Rosuvastatin Improves Vaspin Serum Levels in Obese Patients with Acute Coronary Syndrome
Abstract
1. Introduction
2. Patient and Methods
3. Sample Collections and Anthropometric Profiles
4. Statistical Analysis
5. Results
6. Discussion
7. Conclusions
Author Contributions
Conflicts of Interest
References
- Tsaousi, G.; Pitsis, A.A.; Ioannidis, G.D.; Vasilakos, D.G. A multidisciplinary approach to unplanned conversion from off-pump to on-pump beating heart coronary artery revascularization in patients with compromised left ventricular function. Crit. Care Res. Pract. 2014, 2014, 348021. [Google Scholar] [CrossRef] [PubMed]
- Erne, P.; Radovanovic, D.; Seifert, B.; Bertel, O.; Urban, P.; AMIS Plus Investigators. The outcome of patients admitted with acute coronary syndrome on palliative treatment: Insights from the nationwide AMIS Plus Registry 1997–2014. BMJ Open 2015, 5, e006218. [Google Scholar] [CrossRef] [PubMed]
- Hicks, K.; Cocks, K.; Corbacho Martin, B.; Elton, P.; MacNab, A.; Colecliffe, W.; Furze, G. An intervention to reassure patients about test results in rapid access chest pain clinic: A pilot randomised controlled trial. BMC Cardiovasc. Disord. 2014, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Awad, M.S.; Alrifai, S.B. Assessment of serum prolactin levels in acute myocardial infarction: The role of pharmacotherapy. Indian J. Endocrinol. Metab. 2016, 20, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I. New Insights into the Role of Metformin Effects on Serum Omentin1 Levels in Acute MyocardialInfarction: Cross-Sectional Study. Emerg. Med. Int. 2015, 2015, 283021. [Google Scholar]
- Meyer-Saraei, R.; de Waha, S.; Eitel, I.; Desch, S.; Scheller, B.; Böhm, M.; Lauer, B.; Gawaz, M.; Geisler, T.; Gunkel, O.; et al. Thrombus aspiration in non-ST-elevation myocardial infarction—The 12-month clinical outcome of the randomised TATORT-NSTEMI trial. Eur. Heart J. Acute Cardiovasc. Care 2015, 18, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Ndrepepa, G.; Braun, S.; Tada, T.; Guerra, E.; Schunkert, H.; Laugwitz, K.L.; Kastrati, A. Comparative