Exploring the Interplay of Antioxidants, Inflammation, and Oxidative Stress: Mechanisms, Therapeutic Potential, and Clinical Implications
Abstract
1. Introduction
2. Oxidative Stress: Mechanisms and Pathophysiology
2.1. Endogenous Sources
| Reactive Species/Antioxidants | Primary Production Source (Subcellular Location/Enzyme System) | Reaction/Mechanism | References |
|---|---|---|---|
| Superoxide (O2•−) | Mitochondrial electron transport chain (Complexes I & III), NADPH oxidases (NOX family), xanthine oxidase, and uncoupled NOS. | One-electron reduction of O2 → O2−; rapidly dismutates by SOD to H2O2. | [19,20] |
| Hydrogen peroxide (H2O2) | Product of SOD-mediated dismutation (cytosol/mitochondria), peroxisomal oxidases, and some NOX activity. | Two-electron product (dismutation or direct 2-e− reduction of O2); diffusible signaling oxidant; detoxified by catalase and glutathione peroxidases. | [21,22] |
| Hydroxyl radical (•OH) | Generated locally from H2O2 via iron-mediated Fenton/Haber–Weiss chemistry (labile Fe2+ pools). | H2O2 + Fe2+ → •OH + OH− (Fenton); extremely reactive and non-selective. | [23,24] |
| Nitric oxide (NO) | Nitric oxide synthases (eNOS, iNOS, nNOS)—cytosolic/membrane-associated. | Radical gasotransmitter; reacts rapidly with O2•− to form peroxynitrite (ONOO−). | [25] |
| Peroxynitrite (ONOO−/ONOOH) | Formed by diffusion-limited reaction between NO• and O2•− in cytosol/near membranes. | Potent oxidant/nitrating species; yields secondary radicals (NO2, CO3•−) and modifies proteins/lipids. | [26,27] |
| Lipid peroxyl radical (LOO)/lipid hydroperoxides (LOOH) | Initiated when ROS attack polyunsaturated fatty acids in membranes or lipoproteins (e.g., LDL)—membrane/LDL surface. | Radical chain-propagation (L• → + O2 → LOO → abstracts H → LOOH); leads to reactive aldehydes (MDA, 4-HNE) and ox-LDL formation. | [28,29] |
| Antioxidant enzymes (SOD, Catalase, GPX) | SOD1 (cytosol), SOD2 (mitochondria), catalase (peroxisomes), GPXs (cytosol/mitochondria). | SOD: 2 O2•− + 2 H+ → H2O2 + O2. Catalase/GPX: H2O2 → H2O (via 2 e− reduction or catalase decomposition). | [30,31] |
2.2. Exogenous Sources
2.3. Impact of Oxidative Stress on Cellular Structures and Functions
2.4. Endogenous Antioxidant Defense Systems (Enzymatic and Non-Enzymatic)
2.5. Role of Antioxidants in Organ Protection
3. Inflammation and Its Crosstalk with Oxidative Stress
4. The NRF2–Keap1 Pathway and Its Cross-Talk with Inflammatory Signaling
5. Types and Mechanisms of Antioxidants
5.1. Nutraceuticals
5.1.1. Antioxidant Nutraceuticals
5.1.2. Ascorbic Acid (Vitamin C)
5.1.3. Tocopherols (Vitamin E)
5.1.4. Carotenoids
5.1.5. Phenolics and Flavonoids
6. Fatty Acids as Modulators of Antioxidant and Anti-Inflammatory Pathways
7. Synthetic and Pharmacological Antioxidants: Mechanisms of Action
8. Anti-Inflammatory Agents with Antioxidant Properties
9. Clinical Applications and Evidence of Antioxidants and Anti-Inflammatory Agents
10. Challenges and Limitations
11. Reconciling Discrepancies Between Preclinical and Clinical Outcomes in Oxidative Stress Modulation
12. Emerging Strategies and Future Directions
12.1. Combination Therapies: Synergistic Targeting of Oxidative Stress and Inflammation
12.2. Targeted Delivery Systems: Nanotechnology-Driven Precision in Antioxidant and Anti-Inflammatory Therapy
12.3. Personalized Medicine and Biomarkers: Toward Precision Redox Therapy
12.4. Gut Microbiome as a Modulator of Oxidative Stress and Inflammation
12.5. Synthetic Derivatives of Antioxidants: Advances and Therapeutic Potential
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, Y.; Li, L.; Wang, Y.; Fan, W. Association of dietary inflammatory index and oxidative balance score with all-cause and cardiovascular mortality in US non-diabetic adults. Front. Nutr. 2025, 12, 1607162. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Valko, R.; Fresser, L.; Nepovimova, E.; Kuca, K.; Valko, M. Interplay of oxidative stress and antioxidant mechanisms in cancer development and progression. Arch. Toxicol. 2025. [Google Scholar] [CrossRef]
- Sharma, V.; Sharma, P.; Singh, T.G. Modulation of nitric oxide signaling by flavonoids: Implications for neurodegeneration. Mol. Biol. Rep. 2025, 52, 894. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef] [PubMed]
- Kim, K. Glutathione in the Nervous System as a Potential Therapeutic Target to Control the Development and Progression of Amyotrophic Lateral Sclerosis. Antioxidants 2021, 10, 1011. [Google Scholar] [CrossRef]
- Kumar, S.; Saxena, J.; Srivastava, V.K.; Kaushik, S.; Singh, H.; Abo-El-Sooud, K.; Abdel-Daim, M.M.; Jyoti, A.; Saluja, R. The Interplay of Oxidative Stress and ROS Scavenging: Antioxidants as a Therapeutic Potential in Sepsis. Vaccines 2022, 10, 1575. [Google Scholar] [CrossRef]
- Amin, F.; Bano, B. Damage of cystatin due to ROS-generation and radical-scavenging activity of antioxidants and associated compounds. Int. J. Biol. Macromol. 2018, 119, 369–379. [Google Scholar] [CrossRef]
- Razali, N.A.; Nazarudin, N.A.; Lai, K.S.; Abas, F.; Ahmad, S. Curcumin derivative, 2,6-bis(2-fluorobenzylidene)cyclohexanone (MS65) inhibits interleukin-6 production through suppression of NF-kappaB and MAPK pathways in histamine-induced human keratinocytes cell (HaCaT). BMC Complement. Altern. Med. 2018, 18, 217. [Google Scholar] [CrossRef]
