibodies in fetal and neonatal thrombocytopenia-Antibody specificity analysis using epitope data. J. Reprod. Immunol. 2017, 122, 1–9. [Google Scholar] [CrossRef]
- Refsum, E.; Mörtberg, A.; Dahl, J.; Meinke, S.; Auvinen, M.K.; Westgren, M.; Reilly, M.; Höglund, P.; Wikman, A. Characterisation of maternal human leukocyte antigen class I antibodies in suspected foetal and neonatal alloimmune thrombocytopenia. Transfus. Med. 2017, 27, 43–45. [Google Scholar] [CrossRef]
- Blajchman, M.A.; Slichter, S.J.; Heddle, N.M.; Murphy, M.F. New strategies for the optimal use of platelet transfusions. Hematol. Am. Soc. Hematol. Educ. Program 2008, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Dutcher, J.P.; Schiffer, C.A.; Aisner, J.; Wiernik, P.H. Long-term follow-up of patients with leukemia receiving platelet transfusions: Identification of a large group of patients who do not become alloimmunized. Blood 1981, 58, 1007–1011. [Google Scholar] [PubMed]
- Andreu, G.; Dewailly, J.; Leberre, C.; Quarre, M.C.; Bidet, M.L.; Tardivel, R.; Devers, L.; Lam, Y.; Soreau, E.; Boccaccio, C. Prevention of HLA immunization with leukocyte-poor packed red cells and platelet concentrates obtained by filtration. Blood 1988, 72, 964–969. [Google Scholar]
- Slichter, S.J.; Fish, D.; Abrams, V.K.; Gaur, L.; Nelson, K.; Bolgiano, D. Evaluation of different methods of leukoreduction of donor platelets to prevent alloimmune platelet refractoriness and induce tolerance in a canine transfusion model. Blood 2005, 105, 847–854. [Google Scholar] [CrossRef]
- Slichter, J.S.; Bolgiano, D.; Kao, J.K.; Kickler, K.S.; McFarland, J.; McCullough, J.; Woodson, J. Persistence of Lymphocytotoxic antibodies in patients in the trial to reduce alloimmunization to platelets: Implications for using modified blood products. Transfus. Med. Rev. 2011, 25, 102–110. [Google Scholar] [CrossRef]
- Slichter, S.L.; Davis, K.; Enright, H.; Braine, H.; Gernsheimer, T.; Kao, K.J.; Kickler, T.; Lee, E.; McFarland, J.; McCullough, J.; et al. Factors affecting posttransfusion platelet increments, platelet refractoriness, and platelet transfusion intervals in thrombocytopenic patients. Blood 2005, 105, 4106–4114. [Google Scholar] [CrossRef] [PubMed]
- Andreu, G.; Boccaccio, C.; Klaren, J.; Lecrubier, C.; Pirenne, F.; Garcia, I.; Baudard, M.; Devers, L.; Fournel, J.J. The role of UV radiation in the prevention of human leukocyte antigen alloimmunization. Transfus. Med. Rev. 1992, 6, 212–224. [Google Scholar] [CrossRef]
- Andreu, G.; Perrot, J.Y.; Pirenne, F.; Boccaccio, C. The effect of ultraviolet B light on antigen-presenting cells: Implications for transfusion-induced sensitization. Semin. Hematol. 1992, 29, 122–131. [Google Scholar] [PubMed]
- Curtis, B.R.; Edwards, J.T.; Hessner, M.J.; Klein, J.P.; Aster, R.H. Blood group antigens are strongly expressed on platelets of some individuals. Blood 2000, 96, 1574–1581. [Google Scholar] [PubMed]
- Pavenski, K.; Freedman, J.; Semple, J.W. HLA alloimmunization against platelet transfusions: Pathophysiology, significance, prevention and management. Tissue Antigens 2012, 79, 237–245. [Google Scholar] [CrossRef]
- Stanworth, S.J.; Navarrete, C.; Estcourt, L.; Marsh, J. Platelet refractoriness—Practical approaches and ongoing dilemmas in patient management. Br. J. Haematol. 2015, 171, 297–305. [Google Scholar] [CrossRef]
- Schmidt, A.E.; Refaai, M.A.; Coppage, M. HLA-Mediated Platelet Refractoriness: An ACLPS Critical Review. Am. J. Clin. Pathol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Vu, K.; Leavitt, A.D. Posttransfusion purpura with antibodies against human platelet antigen-4a following checkpoint inhibitor therapy: A case report and review of the literature. Transfusion 2018, 58, 2265–2269. [Google Scholar] [CrossRef] [PubMed]
