Impact of Obesity on Immunity to the Influenza Virus: Gut Microbiota, Mechanisms, and Novel Therapeutic Strategies
Abstract
1. Introduction
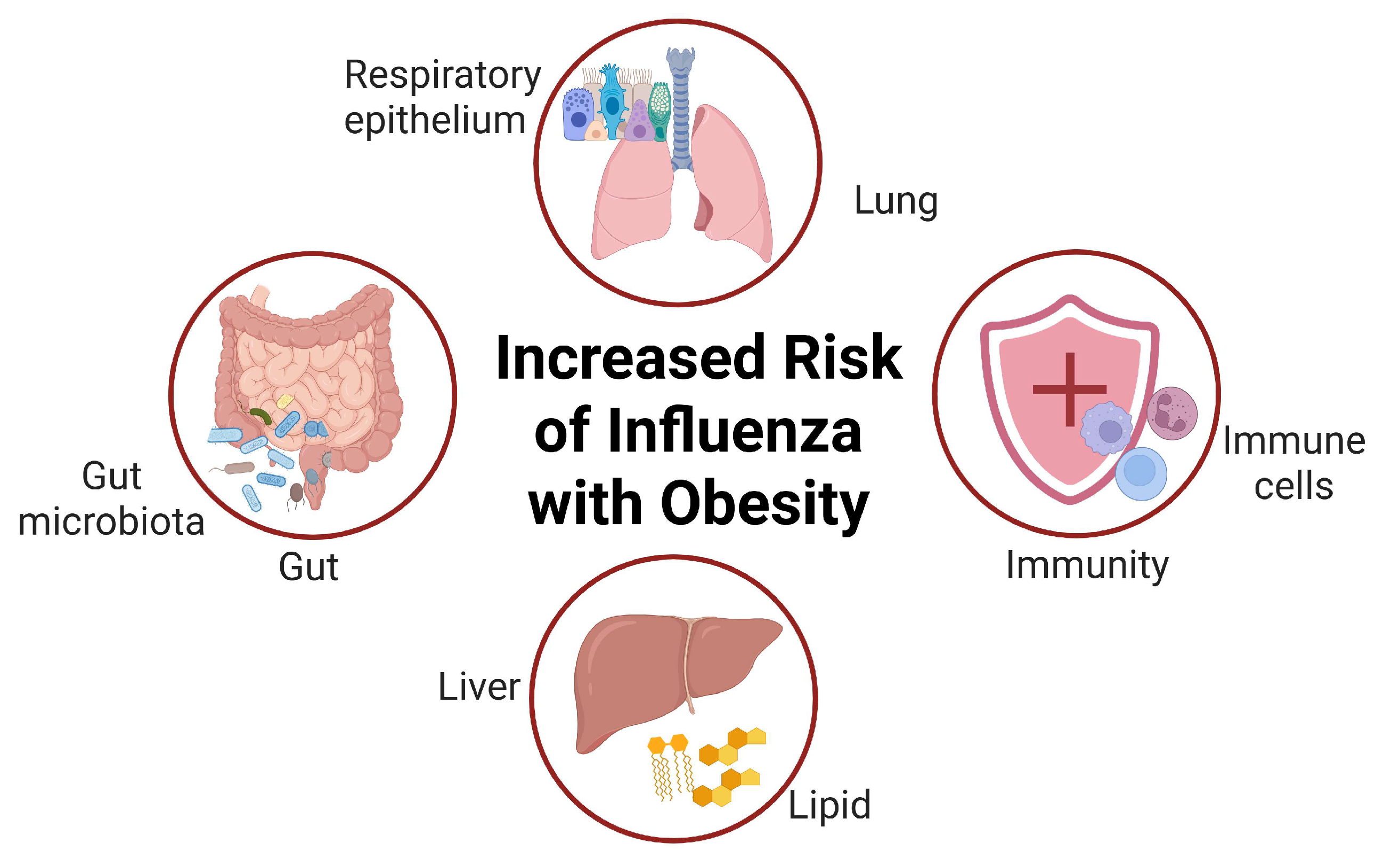
2. The Epidemiological Association Between Obesity and Influenza Infection
3. The Relationship Between Obesity and Gut Microbiota
4. The Impact of Immune Dysfunction on Obese Individuals
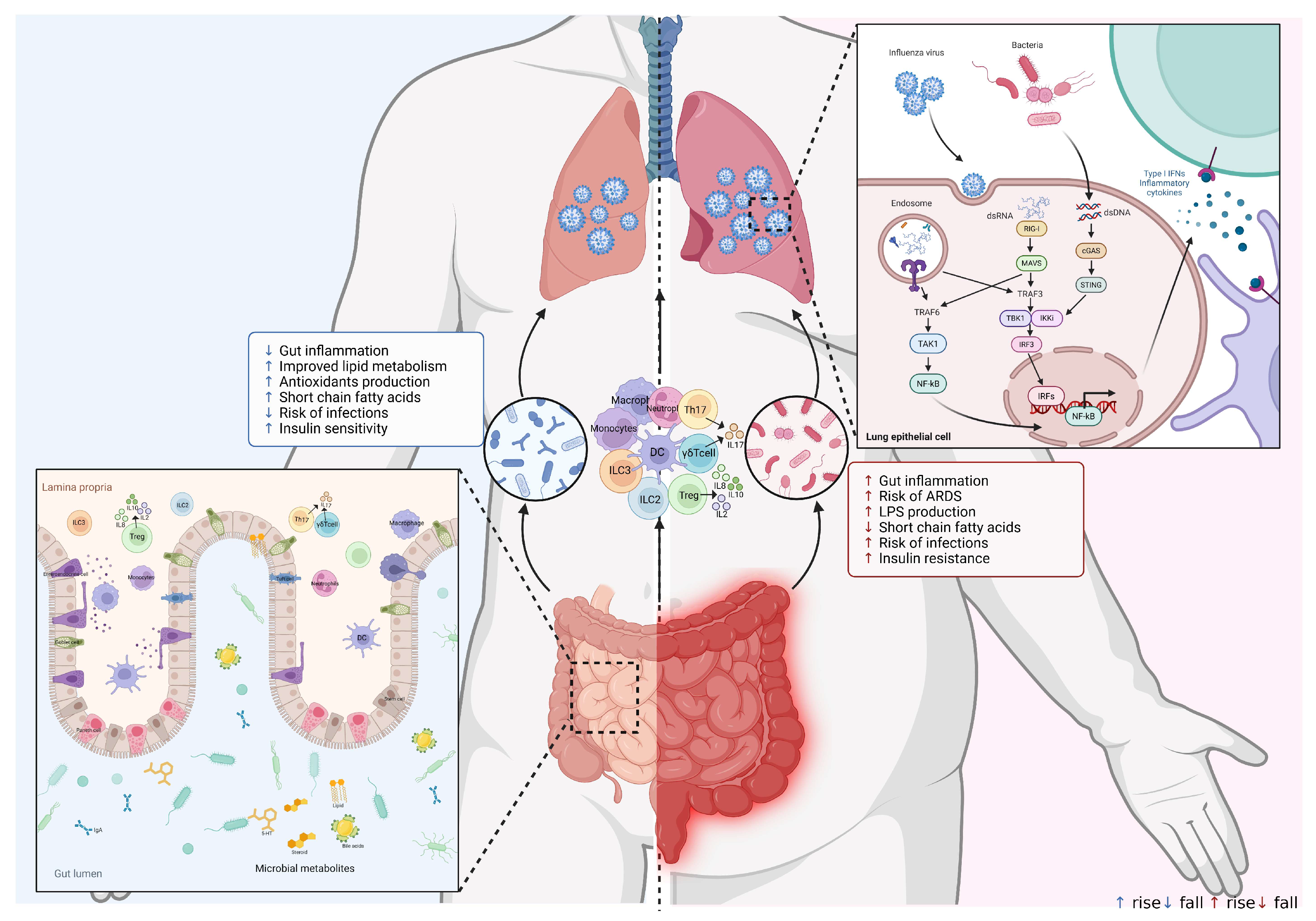
5. Mechanisms of the Interaction Between Gut Microbes and the Respiratory Immune System
6. The Gut–Lung Axis in Influenza Virus Infection and the Immune System
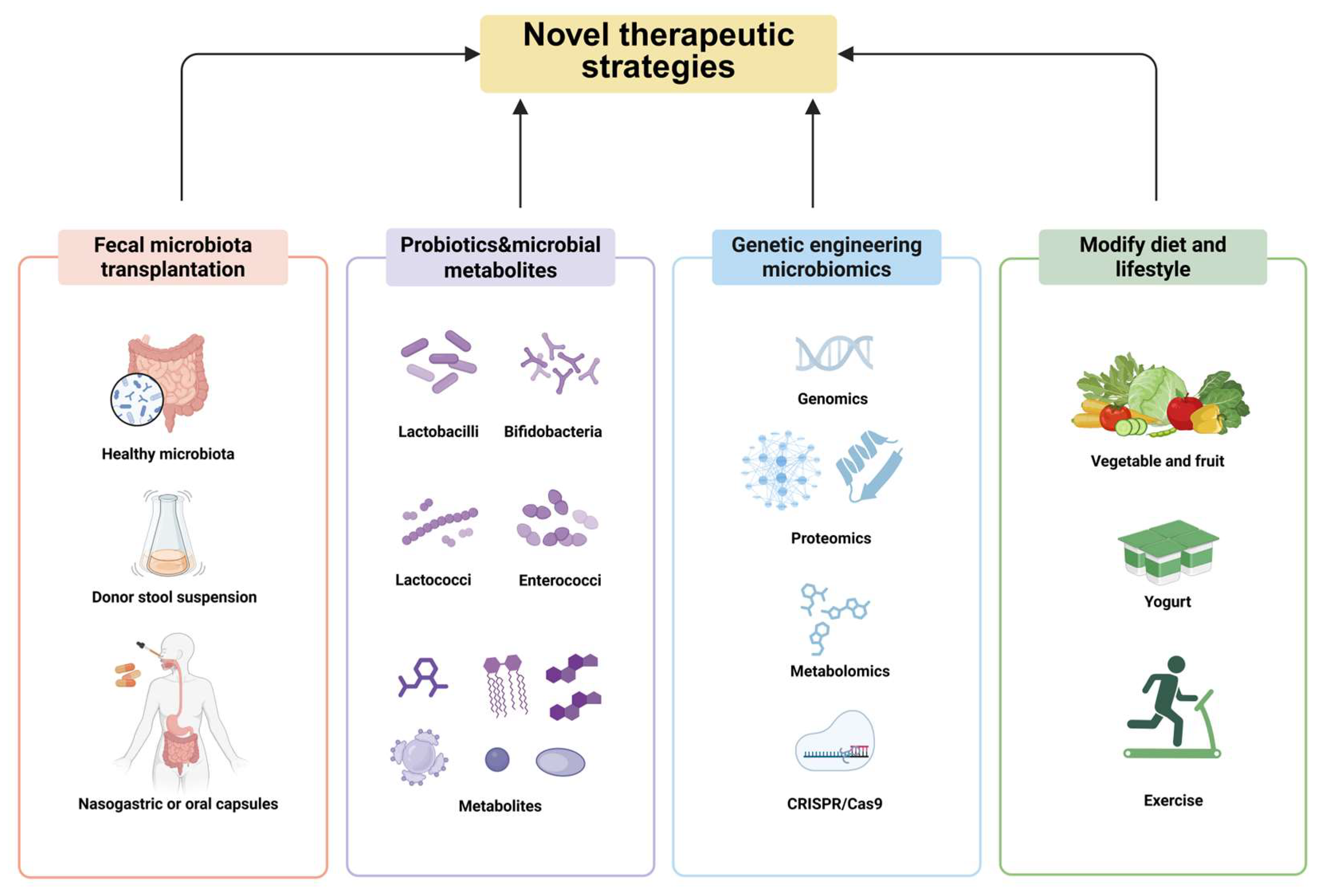
7. Novel Therapeutic Strategies Against Influenza Virus Infection
7.1. Fecal Microbiota Transplantation (FMT)
7.2. Targeted Therapy of Probiotics and Microbial Metabolites
7.3. Transgenic Microbial Therapy
7.4. Modify Diet and Lifestyle
8. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Estimates of deaths associated with seasonal influenza—United States, 1976-2007. MMWR Morb. Mortal. Wkly. Rep. 2010, 59, 1057–1062. [Google Scholar]
- Shrestha, S.S.; Swerdlow, D.L.; Borse, R.H.; Prabhu, V.S.; Finelli, L.; Atkins, C.Y.; Owusu-Edusei, K.; Bell, B.; Mead, P.S.; Biggerstaff, M.; et al. Estimating the burden of 2009 pandemic influenza A (H1N1) in the United States (April 2009–April 2010). Clin. Infect. Dis. 2011, 52 (Suppl. 1), S75–S82. [Google Scholar] [CrossRef]
- Tumpey, T.M.; Basler, C.F.; Aguilar, P.V.; Zeng, H.; Solorzano, A.; Swayne, D.E.; Cox, N.J.; Katz, J.M.; Taubenberger, J.K.; Palese, P.; et al. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science 2005, 310, 77–80. [Google Scholar] [CrossRef]
- Xu, R.; McBride, R.; Paulson, J.C.; Basler, C.F.; Wilson, I.A. Structure, receptor binding, and antigenicity of influenza virus hemagglutinins from the 1957 H2N2 pandemic. J. Virol. 2010, 84, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- West, J.; Roder, J.; Matrosovich, T.; Beicht, J.; Baumann, J.; Mounogou Kouassi, N.; Doedt, J.; Bovin, N.; Zamperin, G.; Gastaldelli, M.; et al. Characterization of changes in the hemagglutinin that accompanied the emergence of H3N2/1968 pandemic influenza viruses. PLoS Pathog. 2021, 17, e1009566. [Google Scholar] [CrossRef] [PubMed]
- Taubenberger, J.K.; Morens, D.M. 1918 Influenza: The mother of all pandemics. Emerg. Infect. Dis. 2006, 12, 15–22. [Google Scholar] [CrossRef]
- Janssen, I.; Katzmarzyk, P.T.; Ross, R. Body mass index, waist circumference, and health risk: Evidence in support of current National Institutes of Health guidelines. Arch. Intern. Med. 2002, 162, 2074–2079. [Google Scholar] [CrossRef]
- Alexopoulos, S.J.; Chen, S.Y.; Brandon, A.E.; Salamoun, J.M.; Byrne, F.L.; Garcia, C.J.; Beretta, M.; Olzomer, E.M.; Shah, D.P.; Philp, A.M.; et al. Mitochondrial uncoupler BAM15 reverses diet-induced obesity and insulin resistance in mice. Nat. Commun. 2020, 11, 2397. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhao, Z.; Ding, C.; Liu, Q.; Ma, T.; Han, X.; Lu, D.; Zhang, L. The association between sarcopenia and cardiovascular disease: An investigative analysis from the NHANES. Nutr. Metab. Cardiovasc. Dis. 2025, 35, 103864. [Google Scholar] [CrossRef]
- Caballero, B. Humans against Obesity: Who Will Win? Adv. Nutr. 2019, 10, S4–S9. [Google Scholar] [CrossRef]
- Hornung, F.; Schulz, L.; Kose-Vogel, N.; Hader, A.; Griesshammer, J.; Wittschieber, D.; Autsch, A.; Ehrhardt, C.; Mall, G.; Loffler, B.; et al. Thoracic adipose tissue contributes to severe virus infection of the lung. Int. J. Obes. 2023, 47, 1088–1099. [Google Scholar] [CrossRef]
- Louie, J.K.; Acosta, M.; Samuel, M.C.; Schechter, R.; Vugia, D.J.; Harriman, K.; Matyas, B.T.; California Pandemic Working, G. A novel risk factor for a novel virus: Obesity and 2009 pandemic influenza A (H1N1). Clin. Infect. Dis. 2011, 52, 301–312. [Google Scholar] [CrossRef]
- Mafort, T.T.; Rufino, R.; Costa, C.H.; Lopes, A.J. Obesity: Systemic and pulmonary complications, biochemical abnormalities, and impairment of lung function. Multidiscip. Respir. Med. 2016, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Athanasoulia, A.P.; Peppas, G.; Karageorgopoulos, D.E. Effect of body mass index on the outcome of infections: A systematic review. Obes. Rev. 2009, 10, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Boden, G. Obesity and free fatty acids. Endocrinol. Metab. Clin. N. Am. 2008, 37, 635–646. [Google Scholar] [CrossRef]
- de Oliveira, S.A.; da Silva, A.A.S.; Hinton, B.T.; Gomes, G.F.; Cunha, T.M.; Cerri, P.S.; Sasso-Cerri, E. SARS-CoV-2 exploits steroidogenic machinery, triggers lipid metabolism for viral replication and induces immune response in Leydig cells of K18-hACE2 mice. Front. Cell. Infect. Microbiol. 2025, 15, 1538461. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Cao, J.; Sun, H.; Niu, W. Clostridium Butyricum 337279 shapes the gut microbiota to attenuate metabolic disorder in diet-induced obese mice. Front. Microbiol. 2025, 16, 1580847. [Google Scholar] [CrossRef]
- Xie, Y.; Pei, F.; Liu, Y.; Liu, Z.; Chen, X.; Xue, D. Fecal fermentation and high-fat diet-induced obesity mouse model confirmed exopolysaccharide from Weissella cibaria PFY06 can ameliorate obesity by regulating the gut microbiota. Carbohydr. Polym. 2023, 318, 121122. [Google Scholar] [CrossRef]
- Arivazhagan, L.; Ruiz, H.H.; Wilson, R.A.; Manigrasso, M.B.; Gugger, P.F.; Fisher, E.A.; Moore, K.J.; Ramasamy, R.; Schmidt, A.M. An Eclectic Cast of Cellular Actors Orchestrates Innate Immune Responses in the Mechanisms Driving Obesity and Metabolic Perturbation. Circ. Res. 2020, 126, 1565–1589. [Google Scholar] [CrossRef]
