Guillain–Barré Syndrome in Older People—A Case Report and Literature Review
Abstract
1. Introduction
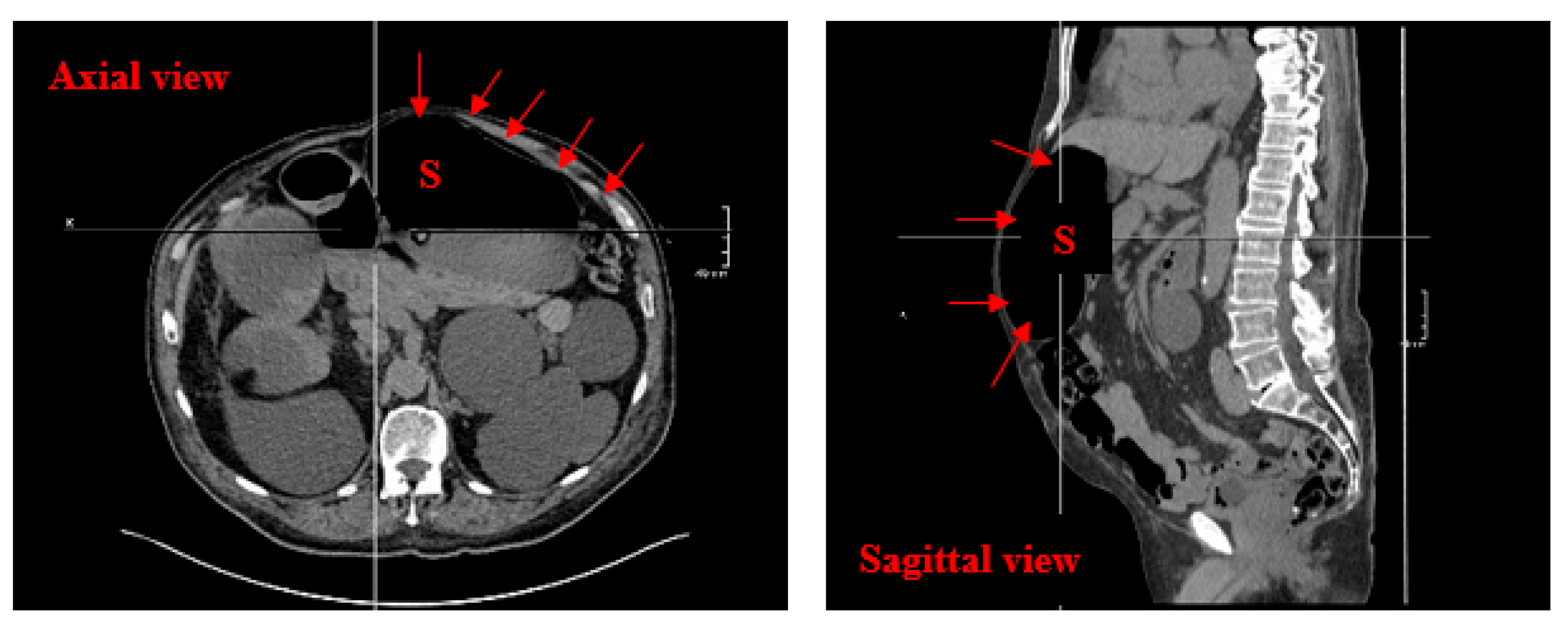
2. Case Report
3. Discussion
- Generally unwell
- Urinary retention or constipation
- Incontinence of urine or stool
- Leg weakness
- Less control of facial muscles
- Autonomic neuropathy
- Inability to climb stairs
- Night pain
- Breathing difficulties
- Ascending paralysis
- Rapid pulse
- Rapid spread of sensorimotor deficits
- Eye movement weakness
- Cardiac
- Vascular
- Gastrointestinal
- Genitourinary
- Sudomotor
- Hyperhidrosis, anhidrosis, hyperthermia, hypothermia
- Increasing respiratory distress
- ➢
- Breathless at rest
- ➢
- Shallow and rapid breathing
- ➢
- Reduced breath sounds at lung bases
- ➢
- Paradoxical breathing
- ➢
- Episodic use of accessory respiratory muscles
- ➢
- Inability to count >10 in one breath
- Imminent respiratory failure
- Symptomatic arrhythmias
- Unstable blood pressure
- Impaired cough reflex
- Difficulty swallowing
- Rapid progression of motor weakness
- Bulbar palsy
- Facial weakness
- Staccato speech with only a few words in one breath
- Mental clouding or somnolence
- Low oxygen saturation < 92%, PO2 < 8 KP, CO2 > 6 KP
- FVC < 20 mL/kg body weight
- Reduction in FVC by >30% from baseline
- Falling trend of FVC
- Inconsistent FVC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sejvar, J.J.; Baughman, A.L.; Wise, M.; Morgan, O.W. Population Incidence of Guillain-Barré Syndrome: A Systematic Review and Meta-Analysis. Neuroepidemiology 2011, 36, 123–133. [Google Scholar] [CrossRef]
- Leonhard, S.E.; Mandarakas, M.R.; Gondim, F.A.A.; Bateman, K.; Ferreiram, M.L.B.; Cornblath, D.R.; van Doorn, P.A.; Dourado, M.E.; Hughes, R.A.C.; Islam, B.; et al. Diagnosis and management of Guillain-Barré syndrome in ten steps. Nat. Rev. Neurol. 2019, 15, 671–683. [Google Scholar] [CrossRef]
- Wakerley, B.R.; Yuki, N. Infectious and noninfectious triggers in Guillain-Barré syndrome. Expert Rev. Clin. Immunol. 2013, 9, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Peric, S.; Berisavac, I.; Stojiljkovic Tamas, O.; Rajic, S.; Babic, M.; Cvijanovic, M.; Dominovic-Kovacevic, A.; Basta, I.; Beslac-Bumbasirevic, L.; Lavrnic, D. Guillain-Barré syndrome in the elderly. J. Peripher. Nerv. Syst. 2016, 21, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wu, X.; Shen, D.; Li, T.; Li, C.; Mao, M.; Zhang, H.-L.; Liu, K. The clinical characteristics and short-term prognosis in elderly patients with Guillain–Barré syndrome. Medicine 2017, 96, e5848. [Google Scholar] [CrossRef] [PubMed]
- Laborde, C.; Bador, J.; Hacquin, A.; Barben, J.; Putot, S.; Manckoundia, P.; Putot, A. Atypical Presentation of Bacteremic Urinary Tract Infection in Older Patients: Frequency and Prognostic Impact. Diagnostics 2021, 11, 523. [Google Scholar] [CrossRef]
- Watson, A.; Wilkinson, T.M.A. Respiratory viral infections in the elderly. Ther. Adv. Respir. Dis. 2021, 15, 1753466621995050. [Google Scholar] [CrossRef]
- Willison, H.J.; Jacobs, B.C.; Van Doorn, P.A. Guillain-Barré syndrome. Lancet 2016, 388, 717–727. [Google Scholar] [CrossRef]
- Levison, L.S.; Thomsen, R.W.; Andersen, H. Hospital-diagnosed morbidities and recent surgery as risk factors for developing Guillain-Barré syndrome. Eur. J. Neurol. 2023, 30, 3277–3285. [Google Scholar] [CrossRef]
- Van den Berg, B.; Walgaard, C.; Drenthen, J.; Fokke, C.; Jacobs, B.C.; van Doorn, P.A. Guillain-Barré syndrome: Pathogenesis, diagnosis, treatment and prognosis. Nat. Rev. Neurol. 2014, 10, 469–482. [Google Scholar] [CrossRef]
- Webb, A.J.S.; Brain, S.A.E.; Wood, R.; Rinaldi, S.; Turner, M.R. Seasonal variation in Guillain-Barré syndrome: A systematic review, meta-analysis and Oxfordshire cohort study. J. Neurol. Neurosurg. Psychiatry 2014, 86, 1196–1201. [Google Scholar] [CrossRef]