- Kang, O.H.; Jang, H.J.; Chae, H.S.; Oh, Y.C.; Choi, J.G.; Lee, Y.S.; Kim, J.H.; Kim, Y.C.; Sohn, D.H.; Park, H.; et al. Anti-inflammatory mechanisms of resveratrol in activated HMC-1 cells: Pivotal roles of NF-kappaB and MAPK. Pharmacol. Res. 2009, 59, 330–337. [Google Scholar] [CrossRef]
- Dama, A.; Shpati, K.; Daliu, P.; Dumur, S.; Gorica, E.; Santini, A. Targeting Metabolic Diseases: The Role of Nutraceuticals in Modulating Oxidative Stress and Inflammation. Nutrients 2024, 16, 507. [Google Scholar] [CrossRef]
- Mohammadi, N.; Farrell, M.; O’Sullivan, L.; Langan, A.; Franchin, M.; Azevedo, L.; Granato, D. Effectiveness of anthocyanin-containing foods and nutraceuticals in mitigating oxidative stress, inflammation, and cardiovascular health-related biomarkers: A systematic review of animal and human interventions. Food Funct. 2024, 15, 3274–3299. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Geicu, O.I.; Bilteanu, L.; Serban, A.I. Antioxidant, anti-inflammatory and immunomodulatory roles of vitamins in COVID-19 therapy. Eur. J. Med. Chem. 2022, 232, 114175. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Juan, C.A.; Perez de la Lastra, J.M.; Plou, F.J.; Perez-Lebena, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Saputra, F.; Kishida, M.; Hu, S.Y. Oxidative stress induced by hydrogen peroxide disrupts zebrafish visual development by altering apoptosis, antioxidant and estrogen related genes. Sci. Rep. 2024, 14, 14454. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, A.; Kozlov, A.V. Biological Activities of Reactive Oxygen and Nitrogen Species: Oxidative Stress versus Signal Transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D. The sites and topology of mitochondrial superoxide production. Exp. Gerontol. 2010, 45, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Veal, E.A.; Day, A.M.; Morgan, B.A. Hydrogen peroxide sensing and signaling. Mol. Cell 2007, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G. Cell signaling. H2O2, a necessary evil for cell signaling. Science 2006, 312, 1882–1883. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Oxygen free radicals and iron in relation to biology and medicine: Some problems and concepts. Arch. Biochem. Biophys. 1986, 246, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. The biological chemistry of hydrogen peroxide. Methods Enzymol. 2013, 528, 3–25. [Google Scholar] [CrossRef]
- Forstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Radi, R. Peroxynitrite, a stealthy biological oxidant. J. Biol. Chem. 2013, 288, 26464–26472. [Google Scholar] [CrossRef]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef]
- Ayala, A.; Munoz, M.F.; Arguelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Brigelius-Flohe, R. Glutathione peroxidases and redox-regulated transcription factors. Biol. Chem. 2006, 387, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Lee, S.W.; Yoon, J.H.; Park, Y.G.; Choi, Y.J.; Nam, S.W.; Lee, J.Y.; Wang, Y.P.; Park, W.S. Association of SOD1 and SOD2 single nucleotide polymorphisms with susceptibility to gastric cancer in a Korean population. APMIS 2013, 121, 246–256. [Google Scholar] [CrossRef]
- Staerck, C.; Gastebois, A.; Vandeputte, P.; Calenda, A.; Larcher, G.; Gillmann, L.; Papon, N.; Bouchara, J.P.; Fleury, M.J.J. Microbial antioxidant defense enzymes. Microb. Pathog. 2017, 110, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Pendurthi, U.R.; Rao, L.V.M. The lipid peroxidation product 4-hydroxy-2-nonenal induces tissue factor decryption via ROS generation and the thioredoxin system. Blood Adv. 2017, 1, 2399–2413. [Google Scholar] [CrossRef]
- Khanam, H.; Ali, A.; Asif, M.; Shamsuzzaman. Neurodegenerative diseases linked to misfolded proteins and their therapeutic approaches: A review. Eur. J. Med. Chem. 2016, 124, 1121–1141. [Google Scholar] [CrossRef]
- Paulis, G. Inflammatory mechanisms and oxidative stress in prostatitis: The possible role of antioxidant therapy. Res. Rep. Urol. 2018, 10, 75–87. [Google Scholar] [CrossRef]
- Karin, M.; Takahashi, T.; Kapahi, P.; Delhase, M.; Chen, Y.; Makris, C.; Rothwarf, D.; Baud, V.; Natoli, G.; Guido, F.; et al. Oxidative stress and gene expression: The AP-1 and NF-kappaB connections. Biofactors 2001, 15, 87–89. [Google Scholar] [CrossRef]
- Stojanovic, B.; Jovanovic, I.; Dimitrijevic Stojanovic, M.; Stojanovic, B.S.; Kovacevic, V.; Radosavljevic, I.; Jovanovic, D.; Miletic Kovacevic, M.; Zornic, N.; Arsic, A.A.; et al. Oxidative Stress-Driven Cellular Senescence: Mechanistic Crosstalk and Therapeutic Horizons. Antioxidants 2025, 14, 987. [Google Scholar] [CrossRef]
- Yan, Z.; Chen, Q.; Xia, Y. Oxidative Stress Contributes to Inflammatory and Cellular Damage in Systemic Lupus Erythematosus: Cellular Markers and Molecular Mechanism. J. Inflamm. Res. 2023, 16, 453–465. [Google Scholar] [CrossRef]
- Silva, B.R.; Silva, J.R.V. Mechanisms of action of non-enzymatic antioxidants to control oxidative stress during in vitro follicle growth, oocyte maturation, and embryo development. Anim. Reprod. Sci. 2023, 249, 107186. [Google Scholar] [CrossRef] [PubMed]