- Xiao, P.; Li, C.; Wu, J.; Dai, J. Unravel the distinct effects of adiposity at different life stages on COVID-19 susceptibility and severity: A life-course Mendelian randomization study. J. Med. Virol. 2024, 96, e29943. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Grantham, M.; Pantelic, J.; Bueno de Mesquita, P.J.; Albert, B.; Liu, F.; Ehrman, S.; Milton, D.K.; Consortium, E. Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community. Proc. Natl. Acad. Sci. USA 2018, 115, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Elliot, J.G.; Donovan, G.M.; Wang, K.C.W.; Green, F.H.Y.; James, A.L.; Noble, P.B. Fatty airways: Implications for obstructive disease. Eur. Respir. J. 2019, 54, 1900857. [Google Scholar] [CrossRef] [PubMed]
- Kwong, J.C.; Campitelli, M.A.; Rosella, L.C. Obesity and respiratory hospitalizations during influenza seasons in Ontario, Canada: A cohort study. Clin. Infect. Dis. 2011, 53, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Maier, H.E.; Lopez, R.; Sanchez, N.; Ng, S.; Gresh, L.; Ojeda, S.; Burger-Calderon, R.; Kuan, G.; Harris, E.; Balmaseda, A.; et al. Obesity Increases the Duration of Influenza A Virus Shedding in Adults. J. Infect. Dis. 2018, 218, 1378–1382. [Google Scholar] [CrossRef]
- Fezeu, L.; Julia, C.; Henegar, A.; Bitu, J.; Hu, F.B.; Grobbee, D.E.; Kengne, A.P.; Hercberg, S.; Czernichow, S. Obesity is associated with higher risk of intensive care unit admission and death in influenza A (H1N1) patients: A systematic review and meta-analysis. Obes. Rev. 2011, 12, 653–659. [Google Scholar] [CrossRef]
- Diaz, E.; Rodriguez, A.; Martin-Loeches, I.; Lorente, L.; Del Mar Martin, M.; Pozo, J.C.; Montejo, J.C.; Estella, A.; Arenzana, A.; Rello, J.; et al. Impact of obesity in patients infected with 2009 influenza A(H1N1). Chest 2011, 139, 382–386. [Google Scholar] [CrossRef]
- Kok, J.; Blyth, C.C.; Foo, H.; Bailey, M.J.; Pilcher, D.V.; Webb, S.A.; Seppelt, I.M.; Dwyer, D.E.; Iredell, J.R. Viral pneumonitis is increased in obese patients during the first wave of pandemic A(H1N1) 2009 virus. PLoS ONE 2013, 8, e55631. [Google Scholar] [CrossRef]
- Vaillant, L.; La Ruche, G.; Tarantola, A.; Barboza, P.; epidemic intelligence team at InVS. Epidemiology of fatal cases associated with pandemic H1N1 influenza 2009. Euro Surveill. 2009, 14, 19309. [Google Scholar] [CrossRef]
- Investigators, A.I.; Webb, S.A.; Pettila, V.; Seppelt, I.; Bellomo, R.; Bailey, M.; Cooper, D.J.; Cretikos, M.; Davies, A.R.; Finfer, S.; et al. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N. Engl. J. Med. 2009, 361, 1925–1934. [Google Scholar] [CrossRef]
- Andrew, M.K.; Pott, H.; Staadegaard, L.; Paget, J.; Chaves, S.S.; Ortiz, J.R.; McCauley, J.; Bresee, J.; Nunes, M.C.; Baumeister, E.; et al. Age Differences in Comorbidities, Presenting Symptoms, and Outcomes of Influenza Illness Requiring Hospitalization: A Worldwide Perspective From the Global Influenza Hospital Surveillance Network. Open Forum Infect. Dis. 2023, 10, ofad244. [Google Scholar] [CrossRef]
- Cohen, L.E.; Hansen, C.L.; Andrew, M.K.; McNeil, S.A.; Vanhems, P.; Kyncl, J.; Domingo, J.D.; Zhang, T.; Dbaibo, G.; Laguna-Torres, V.A.; et al. Predictors of Severity of Influenza-Related Hospitalizations: Results From the Global Influenza Hospital Surveillance Network (GIHSN). J. Infect. Dis. 2024, 229, 999–1009. [Google Scholar] [CrossRef]
- Lau, D.; Tobin, S.; Pribiag, H.; Nakajima, S.; Fisette, A.; Matthys, D.; Franco Flores, A.K.; Peyot, M.L.; Murthy Madiraju, S.R.; Prentki, M.; et al. ABHD6 loss-of-function in mesoaccumbens postsynaptic but not presynaptic neurons prevents diet-induced obesity in male mice. Nat. Commun. 2024, 15, 10652. [Google Scholar] [CrossRef]
- Guan, D.; Men, Y.; Bartlett, A.; Hernandez, M.A.S.; Xu, J.; Yi, X.; Li, H.S.; Kong, D.; Mazitschek, R.; Ozcan, U. Central inhibition of HDAC6 re-sensitizes leptin signaling during obesity to induce profound weight loss. Cell Metab. 2024, 36, 857–876.e10. [Google Scholar] [CrossRef]
- Liang, Y.; Luo, C.; Sun, L.; Feng, T.; Yin, W.; Zhang, Y.; Mulholland, M.W.; Zhang, W.; Yin, Y. Reduction of specific enterocytes from loss of intestinal LGR4 improves lipid metabolism in mice. Nat. Commun. 2024, 15, 4393. [Google Scholar] [CrossRef] [PubMed]
- Sass, F.; Ma, T.; Ekberg, J.H.; Kirigiti, M.; Urena, M.G.; Dollet, L.; Brown, J.M.; Basse, A.L.; Yacawych, W.T.; Burm, H.B.; et al. NK2R control of energy expenditure and feeding to treat metabolic diseases. Nature 2024, 635, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.; Ludwig, M.Q.; Juozaityte, V.; Ranea-Robles, P.; Svendsen, C.; Hwang, E.; Kristensen, A.W.; Fadahunsi, N.; Lund, J.; Breum, A.W.; et al. GLP-1-directed NMDA receptor antagonism for obesity treatment. Nature 2024, 629, 1133–1141. [Google Scholar] [CrossRef]
- Fadahunsi, N.; Petersen, J.; Metz, S.; Jakobsen, A.; Vad Mathiesen, C.; Silke Buch-Rasmussen, A.; Kurgan, N.; Kjaergaard Larsen, J.; Andersen, R.C.; Topilko, T.; et al. Targeting postsynaptic glutamate receptor scaffolding proteins PSD-95 and PICK1 for obesity treatment. Sci. Adv. 2024, 10, eadg2636. [Google Scholar] [CrossRef]
- Wei, W.; Lyu, X.; Markhard, A.L.; Fu, S.; Mardjuki, R.E.; Cavanagh, P.E.; Zeng, X.; Rajniak, J.; Lu, N.; Xiao, S.; et al. PTER is a N-acetyltaurine hydrolase that regulates feeding and obesity. Nature 2024, 633, 182–188. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Li, H.; Xu, P.; Liu, Q.; Sun, Y.; Zhang, Z.; Wu, T.; Tang, Q.; Jia, Q.; et al. B3galt5 functions as a PXR target gene and regulates obesity and insulin resistance by maintaining intestinal integrity. Nat. Commun. 2024, 15, 5919. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, Y.; Wang, Y.; Liu, P.; Liu, K.; Sun, J.; Zhang, P.; Wang, X.; Liu, X.; Xu, X. Influenza A virus infection activates STAT3 to enhance SREBP2 expression, cholesterol biosynthesis, and virus replication. iScience 2024, 27, 110424. [Google Scholar] [CrossRef]