- Huang, W.-C.; Lu, C.-L.; Chen, S.C.-C. A 15-Year Nationwide Epidemiological Analysis of Guillain-Barré Syndrome in Taiwan. Neuroepidemiology 2015, 44, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, R.; Rinaldi, S. Guillain-Barré syndrome: A comprehensive review. Eur. J. Neurol. 2024, 31, e16365. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, K.; Kadkhoda, K. Early Lyme disease-associated Guillain Barre Syndrome: A case report. ID Cases 2022, 27, e01432. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Takahashi, S.; Kanda, A.; Watanabe, T.; Kakinuma, Y.; Yano, S.; Kinno, R. Case Report: Therapeutic effect of efgartigimod in refractory anti-GQ1b antibody syndrome coexisting with myasthenia gravis. Front. Immunol. 2025, 16, 1605985. [Google Scholar] [CrossRef]
- Solodovnikova, Y.; Revurko, A.; Oliinyk, S.; Son, A. Simultaneous development of Guillain–Barre syndrome and bacterial meningitis as complications of pneumonia caused by Staphylococcus aureus: A case report. J. Med. Case Rep. 2025, 19, 217. [Google Scholar] [CrossRef]
- Gómez-Dabó, L.; Llaurado, A.; Sánchez-Tejerina, D.; González, V.; Montalvo-Olmedo, C.; Lázaro-Hernández, C.; Rodrigo-Gisbert, M.; López-Maza, S.; Iza-Achutegui, M.; Giramé-Rizzo, L.; et al. A Rare Guillain-Barré Syndrome Variant with Multi-Ganglioside Reactivity: A Case of Severe Cranial Nerve Involvement. Rev. Neurol. 2025, 80, 37744. [Google Scholar] [CrossRef]
- Thiriveedi, M.; Domingo, F.G.S.; Longley, S.; Patel, S.; Baddam, S.; Chimakurthy, A. Post-COVID-19 Guillain-Barré Syndrome with GM1 and GD1b Antibodies: A Case Study and Literature Review. Am. J. Case Rep. 2025, 26, e947416. [Google Scholar] [CrossRef]
- Ghishan, S.; Shawareb, Y.; Alagha, Z.; Zeid, F. Guillain Barre Syndrome in an Elderly: A Disease of a Different Breed. Am. J. Respir. Crit. Care Med. 2025, 211, A3896. [Google Scholar] [CrossRef]
- Kota, N.K.; Hanchate, A.A.; Avanti, S.; Dhareshwar, B. Clinical profile and outcome of elders with Guillain-Barre syndrome: A Case series. Int. J. Res. Med. Sci. 2025, 7, 170–172. [Google Scholar] [CrossRef]
- Min, X.M.; Feng, H.M.; Zhao, R.M.; Guo, Z.M.; Su, H.M. Anti-sulfatide antibody-positive Guillain–Barré syndrome in adults following off-craniotomy for cerebellar contusion: A case report. Medicine 2024, 103, e40970. [Google Scholar] [CrossRef]
- Ito, S.; Yokoi, S.; Fukami, Y.; Uchibori, A.; Katsuno, M. Guillain-Barré syndrome with overlap between the finger drop variant and acute bulbar palsy: A case report. BMC Neurol. 2024, 24, 411. [Google Scholar] [CrossRef]
- Chen, F.Y.-S.; Hou, W.-H.; Lee, H.-H.; Huang, Y.-C.; Siow, C.Y. Additional Rehabilitative Robot-Assisted Gait Training for Ambulation in Geriatric Individuals with Guillain–Barré Syndrome: A Case Report. Medicina 2024, 60, 1209. [Google Scholar] [CrossRef]
- Reddy, V.; Gongireddy, R.; Pinninty, D.; Mudduluru, P.; Bollam, R. Uncommon Presentation of Guillain-Barre Syndrome in an Elderly Male: A Case Report. Neurology 2024, 103, S30–S31. [Google Scholar] [CrossRef]
- Obara, K. Unique Clinical and Psychiatric Challenges in Elderly Patients With Guillain-Barré Syndrome: A Case Series. Cureus 2024, 16, e69478. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.-Y.; Chen, X.-W.; Zhang, M.; Chu, W.-Z.; Wu, H.-L.; Ren, C. Guillain–Barre syndrome after myocardial infarction: A case report and literature review. BMC Cardiovasc. Disord. 2023, 23, 226. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, H.J.; Heo, J.-H.; Kim, J.H. Guillain-Barré Syndrome Following Spinal Fusion Surgery in an Elderly Patient: A Case Report. Nerve 2023, 9, 203–209. [Google Scholar] [CrossRef]
- Sidoli, C.; Bruni, A.A.; Beretta, S.; Mazzola, P.; Bellelli, G. Guillain-Barré syndrome AMSAN variant in a 90-year-old woman after COVID-19: A case report. BMC Geriatr. 2023, 23, 114. [Google Scholar] [CrossRef]
- Tu, W.-C.; Chang, S.-T.; Huang, C.-H.; Cheng, Y.-Y.; Hsu, C.-S. Guillain-Barré Syndrome with Respiratory Failure following Spine Surgery for Incomplete Cervical Cord Injury: A Case Report and Literature Review. Medicina 2022, 58, 1063. [Google Scholar] [CrossRef]