- Estcourt, L.J.; Stanworth, S.J.; Murphy, M.F. Different platelet count thresholds to guide use of prophylactic platelet transfusions for patients with hematological disorders after myelosuppressive chemotherapy or stem cell transplantation. JAMA Oncol. 2016, 2, 1091–1092. [Google Scholar] [CrossRef]
- Moncharmont, P. Platelet component transfusion and alloimmunization: Where do we stand? Transfus. Clin. Biol. 2018, 25, 72–178. [Google Scholar] [CrossRef]
- Waterman, H.R.; Kapp, L.M.; Munday, A.; Odem-Davis, K.; Zimring, J.C. Transfusion-induced alloimmunization and platelet refractoriness in a mouse model: Mechanisms and interventions. Transfusion 2016, 56, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; van der Wal, D.E.; Zhu, G.; Xu, M.; Yougbare, I.; Ma, L.; Vadasz, B.; Carrim, N.; Grozovsky, R.; Ruan, M.; et al. Desialylation is a mechanism of Fc-independent platelet clearance and a therapeutic target in immune thrombocytopenia. Nat. Commun. 2015, 6, 7737. [Google Scholar] [CrossRef] [PubMed]
- Rijkers, M.; Schmidt, D.; Lu, N.; Kramer, C.S.M.; Heidt, S.; Mulder, A.; Porcelijn, L.; Claas, F.H.J.; Leebeek, F.W.G.; Jansen, A.J.G.; et al. Anti-HLA antibodies with complementary and synergistic interaction geometries promote classical complement activation on platelets. Haematologica 2018. [Google Scholar] [CrossRef] [PubMed]
- Rijkers, M.; Saris, A.; Heidt, S.; Mulder, A.; Porcelijn, L.; Claas, F.H.J.; Bierings, R.; Leebeek, F.W.G.; Jansen, A.J.G.; Vidarsson, G.; et al. A subset of anti-HLA antibodies induces FcγRIIa-dependent platelet activation. Haematologica 2018, 103, 1741–1752. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, E.; Boucheix, C.; Urso, I.; Carroll, R.C. Fc gamma receptor-mediated interplatelet activation by a monoclonal antibody against beta 2 microglobulin. J. Immunol. 1991, 147, 3040–3046. [Google Scholar]
- Garraud, O.; Hamzeh-Cognasse, H.; Pozzetto, B.; Cavaillon, J.M.; Cognasse, F. Bench-to-bedside review: Platelets and active immune functions - new clues for immunopathology? Crit. Care 2013, 17, 236. [Google Scholar] [CrossRef]
- Garraud, O.; Tariket, S.; Sut, C.; Haddad, A.; Aloui, C.; Chakroun, T.; Laradi, S.; Cognasse, F. Transfusion as an Inflammation Hit: Knowns and Unknowns. Front. Immunol. 2016, 7, 534. [Google Scholar] [CrossRef]
- Aloui, C.; Prigent, A.; Sut, C.; Tariket, S.; Hamzeh-Cognasse, H.; Pozzetto, B.; Richard, Y.; Cognasse, F.; Laradi, S.; Garraud, O. The signaling role of CD40 ligand in platelet biology and in platelet component transfusion. Int. J. Mol. Sci. 2014, 15, 22342–22364. [Google Scholar] [CrossRef]
- Garraud, O.; Sut, C.; Haddad, A.; Tariket, S.; Aloui, C.; Laradi, S.; Hamzeh-Cognasse, H.; Bourlet, T.; Zeni, F.; Aubron, C.; et al. Transfusion-associated hazards: A revisit of their presentation. Transfus. Clin. Biol. 2018, 25, 118–135. [Google Scholar] [CrossRef]
- Garraud, O.; Cognasse, F.; Laradi, S.; Hamzeh-Cognasse, H.; Peyrard, T.; Tissot, J.D.; Fontana, S. How to mitigate the risk of inducing transfusion-associated adverse reactions. Transfus. Clin. Biol. 2018, 25, 262–268. [Google Scholar] [CrossRef]
- Cognasse, F.; Hamzeh-Cognasse, H.; Lafarge, S.; Chavarin, P.; Cogné, M.; Richard, Y.; Garraud, O. Human platelets can activate peripheral blood B cells and increase production of immunoglobulins. Exp. Hematol. 2007, 35, 1376–1387. [Google Scholar] [CrossRef] [PubMed]
