Induction of Neuronal Differentiation of Murine N2a Cells by Two Polyphenols Present in the Mediterranean Diet Mimicking Neurotrophins Activities: Resveratrol and Apigenin
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatments
2.2. Evaluation of Neuronal Differentiation with Morphological Criteria
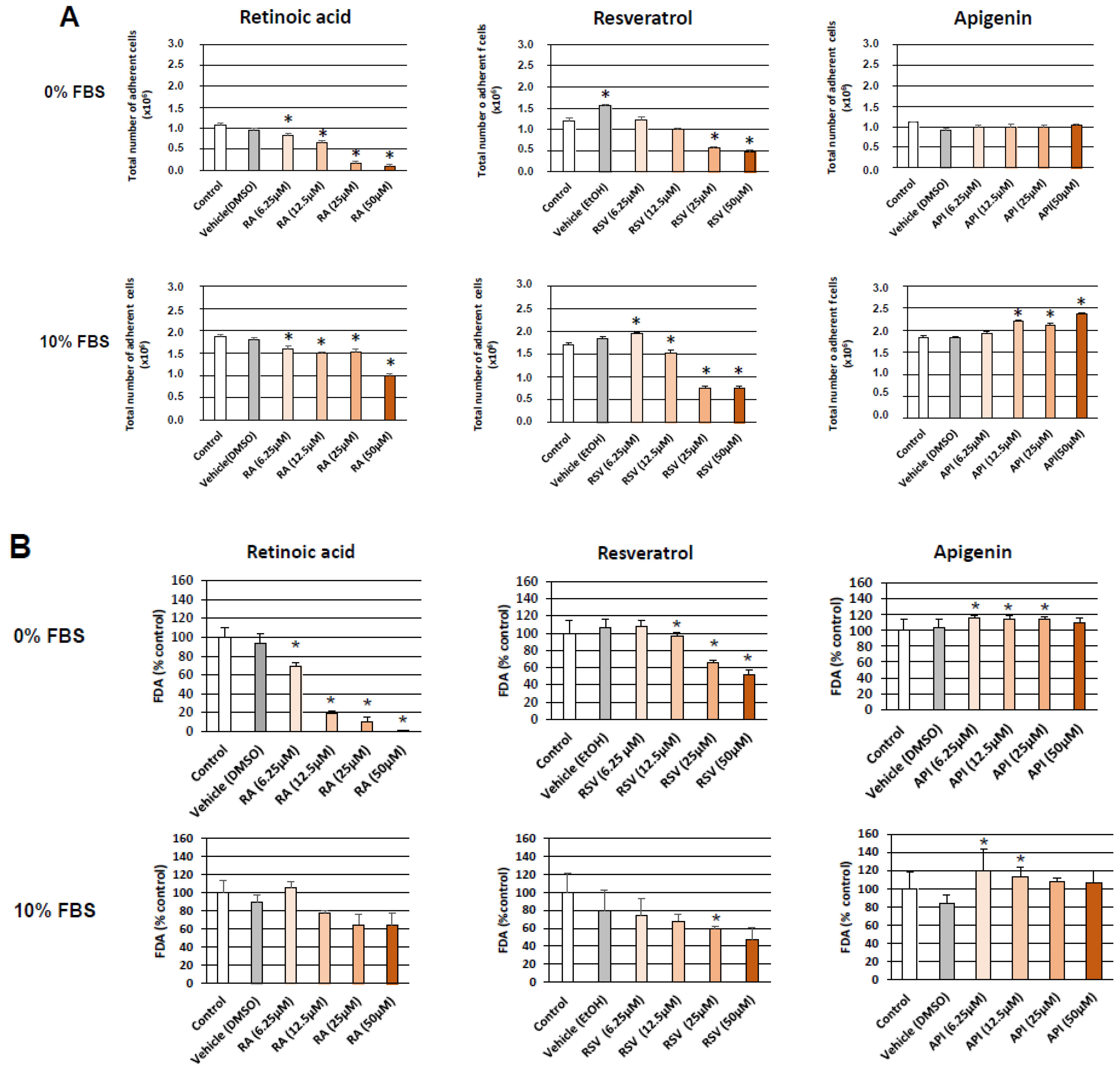
2.2.1. Cell Counting
2.2.2. Measurement of Cell Viability with the Fluorescein Diacetate (FDA) Test
2.2.3. Flow Cytometric Analysis of Cell Cycle
2.2.4. Real-Time PCR Analysis
2.2.5. Statistical Analysis
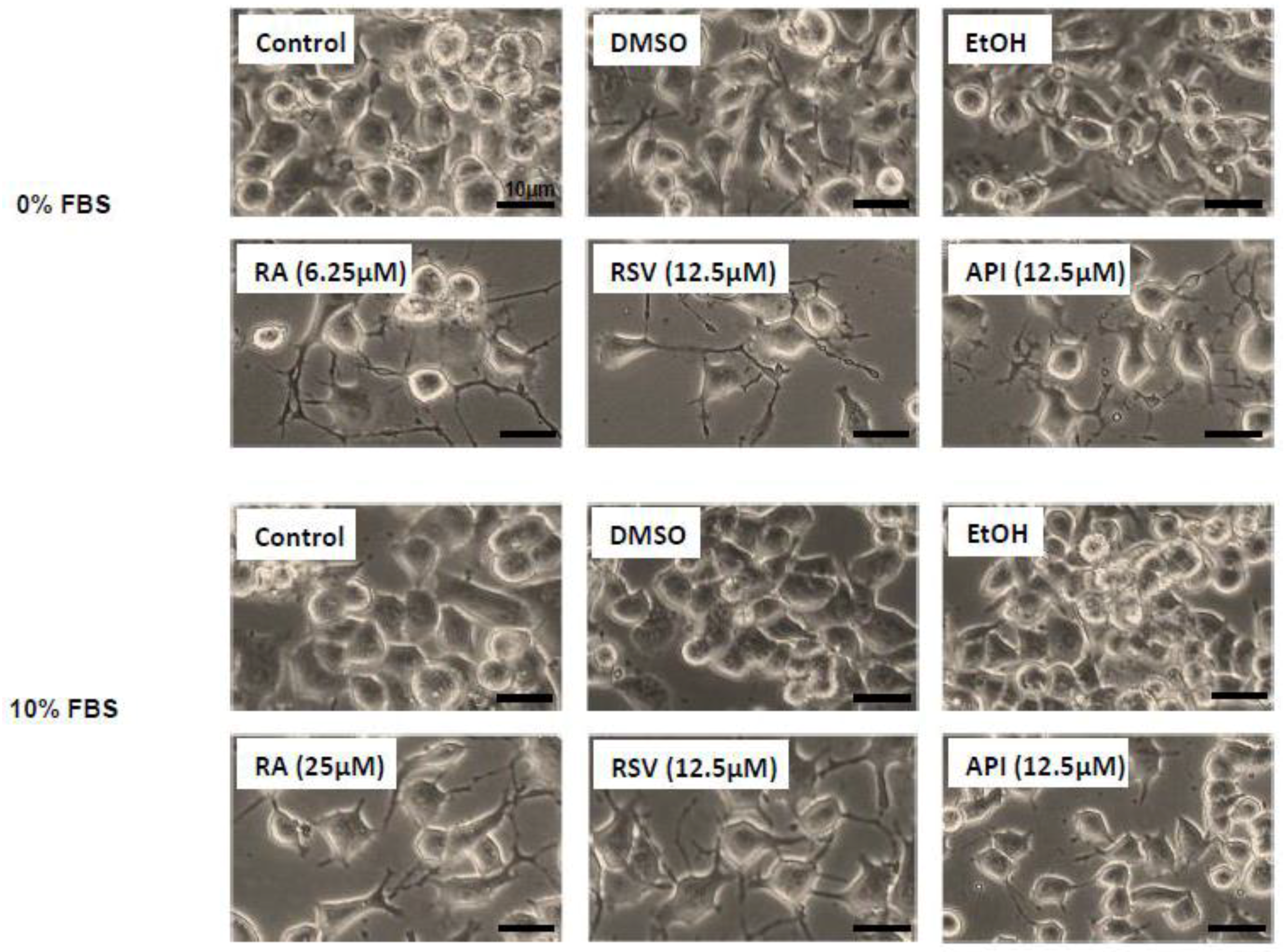
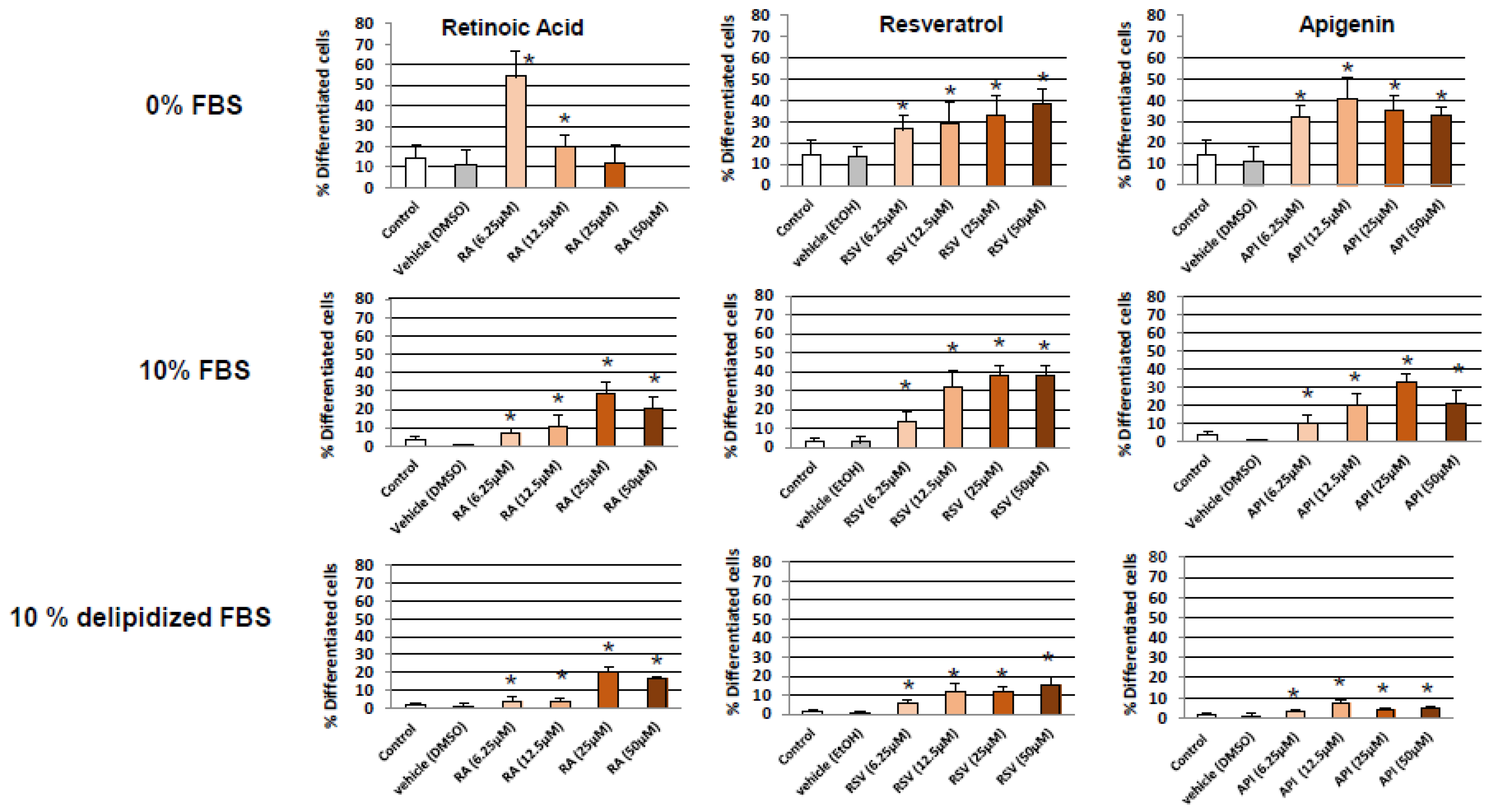
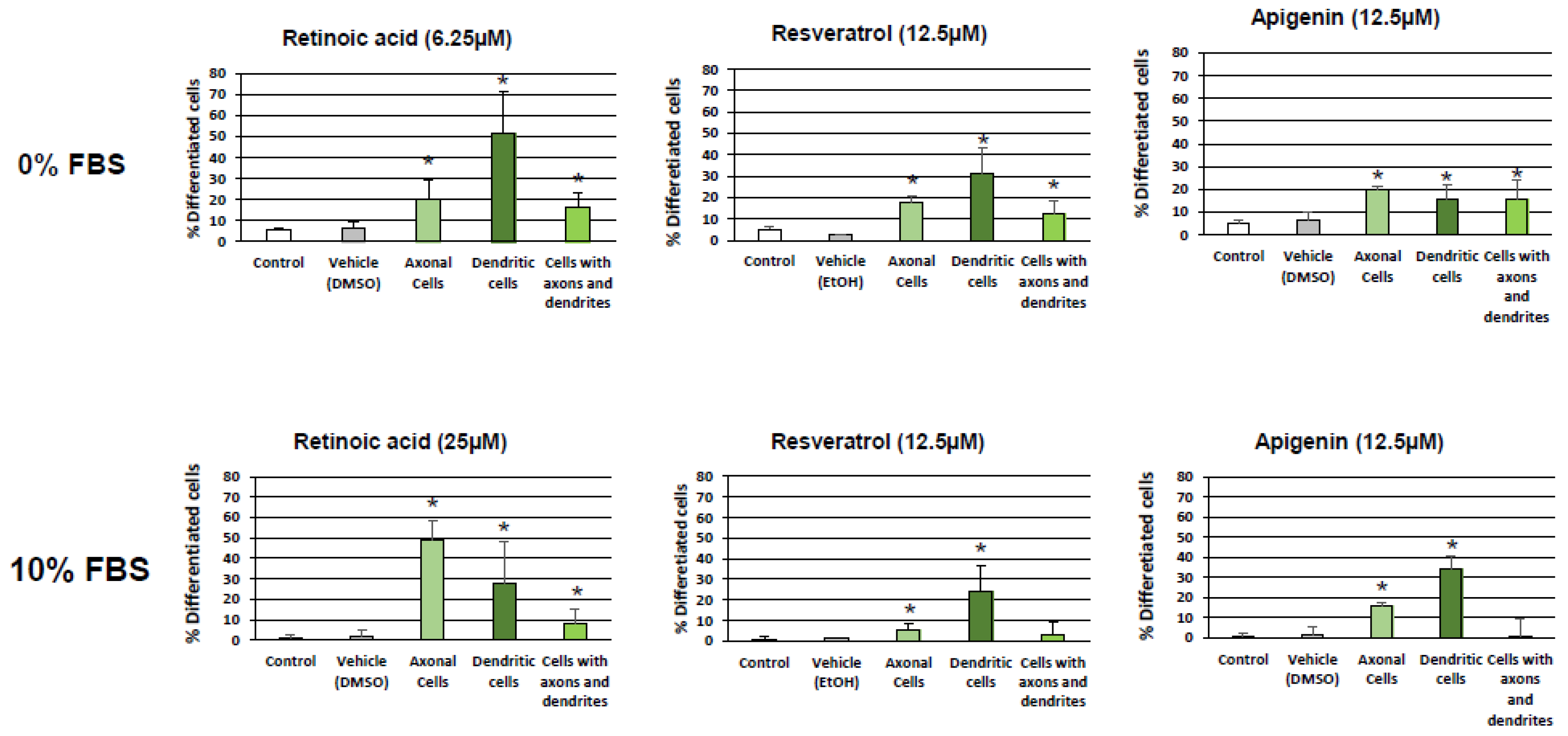
3. Results
3.1. Evaluation and Quantification of Resveratrol- and Apigenin-Induced Neuronal Differentiation of N2a Cells
3.2. Characterization of Resveratrol- and Apigenin-Signaling Pathways Involved in the Neuronal Differentiation of N2a Cells
3.3. Evaluation of the Involvement of Octadecaneuropeptide (ODN) Receptor in the Neuronal Differentiation of N2a Cells Induced by Resveratrol and Apigenin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Santhakumar, A.B.; Battino, M.; Alvarez-Suarez, J.M. Dietary polyphenols: Structures, bioavailability and protective effects against atherosclerosis. Food Chem. Toxicol. 2018, 113, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Zarrouk, A.; Vejux, A.; Mackrill, J.; O’Callaghan, Y.; Hammami, M.; O’Brien, N.; Lizard, G. Involvement of oxysterols in age-related diseases and ageing processes. Ageing Res. Rev. 2014, 18, 148–162. [Google Scholar] [CrossRef] [PubMed]
- Cilla, A.; Alegría, A.; Attanzio, A.; Garcia-Llatas, G.; Tesoriere, L.; Livrea, M.A. Dietary phytochemicals in the protection against oxysterol-induced damage. Chem. Phys. Lipids 2017, 207, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.; Dixit, M. Role of Polyphenols and Other Phytochemicals on Molecular Signaling. Oxid. Med. Cell. Longev. 2015, 2015, 504253. [Google Scholar] [CrossRef] [PubMed]
- Carito, V.; Ceccanti, M.; Tarani, L.; Ferraguti, G.; Chaldakov, G.N.; Fiore, M. Neurotrophins’modulation by olive polyphenols. Curr. Med. Chem. 2016, 23, 3189–3197. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Schubert, D.; Maher, P. Oxytosis: A novel form of programmed cell death. Curr. Top. Med. Chem. 2001, 1, 497–506. [Google Scholar] [PubMed]
- Schaffer, S.; Eckert, G.P.; Schmitt-Schillig, S.; Müller, W.E. Plant foods and brain aging: A critical appraisal. Forum Nutr. 2006, 59, 86–115. [Google Scholar] [PubMed]