- Luvsannyam, E.; Jayaraman, A.; Jain, M.S.; Sharma, K.; Somagutta, M.R.; Yallapragada, R.K. Guillain-Barré syndrome following COVID-19 infection in an elderly patient: A case report. Eur. J. Med. Case Rep. 2021, 5, 242–245. [Google Scholar] [CrossRef]
- Ramakrishna, K.N.; Tambe, V.; Kattamanchi, A.; Dhamoon, A.S. Miller Fisher syndrome with bilateral vocal cord paralysis: A case report. J. Med. Case Rep. 2020, 14, 31. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, S.; Fang, L.; Liu, Y.; Jiang, G.; Ding, X.; Wei, H.; Liu, M. Pulmonary adenocarcinoma associated with Guillain-Barre syndrome: A case report. Medicine 2018, 97, e10737. [Google Scholar] [CrossRef]
- Doctor, G.T.; Alexander, S.K.; Radunovic, A. Guillain-Barré syndrome with exaggerated pleocytosis and anti-GM1 ganglioside antibodies. BMJ Case Rep. 2018, 2018, bcr-2017. [Google Scholar] [CrossRef]
- Helgeson, S.; Heckman, A.J.; Harris, D.M. First reported case of respiratory syncytial virus infection causing guillain–Barré syndrome. Indian J. Crit. Care Med. 2018, 22, 309–310. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.-S.; Choi, J.-Y.; Chung, H.; Kim, Y.; Na, S.-J. Recurrent Guillain-Barré Syndrome Following Urinary Tract Infection by Escherichia coli. J. Korean Med. Sci. 2018, 33, e29. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, T.; Higa, K.; Kido, M.; Ishihara, S.; Nakachi, R.; Suwazono, S. The Sequential Ultrasonographic, Electrophysiological and MRI Findings in a Patient with the Pharyngeal-cervical-brachial Variant of Guillain-Barré Syndrome from the Acute Phase to the Chronic Phase. Intern. Med. 2017, 56, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kimura, T.; Yuki, N.; Yoshioka, A. An Adult Case of Recurrent Guillain-Barré Syndrome with Anti-galactocerebroside Antibodies. Intern. Med. 2018, 57, 409–412. [Google Scholar] [CrossRef]
- Ha, L.D.; Abbas, F.; Rao, M. Guillain-Barré Syndrome Presenting with Sinus Node Dysfunction and Refractory Shock. Am. J. Case Rep. 2017, 18, 251–254. [Google Scholar] [CrossRef]
- Lin, Y.-K.; Yang, F.-C.; Liu, F.-C.; Lee, J.-T.; Sung, Y.-F. Co-Cccurrence of Guillain-Barre Syndrome and Primary Sjögren Syndrome in an Elderly Woman. Acta Neurol. 2016, 25, 83–87. [Google Scholar]
- Jacobs, B.C.; Rothbarth, P.H.; van der Meché, F.G.; Herbrink, P.; Schmitz, P.I.; de Klerk, M.A.; van Doorn, P.A. The spectrum of an-tecedent infections in Guillain-Barré syndrome: A case-control study. Neurology 1998, 51, 1110–1115. [Google Scholar] [CrossRef]
- Gensicke, H.; Datta, A.N.; Dill, P.; Schindler, C.; Fischer, D. Increased incidence of Guillain-Barré syndrome after surgery. Eur. J. Neurol. 2012, 19, 1239–1244. [Google Scholar] [CrossRef]
- Feasby, T.; Hahn, A.; Brown, W.; Bolton, C.; Gilbert, J.; Koopman, W. Severe axonal degeneration in acute Guillain-Barré syndrome: Evidence of two different mechanisms? J. Neurol. Sci. 1993, 116, 185–192. [Google Scholar] [CrossRef]
- Soliven, B. Animal Models of Autoimmune Neuropathy. ILAR J. 2014, 54, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Willison, H.J.; Goodyear, C.S. Glycolipid antigens and autoantibodies in autoimmune neuropathies. Trends Immunol. 2013, 34, 453–459. [Google Scholar] [CrossRef] [PubMed]
- McGonigal, R.; Rowan, E.G.; Greenshields, K.N.; Halstead, S.K.; Humphreys, P.D.; Rother, R.P.; Furukawa, K.; Willison, H.J. Anti-GD1a antibodies activate complement and calpain to injure distal motor nodes of Ranvier in mice. Brain 2010, 133, 1944–1960. [Google Scholar] [CrossRef]
- Rinaldi, S.; Brennan, K.M.; Kalna, G.; Walgaard, C.; van Doorn, P.; Jacobs, B.C.; Yu, R.K.; Mansson, J.-E.; Goodyear, C.S.; Willison, H.J.; et al. Antibodies to Heteromeric Glycolipid Complexes in Guillain-Barré Syndrome. PLoS ONE 2013, 8, e82337. [Google Scholar] [CrossRef]
- Hadden, R.D.M.; Cornblath, D.R.; Hughes, R.A.C.; Zielasek, J.; Hartung, H.; Toyka, K.V.; Swan, A.V. The Plasma Exchange/Sandoglobulin Guillain-Barré Syndrome Trial Group Electrophysiological classification of guillain-barré syndrome: Clinical associations and outcome. Ann. Neurol. 1998, 44, 780–788. [Google Scholar] [CrossRef]
