Abstract
Oxidative stress, resulting from an imbalance between reactive oxygen species (ROS) production and antioxidant defenses, is a major factor in chronic diseases such as cardiovascular disorders, neurodegeneration, diabetes, and cancer. Despite extensive research, current reviews often discuss antioxidants or inflammatory pathways separately, which limits their translational impact. The primary objective of this review is to present an integrated analysis of oxidative stress and inflammation, connecting molecular mechanisms with clinical evidence. We focus on the dual roles of natural and synthetic antioxidants in managing redox balance, regulating inflammatory signaling, and targeting new molecular pathways. Unlike previous work, this review emphasizes recent clinical findings, ongoing therapeutic challenges, and innovative strategies, including combination approaches and synthetic derivatives designed to improve effectiveness. By combining biochemical, preclinical, and clinical perspectives, we highlight both established knowledge and critical gaps. Ultimately, this review highlights the clinical significance of redox biology, clarifies the potential of antioxidant-based treatments, and outlines future research directions essential for translating these insights into effective therapies for chronic disease management.
1. Introduction
In recent decades, the scientific community has paid increasing attention to the complex relationship between oxidative stress, inflammation, and the development of chronic diseases [1,2,3]. Oxidative stress and chronic inflammation have been implicated in a wide range of pathological conditions, including cardiovascular diseases, kidney disease, diabetes, neurodegenerative diseases, and even cancer. As we face an era of rapid environmental changes, unhealthy lifestyles, and population aging, understanding the molecular mechanisms underlying oxidative stress and inflammation is more critical than ever. In this context, the exploration of potential therapeutic agents, particularly nutraceuticals, has gained significant traction. These bioactive compounds, often derived from natural sources, hold promise in modulating oxidative stress and inflammation to prevent or manage various diseases [4,5,6,7].
The main goal of this review is to explore the connections between oxidative stress and inflammation, with an emphasis on how nutraceuticals can influence these processes. We will review recent research on various antioxidant compounds including polyphenols, omega-3 fatty acids, and other bioactive molecules, and their impact on oxidative stress and inflammation. Furthermore, this review will discuss the challenges and opportunities of integrating nutraceuticals into clinical practice, with a focus on their bioavailability, optimal dosages, and potential for personalized treatments.
This review aims to provide a comprehensive understanding of the molecular mechanisms linking oxidative stress and inflammation, while highlighting promising nutraceutical interventions that could lead to new strategies for preventing and managing chronic diseases.
The interplay between oxidative stress, antioxidants, and inflammation forms a dynamic network that influences the progression of disease. On the one hand, ROS are critical for immune signaling and play a significant role in activating inflammatory pathways. However, when ROS production exceeds the body’s ability to neutralize them, it leads to tissue damage, amplifying the inflammatory response and fostering disease development. In this context, antioxidants play a pivotal role in breaking this harmful cycle. By scavenging ROS, antioxidants help mitigate oxidative damage and reduce inflammation, offering a protective mechanism [5,8,9].
Additionally, certain antioxidants have been shown to influence inflammation directly. For example, polyphenolic compounds such as curcumin and resveratrol have been shown to inhibit the activation of key inflammatory pathways, including NF-κB and MAPK, which are involved in cytokine production [10,11]. By modulating both oxidative stress and inflammation, antioxidants may offer a dual strategy to prevent or alleviate chronic diseases, providing a promising approach for therapeutic interventions. Understanding these mechanisms is essential for developing more effective strategies to manage the effects of oxidative stress and inflammation in chronic diseases.
Although traditional pharmacological treatments are common, they often lead to side effects and may not target the underlying causes of these diseases. Consequently, interest in natural compounds with antioxidants and anti-inflammatory properties has grown recently as potential therapeutic options [12,13].
2. Oxidative Stress: Mechanisms and Pathophysiology
2.1. Endogenous Sources
The formation of intracellular free radicals in mitochondria and peroxides occurs due to basic metabolism during normal oxidative phosphorylation, which is essential for ATP generation (Table 1). Cytosolic enzyme systems also produce these radicals. In addition, ROS are enzymatically generated in macrophages to destroy pathogens. However, their excessive generation weakens host cells and organs due to the inability to distinguish between host cells and infectious agents [14].
Highly reactive molecules of ROS and reactive nitrogen species (RNS) promote random damage to cellular structures, leading to cell death by interrupting the physiological functions of key biomolecules [8]. The most common biologically active radicals and molecules that may induce pathophysiological conditions include non-radical hydrogen peroxide (H2O2), superoxide anion radical (O2•−), hydroxyl radical (OH•), nitric oxide radical (NO•), and peroxynitrite (ONOO−).
ATP is produced in mitochondria through energy conversion in a usable form by cells. In this process, oxidative phosphorylation involves the electron transport chain in which hydrogen ions are moved across the inner mitochondrial membrane. Electrons pass through a series of redox-active proteins, each having a higher reduction potential than the last, eventually reducing oxygen to water. However, approximately 1–3% of electrons leak, leading to the premature and partial reduction of oxygen and the formation of O2•− [6,15].
Enzymatic and non-enzymatic reactions produce superoxide radicals and are highly reactive. Their protonated form, HO2•, easily penetrates the phospholipid bilayer, initiating lipid oxidation. This radical can also reduce iron complexes such as Cytochrome c and ferric-EDTA, acting as a potent reducing agent. Conversely, it can oxidize substances such as ascorbic acid and tocopherol which participate in numerous reactions that generate more radical and oxidative species.
Hydroxyl radicals are generated by the reaction of O2•− with H2O2. OH• is regarded as the most harmful ROS, as it reacts aggressively with lipids, proteins, and DNA, causing significant cell damage. It can abstract an electron from polyunsaturated fatty acids, triggering lipid peroxidation [16].
In vivo, H2O2 is formed via a dismutation reaction catalyzed by the enzyme superoxide dismutase (SOD), or by metabolic oxygen consumption in peroxisomes. At low concentrations, H2O2 damages cells, while high concentrations inactivate energy-producing enzymes such as glyceraldehyde-3-phosphate dehydrogenase. It can also affect biological membranes by easily penetrating them [17].
ONOO− is a highly toxic compound that results from the reaction between O2•− and NO•. ONOO− readily reacts with CO2, O2, and water. Its reaction with CO2 produces highly reactive intermediates such as nitroso-peroxy-carboxylate (ONOOCO2−) and peroxynitrous acid (ONOOH), which decompose to form hydroxyl (OH•) and nitrogen dioxide (NO2•) radicals. ONOO− can damage cells by oxidizing lipids, methionine, and tyrosine residues in proteins, and DNA, forming nitroguanine [18].

Table 1.
Major reactive species, their primary cellular sources, and mechanisms.
Table 1.
Major reactive species, their primary cellular sources, and mechanisms.
| Reactive Species/Antioxidants | Primary Production Source (Subcellular Location/Enzyme System) | Reaction/Mechanism | References |
|---|---|---|---|
| Superoxide (O2•−) | Mitochondrial electron transport chain (Complexes I & III), NADPH oxidases (NOX family), xanthine oxidase, and uncoupled NOS. | One-electron reduction of O2 → O2−; rapidly dismutates by SOD to H2O2. | [19,20] |
| Hydrogen peroxide (H2O2) | Product of SOD-mediated dismutation (cytosol/mitochondria), peroxisomal oxidases, and some NOX activity. | Two-electron product (dismutation or direct 2-e− reduction of O2); diffusible signaling oxidant; detoxified by catalase and glutathione peroxidases. | [21,22] |
| Hydroxyl radical (•OH) | Generated locally from H2O2 via iron-mediated Fenton/Haber–Weiss chemistry (labile Fe2+ pools). | H2O2 + Fe2+ → •OH + OH− (Fenton); extremely reactive and non-selective. | [23,24] |
| Nitric oxide (NO) | Nitric oxide synthases (eNOS, iNOS, nNOS)—cytosolic/membrane-associated. | Radical gasotransmitter; reacts rapidly with O2•− to form peroxynitrite (ONOO−). | [25] |
| Peroxynitrite (ONOO−/ONOOH) | Formed by diffusion-limited reaction between NO• and O2•− in cytosol/near membranes. | Potent oxidant/nitrating species; yields secondary radicals (NO2, CO3•−) and modifies proteins/lipids. | [26,27] |
| Lipid peroxyl radical (LOO)/lipid hydroperoxides (LOOH) | Initiated when ROS attack polyunsaturated fatty acids in membranes or lipoproteins (e.g., LDL)—membrane/LDL surface. | Radical chain-propagation (L• → + O2 → LOO → abstracts H → LOOH); leads to reactive aldehydes (MDA, 4-HNE) and ox-LDL formation. | [28,29] |
| Antioxidant enzymes (SOD, Catalase, GPX) | SOD1 (cytosol), SOD2 (mitochondria), catalase (peroxisomes), GPXs (cytosol/mitochondria). | SOD: 2 O2•− + 2 H+ → H2O2 + O2. Catalase/GPX: H2O2 → H2O (via 2 e− reduction or catalase decomposition). | [30,31] |
2.2. Exogenous Sources
Environmental and external factors play a significant role in causing oxidative stress by increasing reactive oxygen and nitrogen species in the body (Figure 1). For instance, cigarette smoke introduces both superoxide (O2•−) and nitric oxide (NO•), directly elevating free radical levels. Similarly, ozone exposure leads to lipid oxidation and promotes neutrophil infiltration into the airway lining. Even low levels of ozone exposure can trigger the release of inflammatory and cytotoxic mediators, including myeloperoxidase (MPO), eosinophil cationic protein, lactate dehydrogenase, and albumin, all of which collectively raise ROS in the lungs and other tissues due to the oxygen-rich environment. Heavy metals such as iron, copper, cadmium, mercury, nickel, lead, and arsenic further exacerbate oxidative stress by catalyzing lipid peroxidation and interacting with proteins and DNA, impairing enzyme activity and damaging cells. Ionizing radiation intensifies these effects through multiple pathways: when oxygen is present, it produces OH, O2•−, and organic radicals, which can then form H2O2 and organic hydroperoxides via Fenton reactions. Moreover, radiation interacts with water, making up ~ 55–60% of the human body, through radiolysis, generating OH, H2O2, O2•−, and O2, all of which contribute to oxidative stress at both cellular and tissue levels. These external sources of oxidative stress highlight the importance of the body’s internal antioxidant defense systems [32], which encompass both enzymatic and non-enzymatic mechanisms by maintaining redox balance and preventing cellular damage.
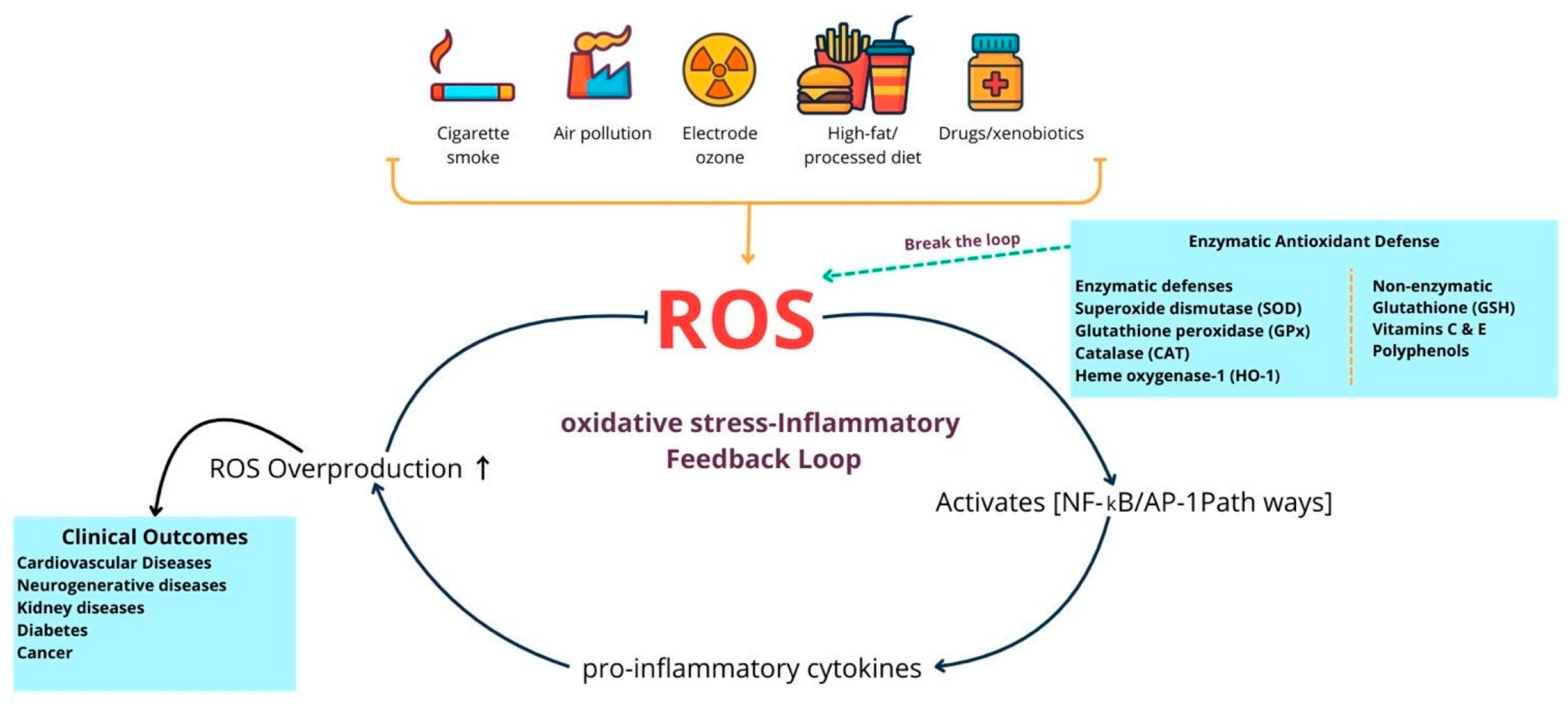
Figure 1.