- Farinetti, A.; Zurlo, V.; Manenti, A.; Coppi, F.; Mattioli, A.V. Mediterranean diet and colorectal cancer: A systematic review. Nutrition 2017, 43–44, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Youn, Y.K.; Hong, M.K.; Kim, L.S. Antiproliferation and redifferentiation in thyroid cancer cell lines by polyphenol phytochemicals. J. Korean Med. Sci. 2011, 26, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, J.; Lançon, A.; Aires, V.; Limagne, E.; Tili, E.; Michaille, J.J.; Latruffe, N. Resveratrol initiates differentiation of mouse skeletal muscle-derived C2C12 myoblasts. Biochem. Pharmacol. 2012, 84, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Latruffe, N.; Rifler, J.P. Bioactive polyphenols from grapes and wine emphasized with resveratrol. Curr. Pharm. Des. 2013, 19, 6053–6063. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Y.; Jiao, Y.; Guo, A.; Xu, X.; Qu, X.; Wang, S.; Zhao, J.; Li, Y.; Cao, Y. Resveratrol sensitizes glioblastoma-initiating cells to temozolomide by inducing cell apoptosis and promoting differentiation. Oncol. Rep. 2016, 35, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, J.L.; Liu, R.; Li, X.X.; Li, J.F.; Zhang, L. Neuroprotective, anti-amyloidogenic and neurotrophic effects of apigenin in an Alzheimer’s disease mouse model. Molecules 2013, 18, 9949–9965. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, L.; Wang, L.; Yun-Peng, S.; Zhou, J.J.; Zhao, Z.; Li, D.P. Resveratrol Induces Differentiation of Human Umbilical Cord Mesenchymal Stem Cells into Neuron-Like Cells. Stem Cells Int. 2017, 2017, 1651325. [Google Scholar] [CrossRef] [PubMed]
- Leonarduzzi, G.; Testa, G.; Sottero, B.; Gamba, P.; Poli, G. Design and development of nanovehicle-based delivery systems for preventive or therapeutic supplementation with flavonoids. Curr. Med. Chem. 2010, 17, 74–95. [Google Scholar] [CrossRef] [PubMed]
- Testa, G.; Gamba, P.; Badilli, U.; Gargiulo, S.; Maina, M.; Guina, T.; Calfapietra, S.; Biasi, F.; Cavalli, R.; Poli, G.; et al. Loading into nanoparticles improves quercetin’s efficacy in preventing neuroinflammation induced by oxysterols. PLoS ONE 2014, 9, e96795. [Google Scholar] [CrossRef] [PubMed]
- Sahni, J.K.; Doggui, S.; Ali, J.; Baboota, S.; Dao, L.; Ramassamy, C. Neurotherapeutic applications of nanoparticles in Alzheimer’s disease. J. Control. Release 2011, 152, 208–231. [Google Scholar] [CrossRef] [PubMed]
- Poulose, S.M.; Miller, M.G.; Scott, T.; Shukitt-Hale, B. Nutritional Factors Affecting Adult Neurogenesis and Cognitive Function. Adv. Nutr. 2017, 8, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, F.; Hosseini, R.; Saso, L.; Firuzi, O. Modulation of neurotrophic signaling pathways by polyphenols. Drug Des. Dev. Ther. 2015, 10, 23–42. [Google Scholar]
- Costa, S.L.; Silva, V.D.; Dos Santos Souza, C.; Santos, C.C.; Paris, I.; Muñoz, P.; Segura-Aguilar, J. Impact of Plant-Derived Flavonoids on Neurodegenerative Diseases. Neurotox. Res. 2016, 30, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, M.; Bashir, R.M.; Heidrick, M.L.; Hamada, F.M.; Refaey, H.E.; Hamed, A.; Helal, G.; Baxin, M.D.; Cerutis, D.R.; Lassi, N.K. Neurotrophins and their receptors in nerve injury and repair. Neurochem. Int. 1997, 30, 347–374. [Google Scholar] [CrossRef]
