Abstract
The incidence of infectious diseases in children may be affected by climate change-related disaster risks that increase as extreme weather events become more frequent. Therefore, this research aims to diagnose the impact of such disaster risks on the disease incidence, focusing on diarrhoea, dengue haemorrhagic fever (DHF), and acute respiratory infection (ARI), commonly experienced by children. To accomplish this task, we construct integer-valued autoregressive (INAR) models for the number of disease cases among children in several age groups, with an overdispersed distributional assumption to account for its variability that exceeds its central tendency. Additionally, we include the numbers of floods, landslides, and extreme weather events at previous times as explanatory variables. In particular, we consider a case study in Indonesia, a tropical country highly vulnerable to the aforementioned climate change-related diseases and disasters. Using monthly data from January 2010 to December 2024, we find that the incidence of diarrhoea in children is positively impacted by landslides (but negatively affected by floods and extreme weather events). Landslides, frequently caused by excessive rainfall, also increase DHF incidence. Furthermore, the increased incidence of ARI is driven by extreme weather conditions, which are more apparent during and after COVID-19. These findings offer insights into how climate scenarios may increase children’s future health risks. This helps shape health strategies and policy responses, highlighting the urgent need for preventive measures to protect future generations.
1. Introduction
Children are particularly vulnerable to infections because their immune systems are still developing, which can lead to severe infectious diseases. This increased vulnerability is also associated with their limited capacity to mitigate the effects of climate change. As climate change drives an increase in the frequency and severity of disasters, infectious diseases become more evident. Post-disaster events due to climate change, such as floods and heatwaves, damage the health infrastructure and worsen sanitation, leading to higher infection rates [1] and, consequently, a higher incidence of infectious diseases, such as diarrhoea, dengue haemorrhagic fever (DHF), and acute respiratory infection (ARI). These three diseases act as the top three causes of death among children in Indonesia for some periods [2,3,4].
Climate-related incidents, such as droughts and extreme temperature fluctuations, increase the risk of respiratory problems in children. When exposed to cold, dry air, or hot air, their bodies may respond with bronchoconstriction and coughing [5]. An increase of 1 °C in temperature can lead to higher respiratory rates, increased incidence of pneumonia, more hospital admissions for pneumonia, and a higher number of emergency department visits due to pneumonia [6]. Furthermore, Chua et al. [7] noted that increased temperatures are associated with an increase in certain infectious diseases caused by bacterial pathogens, such as cholera, salmonella, and Escherichia coli. These diseases are associated with both vector-borne and waterborne transmission.
During droughts, people often use unhygienic water and sanitation facilities, leading to gastrointestinal problems, such as diarrhoea, typically the primary health consequence [1]. When children experience diarrhoea, dehydration is the most common health problem they face. Children are also more susceptible to experiencing water loss through evaporation due to severe temperatures since children have a higher total body water content compared to adults [8].
In attempts to conserve water during droughts, individuals often store water in open containers, such as tubs, unintentionally creating ideal breeding environments for mosquitoes and subsequently elevating the risk of DHF. A study by Wibawa et al. [9] suggested that extreme and anomalous weather patterns have a significant impact on the prevalence of DHF, making its seasonal occurrence increasingly unpredictable. In line with this, Thomson et al. [10] also claimed that extreme weather events increase the risk of vector-borne infectious diseases, such as DHF.
Indonesia, as an archipelagic country, is highly vulnerable to climate change, including rising sea levels and tidal flooding [11,12,13]. Climate change in Indonesia appears to worsen with (i) improper waste management practices, (ii) deforestation, (iii) forest fires, (iv) massive exploitation of natural resources without conservation, (v) industrial and vehicle pollution, and (vi) unsustainable agricultural practices [14,15,16,17]. The potential effects on Indonesian children’s health, threatened by infectious diseases, more specifically diarrhoea, DHF, and ARI, are therefore important to investigate.
In this paper, we aim to diagnose the impact of climate change-related disasters (namely, floods, landslides, and extreme weather events) on the incidence of the above infectious diseases (i.e., diarrhoea, DHF, and ARI) in children. It is worth noting that the number of infectious disease cases is a nonnegative integer, leading many studies (e.g., [18,19]) to accommodate its dynamic values over time using an integer-valued time-series model. More specifically, they employed an integer-valued autoregressive (INAR) model introduced independently by McKenzie [20] and Al-Osh and Alzaid [21]. This model was assumed to have a Poisson distribution whose mean and variance are equal, making it equidispersed. In practice, count time-series data on disease cases often exhibit overdispersion, a phenomenon where the variance exceeds the mean [22,23,24]. This phenomenon can be captured using an INAR model with (i) a Poisson–exponential distribution, commonly known as a geometric distribution [22], or (ii) a Poisson–Lindley distribution [25,26]. To the best of our knowledge, these models have not been implemented for medical data on infectious disease cases in children. Furthermore, their extension, which incorporates some exogenous or explanatory variables related to climate change, has not been proposed by the existing studies, thereby inspiring us to construct the so-called INAR-X model with a Poisson–exponential or Poisson–Lindley INAR-X distribution.
We organise the remainder of this paper as follows. In Section 2, we describe the materials and methods, i.e., (i) the sources and visualisation of datasets, which cover the numbers of infectious disease cases among children and climate change-related disaster events, and (ii) INAR models with overdispersion and explanatory variables required to diagnose the impact of the disaster events on the disease incidence. In Section 3, we provide and discuss the results, i.e., statistical analyses that confirm the presence of (i) an overdispersion phenomenon and (ii) a correlation within and between the datasets, along with the main modelling results. We then draw conclusions in Section 4.
2. Materials and Methods
2.1. Data Sources and Visualisation
2.1.1. Epidemiological Surveillance and Immunisation Information System Database
Data on childhood diseases were obtained from the epidemiological surveillance and immunisation information system database maintained by the Health Office of the Province of the Special Region of Jakarta, Indonesia. Specifically, we focus on three diseases: diarrhoea, dengue haemorrhagic fever (DHF), and acute respiratory infection (ARI). Our focus on Jakarta comes from the fact that this province reflects much of Indonesia through its diverse population and frequent exposure to such diseases in children; see Figure S1 in the Supplementary Materials. This makes our findings more relevant to the whole country. However, we recognise that differences in transmission patterns and population density may affect disease loads in other places, highlighting the necessity of research in provinces that are rural and semi-urban. Furthermore, the selection of the disease variables is based on recent research conducted by Harapan et al. [2], Sulistiyowati et al. [3], and Purnama et al. [4], which revealed that diarrhoea, DHF, and ARI acted as the top three causes of death among children in Indonesia for some periods. See also a study by Helldén et al. [27], which found these to be common diseases faced by children due to climate change.
The above database contains a summary of integrated disease surveillance (IDS). The comprehensive IDS data were compiled from the epidemiological surveillance of both communicable and noncommunicable diseases. These data incorporate information from community health centres, hospitals, laboratories, and the Health Office of Jakarta. In the IDS data, patients’ ages are categorised into twelve distinct groups with nonoverlapping ranges that cover the entire age spectrum, from infancy to old age. The defined age ranges are as follows: 0–7 days, 8–28 days, 29 days–11 months, 12 months–4 years, 5–9 years, 10–14 years, 15–19 years, 20–44 years, 45–54 years, 55–59 years, 60–69 years, and 70 years and older. The first seven are the age groups for children. More detailed IDS data can be accessed, including additional variables such as districts or cities, subdistricts, healthcare centre work units, and sexes.
The primary data source for this study was obtained from the healthcare centres’ IDS database, covering the period from January 2010 to December 2024 and focusing on the Province of the Special Region of Jakarta. It specifically utilises age group information for children of both sexes, within the 0–19 age range. This age range is consistent with the legal definition of a child stated in the Republic of Indonesia Law Number 35 of 2014, which identifies a child as anyone under the age of 18, including those still in the womb [28].