- El Assar, M.; Angulo, J.; Vallejo, S.; Peiro, C.; Sanchez-Ferrer, C.F.; Rodriguez-Manas, L. Mechanisms involved in the aging-induced vascular dysfunction. Front. Physiol. 2012, 3, 132. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, S. Targeting oxidative stress for the treatment of ischemic stroke: Upstream and downstream therapeutic strategies. Brain Circ. 2016, 2, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Li, K.; Chen, Y.; Xie, J.; Wu, C.; Cui, C.; Deng, B. Oxidative Stress in Diabetic Peripheral Neuropathy: Pathway and Mechanism-Based Treatment. Mol. Neurobiol. 2023, 60, 4574–4594. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Rodrigo, R.; Prieto, J.C.; Aguayo, R.; Ramos, C.; Puentes, A.; Gajardo, A.; Panieri, E.; Rojas-Sole, C.; Lillo-Moya, J.; Saso, L. Joint Cardioprotective Effect of Vitamin C and Other Antioxidants against Reperfusion Injury in Patients with Acute Myocardial Infarction Undergoing Percutaneous Coronary Intervention. Molecules 2021, 26, 5702. [Google Scholar] [CrossRef]
- Yim, S.; Malhotra, A.; Veves, A. Antioxidants and CVD in diabetes: Where do we stand now. Curr. Diab Rep. 2007, 7, 8–13. [Google Scholar] [CrossRef][Green Version]
- Almeida, S.; Alves, M.G.; Sousa, M.; Oliveira, P.F.; Silva, B.M. Are Polyphenols Strong Dietary Agents Against Neurotoxicity and Neurodegeneration? Neurotox. Res. 2016, 30, 345–366. [Google Scholar] [CrossRef]
- Verma, V.K.; Ramesh, V.; Tewari, S.; Gupta, R.K.; Sinha, N.; Pandey, C.M. Role of bilirubin, vitamin C and ceruloplasmin as antioxidants in coronary artery disease [CAD]. Indian. J. Clin. Biochem. 2005, 20, 68–74. [Google Scholar] [CrossRef][Green Version]
- Shaikh, Z.A.; Vu, T.T.; Zaman, K. Oxidative stress as a mechanism of chronic cadmium-induced hepatotoxicity and renal toxicity and protection by antioxidants. Toxicol. Appl. Pharmacol. 1999, 154, 256–263. [Google Scholar] [CrossRef]
- Baradaran, A.; Nasri, H.; Rafieian-Kopaei, M. Protection of renal tubular cells by antioxidants: Current knowledge and new trends. Cell J. 2015, 16, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Perez-Torres, I.; Castrejon-Tellez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef]
- D’Souza, L.C.; Kuriakose, N.; Raghu, S.V.; Kabekkodu, S.P.; Sharma, A. ROS-directed activation of Toll/NF-kappaB in the hematopoietic niche triggers benzene-induced emergency hematopoiesis. Free Radic. Biol. Med. 2022, 193, 190–201. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Kojima, R.; Ito, M. Influence of aging on gastric ulcer healing activities of the antioxidants alpha-tocopherol and probucol. Eur. J. Pharmacol. 2008, 601, 143–147. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, H.; Yu, B.; He, J.; Mao, X.; Yu, J.; Zheng, P.; Huang, Z.; Luo, Y.; Luo, J.; et al. Protective Effects of Natural Antioxidants on Inflammatory Bowel Disease: Thymol and Its Pharmacological Properties. Antioxidants 2022, 11, 1947. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, M.; Leal-Campanario, R.; Campos-Esparza, M.R.; Sanchez-Gomez, M.V.; Alberdi, E.; Arranz, A.; Delgado-Garcia, J.M.; Gruart, A.; Matute, C. Neuroprotection by two polyphenols following excitotoxicity and experimental ischemia. Neurobiol. Dis. 2006, 23, 374–386. [Google Scholar] [CrossRef]
- Vicente-Zurdo, D.; Gomez-Mejia, E.; Rosales-Conrado, N.; Leon-Gonzalez, M.E. A Comprehensive Analytical Review of Polyphenols: Evaluating Neuroprotection in Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 5906. [Google Scholar] [CrossRef] [PubMed]
- Bakir, S.; Catalkaya, G.; Ceylan, F.D.; Khan, H.; Guldiken, B.; Capanoglu, E.; Kamal, M.A. Role of Dietary Antioxidants in Neurodegenerative Diseases: Where are We Standing? Curr. Pharm. Des. 2020, 26, 714–729. [Google Scholar] [CrossRef]
- Di Matteo, V.; Esposito, E. Biochemical and therapeutic effects of antioxidants in the treatment of Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis. Curr. Drug Targets CNS Neurol. Disord. 2003, 2, 95–107. [Google Scholar] [CrossRef]
- Akhter, N.; Wilson, A.; Thomas, R.; Al-Rashed, F.; Kochumon, S.; Al-Roub, A.; Arefanian, H.; Al-Madhoun, A.; Al-Mulla, F.; Ahmad, R.; et al. ROS/TNF-alpha Crosstalk Triggers the Expression of IL-8 and MCP-1 in Human Monocytic THP-1 Cells via the NF-kappaB and ERK1/2 Mediated Signaling. Int. J. Mol. Sci. 2021, 22, 10519. [Google Scholar] [CrossRef]
- Liu, J.; Han, X.; Zhang, T.; Tian, K.; Li, Z.; Luo, F. Reactive oxygen species (ROS) scavenging biomaterials for anti-inflammatory diseases: From mechanism to therapy. J. Hematol. Oncol. 2023, 16, 116. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Y.; Xiong, Z.; Xie, W.; Shao, M.; Liu, Z. Broad-Spectrum ROS/RNS Scavenging Catalase-Loaded Microreactors for Effective Oral Treatment of Inflammatory Bowel Diseases. Small 2025, 21, e2501341. [Google Scholar] [CrossRef]
- Feng, X.Q.; Deng, A.P.; Wu, Y.Q.; Cai, C.Z.; Ye, X.Q.; Liu, P.F.; Huang, X.J.; Li, Z.J.; Xu, Z.F. Cardiac-specific overexpression of Klotho attenuates paraquat-induced myocardial injury by enhancing the Nrf2/ARE signaling pathway. J. Cardiovasc. Pharmacol. 2025, 86, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Alruhaimi, R.S.; Hassanein, E.H.M.; Alnasser, S.M.; Ahmeda, A.F.; Althagafy, H.S.; Allam, A.M.T.; Qebesy, H.S.; Mahmoud, A.M. Attenuation of NF-kappaB/NLRP3 inflammasome axis and oxidative stress, and upregulation of Nrf2/HO-1 signaling mediate the protective effect of S-carboxymethylcysteine against cyclophosphamide-induced cardiotoxicity. Tissue Cell 2025, 97, 103092. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhou, D.; Yan, B. Eriocitrin alleviates oxidative stress and inflammatory response in cerebral ischemia reperfusion rats by regulating phosphorylation levels of Nrf2/NQO-1/HO-1/NF-kappaB p65 proteins. Ann. Transl. Med. 2020, 8, 757. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Park, J.E.; Park, J.S.; Leem, Y.H.; Kim, D.Y.; Hyun, J.W.; Kim, H.S. Anti-inflammatory and antioxidant mechanisms of coniferaldehyde in lipopolysaccharide-induced neuroinflammation: Involvement of AMPK/Nrf2 and TAK1/MAPK/NF-kappaB signaling pathways. Eur. J. Pharmacol. 2024, 979, 176850. [Google Scholar] [CrossRef]