- Xing, M.; Li, Y.; Zhang, Y.; Zhou, J.; Ma, D.; Zhang, M.; Tang, M.; Ouyang, T.; Zhang, F.; Shi, X.; et al. Paraventricular hypothalamic RUVBL2 neurons suppress appetite by enhancing excitatory synaptic transmission in distinct neurocircuits. Nat. Commun. 2024, 15, 8939. [Google Scholar] [CrossRef]
- Thingholm, L.B.; Ruhlemann, M.C.; Koch, M.; Fuqua, B.; Laucke, G.; Boehm, R.; Bang, C.; Franzosa, E.A.; Hubenthal, M.; Rahnavard, A.; et al. Obese Individuals with and without Type 2 Diabetes Show Different Gut Microbial Functional Capacity and Composition. Cell Host Microbe 2019, 26, 252–264.e10. [Google Scholar] [CrossRef]
- Menni, C.; Jackson, M.A.; Pallister, T.; Steves, C.J.; Spector, T.D.; Valdes, A.M. Gut microbiome diversity and high-fibre intake are related to lower long-term weight gain. Int. J. Obes. 2017, 41, 1099–1105. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, W.; Huang, W.; Lin, Y.; Chan, F.K.L.; Ng, S.C. Gut microbiota in patients with obesity and metabolic disorders—A systematic review. Genes Nutr. 2022, 17, 2. [Google Scholar] [CrossRef]
- Kasai, C.; Sugimoto, K.; Moritani, I.; Tanaka, J.; Oya, Y.; Inoue, H.; Tameda, M.; Shiraki, K.; Ito, M.; Takei, Y.; et al. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol. 2015, 15, 100. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.J.; West, N.P.; Cripps, A.W. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015, 3, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Jumpertz, R.; Le, D.S.; Turnbaugh, P.J.; Trinidad, C.; Bogardus, C.; Gordon, J.I.; Krakoff, J. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am. J. Clin. Nutr. 2011, 94, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Choi, H.N.; Yim, J.E. Effect of Diet on the Gut Microbiota Associated with Obesity. J. Obes. Metab. Syndr. 2019, 28, 216–224. [Google Scholar] [CrossRef]
- Jiang, Z.; Tabuchi, C.; Gayer, S.G.; Bapat, S.P. Immune Dysregulation in Obesity. Annu. Rev. Pathol. 2025, 20, 483–509. [Google Scholar] [CrossRef]
- Hildreth, A.D.; Ma, F.; Wong, Y.Y.; Sun, R.; Pellegrini, M.; O’Sullivan, T.E. Single-cell sequencing of human white adipose tissue identifies new cell states in health and obesity. Nat. Immunol. 2021, 22, 639–653. [Google Scholar] [CrossRef]
- Tang, Q.; Li, J.; Zhang, L.; Zeng, S.; Bao, Q.; Hu, W.; He, L.; Huang, G.; Wang, L.; Liu, Y.; et al. Orlistat facilitates immunotherapy via AKT-FOXO3a-FOXM1-mediated PD-L1 suppression. J. Immunother. Cancer 2025, 13, e008923. [Google Scholar] [CrossRef]
- Kalil, A.C.; Thomas, P.G. Influenza virus-related critical illness: Pathophysiology and epidemiology. Crit. Care 2019, 23, 258. [Google Scholar] [CrossRef]
- Green, W.D.; Al-Shaer, A.E.; Shi, Q.; Gowdy, K.M.; MacIver, N.J.; Milner, J.J.; Beck, M.A.; Shaikh, S.R. Metabolic and functional impairment of CD8(+) T cells from the lungs of influenza-infected obese mice. J. Leukoc. Biol. 2022, 111, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.H.; Aitken, R.J.; Bromfield, E.G.; Cafe, S.L.; Sutherland, J.M.; Frost, E.R.; Nixon, B.; Lord, T. Investigation into the presence and functional significance of proinsulin C-peptide in the female germlinedagger. Biol. Reprod. 2019, 100, 1275–1289. [Google Scholar] [CrossRef]
- Xia, Y.; Zeng, Y.; Jiang, R. Effect of chronic periodontitis on the endothelial glycocalyx of rat penile corpus cavernosum. Andrology 2024, 1–9. [Google Scholar] [CrossRef]
- Jacobo-Tovar, E.; Medel-Sanchez, A.; Duran-Castillo, C.; Guardado-Mendoza, R. Thematic issue: Obesity-driven cancer: Clinical and molecular aspects. Semin. Cancer Biol. 2025, 114, 73–87. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, Q.; Song, N.; Yan, Z.; Lin, R.; Wu, S.; Jiang, L.; Hong, S.; Xie, J.; Zhou, H.; et al. AdipoR1/AdipoR2 dual agonist recovers nonalcoholic steatohepatitis and related fibrosis via endoplasmic reticulum-mitochondria axis. Nat. Commun. 2020, 11, 5807. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Wu, Q.; Wang, H.; Liu, D.; Chen, C.; Zhu, Z.; Zheng, H.; Yang, G.; Li, L.; Yang, M. AZGP1 in POMC neurons modulates energy homeostasis and metabolism through leptin-mediated STAT3 phosphorylation. Nat. Commun. 2024, 15, 3377. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Wu, L.; Gao, M.; Yang, P.; Yang, J.; Deng, Y. Omentin inhibits the resistin-induced hypertrophy of H9c2 cardiomyoblasts by inhibiting the TLR4/MyD88/NF-kappaB signaling pathway. Exp. Ther. Med. 2022, 23, 292. [Google Scholar] [CrossRef]
- Su, Y.; Sun, J.; Li, X.; Huang, F.; Kong, Y.; Chen, Z.; Zhang, J.; Qin, D.; Chen, X.; Wang, Z.; et al. CD47-blocking antibody confers metabolic benefits against obesity. Cell Rep. Med. 2025, 6, 102089. [Google Scholar] [CrossRef]
- Wang, Z.; Aguilar, E.G.; Luna, J.I.; Dunai, C.; Khuat, L.T.; Le, C.T.; Mirsoian, A.; Minnar, C.M.; Stoffel, K.M.; Sturgill, I.R.; et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat. Med. 2019, 25, 141–151. [Google Scholar] [CrossRef]
- Vazquez-Mateo, C.; Collins, J.; Fleury, M.; Dooms, H. Broad induction of immunoregulatory mechanisms after a short course of anti-IL-7Ralpha antibodies in NOD mice. BMC Immunol. 2017, 18, 18. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, T.; Zhu, Z.; Yang, Y. The association between immune cells and breast cancer: Insights from Mendelian randomization and meta-analysis. Int. J. Surg. 2025, 111, 230–241. [Google Scholar] [CrossRef]
- Huang, Y.; Mao, K.; Chen, X.; Sun, M.A.; Kawabe, T.; Li, W.; Usher, N.; Zhu, J.; Urban, J.F., Jr.; Paul, W.E.; et al. S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science 2018, 359, 114–119. [Google Scholar] [CrossRef]