prognostic value of low-density lipoprotein cholesterol and C-reactive protein in patients with stable coronary artery disease treated with percutaneous coronary intervention and chronic statin therapy. Cardiovasc. Revasc. Med. 2014, 15, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Ndrepepa, G.; Braun, S.; Tada, T.; King, L.; Cassese, S.; Fusaro, M.; Keta, D.; Kastrati, A.; Schmidt, R. Comparative prognostic value of C-reactive protein & fibrinogen in patients with coronary artery disease. Indian J. Med. Res. 2014, 140, 392–400. [Google Scholar] [PubMed]
- Xia, J.; Qu, Y.; Yin, C.; Xu, D. Preoperative rosuvastatin protects patients with coronary artery disease undergoing noncardiac surgery. Cardiology 2015, 131, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Esaki, E.; Adachi, H.; Hirai, Y.; Yamagishi, S.; Kakuma, T.; Enomoto, M.; Fukami, A.; Kumagai, E.; Ohbu, K.; Obuchi, A.; et al. Serum vaspin levels are positively associated with carotid atherosclerosis in a general population. Atherosclerosis 2014, 233, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Shaker, O.G.; Sadik, N.A. Vaspin gene in rat adipose tissue: Relation to obesity-induced insulin resistance. Mol. Cell. Biochem. 2013, 373, 229–239. [Google Scholar] [CrossRef] [PubMed]
- DeClercq, V.; Enns, J.E.; Yeganeh, A.; Taylor, C.G.; Zahradka, P. Modulation of cardiovascular function by adipokines. Cardiovasc. Hematol. Disord. Drug Targets 2013, 13, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.J.; Matsuo, Y.; Aoki, T.; Kwon, T.G.; Prasad, A.; Gulati, R.; Lennon, R.J.; Lerman, L.O.; Lerman, A. Coronary endothelial dysfunction is associated with inflammation and vasa vasorum proliferation in patients with early atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2473–2477. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.; Mirzaei, K.; Abdurahman, A.A.; Keshavarz, S.A.; Hossein-Nezhad, A. Mediatory effect of circulating vaspin on resting metabolic rate in obese individuals. Eur. J. Nutr. 2015, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kêkê, L.M.; Samouda, H.; Jacobs, J.; Di Pompeo, C.; Lemdani, M.; Hubert, H.; Zitouni, D.; Guinhouya, B.C. Body mass index and childhood obesity classification systems: A comparison of the French, International Obesity Task Force (IOTF) and World Health Organization (WHO) references. Rev. Epidemiol. Sante Publique 2015, 63, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Abeles, A.M.; Pillinger, M.H. Statins as antiinflammatory and immunomodulatory agents: A future in rheumatologic therapy? Arthritis Rheumatol. 2006, 54, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I. Effects of Rosuvastatin Alone or in Combination withOmega3 FattyAcid on Adiponectin Levelsand Cardiometabolic Profile. J. Basic Clin. Pharm. 2016, 8, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Abudukeremu, N.; Pan, S.; Ma, Y.; Yang, Y.; Ma, X.; Li, X.; Fu, Z.; Huang, Y.; Xie, X.; Liu, F.; et al. The optimal cutoff point of the waist-to-hip ratio for screening Uyghur population aged 35 years and over at high-risk of cardiovascular diseases in Xinjiang. Zhonghua Xin Xue Guan Bing Za Zhi 2015, 43, 173–178. [Google Scholar] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I. Acylation-stimulating protein is a surrogate biomarker for acute myocardial infarction: Role of statins. J. Lab. Physicians 2017, 9, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Vaspin in obesity and diabetes: Pathophysiological and clinical significance. Endocrine 2012, 41, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Heiker, J.T.; Klöting, N.; Kovacs, P.; Kuettner, E.B.; Sträter, N.; Schultz, S.; Kern, M.; Stumvoll, M.; Blüher, M.; Beck-Sickinger, A.G.; et al. Vaspin inhibits kallikrein 7 by serpin mechanism. Cell. Mol. Life Sci. 2013, 70, 2569–2583. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, S.E.; Gee, L.L.; Wachtel, M.S.; Frezza, E.E. Adipose tissue: The new endocrine organ? A review article. Dig. Dis. Sci. 2009, 54, 1847–1856. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.K.; Han, T.K.; Kang, H.S. Combined effects of body mass index and cardio/respiratory fitness on serum vaspin concentrations in Korean young men. Eur. J. Appl. Physiol. 2010, 108, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Mahdirejei, H.A.; Abadei, S.F.R.; Seidi, A.A.; Gorji, N.E.; Kafshgari, H.R.; Pour, M.E.; Khalili, H.B.; Hajeizad, F.; Khayeri, M. Effects of an eight-week resistance training on plasma vaspin concentrations, metabolic parameters levels and physical fitness in patients with type 2 diabetes. Cell J. 2014, 16, 367–374. [Google Scholar]
- Kim, J.M.; Kim, T.N.; Won, J.C. Association between serum vaspin level and metabolic syndrome in healthy Korean subjects. Metab. Syndr. Relat. Disord. 2013, 11, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Kobat, M.A.; Celik, A.; Balin, M.; Altas, Y.; Baydas, A.; Bulut, M.; Aydin, S.; Dagli, N.; Yavuzkir, M.F.; Ilhan, S. The investigation of serum vaspin level in atherosclerotic coronary artery disease. J. Clin. Med. Res. 2012, 4, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.; Vrabas, I.S.; Kapelouzou, A.; Lampropoulos, S.; Sailer, N.; Kostakis, A.; Liapis, C.D. Impact of atorvastatin on serum vaspin levels in hypercholesterolemic patients with moderate cardiovascular risk. Regul. Pept. 2011, 170, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Takahashi, Y.; Tabuchi, T.; Minami, Y.; Tamada, M.; Takahashi, K.; Itoh, T.; Morino, Y.; Nakamura, M. Cellular and molecular mechanisms of statins: An update on pleiotropic effects. Clin. Sci. 2015, 129, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Vidyarthi, M.; Jacob, P.; Chowdhury, T.A. Oral use of “Low and Slow” Rosuvastatin with Co-Enzyme Q10 in patients with Statin-Induced Myalgia: Retrospective case review. Indian J. Endocrinol. Metab. 2012, 16 (Suppl. S2), S498–S500. [Google Scholar] [PubMed]
- Kavalipati, N.; Shah, J.; Ramakrishan, A.; Vasnawala, H. Pleiotropic effects of statins. Indian J. Endocrinol. Metab. 2015, 19, 554–562. [Google Scholar] [PubMed]