Endogenous oxidative stress and antioxidant defenses in human diseases. This diagram illustrates the balance between external sources of oxidative stress, such as smoking, pollutants, radiation, diet, xenobiotics, and internal sources like chronic inflammation, along with the antioxidant defenses that mitigate their effects. Enzymatic systems, such as superoxide dismutase, catalase, glutathione peroxidase, and heme oxygenase-1, along with non-enzymatic molecules including glutathione, vitamins C and E, and polyphenols, neutralize reactive oxygen species (ROS) and protect macromolecules from damage. When ROS production exceeds antioxidant capacity, it results in oxidative damage to lipids, proteins, and DNA, which in turn increases inflammatory signaling, leading to further ROS production and creating a self-perpetuating cycle of oxidative stress and inflammation. Antioxidant defenses aim to break this cycle and prevent ROS-driven inflammation from escalating. If this balance is not restored, persistent oxidative stress and cellular damage occur, contributing to the development and progression of cardiovascular disease, type 2 diabetes mellitus, neurodegenerative disorders, chronic kidney disease, and cancer. This illustration was created using Canva (https://www.canva.com, accessed on 12 September 2025).
2.3. Impact of Oxidative Stress on Cellular Structures and Functions
The harmful effects of oxidative stress on cellular structures are significant. ROS and RNS can directly damage lipids, proteins, and nucleic acids, resulting in structural and functional abnormalities. Lipid peroxidation, a process in which ROS attack lipid membranes, produces toxic aldehydes that can compromise cell membrane integrity and fluidity. This disruption can hinder cell signaling and lead to cellular dysfunction [33].
Protein oxidation occurs when ROS modifies amino acid side chains, resulting in changes to protein structure and function. This can lead to the loss of enzyme activity, disrupted protein–protein interactions, and the buildup of misfolded proteins, which are often linked to neurodegenerative diseases such as Alzheimer’s and Parkinson’s [34]. DNA damage caused by ROS can cause mutations, chromosomal fragmentation, and even apoptosis. If left unrepaired, these mutations may contribute to the onset and advancement of cancer [35].
Furthermore, oxidative stress has a significant impact on cellular signaling pathways. For example, ROS activates transcription factors such as nuclear factor-kappa B (NF-κB) and activator protein-1 (AP-1), which regulate the expression of genes involved in inflammation, cell survival, and apoptosis [36]. This activation may lead to chronic inflammation, potentially exacerbating tissue damage and disease progression.
Oxidative stress is also a key factor in aging and age-related diseases. As the body’s antioxidant defenses weaken with age, the accumulation of oxidative damage to cellular components plays a significant role in the aging process and the development of age-associated conditions, such as atherosclerosis, cataracts, and neurodegeneration. In particular, mitochondria, which are the primary source of ROS, undergo damage over time, resulting in impaired energy production and increased ROS generation, which creates a vicious cycle of mitochondrial dysfunction and cellular aging [37]. The mechanisms behind oxidative stress are complex, involving multiple sources of ROS and RNS, as well as advanced antioxidant defenses. When these systems fail to maintain redox balance, oxidative stress can cause significant cellular damage, contributing to the development of various chronic diseases [38]. Understanding these processes, along with the role of antioxidants in mitigating oxidative damage, offers promising therapeutic options for preventing and treating diseases associated with oxidative stress.
Future research should continue exploring new antioxidant-based treatments and strategies to enhance the body’s natural defenses, ultimately leading to better health outcomes despite oxidative stress and its related conditions.
2.4. Endogenous Antioxidant Defense Systems (Enzymatic and Non-Enzymatic)
The endogenous antioxidant defense system consists of a carefully regulated balance between enzymatic and non-enzymatic molecules, acting as the first line of defense against redox imbalance and oxidative damage (Table 2). Enzymatic antioxidants, such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidases (GPx), work together to neutralize reactive oxyROS and RNS [39]. SOD converts O2−• into H2O2, which, if not quickly neutralized, can participate in Fenton reactions, producing highly reactive hydroxyl radicals. Then, CAT, mainly found in peroxisomes, and GPx, primarily active in mitochondria and the cytosol, convert H2O2 into harmless water and oxygen, preventing oxidative damage to nucleic acids, proteins, and membrane lipids.

Table 2.
Comparison Between Endogenous Enzymatic and Non-Enzymatic Antioxidants.
Importantly, each enzyme requires specific cofactors to work. SOD types need manganese, copper, or zinc, while CAT contains a heme iron center, and GPx activity depends on selenium. Changes in the levels or activity of these enzymes are associated with various health issues, including neurodegeneration, cancer progression, and heart problems. Recent studies indicate that increasing SOD activity or administering CAT through gene therapy can reduce neuronal damage after ischemia and lower infarct size [40,41]. Additionally, synthetic mimetics of SOD and mitochondrial-targeted antioxidants such as mitoquinone (MitoQ) or plastoquinone (SkQ1) are being tested in clinical settings to regulate redox balance in chronic diseases.
Non-enzymatic antioxidants, including GSH, α-lipoic acid, melatonin, uric acid, bilirubin, and coenzyme Q10, work by directly scavenging free radicals or restoring oxidized antioxidants. GSH, a tripeptide made up of glutamate, cysteine, and glycine, functions as a key redox buffer and serves as a substrate for GPx. In conditions like Parkinson’s and Alzheimer’s diseases, GSH depletion is linked to increased ROS levels and neuronal cell death. Supplementation with N-acetylcysteine (NAC) or liposomal GSH has shown promising results in restoring cellular redox balance [42]. Similarly, α-lipoic acid, through its disulfide/dithiol redox pair, not only neutralizes SOD, OH•, and ROO• radicals but also regenerates other antioxidants like GSH and vitamin C, making it a valuable addition in managing diabetic neuropathy and metabolic syndrome [43].
Melatonin, mainly produced by the pineal gland, has notable antioxidant effects that go beyond regulating the sleep–wake cycle. Its amphiphilic nature enables it to cross cell membranes easily and gather within mitochondria, where it combats oxidative stress and prevents the opening of the mitochondrial permeability transition pore (mPTP). Clinical studies have shown its potential to improve outcomes in cases of sepsis, ischemic injuries, and even post-COVID-19 fatigue [44].
Although substantial experimental evidence supports the effectiveness of endogenous antioxidant systems, their clinical use is limited by pharmacokinetic challenges, including low oral bioavailability, poor tissue targeting, and a short half-life. Additionally, systemic antioxidant administration can disrupt normal redox signaling, emphasizing the need for interventions tailored to specific contexts. Advances include nano-formulated antioxidants, prodrug strategies, and redox phenotype-guided therapies. Personalized antioxidant approaches that consider disease stage, genetic background, and redox status could improve treatment outcomes.
2.5. Role of Antioxidants in Organ Protection
Oxidative stress is a common pathogenic mechanism that connects metabolic disorders, cardiovascular disease, chronic kidney disease, gastrointestinal injury, and neurodegeneration. Antioxidants, both natural and synthetic, exert protective effects by neutralizing ROS, maintaining redox balance, and regulating inflammatory and apoptotic pathways.
The cardiovascular system is particularly vulnerable to oxidative stress due to continuous exposure to oxidized lipoproteins and vascular inflammation. Antioxidants such as vitamin E, vitamin C, polyphenols (resveratrol, curcumin, green tea catechins), and pharmacological agents with antioxidant activity (e.g., statins, probucol) reduce LDL oxidation, improve endothelial nitric oxide bioavailability, and attenuate atherogenesis [45,46,47]. Clinical studies have shown that diets rich in natural antioxidants correlate with a lower incidence of coronary artery disease and improved vascular function [48].
The kidney is also vulnerable to ROS-mediated damage in both acute and chronic injury. Antioxidants, including N-acetylcysteine, curcumin, and green tea polyphenols, reduce ischemia–reperfusion injury, attenuate tubular apoptosis, and decrease proteinuria in experimental nephropathy [49,50]. Synthetic antioxidants have been used to protect against contrast-induced nephropathy and drug-induced renal toxicity. Thus, targeting oxidative stress pathways is increasingly recognized as a potential renoprotective strategy.
The gastrointestinal mucosa is continuously exposed to dietary oxidants, microbial metabolites, and inflammatory mediators. Flavonoids, carotenoids, and vitamins (C and E) strengthen mucosal defenses by enhancing epithelial barrier integrity, scavenging ROS, and suppressing NF-κB activation [51,52]. Experimental studies demonstrate that curcumin and quercetin reduce the severity of colitis, while vitamin C and other antioxidants accelerate gastric ulcer healing [53,54,55].
The brain consumes high amounts of oxygen and is rich in polyunsaturated fatty acids, which makes it very vulnerable to lipid peroxidation and oxidative injury. Natural antioxidants such as polyphenols, omega-3 fatty acids, and coenzyme Q10, as well as synthetic antioxidants, have shown neuroprotective effects by preventing protein aggregation, reducing mitochondrial dysfunction, and suppressing neuroinflammation [56,57]. Furthermore, clinical and preclinical studies support their role in slowing cognitive decline in Alzheimer’s disease and reducing dopaminergic neuronal loss in Parkinson’s disease [58,59].
In summary, antioxidants play a crucial role in protecting vital organs from oxidative stress–induced injury. Their multifaceted actions on redox signaling, inflammation, and apoptosis highlight their potential as supportive therapeutics in preventing and managing metabolic and degenerative diseases.
3. Inflammation and Its Crosstalk with Oxidative Stress
Inflammation and oxidative stress are closely connected biological processes that play a central role in the development of many chronic diseases. Oxidative stress, characterized by an imbalance between the production of ROS and antioxidant defenses, can trigger various inflammatory pathways, including the nuclear factor kappa B (NF-κB) signaling pathway. NF-κB is a key transcription factor that regulates the expression of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β), thereby amplifying the inflammatory response [37,60]. Conversely, inflammation can further increase ROS production by activating immune cells, such as macrophages and neutrophils, which release oxidative molecules as part of the host’s defense mechanisms. This bidirectional interaction creates a vicious cycle that perpetuates cellular damage, tissue dysfunction, and contributes to the development and progression of chronic conditions such as cardiovascular diseases, diabetes mellitus, kidney diseases, neurodegenerative disorders, and cancer [61,62]. Mounting evidence indicates that prolonged activation of inflammatory and oxidative pathways disrupts cellular balance, worsens mitochondrial dysfunction, and ultimately perpetuates disease. Therefore, targeting the molecular interaction between oxidative stress and inflammation presents a promising therapeutic strategy to reduce the impact of chronic inflammatory diseases.
4. The NRF2–Keap1 Pathway and Its Cross-Talk with Inflammatory Signaling
The nuclear factor erythroid 2–related factor 2 (NRF2) is a key transcription factor that controls the expression of a wide range of antioxidant and cytoprotective genes. Under normal conditions, NRF2 is kept in the cytoplasm by Kelch-like ECH-associated protein 1 (Keap1), which marks it for ubiquitination and subsequent degradation by the proteasome. When cells face oxidative stress or electrophilic compounds, critical cysteine residues in Keap1 become modified, causing conformational changes that reduce NRF2 ubiquitination. This stabilization allows NRF2 to accumulate and move into the nucleus, where it binds to antioxidant response elements (ARE) in the promoter regions of target genes [63]. This leads to increased transcription of phase II detoxifying enzymes and antioxidant proteins, such as heme oxygenase-1 (HO-1), NAD(P)H: quinone oxidoreductase 1 (NQO1), the catalytic (GCLC) and modifier (GCLM) subunits of glutamate-cysteine ligase, and glutathione peroxidase (GPx). Collectively, these effectors boost redox balance, protect against lipid peroxidation, and reduce oxidative damage [64].
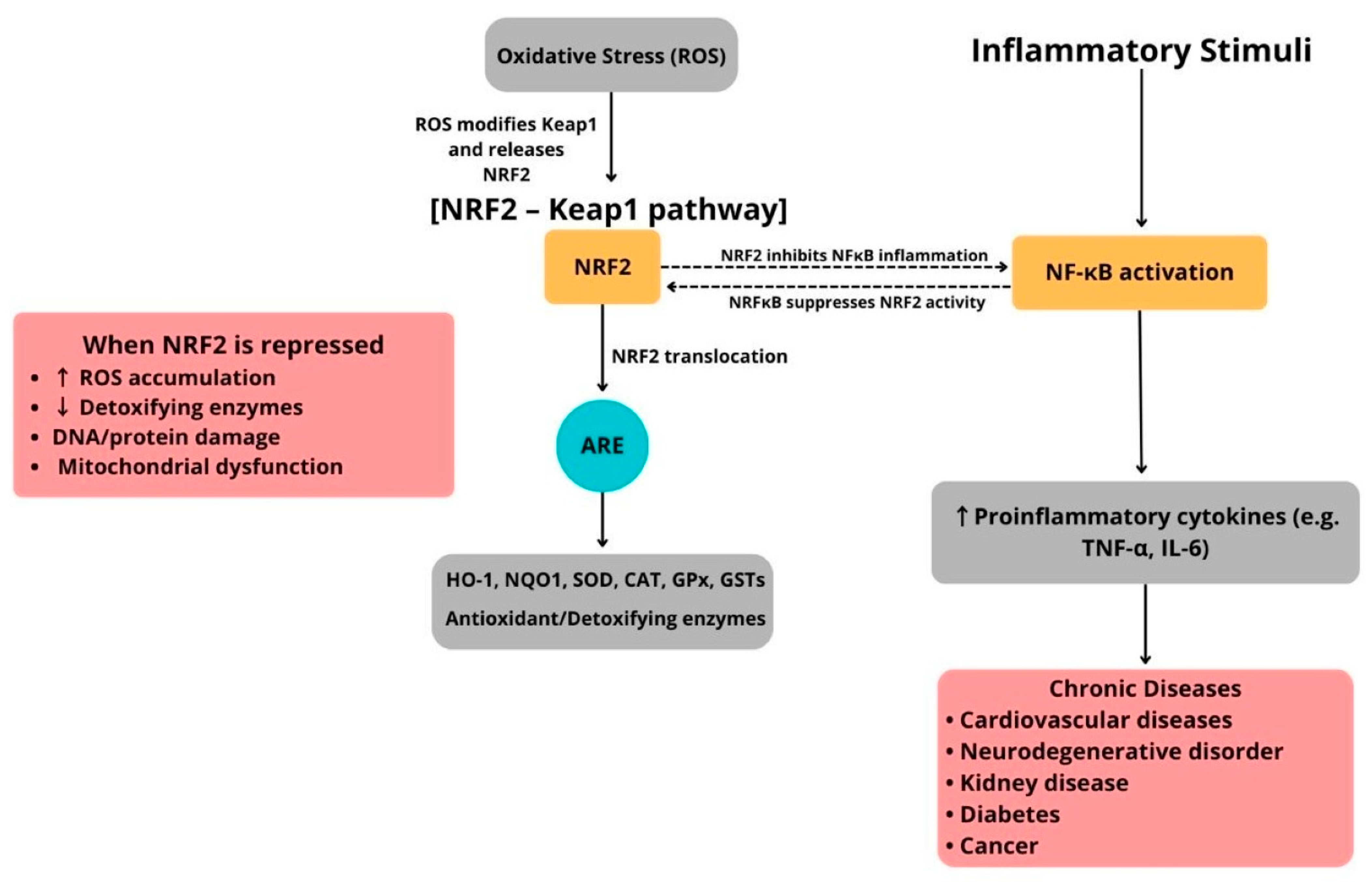
Importantly, the NRF2–Keap1 pathway does not operate alone but interacts with pro-inflammatory signaling pathways, especially the NF-κB pathway. Oxidative stress strongly activates NF-κB, whereas NRF2 activation counteracts this by lowering ROS levels and inhibiting NF-κB–dependent gene expression (Figure 2). Additionally, direct molecular interactions between these pathways have been identified. For example, the NF-κB p65 subunit can suppress NRF2’s transcriptional activity by competing for coactivators, while NRF2-driven HO-1 expression can limit NF-κB activation through carbon monoxide and biliverdin signaling [65,66]. This reciprocal regulation maintains a delicate balance between antioxidant defenses and inflammatory responses (Figure 2).