- Semple, J.W.; Speck, E.R.; Milev, Y.P.; Blanchette, V.; Freedman, J. Indirect allorecognition of platelets by T helper cells during platelet transfusions correlates with anti-major histocompatibility complex antibody and cytotoxic T lymphocyte formation. Blood 1995, 86, 805–812. [Google Scholar] [PubMed]
- Gilson, C.R.; Patel, S.R.; Zimring, J.C. CTLA4-Ig prevents alloantibody production and BMT rejection in response to platelet transfusions in mice. Transfusion 2012, 52, 2209–2219. [Google Scholar] [CrossRef] [PubMed]
- Gilson, C.R.; Zimring, J.C. Alloimmunization to transfused platelets requires priming of CD4+ T cells in the splenic microenvironment in a murine model. Transfusion 2012, 52, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, N.; Hori, T.; Yamamoto, M.; Inazawa, N.; Iesato, K.; Miyazaki, T.; Ikeda, H.; Tsutsumi, H.; Suzuki, N. Platelet transfusion refractoriness attributable to HLA antibodies produced by donor-derived cells after allogeneic bone marrow transplantation from one HLA-antigen-mismatched mother. Pediatr. Transplant. 2011, 15, E177–E182. [Google Scholar] [CrossRef] [PubMed]
- Gibb, D.R.; Liu, J.; Natarajan, P.; Santhanakrishnan, M.; Madrid, D.J.; Eisenbarth, S.C.; Zimring, J.C.; Iwasaki, A.; Hendrickson, J.E. Type I IFN Is Necessary and Sufficient for Inflammation-Induced Red Blood Cell Alloimmunization in Mice. J. Immunol. 2017, 199, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Saris, A.; Peyron, I.; van der Meer, P.F.; Stuge, T.B.; Zwaginga, J.J.; van Ham, S.M.; Ten Brinke, A. Storage-Induced Platelet Apoptosis Is a Potential Risk Factor for Alloimmunization Upon Platelet Transfusion. Front. Immunol. 2018, 9, 1251. [Google Scholar] [CrossRef]
- Loewenthal, R.; Rosenberg, N.; Kalt, R.; Dardik, R.; Landau, M.; Yahalom, V.; Avishai, O.; Frenkel, O.; Gazit, E.; Steinberg, D.M.; et al. Compound heterozygosity of HLA-DRB3*01:01 and HLA-DRB4*01:01 as a potential predictor of fetal neonatal alloimmune thrombocytopenia. Transfusion 2011, 53, 344–352. [Google Scholar] [CrossRef]
- Wienzek-Lischka, S.; König, I.R.; Papenkort, E.M.; Hackstein, H.; Santoso, S.; Sachs, U.J.; Bein, G. HLA-DRB3*01:01 is a predictor of immunization against human platelet antigen-1a but not of the severity of fetal and neonatal alloimmune thrombocytopenia. Transfusion 2017, 57, 533–549. [Google Scholar] [CrossRef]
- Ahlen, M.T.; Husebekk, A.; Killie, M.K.; Skogen, B.; Stuge, T.B. T-cell responses associated with neonatal alloimmune thrombocytopenia: Isolation of HPA-1a-specific, HLA-DRB3*0101-restricted CD4+ T cells. Blood 2009, 113, 3838–3844. [Google Scholar] [CrossRef]
- Ahlen, M.T.; Husebekk, A.; Killie, I.L.; Skogen, B.; Stuge, T.B. T cell responses to human platelet antigen-1a involve a unique form of indirect allorecognition. JCI Insight 2016, 1, e86558. [Google Scholar] [CrossRef] [PubMed]
- Körmöczi, G.F.; Mayr, W.R. Responder individuality in red blood cell alloimmunization. Transfus. Med. Hemother. 2014, 41, 446–451. [Google Scholar] [CrossRef]
- Hickey, M.J.; Valenzuela, N.M.; Reed, E.F. Alloantibody Generation and Effector Function Following Sensitization to Human Leukocyte Antigen. Front. Immunol. 2016, 7, 30. [Google Scholar] [CrossRef]
- McFarland, J. Platelet immunology and alloimmunization. In Rossi’s Principles of Transfusion; Simon, T.L., McCullough, J., Snyder, E.L., Solhheim, B.G., Strauss, R.G.S., Eds.; Wiley: New York, NY, USA, 2016; Available online: https://pdfs.semanticscholar.org/5e4f/f9b99fac53eb198596fc30beeb892e3a9635.pdf (accessed on 5 November 2018).