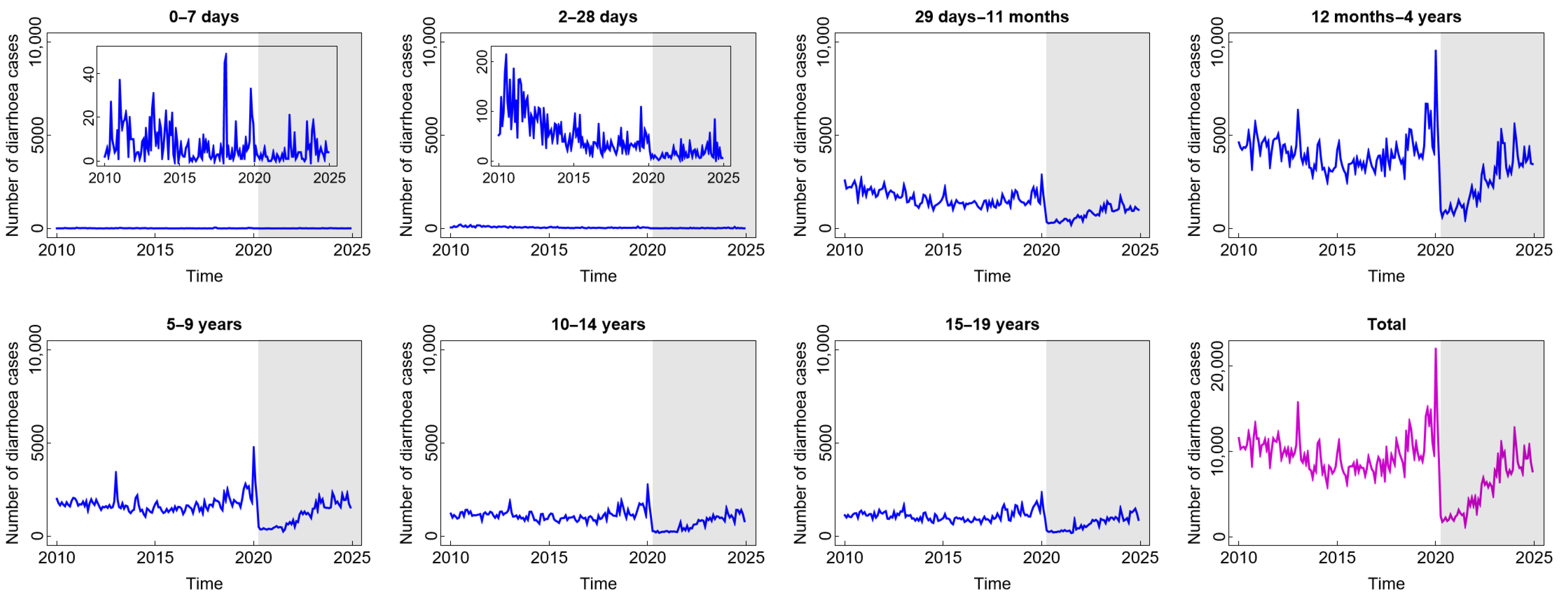
The number of diarrhoea cases, as presented in Figure 1, generally follows the same pattern, with the exception of the age group under one month. Before witnessing a notable uptick in 2019 and a precipitous drop in 2020, the number of diarrhoea cases tended to have a stable pattern until around 2018. The year 2020 marked the beginning of the COVID-19 pandemic era, during which most people stayed at home due to widespread social isolation and lockdown-like policies [29]. Accordingly, less data regarding diarrhoea cases were documented. Following that, people were living in a new normal situation, quite similar to their previous life, and the number of diarrhoea cases, particularly for the age groups above one month, tended to increase. The number of cases of this disease did not exhibit the same pattern in the age groups 0–7 days and 8–28 days, indicating that these age groups may have distinct risk factors for transmission compared to the age groups above them. Furthermore, the disease occurrence in these age groups remained unaffected by the circumstances of the COVID-19 pandemic or the new normal. This condition is understandable considering that newborns often receive constant, extremely stringent, and protective care. Only contact with the main family also contributes to limited pathogen contamination, which means that these two age groups likewise experience the fewest cases of diarrhoea. Exclusive breastfeeding and digestive systems that have not been exposed to solid foods can also contribute to a lower incidence in these two age groups. Furthermore, according to Figure 1, the highest number of diarrhoea cases was found in children aged 12 months–4 years. These children are in the stage of exploring their surrounding environment, making them frequently exposed to microorganisms. Due to their hand-to-mouth habits, children under the age of five also have a less developed immune system, which leaves them vulnerable to infections that can cause diarrhoea [30]. In brief, the incidence of diarrhoea in children during and after COVID-19 tended to increase as in the pre-COVID-19 period, even though it dramatically decreased for a short time at the beginning of the COVID-19 outbreak.
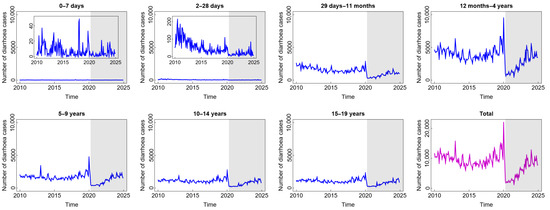
Figure 1.
Number of diarrhoea cases among children in several age groups and in the total child population in Jakarta, Indonesia. Notes: The white and grey areas show Period 1 (before the COVID-19 pandemic) and Period 2 (during and after the COVID-19 pandemic), respectively.
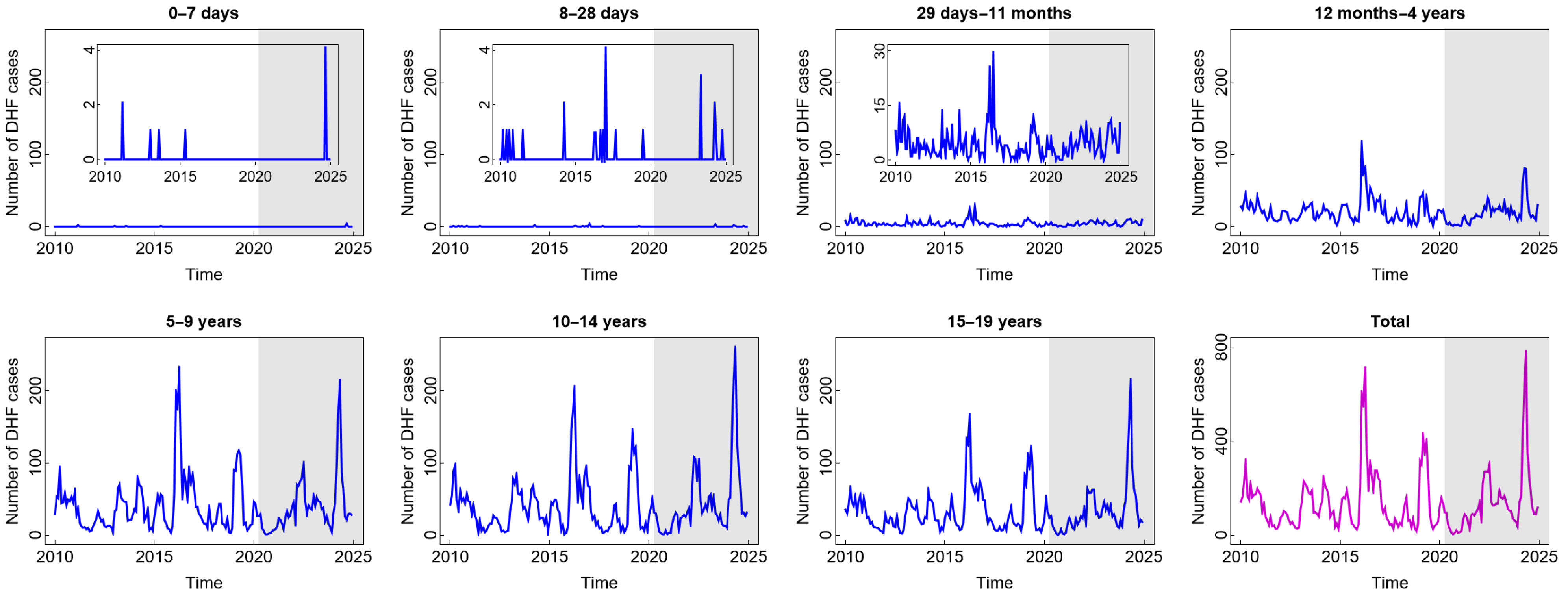
In general, the pattern of DHF cases was almost the same for all age groups and was not impacted by the COVID-19 pandemic situation (see Figure 2). The high number of DHF cases was found in children aged five years and above. Children in these age groups are more likely to be outside their homes and, therefore, more likely to be exposed to vectors that carry dengue viruses (DENV). A study by Liu et al. [31] confirmed that participation in outdoor activities significantly increases the risk of dengue exposure. Furthermore, research has shown that children under five are not at a high risk of contracting dengue. Younger babies are less likely than older children to have dengue because they may have protective levels of anti-DENV antibodies from their mothers [32]. Interestingly, there was a significant spike in 2016 and 2024, in almost all age groups, with the highest numbers of cases reaching 703 in 2016 and 772 in 2024. A study by Mamenun et al. [33] found a change in serotype from DEN-3 to DEN-1 and DEN-2 in 2016 in Indonesia, which increased the risk of more severe secondary DHF infections. Meanwhile, in 2024 during the dry season, Indonesia experienced a warmer rainy season due to the El Niño phenomenon. A temperature shift and an increase in rainfall during the dry season are anomalies resulting from this occurrence. These anomalies, based on the previous studies, are positively correlated with dengue incidence, where they accelerate the mosquito life cycle and shorten the incubation period of the virus in the mosquito’s body, thus increasing the risk of dengue incidence [34,35].
