Factors Associated with Post-Progression Survival in Patients with Advanced Hepatocellular Carcinoma Treated with Sorafenib
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. Results
3.1.1. Patient Characteristics and Outcomes
| Variable | Group A + B n = 55 | Group C n = 21 | p |
|---|---|---|---|
| At initiation of sorafenib treatment | |||
| Sex, n (%) | |||
| Male | 42 (76) | 17 (81) | 0.670 |
| Female | 13 (24) | 4 (19) | |
| Therapy before sorafenib, n (%) | |||
| Surgical resection | 23 (42) | 8 (38) | 0.769 |
| Locoregional ablation | 29 (53) | 12 (57) | 0.732 |
| Transarterial chemoembolization | 45 (82) | 15 (71) | 0.324 |
| Median number of therapies before sorafenib | 4 | 5 | 0.420 |
| At the first rPD | |||
| Median age, years | 74 | 77 | 0.406 |
| ECOG PS, n (%) | |||
| 0, 1 | 51 (93) | 12 (57) | <0.001 |
| ≥2 | 3 (5) | 9 (43) | |
| Unknown | 1 (2) | 0 | |
| Cause of liver disease, n (%) | |||
| HCV | 29 (53) | 17 (80) | 0.074 |
| HBV | 9 (16) | 2 (10) | |
| Others | 17 (31) | 2 (10) | |
| Child–Pugh class, n (%) | |||
| A | 35 (64) | 5 (24) | <0.001 |
| B | 19 (34) | 11 (52) | |
| C | 0 | 5 (24) | |
| Unknown | 1 (2) | 0 | |
| BCLC stage, n (%) | |||
| B | 21 (38) | 5 (24) | 0.241 |
| C | 34 (62) | 16 (76) | |
| Incidence of severe AEs, n (%) | 30 (55) | 10 (48) | 0.591 |
| History of treatment interruption, n (%) | 30 (55) | 10 (48) | 0.591 |
| Best antitumor response †, n (%) | |||
| Partial response | 2 (4) | 1 (5) | 0.424 |
| Stable disease | 22 (40) | 5 (24) | |
| Progression disease | 31 (56) | 15 (71) | |
| Median AFP, ng/mL | 77 | 2,506 | 0.032 |
| AFP >1000 ng/mL, n (%) | 14 (25) | 11 (52) | 0.023 |
3.1.2. Prognostic Factors for PPS
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| p | β | HR (95% CI) | p | |
| Age > 70 years | 0.090 | |||
| Male | 0.646 | |||
| ECOG PS ≥ 2 | <0.001 | 0.943 | 2.568 (1.317–5.006) | 0.006 |
| Child-Pugh Class A | 1 | |||
| B | <0.001 | 0.846 | 2.329 (1.173–4.624) | 0.016 |
| C | <0.001 | 3.200 | 24.525 (5.860–102.635) | <0.001 |
| BCLC Stage C | 0.414 | |||
| PD as the best antitumor response | 0.004 | 1.162 | 3.195 (1.625–6.279) | 0.001 |
| Absence of tumor shrinkage | 0.918 | |||
| Absence of contrast enhancement disappeared lesion | 0.398 | |||
| History of AEs ≥ Grade 3 | 0.468 | |||
| History of interrupted treatment | 0.457 | |||
| AFP > 1000 ng/mL | 0.002 | 0.912 | 2.490 (1.327–4.673) | 0.005 |
3.1.3. Post-rPD Therapy
| Variables at the first rPD | Score | ||
|---|---|---|---|
| 0 | 1 | 2 | |
| Child-Pugh class | A | B | C |
| ECOG PS | 0, 1 | ≥2 | |
| Best response † | CR/PR/SD | PD | |
| AFP | <1000 ng/mL | ≥1000 ng/mL | |
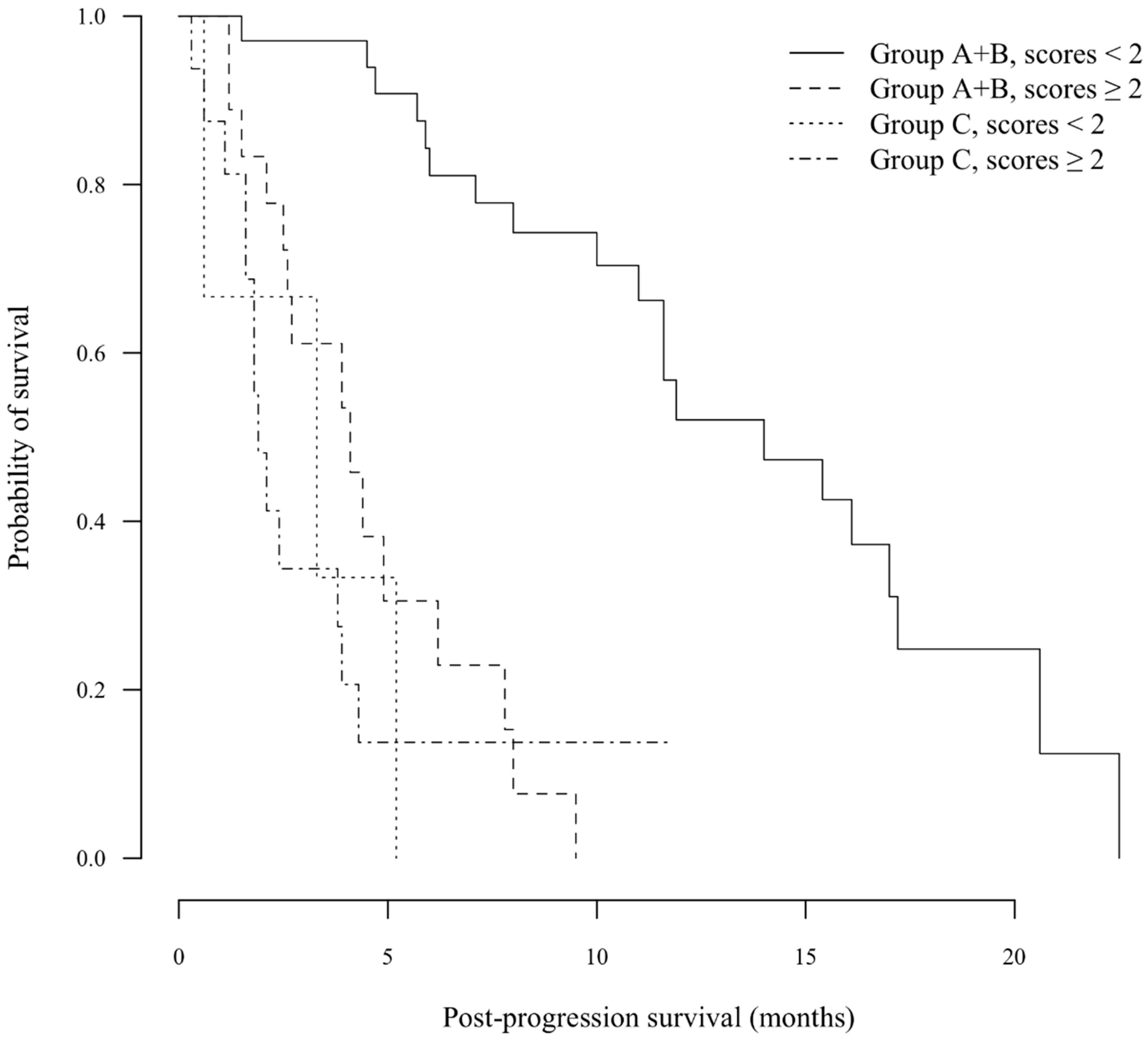
3.1.4. Beyond rPD
3.2. Discussion
4. Conclusions
Author Contributions
Conflicts of Interest
References