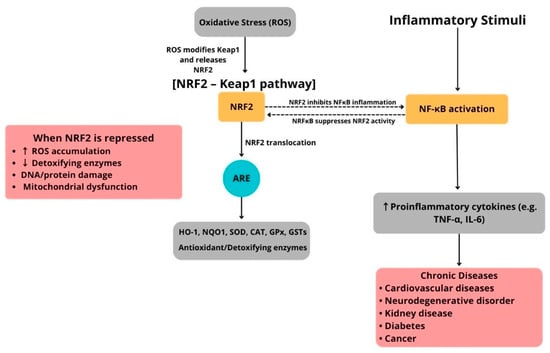
Figure 2.
Schematic illustration of the NRF2–Keap1/ARE pathway and its interaction with NF-κB–mediated inflammation in human diseases.
Dysregulated NRF2–Keap1 signaling has been linked to the development of chronic diseases marked by oxidative stress and low-grade inflammation, including cardiovascular disease, type 2 diabetes mellitus, neurodegeneration, and cancer. Therefore, therapeutic approaches aimed at activating NRF2, such as electrophilic compounds, natural antioxidants like curcumin and sulforaphane, and synthetic derivatives, are actively being explored for their potential to restore redox balance and reduce inflammatory injury [64,67,68].
Under resting conditions, nuclear factor erythroid 2–related factor 2 (NRF2) is held in the cytoplasm by Kelch-like ECH-associated protein 1 (Keap1). Oxidative or conformational changes in Keap1 prevent NRF2 from being ubiquitinated, allowing NRF2 to stabilize and accumulate in the nucleus. Once inside, NRF2 forms a heterodimer with small Maf proteins and binds to antioxidant response element (ARE) sequences to activate a wide range of cytoprotective genes, including heme oxygenase-1 (HO-1), NAD(P)H quinone oxidoreductase 1 (NQO1), glutathione S-transferases (GSTs), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and enzymes involved in glutathione synthesis. These gene products work together to neutralize reactive oxygen species (ROS), repair oxidative damage, and facilitate detoxification, thus maintaining redox and metabolic balance. Simultaneously, inflammatory signals activate nuclear factor kappa B (NF-κB), promoting the transcription of pro-inflammatory cytokines and mediators. The NRF2 and NF-κB pathways interact through bidirectional cross-talk. Activation of NRF2 reduces NF-κB–driven inflammation, whereas chronic inflammatory signals can impair NRF2 activity through post-translational and transcriptional mechanisms. Disruption of this balance due to abnormal Keap1 activity, genetic mutations, persistent oxidative or inflammatory stress, or mitochondrial dysfunction weakens ARE-dependent defenses, leading to excessive ROS buildup, lipid peroxidation, protein carbonylation, DNA strand breaks, and compromised detoxification capacity. These processes drive the development and progression of chronic diseases, such as cardiovascular disease, type 2 diabetes mellitus, neurodegenerative disorders, chronic kidney disease, and cancer. This illustration was created using Canva (https://www.canva.com, accessed on 12 September 2025).
5. Types and Mechanisms of Antioxidants
Nutraceutical and pharmacological antioxidants are crucial in combating oxidative stress, a condition associated with the development of numerous chronic diseases. Nutraceutical antioxidants, derived from natural dietary sources such as flavonoids, vitamins, and carotenoids, help neutralize free radicals and support the body’s natural defense mechanisms. Conversely, pharmacological antioxidants are synthetically created to enhance their antioxidant effects through improved stability, increased bioavailability, and enhanced cellular targeting. These agents have demonstrated promising results in managing oxidative damage related to aging, neurodegenerative diseases, and cardiovascular conditions [69,70].
5.1. Nutraceuticals
Nutraceuticals are substances that offer medicinal or health benefits beyond their nutritional content. The term “nutraceutical” is derived from the combination of “nutrition” and “pharmaceuticals.” These compounds, found in functional foods, contain biologically active molecules that interact with various cellular targets across organ systems. Their effects include antioxidant, anti-inflammatory, anti-proliferative, antimicrobial, and cholesterol-lowering properties [71,72,73]. They can encompass a range of ingredients, including isolated nutrients, phytochemicals, vitamins, minerals, amino acids, fatty acids, herbal remedies, and even genetically modified foods. Research has shown that regular intake of nutraceuticals as part of a diverse diet can help lower the risk of diseases related to oxidative stress, such as Alzheimer’s, cardiovascular diseases, diabetes, and obesity [55,74,75,76].
5.1.1. Antioxidant Nutraceuticals
Dietary antioxidants work synergistically with endogenous antioxidants to neutralize ROS and RNS. Their deficiency is associated with chronic and degenerative diseases. These compounds are structurally diverse and include a range of various molecules.
5.1.2. Ascorbic Acid (Vitamin C)
Ascorbic acid, commonly known as vitamin C, is an essential water-soluble antioxidant that humans cannot synthesize endogenously, unlike most animals and plants, making its dietary intake essential [77]. Rich dietary sources of vitamin C include citrus fruits such as lemons and oranges, as well as parsley and various edible flowers.
Biologically, it plays a crucial role in normal growth and development and is involved in multiple physiological functions [78]. It is characterized as one of the primary antioxidants, directly scavenging ROS and RNS, including superoxide anions, hydroxyl radicals, alkoxyl radicals, hydrogen peroxide, and singlet oxygen [79].
Vitamin C also regenerates other antioxidants such as vitamin E, contributes to collagen biosynthesis, improves iron absorption, and modulates cellular signal transduction. Donating hydrogen reduces tocopherol radicals, thereby restoring the antioxidant function of tocopherol and protecting membrane lipids. Moreover, it reduces the Fenton reaction by neutralizing metal ions that would otherwise promote the formation of free radicals. In plants, ascorbic acid serves as a substrate for ascorbate peroxidase (APX), playing a crucial role in resistance to oxidative stress [80].
5.1.3. Tocopherols (Vitamin E)
It is a group of phenolic compounds containing eight related tocopherols and tocotrienols known as vitamin E. It is a family of lipid-soluble vitamins. The most famous compound of this class is alpha-tocopherol, which has the highest bioavailability, allowing the body to absorb and metabolize it easily. The primary function of alpha-tocopherol is to protect membranes from oxidation, as it inhibits lipid peroxidation by interacting with lipid radicals generated from peroxynitrite and inflammatory reactions [81]. Moreover, tocopherols react with alkyl or alkyl peroxyl radicals by donating hydrogen and in turn produce a tocopherol radical, while ascorbic acid regenerates the reduced form of tocopherol. At low peroxyl radical concentrations and high tocopherol radical concentrations, the tocopherol radical can take hydrogen from the lipid substrate, thereby generating lipid radicals and regenerating tocopherol. In this scenario, the lipid radical contributes to lipid oxidation by interacting with triplet oxygen; thus, the tocopherol radicals may act as prooxidants instead of antioxidants [82,83].
5.1.4. Carotenoids
Carotenoids fall under a large group of molecules called terpenes that are formed by isoprene chains with conjugated double bonds and are fat-soluble [84]. They are divided into two classes based on their structure: simple polyene hydrocarbons, such as β-carotene and lycopene, and xanthophylls, which contain oxidizing groups, including hydroxy, oxo, or epoxy. The broad interest in carotenoids is due to their roles as antioxidants. They act as ROS and RNS scavengers or singlet-oxygen quenchers during lipid oxidation through physical and chemical processes. Tocopherols, known as powerful lipid antioxidants, enhance the presence of carotenoids in the lipid phase of membranes. Their antioxidant activity depends on scavenging singlet oxygen, peroxyl radicals, sulfonyl, thiyl, and NO2 radicals, which leads to protection against hydroxyl attack and superoxide radicals.
Carotenoids are also known for their ability to reduce radicals by donating electrons and hydrogen. They form when hydrogen atoms are donated to lipid peroxyl radicals. These radicals react with lipid peroxyl radicals, leading to the formation of non-radical carotene peroxides. Carotene radicals are also prone to molecular-oxygen addition and subsequent reaction with another carotene molecule, producing carotene epoxides and carbonylated carotene compounds. One of the most stable carotene radicals is the β-carotene radical because of the delocalization of its unpaired electron within the conjugated polyene structure [85]. Carotene is a key member of the carotenoid family that can be converted into active vitamin A and into zeaxanthin, which gives the retina its yellow pigment. It has been reported that carotenoids can inhibit cell renewal and transformation, as well as regulate gene expression involved in the development of certain cancers [86].
5.1.5. Phenolics and Flavonoids
Plant polyphenols form a broad class of phenolic compounds, the most prominent being flavonoids flavones and flavonols. These molecules exhibit strong antioxidant activity by effectively scavenging superoxide anions, nitric oxide, and hydroxyl radicals [87]. In edible fruits and vegetables, they also regulate redox-related enzymes, reducing cellular free-radical levels, lowering hyperglycemia, and exerting antiproliferative effects. For phenolic acids, antioxidant strength increases with both the number and the ring position of hydroxyl groups.
Once incorporated into biological membranes, polyphenols insert into the hydrophobic core and slow the oxidation of lipids and proteins; membrane-embedded flavonoids further protect bilayer integrity by physically blocking the entry of ROS and RNS. Dietary sources such as green tea, red grape skins (used in red wine), apples, cocoa, ginkgo biloba, soy, turmeric, various berries, onions, and broccoli are all associated with decreased inflammation and oxidative stress [88].
6. Fatty Acids as Modulators of Antioxidant and Anti-Inflammatory Pathways
Fatty acids exert important regulatory effects on oxidative stress and inflammation through multiple mechanisms [89], including activation of nuclear receptors (peroxisome proliferator–activated receptors, PPARs), engagement of membrane G-protein–coupled receptors (GPR120), enzymatic conversion into specialized pro-resolving mediators (SPMs), and formation of bioactive electrophilic derivatives such as nitro-fatty acids. Collectively, these actions converge to inhibit NF-κB–driven pro-inflammatory signaling while enhancing antioxidant defenses [90].
Long-chain polyunsaturated fatty acids (PUFAs), especially omega-3 PUFAs such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), serve as natural ligands for PPARα, PPARγ, and PPARδ. Upon activation, PPARs heterodimerize with retinoid X receptor (RXR) and regulate target genes involved in lipid metabolism and redox control. In addition, PPARs exert anti-inflammatory effects by trans-repression of NF-κB signaling. Mechanisms include sequestration of coactivators such as CBP/p300, stabilization of the NF-κB inhibitor IκBα, and recruitment of corepressors to NF-κB target promoters. Experimental studies show that oxidized omega-3 fatty acids inhibit NF-κB activation via a PPARα-dependent mechanism, underscoring their dual role in lipid and inflammatory homeostasis [91].
Moreover, PPAR activation complements NRF2 signaling. Both pathways work together to increase antioxidant enzymes, including heme oxygenase-1 (HO-1), NAD(P)H: quinone oxidoreductase 1 (NQO1), and glutamate-cysteine ligase. Studies in metabolic and toxicological models indicate that co-activation of PPARγ and NRF2 provides synergistic protection against oxidative damage, thereby reducing secondary NF-κB activation [92].
Omega-3 PUFAs also activate GPR120 (also known as FFAR4), a membrane receptor highly expressed in macrophages and adipocytes. GPR120 signaling inhibits NF-κB and NLRP3 inflammasome activation while promoting a shift toward an anti-inflammatory M2 macrophage phenotype [93]. In parallel, enzymatic conversion of EPA and DHA produce SPMs such as resolvins, protectins, and maresins, which actively promote resolution of inflammation and limit the oxidative injury [94].
Electrophilic derivatives of unsaturated fatty acids, such as nitro-oleic acid and other nitro-fatty acids, activate both PPARs and NRF2 by covalently modifying cysteine residues in Keap1. This results in the upregulation of antioxidant genes and inhibition of NF-κB signaling, highlighting a direct link between lipid electrophiles and cytoprotective transcriptional responses [95].