- Stoian, A.; Bălașa, R.; Grigorescu, B.L.; Maier, S.; Andone, S.; Cocuz, I.G.; Bajko, Z.; Filep, C.R.; Stoian, M. Guillain-Barré syndrome associated with COVID-19: A close relationship or just a coincidence? Exp. Ther. Med. 2021, 22, 916. [Google Scholar] [CrossRef]
- Hagen, K.M.; Ousman, S.S. The Neuroimmunology of Guillain-Barré Syndrome and the Potential Role of an Aging Immune System. Front. Aging Neurosci. 2021, 12, 613628. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, K.; Deng, F.; Xing, Y. Electrophysiological subtypes and prognosis of Guillain-Barré syndrome in North Eastern China. Muscle Nerve 2013, 47, 68–71. [Google Scholar] [CrossRef]
- Zochodne, D.W. Autonomic involvement in Guillain-Barré syndrome: A review. Muscle Nerve 1994, 17, 1145–1155. [Google Scholar] [CrossRef]
- Bao, L.; Chen, X.; Li, Q.; Zhang, R.; Shi, H.; Cui, G. Surgery and Guillain-Barré Syndrome: A Single-Center Retrospective Study Focused on Clinical and Electrophysiological Subtypes. Neuropsychiatr. Dis. Treat. 2020, 16, 969–974. [Google Scholar] [CrossRef]
- Pagaling, G.T.; Dichoso, L.P.C.; Reyes, N.G.D.; Prado, M.B. Association of dysautonomia and different risk factors in patients with Guillain-Barré syndrome in a tertiary hospital in the Philippines. BMC Neurol. 2025, 25, 29. [Google Scholar] [CrossRef]
- Keir, D.A.; Badrov, M.B.; Tomlinson, G.; Notarius, C.F.; Kimmerly, D.S.; Millar, P.J.; Shoemaker, J.K.; Floras, J.S. Influence of Sex and Age on Muscle Sympathetic Nerve Activity of Healthy Normotensive Adults. Hypertension 2020, 76, 997–1005. [Google Scholar] [CrossRef]
- Jiang, Y.; Yabluchanskiy, A.; Deng, J.; Amil, F.A.; Po, S.S.; Dasari, T.W. The role of age-associated autonomic dysfunction in inflammation and endothelial dysfunction. GeroScience 2022, 44, 2655–2670. [Google Scholar] [CrossRef]
- Chakraborty, T.; Kramer, C.L.; Wijdicks, E.F.M.; Rabinstein, A.A. Dysautonomia in Guillain-Barré Syndrome: Prevalence, Clinical Spectrum, and Outcomes. Neurocritical Care 2020, 32, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J. Triggers of Guillain–Barré Syndrome: Campylobacter jejuni Predominates. Int. J. Mol. Sci. 2022, 23, 14222. [Google Scholar] [CrossRef] [PubMed]
- Stoian, A.; Bajko, Z.; Maier, S.; Cioflinc, R.A.; Grigorescu, B.L.; Moțățăianu, A.; Bărcuțean, L.; Balașa, R.; Stoian, M. High-dose intravenous immunoglobulins as a therapeutic option in critical illness polyneuropathy accompanying SARS-CoV-2 infection: A case-based review of the literature. Exp. Ther. Med. 2021, 22, 1182. [Google Scholar] [CrossRef] [PubMed]
- Yadegari, S.; Nafissi, S.; Kazemi, N. Comparison of electrophysiological findings in axonal and demyelinating Guillain-Barre syndrome. Iran J. Neurol. 2014, 13, 138–143. [Google Scholar]
- França, M.C., Jr.; Deus-Silva, L.; de Castro, R.; Garibaldi, S.G.; Pfeilsticker, B.H.; Nucci, A.; Marques, J.F., Jr. Guillain-Barré syndrome in the elderly: Clinical, electrophysiological, therapeutic and outcome features. Arq. Neuro-Psiquiatr. 2005, 63, 772–775. [Google Scholar] [CrossRef]
- Kim, S.; Han, H.J.; Shin, H.Y.; Kim, S.W. Old age and multiple comorbidity are associated with delayed diagnosis of Guillain–Barre syndrome. Sci. Rep. 2022, 12, 9913. [Google Scholar] [CrossRef]
- Samadi, M.; Kazemi, B.; Oskoui, S.G.; Barzegar, M. Assessment of Autonomic Dysfunction in Childhood Guillain-Barré Syndrome. J. Cardiovasc. Thorac. Res. 2013, 5, 81–85. [Google Scholar] [CrossRef]
- Zaeem, Z.; Siddiqi, Z.A.; Zochodne, D.W. Autonomic involvement in Guillain–Barré syndrome: An update. Clin. Auton. Res. 2019, 29, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Jannat, M.; Hannan, M.A.; Alam, S.M. Pattern of Autonomic Involvement in Adult Patients with Guillain Barre Syndrome in a Tertiary Hospital. Bangladesh Med. Res. Counc. Bull. 2022, 48, 174–179. [Google Scholar] [CrossRef]