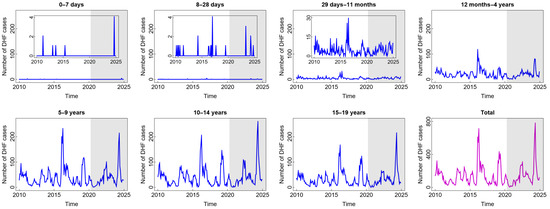
Figure 2.
Number of dengue haemorrhagic fever (DHF) cases among children in several age groups and in the total child population in Jakarta, Indonesia. Notes: The white and grey areas show Period 1 (before the COVID-19 pandemic) and Period 2 (during and after the COVID-19 pandemic), respectively.
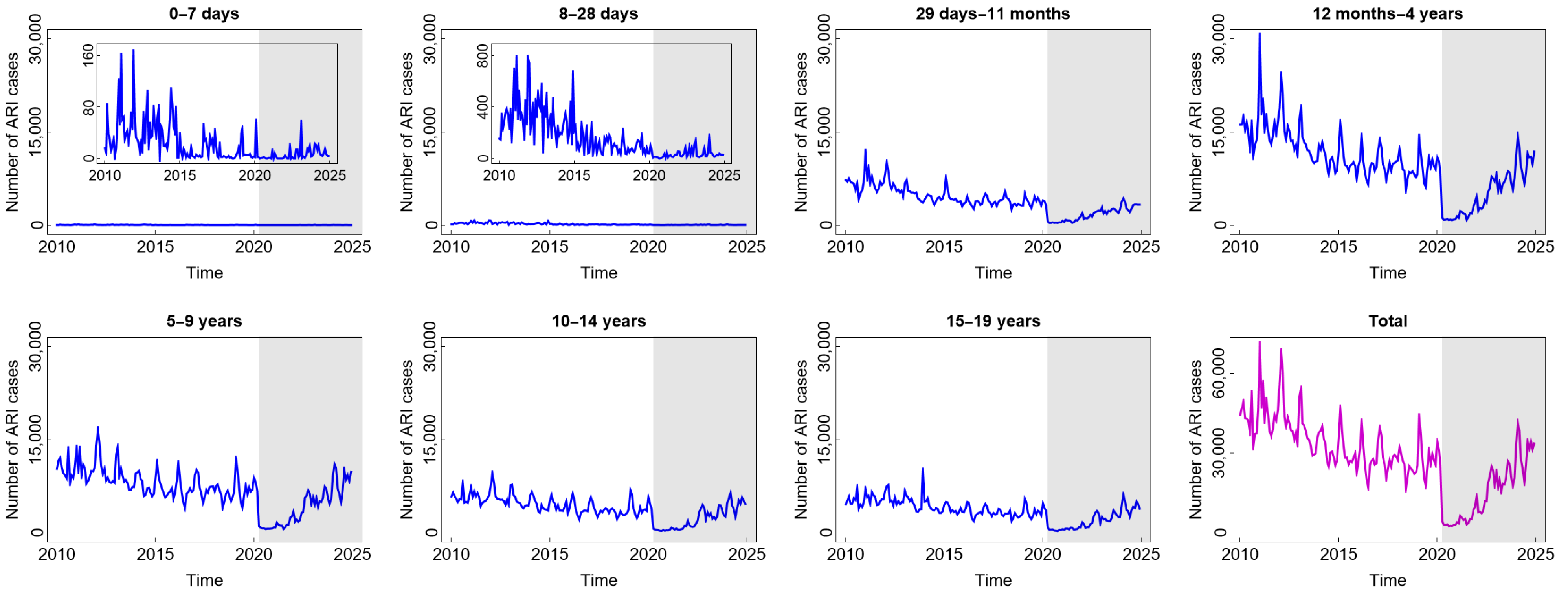
Compared to diarrhoea and DHF, ARI is the most common disease experienced by children in all age groups. From Figure 3, we find that the highest number of total ARI cases in the last 15 years was almost 70,000. The incidence decreased slowly before COVID-19 and then decreased significantly at the beginning of the COVID-19 outbreak. The reason for this phenomenon was similar to the sharp decrease in diarrhoea cases during such a short period. However, the incidence gradually increased from 2022 to 2025 after the new normal phase began to be implemented. Children aged 12 months to 4 years were the group with the highest number of ARI cases. This was also related to the highest mortality rate in children under five due to respiratory infections.
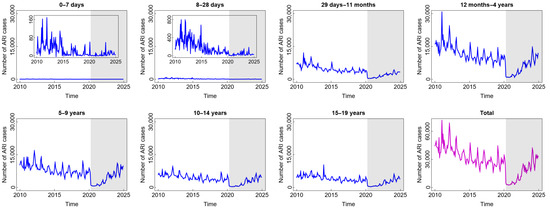
Figure 3.
Number of acute respiratory infection (ARI) cases among children in several age groups and in the total child population in Jakarta, Indonesia. Notes: The white and grey areas show Period 1 (before the COVID-19 pandemic) and Period 2 (during and after the COVID-19 pandemic), respectively.
2.1.2. Indonesia Disaster Information Database
The disaster data analysed in this study were derived from the Indonesia Disaster Information Database (DIBI), managed by the National Disaster Management Agency (BNPB) of the Republic of Indonesia. This database provides detailed information about disasters in Indonesia, including their impacts, such as fatalities and economic losses. It organises this information by region, date of occurrence, and types of disaster. The DIBI report encompasses a comprehensive dataset of disasters in Indonesia, spanning from the year 2000, which covers eleven distinct categories of disasters: floods, landslides, wave surges and abrasion, extreme weather, droughts, forest fires, earthquakes, tsunamis, volcanic eruptions, and volcanic explosions. BNPB assigns a unique code to each category of disaster, along with related subsidiary incidents. Users can access DIBI by province and district/city, as well as by year, month, and specific type of disaster.
This research focuses on the Province of the Special Region of Jakarta and considers disasters that occurred in the area from January 2010 to December 2024. In particular, we consider the monthly occurrence of three disasters believed related to climate change: floods, landslides, and extreme weather events; see their recent distributions in Figure S2 in the Supplementary Materials. BNPB classifies various floods under code 101, including coastal floods, flash floods, drainage and trench floods, reservoir floods, stagnant floods, and breaches of levees. Landslides and soil movement are classified as landslide disasters under code 102. Extreme weather events, such as tornadoes, strong windstorms, hurricanes, hailstorms, tropical cyclones, and significant temperature fluctuations, fall under category 104. The Head of BNPB regulates naming conventions and codes for other disaster events in accordance with the Technical Guidelines for Standard Data on Disaster Events and Impacts (Number 7 of 2023) [36].