- Rizk, S.K.; Ali, E.A.; Sheref, A.A.M.; Tayel, S.G.; El Derbaly, S.A. Vitamin D and canagliflozin combination alleviates Parkinson’s disease in rats through modulation of RAC1/NF-kappaB/Nrf2 interaction. Immunopharmacol. Immunotoxicol. 2025, 47, 328–344. [Google Scholar] [CrossRef]
- Yoon, Y.E.; Ju, S.H.; Kim, Y.; Lee, S.J. Natural Flavonoids for the Prevention of Sarcopenia: Therapeutic Potential and Mechanisms. Int. J. Mol. Sci. 2025, 26, 7458. [Google Scholar] [CrossRef]
- Cadar, E.; Popescu, A.; Dragan, A.M.; Pesterau, A.M.; Pascale, C.; Anuta, V.; Prasacu, I.; Velescu, B.S.; Tomescu, C.L.; Bogdan-Andreescu, C.F.; et al. Bioactive Compounds of Marine Algae and Their Potential Health and Nutraceutical Applications: A Review. Mar. Drugs 2025, 23, 152. [Google Scholar] [CrossRef]
- Khan, S.; Ali, A.; Khan, S.; Bakillah, A.; Damanhouri, G.; Khan, A.; Makki, A.; AlAnsari, I.; Banu, N. Current therapies in alleviating liver disorders and cancers with a special focus on the potential of vitamin D. Nutr. Metab. 2018, 15, 13. [Google Scholar] [CrossRef]
- Caponio, G.R.; Lippolis, T.; Tutino, V.; Gigante, I.; De Nunzio, V.; Milella, R.A.; Gasparro, M.; Notarnicola, M. Nutraceuticals: Focus on Anti-Inflammatory, Anti-Cancer, Antioxidant Properties in Gastrointestinal Tract. Antioxidants 2022, 11, 1274. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; D’Angelo, A.; Maffioli, P. The role of selected nutraceuticals in management of prediabetes and diabetes: An updated review of the literature. Phytother. Res. 2022, 36, 3709–3765. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Dewapriya, P. Bioactive compounds from marine sponges and their symbiotic microbes: A potential source of nutraceuticals. Adv. Food Nutr. Res. 2012, 65, 137–151. [Google Scholar] [CrossRef]
- Kumari, A.; Garima; Bharadvaja, N. A comprehensive review on algal nutraceuticals as prospective therapeutic agent for different diseases. 3 Biotech 2023, 13, 44. [Google Scholar] [CrossRef]
- Garza-Juarez, A.; Perez-Carrillo, E.; Arredondo-Espinoza, E.U.; Islas, J.F.; Benitez-Chao, D.F.; Escamilla-Garcia, E. Nutraceuticals and Their Contribution to Preventing Noncommunicable Diseases. Foods 2023, 12, 3262. [Google Scholar] [CrossRef]
- Kocsis, A.E.; Kucsapszky, N.; Santa-Maria, A.R.; Hunyadi, A.; Deli, M.A.; Walter, F.R. Much More than Nutrients: The Protective Effects of Nutraceuticals on the Blood-Brain Barrier in Diseases. Nutrients 2025, 17, 766. [Google Scholar] [CrossRef]
- Basegmez, M.; Eryavuz, A.; Demirel, H.H. Effects of Vitamin C Supplementation on Total Antioxidant Status, Inflammation, and Histopathological Changes in Aged Rats. J. Biochem. Mol. Toxicol. 2025, 39, e70324. [Google Scholar] [CrossRef]
- Sasidharan Nair, V.; Huehn, J. Impact of vitamin C on the development, differentiation and functional properties of T cells. Eur. J. Microbiol. Immunol. 2024, 14, 67–74. [Google Scholar] [CrossRef]
- Zhang, P.; Zang, M.; Sang, Z.; Wei, Y.; Yan, Y.; Bian, X.; Dong, S. Vitamin C alleviates LPS-induced myocardial injury by inhibiting pyroptosis via the ROS-AKT/mTOR signalling pathway. BMC Cardiovasc. Disord. 2022, 22, 561. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, K.; Venkataraman, G.; Parida, A. An oxidative and salinity stress induced peroxisomal ascorbate peroxidase from Avicennia marina: Molecular and functional characterization. Plant Physiol. Biochem. 2008, 46, 794–804. [Google Scholar] [CrossRef]
- Vineetha, R.C.; Hariharan, S.; Jaleel, A.; Chandran, M.; Nair, R.H. L-Ascorbic Acid and alpha-Tocopherol Synergistically Triggers Apoptosis Inducing Antileukemic Effects of Arsenic Trioxide via Oxidative Stress in Human Acute Promyelocytic Leukemia Cells. Front. Oncol. 2020, 10, 65. [Google Scholar] [CrossRef]
- Kontush, A.; Finckh, B.; Karten, B.; Kohlschutter, A.; Beisiegel, U. Antioxidant and prooxidant activity of alpha-tocopherol in human plasma and low density lipoprotein. J. Lipid Res. 1996, 37, 1436–1448. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Bohn, T. Carotenoids and Markers of Oxidative Stress in Human Observational Studies and Intervention Trials: Implications for Chronic Diseases. Antioxidants 2019, 8, 179. [Google Scholar] [CrossRef]
- Mordi, R.C.; Ademosun, O.T.; Ajanaku, C.O.; Olanrewaju, I.O.; Walton, J.C. Free Radical Mediated Oxidative Degradation of Carotenes and Xanthophylls. Molecules 2020, 25, 1038. [Google Scholar] [CrossRef] [PubMed]
- Cantin, L.; Daignault-Gelinas, M.; Latreille, J.; Bhat, P.V.; Lacroix, A. [Retinol, carotenes and nutritional status in metastatic breast cancer]. Union. Med. Can. 1988, 117, 29–36. [Google Scholar] [PubMed]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Futur. J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef]