- Olszak, T.; An, D.; Zeissig, S.; Vera, M.P.; Richter, J.; Franke, A.; Glickman, J.N.; Siebert, R.; Baron, R.M.; Kasper, D.L.; et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science 2012, 336, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Alon, R.; Sportiello, M.; Kozlovski, S.; Kumar, A.; Reilly, E.C.; Zarbock, A.; Garbi, N.; Topham, D.J. Leukocyte trafficking to the lungs and beyond: Lessons from influenza for COVID-19. Nat. Rev. Immunol. 2021, 21, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Pick, R.; He, W.; Chen, C.S.; Scheiermann, C. Time-of-Day-Dependent Trafficking and Function of Leukocyte Subsets. Trends Immunol. 2019, 40, 524–537. [Google Scholar] [CrossRef]
- Zundler, S.; Gunther, C.; Kremer, A.E.; Zaiss, M.M.; Rothhammer, V.; Neurath, M.F. Gut immune cell trafficking: Inter-organ communication and immune-mediated inflammation. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.S.; Wang, J.; Yannie, P.J.; Ghosh, S. Intestinal Barrier Dysfunction, LPS Translocation, and Disease Development. J. Endocr. Soc. 2020, 4, bvz039. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Janssen, A.W.; Kersten, S. Potential mediators linking gut bacteria to metabolic health: A critical view. J. Physiol. 2017, 595, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Zhang, Z.; Hang, X.; Jiang, Y.L. plantarum prevents enteroinvasive Escherichia coli-induced tight junction proteins changes in intestinal epithelial cells. BMC Microbiol. 2009, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Song, W.S.; Jeon, Y.J.; Namgung, B.; Hong, M.; Yoon, S.I. A conserved TLR5 binding and activation hot spot on flagellin. Sci. Rep. 2017, 7, 40878. [Google Scholar] [CrossRef]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
- Uchimura, Y.; Fuhrer, T.; Li, H.; Lawson, M.A.; Zimmermann, M.; Yilmaz, B.; Zindel, J.; Ronchi, F.; Sorribas, M.; Hapfelmeier, S.; et al. Antibodies Set Boundaries Limiting Microbial Metabolite Penetration and the Resultant Mammalian Host Response. Immunity 2018, 49, 545–559.e5. [Google Scholar] [CrossRef]
- Trompette, A.; Pernot, J.; Perdijk, O.; Alqahtani, R.A.A.; Domingo, J.S.; Camacho-Munoz, D.; Wong, N.C.; Kendall, A.C.; Wiederkehr, A.; Nicod, L.P.; et al. Gut-derived short-chain fatty acids modulate skin barrier integrity by promoting keratinocyte metabolism and differentiation. Mucosal. Immunol. 2022, 15, 908–926. [Google Scholar] [CrossRef]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Clarke, T.B.; Davis, K.M.; Lysenko, E.S.; Zhou, A.Y.; Yu, Y.; Weiser, J.N. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat. Med. 2010, 16, 228–231. [Google Scholar] [CrossRef]
- Trompette, A.; Gollwitzer, E.S.; Pattaroni, C.; Lopez-Mejia, I.C.; Riva, E.; Pernot, J.; Ubags, N.; Fajas, L.; Nicod, L.P.; Marsland, B.J. Dietary Fiber Confers Protection against Flu by Shaping Ly6c(−) Patrolling Monocyte Hematopoiesis and CD8(+) T Cell Metabolism. Immunity 2018, 48, 992–1005.e8. [Google Scholar] [CrossRef]
- Hill, D.A.; Siracusa, M.C.; Abt, M.C.; Kim, B.S.; Kobuley, D.; Kubo, M.; Kambayashi, T.; Larosa, D.F.; Renner, E.D.; Orange, J.S.; et al. Commensal bacteria-derived signals regulate basophil hematopoiesis and allergic inflammation. Nat. Med. 2012, 18, 538–546. [Google Scholar] [CrossRef]
- Maurice, C.F.; Haiser, H.J.; Turnbaugh, P.J. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell 2013, 152, 39–50. [Google Scholar] [CrossRef]
- Zuo, T.; Zhang, F.; Lui, G.C.Y.; Yeoh, Y.K.; Li, A.Y.L.; Zhan, H.; Wan, Y.; Chung, A.C.K.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients with COVID-19 During Time of Hospitalization. Gastroenterology 2020, 159, 944–955.e8. [Google Scholar] [CrossRef]
- Sencio, V.; Barthelemy, A.; Tavares, L.P.; Machado, M.G.; Soulard, D.; Cuinat, C.; Queiroz-Junior, C.M.; Noordine, M.L.; Salome-Desnoulez, S.; Deryuter, L.; et al. Gut Dysbiosis during Influenza Contributes to Pulmonary Pneumococcal Superinfection through Altered Short-Chain Fatty Acid Production. Cell Rep. 2020, 30, 2934–2947.e6. [Google Scholar] [CrossRef]
- Wang, J.; Li, F.; Wei, H.; Lian, Z.X.; Sun, R.; Tian, Z. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J. Exp. Med. 2014, 211, 2397–2410. [Google Scholar] [CrossRef]
- Laidlaw, B.J.; Zhang, N.; Marshall, H.D.; Staron, M.M.; Guan, T.; Hu, Y.; Cauley, L.S.; Craft, J.; Kaech, S.M. CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity 2014, 41, 633–645. [Google Scholar] [CrossRef]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-chain fatty acids: Linking diet, the microbiome and immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef]
- Bachem, A.; Makhlouf, C.; Binger, K.J.; de Souza, D.P.; Tull, D.; Hochheiser, K.; Whitney, P.G.; Fernandez-Ruiz, D.; Dahling, S.; Kastenmuller, W.; et al. Microbiota-Derived Short-Chain Fatty Acids Promote the Memory Potential of Antigen-Activated CD8(+) T Cells. Immunity 2019, 51, 285–297.e5. [Google Scholar] [CrossRef]
- Moriyama, M.; Ichinohe, T. High ambient temperature dampens adaptive immune responses to influenza A virus infection. Proc. Natl. Acad. Sci. USA 2019, 116, 3118–3125. [Google Scholar] [CrossRef]
- Alarcon, P.C.; Ulanowicz, C.J.; Damen, M.; Eom, J.; Sawada, K.; Chung, H.; Alahakoon, T.; Oates, J.R.; Wayland, J.L.; Stankiewicz, T.E.; et al. Obesity Uncovers the Presence of Inflammatory Lung Macrophage Subsets with an Adipose Tissue Transcriptomic Signature in Influenza Virus Infection. J. Infect. Dis. 2025, 231, e317–e327. [Google Scholar] [CrossRef]