- El-Serag, H.B.; Rudolph, K.L. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Tsao, L.; Hsing, A.W.; Devesa, S.S.; Fraumeni, J.F., Jr. International trends and patterns of primary liver cancer. Int. J. Cancer 2001, 94, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Saad, E.D.; Katz, A.; Buyse, M. Overall survival and post-progression survival in advanced breast cancer: A review of recent randomized clinical trials. J. Clin. Oncol. 2010, 28, 1958–1962. [Google Scholar] [CrossRef] [PubMed]
- Hotta, K.; Kiura, K.; Fujiwara, Y.; Takigawa, N.; Hisamoto, A.; Ichihara, E.; Tabata, M.; Tanimoto, M. Role of survival post-progression in phase III trials of systemic chemotherapy in advanced non-small-cell lung cancer: A systematic review. PLoS ONE 2011, 6, e26646. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Barni, S. Correlation of progression-free and post-progression survival with overall survival in advanced colorectal cancer. Ann. Oncol. 2013, 24, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Oxnard, G.R.; Morris, M.J.; Hodi, F.S.; Baker, L.H.; Kris, M.G.; Venook, A.P.; Schwartz, L.H. When progressive disease does not mean treatment failure: Reconsidering the criteria for progression. J. Natl. Cancer Inst. 2012, 104, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Therasse, P. Measuring the clinical response. What does it mean? Eur. J. Cancer 2002, 38, 1817–1823. [Google Scholar] [CrossRef] [PubMed]
- Ratain, M.J.; Eckhardt, S.G. Phase II studies of modern drugs directed against new targets: If you are fazed, too, then resist RECIST. J. Clin. Oncol. 2004, 22, 4442–4445. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Heagerty, P.J.; Lumley, T.; Pepe, M.S. Time-dependent ROC curves for censored survival data and a diagnostic marker. Bilmetrics 2000, 56, 337–344. [Google Scholar] [CrossRef]
- Kim, H.Y.; Park, J.W.; Nam, B.H.; Kim, H.K.; Choi, J.I.; Kim, T.H.; Kim, H.B.; Kim, C.M. Survival of patients with advanced hepatocellular carcinoma: Sorafenib versus other treatments. J. Gastroenterol. Hepatol. 2011, 26, 1612–1618. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Lencioni, R.; Kudo, M.; Ye, S.; Nakajima, K.; Cihon, F.; Venook, A.P. Global Investigation of Therapeutic Decisions in Hepatocellular Carcinoma and of its Treatment with Sorafenib (GIDEON) second interim analysis in more than 1,500 patients: Clinical findings in patients with liver dysfunction. J. Clin. Oncol. 2011, 29. Abstract 4001. [Google Scholar]
- Bruix, J.; Raoul, J.L.; Sherman, M.; Mazzaferro, V.; Bolondi, L.; Craxi, A.; Galle, P.R.; Santoro, A.; Beaugrand, M.; Sangiovanni, A.; et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: Subanalyses of a phase III trial. J. Hepatol. 2012, 57, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.Y.; Lin, Z.Z.; Hsu, C.; Shen, Y.C.; Hsu, C.H.; Cheng, A.L. Early alpha-fetoprotein response predicts treatment efficacy of antiangiogenic systemic therapy in patients with advanced hepatocellular carcinoma. Cancer 2010, 116, 4590–4596. [Google Scholar] [CrossRef] [PubMed]