- Kuljarusnont, S.; Iwakami, S.; Iwashina, T.; Tungmunnithum, D. Flavonoids and Other Phenolic Compounds for Physiological Roles, Plant Species Delimitation, and Medical Benefits: A Promising View. Molecules 2024, 29, 5351. [Google Scholar] [CrossRef]
- Aiello, A.; Medoro, A.; Accardi, G.; Calabro, A.; Carru, C.; Cannavo, A.; Caruso, C.; Candore, G.; Scapagnini, G.; Corbi, G.; et al. Polyunsaturated fatty acid status and markers of oxidative stress and inflammation across the lifespan: A cross-sectional study in a cohort with long-lived individuals. Exp. Gerontol. 2024, 195, 112531. [Google Scholar] [CrossRef]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Chaudhary, A.; Sethi, S. Oxidized omega-3 fatty acids inhibit NF-kappaB activation via a PPARalpha-dependent pathway. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1621–1627. [Google Scholar] [CrossRef]
- Lee, C. Collaborative Power of Nrf2 and PPARgamma Activators against Metabolic and Drug-Induced Oxidative Injury. Oxid. Med. Cell Longev. 2017, 2017, 1378175. [Google Scholar] [CrossRef]
- Szukiewicz, D. Potential Therapeutic Exploitation of G Protein-Coupled Receptor 120 (GPR120/FFAR4) Signaling in Obesity-Related Metabolic Disorders. Int. J. Mol. Sci. 2025, 26, 2501. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef]
- Shilovsky, G.A.; Dibrova, D.V. Regulation of Cell Proliferation and Nrf2-Mediated Antioxidant Defense: Conservation of Keap1 Cysteines and Nrf2 Binding Site in the Context of the Evolution of KLHL Family. Life 2023, 13, 1045. [Google Scholar] [CrossRef] [PubMed]
- Norris, P.C.; Skulas-Ray, A.C.; Riley, I.; Richter, C.K.; Kris-Etherton, P.M.; Jensen, G.L.; Serhan, C.N.; Maddipati, K.R. Identification of specialized pro-resolving mediator clusters from healthy adults after intravenous low-dose endotoxin and omega-3 supplementation: A methodological validation. Sci. Rep. 2018, 8, 18050. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Tokgozoglu, L.; Parhofer, K.G.; Handelsman, Y.; Leiter, L.A.; Landmesser, U.; Brinton, E.A.; Catapano, A.L. Icosapent ethyl for reduction of persistent cardiovascular risk: A critical review of major medical society guidelines and statements. Expert. Rev. Cardiovasc. Ther. 2022, 20, 609–625. [Google Scholar] [CrossRef]
- Huston, J.; Schaffner, H.; Cox, A.; Sperry, A.; McGee, S.; Lor, P.; Langley, L.; Skrable, B.; Ashchi, M.; Bisharat, M.; et al. A Critical Review of Icosapent Ethyl in Cardiovascular Risk Reduction. Am. J. Cardiovasc. Drugs 2023, 23, 393–406. [Google Scholar] [CrossRef]
- Zhang, W.; Gan, D.; Huo, S.; Chen, P. Unraveling the discrepancies between REDUCE-IT and STRENGTH trials with omega-3 fatty acids: New analytical approaches. Front. Nutr. 2024, 11, 1490953. [Google Scholar] [CrossRef] [PubMed]
- Tauchen, J.; Huml, L.; Jurasek, M.; Regenstein, J.M.; Ozogul, F. Synthetic and semi-synthetic antioxidants in medicine and food industry: A review. Front. Pharmacol. 2025, 16, 1599816. [Google Scholar] [CrossRef]
- Wilcox, C.S. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol. Ther. 2010, 126, 119–145. [Google Scholar] [CrossRef]
- Pant, T.; Uche, N.; Juric, M.; Zielonka, J.; Bai, X. Regulation of immunomodulatory networks by Nrf2-activation in immune cells: Redox control and therapeutic potential in inflammatory diseases. Redox Biol. 2024, 70, 103077. [Google Scholar] [CrossRef] [PubMed]
- Mucha, P.; Skoczynska, A.; Malecka, M.; Hikisz, P.; Budzisz, E. Overview of the Antioxidant and Anti-Inflammatory Activities of Selected Plant Compounds and Their Metal Ions Complexes. Molecules 2021, 26, 4886. [Google Scholar] [CrossRef] [PubMed]
- Shahcheraghi, S.H.; Salemi, F.; Peirovi, N.; Ayatollahi, J.; Alam, W.; Khan, H.; Saso, L. Nrf2 Regulation by Curcumin: Molecular Aspects for Therapeutic Prospects. Molecules 2021, 27, 167. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, T.; Menon, S.N.; Pandey, A.; Siddiqua, S.; Kuddus, S.A.; Rahman, M.M.; Khan, F.; Hoque, N.; Rana, M.S.; Subhan, N.; et al. Resveratrol attenuates hepatic oxidative stress and preserves gut mucosal integrity in high-fat diet-fed rats by modulating antioxidant and anti-inflammatory pathways. Sci. Rep. 2025, 15, 25162. [Google Scholar] [CrossRef]
- Truong, V.L.; Jun, M.; Jeong, W.S. Role of resveratrol in regulation of cellular defense systems against oxidative stress. Biofactors 2018, 44, 36–49. [Google Scholar] [CrossRef]
- Malaguarnera, L. Influence of Resveratrol on the Immune Response. Nutrients 2019, 11, 946. [Google Scholar] [CrossRef]
- Cho, S.Y.; Park, S.J.; Kwon, M.J.; Jeong, T.S.; Bok, S.H.; Choi, W.Y.; Jeong, W.I.; Ryu, S.Y.; Do, S.H.; Lee, C.S.; et al. Quercetin suppresses proinflammatory cytokines production through MAP kinases andNF-kappaB pathway in lipopolysaccharide-stimulated macrophage. Mol. Cell Biochem. 2003, 243, 153–160. [Google Scholar] [CrossRef]
- Cheng, S.C.; Huang, W.C.; JH, S.P.; Wu, Y.H.; Cheng, C.Y. Quercetin Inhibits the Production of IL-1beta-Induced Inflammatory Cytokines and Chemokines in ARPE-19 Cells via the MAPK and NF-kappaB Signaling Pathways. Int. J. Mol. Sci. 2019, 20, 2957. [Google Scholar] [CrossRef]
- Wang, P.; Li, Z.; Song, Y.; Zhang, B.; Fan, C. Resveratrol-driven macrophage polarization: Unveiling mechanisms and therapeutic potential. Front. Pharmacol. 2024, 15, 1516609. [Google Scholar] [CrossRef]