Clinical data support the anti-inflammatory potential of omega-3 PUFAs, though outcomes vary by formulation and population. A randomized dose–response trial demonstrated that EPA and DHA supplementation modulate innate immune responses and reduce ex vivo endotoxin-stimulated cytokine release [96]. Large cardiovascular outcome trials produced divergent results: REDUCE-IT showed that high-dose Icosapent ethyl (EPA-only) significantly reduced cardiovascular events, whereas the STRENGTH trial using combined EPA + DHA failed to demonstrate benefit [97,98,99]. These findings suggest that clinical efficacy depends on the specific fatty acid formulation, dosage, and patient context [99].
7. Synthetic and Pharmacological Antioxidants: Mechanisms of Action
Synthetic and pharmacological antioxidants have been developed to imitate or enhance the body’s natural antioxidant defenses, especially in cases of diseases related to oxidative stress [100]. These compounds include well-studied agents such as N-acetylcysteine (NAC), edaravone, tempol, and synthetic versions of natural antioxidants, including Trolox, a vitamin E analog. These molecules are used clinically or experimentally to reduce oxidative damage, particularly in conditions like neurodegenerative disorders, ischemia–reperfusion injury, and inflammation-related diseases [100].
The mechanisms of action of these antioxidants are complex. One primary mechanism is free radical scavenging, in which these compounds donate electrons to neutralize ROS and RNS, thereby preventing lipid peroxidation and DNA damage [16]. Studies have demonstrated that ROS acts as a direct scavenger and also serves as a precursor to glutathione, a major endogenous antioxidant [8]. In addition to scavenging, some synthetic antioxidants modulate antioxidant enzyme systems. For example, molecules like tempol and specific pharmacological activators stimulate the expression or activity of SOD and catalase, thereby helping to maintain cellular redox balance [101].
Furthermore, recent studies have shown that certain pharmacological antioxidants exert their effects by modulating gene expression. Compounds such as dimethyl fumarate and resveratrol analogs activate the Nrf2 pathway, a transcription factor that upregulates ARE-driven genes, including those encoding for glutathione peroxidase and NAD(P)H: quinone oxidoreductase [102]. This gene-regulatory mechanism provides a more sustained antioxidant effect by enhancing the body’s defense capacity at the transcriptional level.
These diverse mechanisms underscore the potential of synthetic and pharmacological antioxidants, not only as direct radical scavengers but also as regulators of cellular antioxidant systems and gene expression pathways.
8. Anti-Inflammatory Agents with Antioxidant Properties
In recent years, scientific interest has grown in bioactive phytochemicals that have both anti-inflammatory and antioxidant properties. Notably, compounds like curcumin, resveratrol, and quercetin have gained significant attention for their multiple effects in regulating immune responses and redox homeostasis [103].
Curcumin, a polyphenol from Curcuma longa, is well-known for its diverse biological effects. It serves as a potent scavenger of ROS and RNS, thereby decreasing cellular oxidative damage. At the same time, curcumin reduces pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-1β, primarily by inhibiting the NF-κB signaling pathway. This inhibition lessens the activation of inflammation-related genes and helps regulate the immune response to harmful stimuli. Additionally, curcumin stimulates the Nrf2 pathway, promoting the production of protective enzymes such as heme oxygenase-1 (HO-1) and glutathione S-transferases (GSTs), which play crucial roles in mitigating oxidative stress [104].
Resveratrol, a stilbene compound predominantly found in grapes and red wine, exhibits similar dual activities. In vitro and in vivo studies have demonstrated that resveratrol reduces oxidative stress by enhancing the activity of SOD, CAT, and GPx [105,106]. Its anti-inflammatory effects are primarily attributed to inhibition of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS), as well as attenuation of NF-κB signaling. Importantly, resveratrol also influences the behavior of immune cells, including the suppression of T-cell proliferation and promotion of regulatory T-cells (Treg) differentiation, thereby maintaining immune homeostasis [107].
Quercetin, a flavonoid abundantly present in onions, apples, and tea, also exerts dual functionality by influencing oxidative and inflammatory pathways. It effectively inhibits lipid peroxidation and ROS generation, while concurrently suppressing the expression of key inflammatory mediators. Similar to curcumin and resveratrol, quercetin inhibits the NF-κB pathway and downregulates MAPK (mitogen-activated protein kinase) signaling cascades, including ERK, JNK, and p38, leading to reduced production of pro-inflammatory cytokines [108]. Moreover, quercetin enhances mitochondrial function and biogenesis through activation of AMP-activated protein kinase (AMPK), a central regulator of energy and oxidative balance in cells [109].
The immunomodulatory effects of these compounds are not restricted to cytokine modulation but also extend to cellular interactions within the immune system. For instance, curcumin inhibits dendritic cell maturation and antigen presentation, while promoting the expansion of Tregs. Resveratrol enhances macrophage polarization toward the anti-inflammatory M2 phenotype [110], thereby contributing to tissue repair and resolution of inflammation. Quercetin inhibits mast cell degranulation and modulate neutrophil infiltration in inflamed tissues [111]. These mechanisms collectively contribute to the restoration of immune balance and attenuation of chronic inflammation.
Furthermore, these dual-function agents interfere with intracellular signaling pathways that control cellular responses to stress and inflammation. The interplay between NF-κB, Nrf2, and MAPK pathways constitutes a central node targeted by these compounds. By inhibiting NF-κB and activating Nrf2, these agents shift the cellular response from a pro-inflammatory to an antioxidative and cytoprotective state. This dual modulation not only reduces inflammatory damage but also improves resilience against oxidative injury.
In conclusion, dual-function compounds such as curcumin, resveratrol, and quercetin show significant potential as therapeutic agents due to their ability to modulate both inflammation and oxidative stress simultaneously. Their influence on key signaling pathways and immune functions highlights the importance of these natural compounds in preventing and managing chronic inflammatory diseases. Future research should focus on improving their bioavailability and investigating the synergistic effects through combination therapy strategies.
9. Clinical Applications and Evidence of Antioxidants and Anti-Inflammatory Agents
An increasing number of preclinical and clinical studies highlight the significant therapeutic potential of antioxidants and anti-inflammatory agents in combating diseases mediated by oxidative stress and chronic inflammation. These compounds, particularly polyphenols such as curcumin, resveratrol, and quercetin, are of high interest due to their ability to act on both oxidative and inflammatory pathways. For instance, curcumin demonstrates significant anti-inflammatory effects by inhibiting NF-κB activation and reducing the expression of COX-2, IL-1β, and TNF-α. In animal models of atherosclerosis, curcumin has been shown to decrease oxidative stress markers such as MDA and boost antioxidant enzyme activities, including SOD and catalase [112].
Similarly, resveratrol exerts cardioprotective effects by activating SIRT1 and AMPK, promoting mitochondrial biogenesis, and reducing ROS generation. Preclinical models of myocardial infarction and cerebral ischemia have demonstrated that resveratrol attenuates infarct size, improves neurological function, and inhibits the release of pro-apoptotic factors. Quercetin has been extensively studied for its ability to restore redox balance by modulating glutathione levels, reducing lipid peroxidation, and suppressing inflammatory cytokines via inhibition of MAPK and JAK-STAT signaling pathways [113].
Clinical evidence, although still emerging, reflects many of these beneficial outcomes (Table 3). For example, curcumin supplementation (500 mg twice daily for 8 weeks) in patients with metabolic syndrome led to significant improvements in endothelial-dependent vasodilation, a reduction in C-reactive protein (CRP), and decreased serum levels of IL-6. In another trial, resveratrol was shown to enhance cerebral blood flow and cognitive performance in patients with mild to moderate Alzheimer’s disease, possibly through its vasodilatory and anti-aggregatory effects. Additionally, Quercetin demonstrated efficacy in lowering systolic blood pressure and improving HDL levels in type 2 diabetic patients with hypertension [114].

Table 3.
Examples of Clinical Trials Investigating Anti-Inflammatory and Antioxidant Therapies Targeting Oxidative Stress and Inflammation.
Beyond curcumin, resveratrol, and quercetin, several other agents with dual antioxidant and anti-inflammatory properties are currently being evaluated in clinical settings (Table 3). Astaxanthin, a potent carotenoid with strong radical-scavenging activity, is under investigation in heart failure patients, where it is expected to modulate both oxidative stress and inflammatory markers. Similarly, ellagic acid, a polyphenol abundant in pomegranates and berries, has demonstrated clinical benefits in patients with irritable bowel syndrome by increasing total antioxidant capacity and reducing markers of IL-6, CRP, and lipid peroxidation, along with improvements in quality of life.
L-carnitine supplementation combined with exercise has also shown synergistic effects in overweight/obese adults, enhancing antioxidant enzyme activity (CAT, SOD) and reducing ROS, MDA, and IL-6 levels, highlighting the importance of lifestyle-drug combinations. Moreover, eriocitrin, a citrus-derived flavonoid, demonstrated significant reductions in glycemia, systemic inflammation, and oxidative stress, while also enhancing GLP-1 secretion in prediabetic individuals.
In addition to nutraceuticals, novel pharmacological agents are emerging. Sonlicromanol (KH176), a redox-modulating drug, has been shown to improve mitochondrial redox balance and mood in patients with mitochondrial disease. In contrast, GC-4419, an SOD mimetic, is currently being tested in oncology for its dual antioxidant and anti-tumor potential. Crisdesalazine and AT-001 are also being investigated for neurodegenerative and brain oxidative stress–related disorders, respectively, underscoring a growing pipeline of clinically oriented redox-active compounds.
Together, these examples demonstrate that while classical polyphenols remain central to antioxidant–anti-inflammatory research, newer compounds, pharmacological agents, and combination approaches are expanding the therapeutic landscape. However, challenges such as poor bioavailability, short half-life, and inter-individual variability remain common obstacles across these agents, necessitating improved formulations and more rigorous translational efforts.
10. Challenges and Limitations
Despite promising results, several challenges limit the clinical translation of antioxidant and anti-inflammatory agents. Classical compounds such as curcumin, resveratrol, and quercetin are constrained by poor bioavailability, rapid metabolism, and inconsistent outcomes across clinical trials. Similarly, newer agents face their own barriers. Astaxanthin, although a potent radical scavenger, has also some bioavailability issues, and its long-term cardiovascular safety remains unclear [115]. Ellagic acid shows benefits in irritable bowel syndrome (IBS) patients, but its effects are highly dependent on gut microbiota metabolism, leading to significant variability in response. Additionally, when L-carnitine is combined with exercise, it exhibits synergistic effects; however, its efficacy is confounded by adherence to lifestyle factors. Eriocitrin has shown improvements in glycemia, oxidative stress, and GLP-1 secretion in prediabetic individuals, but evidence remains limited to short-duration studies with small cohorts [116]. Omega-3 fatty acids, widely studied in metabolic and cardiovascular diseases, often require high doses and show variable outcomes, including paradoxical reductions in insulin sensitivity in specific populations [117]. Novel pharmacological agents such as sonlicromanol and GC-4419, though mechanistically compelling, still face uncertainties regarding long-term safety, patient selection, and cost-effectiveness [118], while compounds like crisdesalazine and AT-001 remain at early investigational stages with limited efficacy data [119]. To address these limitations, several promising strategies have emerged. For example, solid lipid nanoparticles and micelles have been used to enhance curcumin’s dissolution rate, stability, and systemic absorption [120,121,122]. Additionally, prodrugs or analogs were used to improve metabolic stability and pharmacokinetics, although these are more commonly explored in preclinical settings [123]. Enhancers like piperine, an alkaloid from black pepper, substantially improved curcumin exposure by inhibiting hepatic and intestinal glucuronidation and efflux transporters [124].
Collectively, these limitations highlight the need for improved delivery systems, precision-medicine strategies, and well-designed long-term clinical trials to establish reliable therapeutic roles for these agents. Beyond pharmacological limitations, individual variability in therapeutic response is also a critical factor affecting clinical outcomes. Genetic polymorphisms in drug-metabolizing enzymes (e.g., CYP450 isoforms), variation in gut microbiota composition, and differing baseline levels of oxidative stress or inflammation may influence the effectiveness and safety of antioxidant interventions [125]. Some individuals, particularly those with elevated oxidative markers, may respond favorably to treatment, whereas others might experience negligible benefits or even adverse pro-oxidant effects. This reinforces the need for personalized antioxidant therapy, where interventions are tailored based on the individual’s genetic, metabolic, and microbiomic profiles. Moreover, the translational gap from preclinical research to clinical practice remains wide. Although many in vitro and animal studies demonstrate robust anti-inflammatory and antioxidant effects, human trials frequently yield mixed or inconclusive results. These discrepancies often arise from differences in disease complexity, inconsistent dosing regimens, heterogeneous patient populations, and varied endpoints. For example, while polyphenol-based treatments may significantly reduce inflammatory cytokines in animal models, equivalent clinical trials may show only marginal improvements in human subjects [126,127,128]. This gap underscores the importance of standardizing clinical trial methodologies, developing predictive biomarkers, and conducting large-scale, long-term studies to assess real-world efficacy.
11. Reconciling Discrepancies Between Preclinical and Clinical Outcomes in Oxidative Stress Modulation
Despite the strong evidence that antioxidant interventions are effective in preclinical models, clinical trials often show minimal or inconsistent effects on oxidative stress biomarkers. Several connected factors contribute to this gap in translation. First, inadequate dosing and pharmacokinetic limitations restrict clinical effectiveness. Many trials use doses that do not reach tissue levels comparable to those in animal or in vitro studies. Non-enzymatic antioxidants often react more slowly with reactive species, such as superoxide, at rates significantly lower than those of nitric oxide, which substantially reduces their effectiveness in living organisms [6]. Second, patient heterogeneity and disease complexity contribute to variability in outcomes. Clinical populations include patients at different disease stages, with diverse comorbidities and genetic backgrounds, making uniform responses unlikely. Trials are often underpowered or lack stratified designs to account for this variability [129]. Third, insensitive or non-specific biomarkers can obscure subtle effects. Standard plasma measures, such as MDA, SOD, or 8-isoprostanes, may not capture localized oxidative stress, and methodological inconsistencies further complicate interpretation. Fourth, treatment duration and study design issues play a role. Chronic diseases evolve over decades, whereas most clinical studies are conducted over weeks or months, limiting the ability to detect long-term molecular or clinical benefits [130,131]. Finally, mechanistic discordance between preclinical and clinical settings contributes to outcome discrepancies. Preclinical models often rely on severe oxidative insults or controlled environments, while human disease involves multifactorial, chronic processes. Moreover, some antioxidants exert effects through non-redox pathways, complicating biomarker-based assessments [132]. Together, these considerations emphasize the need for optimized dosing strategies, patient stratification, longer follow-up, and deployment of sensitive biomarker panels to bridge the gap between promising preclinical results and variable clinical outcomes.