- Scalbert, A.; Morand, C.; Manach, C.; Rémésy, C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed. Pharmacother. 2002, 56, 276–282. [Google Scholar] [CrossRef]
- Figueira, I.; Garcia, G.; Pimpão, R.C.; Terrasso, A.P.; Costa, I.; Almeida, A.F.; Tavares, L.; Pais, T.F.; Pinto, P.; Ventura, M.R.; et al. Polyphenols journey through blood-brain barrier towards neuronal protection. Sci. Rep. 2017, 7, 11456. [Google Scholar] [CrossRef] [PubMed]
- Akagi, M.; Matsui, N.; Akae, H.; Hirashima, N.; Fukuishi, N.; Fukuyama, Y.; Akagi, R. Nonpeptide neurotrophic agents useful in the treatment of neurodegenerative diseases such as Alzheimer’s disease. J. Pharmacol. Sci. 2015, 127, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.C.; Xu, H.; Yuan, Y.; Chen, J.Y.; Zhang, Y.J.; Lin, Y.; Yuan, S.Y. Delayed Treatment with green tea polyphenol egcg promotes neurogenesis after ischemic stroke in adult mice. Mol. Neurobiol. 2017, 54, 3652–3664. [Google Scholar] [CrossRef] [PubMed]
- Dias, G.P.; Cocks, G.; do Nascimento Bevilaqua, M.C.; Nardi, A.E.; Thuret, S. Resveratrol: A Potential Hippocampal Plasticity Enhancer. Oxid. Med. Cell. Longev. 2016, 2016, 9651236. [Google Scholar] [CrossRef] [PubMed]
- Farzaei, M.H.; Abbasabadi, Z.; Ardekani, M.R.; Rahimi, R.; Farzaei, F. Parsley: A review of ethnopharmacology, phytochemistry and biological activities. J. Tradit. Chin. Med. 2013, 33, 815–826. [Google Scholar] [CrossRef]
- Khalil, M.I.; Sulaiman, S.A.; Boukraa, L. Antioxidant properties of honey and its role in preventing health disorders. Open Nutraceut. J. 2010, 3, 6–16. [Google Scholar] [CrossRef]
- Ricciutelli, M.; Marconi, S.; Boarelli, M.C.; Caprioli, G.; Sagratini, G.; Ballini, R.; Fiorini, D. Olive oil polyphenols: A quantitative method by high-performance liquid-chromatography-diode-array detection for their determination and the assessment of the related health claim. J. Chromatogr. A 2017, 1481, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Losi, G.; Puia, G.; Garzon, G.; Vuono, M.C.; Baraldi, M. Apigenin modulates GABAergic and glutamatergic transmission in cultured cortical neurons. Eur. J. Pharmacol. 2004, 502, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Kojima, N.; Kurosawa, N.; Nishi, T.; Hanai, N.; Tsuji, S. Induction of cholinergic differentiation with neurite sprouting by de novo biosynthesis and expression of GD3 and b-series gangliosides in Neuro2a cells. J. Biol. Chem. 1994, 269, 30451–30456. [Google Scholar] [PubMed]
- Tremblay, R.G.; Sikorska, M.; Sandhu, J.K.; Lanthier, P.; Ribecco-Lutkiewicz, M.; Bani-Yaghoub, M. Differentiation of mouse Neuro 2A cells into dopamine neurons. J. Neurosci. Methods 2010, 186, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.; Guidotti, A. Diazepam binding inhibitor (DBI): A peptide with multiple biological actions. Life Sci. 1991, 49, 325–344. [Google Scholar] [CrossRef]