- Zhao, C.; Ding, Y.; Huang, Y.; Wang, C.; Guo, B.; Zhang, T. Quercetin Attenuates MRGPRX2-Mediated Mast Cell Degranulation via the MyD88/IKK/NF-kappaB and PI3K/AKT/ Rac1/Cdc42 Pathway. J. Inflamm. Res. 2024, 17, 7099–7110. [Google Scholar] [CrossRef]
- Ahmadabady, S.; Beheshti, F.; Shahidpour, F.; Khordad, E.; Hosseini, M. A protective effect of curcumin on cardiovascular oxidative stress indicators in systemic inflammation induced by lipopolysaccharide in rats. Biochem. Biophys. Rep. 2021, 25, 100908. [Google Scholar] [CrossRef]
- Zalpoor, H.; Nabi-Afjadi, M.; Forghaniesfidvajani, R.; Tavakol, C.; Farahighasreaboonasr, F.; Pakizeh, F.; Dana, V.G.; Seif, F. Quercetin as a JAK-STAT inhibitor: A potential role in solid tumors and neurodegenerative diseases. Cell Mol. Biol. Lett. 2022, 27, 60. [Google Scholar] [CrossRef] [PubMed]
- Serban, M.C.; Sahebkar, A.; Zanchetti, A.; Mikhailidis, D.P.; Howard, G.; Antal, D.; Andrica, F.; Ahmed, A.; Aronow, W.S.; Muntner, P.; et al. Effects of Quercetin on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2016, 5, e002713. [Google Scholar] [CrossRef]
- Nishida, Y.; Berg, P.C.; Shakersain, B.; Hecht, K.; Takikawa, A.; Tao, R.; Kakuta, Y.; Uragami, C.; Hashimoto, H.; Misawa, N.; et al. Astaxanthin: Past, Present, and Future. Mar. Drugs 2023, 21, 514. [Google Scholar] [CrossRef]
- Cesar, T.B.; Ramos, F.M.M.; Ribeiro, C.B. Nutraceutical Eriocitrin (Eriomin) Reduces Hyperglycemia by Increasing Glucagon-Like Peptide 1 and Downregulates Systemic Inflammation: A Crossover-Randomized Clinical Trial. J. Med. Food 2022, 25, 1050–1058. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Smeitink, J.; van Es, J.; Bosman, B.; Janssen, M.C.H.; Klopstock, T.; Gorman, G.; Vissing, J.; Ruiterkamp, G.; Edgar, C.J.; Abbink, E.J.; et al. Phase 2b program with sonlicromanol in patients with mitochondrial disease due to m.3243A>G mutation. Brain 2025, 148, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Oh, Y.H.; Lim, G.H.; An, J.H.; Lee, J.H.; Gwag, B.J.; Won, S.J.; Seo, K.W.; Youn, H.Y. Crisdesalazine alleviates inflammation in an experimental autoimmune encephalomyelitis multiple sclerosis mouse model by regulating the immune system. BMC Neurosci. 2025, 26, 1. [Google Scholar] [CrossRef]
- Tousson, E.; Beltagy, D.M.; Nawar, N.F.; Dora, M.A.; Bari, H.M.A.; El-Sayed, I.E.T. Curcumin nanoparticles ameliorates cardiac toxicity through modulation of oxidative stress, apoptosis, inflammation, and DNA damage in rat. Toxicol. Res. 2025, 14, tfaf112. [Google Scholar] [CrossRef]
- Yeo, S.; Kim, M.J.; Shim, Y.K.; Yoon, I.; Lee, W.K. Solid Lipid Nanoparticles of Curcumin Designed for Enhanced Bioavailability and Anticancer Efficiency. ACS Omega 2022, 7, 35875–35884. [Google Scholar] [CrossRef]
- Zhao, W.; Zeng, M.; Li, K.; Pi, C.; Liu, Z.; Zhan, C.; Yuan, J.; Su, Z.; Wei, Y.; Wen, J.; et al. Solid lipid nanoparticle as an effective drug delivery system of a novel curcumin derivative: Formulation, release in vitro and pharmacokinetics in vivo. Pharm. Biol. 2022, 60, 2300–2307. [Google Scholar] [CrossRef]
- Markovic, M.; Ben-Shabat, S.; Dahan, A. Prodrugs for Improved Drug Delivery: Lessons Learned from Recently Developed and Marketed Products. Pharmaceutics 2020, 12, 1031. [Google Scholar] [CrossRef]
- Bertoncini-Silva, C.; Vlad, A.; Ricciarelli, R.; Giacomo Fassini, P.; Suen, V.M.M.; Zingg, J.M. Enhancing the Bioavailability and Bioactivity of Curcumin for Disease Prevention and Treatment. Antioxidants 2024, 13, 331. [Google Scholar] [CrossRef]
- Zhao, M.; Ma, J.; Li, M.; Zhang, Y.; Jiang, B.; Zhao, X.; Huai, C.; Shen, L.; Zhang, N.; He, L.; et al. Cytochrome P450 Enzymes and Drug Metabolism in Humans. Int. J. Mol. Sci. 2021, 22, 12808. [Google Scholar] [CrossRef]
- Martin, D.A.; Bolling, B.W. A review of the efficacy of dietary polyphenols in experimental models of inflammatory bowel diseases. Food Funct. 2015, 6, 1773–1786. [Google Scholar] [CrossRef] [PubMed]
- Taslim, N.A.; Yusuf, M.; Ambari, A.M.; Del Rosario Puling, I.M.; Ibrahim, F.Z.; Hardinsyah, H.; Kurniawan, R.; Gunawan, W.B.; Mayulu, N.; Joseph, V.F.F.; et al. Anti-Inflammatory, Antioxidant, Metabolic and Gut Microbiota Modulation Activities of Probiotic in Cardiac Remodeling Condition: Evidence from Systematic Study and Meta-Analysis of Randomized Controlled Trials. Probiotics Antimicrob. Proteins 2023, 15, 1049–1061. [Google Scholar] [CrossRef]
- Jalouli, M.; Rahman, M.A.; Biswas, P.; Rahman, H.; Harrath, A.H.; Lee, I.S.; Kang, S.; Choi, J.; Park, M.N.; Kim, B. Targeting natural antioxidant polyphenols to protect neuroinflammation and neurodegenerative diseases: A comprehensive review. Front. Pharmacol. 2025, 16, 1492517. [Google Scholar] [CrossRef]
- Al-Madhagi, H.; Masoud, A. Limitations and Challenges of Antioxidant Therapy. Phytother. Res. 2024, 38, 5549–5566. [Google Scholar] [CrossRef] [PubMed]
- Ohlow, M.J.; Sohre, S.; Granold, M.; Schreckenberger, M.; Moosmann, B. Why Have Clinical Trials of Antioxidants to Prevent Neurodegeneration Failed?—A Cellular Investigation of Novel Phenothiazine-Type Antioxidants Reveals Competing Objectives for Pharmaceutical Neuroprotection. Pharm. Res. 2017, 34, 378–393. [Google Scholar] [CrossRef] [PubMed]
- Steinhubl, S.R. Why have antioxidants failed in clinical trials? Am. J. Cardiol. 2008, 101, 14D–19D. [Google Scholar] [CrossRef] [PubMed]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxid. Med. Cell Longev. 2017, 2017, 6501046. [Google Scholar] [CrossRef] [PubMed]