12. Emerging Strategies and Future Directions
12.1. Combination Therapies: Synergistic Targeting of Oxidative Stress and Inflammation
Given the complex interplay between oxidative stress and inflammation in the development of chronic diseases, combination therapies that target both pathways simultaneously have shown promising results. Using antioxidants and anti-inflammatory agents together can create synergistic effects, resulting in increased effectiveness and fewer side effects compared to using either therapy alone. For instance, a combination of N-acetylcysteine (NAC) and curcumin significantly reduced oxidative damage and neuroinflammation in models of Alzheimer’s disease by lowering neuronal apoptosis, lipid peroxidation, and pro-inflammatory cytokines such as TNF-α and IL-1β [133]. Similarly, the co-administration of resveratrol and omega-3 fatty acids has been shown to improve insulin sensitivity and decrease systemic inflammation in metabolic syndrome by modulating key signaling pathways like NF-κB and MAPK [134,135].
12.2. Targeted Delivery Systems: Nanotechnology-Driven Precision in Antioxidant and Anti-Inflammatory Therapy
Pharmacokinetic limitations, including poor aqueous solubility, rapid degradation, and nonspecific tissue distribution, often hinder the clinical translation of antioxidant and anti-inflammatory therapies. To address these challenges, nanotechnology-based delivery systems have emerged as a highly promising strategy, offering enhanced bioavailability, sustained release, and targeted delivery of therapeutic agents.
Liposomal delivery systems, composed of biocompatible phospholipid bilayers, have demonstrated strong potential for encapsulating both hydrophilic and lipophilic antioxidants. For example, curcumin-loaded liposomes have been shown to significantly improve therapeutic efficacy in experimental colitis by enhancing mucosal retention and reducing systemic toxicity compared to free curcumin [136].
Polymeric nanoparticles, such as those made from poly(lactic-co-glycolic acid) (PLGA), enable controlled release and protection of unstable bioactive compounds. Quercetin has demonstrated improved solubility, bioavailability, and cellular uptake when delivered via PLGA nanoparticles, resulting in better outcomes in models of hepatic oxidative stress [137].
Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) are lipid-based nanocarriers offering enhanced encapsulation efficiency, stability, and scalability. SLNs loaded with resveratrol were found to effectively protect neuronal cells from ROS-induced apoptosis by facilitating mitochondrial delivery and sustained intracellular release [138]. Emerging stimuli-responsive nanocarriers, designed to respond to specific pathological environments such as oxidative stress or acidic pH, provide even greater precision. For instance, glutathione-sensitive nanoparticles have been engineered to release their antioxidant cargo selectively in cells exhibiting high oxidative stress, thereby minimizing damage to healthy tissues [139]. These advances in nanotechnology enhance the therapeutic index of antioxidant and anti-inflammatory agents, offering a new frontier for precision medicine in managing chronic diseases associated with oxidative stress and inflammation.
12.3. Personalized Medicine and Biomarkers: Toward Precision Redox Therapy
Advancements in personalized medicine have opened new ways to customize antioxidant and anti-inflammatory treatments based on individual biomarker profiles. Biomarkers such as 8-hydroxy-2′-deoxyguanosine (8-OHdG), malondialdehyde (MDA), C-reactive protein (CRP), IL-6, and TNF-α serve as indicators of oxidative damage and inflammatory status. Multi-omics technologies now enable the integration of genomic, transcriptomic, and metabolomic data to guide personalized therapy. Elevated levels of urinary 8-OHdG, for example, have been used to predict oxidative DNA damage and assess response to antioxidant therapies for cardiovascular patients. The combination of biomarker monitoring and computational tools can further refine treatment protocols, leading to more effective and individualized therapies [140,141,142].
12.4. Gut Microbiome as a Modulator of Oxidative Stress and Inflammation
Recent advances identify the gut microbiome as a promising therapeutic target to break the cycle of oxidative stress and inflammation in chronic and metabolic diseases. Dysbiosis is associated with the excessive production of pro-oxidant metabolites, weakened antioxidant defenses, and the release of microbial products, such as lipopolysaccharides, that activate the NF-κB and inflammasome pathways [143]. These changes promote systemic inflammation and oxidative damage, contributing to conditions like cardiovascular disorders, type 2 diabetes, neurodegeneration, and cancer. Interventions that restore microbial balance are an emerging strategy to counteract these processes. Probiotics and prebiotics have demonstrated effectiveness in reducing circulating markers of oxidative stress and inflammation in metabolic syndrome and diabetes [144,145]. Dietary polyphenols not only boost microbial diversity but also work with microbial metabolism to produce metabolites that activate NRF2 signaling and reduce NF-κB activity [47,146]. Additional experimental approaches, such as fecal microbiota transplantation, have shown promise in improving insulin sensitivity and reducing low-grade inflammation in metabolic disorders [147,148,149].
Positioning the microbiome as a therapeutic target opens novel opportunities to complement existing antioxidant and anti-inflammatory therapies. By modulating host redox balance and immune signaling at their source, microbiome-directed interventions may offer a durable and systemic way to break the vicious cycle of oxidative stress and inflammation.
12.5. Synthetic Derivatives of Antioxidants: Advances and Therapeutic Potential
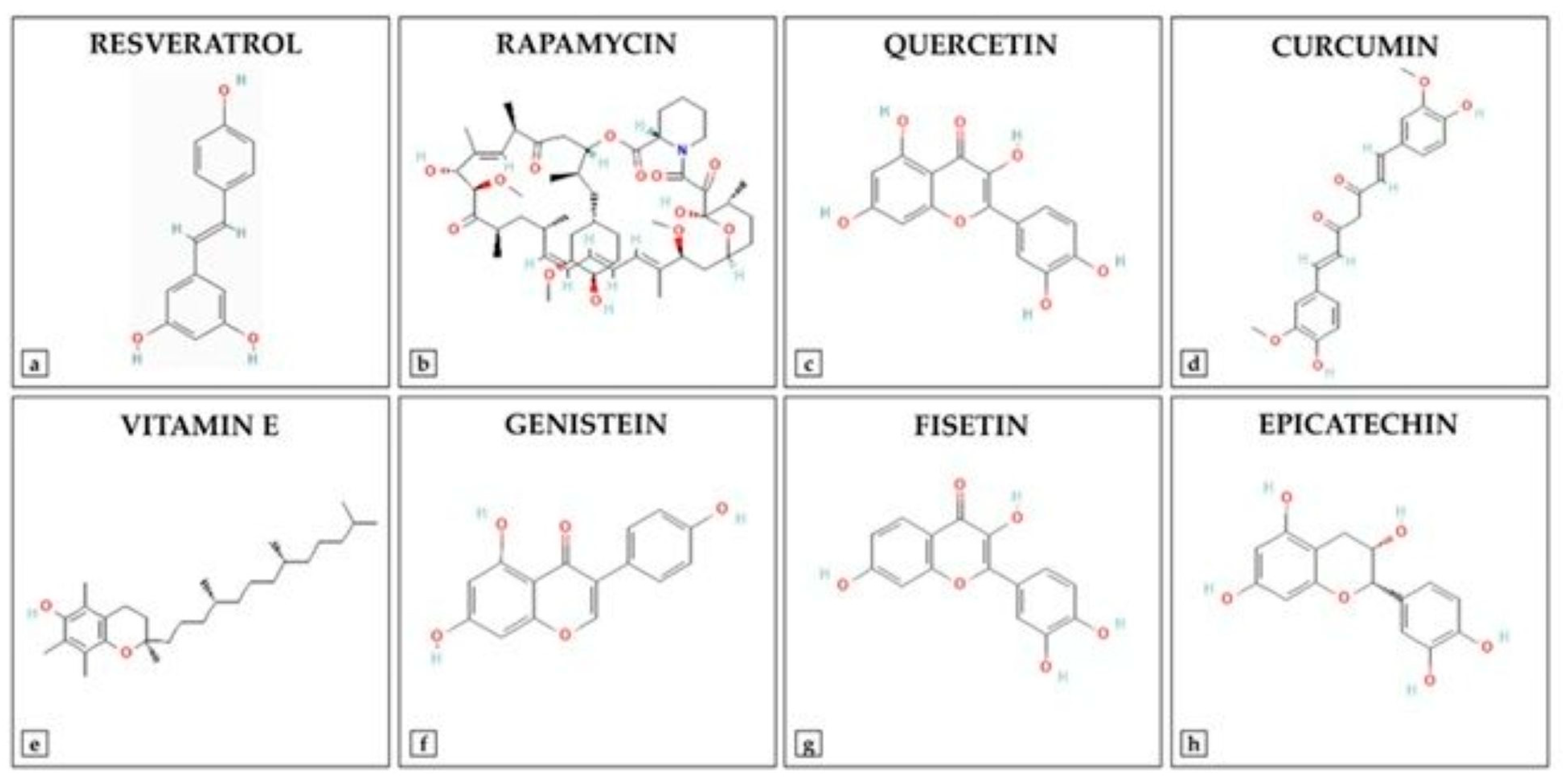
Advancements in medicinal chemistry have led to the development of synthetic derivatives of natural antioxidants (Figure 3), aiming to overcome limitations such as poor bioavailability, rapid metabolism, and limited stability. To enhance the therapeutic potential of natural antioxidants, researchers have developed various synthetic derivatives aimed at improving bioavailability, stability, and efficacy. These modifications have resulted in compounds with superior antioxidant, anti-inflammatory, and anticancer properties compared to their natural counterparts. For example, Curcumin has demonstrated multiple bioactivities. However, its clinical application is limited due to poor bioavailability and rapid metabolism. To address these limitations, several curcumin analogs have been synthesized. EF24 (3,5-bis[(2-fluorophenyl)methylidene]piperidin-4-one): EF24 is a synthetic analog of curcumin that exhibits enhanced bioavailability and potent bioactivity, including anti-cancer, anti-inflammatory, and anti-bacterial effects [150]. It induces cell cycle arrest and apoptosis via a redox-dependent mechanism, primarily through its inhibitory effect on the NF-κB pathway. Recent studies have reported the synthesis of twelve asymmetric curcumin analogs using Pabon’s method [151]. These compounds have shown improved antioxidant abilities compared to curcumin, with some exhibiting low to moderate yields. Resveratrol Derivatives have been extensively studied for their antioxidant properties. To enhance its efficacy, several synthetic derivatives have been developed. Various resveratrol derivatives have been synthesized to improve their biological activities, including anticancer, antioxidant, and anti-inflammatory effects. These modifications aim to overcome the limitations of resveratrol’s bioavailability and stability [152]. Flavonoid-based synthetic antioxidants possess significant antioxidant properties. Synthetic modifications of flavonoids have led to compounds with enhanced stability and bioactivity [153]. Synthesis of various flavonoid derivatives has been explored to improve their antioxidant and therapeutic properties. These derivatives have been synthesized to enhance the pharmacological activities of flavonoids while overcoming their limitations. These synthetic derivatives represent promising strategies to increase the therapeutic potential of natural antioxidants, addressing challenges such as poor bioavailability and rapid metabolism. Additionally, other derivatives, including sulfonamide-modified gallic acid and Trolox-based compounds, exhibit enhanced antioxidant and anti-inflammatory properties, offering improved therapeutic potential [154]. For instance, gallic acid sulfonamide derivatives demonstrate increased oral bioavailability and sustained antioxidant activity, while Trolox derivatives have shown promising anti-inflammatory effects [154,155]. Such modifications not only enhance the pharmacokinetic profiles of these compounds but also expand their therapeutic applications in treating disorders related to oxidative stress. The ongoing research on these synthetic derivatives highlights their important role in developing new therapeutic agents that target oxidative stress and inflammation.
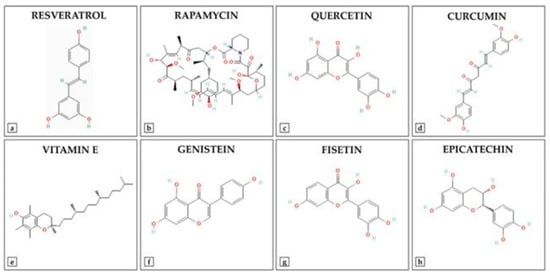
Figure 3.
Chemical Structures of Key Antioxidant Compounds.
13. Conclusions
This review has explored the complex link between oxidative stress and inflammation, highlighting their key roles in the development and progression of various chronic diseases. The evidence shows that oxidative stress not only causes cellular damage but also worsens inflammatory responses, sustaining pathological processes in conditions such as cardiovascular disease, neurodegeneration, chronic kidney disease, diabetes, and cancer. Natural and synthetic antioxidants are promising in reducing these harmful effects by neutralizing reactive oxygen species and affecting pro-inflammatory pathways. Importantly, combination therapies that include antioxidant and anti-inflammatory agents demonstrate synergistic effects, providing better results and fewer side effects than using single drugs. Recent advances in nanotechnology and targeted delivery systems have significantly improved the pharmacokinetics of these agents, enabling precise, site-specific treatments with minimal systemic side effects. The development of personalized medicine and the use of biomarkers have further enhanced therapeutic strategies, allowing for tailored treatments based on individual oxidative and inflammatory profiles. This move toward precision medicine offers great hope for improved clinical outcomes and a reduction in the burden of chronic diseases associated with oxidative stress.