- Hamdi, Y.; Kaddour, H.; Vaudry, D.; Bahdoudi, S.; Douiri, S.; Leprince, J.; Castel, H.; Vaudry, H.; Tonon, M.C.; Amri, M.; et al. The octadecaneuropeptide ODN protects astrocytes against hydrogen peroxide-induced apoptosis via a PKA/MAPK-dependent mechanism. PLoS ONE 2012, 7, e42498. [Google Scholar] [CrossRef] [PubMed]
- Dickson, L.; Finlayson, K. VPAC and PAC receptors: From ligands to function. Pharmacol. Ther. 2009, 12, 294–316. [Google Scholar] [CrossRef] [PubMed]
- Vaudry, D.; Falluel-Morel, A.; Bourgault, S.; Basille, M.; Burel, D.; Wurtz, O.; Fournier, A.; Chow, B.K.; Hashimoto, H.; Galas, L.; et al. Pituitary adenylate cyclase activating polypeptide and its receptors: 20 years after the discovery. Pharmacol. Rev. 2009, 61, 283–357. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, T.K.; Mackenzie, C.J.; Plevin, R.; Lutz, E.M. PACAP-38 induces neuronal differentiation of human SH-SY5Y neuroblastoma cells via cAMP-mediated activation of ERK and p38 MAP kinases. J. Neurochem. 2008, 104, 74–88. [Google Scholar] [CrossRef] [PubMed]
- May, V.; Lutz, E.; MacKenzie, C.; Schutz, K.C.; Dozark, K.; Braas, K.M. Pituitary adenylate cyclase-activating polypeptide (PACAP)/PAC1HOP1 receptor activation coordinates multiple neurotrophic signaling pathways: Akt activation through phosphatidylinositol 3-kinase gamma and vesicle endocytosis for neuronal survival. J. Biol. Chem. 2010, 285, 9749–9761. [Google Scholar] [CrossRef] [PubMed]
- Castorina, A.; Scuderi, S.; D’Amico, A.G.; Drago, F.; D’Agata, V. PACAP and VIP increase the expression of myelin-related proteins in rat schwannoma cells: Involvement of PAC1/VPAC2 receptor-mediated activation of PI3K/Akt signaling pathways. Exp. Cell Res. 2014, 322, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, T.; Nakamachi, T.; Shioda, S. Discovery of PACAP and its receptors in the brain. J. Headache Pain 2018, 19, 28. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, B.; Milbrandt, J. Resveratrol stimulates AMP kinase activity in neurons. Proc. Natl. Acad. Sci. USA 2007, 104, 7217–7222. [Google Scholar] [CrossRef] [PubMed]
- Marzinke, M.A.; Clagett-Dame, M. The all-trans retinoic acid (atRA)-regulated gene Calmin (Clmn) regulates cell cycle exit and neurite outgrowth in murine neuroblastoma (Neuro2a) cells. Exp. Cell Res. 2012, 318, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, B.J.; Basille, M.; Vaudry, D.; Fournier, A.; Vaudry, H. Pituitary adenylate cyclase-activating polypeptide promotes cell survival and neurite outgrowth in rat cerebellar neuroblasts. Neuroscience 1997, 78, 419–430. [Google Scholar] [CrossRef]