- Castro, I.A.; Jorge, D.M.M.; Ferreri, L.M.; Martins, R.B.; Pontelli, M.C.; Jesus, B.L.S.; Cardoso, R.S.; Criado, M.F.; Carenzi, L.; Valera, F.C.P.; et al. Silent Infection of B and CD8(+) T Lymphocytes by Influenza A Virus in Children with Tonsillar Hypertrophy. J. Virol. 2020, 94, e01969-19. [Google Scholar] [CrossRef]
- Hensen, L.; Illing, P.T.; Bridie Clemens, E.; Nguyen, T.H.O.; Koutsakos, M.; van de Sandt, C.E.; Mifsud, N.A.; Nguyen, A.T.; Szeto, C.; Chua, B.Y.; et al. CD8(+) T cell landscape in Indigenous and non-Indigenous people restricted by influenza mortality-associated HLA-A*24:02 allomorph. Nat. Commun. 2021, 12, 2931. [Google Scholar] [CrossRef]
- Koutsakos, M.; Illing, P.T.; Nguyen, T.H.O.; Mifsud, N.A.; Crawford, J.C.; Rizzetto, S.; Eltahla, A.A.; Clemens, E.B.; Sant, S.; Chua, B.Y.; et al. Human CD8(+) T cell cross-reactivity across influenza A, B and C viruses. Nat. Immunol. 2019, 20, 613–625. [Google Scholar] [CrossRef]
- van de Wall, S.; Anthony, S.M.; Hancox, L.S.; Pewe, L.L.; Langlois, R.A.; Zehn, D.; Badovinac, V.P.; Harty, J.T. Dynamic landscapes and protective immunity coordinated by influenza-specific lung-resident memory CD8(+) T cells revealed by intravital imaging. Immunity 2024, 57, 1878–1892.e5. [Google Scholar] [CrossRef]
- Schmidt, A.; Fuchs, J.; Dedden, M.; Kocher, K.; Schulein, C.; Hubner, J.; Vieira Antao, A.; Irrgang, P.; Oltmanns, F.; Viherlehto, V.; et al. Inflammatory conditions shape phenotypic and functional characteristics of lung-resident memory T cells in mice. Nat. Commun. 2025, 16, 3612. [Google Scholar] [CrossRef]
- Pritzl, C.J.; Luera, D.; Knudson, K.M.; Quaney, M.J.; Calcutt, M.J.; Daniels, M.A.; Teixeiro, E. IKK2/NFkB signaling controls lung resident CD8(+) T cell memory during influenza infection. Nat. Commun. 2023, 14, 4331. [Google Scholar] [CrossRef]
- Lyu, Z.; Yuan, G.; Zhang, Y.; Zhang, F.; Liu, Y.; Li, Y.; Li, G.; Wang, Y.; Zhang, M.; Hu, Y.; et al. Anaerostipes caccae CML199 enhances bone development and counteracts aging-induced bone loss through the butyrate-driven gut-bone axis: The chicken model. Microbiome 2024, 12, 215. [Google Scholar] [CrossRef]
- Dang, A.T.; Begka, C.; Pattaroni, C.; Caley, L.R.; Floto, R.A.; Peckham, D.G.; Marsland, B.J. Butyrate regulates neutrophil homeostasis and impairs early antimicrobial activity in the lung. Mucosal. Immunol. 2023, 16, 476–485. [Google Scholar] [CrossRef]
- Gough, D.J.; Messina, N.L.; Clarke, C.J.; Johnstone, R.W.; Levy, D.E. Constitutive type I interferon modulates homeostatic balance through tonic signaling. Immunity 2012, 36, 166–174. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef]
- Loo, Y.M.; Gale, M., Jr. Immune signaling by RIG-I-like receptors. Immunity 2011, 34, 680–692. [Google Scholar] [CrossRef]
- Sun, L.; Wu, J.; Du, F.; Chen, X.; Chen, Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 2013, 339, 786–791. [Google Scholar] [CrossRef]
- Ichinohe, T.; Pang, I.K.; Kumamoto, Y.; Peaper, D.R.; Ho, J.H.; Murray, T.S.; Iwasaki, A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl. Acad. Sci. USA 2011, 108, 5354–5359. [Google Scholar] [CrossRef]
- Abt, M.C.; Osborne, L.C.; Monticelli, L.A.; Doering, T.A.; Alenghat, T.; Sonnenberg, G.F.; Paley, M.A.; Antenus, M.; Williams, K.L.; Erikson, J.; et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity 2012, 37, 158–170. [Google Scholar] [CrossRef]
- McFarlane, A.J.; McSorley, H.J.; Davidson, D.J.; Fitch, P.M.; Errington, C.; Mackenzie, K.J.; Gollwitzer, E.S.; Johnston, C.J.C.; MacDonald, A.S.; Edwards, M.R.; et al. Enteric helminth-induced type I interferon signaling protects against pulmonary virus infection through interaction with the microbiota. J. Allergy Clin. Immunol. 2017, 140, 1068–1078.e6. [Google Scholar] [CrossRef]
- Stefan, K.L.; Kim, M.V.; Iwasaki, A.; Kasper, D.L. Commensal Microbiota Modulation of Natural Resistance to Virus Infection. Cell 2020, 183, 1312–1324.e10. [Google Scholar] [CrossRef]
- Schaupp, L.; Muth, S.; Rogell, L.; Kofoed-Branzk, M.; Melchior, F.; Lienenklaus, S.; Ganal-Vonarburg, S.C.; Klein, M.; Guendel, F.; Hain, T.; et al. Microbiota-Induced Type I Interferons Instruct a Poised Basal State of Dendritic Cells. Cell 2020, 181, 1080–1096.e19. [Google Scholar] [CrossRef]
- Winkler, E.S.; Shrihari, S.; Hykes, B.L., Jr.; Handley, S.A.; Andhey, P.S.; Huang, Y.S.; Swain, A.; Droit, L.; Chebrolu, K.K.; Mack, M.; et al. The Intestinal Microbiome Restricts Alphavirus Infection and Dissemination through a Bile Acid-Type I IFN Signaling Axis. Cell 2020, 182, 901–918.e18. [Google Scholar] [CrossRef]
- Hu, M.M.; He, W.R.; Gao, P.; Yang, Q.; He, K.; Cao, L.B.; Li, S.; Feng, Y.Q.; Shu, H.B. Virus-induced accumulation of intracellular bile acids activates the TGR5-beta-arrestin-SRC axis to enable innate antiviral immunity. Cell Res. 2019, 29, 193–205. [Google Scholar] [CrossRef]
- Steed, A.L.; Christophi, G.P.; Kaiko, G.E.; Sun, L.; Goodwin, V.M.; Jain, U.; Esaulova, E.; Artyomov, M.N.; Morales, D.J.; Holtzman, M.J.; et al. The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science 2017, 357, 498–502. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, J.; Liu, J.; Li, M.; Xie, J.; Lv, Q.; Deng, W.; Zhou, N.; Zhou, Y.; Song, J.; et al. Mucus production stimulated by IFN-AhR signaling triggers hypoxia of COVID-19. Cell Res. 2020, 30, 1078–1087. [Google Scholar] [CrossRef]
- Giovannoni, F.; Li, Z.; Remes-Lenicov, F.; Davola, M.E.; Elizalde, M.; Paletta, A.; Ashkar, A.A.; Mossman, K.L.; Dugour, A.V.; Figueroa, J.M.; et al. AHR signaling is induced by infection with coronaviruses. Nat. Commun. 2021, 12, 5148. [Google Scholar] [CrossRef]
- Major, J.; Crotta, S.; Finsterbusch, K.; Chakravarty, P.; Shah, K.; Frederico, B.; D’Antuono, R.; Green, M.; Meader, L.; Suarez-Bonnet, A.; et al. Endothelial AHR activity prevents lung barrier disruption in viral infection. Nature 2023, 621, 813–820. [Google Scholar] [CrossRef]