- Kadoglou, N.P.; Gkontopoulos, A.; Kapelouzou, A.; Fotiadis, G.; Theofilogiannakos, E.K.; Kottas, G.; Lampropoulos, S. Serum levels of vaspin and visfatin in patients with the coronary artery disease-Kozani study. Clin. Chim. Acta 2011, 412, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Phalitakul, S.; Okada, M.; Hara, Y.; Yamawaki, H. Vaspin prevents TNF-α-induced intracellular adhesion molecule-1 via inhibiting reactive oxygen species-dependent NF-κB and PKC activation in cultured rat vascular smooth muscle cells. Pharmacol. Res. 2011, 64, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Phalitakul, S.; Okada, M.; Hara, Y.; Yamawaki, H. A novel adipocytokine, vaspin inhibits platelet-derived growth factor-BB-induced migration of vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2012, 423, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Al-Azzam, S.I.; Alzoubi, K.H.; Abeeleh, J.A.; Mhaidat, N.M.; Abu-Abeeleh, M. Effect of statin therapy on vaspin levels in type 2 diabetic patients. Clin. Pharmacol. 2013, 5, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Teshigawara, S.; Wada, J.; Hida, K.; Nakatsuka, A.; Eguchi, J.; Murakami, K.; Kanzaki, M.; Inoue, K.; Terami, T.; Katayama, A.; et al. Serum vaspin concentrations are closely related to insulin resistance, with higher serum levels in Japanese population. J. Clin. Endocrinol. Metab. 2012, 97, E1202–E1207. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, H.; Honda, T.; Maekawa, T.; Takahashi, N.; Aoki, Y.; Nakajima, T.; Tabeta, K.; Yamazaki, K. Relationship between serum antibody titers to Porphyromonas gingivalis and hs-CRP levels as inflammatory markers of periodontitis. Arch. Oral Biol. 2012, 57, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Motawi, T.M.K.; Mahdy, S.G.; El-Sawalhi, M.M.; Ali, E.N.; El-Telbany, R.F.A. Serum levels of chemerin, apelin, vaspin, and omentin-1 in obese type 2 diabetic Egyptian patients with coronary artery stenosis. Can. J. Physiol. Pharmacol. 2017, 28, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sathyaseelan, A.J.; Adole, P.S.; Wyawahare, M.; Saya, R.P. Assessment of Serum VASPIN Levels among Type 2 Diabetes Mellitus Patients with or without Acute Coronary Syndrome. J. Clin. Diagn. Res. 2016, 10, BC07–BC10. [Google Scholar] [CrossRef] [PubMed]