- Botia, B.; Basille, M.; Allais, A.; Raoult, E.; Falluel-Morel, A.; Galas, L.; Jolivel, V.; Wurtz, O.; Komuro, H.; Fournier, A.; et al. Neurotrophic effects of PACAP in the cerebellar cortex. Peptides 2007, 28, 1746–1752. [Google Scholar] [CrossRef] [PubMed]
- Ogata, K.; Shintani, N.; Hayata-Takano, A.; Kamo, T.; Higashi, S.; Seiriki, K.; Momosaki, H.; Vaudry, D.; Vaudry, H.; Galas, L.; et al. PACAP enhances axon outgrowth in cultured hippocampal neurons to a comparable extent as BDNF. PLoS ONE 2015, 10, e0120526. [Google Scholar] [CrossRef] [PubMed]
- Kaddour, H.; Hamdi, Y.; Vaudry, D.; Basille, M.; Desrues, L.; Leprince, J.; Castel, H.; Vaudry, H.; Tonon, M.C.; Amri, M.; et al. The octadecaneuropeptide ODN prevents 6-hydroxydopamine-induced apoptosis of cerebellar granule neurons through a PKC-MAPK-dependent pathway. J. Neurochem. 2013, 125, 620–633. [Google Scholar] [CrossRef] [PubMed]
- Marel, A.K.; Lizard, G.; Izard, J.C.; Latruffe, N.; Delmas, D. Inhibitory effects of trans-resveratrol analogs molecules on the proliferation and the cell cycle progression of human colon tumoral cells. Mol. Nutr. Food Res. 2008, 52, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Colin, D.; Lancon, A.; Delmas, D.; Lizard, G.; Abrossinow, J.; Kahn, E.; Jannin, B.; Latruffe, N. Antiproliferative activities of resveratrol and related compounds in human hepatocyte derived HepG2 cells are associated with biochemical cell disturbance revealed by fluorescence analyses. Biochimie 2008, 90, 1674–1684. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Heideman, L.; Chung, C.S.; Pelling, J.C.; Koehler, K.J.; Birt, D.F. Cell-cycle arrest at G2/M and growth inhibition by apigenin in human colon carcinoma cell lines. Mol. Carcinog. 2000, 28, 102–110. [Google Scholar] [CrossRef]
- Elsisi, N.S.; Darling-Reed, S.; Lee, E.Y.; Oriaku, E.T.; Soliman, K.F. Ibuprofen and apigenin induce apoptosis and cell cycle arrest in activated microglia. Neurosci. Lett. 2005, 375, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Latruffe, N. Vin, Nutrition Méditerranéenne et Santé: Une Association Vertueuse; Editions Universitaires de Dijon, Collection Sciences: Dijon, France, 2017. [Google Scholar]
- Dugas, B.; Charbonnier, S.; Baarine, M.; Ragot, K.; Delmas, D.; Ménétrier, F.; Lherminier, J.; Malvitte, L.; Khalfaoui, T.; Bron, A.; et al. Effects of oxysterols on cell viability, inflammatory cytokines, VEGF, and reactive oxygen species production on human retinal cells: Cytoprotective effects and prevention of VEGF secretion by resveratrol. Eur. J. Nutr. 2010, 49, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Lançon, A.; Michaille, J.J.; Latruffe, N. Effects of dietary phytophenols on the expression of microRNAs involved in mammalian cell homeostasis. J. Sci. Food Agric. 2013, 93, 3155–3164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, C.; Zha, X.; Xu, Z.; Li, L.; Liu, Y.; Xu, L.; Cui, L.; Xu, D.; Zhu, B. Apigenin promotes osteogenic differentiation of human mesenchymal stem cells through JNK and p38 MAPK pathways. Mol. Cell. Biochem. 