- Antunes, K.H.; Fachi, J.L.; de Paula, R.; da Silva, E.F.; Pral, L.P.; Dos Santos, A.A.; Dias, G.B.M.; Vargas, J.E.; Puga, R.; Mayer, F.Q.; et al. Microbiota-derived acetate protects against respiratory syncytial virus infection through a GPR43-type 1 interferon response. Nat. Commun. 2019, 10, 3273. [Google Scholar] [CrossRef]
- Antunes, K.H.; Singanayagam, A.; Williams, L.; Faiez, T.S.; Farias, A.; Jackson, M.M.; Faizi, F.K.; Aniscenko, J.; Kebadze, T.; Chander Veerati, P.; et al. Airway-delivered short-chain fatty acid acetate boosts antiviral immunity during rhinovirus infection. J. Allergy Clin. Immunol. 2023, 151, 447–457.e5. [Google Scholar] [CrossRef]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef]
- He, Y.; Zhao, C.; Su, N.; Yang, W.; Yang, H.; Yuan, C.; Zhang, N.; Hu, X.; Fu, Y. Disturbances of the gut microbiota-derived tryptophan metabolites as key actors in vagotomy-induced mastitis in mice. Cell Rep. 2024, 43, 114585. [Google Scholar] [CrossRef]
- Martin, A.M.; Yabut, J.M.; Choo, J.M.; Page, A.J.; Sun, E.W.; Jessup, C.F.; Wesselingh, S.L.; Khan, W.I.; Rogers, G.B.; Steinberg, G.R.; et al. The gut microbiome regulates host glucose homeostasis via peripheral serotonin. Proc. Natl. Acad. Sci. USA 2019, 116, 19802–19804. [Google Scholar] [CrossRef]
- Griffin, M.E.; Hespen, C.W.; Wang, Y.C.; Hang, H.C. Translation of peptidoglycan metabolites into immunotherapeutics. Clin. Transl. Immunol. 2019, 8, e1095. [Google Scholar] [CrossRef]
- Yang, M.; Qi, X.; Li, N.; Kaifi, J.T.; Chen, S.; Wheeler, A.A.; Kimchi, E.T.; Ericsson, A.C.; Rector, R.S.; Staveley-O’Carroll, K.F.; et al. Western diet contributes to the pathogenesis of non-alcoholic steatohepatitis in male mice via remodeling gut microbiota and increasing production of 2-oleoylglycerol. Nat. Commun. 2023, 14, 228. [Google Scholar] [CrossRef]
- Yoo, W.; Zieba, J.K.; Foegeding, N.J.; Torres, T.P.; Shelton, C.D.; Shealy, N.G.; Byndloss, A.J.; Cevallos, S.A.; Gertz, E.; Tiffany, C.R.; et al. High-fat diet-induced colonocyte dysfunction escalates microbiota-derived trimethylamine N-oxide. Science 2021, 373, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Hagihara, M.; Yamashita, M.; Ariyoshi, T.; Eguchi, S.; Minemura, A.; Miura, D.; Higashi, S.; Oka, K.; Nonogaki, T.; Mori, T.; et al. Clostridium butyricum-induced omega-3 fatty acid 18-HEPE elicits anti-influenza virus pneumonia effects through interferon-lambda upregulation. Cell Rep. 2022, 41, 111755. [Google Scholar] [CrossRef]
- Erttmann, S.F.; Swacha, P.; Aung, K.M.; Brindefalk, B.; Jiang, H.; Hartlova, A.; Uhlin, B.E.; Wai, S.N.; Gekara, N.O. The gut microbiota prime systemic antiviral immunity via the cGAS-STING-IFN-I axis. Immunity 2022, 55, 847–861.e10. [Google Scholar] [CrossRef]
- Platt, D.J.; Lawrence, D.; Rodgers, R.; Schriefer, L.; Qian, W.; Miner, C.A.; Menos, A.M.; Kennedy, E.A.; Peterson, S.T.; Stinson, W.A.; et al. Transferrable protection by gut microbes against STING-associated lung disease. Cell Rep. 2021, 35, 109113. [Google Scholar] [CrossRef]
- Broggi, A.; Ghosh, S.; Sposito, B.; Spreafico, R.; Balzarini, F.; Lo Cascio, A.; Clementi, N.; De Santis, M.; Mancini, N.; Granucci, F.; et al. Type III interferons disrupt the lung epithelial barrier upon viral recognition. Science 2020, 369, 706–712. [Google Scholar] [CrossRef]
- Canfora, E.E.; Meex, R.C.R.; Venema, K.; Blaak, E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019, 15, 261–273. [Google Scholar] [CrossRef]
- Lopez, C.E.; Zacharias, Z.R.; Ross, K.A.; Narasimhan, B.; Waldschmidt, T.J.; Legge, K.L. Polyanhydride nanovaccine against H3N2 influenza A virus generates mucosal resident and systemic immunity promoting protection. NPJ Vaccines 2024, 9, 96. [Google Scholar] [CrossRef]
- Gaisina, I.; Li, P.; Du, R.; Cui, Q.; Dong, M.; Zhang, C.; Manicassamy, B.; Caffrey, M.; Moore, T.; Cooper, L.; et al. An orally active entry inhibitor of influenza A viruses protects mice and synergizes with oseltamivir and baloxavir marboxil. Sci. Adv. 2024, 10, eadk9004. [Google Scholar] [CrossRef]
- Ou, G.; Xu, H.; Wu, J.; Wang, S.; Chen, Y.; Deng, L.; Chen, X. The gut-lung axis in influenza A: The role of gut microbiota in immune balance. Front. Immunol. 2023, 14, 1147724. [Google Scholar] [CrossRef] [PubMed]
- Ross, F.C.; Patangia, D.; Grimaud, G.; Lavelle, A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. The interplay between diet and the gut microbiome: Implications for health and disease. Nat. Rev. Microbiol. 2024, 22, 671–686. [Google Scholar] [CrossRef]
- Johnsen, P.H.; Hilpusch, F.; Cavanagh, J.P.; Leikanger, I.S.; Kolstad, C.; Valle, P.C.; Goll, R. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: A double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol. Hepatol. 2018, 3, 17–24. [Google Scholar] [CrossRef]
- Van Lier, Y.F.; Davids, M.; Haverkate, N.J.E.; de Groot, P.F.; Donker, M.L.; Meijer, E.; Heubel-Moenen, F.; Nur, E.; Zeerleder, S.S.; Nieuwdorp, M.; et al. Donor fecal microbiota transplantation ameliorates intestinal graft-versus-host disease in allogeneic hematopoietic cell transplant recipients. Sci. Transl. Med. 2020, 12, eaaz8926. [Google Scholar] [CrossRef]
- Ghani, R.; Mullish, B.H.; McDonald, J.A.K.; Ghazy, A.; Williams, H.R.T.; Brannigan, E.T.; Mookerjee, S.; Satta, G.; Gilchrist, M.; Duncan, N.; et al. Disease Prevention Not Decolonization: A Model for Fecal Microbiota Transplantation in Patients Colonized with Multidrug-resistant Organisms. Clin. Infect. Dis. 2021, 72, 1444–1447. [Google Scholar] [CrossRef]