Author Contributions
Conceptualization, S.Y.A. and A.B.; methodology, S.Y.A. and A.B.; writing—original draft preparation, S.Y.A. and A.B.; writing—review and editing, S.Y.A., N.D., and A.B.; supervision, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no funding. The content of this review reflects the authors’ own opinions, views, and responsibilities. It does not represent the official position or opinions of KFU, Rochester General Hospital, or KAIMRC/KSAU-MNGHA.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Han, Y.; Li, L.; Wang, Y.; Fan, W. Association of dietary inflammatory index and oxidative balance score with all-cause and cardiovascular mortality in US non-diabetic adults. Front. Nutr. 2025, 12, 1607162. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Valko, R.; Fresser, L.; Nepovimova, E.; Kuca, K.; Valko, M. Interplay of oxidative stress and antioxidant mechanisms in cancer development and progression. Arch. Toxicol. 2025. [Google Scholar] [CrossRef]
- Sharma, V.; Sharma, P.; Singh, T.G. Modulation of nitric oxide signaling by flavonoids: Implications for neurodegeneration. Mol. Biol. Rep. 2025, 52, 894. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef] [PubMed]
- Kim, K. Glutathione in the Nervous System as a Potential Therapeutic Target to Control the Development and Progression of Amyotrophic Lateral Sclerosis. Antioxidants 2021, 10, 1011. [Google Scholar] [CrossRef]
- Kumar, S.; Saxena, J.; Srivastava, V.K.; Kaushik, S.; Singh, H.; Abo-El-Sooud, K.; Abdel-Daim, M.M.; Jyoti, A.; Saluja, R. The Interplay of Oxidative Stress and ROS Scavenging: Antioxidants as a Therapeutic Potential in Sepsis. Vaccines 2022, 10, 1575. [Google Scholar] [CrossRef]
- Amin, F.; Bano, B. Damage of cystatin due to ROS-generation and radical-scavenging activity of antioxidants and associated compounds. Int. J. Biol. Macromol. 2018, 119, 369–379. [Google Scholar] [CrossRef]
- Razali, N.A.; Nazarudin, N.A.; Lai, K.S.; Abas, F.; Ahmad, S. Curcumin derivative, 2,6-bis(2-fluorobenzylidene)cyclohexanone (MS65) inhibits interleukin-6 production through suppression of NF-kappaB and MAPK pathways in histamine-induced human keratinocytes cell (HaCaT). BMC Complement. Altern. Med. 2018, 18, 217. [Google Scholar] [CrossRef]
- Kang, O.H.; Jang, H.J.; Chae, H.S.; Oh, Y.C.; Choi, J.G.; Lee, Y.S.; Kim, J.H.; Kim, Y.C.; Sohn, D.H.; Park, H.; et al. Anti-inflammatory mechanisms of resveratrol in activated HMC-1 cells: Pivotal roles of NF-kappaB and MAPK. Pharmacol. Res. 2009, 59, 330–337. [Google Scholar] [CrossRef]
- Dama, A.; Shpati, K.; Daliu, P.; Dumur, S.; Gorica, E.; Santini, A. Targeting Metabolic Diseases: The Role of Nutraceuticals in Modulating Oxidative Stress and Inflammation. Nutrients 2024, 16, 507. [Google Scholar] [CrossRef]
- Mohammadi, N.; Farrell, M.; O’Sullivan, L.; Langan, A.; Franchin, M.; Azevedo, L.; Granato, D. Effectiveness of anthocyanin-containing foods and nutraceuticals in mitigating oxidative stress, inflammation, and cardiovascular health-related biomarkers: A systematic review of animal and human interventions. Food Funct. 2024, 15, 3274–3299. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Geicu, O.I.; Bilteanu, L.; Serban, A.I. Antioxidant, anti-inflammatory and immunomodulatory roles of vitamins in COVID-19 therapy. Eur. J. Med. Chem. 2022, 232, 114175. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Juan, C.A.; Perez de la Lastra, J.M.; Plou, F.J.; Perez-Lebena, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Saputra, F.; Kishida, M.; Hu, S.Y. Oxidative stress induced by hydrogen peroxide disrupts zebrafish visual development by altering apoptosis, antioxidant and estrogen related genes. Sci. Rep. 2024, 14, 14454. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, A.; Kozlov, A.V. Biological Activities of Reactive Oxygen and Nitrogen Species: Oxidative Stress versus Signal Transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D. The sites and topology of mitochondrial superoxide production. Exp. Gerontol. 2010, 45, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Veal, E.A.; Day, A.M.; Morgan, B.A. Hydrogen peroxide sensing and signaling. Mol. Cell 2007, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G. Cell signaling. H2O2, a necessary evil for cell signaling. Science 2006, 312, 1882–1883. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Oxygen free radicals and iron in relation to biology and medicine: Some problems and concepts. Arch. Biochem. Biophys. 1986, 246, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. The biological chemistry of hydrogen peroxide. Methods Enzymol. 2013, 528, 3–25. [Google Scholar] [CrossRef]
- Forstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Radi, R. Peroxynitrite, a stealthy biological oxidant. J. Biol. Chem. 2013, 288, 26464–26472. [Google Scholar] [CrossRef]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef]
- Ayala, A.; Munoz, M.F.; Arguelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Brigelius-Flohe, R. Glutathione peroxidases and redox-regulated transcription factors. Biol. Chem. 2006, 387, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Lee, S.W.; Yoon, J.H.; Park, Y.G.; Choi, Y.J.; Nam, S.W.; Lee, J.Y.; Wang, Y.P.; Park, W.S. Association of SOD1 and SOD2 single nucleotide polymorphisms with susceptibility to gastric cancer in a Korean population. APMIS 2013, 121, 246–256. [Google Scholar] [CrossRef]
- Staerck, C.; Gastebois, A.; Vandeputte, P.; Calenda, A.; Larcher, G.; Gillmann, L.; Papon, N.; Bouchara, J.P.; Fleury, M.J.J. Microbial antioxidant defense enzymes. Microb. Pathog. 2017, 110, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Pendurthi, U.R.; Rao, L.V.M. The lipid peroxidation product 4-hydroxy-2-nonenal induces tissue factor decryption via ROS generation and the thioredoxin system. Blood Adv. 2017, 1, 2399–2413. [Google Scholar] [CrossRef]
- Khanam, H.; Ali, A.; Asif, M.; Shamsuzzaman. Neurodegenerative diseases linked to misfolded proteins and their therapeutic approaches: A review. Eur. J. Med. Chem. 2016, 124, 1121–1141. [Google Scholar] [CrossRef]
- Paulis, G. Inflammatory mechanisms and oxidative stress in prostatitis: The possible role of antioxidant therapy. Res. Rep. Urol. 2018, 10, 75–87. [Google Scholar] [CrossRef]
- Karin, M.; Takahashi, T.; Kapahi, P.; Delhase, M.; Chen, Y.; Makris, C.; Rothwarf, D.; Baud, V.; Natoli, G.; Guido, F.; et al. Oxidative stress and gene expression: The AP-1 and NF-kappaB connections. Biofactors 2001, 15, 87–89. [Google Scholar] [CrossRef]
- Stojanovic, B.; Jovanovic, I.; Dimitrijevic Stojanovic, M.; Stojanovic, B.S.; Kovacevic, V.; Radosavljevic, I.; Jovanovic, D.; Miletic Kovacevic, M.; Zornic, N.; Arsic, A.A.; et al. Oxidative Stress-Driven Cellular Senescence: Mechanistic Crosstalk and Therapeutic Horizons. Antioxidants 2025, 14, 987. [Google Scholar] [CrossRef]
- Yan, Z.; Chen, Q.; Xia, Y. Oxidative Stress Contributes to Inflammatory and Cellular Damage in Systemic Lupus Erythematosus: Cellular Markers and Molecular Mechanism. J. Inflamm. Res. 2023, 16, 453–465. [Google Scholar] [CrossRef]
- Silva, B.R.; Silva, J.R.V. Mechanisms of action of non-enzymatic antioxidants to control oxidative stress during in vitro follicle growth, oocyte maturation, and embryo development. Anim. Reprod. Sci. 2023, 249, 107186. [Google Scholar] [CrossRef] [PubMed]
- El Assar, M.; Angulo, J.; Vallejo, S.; Peiro, C.; Sanchez-Ferrer, C.F.; Rodriguez-Manas, L. Mechanisms involved in the aging-induced vascular dysfunction. Front. Physiol. 2012, 3, 132. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, S. Targeting oxidative stress for the treatment of ischemic stroke: Upstream and downstream therapeutic strategies. Brain Circ. 2016, 2, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Li, K.; Chen, Y.; Xie, J.; Wu, C.; Cui, C.; Deng, B. Oxidative Stress in Diabetic Peripheral Neuropathy: Pathway and Mechanism-Based Treatment. Mol. Neurobiol. 2023, 60, 4574–4594. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Rodrigo, R.; Prieto, J.C.; Aguayo, R.; Ramos, C.; Puentes, A.; Gajardo, A.; Panieri, E.; Rojas-Sole, C.; Lillo-Moya, J.; Saso, L. Joint Cardioprotective Effect of Vitamin C and Other Antioxidants against Reperfusion Injury in Patients with Acute Myocardial Infarction Undergoing Percutaneous Coronary Intervention. Molecules 2021, 26, 5702. [Google Scholar] [CrossRef]
- Yim, S.; Malhotra, A.; Veves, A. Antioxidants and CVD in diabetes: Where do we stand now. Curr. Diab Rep. 2007, 7, 8–13. [Google Scholar] [CrossRef][Green Version]
- Almeida, S.; Alves, M.G.; Sousa, M.; Oliveira, P.F.; Silva, B.M. Are Polyphenols Strong Dietary Agents Against Neurotoxicity and Neurodegeneration? Neurotox. Res. 2016, 30, 345–366. [Google Scholar] [CrossRef]
- Verma, V.K.; Ramesh, V.; Tewari, S.; Gupta, R.K.; Sinha, N.; Pandey, C.M. Role of bilirubin, vitamin C and ceruloplasmin as antioxidants in coronary artery disease [CAD]. Indian. J. Clin. Biochem. 2005, 20, 68–74. [Google Scholar] [CrossRef][Green Version]
- Shaikh, Z.A.; Vu, T.T.; Zaman, K. Oxidative stress as a mechanism of chronic cadmium-induced hepatotoxicity and renal toxicity and protection by antioxidants. Toxicol. Appl. Pharmacol. 1999, 154, 256–263. [Google Scholar] [CrossRef]
- Baradaran, A.; Nasri, H.; Rafieian-Kopaei, M. Protection of renal tubular cells by antioxidants: Current knowledge and new trends. Cell J. 2015, 16, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Perez-Torres, I.; Castrejon-Tellez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef]
- D’Souza, L.C.; Kuriakose, N.; Raghu, S.V.; Kabekkodu, S.P.; Sharma, A. ROS-directed activation of Toll/NF-kappaB in the hematopoietic niche triggers benzene-induced emergency hematopoiesis. Free Radic. Biol. Med. 2022, 193, 190–201. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Kojima, R.; Ito, M. Influence of aging on gastric ulcer healing activities of the antioxidants alpha-tocopherol and probucol. Eur. J. Pharmacol. 2008, 601, 143–147. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, H.; Yu, B.; He, J.; Mao, X.; Yu, J.; Zheng, P.; Huang, Z.; Luo, Y.; Luo, J.; et al. Protective Effects of Natural Antioxidants on Inflammatory Bowel Disease: Thymol and Its Pharmacological Properties. Antioxidants 2022, 11, 1947. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, M.; Leal-Campanario, R.; Campos-Esparza, M.R.; Sanchez-Gomez, M.V.; Alberdi, E.; Arranz, A.; Delgado-Garcia, J.M.; Gruart, A.; Matute, C. Neuroprotection by two polyphenols following excitotoxicity and experimental ischemia. Neurobiol. Dis. 2006, 23, 374–386. [Google Scholar] [CrossRef]
- Vicente-Zurdo, D.; Gomez-Mejia, E.; Rosales-Conrado, N.; Leon-Gonzalez, M.E. A Comprehensive Analytical Review of Polyphenols: Evaluating Neuroprotection in Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 5906. [Google Scholar] [CrossRef] [PubMed]
- Bakir, S.; Catalkaya, G.; Ceylan, F.D.; Khan, H.; Guldiken, B.; Capanoglu, E.; Kamal, M.A. Role of Dietary Antioxidants in Neurodegenerative Diseases: Where are We Standing? Curr. Pharm. Des. 2020, 26, 714–729. [Google Scholar] [CrossRef]
- Di Matteo, V.; Esposito, E. Biochemical and therapeutic effects of antioxidants in the treatment of Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis. Curr. Drug Targets CNS Neurol. Disord. 2003, 2, 95–107. [Google Scholar] [CrossRef]
- Akhter, N.; Wilson, A.; Thomas, R.; Al-Rashed, F.; Kochumon, S.; Al-Roub, A.; Arefanian, H.; Al-Madhoun, A.; Al-Mulla, F.; Ahmad, R.; et al. ROS/TNF-alpha Crosstalk Triggers the Expression of IL-8 and MCP-1 in Human Monocytic THP-1 Cells via the NF-kappaB and ERK1/2 Mediated Signaling. Int. J. Mol. Sci. 2021, 22, 10519. [Google Scholar] [CrossRef]