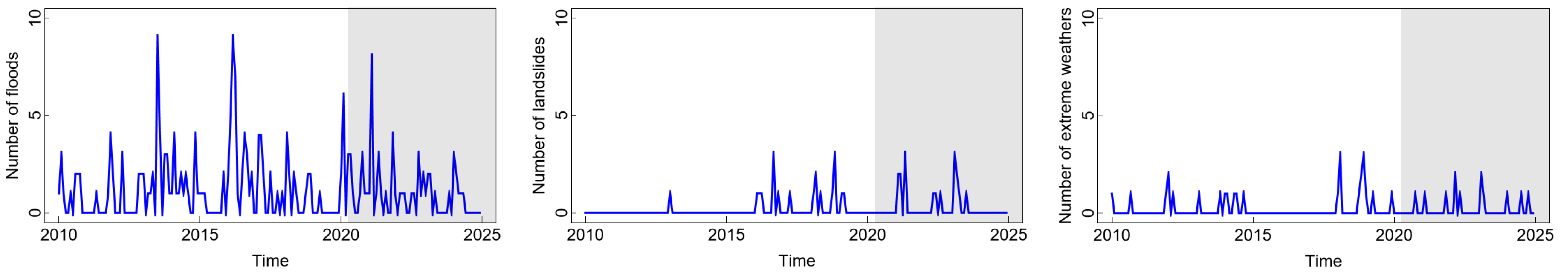
In Figure 4, we compare the numbers of floods, landslides, and extreme weather events in Jakarta over the aforementioned period. We observe from the figure that floods occurred more frequently compared to other natural disasters. Floods in Jakarta have become a major issue, not only related to rainfall but also to the management of the water system. The relatively high flood incidents occurred around 2013, 2016, and 2021, with the highest number of cases being nine incidents in both 2013 and 2016. This could be an early indication of its influence on the spike in DHF cases among children in 2016 and the relatively high number of ARI cases in 2013. Meanwhile, although the number of floods in 2021 was also relatively high, the COVID-19 pandemic conditions made this relationship anomalous. On the other hand, the relatively high number of landslides and extreme weather events between 2015 and 2020 can be an early indication of their impact on the number of diarrhoea cases among children approaching 2020. Landslides can worsen sanitation and clean water quality, which can impact the emergence of diseases, such as diarrhoea and cholera [37]. Meanwhile, extreme weather conditions contribute to the possibility of high temperature fluctuations, which accelerate the growth of microorganisms. This is closely related to the increase in cases of waterborne diseases, such as diarrhoea and DHF [38]. It is worth highlighting that although other climate variables, such as rainfall, temperature, and humidity, are recognised as potential factors influencing infectious diseases, they were not included in our analysis and remain important directions for future research.
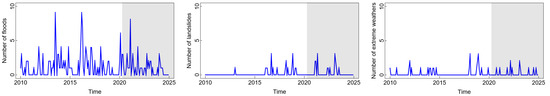
Figure 4.
Number of disasters in Jakarta, Indonesia. Notes: The white and grey areas show Period 1 (before the COVID-19 pandemic) and Period 2 (during and after the COVID-19 pandemic), respectively.
2.2. Integer-Valued Time-Series Models
The widely used model for a time series {Yt, t = 0, 1,…} is an autoregressive (AR) model. Using this model of order one, AR(1), Yt’s can be expressed as
where β denotes a constant satisfying −1 < β < 1, {εt, t = 0, 1,…} denotes a sequence of independent and identical errors commonly assumed to follow a normal distribution, and εt is independent of Yt−1. If we deal with nonnegative Yt’s, then β must satisfy 0 ≤ β < 1, and εt’s must be nonnegatively distributed according to, for instance, an exponential distribution, as proposed by Gaver and Lewis [39].
In this study, we work with nonnegative integer-valued Yt’s that specifically represent the numbers of infectious disease cases among children. Hence, we need to replace Equation (1) with the so-called integer-valued autoregressive (INAR) model, as introduced by McKenzie [20] and Al-Osh and Alzaid [21]. This model can express the number of disease cases at a certain time point as the sum of (i) the number of such cases surviving from the previous time point and (ii) the number of new cases at the time point under investigation. The latter term, also taking the value of nonnegative integers, is commonly assumed to have a Poisson distribution whose mean and variance are equal, resulting in a Poisson INAR model that exhibits equidispersion. It was implemented by Cardinal et al. [18] to model the number of meningococcal disease cases reported in the Montréal-Centre region, Canada, and recently by Ahdika and Lusiyana [19] to model the number of DHF patients in West Java, Indonesia. It has also been extended by Brännäs [40] by incorporating some explanatory variables potentially affecting its outcomes. The resulting Poisson INAR-X model was then adopted by Enciso-Mora et al. [41] to model (i) the number of poliomyelitis cases in the United States by involving a time trend or periodicity and (ii) the score achieved by a schizophrenic patient on a test of perceptual speed by including time trend and drug treatment effects. See also similar works by Moriña [42] on modelling the number of hospital emergency service arrivals with influenza symptoms that presented a periodic behaviour; by Syuhada et al. [43] on modelling the health status score of children in Indonesia with consideration of the income of their parents, the education of their mother, and so on; and by Wang [44] on modelling the number of disease cases caused by Escherichia coli in North Rhine-Westphalia, Germany, with the inclusion of a time trend.
In practice, count time-series data on disease cases frequently show overdispersion, a phenomenon where the variance exceeds the mean. This evidence was highlighted by Maiti and Biswas [22] when analysing the numbers of poliomyelitis cases in the United States (see also [23]) and submissions with skin lesions from a region in New Zealand to animal health laboratories and by Mojica and Co [24] when detecting measles cases during the largest measles outbreak in the Philippines. The overdispersion in these data cannot be captured by the classical Poisson INAR model, thereby motivating the use of a geometric INAR model as an alternative. They demonstrated that using a geometric distributional assumption resulted in an overdispersed INAR model with superior performance compared to the Poisson INAR model. Basically, a geometric distribution can be viewed as a mixed Poisson distribution, namely, a Poisson–exponential distribution, where the Poisson parameter is assumed to follow an exponential distribution. Another important one is Sankaran’s [45] Poisson–Lindley distribution, which is also overdispersed. Its use to construct an INAR model has been taken into consideration by Lívio et al. [25] and Mohammadpour et al. [26]. (It has also been utilised to construct a continuous-time stochastic model by Cha [46] and Syuhada et al. [47]). In particular, Mohammadpour et al. [26] implemented the resulting Poisson–Lindley INAR model for count time-series data on submissions with skin lesions and anorexia from a region in New Zealand to animal health laboratories.
2.2.1. INAR Models
An INAR model of order one, INAR(1), is formulated as follows [20,21,48]:
where for some sequence {Bi, i = 1, 2,…} of independent and identical binary random variables with probability distribution and , . This means that at time , there exist different children diagnosed with a certain disease. At time t, each of them may still have the disease with probability or may not have the disease with probability . Thus, at time t, there are children who have this disease along with additional children diagnosed with the same disease. Their sum, , given in Equation (2), has a mean, a variance, and a dispersion index written, respectively, as follows [49,50]:
where , , and , respectively, denote the mean, variance, and dispersion index of . This indicates that implies (equidispersion), and implies (overdispersion).
2.2.2. INAR Models with Overdispersion
One commonly assumes that the error term follows a Poisson distribution with parameter , which is equal to its mean and variance. Consequently, the resulting Poisson INAR(1) model for is equidispersed. In this study, we consider to have a mixed Poisson distribution by assuming that the Poisson parameter is a value of a nonnegative random variable . In particular, if follows an exponential distribution with parameter , then possesses a Poisson–exponential distribution, well known as a geometric distribution. Under this distributional assumption, the resulting Poisson–exponential INAR(1) model is overdispersed. Alternatively, if obeys a Lindley distribution with parameter [51], then has a Poisson–Lindley distribution [45]. In this case, we obtain a Poisson–Lindley INAR(1) model that is also overdispersed. See Table 1 for more details.

Table 1.
Distributional assumptions for in the INAR(1) model for .
2.2.3. INAR Models with Explanatory Variables
We now assume that there are some explanatory variables X1,t−1, X2,t−1,…, Xm,t−1 that specifically denote the numbers of m different disasters at the previous time t − 1. To incorporate them, we adopt an approach by Brännäs [40] and Enciso-Mora et al. [41] to extend the classical INAR(1) model to become an INAR(1)-X model. We can express the latter model as
with a time-varying probability parameter
for some constants b0, b1,…, bm. In particular, we propose Poisson–exponential INAR(1)-X and Poisson–Lindley INAR(1)-X models, each of which has a time-varying parameter
for some constants c0, c1,…, cm. It is worth mentioning that in their original works, Brännäs [40] and Enciso-Mora et al. [41] considered a time-varying probability parameter of the form , which depends on the explanatory variables at time t and is a decreasing function of bj, j = 0, 1,…, m; they also assumed that the error term εt has a Poisson distribution with time-varying parameter , which is an increasing function of bj, j = 0, 1,…, m. Meanwhile, our proposed logistic link function for in Equation (5) is set to ensure that it depends on the explanatory variables at the previous time t − 1 and is increasing with respect to bj, j = 0, 1,…, m. Similarly, Equation (6) results in a time-varying that is increasing with respect to cj, j = 0, 1,…, m. As a consequence, the time-varying conditional mean of , i.e., , is also increasing with respect to both bj and cj, j = 0, 1,…, m.