- Surawicz, C.M.; Brandt, L.J.; Binion, D.G.; Ananthakrishnan, A.N.; Curry, S.R.; Gilligan, P.H.; McFarland, L.V.; Mellow, M.; Zuckerbraun, B.S. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am. J. Gastroenterol. 2013, 108, 478–498; quiz 499. [Google Scholar] [CrossRef]
- Liu, F.; Ye, S.; Zhu, X.; He, X.; Wang, S.; Li, Y.; Lin, J.; Wang, J.; Lin, Y.; Ren, X.; et al. Gastrointestinal disturbance and effect of fecal microbiota transplantation in discharged COVID-19 patients. J. Med. Case Rep. 2021, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Bilinski, J.; Winter, K.; Jasinski, M.; Szczes, A.; Bilinska, N.; Mullish, B.H.; Malecka-Panas, E.; Basak, G.W. Rapid resolution of COVID-19 after faecal microbiota transplantation. Gut 2022, 71, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.; Hock, Q.S.; Kadim, M.; Mohan, N.; Ryoo, E.; Sandhu, B.; Yamashiro, Y.; Jie, C.; Hoekstra, H.; Guarino, A. Probiotics for gastrointestinal disorders: Proposed recommendations for children of the Asia-Pacific region. World J. Gastroenterol. 2017, 23, 7952–7964. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, L.; Lin, W.; Tang, W.; Chan, F.K.L.; Ng, S.C. Review article: Probiotics, prebiotics and dietary approaches during COVID-19 pandemic. Trends Food Sci. Technol. 2021, 108, 187–196. [Google Scholar] [CrossRef]
- Xavier-Santos, D.; Padilha, M.; Fabiano, G.A.; Vinderola, G.; Gomes Cruz, A.; Sivieri, K.; Costa Antunes, A.E. Evidences and perspectives of the use of probiotics, prebiotics, synbiotics, and postbiotics as adjuvants for prevention and treatment of COVID-19: A bibliometric analysis and systematic review. Trends Food Sci. Technol. 2022, 120, 174–192. [Google Scholar] [CrossRef]
- Ceccarelli, G.; Borrazzo, C.; Pinacchio, C.; Santinelli, L.; Innocenti, G.P.; Cavallari, E.N.; Celani, L.; Marazzato, M.; Alessandri, F.; Ruberto, F.; et al. Oral Bacteriotherapy in Patients with COVID-19: A Retrospective Cohort Study. Front. Nutr. 2020, 7, 613928. [Google Scholar] [CrossRef] [PubMed]
- d’Ettorre, G.; Ceccarelli, G.; Marazzato, M.; Campagna, G.; Pinacchio, C.; Alessandri, F.; Ruberto, F.; Rossi, G.; Celani, L.; Scagnolari, C.; et al. Challenges in the Management of SARS-CoV2 Infection: The Role of Oral Bacteriotherapy as Complementary Therapeutic Strategy to Avoid the Progression of COVID-19. Front. Med. 2020, 7, 389. [Google Scholar] [CrossRef]
- McCarville, J.L.; Chen, G.Y.; Cuevas, V.D.; Troha, K.; Ayres, J.S. Microbiota Metabolites in Health and Disease. Annu. Rev. Immunol. 2020, 38, 147–170. [Google Scholar] [CrossRef] [PubMed]
- Van Treuren, W.; Dodd, D. Microbial Contribution to the Human Metabolome: Implications for Health and Disease. Annu. Rev. Pathol. 2020, 15, 345–369. [Google Scholar] [CrossRef]
- Gu, S.; Chen, Y.; Wu, Z.; Chen, Y.; Gao, H.; Lv, L.; Guo, F.; Zhang, X.; Luo, R.; Huang, C.; et al. Alterations of the Gut Microbiota in Patients with Coronavirus Disease 2019 or H1N1 Influenza. Clin. Infect. Dis. 2020, 71, 2669–2678. [Google Scholar] [CrossRef]
- Zuo, T.; Liu, Q.; Zhang, F.; Lui, G.C.; Tso, E.Y.; Yeoh, Y.K.; Chen, Z.; Boon, S.S.; Chan, F.K.; Chan, P.K.; et al. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut 2021, 70, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wan, Y.; Zuo, T.; Yeoh, Y.K.; Liu, Q.; Zhang, L.; Zhan, H.; Lu, W.; Xu, W.; Lui, G.C.Y.; et al. Prolonged Impairment of Short-Chain Fatty Acid and L-Isoleucine Biosynthesis in Gut Microbiome in Patients with COVID-19. Gastroenterology 2022, 162, 548–561.e4. [Google Scholar] [CrossRef]
- Piscotta, F.J.; Hoffmann, H.H.; Choi, Y.J.; Small, G.I.; Ashbrook, A.W.; Koirala, B.; Campbell, E.A.; Darst, S.A.; Rice, C.M.; Brady, S.F. Metabolites with SARS-CoV-2 Inhibitory Activity Identified from Human Microbiome Commensals. mSphere 2021, 6, e0071121. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef]
- Mitragotri, S.; Burke, P.A.; Langer, R. Overcoming the challenges in administering biopharmaceuticals: Formulation and delivery strategies. Nat. Rev. Drug Discov. 2014, 13, 655–672. [Google Scholar] [CrossRef]
- Chen, S.W.; Zhang, W. Current trends and challenges in the downstream purification of bispecific antibodies. Antib. Ther. 2021, 4, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Charbonneau, M.R.; Isabella, V.M.; Li, N.; Kurtz, C.B. Developing a new class of engineered live bacterial therapeutics to treat human diseases. Nat. Commun. 2020, 11, 1738. [Google Scholar] [CrossRef]
- Roslan, M.A.M.; Omar, M.N.; Sharif, N.A.M.; Raston, N.H.A.; Arzmi, M.H.; Neoh, H.M.; Ramzi, A.B. Recent advances in single-cell engineered live biotherapeutic products research for skin repair and disease treatment. NPJ Biofilms Microbiomes 2023, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Vigouroux, A.; Rousset, F.; Varet, H.; Khanna, V.; Bikard, D. A CRISPRi screen in E. coli reveals sequence-specific toxicity of dCas9. Nat. Commun. 2018, 9, 1912. [Google Scholar] [CrossRef] [PubMed]
- Crook, N.; Ferreiro, A.; Gasparrini, A.J.; Pesesky, M.W.; Gibson, M.K.; Wang, B.; Sun, X.; Condiotte, Z.; Dobrowolski, S.; Peterson, D.; et al. Adaptive Strategies of the Candidate Probiotic E. coli Nissle in the Mammalian Gut. Cell Host Microbe 2019, 25, 499–512.e8. [Google Scholar] [CrossRef]
- Xin, Y.; Liu, C.G.; Zang, D.; Chen, J. Gut microbiota and dietary intervention: Affecting immunotherapy efficacy in non-small cell lung cancer. Front. Immunol. 2024, 15, 1343450. [Google Scholar] [CrossRef]
- McQuade, J.L.; Daniel, C.R.; Helmink, B.A.; Wargo, J.A. Modulating the microbiome to improve therapeutic response in cancer. Lancet Oncol. 2019, 20, e77–e91. [Google Scholar] [CrossRef]