- Mursaleen, L.; Chan, S.H.Y.; Noble, B.; Somavarapu, S.; Zariwala, M.G. Curcumin and N-Acetylcysteine Nanocarriers Alone or Combined with Deferoxamine Target the Mitochondria and Protect against Neurotoxicity and Oxidative Stress in a Co-Culture Model of Parkinson’s Disease. Antioxidants 2023, 12, 130. [Google Scholar] [CrossRef]
- Kakoti, B.B.; Hernandez-Ontiveros, D.G.; Kataki, M.S.; Shah, K.; Pathak, Y.; Panguluri, S.K. Resveratrol and Omega-3 Fatty Acid: Its Implications in Cardiovascular Diseases. Front. Cardiovasc. Med. 2015, 2, 38. [Google Scholar] [CrossRef]
- Li, M.; Ding, L.; Cao, L.; Zhang, Z.; Li, X.; Li, Z.; Xia, Q.; Yin, K.; Song, S.; Wang, Z.; et al. Natural products targeting AMPK signaling pathway therapy, diabetes mellitus and its complications. Front. Pharmacol. 2025, 16, 1534634. [Google Scholar] [CrossRef]
- Wang, C.; Han, Z.; Wu, Y.; Lu, X.; Tang, X.; Xiao, J.; Li, N. Enhancing stability and anti-inflammatory properties of curcumin in ulcerative colitis therapy using liposomes mediated colon-specific drug delivery system. Food Chem. Toxicol. 2021, 151, 112123. [Google Scholar] [CrossRef]
- Baiomy, R.F.E. Quercetin nanoparticles as a therapeutic approach: Pharmacological actions and potential applications in therapy. BioTechnologia 2024, 105, 377–393. [Google Scholar] [CrossRef]
- Chung, J.H.; Lee, J.S.; Lee, H.G. Resveratrol-loaded chitosan-gamma-poly(glutamic acid) nanoparticles: Optimization, solubility, UV stability, and cellular antioxidant activity. Colloids Surf. B Biointerfaces 2020, 186, 110702. [Google Scholar] [CrossRef]
- Cheng, R.; Feng, F.; Meng, F.; Deng, C.; Feijen, J.; Zhong, Z. Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J. Control. Release 2011, 152, 2–12. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Kloska, D.; Grochot-Przeczek, A.; Feelisch, M.; Cuadrado, A.; van Goor, H. Personalized redox medicine in inflammatory bowel diseases: An emerging role for HIF-1alpha and NRF2 as therapeutic targets. Redox Biol. 2023, 60, 102603. [Google Scholar] [CrossRef] [PubMed]
- Moustakli, E.; Grigoriadis, T.; Stavros, S.; Potiris, A.; Zikopoulos, A.; Gerede, A.; Tsimpoukis, I.; Papageorgiou, C.; Louis, K.; Domali, E. Artificial Intelligence in Assessing Reproductive Aging: Role of Mitochondria, Oxidative Stress, and Telomere Biology. Diagnostics 2025, 15, 2075. [Google Scholar] [CrossRef]
- Pokushalov, E.; Ponomarenko, A.; Shrainer, E.; Kudlay, D.; Miller, R. Biomarker-Guided Dietary Supplementation: A Narrative Review of Precision in Personalized Nutrition. Nutrients 2024, 16, 4033. [Google Scholar] [CrossRef]
- Shen, Y.; Fan, N.; Ma, S.X.; Cheng, X.; Yang, X.; Wang, G. Gut Microbiota Dysbiosis: Pathogenesis, Diseases, Prevention, and Therapy. MedComm 2025, 6, e70168. [Google Scholar] [CrossRef]
- Chen, X.; Yan, L.; Yang, J.; Xu, C.; Yang, L. The impact of probiotics on oxidative stress and inflammatory markers in patients with diabetes: A meta-research of meta-analysis studies. Front. Nutr. 2025, 12, 1552358. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Kim, S.S. Probiotics and Prebiotics: Present Status and Future Perspectives on Metabolic Disorders. Nutrients 2016, 8, 173. [Google Scholar] [CrossRef]
- Scapagnini, G.; Caruso, C.; Calabrese, V. Therapeutic potential of dietary polyphenols against brain ageing and neurodegenerative disorders. Adv. Exp. Med. Biol. 2010, 698, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Boicean, A.; Ichim, C.; Sasu, S.M.; Todor, S.B. Key Insights into Gut Alterations in Metabolic Syndrome. J. Clin. Med. 2025, 14, 2678. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Liu, Y.; Shi, M.; Zhang, M.; Zhang, H.; Chen, J. Advances in fecal microbiota transplantation for the treatment of diabetes mellitus. Front. Cell. Infect. Microbiol. 2024, 14, 1370999. [Google Scholar] [CrossRef]
- Zhang, S.; Deng, F.; Chen, J.; Chen, F.; Wu, Z.; Li, L.; Hou, K. Fecal microbiota transplantation treatment of autoimmune-mediated type 1 diabetes: A systematic review. Front. Cell. Infect. Microbiol. 2022, 12, 1075201. [Google Scholar] [CrossRef]
- Sazdova, I.; Keremidarska-Markova, M.; Dimitrova, D.; Mitrokhin, V.; Kamkin, A.; Hadzi-Petrushev, N.; Bogdanov, J.; Schubert, R.; Gagov, H.; Avtanski, D.; et al. Anticarcinogenic Potency of EF24: An Overview of Its Pharmacokinetics, Efficacy, Mechanism of Action, and Nanoformulation for Drug Delivery. Cancers 2023, 15, 5478. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.J.; Li, C.W.; Kuo, C.L.; Shih, T.L.; Chen, J.J. Improved Synthesis of Asymmetric Curcuminoids and Their Assessment as Antioxidants. Molecules 2022, 27, 2547. [Google Scholar] [CrossRef]
- Gambini, J.; Ingles, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid. Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef]
- Xiao, J.; Kai, G.; Yamamoto, K.; Chen, X. Advance in dietary polyphenols as alpha-glucosidases inhibitors: A review on structure-activity relationship aspect. Crit. Rev. Food Sci. Nutr. 2013, 53, 818–836. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Gao, S.; Zhou, L.; Rong, K.; Zuo, F.; Tang, W.; Zhu, L. Trolox derivatives: Synthesis, structure-activity relationship and promote wound healing by regulating oxidative stress and inflammation. Bioorg. Chem. 2025, 154, 108045. [Google Scholar] [CrossRef] [PubMed]