- Seftel, M.D.; Growe, G.H.; Petraszko, T.; Benny, W.B.; Le, A.; Lee, C.Y.; Spinelli, J.J.; Sutherland, H.J.; Tsang, P.; Hogge, D.E. Universal prestorage leukoreduction in Canada decreases platelet alloimmunization and refractoriness. Blood 2004, 103, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Lannan, K.L.; Sahler, J.; Spinelli, S.L.; Phipps, R.P.; Blumberg, N. Transfusion immunomodulation—The case for leukoreduced and (perhaps) washed transfusions. Blood Cells Mol. Dis. 2013, 50, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Garraud, O.; Cognasse, F.; Hamzeh-Cognasse, H.; Spinelli, S.; Phipps, R.P.; Blumberg, N. Removal of biologic response modifiers associated with platelet transfusion reactions: Strategies worth considering? Transfusion 2014, 54, 2583. [Google Scholar] [CrossRef]
- Aloui, C.; Chakroun, T.; Prigent, A.; Jemni-Yacoub, S.; Cognasse, F.; Laradi, S.; Garraud, O. Leucocyte cytokines dominate platelet cytokines overtime in non-leucoreduced platelet components. Blood Transfus. 2018, 16, 63–72. [Google Scholar] [PubMed]
- Garraud, O.; Lozano, M. Pathogen inactivation/reduction technologies for platelet transfusion: Where do we stand? Transfus. Clin. Biol. 2018, 25, 165–171. [Google Scholar] [CrossRef]
- Slichter, S.J.; Pellham, E.; Bailey, S.L.; Christoffel, T.; Gettinger, I.; Gaur, L.; Latchman, Y.; Nelson, K.; Bolgiano, D. Leukofiltration plus pathogen reduction prevents alloimmune platelet refractoriness in a dog transfusion model. Blood 2017, 130, 1052–1061. [Google Scholar] [CrossRef]
- Van der Meer, P.F.; Ypma, P.F.; van Geloven, N.; van Hilten, J.A.; van Wordragen-Vlaswinkel, R.J.; Eissen, O.; Zwaginga, J.J.; Trus, M.; Beckers, E.A.M.; Te Boekhorst, P.; et al. Hemostatic efficacy of pathogen-inactivated vs untreated platelets: A randomized controlled trial. Blood 2018, 132, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Garraud, O. Pathogen reduction or inactivation technologies for platelet components: Does decision making have to await further clinical trials? Transfus. Apher. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kopko, P.M.; Warner, P.; Kresie, L.; Pancoska, C. Methods for the selection of platelet products for alloimmune-refractory patients. Transfusion 2015, 55, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Vassalo, R.R.; Fung, M.; Rebulla, P.; Duquesnoy, R.; Saw, C.L.; Slichter, S.J.; Tanael, S.; Shehata, N.; International Collaboration for Guideline Development, Implementation and Evaluation for Transfusion Therapies. Utility of cross-matched platelet transfusions in patients with hypoproliferative thrombocytopenia: A systematic review. Transfusion 2014, 54, 1180–1191. [Google Scholar] [CrossRef] [PubMed]