3. Results and Discussion
3.1. Descriptive Statistics
In Table 2, we provide a summary of the statistics for infectious disease cases among children, including the mean, variance, skewness, kurtosis, and dispersion index. According to the mean value, the most cases of diarrhoea and ARI occurred in children aged 1–4 years, whereas the majority of DHF cases occurred in the 5–14-year age group. Similarly, the highest variance value was seen in the same age groups for each kind of disease. This demonstrates that, in addition to having the largest number of cases, the disease occurrences vary significantly in such age groups. This suggests that in these age groups, changes in environmental and behavioural factors are more susceptible to effect. While most cases of diarrhoea and ARI occurred in children aged 1–4 years, they decreased almost by half over the period during and after the COVID-19 pandemic. A different pattern was found in DHF cases, where it increased in several age groups due to the pandemic. Meanwhile, a dispersion index value larger than one (which is in line with a positive coefficient of skewness) in all disease categories and age groups indicates that the data are overdispersed, with extremely large dispersion in the diarrhoea and ARI case datasets. This evidence tends to be more pronounced during and after the pandemic, making a Poisson distribution less applicable. Floods were the most common disaster, followed by landslides and extreme weather events (see Table 3). When compared to the other two disasters, floods also had the comparatively highest case variability and dispersion.

Table 2.
Descriptive statistics of count time-series data on infectious disease cases among children over Period 1 (before the COVID-19 pandemic) and Period 2 (during and after the COVID-19 pandemic).

Table 3.
Descriptive statistics of count time-series data on natural disasters over Period 1 (before the COVID-19 pandemic) and Period 2 (during and after the COVID-19 pandemic).
3.2. Correlation Analysis
In Figures S3–S6 in the Supplementary Materials, we depict the autocorrelation and partial autocorrelation functions for diarrhoea, DHF, and ARI cases as well as disaster events, respectively. For diarrhoea and ARI, there was evidence of strongly positive autocorrelations between the number of cases in a certain month and those in previous months for all age groups, with the strongest autocorrelation occurring at a one-month lag. The autocorrelations for age groups beyond 28 days became stronger as the COVID-19 pandemic progressed. Meanwhile, in contrast to the other two types of disease, the numbers of DHF cases were weakly positively autocorrelated at large lags, with the weakest autocorrelations occurring in children aged below one month. Due to evidence from Figure 2 that DHF cases were not impacted by the COVID-19 pandemic, their autocorrelations tended to remain unchanged. For all disease types, the significance of their partial autocorrelation only at a one-month lag led us to consider an integer-valued autoregressive model of order one, INAR(1). For disaster events, despite being unclearly apparent, their autocorrelation at a one-month lag is sufficient to provide an argument that each disaster event in a certain month t was influenced by the same event in the previous month t − 1.
Furthermore, in Figures S7–S9 in the Supplementary Materials, we provide the cross-correlation function between the number of infectious disease cases in children in month t and the number of natural disasters in the previous month t − 1. We observe from the figures that flood and landslide occurrences tended to be positively cross-correlated with DHF cases, although the cross-correlation became weaker following the COVID-19 outbreak. In contrast, they were negatively cross-correlated with diarrhoea and ARI cases. Meanwhile, extreme weather events tended to have positive but weak cross-correlations with all disease cases. The same patterns appeared even when the numbers of disaster events were transformed using an exponential or logistic function. This suggests the presence of nonlinear relationships between disease cases and disaster occurrences, thereby allowing us to incorporate the latter as explanatory variables nonlinearly impacting the former in our proposed INAR(1)-X model.
3.3. Time-Series Modelling
Using the available data described in previous subsections, we estimate the parameters b0, b1, b2, b3, c0, c1, c2, c3 of the Poisson–exponential INAR(1)-X and Poisson–Lindley INAR(1)-X models for the numbers of cases of each infectious disease in children. We accomplish this task by involving the numbers of the three disasters—floods, landslides, and extreme weather events—as explanatory variables. We provide the parameter estimation results in Table 4, Table 5 and Table 6. Through a comparative analysis with the Nash–Sutcliffe efficiency (NSE), we reveal that the two models have similar accuracy and interchangeably perform best. Furthermore, as shown in Figures S10–S15 in the Supplementary Materials, the resulting errors or residuals have no (partial) autocorrelation, indicating that they are randomly and independently distributed.

Table 4.
Estimated parameters and Nash–Sutcliffe efficiency of INAR(1)-X models for the number of diarrhoea cases among children over Period 1 (before the COVID-19 pandemic) and Period 2 (during and after the COVID-19 pandemic).

Table 5.
Estimated parameters and Nash–Sutcliffe efficiency of INAR(1)-X models for the number of DHF cases among children over Period 1 (before the COVID-19 pandemic) and Period 2 (during and after the COVID-19 pandemic).

Table 6.
Estimated parameters and Nash–Sutcliffe efficiency of INAR(1)-X models for the number of ARI cases among children over Period 1 (before the COVID-19 pandemic) and Period 2 (during and after the COVID-19 pandemic).
According to Table 4, regardless of the effects of natural disasters, values, which are positive for almost all age groups, indicate that children diagnosed with diarrhoea tend to remain diagnosed with the same disease in the following month with probability greater than 0.5. When examining the relationships between diarrhoea incidences and disaster occurrences affecting children, the statistical analysis indicates that disaster events have a relatively significant impact on the probability that a child will remain diagnosed with diarrhoea (see the columns) and on the additional new children diagnosed with diarrhoea in the following month (see the columns). However, floods (and extreme weather events) tend to have relatively negative effects. This finding is contrary to other studies, which demonstrated that flooding is associated with increased diarrhoea cases due to a lack of access to clean water [54] and a higher risk of waterborne vector transmission [55]. Moreover, Wang et al. [56] noted that a heightened risk of diarrhoea can occur during severe flooding events that last for extended periods. This scenario does not align with conditions in Indonesia, where nonextreme floods occur ten times more frequently than flash floods. Furthermore, the average duration of flood inundation is approximately three days, which starkly contrasts with flood events that occur on a weekly or monthly basis [57,58].
Table 5 unequivocally shows that even without the influence of disasters, children remain at a significant risk of contracting dengue haemorrhagic fever (DHF). The incidence of DHF can be influenced by several environmental factors, which tend to play a more dominant role. These factors include geographical variation, climate, mosquito breeding sites, and preventive measures, such as mosquito control practice [59]. Statistical evidence clearly establishes a significantly positive relationship between floods/landslides and the increased risk of DHF. While landslides do not directly lead to DHF, they are frequently triggered by excessive rainfall [60]. Excessive rainfall with inadequate water absorption often creates puddles that serve as an ideal breeding site for mosquitoes [61]. Additionally, high rainfall increases air humidity, a key factor that facilitates the breeding of Aedes aegypti mosquitoes [62]. Additionally, the forced relocation of residents affected by landslides to other areas significantly escalates the risk of DHF, as it leads to increased population density and the establishment of new mosquito habitats.
The statistical modelling results in Table 6 clearly demonstrate that, even in the absence of disaster variables, a baseline risk of acute respiratory infections (ARIs) remains significant and is likely to increase. These results also show the statistical significance of a strongly positive relationship between extreme weather conditions and the likelihood of ARI cases in children, particularly during and after COVID-19. This heightened risk can be directly attributed to their weak respiratory systems that increase susceptibility to infections [63]. Therefore, immunisation programs become extremely important to be applied in Indonesia. Vaccines can save lives by imitating an infection triggered by weakened or killed microorganisms [64,65].
Furthermore, maternal demographic factors significantly affect the risk of ARI, as young mothers (under 20 years of age) are 2.02 times more likely to give birth to babies with low birth weight (LBW). Agho et al. [66] and Ejigu et al. [67] mentioned that young mothers tend not to feed their infants and often give their infants formula milk due to a lack of experience, education, attitudes, and social support. Nevertheless, given that breast milk contains vital natural antibodies crucial for combating infections and enhancing immune responses, inadequate breastfeeding further exacerbates the risk [68]. Moreover, socioeconomic conditions and geographic factors undeniably contribute to the prevalence of ARI. Families with low socioeconomic status typically live in poorly ventilated and densely populated environments, which worsen health outcomes [69].