- Shang, P.; Feng, J.; Wu, W.; Zhang, H.-L. Intensive Care and Treatment of Severe Guillain–Barré Syndrome. Front. Pharmacol. 2021, 12, 608130. [Google Scholar] [CrossRef] [PubMed]
- Raphaël, J.C.; Chevret, S.; Hughes, R.A.; Annane, D. Plasma exchange for Guillain-Barré syndrome. Cochrane Database Syst. Rev. 2012, 7, CD001798. [Google Scholar] [CrossRef]
- Stoian, A.; Moțățăianu, A.; Bărcuțean, L.; Maier, S.; Bajko, Z.; Voidazan, S.; Farcas, A.; Balasa, R. Understanding the mechanism of action of intravenous immunoglobulins: A ten-year experience in treating Guillain-Barré Syndrome. Farmacia 2020, 68, 426–435. [Google Scholar] [CrossRef]
- Zaki, H.A.; Iftikhar, H.; Najam, M.; Masood, M.; Al-Marri, N.D.R.; Elgassim, M.A.M.; Fayed, M.; Shaban, E.E. Plasma exchange (PE) versus intravenous immunoglobulin (IVIG) for the treatment of Guillain-Barré syndrome (GBS) in patients with severe symptoms: A systematic review and meta-analysis. eNeurologicalSci 2023, 31, 100468. [Google Scholar] [CrossRef]
- Korinthenberg, R.; Schessl, J.; Kirschner, J.; Mönting, J.S. Intravenously Administered Immunoglobulin in the Treatment of Childhood Guillain-Barré Syndrome: A Randomized Trial. Pediatrics 2005, 116, 8–14. [Google Scholar] [CrossRef]
- Hughes, R.A.C.; Swan, A.V.; Raphaël, J.-C.; Annane, D.; van Koningsveld, R.; van Doorn, P.A. Immunotherapy for Guillain-Barre syndrome: A systematic review. Brain 2007, 130, 2245–2257. [Google Scholar] [CrossRef]
- Plasma Exchange/Sandoglobulin Guillain-Barré Syndrome Trial Group. Randomised trial of plasma exchange, intravenous immunoglobulin, and combined treatments in Guillain-Barré syndrome. Lancet 1997, 349, 225–230. [Google Scholar] [CrossRef]
- Van Doorn, P.A. Diagnosis, treatment and prognosis of Guillain-Barré syndrome (GBS). Presse Med. 2013, 42, e193–e201. [Google Scholar] [CrossRef] [PubMed]
- Ruts, L.; Drenthen, J.; Jacobs, B.C.; van Doorn, P.A.; Dutch GBS Study Group. Distinguishing acute-onset CIDP from fluctuating Guillain-Barre syndrome: A prospective study. Neurology 2010, 74, 1680–1686. [Google Scholar] [CrossRef] [PubMed]
- Walgaard, C.; Lingsma, H.F.; van Doorn, P.A.; van der Jagt, M.; Steyerberg, E.W.; Jacobs, B.C. Tracheostomy or Not: Prediction of Prolonged Mechanical Ventilation in Guillain–Barré Syndrome. Neurocritical Care 2016, 26, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.A.; Brassington, R.; Gunn, A.A.; van Doorn., P.A. Corticosteroids for Guillain-Barré syndrome. Cochrane Database Syst. Rev. 2016, 10, CD001446. [Google Scholar] [CrossRef]
- Hughes, R.; Newsom-Davis, J.; Perkin, G.; Pierce, J. Controlled trial of prednisolone in acute polyneuropathy. Lancet 1978, 312, 750–753. [Google Scholar] [CrossRef]
- Rajabally, Y.A.; Uncini, A. Outcome and its predictors in GuillainBarre syndrome. J. Neurol. Neurosurg. Psychiatry 2012, 83, 711–718. [Google Scholar] [CrossRef]
- Van den Berg, B.; Bunschoten, C.; van Doorn, P.A.; Jacobs, B.C. Mortality in Guillain-Barre syndrome. Neurology 2013, 80, 1650–1654. [Google Scholar] [CrossRef]
- Nagappa, M.; Rahul, W.; Sinha, S.; Bindu, P.S.; Mathuranath, P.S.; Rao, S.; Periyavan, S.; Umamaheshwara Rao, G.S.; Taly, A.B. Guillain Barre Syndrome in the elderly: Experience from a tertiary-care hospital in India. J. Clin. Neurosci. 2017, 46, 45–49. [Google Scholar] [CrossRef]
- Briseño-Godínez, M.E.; Arauz, A.; López-Hernández, J.C.; de Saráchaga, A.J.; Pérez-Valdez, E.Y.; May-Más, R.N.; López-Hernández, G.; Bazán-Rodriguez, L.; Galnares-Olalde, J.A.; León-Manríquez, E.; et al. Prognostic Factors in Elderly Patients With Guillain-Barré Syndrome: Does Age Matter? Neurohospitalist 2021, 11, 303–309. [Google Scholar] [CrossRef]
| Study | Patient/Presentation | Examination | Investigations | Treatment | Outcome |
|---|---|---|---|---|---|