- Juskewitch, J.E.; Norgan, A.P.; De Goey, S.R.; Duellman, P.M.; Wakefield, L.L.; Gandhi, M.G.; Stubbs, J.R.; Kreuter, J.D. How do I … manage the platelet transfusion-refractory patient? Transfusion 2017, 57, 2828–2835. [Google Scholar] [CrossRef] [PubMed]
- Pavenski, K.; Rebulla, P.; Duquesnoy, R.; Saw, C.L.; Slichter, S.J.; Tanael, S.; Shehata, N.; International Collaboration for Guideline Development, Implementation and Evaluation for Transfusion Therapies (ICTMG) Collaborators. Efficacy of HLA-matched platelet transfusions for patients with hypoproliferative thrombocytopenia: A systematic review. Transfusion 2013, 53, 2230–2242. [Google Scholar] [CrossRef]
- Garraud, O.; Coppo, P. Plasma types with focus on therapeutic plasma exchange. Transfus. Apher. Sci. 2018, in press. [Google Scholar]
- Duquesnoy, R.J. Antibody-reactive epitope determination with HLAMatchmaker and its clinical applications. Tissue Antigens 2011, 77, 525–534. [Google Scholar] [CrossRef]
- Nambiar, A.; Duquesnoy, R.J.; Adams, S.; Zhao, Y.; Oblitas, J.; Leitman, S.; Stroncek, D.; Marincola, F. HLAMatchmaker-driven analysis of responses to HLA-typed platelet transfusions in alloimmunized thrombocytopenic patients. Blood 2006, 107, 1680–1687. [Google Scholar] [CrossRef]
- Brooks, E.G.; MacPherson, B.R.; Fung, M.K. Validation of HLAMatchmaker algorithm in identifying acceptable HLA mismatches for thrombocytopenic patients refractory to platelet transfusions. Transfusion 2008, 48, 2159–2166. [Google Scholar] [CrossRef]
- Pai, S.C.; Lo, S.C.; Lin Tsai, S.J.; Lin, D.T.; Lin, K.S.; Lin, L.I. Epitope-based matching for HLA-alloimmunized platelet refractoriness in patients with hematological diseases. Transfusion 2010, 50, 2318–2327. [Google Scholar] [CrossRef]
- O’Rafferty, C.; Rooney, G.; Hagan, R.; Woolfson, M.; O’Donghaile, D.; Fitgerald, G. HLAMatchmaker is effective for selecting appropriate platelet units for alloimmunised thrombocytopaenic patients who are refractory to random donor platelets. Transfus. Med. 2017, 27, 369–374. [Google Scholar] [CrossRef]
- Estcourt, L.; Stanworth, S.; Doree, C.; Hopewell, S.; Murphy, M.F.; Tinmouth, A.; Heddle, N. Prophylactic platelet transfusion for prevention of bleeding in patients with haematological disorders after chemotherapy and stem cell transplantation. Cochrane Database Syst. Rev. 2012, 16, CD004269. [Google Scholar] [CrossRef] [PubMed]
- Petraszko, T.; Zeller, M. Canadian Blood Services. Platelet Transfusion, Alloimmunization and Management of Platelet Refractoriness. 2018. Available online: https://professionaleducation.blood.ca/en/transfusion/guide-clinique/platelet-transfusion-alloimmunization-and-management-platelet (accessed on 23 December 2018).