In comparing our time-series model with those reported in previous studies, we note that, unlike many models that rely primarily on climate variables, our approach uses disaster events to diagnose disease outcomes, offering practical value in disaster-prone settings. Although validation and local adaptation are still required, this highlights the model’s contribution to improving diagnostic tools for climate-related health risks.
The utilisation of the time-series model’s framework for data from Jakarta is adaptable and could be applied in other regions with comparable disaster and disease surveillance data. Its use elsewhere will require validation and adjustment to local epidemiological, demographic, environmental, and health system conditions and may include spatial or spatiotemporal effects among different regions, as in recent studies by, e.g., Alene et al. [70] and Silveira et al. [71]. Such a work would confirm its transferability and strengthen its value for global climate-related health risk assessment.
4. Conclusions
This study highlights the urgent need for diagnostic tools to protect children in Indonesia from the health impacts of climate-related disasters. The results demonstrate that landslides (floods and extreme weather events) tend to increase (decrease) the incidence of diarrhoea in children. Floods and landslides, linked to heavy rainfall, are also expected to drive dengue haemorrhagic fever (DHF), whereas extreme weather events sharply elevate acute respiratory infection (ARI), particularly during and after the COVID-19 pandemic.
Our findings suggest that disaster events such as floods, landslides, and extreme weather events are often followed by short-term rises in certain infectious diseases such as diarrhoea, DHF, and ARI in children. While these increases may appear temporary, they add to the overall disease burden, place additional pressure on health services, and, in settings with high vulnerability or limited capacity, can escalate into severe public health emergencies. Recognising these patterns provides an important evidence base for strengthening early warning systems, improving the allocation of health resources, and shaping disaster risk reduction and public health strategies in Indonesia.
While this study centres on Jakarta, the city’s diverse population, frequent exposure to infectious diseases in children, and frequent encounters with climate-related disasters make it useful as a reflection for wider Indonesia or even more broadly to other cities in the world that have similar conditions. We acknowledge that patterns of disease transmission, differences in population density, and the varied impacts of disasters may shape health outcomes differently in other settings. This underscores the need for further research in rural and semi-urban provinces, where the risks of disasters differ and where there is an even greater urgency to design interventions that protect the health of children.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/diseases13090303/s1.
Author Contributions
Conceptualisation, D.W., H.A.J., A.R.H., and S.S.S.; methodology, A.R.H., A.A., and K.S.; software, A.R.H.; validation, D.W., H.A.J, A.R.H., and A.A.; formal analysis, A.R.H., A.A., and K.S.; investigation, D.W., H.A.J., and S.S.S.; resources, D.W. and S.S.S.; data curation, H.A.J.; writing—original draft preparation, D.W., H.A.J., A.R.H., A.A., S.S.S., and K.S.; writing—review and editing, D.W., H.A.J., and A.R.H.; visualisation, A.R.H. and A.A.; supervision, D.W., S.S.S., and K.S.; project administration, S.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
No external funding was received for this research.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data on infectious disease cases among children analysed in this study were retrieved from the epidemiological surveillance and immunisation information system database, particularly the healthcare centres’ integrated disease surveillance database, maintained by the Health Office of the Province of the Special Region of Jakarta, Indonesia (https://surveilans-dinkes.jakarta.go.id, accessed on 17 March 2025). Meanwhile, the disaster data for this province were collected from the Indonesia Disaster Information Database managed by the National Disaster Management Agency (BNPB) of the Republic of Indonesia (https://dibi.bnpb.go.id, accessed on 17 March 2025).
Acknowledgments
This paper was presented at the 45th International Symposium on Forecasting (ISF 2025) in Beijing, China. The authors would like to thank the conference committees and participants as well as the Diseases journal’s editor and anonymous reviewers for their constructive comments that greatly improved the quality of this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ARI | Acute respiratory infection |
| DHF | Dengue haemorrhagic fever |
| INAR | Integer-valued autoregressive |
| INAR-X | Integer-valued autoregressive with explanatory variables |
| NSE | Nash–Sutcliffe efficiency |
| PE | Poisson–exponential |
| PL | Poisson–Lindley |
Appendix A. Estimation for INAR-X Models
Let {Y1, Y2,…, Yn} denote a sample of size n with Yt denoting the actual number of infectious disease cases among children at time t. Suppose that denotes its fitted value according to an INAR(1)-X model, that is, . The corresponding error is thus given by
Using the conditional least-squares estimation method, we can determine an estimator for the parameter vector of the INAR(1)-X model by minimising the following sum of squared errors: . This means that it can be derived by finding a solution to
evaluated at . It is worth mentioning that its asymptotic normality is given by as n approaches infinity. By adopting M-estimation theory in van der Vaart [72], we can estimate the covariance matrix ∑ by , with
In particular, for some or , we have , with
Accordingly, we can examine the null hypothesis H0: θj = 0 using a t-test with test statistic , which asymptotically follows a t-distribution with degrees of freedom. To compare some INAR(1)-X models under consideration, we can compare their Nash–Sutcliffe efficiency (NSE) defined as
where denotes the sample mean of {Y1, Y2,…, Yn}.
References
- Proulx, K.; Daelmans, B.; Baltag, V.; Banati, P. Climate change impacts on child and adolescent health and well-being: A narrative review. J. Glob. Health 2024, 14, 04061. [Google Scholar] [CrossRef]
- Harapan, H.; Michie, A.; Mudatsir, M.; Sasmono, R.T.; Imrie, A. Epidemiology of dengue hemorrhagic fever in Indonesia: Analysis of five decades data from the National Disease Surveillance. BMC Res. Notes 2019, 12, 350. [Google Scholar] [CrossRef]
- Sulistiyowati, N.; Tjandrarini, D.H.; Titaley, C.R.; Que, B.J.; Hidayangsih, P.S.; Suparmi; Sudikno, S.; Purwatiningsih, Y.; Indrawati, L.; Siahaan, S.; et al. Suboptimal child healthcare practices and the development of multiple infectious diseases in children aged 24–59 months. Front. Public Health 2024, 12, 1340559. [Google Scholar] [CrossRef]
- Purnama, T.B.; Wagatsuma, K.; Saito, R. Prevalence and risk factors of acute respiratory infection and diarrhea among children under 5 years old in low-middle wealth household, Indonesia. Infect. Dis. Poverty 2025, 14, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Bignier, C.; Havet, L.; Brisoux, M.; Omeiche, C.; Misra, S.; Gonsard, A.; Drummond, D. Climate change and children’s respiratory health. Paediatr. Respir. Rev. 2024, 53, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Makrufardi, F.; Triasih, R.; Nurnaningsih, N.; Chung, K.F.; Lin, S.C.; Chuang, H.C. Extreme temperatures increase the risk of pediatric pneumonia: A systematic review and meta-analysis. Front. Pediatr. 2024, 12, 1329918. [Google Scholar] [CrossRef] [PubMed]