| Watanabe K, et al., case report, Japan, 2025 [13]. | 84 M, weakness in right upper limb, gastroenteritis 4 days previously. | Progression of weakness to all limbs, areflexia, ↓ touch sense. | NCS, axonal neuropathy, progressed to respiratory failure. | IVIG, mechanical ventilation, PE. | Partial recovery after rehabilitation for 187 days. |
| Solodovnikova Y, et al., case report, Ukraine, 2025 [14]. | 77 M, acute symmetric ascending flaccid tetraparesis, recent history of pneumonia. | Areflexia, glove-stocking–glove sensory loss, urine retention. | CSF consistent with meningitis, progression of neurological symptoms. | IV antibiotics, IVIG 5 days. | Partial recovery of neurological symptoms. |
| Gómez-Dabó L, et al., case report, Spain, 2025 [15]. | 75 F, progressive impairment of bilateral III, IV, VI, VII, IX, X XII cranial nerves, recent gastroenteritis. | Bilateral ophthalmoplegia, ptosis, mydriasis, facial, tongue and palate palsy, ↓ pharyngeal reflex, flaccid dysarthria, brisk tendon reflexes, all limb dysmetria. | NCS, acute inflammatory demyelinating polyneuropathy. | Mechanical ventilation, IVIG 5 days. | Partial recovery after 5 months. |
| Thiriveedi M, et al., case report, US, 2025 [16]. | 86 M, progressive generalised weakness, ↓ mobility, recent COVID-19 infection. | Weakness in all limbs, ↓ pain and vibration sense, hyporeflexia | CSF, ↑ protein 95.9 mg/L, leukocytes 1/µL. | IVIG 5 days, then a second course given, NCS, axonal demyelinating polyneuropathy. | Minimal motor recovery, discharged to care home after 24 days. |
| Ghishan S, et al., case report, US, 2025 [17]. | 83 M, fall, acute lower extremity weakness, dysphonia. | Symmetrical weakness, intact sensation, absent reflexes. | CSF, ↑ protein 179 mg/L, mild leucocytosis, progressed to respiratory failure. | Mechanical ventilation, PE. | Partial recovery. |
| Kota NK, et al., case report, India, 2025 [18]. | 81 M, acute onset bilateral lower limb weakness followed by upper limb over 1 day. | Complete areflexia with reduced power in four limbs. | NCS, motor peripheral neuropathy, CSF ↑ protein 1261.3 mg/dl, leukocytes 2/µL. | IVIG 5 days. | Full recovery in 2 weeks. |
| Min X, et al., case report, China, 2024 [19]. | 79 F, progressive limb weakness 7 days after craniotomy for cerebellar contusion. | Quadriplegia, autonomic dysfunction, dilated pupils, respiratory failure. | CSF, ↑ protein 1.3 g/L, leukocytes 3/µL. | Mechanical ventilation, IVIG 5 days. | Good recovery after 6 weeks rehabilitation. |
| Ito S, et al., case report, Japan, 2024 [20]. | 81 M, dysarthria, dysphagia, upper limb weakness. | Inability to protrude tongue beyond the dental arch, muscle weakness in distal upper limbs. | CSF, ↑ protein 48 mg/dl, leukocytes 5/mm3, NCS, reduced amplitudes and velocities in median and ulnar nerves. | IVIG 5 days. | Partial recovery after 31 days rehabilitation. |
| Chen FY, et al., Taiwan, 2024 [21]. | 75 F, symmetric weakness in both distal lower limbs and ataxia. | Absent deep tendon reflexes in knee and ankle, ascending weakness, constipation and urine retention. | NCS, acute inflammatory demyelinating polyradiculoneuropathy, CSF, albuminocytologic dissociation. | IVIG 5 days. | Partial recovery one month after rehabilitation. |
| Reddy V, et al., case report, India, 2024 [22]. | 79 M, cough, malaise, fever, diplopia, watery diarrhoea 5 days previously. | Left-beating nystagmus, postural tremor in both arms, intermittent leg jerking, exaggerated reflexes in biceps and patella. | CSF, ↑ protein 82 mg/dl, leukocytes 13–20/mm3, blood cultures campylobacter jejuni. | IV antibiotics. | Mobility improved after three months of physical therapy. |
| Obara K. case series, Japan, 2024 [23]. | 1. 81 M, diplopia, unsteady standing, URTI 3 days previously. 2. 78 M, diplopia, tingling in both arms, enterocolitis 4 days previously. | 1. Dysarthria, bilateral ptosis, ataxia, weakness in upper limbs, progressed to flaccid tetraplegia, areflexia. 2. Eyes, fixed, flaccid tetraplegia. | 1. NCS, consistent with axonal neuropathy with demyelinating features., CSF, ↑ protein 1216 mg/dl, leukocytes 6/mm3 2. NCS, axonal neuropathy with demyelinating features, CSF, albuminocytologic dissociation. | 1. Mechanical ventilated and intubation, PE and IVIG. 2. Mechanical ventilation and intubation, PE and IVIG. | Only slight recovery with residual weakness. 2. Only slight recovery with residual weakness. |