- Personeni, N.; Bozzarelli, S.; Pressiani, T.; Rimassa, L.; Tronconi, M.C.; Sclafani, F.; Carnaghi, C.; Pedicini, V.; Giordano, L.; Santoro, A. Usefulness of alpha-fetoprotein response in patients treated with sorafenib for advanced hepatocellular carcinoma. J. Hepatol. 2012, 57, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Ueshima, K.; Arizumi, T. Real-life clinical practice with sorafenib in advanced hepatocellular carcinoma: A single-center experience. Digit. Distrib. 2012, 30, 609–616. [Google Scholar]
- Nojiri, S.; Kusakabe, A.; Fujiwara, K.; Shinkai, N.; Matsuura, K.; Iio, E.; Miyaki, T.; Nomura, T.; Sobue, S.; Sano, H.; et al. Clinical factors related to long-term administration of sorafenib in patients with hepatocellular carcinoma. Cancer Manag. Res. 2012, 4, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Pressiani, T.; Boni, C.; Rimassa, L.; Labianca, R.; Fagiuoli, S.; Salvagni, S.; Ferrari, D.; Cortesi, E.; Porta, C.; Mucciarini, C.; et al. Sorafenib in patients with Child-Pugh class A and B advanced hepatocellular carcinoma: A prospective feasibility analysis. Ann. Oncol. 2013, 24, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, T.; Eguchi, Y.; Kawazoe, S.; Yanagita, K.; Ario, K.; Kitahara, K.; Kawasoe, H.; Kato, H.; Mizuta, T.; the Saga Liver Cancer Study Group. Skin toxicities and survival in advanced hepatocellular carcinoma patients treated with sorafenib. Hepatol. Res. 2012, 42, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A.; Sugrue, M.M.; Purdie, D.M.; Dong, W.; Sargent, D.; Hedrick, E.; Kozloff, M. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: Results from a large observational cohort study (BRiTE). J. Clin. Oncol. 2008, 26, 5326–5334. [Google Scholar] [CrossRef] [PubMed]
- Cohn, A.L.; Bekaii-Saab, T.; Bendell, J.C.; Hurwitz, H.; Kozloff, M.; Roach, N.; Tezcan, H.; Feng, S.; Sing, A.; Grothey, A.; et al. Clinical outcomes in bevacizumab (BV)-treated patients (pts) with metastatic colorectal cancer (mCRC): Results from ARIES observational cohort study (OCS) and confirmation of BRiTE data on BV beyond progression (BBP). J. Clin. Oncol. 2010, 28. Abstract 3596. [Google Scholar]
- Bennouna, J.; Sastre, J.; Arnold, D.; Österlund, P.; Greil, R.; Van Cutsem, E.; von Moos, R.; Viéitez, J.M.; Bouché, O.; Borg, C.; et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): A randomised phase 3 trial. Lancet Oncol. 2013, 14, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, M.R.; Davis, R.; Norberg, S.M.; O'Brien, S.; Sennino, B.; Nakahara, T.; Yao, V.J.; Inai, T.; Brooks, P.; Freimark, B.; et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J. Clin. Investig. 2006, 116, 2610–2621. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, T.T.; Sorensen, A.G.; di Tomaso, E.; Zhang, W.T.; Duda, D.G.; Cohen, K.S.; Kozak, K.R.; Cahill, D.P.; Chen, P.J.; Zhu, M.; et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 2007, 11, 83–95. [Google Scholar] [CrossRef]