| Variables | Rosuvastatin Group | Control Group | p Value |
|---|---|---|---|
| (n = 70) | (n = 40) | ||
| Age | 47.87 ± 12.72 | 49.85 ± 11.83 | 0.52 |
| Gender | |||
| M:F ratio | (71.42:28.57)% | (22:18) % | - |
| BMI | 31.44 ± 7.81 | 32.39 ± 6.77 | 0.59 |
| W-H ratio | |||
| Men | 1.32 ± 0.67 | 1.39 ± 0.77 | 0.71 |
| Women | 0.88 ± 0.31 | 0.91 ± 0.11 | 0.67 |
| Hypertension | 70 (100%) | 20 (100%) | <0.0001 ** |
| ACS | |||
| STEMI | 25 (35.71%) | 8 (40%) | 0.72 |
| NSTEMI | 25 (35.71%) | 10 (50%) | 0.24 |
| UA | 20 (28.57%) | 2 (10%) | 0.08 |
| Troponin positive | 67 (95.71%) | 18 (90%) | 0.32 |
| Troponin negative | 3 (4.28%) | 2 (10%) | 0.32 |
| Dyslipidemia | 64 (44.8%) | 18 (90%) | 0.84 |
| Duration of IHD (years) | 8.42 ± 2.28 | 7.44 ± 2.39 | 0.11 |
| Diabetes mellitus | 3 (4.28%) | 2 (10%) | 0.32 |
| Pharmacotherapy | |||
| Anticoagulant | 44 (62.58%) | 17 (85%) | 0.06 |
| Antiplatelet | 70 (100%) | 18 (90%) | 0.0072 |
| ACEIs | 44 (62.58%) | 18 (90%) | 0.02 * |
| Statins | 70 (100%) | - | <0.0001 ** |
| CCB | 22 (31.42%) | 12 (60%) | 0.02 * |
| β-blockers | 6 (8.57%) | 5 (25%) | 0.04 * |
| Insulin | 3 (4.28%) | 2 (10%) | 0.32 |
| Complications | 10 (15.71%) | 4 (20%) | 0.53 |
| Shock | 2 (2.85%) | 1 (5%) | 0.63 |
| Heart failure | 5 (3.5%) | 2 (10%) | 0.67 |
| Cardiac aneurysm | 1 (1.42%) | 1 (5%) | 0.33 |
| Death | 2 (2.85%) | 1 (5%) | 0.33 |
| Cardio-Metabolic Variables | Study Group (n = 70) | Control Group (n = 40) | t Value | 95% CI Upper-Lower Limits | p Value |
|---|---|---|---|---|---|
| Serum cTn-I pg/mL) | 74.54 ± 13.32 | 77.64 ± 12.22 | −0.98 | 3.33–9.53 | 0.33 |
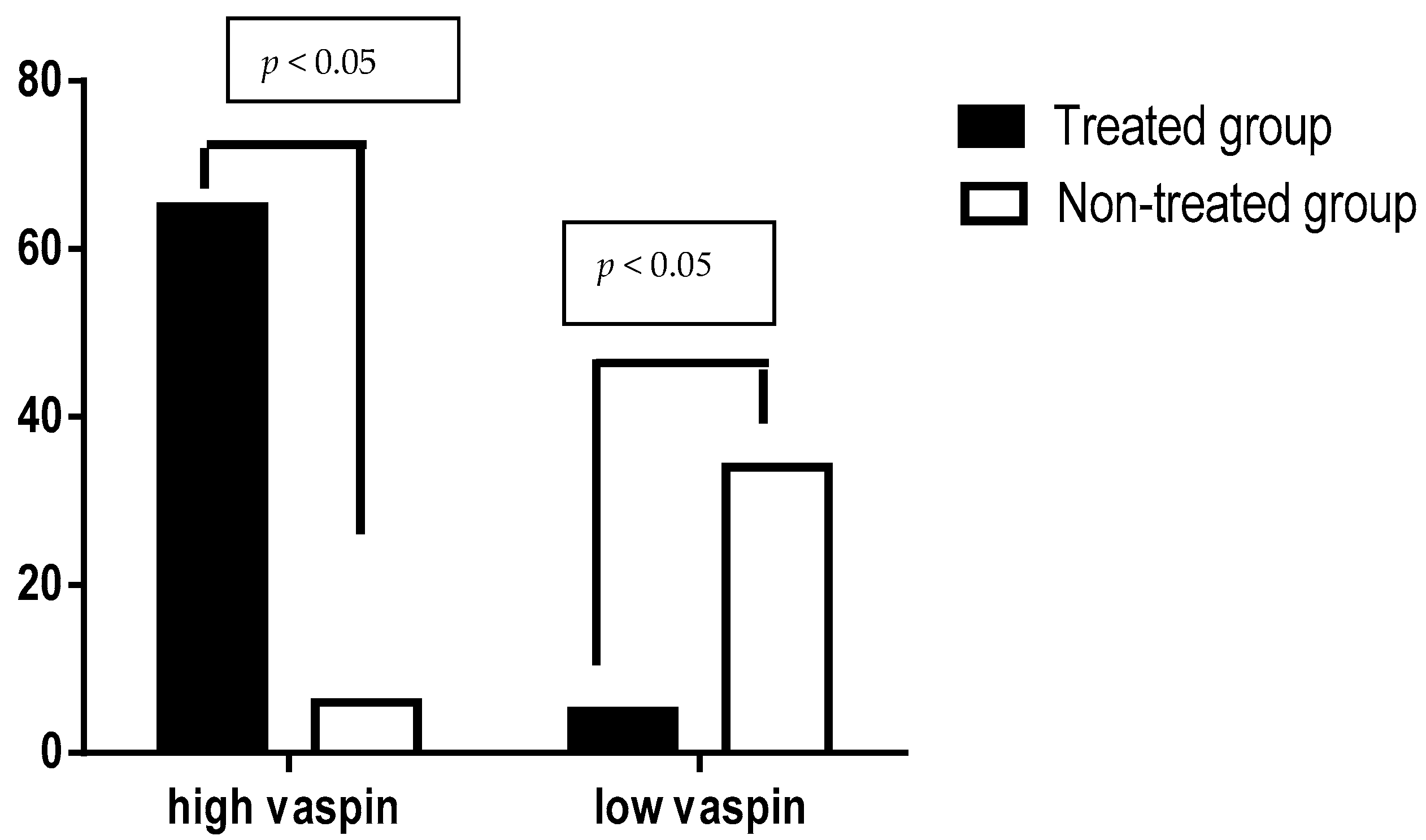
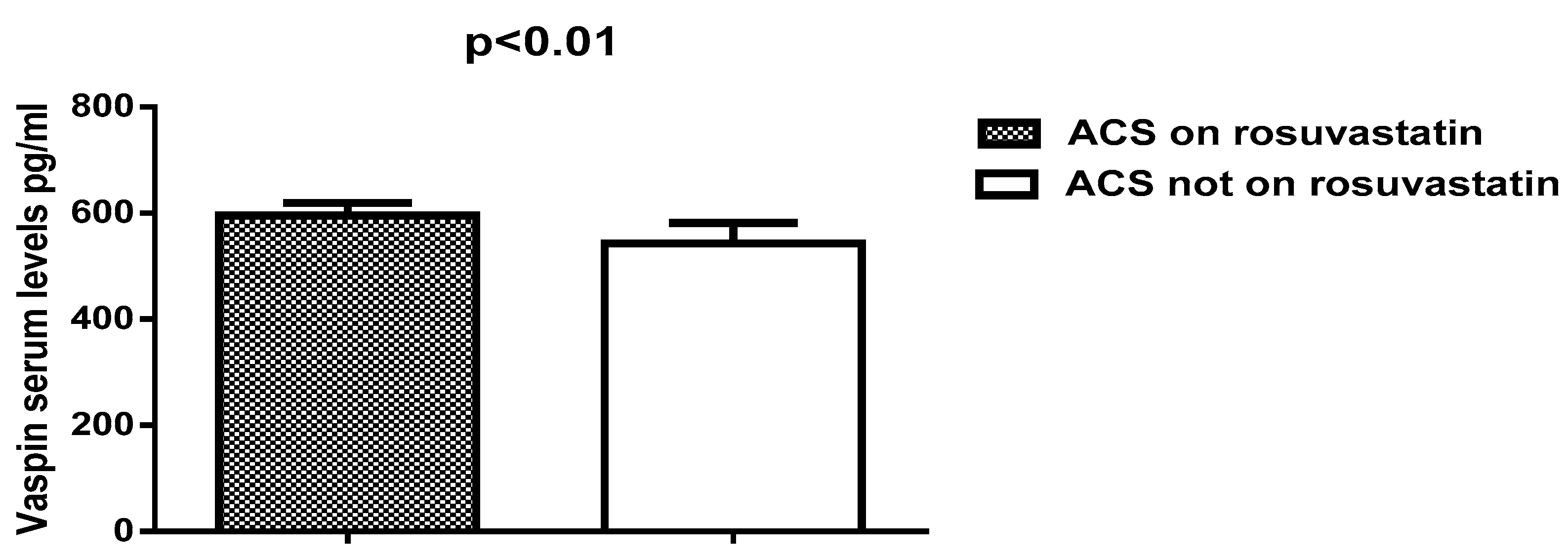
| Serum vaspin(pg/mL) | 603.83 ± 18.13 | 542.75 ± 38.95 | 6.8 | 79.72–42.33 | <0.0001 ** |
| TC (mg/dL) | 199.28 ± 20.49 | 266.43 ± 16.59 | −15.1 | −134.29 | <0.0001 ** |
| TG (mg/dL) | 166.83 ± 17.34 | 254.73 ± 22.82 | −15.95 | −175.78 | <0.0001 ** |