- Chua, P.L.C.; Ng, C.F.S.; Tobias, A.; Seposo, X.T.; Hashizume, M. Associations between ambient temperature and enteric infections by pathogen: A systematic review and meta-analysis. Lancet Planet. Health 2022, 6, e202–e218. [Google Scholar] [CrossRef]
- Lu, H.; Ayers, E.; Patel, P.; Mattoo, T.K. Body water percentage from childhood to old age. Kidney Res. Clin. Pract. 2023, 42, 340–348. [Google Scholar] [CrossRef]
- Wibawa, B.S.S.; Wang, Y.C.; Andhikaputra, G.; Lin, Y.K.; Hsieh, L.H.C.; Tsai, K.H. The impact of climate variability on dengue fever risk in central java, Indonesia. Clim. Serv. 2024, 33, 100433. [Google Scholar] [CrossRef]
- Thomson, M.C.; Stanberry, L.R. Climate change and vectorborne diseases. N. Engl. J. Med. 2022, 387, 1969–1978. [Google Scholar] [CrossRef]
- Ryco, F.A.; Syafararisa, D.P.; Suvany, A.; Sabilla, C.J.; Revia, M.; Ikrom, M. Tidal flood hazard assessment in Pekalongan City, Central Java. IOP Conf. Ser. Earth Environ. Sci. 2023, 012058, 012058. [Google Scholar]
- Herbanu, P.S.; Nurmaya, A.; Nisaa, R.M.; Wardana, R.A.; Sahid. The zoning of flood disasters by combining tidal flood and urban flood in Semarang City, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2024, 1314, 012028. [Google Scholar] [CrossRef]
- Arhatin, R.E.; Gaol, J.L.; Nurjaya, I.W.; Susilo, S.B.; Kushardono, D.; Nugroho, U.C.; Jumarang, M.I.; Sinurat, M.E.; Balbeid, N. Assessing land subsidence and analyzing tidal flooding in Tangerang, Banten. BIO Web Conf. 2024, 106, 04010. [Google Scholar] [CrossRef]
- Kumar, P.G.; Lekhana, P.; Tejaswi, M.; Chandrakala, S. Effects of vehicular emissions on the urban environment- a state of the art. Mater. Today Proc. 2021, 45, 6314–6320. [Google Scholar] [CrossRef]
- Sukayat, Y.; Setiawan, I.; Suharfaputra, U.; Kurnia, G. Determining factors for farmers to engage in sustainable agricultural practices: A case from Indonesia. Sustainability 2023, 15, 10548. [Google Scholar] [CrossRef]
- Suprapto, S. Waste management laws and policies in Indonesia: Challenges and opportunities. J. Appl. Phys. Sci. 2023, 8, 1–8. [Google Scholar] [CrossRef]
- Yahman, Y.; Setyagama, A. Government policy in regulating the environment for development of sustainable environment in Indonesia. Environ. Dev. Sustain. 2023, 25, 12829–12840. [Google Scholar] [CrossRef]
- Cardinal, M.; Roy, R.; Lambert, J. On the application of integer-valued time series models for the analysis of disease incidence. Stat. Med. 1999, 18, 2025–2039. [Google Scholar] [CrossRef]
- Ahdika, A.; Lusiyana, N. Comparison of INAR(1)-Poisson model and Markov prediction model in forecasting the number of DHF patients in west java Indonesia. J. Phys. Conf. Ser. 2017, 814, 012002. [Google Scholar] [CrossRef]
- McKenzie, E. Some simple models for discrete variate time series. JAWRA J. Am. Water Resour. Assoc. 1985, 21, 645–650. [Google Scholar] [CrossRef]
- Al-Osh, M.A.; Alzaid, A.A. First-order integer-valued autoregressive (INAR(1)) process. J. Time Ser. Anal. 1987, 8, 261–275. [Google Scholar] [CrossRef]
- Maiti, R.; Biswas, A. Coherent forecasting for over-dispersed time series of count data. Braz. J. Probab. Stat. 2015, 29, 747–766. [Google Scholar] [CrossRef]
- Tian, S.; Wang, D.; Cui, S. A seasonal geometric INAR process based on negative binomial thinning operator. Stat. Pap. 2020, 61, 2561–2581. [Google Scholar] [CrossRef]
- Mojica, V.J.C.; Co, F.F. Performance evaluation and comparison of integer-valued time series models for measles outbreak detection in Cavite. Philipp. Stat. 2019, 68, 1–25. [Google Scholar]
- Lívio, T.; Khan, N.M.; Bourguignon, M.; Bakouch, H.S. An INAR(1) model with Poisson–Lindley innovations. Econ. Bull. 2018, 38, 1505–1513. [Google Scholar]
- Mohammadpour, M.; Bakouch, H.S.; Shirozhan, M. Poisson–Lindley INAR(1) model with applications. Braz. J. Probab. Stat. 2018, 32, 262–280. [Google Scholar] [CrossRef]
- Helldén, D.; Andersson, C.; Nilsson, M.; Ebi, K.L.; Friberg, P.; Alfvén, T. Climate change and child health: A scoping review and an expanded conceptual framework. Lancet Planet. Health 2021, 5, e164–e175. [Google Scholar] [CrossRef]
- President of the Republic of Indonesia. Undang-Undang Republik Indonesia Nomor 35 Tahun 2014 Tentang Perubahan Atas Undang-Undang Nomor 23 Tahun 2002 Tentang Perlindungan Anak. 2014. Available online: https://peraturan.bpk.go.id/Details/38723/uu-no-35-tahun-2014 (accessed on 8 April 2025).
- Syuhada, K.; Wibisono, A.; Hakim, A.; Addini, F. COVID-19 risk data during lockdown-like policy in Indonesia. Data Brief 2021, 35, 106801. [Google Scholar] [CrossRef] [PubMed]
- George, C.M.; Cirhuza, L.B.; Kuhl, J.; Williams, C.; Coglianese, N.; Thomas, E.; Bauler, S.; François, R.; Saxton, R.; Presence, A.S.; et al. Child mouthing of feces and fomites and animal contact are associated with diarrhea and impaired growth among young children in the Democratic Republic of the Congo: A prospective cohort study (REDUCE Program). J. Pediatr. 2021, 228, 110–116. [Google Scholar] [CrossRef]
- Liu, J.; Tian, X.; Deng, Y.; Du, Z.; Liang, T.; Hao, Y.; Zhang, D. Risk factors associated with dengue virus infection in Guangdong Province: A community-based case-control study. Int. J. Environ. Res. Public Health 2019, 16, 617. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zhang, H.; Wang, Y.; Liu, Q.; Du, M.; Yan, W.; Qin, C.; Zhang, S.; Chen, W.; Zhou, L.; et al. Global, regional, and national burden of dengue infection in children and adolescents: An analysis of the Global Burden of Disease Study 2021. EClinicalMedicine 2024, 78, 102943. [Google Scholar] [CrossRef] [PubMed]
- Mamenun; Koesmaryono, Y.; Sopaheluwakan, A.; Hidayati, R.; Dasanto, B.D.; Aryati, R. Spatiotemporal characterization of dengue incidence and its correlation to climate parameters in Indonesia. Insects 2024, 15, 366. [Google Scholar] [CrossRef]
- Mokhtar, S.; Ratterree, D.C.P.; Britt, A.F.; Fisher, R.; Ndeffo-Mbah, M.L. Global risk of dengue outbreaks and the impact of El Niño events. Environ. Res. 2024, 262, 119830. [Google Scholar] [CrossRef]
- Pirani, M.; Lorenz, C.; de Azevedo, T.S.; Barbosa, G.L.; Blangiardo, M.; Chiaravalloti-Neto, F. Effects of the El Niño-Southern Oscillation and seasonal weather conditions on Aedes aegypti infestation in the State of São Paulo (Brazil): A Bayesian spatio-temporal study. PLoS Neglected Trop. Dis. 2024, 18, e0012397. [Google Scholar] [CrossRef]
- National Disaster Management Agency (BNPB) of the Republic of Indonesia. Peraturan BNPB Nomor 7 Tahun 2023 tentang Petunjuk Pelaksanaan Standar Data Kejadian dan Dampak Bencana. 2023. Available online: https://data.bnpb.go.id/dataset/juklak-standar-data-kejadian-dan-dampak-bencana (accessed on 10 June 2025).
- Atuyambe, L.M.; Ediau, M.; Orach, C.G.; Musenero, M.; Bazeyo, W. Land slide disaster in eastern Uganda: Rapid assessment of water, sanitation and hygiene situation in Bulucheke camp, Bududa district. Environ. Health 2011, 10, 38. [Google Scholar] [CrossRef]
- Davies, G.I.; McIver, L.; Kim, Y.; Hashizume, M.; Iddings, S.; Chan, V. Water-borne diseases and extreme weather events in Cambodia: Review of impacts and implications of climate change. Int. J. Environ. Res. Public Health 2015, 12, 191–213. [Google Scholar] [CrossRef]
- Gaver, D.P.; Lewis, P.A. First-order autoregressive gamma sequences and point processes. Adv. Appl. Probab. 1980, 12, 727–745. [Google Scholar] [CrossRef]
- Brännäs, K. Explanatory variables in the AR(1) count data model. Umeå Econ. Stud. 1995. Available online: https://www.usbe.umu.se/digitalAssets/39/39103_ues381.pdf (accessed on 1 April 2025).