- Liu, J.; Han, X.; Zhang, T.; Tian, K.; Li, Z.; Luo, F. Reactive oxygen species (ROS) scavenging biomaterials for anti-inflammatory diseases: From mechanism to therapy. J. Hematol. Oncol. 2023, 16, 116. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Y.; Xiong, Z.; Xie, W.; Shao, M.; Liu, Z. Broad-Spectrum ROS/RNS Scavenging Catalase-Loaded Microreactors for Effective Oral Treatment of Inflammatory Bowel Diseases. Small 2025, 21, e2501341. [Google Scholar] [CrossRef]
- Feng, X.Q.; Deng, A.P.; Wu, Y.Q.; Cai, C.Z.; Ye, X.Q.; Liu, P.F.; Huang, X.J.; Li, Z.J.; Xu, Z.F. Cardiac-specific overexpression of Klotho attenuates paraquat-induced myocardial injury by enhancing the Nrf2/ARE signaling pathway. J. Cardiovasc. Pharmacol. 2025, 86, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Alruhaimi, R.S.; Hassanein, E.H.M.; Alnasser, S.M.; Ahmeda, A.F.; Althagafy, H.S.; Allam, A.M.T.; Qebesy, H.S.; Mahmoud, A.M. Attenuation of NF-kappaB/NLRP3 inflammasome axis and oxidative stress, and upregulation of Nrf2/HO-1 signaling mediate the protective effect of S-carboxymethylcysteine against cyclophosphamide-induced cardiotoxicity. Tissue Cell 2025, 97, 103092. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhou, D.; Yan, B. Eriocitrin alleviates oxidative stress and inflammatory response in cerebral ischemia reperfusion rats by regulating phosphorylation levels of Nrf2/NQO-1/HO-1/NF-kappaB p65 proteins. Ann. Transl. Med. 2020, 8, 757. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Park, J.E.; Park, J.S.; Leem, Y.H.; Kim, D.Y.; Hyun, J.W.; Kim, H.S. Anti-inflammatory and antioxidant mechanisms of coniferaldehyde in lipopolysaccharide-induced neuroinflammation: Involvement of AMPK/Nrf2 and TAK1/MAPK/NF-kappaB signaling pathways. Eur. J. Pharmacol. 2024, 979, 176850. [Google Scholar] [CrossRef]
- Rizk, S.K.; Ali, E.A.; Sheref, A.A.M.; Tayel, S.G.; El Derbaly, S.A. Vitamin D and canagliflozin combination alleviates Parkinson’s disease in rats through modulation of RAC1/NF-kappaB/Nrf2 interaction. Immunopharmacol. Immunotoxicol. 2025, 47, 328–344. [Google Scholar] [CrossRef]
- Yoon, Y.E.; Ju, S.H.; Kim, Y.; Lee, S.J. Natural Flavonoids for the Prevention of Sarcopenia: Therapeutic Potential and Mechanisms. Int. J. Mol. Sci. 2025, 26, 7458. [Google Scholar] [CrossRef]
- Cadar, E.; Popescu, A.; Dragan, A.M.; Pesterau, A.M.; Pascale, C.; Anuta, V.; Prasacu, I.; Velescu, B.S.; Tomescu, C.L.; Bogdan-Andreescu, C.F.; et al. Bioactive Compounds of Marine Algae and Their Potential Health and Nutraceutical Applications: A Review. Mar. Drugs 2025, 23, 152. [Google Scholar] [CrossRef]
- Khan, S.; Ali, A.; Khan, S.; Bakillah, A.; Damanhouri, G.; Khan, A.; Makki, A.; AlAnsari, I.; Banu, N. Current therapies in alleviating liver disorders and cancers with a special focus on the potential of vitamin D. Nutr. Metab. 2018, 15, 13. [Google Scholar] [CrossRef]
- Caponio, G.R.; Lippolis, T.; Tutino, V.; Gigante, I.; De Nunzio, V.; Milella, R.A.; Gasparro, M.; Notarnicola, M. Nutraceuticals: Focus on Anti-Inflammatory, Anti-Cancer, Antioxidant Properties in Gastrointestinal Tract. Antioxidants 2022, 11, 1274. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; D’Angelo, A.; Maffioli, P. The role of selected nutraceuticals in management of prediabetes and diabetes: An updated review of the literature. Phytother. Res. 2022, 36, 3709–3765. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Dewapriya, P. Bioactive compounds from marine sponges and their symbiotic microbes: A potential source of nutraceuticals. Adv. Food Nutr. Res. 2012, 65, 137–151. [Google Scholar] [CrossRef]
- Kumari, A.; Garima; Bharadvaja, N. A comprehensive review on algal nutraceuticals as prospective therapeutic agent for different diseases. 3 Biotech 2023, 13, 44. [Google Scholar] [CrossRef]
- Garza-Juarez, A.; Perez-Carrillo, E.; Arredondo-Espinoza, E.U.; Islas, J.F.; Benitez-Chao, D.F.; Escamilla-Garcia, E. Nutraceuticals and Their Contribution to Preventing Noncommunicable Diseases. Foods 2023, 12, 3262. [Google Scholar] [CrossRef]
- Kocsis, A.E.; Kucsapszky, N.; Santa-Maria, A.R.; Hunyadi, A.; Deli, M.A.; Walter, F.R. Much More than Nutrients: The Protective Effects of Nutraceuticals on the Blood-Brain Barrier in Diseases. Nutrients 2025, 17, 766. [Google Scholar] [CrossRef]
- Basegmez, M.; Eryavuz, A.; Demirel, H.H. Effects of Vitamin C Supplementation on Total Antioxidant Status, Inflammation, and Histopathological Changes in Aged Rats. J. Biochem. Mol. Toxicol. 2025, 39, e70324. [Google Scholar] [CrossRef]
- Sasidharan Nair, V.; Huehn, J. Impact of vitamin C on the development, differentiation and functional properties of T cells. Eur. J. Microbiol. Immunol. 2024, 14, 67–74. [Google Scholar] [CrossRef]
- Zhang, P.; Zang, M.; Sang, Z.; Wei, Y.; Yan, Y.; Bian, X.; Dong, S. Vitamin C alleviates LPS-induced myocardial injury by inhibiting pyroptosis via the ROS-AKT/mTOR signalling pathway. BMC Cardiovasc. Disord. 2022, 22, 561. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, K.; Venkataraman, G.; Parida, A. An oxidative and salinity stress induced peroxisomal ascorbate peroxidase from Avicennia marina: Molecular and functional characterization. Plant Physiol. Biochem. 2008, 46, 794–804. [Google Scholar] [CrossRef]
- Vineetha, R.C.; Hariharan, S.; Jaleel, A.; Chandran, M.; Nair, R.H. L-Ascorbic Acid and alpha-Tocopherol Synergistically Triggers Apoptosis Inducing Antileukemic Effects of Arsenic Trioxide via Oxidative Stress in Human Acute Promyelocytic Leukemia Cells. Front. Oncol. 2020, 10, 65. [Google Scholar] [CrossRef]
- Kontush, A.; Finckh, B.; Karten, B.; Kohlschutter, A.; Beisiegel, U. Antioxidant and prooxidant activity of alpha-tocopherol in human plasma and low density lipoprotein. J. Lipid Res. 1996, 37, 1436–1448. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Bohn, T. Carotenoids and Markers of Oxidative Stress in Human Observational Studies and Intervention Trials: Implications for Chronic Diseases. Antioxidants 2019, 8, 179. [Google Scholar] [CrossRef]
- Mordi, R.C.; Ademosun, O.T.; Ajanaku, C.O.; Olanrewaju, I.O.; Walton, J.C. Free Radical Mediated Oxidative Degradation of Carotenes and Xanthophylls. Molecules 2020, 25, 1038. [Google Scholar] [CrossRef] [PubMed]
- Cantin, L.; Daignault-Gelinas, M.; Latreille, J.; Bhat, P.V.; Lacroix, A. [Retinol, carotenes and nutritional status in metastatic breast cancer]. Union. Med. Can. 1988, 117, 29–36. [Google Scholar] [PubMed]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Futur. J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef]
- Kuljarusnont, S.; Iwakami, S.; Iwashina, T.; Tungmunnithum, D. Flavonoids and Other Phenolic Compounds for Physiological Roles, Plant Species Delimitation, and Medical Benefits: A Promising View. Molecules 2024, 29, 5351. [Google Scholar] [CrossRef]
- Aiello, A.; Medoro, A.; Accardi, G.; Calabro, A.; Carru, C.; Cannavo, A.; Caruso, C.; Candore, G.; Scapagnini, G.; Corbi, G.; et al. Polyunsaturated fatty acid status and markers of oxidative stress and inflammation across the lifespan: A cross-sectional study in a cohort with long-lived individuals. Exp. Gerontol. 2024, 195, 112531. [Google Scholar] [CrossRef]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Chaudhary, A.; Sethi, S. Oxidized omega-3 fatty acids inhibit NF-kappaB activation via a PPARalpha-dependent pathway. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1621–1627. [Google Scholar] [CrossRef]
- Lee, C. Collaborative Power of Nrf2 and PPARgamma Activators against Metabolic and Drug-Induced Oxidative Injury. Oxid. Med. Cell Longev. 2017, 2017, 1378175. [Google Scholar] [CrossRef]
- Szukiewicz, D. Potential Therapeutic Exploitation of G Protein-Coupled Receptor 120 (GPR120/FFAR4) Signaling in Obesity-Related Metabolic Disorders. Int. J. Mol. Sci. 2025, 26, 2501. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef]
- Shilovsky, G.A.; Dibrova, D.V. Regulation of Cell Proliferation and Nrf2-Mediated Antioxidant Defense: Conservation of Keap1 Cysteines and Nrf2 Binding Site in the Context of the Evolution of KLHL Family. Life 2023, 13, 1045. [Google Scholar] [CrossRef] [PubMed]
- Norris, P.C.; Skulas-Ray, A.C.; Riley, I.; Richter, C.K.; Kris-Etherton, P.M.; Jensen, G.L.; Serhan, C.N.; Maddipati, K.R. Identification of specialized pro-resolving mediator clusters from healthy adults after intravenous low-dose endotoxin and omega-3 supplementation: A methodological validation. Sci. Rep. 2018, 8, 18050. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Tokgozoglu, L.; Parhofer, K.G.; Handelsman, Y.; Leiter, L.A.; Landmesser, U.; Brinton, E.A.; Catapano, A.L. Icosapent ethyl for reduction of persistent cardiovascular risk: A critical review of major medical society guidelines and statements. Expert. Rev. Cardiovasc. Ther. 2022, 20, 609–625. [Google Scholar] [CrossRef]
- Huston, J.; Schaffner, H.; Cox, A.; Sperry, A.; McGee, S.; Lor, P.; Langley, L.; Skrable, B.; Ashchi, M.; Bisharat, M.; et al. A Critical Review of Icosapent Ethyl in Cardiovascular Risk Reduction. Am. J. Cardiovasc. Drugs 2023, 23, 393–406. [Google Scholar] [CrossRef]
- Zhang, W.; Gan, D.; Huo, S.; Chen, P. Unraveling the discrepancies between REDUCE-IT and STRENGTH trials with omega-3 fatty acids: New analytical approaches. Front. Nutr. 2024, 11, 1490953. [Google Scholar] [CrossRef] [PubMed]
- Tauchen, J.; Huml, L.; Jurasek, M.; Regenstein, J.M.; Ozogul, F. Synthetic and semi-synthetic antioxidants in medicine and food industry: A review. Front. Pharmacol. 2025, 16, 1599816. [Google Scholar] [CrossRef]
- Wilcox, C.S. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol. Ther. 2010, 126, 119–145. [Google Scholar] [CrossRef]
- Pant, T.; Uche, N.; Juric, M.; Zielonka, J.; Bai, X. Regulation of immunomodulatory networks by Nrf2-activation in immune cells: Redox control and therapeutic potential in inflammatory diseases. Redox Biol. 2024, 70, 103077. [Google Scholar] [CrossRef] [PubMed]
- Mucha, P.; Skoczynska, A.; Malecka, M.; Hikisz, P.; Budzisz, E. Overview of the Antioxidant and Anti-Inflammatory Activities of Selected Plant Compounds and Their Metal Ions Complexes. Molecules 2021, 26, 4886. [Google Scholar] [CrossRef] [PubMed]
- Shahcheraghi, S.H.; Salemi, F.; Peirovi, N.; Ayatollahi, J.; Alam, W.; Khan, H.; Saso, L. Nrf2 Regulation by Curcumin: Molecular Aspects for Therapeutic Prospects. Molecules 2021, 27, 167. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, T.; Menon, S.N.; Pandey, A.; Siddiqua, S.; Kuddus, S.A.; Rahman, M.M.; Khan, F.; Hoque, N.; Rana, M.S.; Subhan, N.; et al. Resveratrol attenuates hepatic oxidative stress and preserves gut mucosal integrity in high-fat diet-fed rats by modulating antioxidant and anti-inflammatory pathways. Sci. Rep. 2025, 15, 25162. [Google Scholar] [CrossRef]
- Truong, V.L.; Jun, M.; Jeong, W.S. Role of resveratrol in regulation of cellular defense systems against oxidative stress. Biofactors 2018, 44, 36–49. [Google Scholar] [CrossRef]
- Malaguarnera, L. Influence of Resveratrol on the Immune Response. Nutrients 2019, 11, 946. [Google Scholar] [CrossRef]
- Cho, S.Y.; Park, S.J.; Kwon, M.J.; Jeong, T.S.; Bok, S.H.; Choi, W.Y.; Jeong, W.I.; Ryu, S.Y.; Do, S.H.; Lee, C.S.; et al. Quercetin suppresses proinflammatory cytokines production through MAP kinases andNF-kappaB pathway in lipopolysaccharide-stimulated macrophage. Mol. Cell Biochem. 2003, 243, 153–160. [Google Scholar] [CrossRef]