2015, 407, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Melguizo-Rodríguez, L.; Manzano-Moreno, F.J.; De Luna-Bertos, E.; Rivas, A.; Ramos-Torrecillas, J.; Ruiz, C.; García-Martínez, O. Effect of olive oil phenolic compounds on osteoblast differentiation. Eur. J. Clin. Investig. 2018, 48, e12904. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.J.; Son, H.J.; Choi, Y.M.; Ahn, J.; Jung, C.H.; Ha, T.Y. Apigenin enhances skeletal muscle hypertrophy and myoblast differentiation by regulating Prmt7. Oncotarget 2017, 8, 78300–78311. [Google Scholar] [CrossRef] [PubMed]
- Nakazaki, E.; Tsolmon, S.; Han, J.; Isoda, H. Proteomic study of granulocytic differentiation induced by apigenin 7-glucoside in human promyelocytic leukemia HL-60 cells. Eur. J. Nutr. 2013, 52, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Sato, F.; Matsukawa, Y.; Matsumoto, K.; Nishino, H.; Sakai, T. Apigenin induces morphological differentiation and G2-M arrest in rat neuronal cells. Biochem. Biophys. Res. Commun. 1994, 204, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.L.; Oliveira, M.N.; da Silva, A.B.; Pitanga, B.P.; Silva, V.D.; Faria, G.P.; Sampaio, G.P.; Costa, M.F.; Braga-de-Souza, S.; Costa, S.L. The flavonoid apigenin from Croton betulaster Mull inhibits proliferation, induces differentiation and regulates the inflammatory profile of glioma cells. Anticancer Drugs 2016, 27, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wang, N.; Liu, X. Resveratrol and Amyloid-Beta: Mechanistic Insights. Nutrients 2017, 9, 1122. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, M.P.; Roberts, C.; Waseem, M.; Tyagi, P. Drug Targets in Neurotrophin Signaling in the Central and Peripheral Nervous System. Mol. Neurobiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bahdoudi, S.; Ghouili, I.; Hmiden, M.; do Rego, J.L.; Lefranc, B.; Leprince, J.; Chuquet, J.; do Rego, J.C.; Marcher, A.B.; Mandrup, S.; et al. Neuroprotective effects of the gliopeptide ODN in an in vivo model of Parkinson’s disease. Cell. Mol. Life Sci. 2018, 75, 2075–2091. [Google Scholar] [CrossRef] [PubMed]
- Schiborr, C.; Eckert, G.P.; Rimbach, G.; Frank, J. A validated method for the quantification of curcumin in plasma and brain tissue by fast narrow-bore high-performance liquid chromatography with fluorescence detection. Anal. Bioanal. Chem. 2010, 397, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Ferri, P.; Angelino, D.; Gennari, L.; Benedetti, S.; Ambrogini, P.; Del Grande, P.; Ninfali, P. Enhancement of flavonoid ability to cross the blood-brain barrier of rats by co-administration with α-tocopherol. Food Funct. 2015, 6, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, R.G. The toxicogenomics of nuclear receptor agonists. Curr. Opin. Chem. Biol. 2003, 7, 505–510. [Google Scholar] [CrossRef]