- Enciso-Mora, V.; Neal, P.; Rao, T.S. Integer valued AR processes with explanatory variables. Sankhyā Indian J. Stat. Ser. B 2009, 71, 248–263. [Google Scholar]
- Moriña, D.; Puig, P.; Ríos, J.; Vilella, A.; Trilla, A. A statistical model for hospital admissions caused by seasonal diseases. Stat. Med. 2011, 30, 3125–3136. [Google Scholar] [CrossRef]
- Syuhada, K.; Wanda, D.; Hartati, S. A simple statistical method for measuring health status for a child: The case of Indonesia. J. Appl. Probab. Stat. 2015, 10, 23–32. [Google Scholar]
- Wang, X. Variable selection for first-order Poisson integer-valued autoregressive model with covariables. Aust. N. Z. J. Stat. 2020, 62, 278–295. [Google Scholar] [CrossRef]
- Sankaran, M. The discrete Poisson–Lindley distribution. Biometrics 1970, 26, 145–149. [Google Scholar] [CrossRef]
- Cha, J.H. Poisson Lindley process and its main properties. Stat. Probab. Lett. 2019, 152, 74–81. [Google Scholar] [CrossRef]
- Syuhada, K.; Tjahjono, V.; Hakim, A. Compound Poisson–Lindley process with Sarmanov dependence structure and its application for premium-based spectral risk forecasting. Appl. Math. Comput. 2024, 467, 128492. [Google Scholar] [CrossRef]
- Syuhada, K.; Alzaid, A.; Salah, D. Prediction limits for Poisson INAR(1) process. J. Math. Fundam. Sci. 2015, 47, 117–125. [Google Scholar]
- Weiss, C.H. Serial dependence and regression of Poisson INARMA models. J. Stat. Plan. Inference 2008, 138, 2975–2990. [Google Scholar] [CrossRef]
- Schweer, S.; Weiss, C.H. Compound Poisson INAR(1) processes: Stochastic properties and testing for overdispersion. Comput. Stat. Data Anal. 2014, 77, 267–284. [Google Scholar] [CrossRef]
- Lindley, D.V. Fiducial distributions and Bayes’ theorem. J. R. Stat. Soc. Ser. B (Methodol.) 1958, 20, 102–107. [Google Scholar] [CrossRef]
- Jazi, M.A.; Jones, G.; Lai, C.D. Integer valued AR(1) with geometric innovations. J. Iran. Stat. Soc. 2012, 11, 173–190. [Google Scholar]
- Ghitany, M.E.; Al-Mutairi, D.K. Estimation methods for the discrete Poisson–Lindley distribution. J. Stat. Comput. Simul. 2009, 79, 1–9. [Google Scholar] [CrossRef]
- ul Haq, I.; Mehmood, Z.; Ahmed, B.; Shah, J.; Begum, N.; Hajira, B.; Xu, J.; Wang, S. Determinants of diarrhea among children aged 1 to 6 years in flood-affected areas of Pakistan: A cross-sectional study. Am. J. Trop. Med. Hyg. 2023, 110, 323–330. [Google Scholar] [CrossRef]
- Yazdi, M.S.; Ardalan, M.A.; Hosseini, M.; Zoshk, M.Y.; Hami, Z.; Heidari, R.; Mosaed, R.; Chamanara, M. Infectious diarrhea risks as a public health emergency in floods; a systematic review and meta-analysis. Arch. Acad. Emerg. Med. 2024, 12, e46. [Google Scholar] [PubMed]
- Wang, P.; Asare, E.O.; Pitzer, V.E.; Dubrow, R.; Chen, K. Floods and diarrhea risk in young children in low- and middle-income countries. JAMA Pediatr. 2023, 177, 1206–1214. [Google Scholar] [CrossRef]
- National Disaster Management Agency (BNPB) of the Republic of Indonesia. Indeks Risiko Bencana Indonesia Tahun 2024. 2025. Available online: https://inarisk.bnpb.go.id/IRBI-2024/mobile/index.html (accessed on 11 June 2025).
- Government of the Province of the Special Region of Jakarta. Pantau Banjir Jakarta. 2025. Available online: https://pantaubanjir.jakarta.go.id (accessed on 11 June 2025).
- Al-Manji, A.; Wirayuda, A.A.B.; Eltayib, R.A.A.; Al-Azri, M.; Chan, M.F. Factors contributing to mosquito-borne disease: A systematic review in the Middle East and North Africa (MENA) region. Curr. Res. Parasitol. Vector-Borne Dis. 2025, 8, 100281. [Google Scholar] [CrossRef] [PubMed]
- Jaelani, L.M.; Fahlefi, R.; Khoiri, M.; Rochman, J.P.G.N. The rainfall effect analysis of landslide occurrence on Mount Slopes of Wilis. Int. J. Adv. Sci. Eng. Inf. Technol. 2020, 10, 298–303. [Google Scholar] [CrossRef]
- Herath, J.M.M.K.; De Silva, W.A.P.P.; Weeraratne, T.C.; Karunaratne, S.H.P.P. Breeding habitat preference of the dengue vector mosquitoes Aedes aegypti and Aedes albopictus from urban, semiurban, and rural areas in Kurunegala district, Sri Lanka. J. Trop. Med. 2024, 2024, 4123543. [Google Scholar] [CrossRef]
- Valdez, L.D.; Sibona, G.J.; Condat, C.A. Impact of rainfall on Aedes aegypti populations. Ecol. Model. 2018, 385, 96–105. [Google Scholar] [CrossRef]
- Anagnostopoulou, P.; Schittny, J.C. Anatomy and development of the respiratory system. In ERS Handbook of Paediatric Respiratory Medicine, 2nd ed.; Eber, E., Midulla, F., Eds.; European Respiratory Society: Lausanne, Switzerland, 2021; pp. 1–13. [Google Scholar]
- Luby, S.P.; Brooks, W.A.; Zaman, K.; Hossain, S.; Ahmed, T. Infectious diseases and vaccine sciences: Strategic directions. J. Health Popul. Nutr. 2008, 6, 295–310. [Google Scholar] [CrossRef][Green Version]
- U.S. Centers for Diseases Control and Prevention. Explaining How Vaccines Work. 2024. Available online: https://www.cdc.gov/vaccines/basics/explaining-how-vaccines-work.html (accessed on 3 September 2025).
- Agho, K.E.; Ahmed, T.; Fleming, C.; Dhami, M.V.; Miner, C.A.; Torome, R.; Ogbo, F.A. Breastfeeding practices among adolescent mothers and associated factors in Bangladesh (2004–2014). Nutrients 2021, 13, 557. [Google Scholar] [CrossRef]
- Ejigu, N.; Sarbecha, N.; Seyoum, K.; Gomora, D.; Geta, G.; Chele, K.; Mengistu, S.; Eshetu, D.; Admasu, Y.; Mesfin, T.; et al. Prevalence of low birth weight and associated factors in Ethiopia: An umbrella review of systematic review and meta-analyses. PLoS Glob. Public Health 2025, 5, e0004556. [Google Scholar] [CrossRef]
- Camacho-Morales, A.; Caba, M.; García-Juárez, M.; Caba-Flores, M.D.; Viveros-Contreras, R.; Martínez-Valenzuela, C. Breastfeeding contributes to physiological immune programming in the newborn. Front. Pediatr. 2021, 9, 744104. [Google Scholar] [CrossRef]
- Mathiarasan, S.; Hüls, A. Impact of environmental injustice on children’s health—Interaction between air pollution and socioeconomic status. Int. J. Environ. Res. Public Health 2021, 18, 795. [Google Scholar] [CrossRef] [PubMed]
- Alene, K.A.; Gordon, C.A.; Clements, A.C.; Williams, G.M.; Gray, D.J.; Zhou, X.N.; Li, Y.; Utzinger, J.; Kurscheid, J.; Forsyth, S.; et al. Spatial analysis of schistosomiasis in Hunan and Jiangxi provinces in the People’s Republic of China. Diseases 2022, 10, 93. [Google Scholar] [CrossRef] [PubMed]
- Silveira, J.R.S.; Lima, S.V.M.A.; Dos Santos, A.D.; Siqueira, L.S.; Santos, G.R.d.S.; Sousa, Á.F.L.d.; de Oliveira, L.B.; Mendes, I.A.C.; Ribeiro, C.J.N. Impact of the COVID-19 pandemic surveillance of visceral leishmaniasis in Brazil: An ecological study. Infect. Dis. Rep. 2024, 16, 116–127. [Google Scholar] [CrossRef] [PubMed]
- van der Vaart, A.W. Asymptotic Statistics; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).