| Wen PY, et al., case report, China, 2023 [24]. | 75 F, sub-acute upper limb weakness, progressed to lower limbs, recent myocardial infarction. | Hypotonia in limbs, quadriplegia, hypoesthesia, bulbar palsy, tendon areflexia. | Declined investigations, progressed to respiratory failure. | Mechanical ventilation, IVIG 5 days. | Died after 25 days. |
| Lee J, et al., Korea, 2023 [25]. | 79 M, inability to move both legs, numbness in feet, weakness and tingling in both upper extremities after spinal fusion surgery. | Weak legs, decreased tendon reflexes in upper and lower extremities. | NCS, sensory–motor peripheral polyneuropathy in upper and lower limbs, CSF ↑ protein 381 mg/dl, leukocytes 96/mm3 | IVIG 5 days. | Improved after 6 weeks, fully recovered after 1 year. |
| Sidoli C, et al., case report, Italy, 2023 [26]. | 90 F, fatigue, worsening gait and leg strength, dysphonia, dysarthria and dysphagia, COVID-19 positive previous 3 W. | Dysarthria, dysphonia, dysphagia, symmetric weakness in upper limbs, asymmetric weakness in lower limbs, asymmetric sensory signs in lower limbs, absent tendon reflexes in lower limbs, normal plantar response. | NCS acute motor and sensory axonal neuropathy, CSF ↑ protein 57 mg/dl and cell count 21/µL. | IVIG 5 days. | Poor recovery, died after 2 months. |
| Tu WC, et al., case report, Taiwan, 2022 [27]. | 87 M, weakness in lower limbs, surgery for neck injury 2 weeks previously. | Limb weakness. | CSF, ↑ protein 167 mg/dl, absent leukocytes, NCS, acute axonal polyneuropathy. | Mechanical ventilation, supportive care, rehabilitation. | Remained dependent, died after 3 years. |
| Luvsannyam E, et al., case report, Netherlands, 2021 [28]. | 83 F, bilateral numbness in lower extremities, ↓ mobility, COVID-19 positive previous few weeks. | ↓ Sensations, 0/5 strength, diffuse areflexia in lower limbs. | NCS acute inflammatory demyelinating polyneuropathy, CSF, no leukocytes, ↑ protein 64 mg/dl consistent with albuminocytologic dissociation. | IVIG 5 days and three courses PE. | Improved significantly after rehabilitation. |
| Ramakrishna KN, case report, US, 2020 [29]. | 76 M, unsteadiness, poor oral intake, dysarthria, dizziness. | Respiratory distress, drowsy, impaired gag reflex, limb weakness, reduced reflexes. | NCS, acute inflammatory demyelinating polyneuropathy, CSF, ↑ protein 62 mg/dl, leukocytes < 3/mm3. | Mechanical ventilation, IVIG 5 days. | Tracheostomy-dependent after 6 months, mobilising with walker. |
| Wang Y, et al., case report, China, 2018 [30]. | 80 M, 10-day progressive ascending limb weakness, fever 2 weeks previously. | Weakness in limbs, areflexia. | NCS, peripheral nerve demyelination, CT lung cancer. | Methylprednisolone 80 mg QD for 10 days, then 40 mg QD for 10 days, then prednisone 10 mg QD for 3 months. | Progression, bedbound after 6 months. |
| Doctor GT, et al., case report, UK, 2018 [31]. | 81 F, confusion, fever, mild headache and photophobia, diarrhoea, vomiting 2 weeks previously. | New symmetrical limb weakness, areflexia. | CSF, ↑ protein 72 mg/dl, leukocytes 14/mm3, NCS, axonal polyneuropathy. | IVIG three times at 4 week intervals. | Partial recovery after 3 months rehabilitation. |
| Helgeson SA, et al., case report, US, 2018 [32]. | 81 F, weakness lower limbs, paraesthesia in feet after extubation from pneumonia. | Absent tendon reflexes, progressive weakness to upper limbs. | NCS, axonal sensorimotor peripheral neuropathy. | PE, Mechanical ventilation. | Died after 11 days. |
| Jo YS, et al., case report, Korea, 2018 [33]. | 75 F, weakness in upper and lower limbs, fever, frequency, dysuria 10 days previously. | Limb weakness and areflexia. | NCS, axonal motor type polyneuropathy. | IVIG | Almost full recovery, mild residual weakness. |