- Schubert, D.; Humphreys, S.; Baroni, C.; Cohn, M. In vitro differentiation of a mouse neuroblastoma. Proc. Natl. Acad. Sci. USA 1969, 64, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Seeds, N.W.; Gilman, A.G.; Amano, T.; Nirenberg, M.W. Regulation of axon formation by clonal lines of a neural tumor. Proc. Natl. Acad. Sci. USA 1970, 66, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Evangelopoulos, M.E.; Weis, J.; Krüttgen, A. Signalling pathways leading to neuroblastoma differentiation after serum withdrawal: HDL blocks neuroblastoma differentiation by inhibition of EGFR. Oncogene 2005, 24, 3309–3318. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, Y.; Kaddour, H.; Vaudry, D.; Leprince, J.; Zarrouk, A.; Hammami, M.; Vaudry, H.; Tonon, M.C.; Amri, M.; Masmoudi-Kouki, O. Octadecaneuropeptide ODN prevents hydrogen peroxide-induced oxidative damage of biomolecules in cultured rat astrocytes. Peptides 2015, 71, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Ghouili, I.; Bahdoudi, S.; Morin, F.; Amri, F.; Hamdi, Y.; Coly, P.M.; Walet-Balieu, M.L.; Leprince, J.; Zekri, S.; Vaudry, H.; et al. Endogenous Expression of ODN-Related Peptides in Astrocytes Contributes to Cell Protection against Oxidative Stress: Astrocyte-Neuron Crosstalk Relevance for Neuronal Survival. Mol. Neurobiol. 2018, 55, 4596–4611. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.S.; Vieira, H.L.A. Role of cell metabolism and mitochondrial function during adult neurogenesis. Neurochem. Res. 2017, 42, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Sheppard, A. Dietary micronutrients promote neuronal differentiation by modulating the mitochondrial-nuclear dialogue. Bioessays 2018, 40, e1800051. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.R.; Worrall, J.T.; Mahad, D.J. The central role of mitochondria in axonal degeneration in multiple sclerosis. Mult. Scler. J. 2014, 20, 1806–1813. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Acuña, C.; Ferreira, J.; Speisky, H. Polyphenols and mitochondria: An update on their increasingly emerging ROS-scavenging independent actions. Arch. Biochem. Biophys. 2014, 559, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Sedel, F.; Bernard, D.; Mock, D.M.; Tourbah, A. Targeting demyelination and virtual hypoxia with high-dose biotin as a treatment for progressive multiple sclerosis. Neuropharmacology 2016, 110, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Notario, B.; Zamora, M.; Viñas, O.; Mampel, T. All-trans-retinoic acid binds to and inhibits adenine nucleotide translocase and induces mitochondrial permeability transition. Mol. Pharmacol. 