- Alhyari, D.; Qinna, N.A.; Sheldrake, H.M.; Kantamneni, S.; Ghanem, B.Y.; Paluch, K.J. Antioxidant, Anti-Inflammatory, and Oral Bioavailability of Novel Sulfonamide Derivatives of Gallic Acid. Antioxidants 2025, 14, 374. [Google Scholar] [CrossRef] [PubMed]
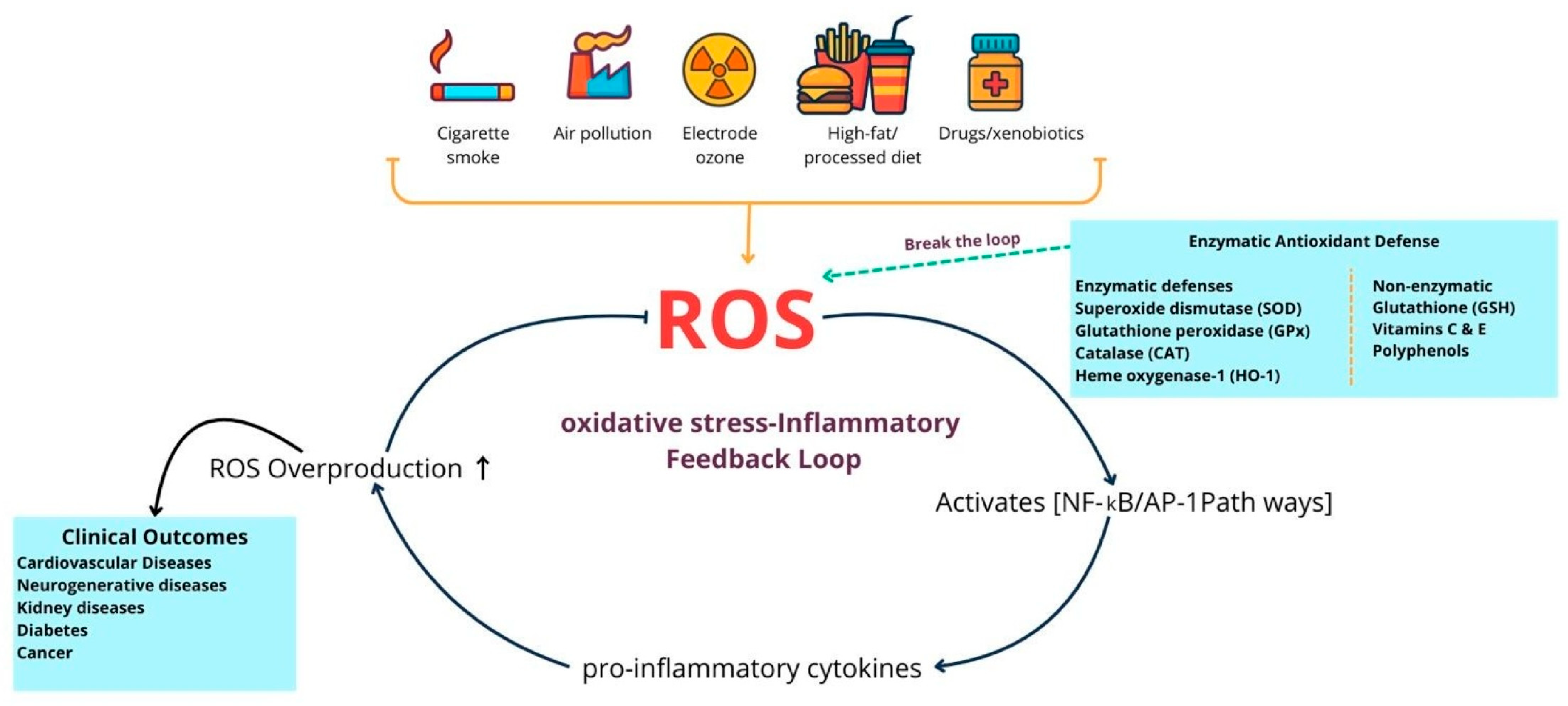
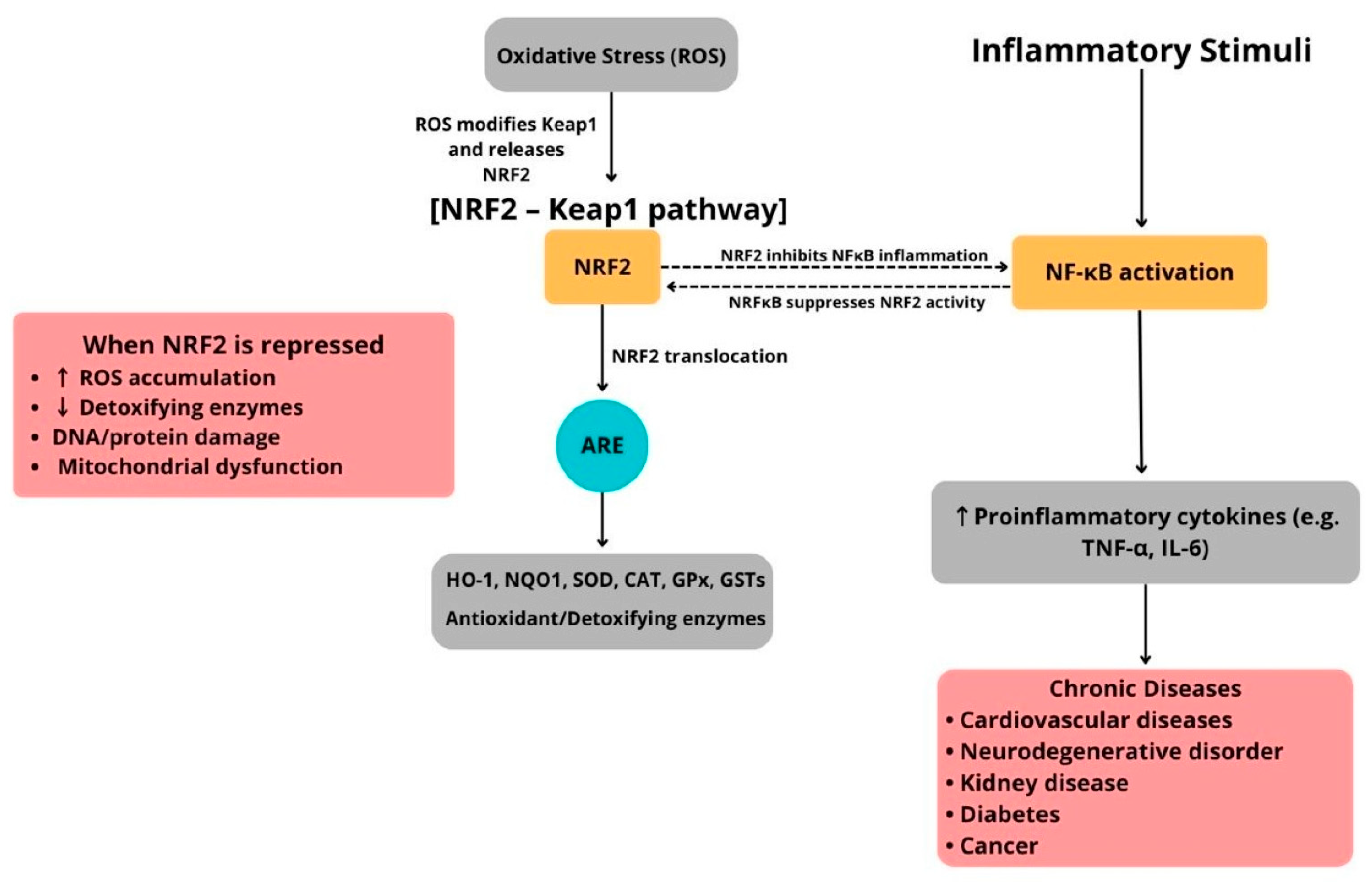
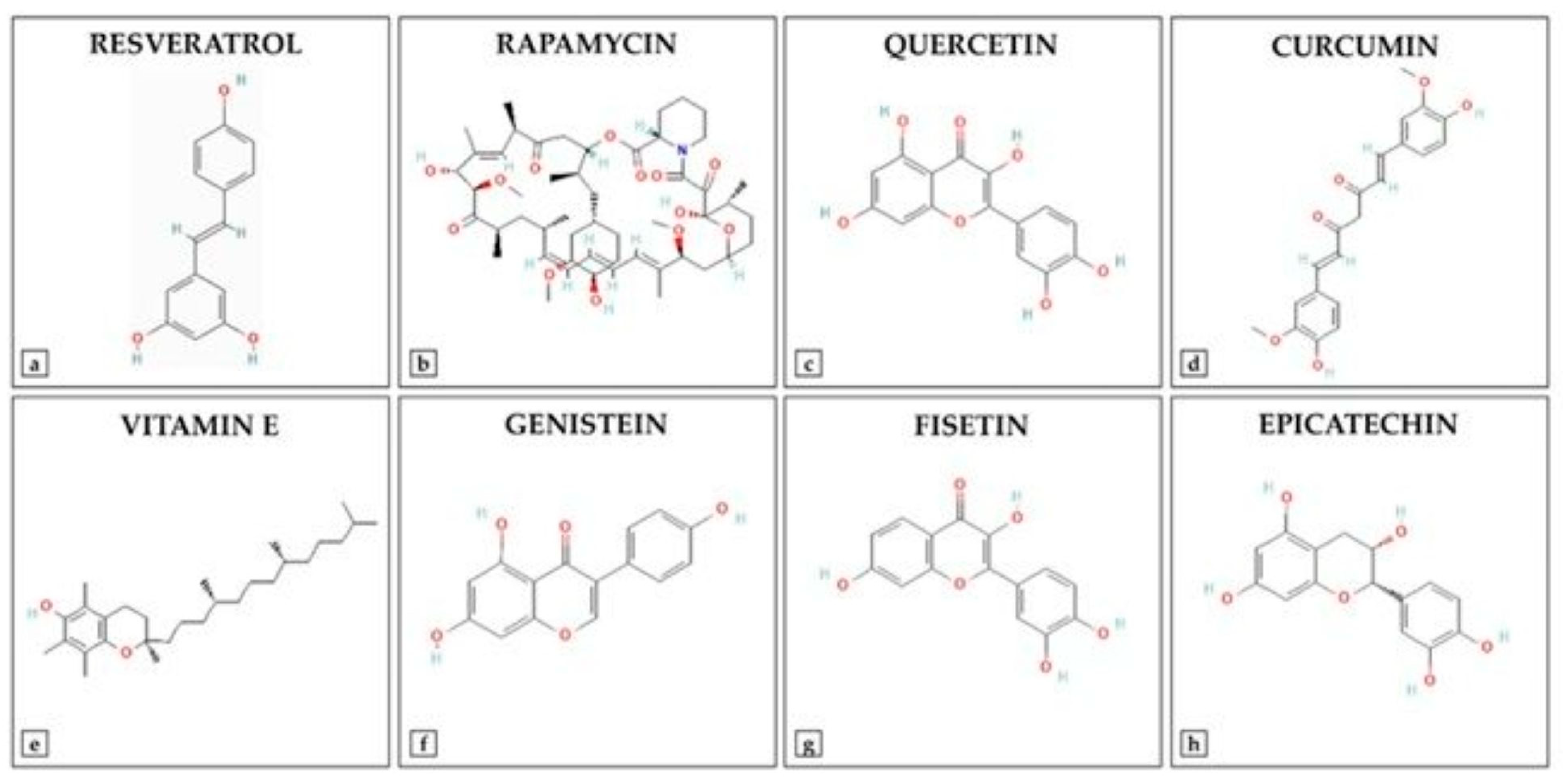
| Category | Enzymatic Antioxidants | Non-Enzymatic Antioxidants |
|---|---|---|
| Definition | Enzymes produced by the body catalytically neutralize reactive oxygen and nitrogen species (ROS/RNS) | Small, naturally occurring molecules that directly interact with and neutralize free radicals |
| Mechanism of Action | Convert ROS/RNS into less harmful substances through stepwise enzymatic reactions | Directly scavenge and deactivate ROS/RNS or regenerate other antioxidant molecules |
| Key Examples | Superoxide dismutase (SOD) Catalase (CAT) Glutathione peroxidase (GPx | Glutathione (GSH) Uric acid Melatonin Alpha-lipoic acid |
| Localization | SOD1: Cytosol SOD2: Mitochondria SOD3: Extracellular CAT: Liver, kidneys, RBCs GPx: Mitochondria and cytosol | GSH: Cytosol and mitochondria Uric acid: Plasma Melatonin: All cellular compartments Lipoic acid: Both intra- and extracellular |
| Distinct Features | Require metal cofactors (e.g., Cu, Zn, Mn, Fe) Function as an integrated antioxidant system | React with multiple ROS types Capable of crossing membranes (e.g., melatonin) Support vitamin regeneration |
| Additional Functions | Decompose hydrogen peroxide Protect proteins, DNA, and lipids from oxidative damage | Regulate circadian rhythm (melatonin) Detoxify peroxynitrite and hydroxyl radicals Maintain redox balance |
| Compound/Drug | Condition/ Population | Phase & Design | Dose | Duration | Key Outcomes | Trial ID | Year |
|---|---|---|---|---|---|---|---|
| Antioxidant cocktail (Vitamins E/C + Tocopherol, Ascorbic Acid, Selenium) | Overweight children | RCT, placebo-controlled | TP,400 IU; 500 mg, SE 500 mg, AA 50 µg daily | 4 months | Reduction of 8-iso-PGF2α; ↑ GPx and ↑ SOD activity | NCT01316081 | 2011 |
| Astaxanthin | Heart failure patients | RCT (protocol stage) | 20 mg of astaxanthin per day | 8 weeks | No results posted | IRCT20200429047235N3 | 2024 |
| Resveratrol | Healthy adult smokers | Phase III, crossover RCT | 500 mg/day | 30 days per arm (3 months) | No results posted | NCT01492114 | 2012 |
| Curcuminoids | Hemodialysis patients | RCT, double-blind, placebo-controlled | 500 mg/8 h | 12 weeks | No posted results yet | NCT06829186 | 2025 |
| Ellagic Acid (polyphenol) | IBS patients | RCT | 180 mg of EA per day | 8 weeks | Antioxidant index improved. ↑ TAC; ↓ MDA | IRCT20141025019669N11 | 2019 |
| L-carnitine + exercise | Overweight/obese adults | RCT | 1 g/day + exercise | 12 weeks | Increase in CAT and SOD; Decrease in ROS, MDA, and IL-6 | NR | NR |
| ω-3 fatty acids | Type 2 diabetes patients | RCT | marine n-3 fatty acids in 100 mL Omegaven (1.25–2.82 g EPA + 1.44–3.09 g DHA) | 9 weeks | Reduced insulin sensitivity and altered proportion of carbohydrate vs. fat oxidation No enzyme/cytokine results posted on the registry | NCT00829569 | 2011 |
| Eriocitrin (Eriomin) | Prediabetic individuals | Crossover RCT | 200 mg/day | 12 weeks | Reduced glycemia, systemic inflammation, and oxidative stress, and increased GLP1 No results for cytokines | NCT03928249 | 2020 |
| Crisdesalazine | Neurodegenerative disorders | Phase I (ongoing) | Not disclosed | NS | Free-radical scavenger; safety & PK data | NR | NR |
| Sonlicromanol (KH176) | Mitochondrial disease patients | Phase II RCT | 50 mg twice daily | 1 month | Improved safety, mood, mitochondrial redox balance | NCT04165239 | 2022 |
| GC-4419 (SOD mimetic) | Head & Neck cancer | Phase I dose escalation | 15–170 mg | NS | No data for plasma SOD/GPx/CAT | NCT01921426 | 2013 |
| AT-001 | Brain oxidative stress | Phase I | NS | 12 weeks | no published enzyme/cytokine panel | NCT01731093 | 2012 |
| Lutein supplementation | Healthy adult nonsmokers | RCT | 200 mg | 12 weeks | No data for SOD/GPx/CAT No IL-6/TNF-α/IL-1β results posted on the registry | NCT01056094 | 2010 |
| Oxytocin | Healthy adult | Phase II | 48 IU intranasal 4×/day (QID) | NS | No results posted for SOD/GPx/CAT No cytokine results posted | NCT04732247 | 2022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altanam, S.Y.; Darwish, N.; Bakillah, A. Exploring the Interplay of Antioxidants, Inflammation, and Oxidative Stress: Mechanisms, Therapeutic Potential, and Clinical Implications. Diseases 2025, 13, 309. https://doi.org/10.3390/diseases13090309
Altanam SY, Darwish N, Bakillah A. Exploring the Interplay of Antioxidants, Inflammation, and Oxidative Stress: Mechanisms, Therapeutic Potential, and Clinical Implications. Diseases. 2025; 13(9):309. https://doi.org/10.3390/diseases13090309
Chicago/Turabian StyleAltanam, Sumayyah Yousef, Nedal Darwish, and Ahmed Bakillah. 2025. "Exploring the Interplay of Antioxidants, Inflammation, and Oxidative Stress: Mechanisms, Therapeutic Potential, and Clinical Implications" Diseases 13, no. 9: 309. https://doi.org/10.3390/diseases13090309
APA StyleAltanam, S. Y., Darwish, N., & Bakillah, A. (2025). Exploring the Interplay of Antioxidants, Inflammation, and Oxidative Stress: Mechanisms, Therapeutic Potential, and Clinical Implications. Diseases, 13(9), 309. https://doi.org/10.3390/diseases13090309