| LDL (mg/dL) | 118.80 ± 8.75 | 164.71 ± 13.59 | −14.28 | −81.81 | <0.0001 ** |
| HDL(mg/dL) | 47.11 ± 9.86 | 50.76 ± 7.34 | −1.8 | 0.43–7.73 | 0.078 |
| VLDL (mg/dL) | 33.36 ± 5.83 | 50.94 ± 6.42 | −11.01 | −35.15 | <0.0001 ** |
| AI | 0.189 ± 0.012 | 0.341 ± 0.021 | −30.95 | −0.3 | <0.0001 ** |
| CRR | 4.23 ± 1.44 | 5.24 ± 1.98 | −2.12 | −2.01 | 0.04 * |
| SBP (mmHg) | 166.54 ± 21.54 | 155.87 ± 19.63 | 2.09 | 21.02–0.31 | 0.043 * |
| DBP (mmHg) | 92.23 ± 22.69 | 87.67 ± 13.75 | 1.11 | 12.78–3.66 | 0.27 |
| FBG (mg/dL) | 101.65 ± 11.93 | 97.87 ± 8.97 | 1.53 | 8.75–1.19 | 0.132 |
| PPG(mg/dL) | 128.64 ± 11.83 | 132.64 ± 12.74 | −1.25 | 2.50–10.50 | 0.21 |
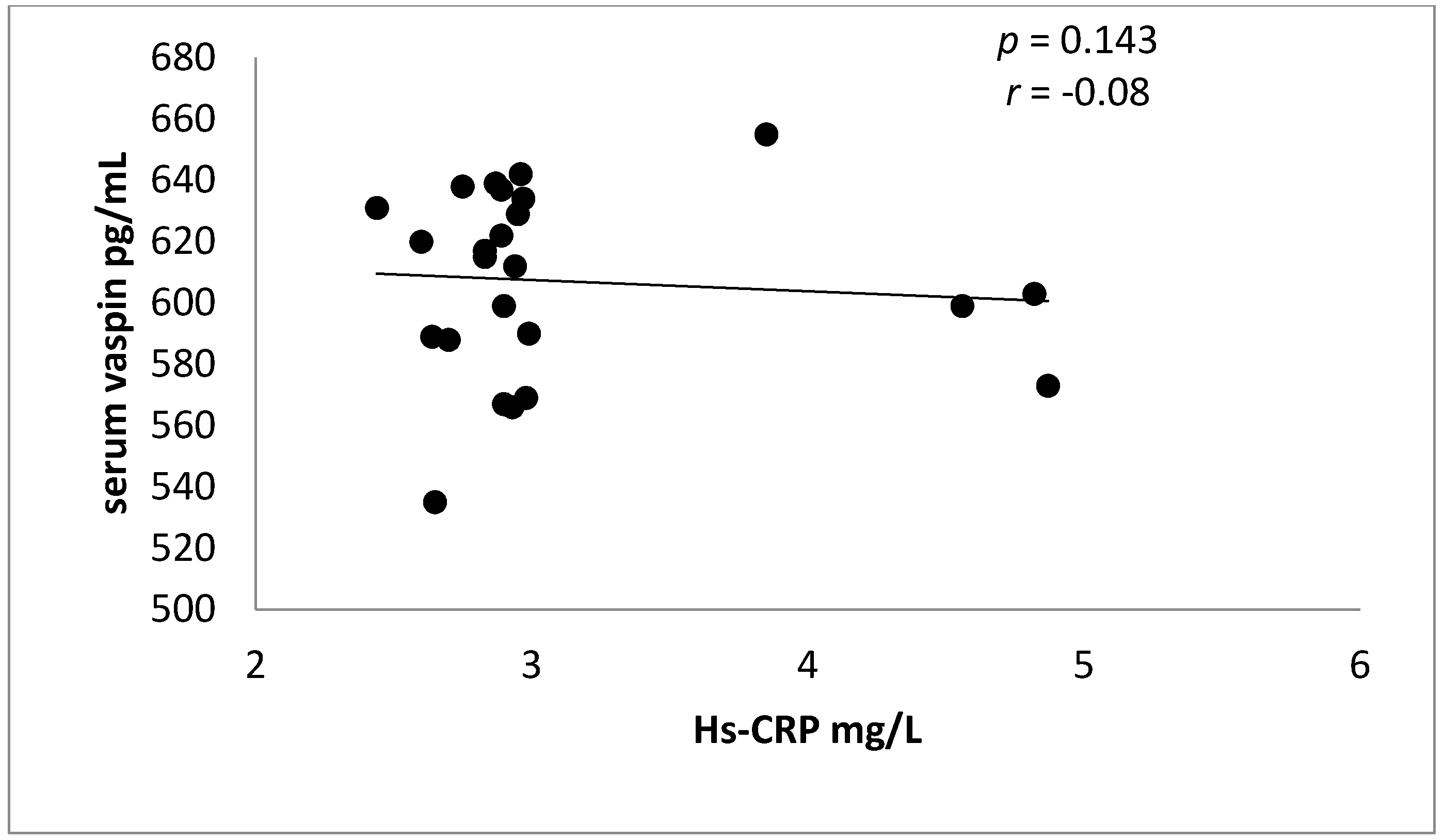
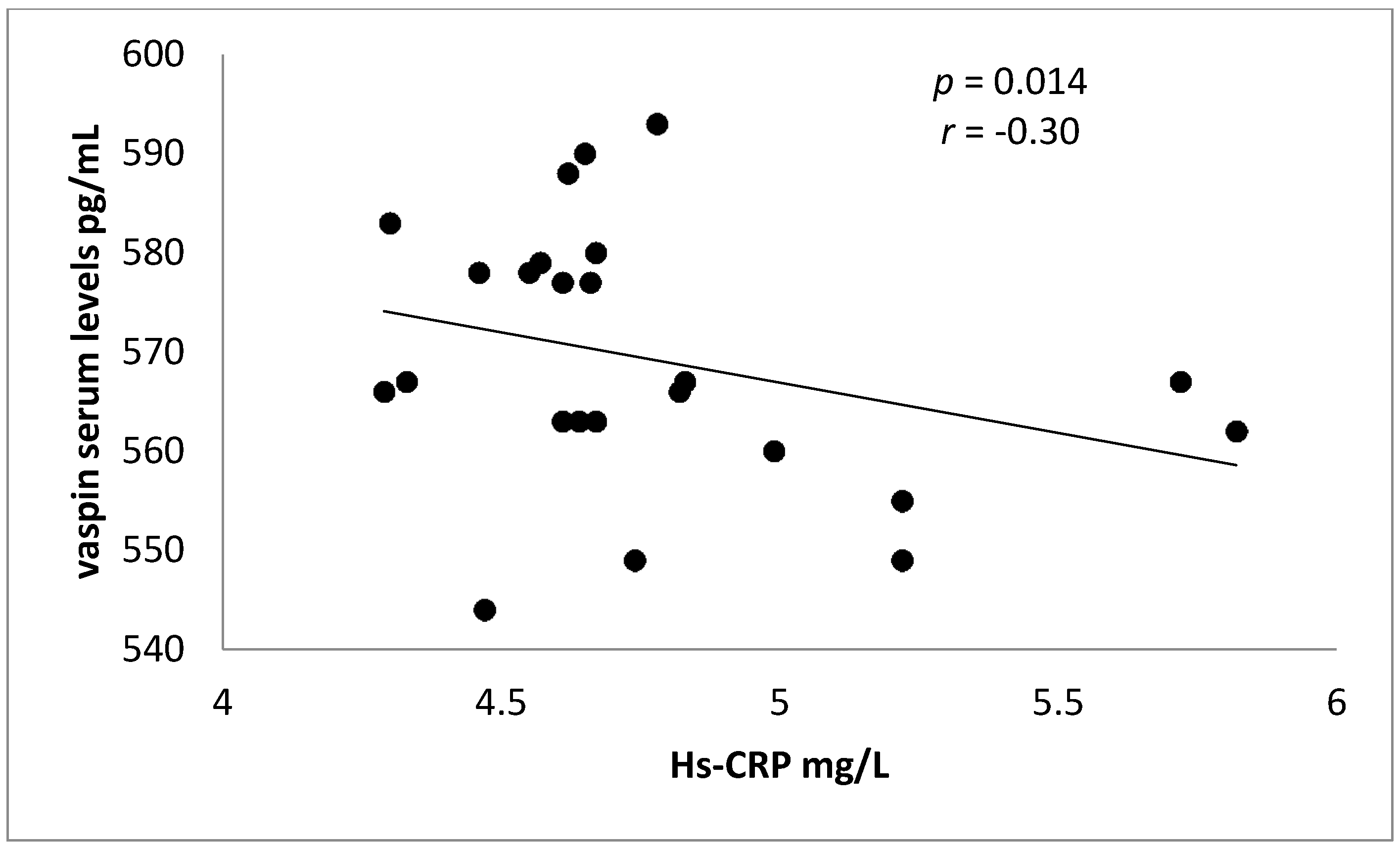
| Hs-CRP (mg/L) | 2.95 ± 0.45 | 4.65 ± 1.84 | −4.09 | −3.39 | 0.0006 ** |
| Cardio-Metabolic Variables | STEMI (n = 25) | NSTEMI (n = 25) | Unstable Angina (n = 20) | Control (n = 40) |
|---|---|---|---|---|
| Serum cTn-I (pg/mL) | 74.12 ± 12.45 | 72.65 ± 22.49 | 54.45 ± 23.53 ** | 77.64 ± 12.22 |
| Serum vaspin (pg/mL) | 611.32 ± 33.64 ** | 588.67 ± 22.29 ** | 586.87 ± 33.72 ** | 542.75 ± 38.95 |
| TC (mg/dL) | 197.28 ± 21.77 ** | 192.99 ± 19.64 ** | 193.64 ± 20.65 ** | 266.43 ± 16.59 |
| TG (mg/dL) | 160.83 ± 19.42 ** | 167.54 ± 18.74 ** | 166.83 ± 16.84 ** | 254.73 ± 22.82 |
| LDL (mg/dL) | 117.78 ± 8.75 ** | 113.85 ± 9.98 ** | 112.63 ± 13.83 ** | 164.71 ± 13.59 |
| HDL (mg/dL) | 47.33 ± 9.77 | 45.63 ± 8.99 | 45.55 ± 8.33 * | 31.76 ± 7.34 |
| VLDL (mg/dL) | 32.16 ± 5.88 ** | 33.50 ± 4.34 ** | 33.57 ± 4.22 ** | 50.94 ± 6.42 |
| AI | 0.171 ± 0.011 ** | 0.205 ± 0.014 ** | 0.204 ± 0.012 ** | 0.341 ± 0.021 |
| CRR | 4.16 ± 1.65 | 4.22 ± 1.99 | 4.25 ± 1.86 | 5.24 ± 1.98 |
| SBP (mmHg) | 162.66 ± 20.33 | 165.62 ± 19.82 | 164.76 ± 18.93 | 155.87 ± 19.63 |
| DBP (mmHg) | 91.20 ± 20.39 | 93.53 ± 20.37 | 93.77 ± 20.53 | 87.67 ± 13.75 |
| Pulse pressure (mmHg) | 71.46 ± 11.76 | 72.09 ± 10.74 | 70.99 ± 11.55 | 68.20 ± 9.33 |
| FBG (mg/dL) | 101.44 ± 10.98 | 100.54 ± 10.71 | 104.61 ± 9.88 | 97.87 ± 8.97 |