2003, 63, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.P.; Jain, P.D.; Sancheti, J.S.; Ghumatkar, P.J.; Tambe, R.; Sathaye, S. Neuroprotective and neurotrophic effects of Apigenin and Luteolin in MPTP induced parkinsonism in mice. Neuropharmacology 2014, 86, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Mi, Y.; Wang, Y.; Li, R.; Huang, S.; Li, X.; Liu, X. Neuroprotective action of tea polyphenols on oxidative stress-induced apoptosis through the activation of the TrkB/CREB/BDNF pathway and Keap1/Nrf2 signaling pathway in SH-SY5Y cells and mice brain. Food Funct. 2017, 8, 4421–4432. [Google Scholar] [CrossRef] [PubMed]
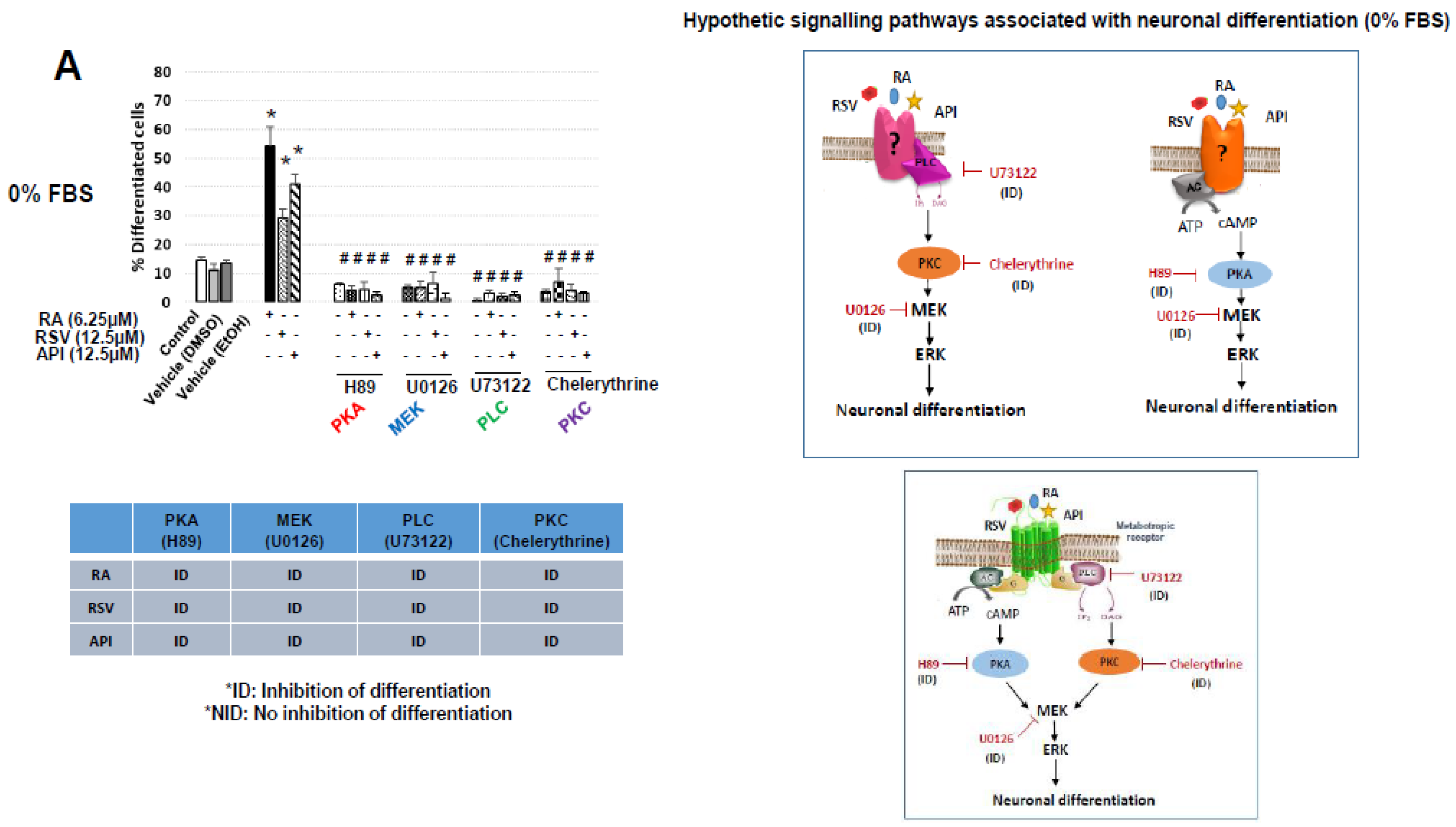
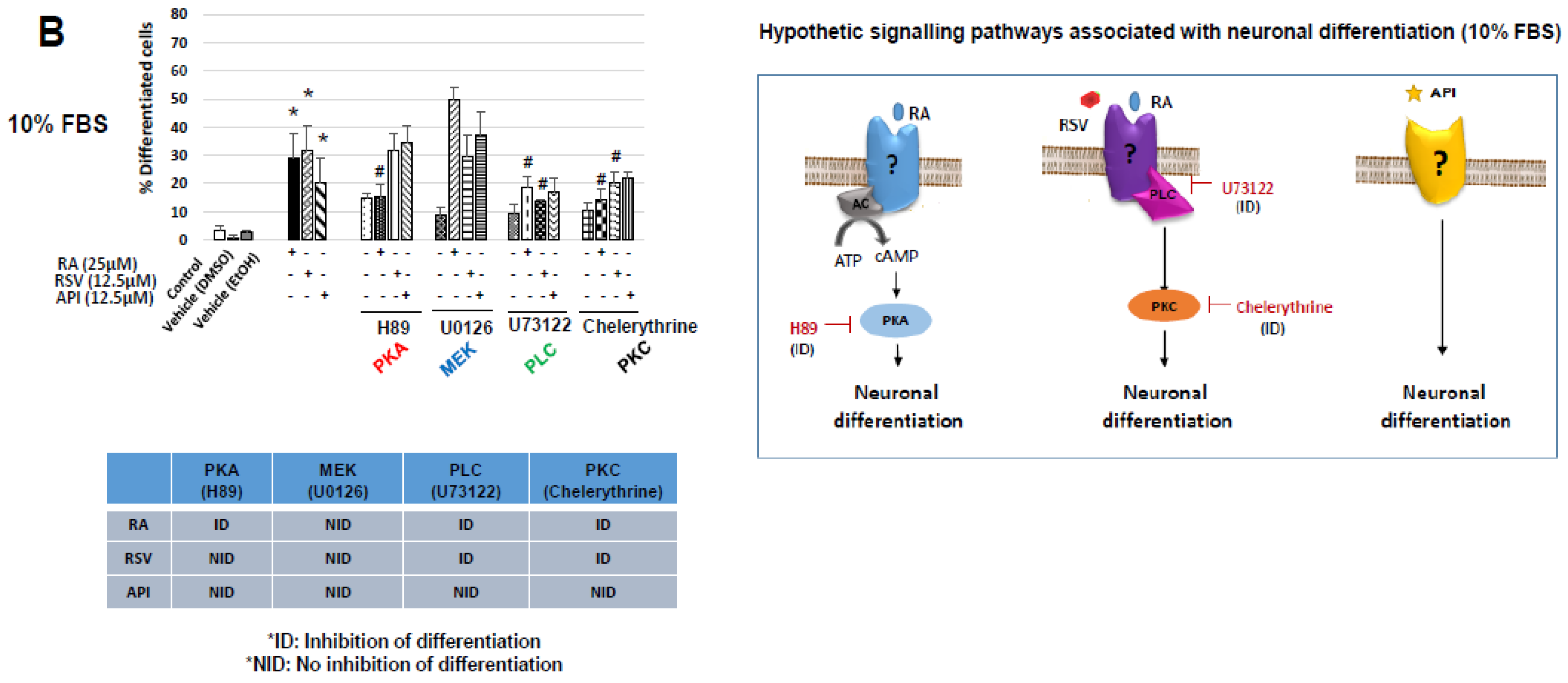
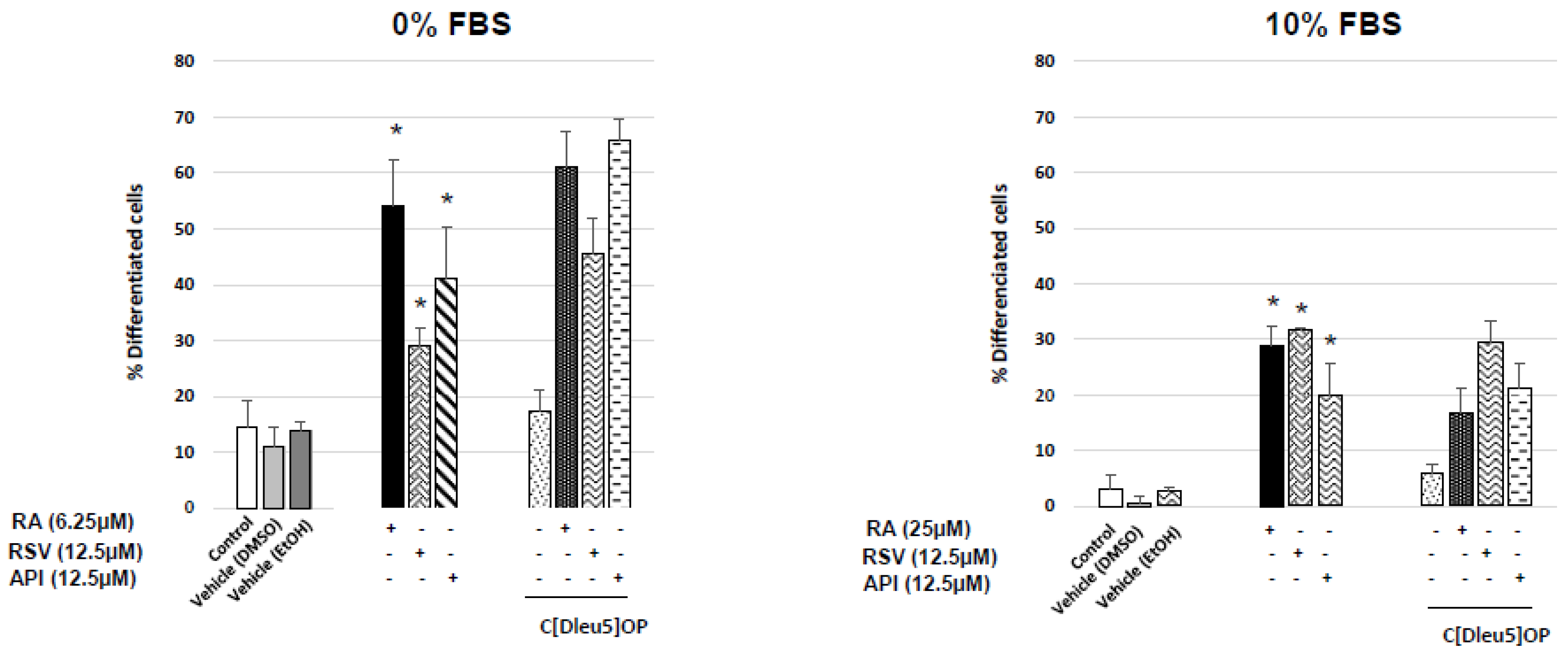
| Neuropeptides | % FBS | Ct | |||||
|---|---|---|---|---|---|---|---|
| Control | EtOH (0.02 %) | DMSO (0.12 %) | RA | RSV | API | ||
| DBI | 0 % | 33.5 ± 0.1 | 33.5 ± 1.6 | 30.6 ± 0.3 | 33.6 ± 1.0 | 33.4 ± 1.2 | 31.9 ± 0.1 |
| 10 % | 30.8 ± 0.5 | 28.4 ± 0.3 | 28.2 ± 0.3 | 32.2 ± 0.2 | 28.9 ± 2.4 | 28.1 ± 0.5 | |
| NGF | 0 % | 32.9 ± 3.7 | 31.3 ± 1.3 | 28.1 ± 0.6 | 29.7 ± 0.2 | 30.0 ± 0.3 | 28.5 ± 0.1 |
| 10 % | 27.8 ± 0.5 | 25.4 ± 1.6 | 25.6 ± 1.8 | 28.6 ± 0.2 | 24.9 ± 0.2 | 25.3 ± 0.2 | |
| BDNF | 0 % | 34.8 ± 0.8 | 33.4 ± 1.1 | 29.7 ± 1.1 | 32.6 ± 0.7 | 35.5 ± 2.3 | 31.3 ± 1.7 |
| 10 % | 31.0 ± 0.6 | 28.1 ± 0.2 | 27.4 ± 0.5 | 31.1 ± 0.5 | 37.6 ± 0.5* | 27.6 ± 0.1 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namsi, A.; Nury, T.; Hamdouni, H.; Yammine, A.; Vejux, A.; Vervandier-Fasseur, D.; Latruffe, N.; Masmoudi-Kouki, O.; Lizard, G. Induction of Neuronal Differentiation of Murine N2a Cells by Two Polyphenols Present in the Mediterranean Diet Mimicking Neurotrophins Activities: Resveratrol and Apigenin. Diseases 2018, 6, 67. https://doi.org/10.3390/diseases6030067
Namsi A, Nury T, Hamdouni H, Yammine A, Vejux A, Vervandier-Fasseur D, Latruffe N, Masmoudi-Kouki O, Lizard G. Induction of Neuronal Differentiation of Murine N2a Cells by Two Polyphenols Present in the Mediterranean Diet Mimicking Neurotrophins Activities: Resveratrol and Apigenin. Diseases. 2018; 6(3):67. https://doi.org/10.3390/diseases6030067
Chicago/Turabian StyleNamsi, Amira, Thomas Nury, Haithem Hamdouni, Aline Yammine, Anne Vejux, Dominique Vervandier-Fasseur, Norbert Latruffe, Olfa Masmoudi-Kouki, and Gérard Lizard. 2018. "Induction of Neuronal Differentiation of Murine N2a Cells by Two Polyphenols Present in the Mediterranean Diet Mimicking Neurotrophins Activities: Resveratrol and Apigenin" Diseases 6, no. 3: 67. https://doi.org/10.3390/diseases6030067
APA StyleNamsi, A., Nury, T., Hamdouni, H., Yammine, A., Vejux, A., Vervandier-Fasseur, D., Latruffe, N., Masmoudi-Kouki, O., & Lizard, G. (2018). Induction of Neuronal Differentiation of Murine N2a Cells by Two Polyphenols Present in the Mediterranean Diet Mimicking Neurotrophins Activities: Resveratrol and Apigenin. Diseases, 6(3), 67. https://doi.org/10.3390/diseases6030067