- Cheng, S.C.; Huang, W.C.; JH, S.P.; Wu, Y.H.; Cheng, C.Y. Quercetin Inhibits the Production of IL-1beta-Induced Inflammatory Cytokines and Chemokines in ARPE-19 Cells via the MAPK and NF-kappaB Signaling Pathways. Int. J. Mol. Sci. 2019, 20, 2957. [Google Scholar] [CrossRef]
- Wang, P.; Li, Z.; Song, Y.; Zhang, B.; Fan, C. Resveratrol-driven macrophage polarization: Unveiling mechanisms and therapeutic potential. Front. Pharmacol. 2024, 15, 1516609. [Google Scholar] [CrossRef]
- Zhao, C.; Ding, Y.; Huang, Y.; Wang, C.; Guo, B.; Zhang, T. Quercetin Attenuates MRGPRX2-Mediated Mast Cell Degranulation via the MyD88/IKK/NF-kappaB and PI3K/AKT/ Rac1/Cdc42 Pathway. J. Inflamm. Res. 2024, 17, 7099–7110. [Google Scholar] [CrossRef]
- Ahmadabady, S.; Beheshti, F.; Shahidpour, F.; Khordad, E.; Hosseini, M. A protective effect of curcumin on cardiovascular oxidative stress indicators in systemic inflammation induced by lipopolysaccharide in rats. Biochem. Biophys. Rep. 2021, 25, 100908. [Google Scholar] [CrossRef]
- Zalpoor, H.; Nabi-Afjadi, M.; Forghaniesfidvajani, R.; Tavakol, C.; Farahighasreaboonasr, F.; Pakizeh, F.; Dana, V.G.; Seif, F. Quercetin as a JAK-STAT inhibitor: A potential role in solid tumors and neurodegenerative diseases. Cell Mol. Biol. Lett. 2022, 27, 60. [Google Scholar] [CrossRef] [PubMed]
- Serban, M.C.; Sahebkar, A.; Zanchetti, A.; Mikhailidis, D.P.; Howard, G.; Antal, D.; Andrica, F.; Ahmed, A.; Aronow, W.S.; Muntner, P.; et al. Effects of Quercetin on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2016, 5, e002713. [Google Scholar] [CrossRef]
- Nishida, Y.; Berg, P.C.; Shakersain, B.; Hecht, K.; Takikawa, A.; Tao, R.; Kakuta, Y.; Uragami, C.; Hashimoto, H.; Misawa, N.; et al. Astaxanthin: Past, Present, and Future. Mar. Drugs 2023, 21, 514. [Google Scholar] [CrossRef]
- Cesar, T.B.; Ramos, F.M.M.; Ribeiro, C.B. Nutraceutical Eriocitrin (Eriomin) Reduces Hyperglycemia by Increasing Glucagon-Like Peptide 1 and Downregulates Systemic Inflammation: A Crossover-Randomized Clinical Trial. J. Med. Food 2022, 25, 1050–1058. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Smeitink, J.; van Es, J.; Bosman, B.; Janssen, M.C.H.; Klopstock, T.; Gorman, G.; Vissing, J.; Ruiterkamp, G.; Edgar, C.J.; Abbink, E.J.; et al. Phase 2b program with sonlicromanol in patients with mitochondrial disease due to m.3243A>G mutation. Brain 2025, 148, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Oh, Y.H.; Lim, G.H.; An, J.H.; Lee, J.H.; Gwag, B.J.; Won, S.J.; Seo, K.W.; Youn, H.Y. Crisdesalazine alleviates inflammation in an experimental autoimmune encephalomyelitis multiple sclerosis mouse model by regulating the immune system. BMC Neurosci. 2025, 26, 1. [Google Scholar] [CrossRef]
- Tousson, E.; Beltagy, D.M.; Nawar, N.F.; Dora, M.A.; Bari, H.M.A.; El-Sayed, I.E.T. Curcumin nanoparticles ameliorates cardiac toxicity through modulation of oxidative stress, apoptosis, inflammation, and DNA damage in rat. Toxicol. Res. 2025, 14, tfaf112. [Google Scholar] [CrossRef]
- Yeo, S.; Kim, M.J.; Shim, Y.K.; Yoon, I.; Lee, W.K. Solid Lipid Nanoparticles of Curcumin Designed for Enhanced Bioavailability and Anticancer Efficiency. ACS Omega 2022, 7, 35875–35884. [Google Scholar] [CrossRef]
- Zhao, W.; Zeng, M.; Li, K.; Pi, C.; Liu, Z.; Zhan, C.; Yuan, J.; Su, Z.; Wei, Y.; Wen, J.; et al. Solid lipid nanoparticle as an effective drug delivery system of a novel curcumin derivative: Formulation, release in vitro and pharmacokinetics in vivo. Pharm. Biol. 2022, 60, 2300–2307. [Google Scholar] [CrossRef]
- Markovic, M.; Ben-Shabat, S.; Dahan, A. Prodrugs for Improved Drug Delivery: Lessons Learned from Recently Developed and Marketed Products. Pharmaceutics 2020, 12, 1031. [Google Scholar] [CrossRef]
- Bertoncini-Silva, C.; Vlad, A.; Ricciarelli, R.; Giacomo Fassini, P.; Suen, V.M.M.; Zingg, J.M. Enhancing the Bioavailability and Bioactivity of Curcumin for Disease Prevention and Treatment. Antioxidants 2024, 13, 331. [Google Scholar] [CrossRef]
- Zhao, M.; Ma, J.; Li, M.; Zhang, Y.; Jiang, B.; Zhao, X.; Huai, C.; Shen, L.; Zhang, N.; He, L.; et al. Cytochrome P450 Enzymes and Drug Metabolism in Humans. Int. J. Mol. Sci. 2021, 22, 12808. [Google Scholar] [CrossRef]
- Martin, D.A.; Bolling, B.W. A review of the efficacy of dietary polyphenols in experimental models of inflammatory bowel diseases. Food Funct. 2015, 6, 1773–1786. [Google Scholar] [CrossRef] [PubMed]
- Taslim, N.A.; Yusuf, M.; Ambari, A.M.; Del Rosario Puling, I.M.; Ibrahim, F.Z.; Hardinsyah, H.; Kurniawan, R.; Gunawan, W.B.; Mayulu, N.; Joseph, V.F.F.; et al. Anti-Inflammatory, Antioxidant, Metabolic and Gut Microbiota Modulation Activities of Probiotic in Cardiac Remodeling Condition: Evidence from Systematic Study and Meta-Analysis of Randomized Controlled Trials. Probiotics Antimicrob. Proteins 2023, 15, 1049–1061. [Google Scholar] [CrossRef]
- Jalouli, M.; Rahman, M.A.; Biswas, P.; Rahman, H.; Harrath, A.H.; Lee, I.S.; Kang, S.; Choi, J.; Park, M.N.; Kim, B. Targeting natural antioxidant polyphenols to protect neuroinflammation and neurodegenerative diseases: A comprehensive review. Front. Pharmacol. 2025, 16, 1492517. [Google Scholar] [CrossRef]
- Al-Madhagi, H.; Masoud, A. Limitations and Challenges of Antioxidant Therapy. Phytother. Res. 2024, 38, 5549–5566. [Google Scholar] [CrossRef] [PubMed]
- Ohlow, M.J.; Sohre, S.; Granold, M.; Schreckenberger, M.; Moosmann, B. Why Have Clinical Trials of Antioxidants to Prevent Neurodegeneration Failed?—A Cellular Investigation of Novel Phenothiazine-Type Antioxidants Reveals Competing Objectives for Pharmaceutical Neuroprotection. Pharm. Res. 2017, 34, 378–393. [Google Scholar] [CrossRef] [PubMed]
- Steinhubl, S.R. Why have antioxidants failed in clinical trials? Am. J. Cardiol. 2008, 101, 14D–19D. [Google Scholar] [CrossRef] [PubMed]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxid. Med. Cell Longev. 2017, 2017, 6501046. [Google Scholar] [CrossRef] [PubMed]
- Mursaleen, L.; Chan, S.H.Y.; Noble, B.; Somavarapu, S.; Zariwala, M.G. Curcumin and N-Acetylcysteine Nanocarriers Alone or Combined with Deferoxamine Target the Mitochondria and Protect against Neurotoxicity and Oxidative Stress in a Co-Culture Model of Parkinson’s Disease. Antioxidants 2023, 12, 130. [Google Scholar] [CrossRef]
- Kakoti, B.B.; Hernandez-Ontiveros, D.G.; Kataki, M.S.; Shah, K.; Pathak, Y.; Panguluri, S.K. Resveratrol and Omega-3 Fatty Acid: Its Implications in Cardiovascular Diseases. Front. Cardiovasc. Med. 2015, 2, 38. [Google Scholar] [CrossRef]
- Li, M.; Ding, L.; Cao, L.; Zhang, Z.; Li, X.; Li, Z.; Xia, Q.; Yin, K.; Song, S.; Wang, Z.; et al. Natural products targeting AMPK signaling pathway therapy, diabetes mellitus and its complications. Front. Pharmacol. 2025, 16, 1534634. [Google Scholar] [CrossRef]
- Wang, C.; Han, Z.; Wu, Y.; Lu, X.; Tang, X.; Xiao, J.; Li, N. Enhancing stability and anti-inflammatory properties of curcumin in ulcerative colitis therapy using liposomes mediated colon-specific drug delivery system. Food Chem. Toxicol. 2021, 151, 112123. [Google Scholar] [CrossRef]
- Baiomy, R.F.E. Quercetin nanoparticles as a therapeutic approach: Pharmacological actions and potential applications in therapy. BioTechnologia 2024, 105, 377–393. [Google Scholar] [CrossRef]
- Chung, J.H.; Lee, J.S.; Lee, H.G. Resveratrol-loaded chitosan-gamma-poly(glutamic acid) nanoparticles: Optimization, solubility, UV stability, and cellular antioxidant activity. Colloids Surf. B Biointerfaces 2020, 186, 110702. [Google Scholar] [CrossRef]
- Cheng, R.; Feng, F.; Meng, F.; Deng, C.; Feijen, J.; Zhong, Z. Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J. Control. Release 2011, 152, 2–12. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Kloska, D.; Grochot-Przeczek, A.; Feelisch, M.; Cuadrado, A.; van Goor, H. Personalized redox medicine in inflammatory bowel diseases: An emerging role for HIF-1alpha and NRF2 as therapeutic targets. Redox Biol. 2023, 60, 102603. [Google Scholar] [CrossRef] [PubMed]
- Moustakli, E.; Grigoriadis, T.; Stavros, S.; Potiris, A.; Zikopoulos, A.; Gerede, A.; Tsimpoukis, I.; Papageorgiou, C.; Louis, K.; Domali, E. Artificial Intelligence in Assessing Reproductive Aging: Role of Mitochondria, Oxidative Stress, and Telomere Biology. Diagnostics 2025, 15, 2075. [Google Scholar] [CrossRef]
- Pokushalov, E.; Ponomarenko, A.; Shrainer, E.; Kudlay, D.; Miller, R. Biomarker-Guided Dietary Supplementation: A Narrative Review of Precision in Personalized Nutrition. Nutrients 2024, 16, 4033. [Google Scholar] [CrossRef]
- Shen, Y.; Fan, N.; Ma, S.X.; Cheng, X.; Yang, X.; Wang, G. Gut Microbiota Dysbiosis: Pathogenesis, Diseases, Prevention, and Therapy. MedComm 2025, 6, e70168. [Google Scholar] [CrossRef]
- Chen, X.; Yan, L.; Yang, J.; Xu, C.; Yang, L. The impact of probiotics on oxidative stress and inflammatory markers in patients with diabetes: A meta-research of meta-analysis studies. Front. Nutr. 2025, 12, 1552358. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Kim, S.S. Probiotics and Prebiotics: Present Status and Future Perspectives on Metabolic Disorders. Nutrients 2016, 8, 173. [Google Scholar] [CrossRef]
- Scapagnini, G.; Caruso, C.; Calabrese, V. Therapeutic potential of dietary polyphenols against brain ageing and neurodegenerative disorders. Adv. Exp. Med. Biol. 2010, 698, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Boicean, A.; Ichim, C.; Sasu, S.M.; Todor, S.B. Key Insights into Gut Alterations in Metabolic Syndrome. J. Clin. Med. 2025, 14, 2678. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Liu, Y.; Shi, M.; Zhang, M.; Zhang, H.; Chen, J. Advances in fecal microbiota transplantation for the treatment of diabetes mellitus. Front. Cell. Infect. Microbiol. 2024, 14, 1370999. [Google Scholar] [CrossRef]
- Zhang, S.; Deng, F.; Chen, J.; Chen, F.; Wu, Z.; Li, L.; Hou, K. Fecal microbiota transplantation treatment of autoimmune-mediated type 1 diabetes: A systematic review. Front. Cell. Infect. Microbiol. 2022, 12, 1075201. [Google Scholar] [CrossRef]
- Sazdova, I.; Keremidarska-Markova, M.; Dimitrova, D.; Mitrokhin, V.; Kamkin, A.; Hadzi-Petrushev, N.; Bogdanov, J.; Schubert, R.; Gagov, H.; Avtanski, D.; et al. Anticarcinogenic Potency of EF24: An Overview of Its Pharmacokinetics, Efficacy, Mechanism of Action, and Nanoformulation for Drug Delivery. Cancers 2023, 15, 5478. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.J.; Li, C.W.; Kuo, C.L.; Shih, T.L.; Chen, J.J. Improved Synthesis of Asymmetric Curcuminoids and Their Assessment as Antioxidants. Molecules 2022, 27, 2547. [Google Scholar] [CrossRef]
- Gambini, J.; Ingles, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid. Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef]
- Xiao, J.; Kai, G.; Yamamoto, K.; Chen, X. Advance in dietary polyphenols as alpha-glucosidases inhibitors: A review on structure-activity relationship aspect. Crit. Rev. Food Sci. Nutr. 2013, 53, 818–836. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Gao, S.; Zhou, L.; Rong, K.; Zuo, F.; Tang, W.; Zhu, L. Trolox derivatives: Synthesis, structure-activity relationship and promote wound healing by regulating oxidative stress and inflammation. Bioorg. Chem. 2025, 154, 108045. [Google Scholar] [CrossRef] [PubMed]
- Alhyari, D.; Qinna, N.A.; Sheldrake, H.M.; Kantamneni, S.; Ghanem, B.Y.; Paluch, K.J. Antioxidant, Anti-Inflammatory, and Oral Bioavailability of Novel Sulfonamide Derivatives of Gallic Acid. Antioxidants 2025, 14, 374. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).