| Miyagi T, et al., case report, Japan, 2017 [34]. | 77 M, progressive dysarthria, dysphagia, bilateral upper limb weakness, diarrhoea 10 days previously. | Weak facial, tongue, cervical, pharyngeal, palatal upper limb muscles, dysphonia, dysphagia. | CSF, ↑ protein 57.4 mg/dl, normal leukocytes, NCS, reduced compound muscle action potential. | IVIG 5 days, IVIG 5 days on day 33. | Good recovery 63 days after rehabilitation. |
| Takahashi H, et al., case report, Japan, 2017 [35]. | 79 F, progressive tetraparesis, cough and fever 7 days previously. | Limbs, ocular and facial muscle weakness, reduced limb sensation. | CSF, ↑ protein 65 mg/dl, leukocytes 40/mm3, NCS, reduced compound muscle action potential. | IVIG 5 days. | Full recovery 37 days after rehabilitation. |
| Ha LD, et al., case report, US, 2017 [36]. | 76 M, diaphoresis, slurred speech, urinary and faecal incontinence. | Lethargic, altered mental state, bradycardia, tachypnoea, areflexia in four limbs. | CSF, ↑ protein 62 mg/dL, normal leukocytes. | PE, supportive care. | Died of multi-organ failure. |
| Lin YK, et al., case report, Taiwan, 2016 [37]. | 82 F, acute ascending weakness, distal numbness in four limbs, URTI previous 1 W. | Flaccid weakness, absent tendon reflexes in four limbs, absent plantars, ↓ peripheral sensation. | CSF protein 161 mg/dl (N = 15–45 mg/dl), leukocytes 1 (N = 0–5 cell/µL), NCS ↓ motor and sensory action potentials in all limbs, positive for SS. | Ventilation, six courses PE, IVIG for 5 days, immunosuppressant hydroxychloroquine. | Full recovery, discharged after 1 month, remained well after 1 year follow-up. |
| Mimic | Diagnostic Investigations |
|---|---|
| Central nervous system Encephalitis, encephalomyelitis, brain stem stroke, brain stem compression, leptomeningeal tumours, Wernicke’s encephalopathy, transvers myelitis. | Clinical picture, CT, MRI, CSF, serum thiamine. |
| Motor neurons Amyotrophic lateral sclerosis, progressive spinal muscular atrophy, poliomyelitis, West Nile virus anterior myelitis. | Clinical picture, MRI, NCS, CSF, viral serology. |
| Nerve roots Acute onset CIDP, autoimmune nodopathy, Lyme disease, viral-related radiculitis, leptomeningeal malignancy. | Clinical picture, NCS, CSF, serology, MRI. |
| Nerve plexus Diabetes mellitus, amyotrophic neuralgia. | Clinical picture, NCS, blood glucose. |
| Peripheral nerves Acute onset CIDP, diphtheria, vasculitis, porphyria, thiamine deficiency, electrolytes disturbance, Lyme disease, toxin-induced neuropathy, critical illness polyneuropathy. | Clinical picture, autoimmune and vasculitic screen, serology, serum electrolytes, NCS. |
| Neuromuscular junction Myasthenia gravis, Lambert–Eaton myathenic syndrome, botulism, organophosphorus poisoning. | Clinical picture, NCS, serum antibodies. |
| Muscles Hypokalaemic periodic paralysis, acute myositis, acute colchicine myopathy, critical illness myopathy. | Clinical picture, serum K, serum CK, NCS. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Ko, W.; Waseem, F.; Cilcic, L.; Kazi, N.; Abdelhafiz, A. Guillain–Barré Syndrome in Older People—A Case Report and Literature Review. Diseases 2025, 13, 306. https://doi.org/10.3390/diseases13090306
Chen X, Ko W, Waseem F, Cilcic L, Kazi N, Abdelhafiz A. Guillain–Barré Syndrome in Older People—A Case Report and Literature Review. Diseases. 2025; 13(9):306. https://doi.org/10.3390/diseases13090306
Chicago/Turabian StyleChen, Xiaomei, Win Ko, Fiza Waseem, Lidia Cilcic, Nahian Kazi, and Ahmed Abdelhafiz. 2025. "Guillain–Barré Syndrome in Older People—A Case Report and Literature Review" Diseases 13, no. 9: 306. https://doi.org/10.3390/diseases13090306
APA StyleChen, X., Ko, W., Waseem, F., Cilcic, L., Kazi, N., & Abdelhafiz, A. (2025). Guillain–Barré Syndrome in Older People—A Case Report and Literature Review. Diseases, 13(9), 306. https://doi.org/10.3390/diseases13090306