- Van Malenstein, H.; Dekervel, J.; Verslype, C.; Van Cutsem, E.; Windmolders, P.; Nevens, F.; van Pelt, J. Long-term exposure to sorafenib of liver cancer cells induces resistance with epithelial-to-mesenchymal transition, increased invasion and risk of rebound growth. Cancer Lett. 2013, 329, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A.; Llovet, J.M. Targeted therapies for hepatocellular carcinoma. Gastroenterology 2011, 140, 1410–1426. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.L.; Yeo, W. Targeted therapy of hepatocellular carcinoma: Present and future. J. Gastroenterol. Hepatol. 2012, 27, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Rimassa, L.; Borbath, I.; Daniele, B.; Salvagni, S.; van Laethem, J.L.; van Vlierberghe, H.; Trojan, J.; Kolligs, F.T.; Weiss, A.; et al. Tivantinib for second-line treatment of advanced hepatocellular carcinoma: A randomised, placebo-controlled phase 2 study. Lancet Oncol. 2013, 14, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Rimassa, L.; Pressiani, T.; Boni, C.; Carnaghi, C.; Rota Caremoli, E.; Fagiuoli, S.; Foa, P.; Salvagni, S.; Cortesi, E.; Chiara Tronconi, M.; et al. A phase II randomized dose escalation trial of sorafenib in patients with advanced hepatocellular carcinoma. Oncologist 2013, 18, 379–380. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otsuka, T.; Nakashita, S.; Yanagita, K.; Ario, K.; Kawasoe, H.; Kawazoe, S.; Eguchi, Y.; Mizuta, T. Factors Associated with Post-Progression Survival in Patients with Advanced Hepatocellular Carcinoma Treated with Sorafenib. Diseases 2015, 3, 68-77. https://doi.org/10.3390/diseases3020068
Otsuka T, Nakashita S, Yanagita K, Ario K, Kawasoe H, Kawazoe S, Eguchi Y, Mizuta T. Factors Associated with Post-Progression Survival in Patients with Advanced Hepatocellular Carcinoma Treated with Sorafenib. Diseases. 2015; 3(2):68-77. https://doi.org/10.3390/diseases3020068
Chicago/Turabian StyleOtsuka, Taiga, Shunya Nakashita, Kimihiko Yanagita, Keisuke Ario, Hiroaki Kawasoe, Seiji Kawazoe, Yuichiro Eguchi, and Toshihiko Mizuta. 2015. "Factors Associated with Post-Progression Survival in Patients with Advanced Hepatocellular Carcinoma Treated with Sorafenib" Diseases 3, no. 2: 68-77. https://doi.org/10.3390/diseases3020068
APA StyleOtsuka, T., Nakashita, S., Yanagita, K., Ario, K., Kawasoe, H., Kawazoe, S., Eguchi, Y., & Mizuta, T. (2015). Factors Associated with Post-Progression Survival in Patients with Advanced Hepatocellular Carcinoma Treated with Sorafenib. Diseases, 3(2), 68-77. https://doi.org/10.3390/diseases3020068