| PPG (mg/dL) | 129.60 ± 10.82 | 127.55 ± 9.76 | 126.83 ± 9.38 | 132.64 ± 12.74 |
| Hs-CRP (mg/L) | 2.93 ± 0.44 ** | 2.89 ± 0.45 ** | 1.44 ± 0.11 ** | 4.65 ± 1.84 |
| Variables | STEMI (n = 25) | NSTEMI (n = 25) | UA (n = 20) | Control (n = 40) | ||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| Serum cTn-I (pg/mL) | 0.87 | 0.001 * | 0.77 | 0.0001 * | 0.44 | NS | 0.94 | 0.0001 * |
| TC (mg/dL) | −0.52 | 0.007 * | −0.67 | 0.0002 * | −0.67 | 0.001 * | −0.56 | 0.007 * |
| TG (mg/dL) | −0.41 | 0.04 ! | −0.32 | NS | −0.36 | NS | −0.75 | 0.001 * |
| LDL (mg/dL) | −0.33 | NS | −0.32 | NS | 0.38 | NS | −0.63 | 0.0002 * |
| HDL (mg/dL) | 0.66 | 0.0003 * | 0.69 | 0.0002 * | 0.71 | 0.0004 * | 0.51 | 0.008 * |
| VLDL (mg/dL) | −0.31 | NS | −0.34 | NS | −0.38 | NS | −0.42 | 0.006 * |
| AI | −0.41 | 0.04 ! | −0.35 | NS | −0.41 | NS | −0.51 | 0.0007 * |
| CRR | −0.34 | NS | −0.31 | NS | −0.37 | NS | −0.41 | 0.008 * |
| SBP (mmHg) | −0.33 | NS | −0.36 | NS | −0.32 | NS | −0.42 | 0.006 * |
| DBP (mmHg) | −0.37 | NS | −0.30 | NS | −0.22 | NS | −0.41 | 0.008 * |
| FBG (mg/dL) | −0.28 | NS | −0.32 | NS | −0.30 | NS | −0.33 | 0.03 ! |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-kuraishy, H.M.; Al-Gareeb, A.I.; Al-Buhadilly, A.K. Rosuvastatin Improves Vaspin Serum Levels in Obese Patients with Acute Coronary Syndrome. Diseases 2018, 6, 9. https://doi.org/10.3390/diseases6010009
Al-kuraishy HM, Al-Gareeb AI, Al-Buhadilly AK. Rosuvastatin Improves Vaspin Serum Levels in Obese Patients with Acute Coronary Syndrome. Diseases. 2018; 6(1):9. https://doi.org/10.3390/diseases6010009
Chicago/Turabian StyleAl-kuraishy, Hayder M., Ali I. Al-Gareeb, and Ali K. Al-Buhadilly. 2018. "Rosuvastatin Improves Vaspin Serum Levels in Obese Patients with Acute Coronary Syndrome" Diseases 6, no. 1: 9. https://doi.org/10.3390/diseases6010009
APA StyleAl-kuraishy, H. M., Al-Gareeb, A. I., & Al-Buhadilly, A. K. (2018). Rosuvastatin Improves Vaspin Serum Levels in Obese Patients with Acute Coronary Syndrome. Diseases, 6(1), 9. https://doi.org/10.3390/diseases6010009