- Comont, T.; Tavitian, S.; Bardiaux, L.; Fort, M.; Debiol, B.; Morère, D.; Bérard, E.; Delabesse, E.; Luquet, I.; Martinez, S.; et al. Platelet transfusion refractoriness in patients with acute myeloid leukemia treated by intensive chemotherapy. Leuk. Res. 2017, 61, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Nahirniak, S.; Slichter, S.J.; Tanael, S.; Rebulla, P.; Pavenski, K.; Vassallo, R.; Fung, M.; Duquesnoy, R.; Saw, C.L.; Stanworth, S.; et al. Guidance on platelet transfusion for patients with hypoproliferative thrombocytopenia. Transfus. Med. Rev. 2015, 29, 3–13. [Google Scholar] [CrossRef]
- Carter, J.; Szymanski, J.; Cantilena, C.; Adams, S.; Sahu, S. Continuous platelet transfusion as a potential desensitization regimen in HLA class I alloimmune-mediated platelet refractoriness. Blood 2017, 130 (Suppl. 1), 4925. [Google Scholar]
- Doughty, H.A.; Murphy, M.F.; Metcalf, P.; Rohatiner, A.Z.S.; Lister, T.A.; Waters, A.H. Relative importance of immune and non-immune causes of platelet refractoriness. Vox Sang. 1994, 66, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Kickler, T.; Kennedy, S.D.; Braine, H.G. Alloimmunization to platelet-specific antigens on glycoproteins IIb-IIIa and Ib/IX in multiply transfused thrombocytopenic patients. Transfusion 1990, 30, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Schnaidt, M.; Northoff, H.; Wernet, D. Frequency and specificity of platelet-specific alloantibodies in HLA-immunized haematologic-oncologic patients. Transfus. Med. 1996, 6, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Kiefel, V.; König, C.; Kroll, H.; Santoso, S. Platelet alloantibodies in transfused patients. Transfusion 2001, 41, 766–770. [Google Scholar] [CrossRef]
- Sanz, C.; Freire, C.; Alcorta, I.; Ordinas, A.; Pereira, A. Platelet-specific antibodies in HLA-immunized patients receiving chronic platelet support. Transfusion 2001, 41, 762–765. [Google Scholar] [CrossRef]
- Wang, J.; Xia, W.; Deng, J.; Xu, X.; Shao, Y.; Ding, H.; Chen, Y.; Liu, J.; Chen, D.; Ye, X.; et al. Analysis of platelet-reactive alloantibodies and evaluation of cross-match-compatible platelets for management of patients with transfusion refractoriness. Transfus. Med. 2017. [Google Scholar] [CrossRef]
- Blumberg, N.; Refaai, M.; Heal, J. ABO matching of platelet transfusions—“Start Making Sense”. “As we get older, and stop making sense…:—The Talking Heads (1984). Blood Transfus. 2015, 13, 347–350. [Google Scholar] [PubMed]
- Sahai, T.; Henrichs, K.; Refaai, M.; Heal, J.M.; Kirkley, S.A.; Schmidt, A.E.; Mendler, J.H.; Masel, D.; Liesveld, J.; Aquina, C.; et al. ABO identical and washed blood transfusions as candidate strategies to reduce early mortality in acute promyelocytic leukemia. Leuk. Res. 2017, 62, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Cid, J.; Yazer, M.H.; Lozano, M. Platelet transfusion and respecting patient D type. Curr. Opin. Hematol. 2015, 22, 540–546. [Google Scholar] [CrossRef]
- Garraud, O.; Cognasse, F.; Tissot, J.D.; Chavarin, P.; Laperche, S.; Morel, P.; Lefrère, J.J.; Pozzetto, B.; Lozano, M.; Blumberg, N.; et al. Improving platelet transfusion safety: Biomedical and technical considerations. Blood Transfus. 2016, 14, 109–122. [Google Scholar] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garraud, O.; Cognasse, F.; Moncharmont, P. Immunological Features in the Process of Blood Platelet-Induced Alloimmunisation, with a Focus on Platelet Component Transfusion. Diseases 2019, 7, 7. https://doi.org/10.3390/diseases7010007
Garraud O, Cognasse F, Moncharmont P. Immunological Features in the Process of Blood Platelet-Induced Alloimmunisation, with a Focus on Platelet Component Transfusion. Diseases. 2019; 7(1):7. https://doi.org/10.3390/diseases7010007
Chicago/Turabian StyleGarraud, Olivier, Fabrice Cognasse, and Pierre Moncharmont. 2019. "Immunological Features in the Process of Blood Platelet-Induced Alloimmunisation, with a Focus on Platelet Component Transfusion" Diseases 7, no. 1: 7. https://doi.org/10.3390/diseases7010007
APA StyleGarraud, O., Cognasse, F., & Moncharmont, P. (2019). Immunological Features in the Process of Blood Platelet-Induced Alloimmunisation, with a Focus on Platelet Component Transfusion. Diseases, 7(1), 7. https://doi.org/10.3390/diseases7010007