- Gouez, M.; Raynard, B.; Marijnen, P.; Ho Hio Hen, N.; Fervers, B. [Nutrition and physical activity (PA) during and after cancer treatment: Therapeutic benefits, pathophysiology, recommendations, clinical management]. Bull. Cancer 2022, 109, 516–527. [Google Scholar] [CrossRef]
- Shan, G.; Minchao, K.; Jizhao, W.; Rui, Z.; Guangjian, Z.; Jin, Z.; Meihe, L. Resveratrol improves the cytotoxic effect of CD8+T cells in the tumor microenvironment by regulating HMMR/Ferroptosis in lung squamous cell carcinoma. J. Pharm. Biomed. Anal. 2023, 229, 115346. [Google Scholar] [CrossRef]
- Saraiva, A.; Raheem, D.; Roy, P.R.; BinMowyna, M.N.; Romao, B.; Alarifi, S.N.; Albaridi, N.A.; Alsharari, Z.D.; Raposo, A. Probiotics and Plant-Based Foods as Preventive Agents of Urinary Tract Infection: A Narrative Review of Possible Mechanisms Related to Health. Nutrients 2025, 17, 986. [Google Scholar] [CrossRef] [PubMed]
| Target Protein/Pathway | Main Distribution | Function | References |
|---|---|---|---|
| ABHD6 | Brain, intestine, and immune system | α/β-hydrolase domain 6 (ABHD6) is a lipase affecting energy metabolism. | [32] |
| HDAC6 | Liver and brain | Histone deacetylase 6 (HDAC6) resensitizes leptin signaling during obesity. | [33] |
| LGR4 | Liver | G-protein-coupled receptor 4 (LGR4) impacts long-chain fatty acid-absorption. | [34] |
| NK2R | Colon and immune system | Neurokinin 2 receptor (NK2R) can increase energy expenditure peripherally. | [35] |
| NMDA receptor | Brain | The N-methyl-D-aspartate (NMDA) receptor antagonism can treat obesity. | [36] |
| PICK1, PSD95 | Brain | Protein interacting with C kinase 1 and postsynaptic density protein-95 targeting postsynaptic glutamate receptors for obesity treatment. | [37] |
| PTER | Liver and brain | Orphan enzyme phosphotriesterase-related (PTER) is a N-acetyltaurine hydrolase. | [38] |
| PXR | Liver | Pregnane X receptor (PXR) can regulate glycolipid metabolism. | [39] |
| SREBP2, RORγ | Liver and immune system | Sterol regulatory element-binding protein 2 (SREBP2) and the retinoid acid receptor-related orphan receptor gamma (RORγ) regulate cholesterol metabolism. | [40] |
| RUVBL2 | Liver and brain | Knockout of PVH RUVBL2 results in hyperphagic obesity. | [41] |
| Type | Biomarker | Association with Chronic Disease | Function | Signaling Pathway | References |
|---|---|---|---|---|---|
| Insulin/IGF axis | Insulin/C-peptide | Cardiovascular disease | Inhibit hepatic gluconeogenesis and promote fat synthesis | GPR146-PLC/PKC-PI3K | [55] |
| CRP | Cardiovascular disease, vascular and non-vascular mortality, colorectal cancer | Promote endothelial dysfunction | eNOS | [56] | |
| IGF-1 | Cancer and cardiovascular diseases | Inhibit catabolism | PI3K/AKT/mTOR | [57] | |
| Adipokines | Adiponectin | NASH | Promote fatty acid oxidation | AdipoR1/R2-AMPK/PPARα | [58] |
| Leptin | NASH | Increase energy consumption (promote brown fat thermogenesis) | LepR-JAK2-STAT3 | [59] | |
| Resistin | T2DM | Participate in insulin resistance | MyD88-NF-κB | [60] | |
| Immune checkpoint | CD47 | Cancer | Reduce metabolic rate | isoQC-pGlu-SIRPα | [61] |
| PD-L1 | Cancer | Inhibit T cell activation | PD-1/SHP1/2-TCR | [62] | |
| LAG-3 | Cancer | Inhibit inflammation | IL-7Rα | [63] | |
| Tim-3 | Cancer, autoimmune disease | Mediating insulin resistance in adipose tissue | Gal-9-Bat3/c | [64] |
| Markers | Cell Types and Distribution | Efficacy | References |
|---|---|---|---|
| CD4 | T-cell receptor | CD4 T cells play a multiplicity of roles in protective immunity to influenza, including viral antigen specificity. | [86] |
| CD8 | T-cell receptor | CD8 T cells provide broad cross-reactive immunity and alleviate disease severity by recognizing conserved epitopes. | [90] |
| CD11 | T cells, B cells, monocytes, macrophages, neutrophils, basophilic granulocytes, and eosinophilic granulocytes | CD11b+ cDC2 subsets present in mice are regulated by IRF4 during IAV infection. | [91,92] |
| CD27 | Lymphoid cells (naive T cells, activated B cells, NK cells) | CD45RA−CD27− effector memory-like T cells are increased in IAV- and IBV-infected patients. | [93] |
| CD38 | Lymphocytes, plasma cells, NK cells, and non-hematopoietic tissues | CD38+Ki67+CD8+ effector T cells are increased in IAV infected pediatric and adult subjects. | [94] |
| CD45 | Hematopoietic cells | The CD45-positive macrophages expressing mCherry are increased in IAV-infected patients. | [91,93] |
| CD64 | Monocyte and macrophage | Mice lacking myeloid TBK1 showed less recruitment of CD64+SiglecF−Ly6C inflammatory macrophages. | [91] |
| CD69 | T cells, B cells, natural killer (NK) cells, neutrophils, and eosinophils | CD69+CD103+ TRM cells preferentially localized to lung sites of prior IAV infection. | [95] |
| CD103 | T cells, B cells, lymphocytes, and dendritic cells | Vaccine can induced lung tissue-resident memory T cells expressing high levels of CD103. | [95,96] |
| CD122 | NK cells and activated T cells | Once memory to influenza is established, enhanced NF-κB signaling in T cells can increase CD122 levels. | [97] |
| Microbial Metabolites | Bacteria | Efficacy | References |
|---|---|---|---|
| Acetate | Acetobacter and Bifidobacterium pseudolongum | Acetate can trigger antiviral immunity. | [115,116] |
| Butyrate | Clostridium butyricum and Butyrivibrio | Butyrate reprograms CD8+ T cells by promoting glutamine utilization and fatty acid oxidation. | [90] |
| LPS | Gram-negative bacteria | LPS can activate the TLR4 pathway to trigger the NF-κB signaling pathway and regulate the inflammatory response. | [46] |
| BCAAs | Prevotellacopri and Bacteroides vulgatus | Branched-chain amino acids can induce insulin resistance. | [117] |
| Indole derivatives (e.g., IAA, IPA, 5-HIAA) | Escherichia coli, Proteus and Vibrio cholerae | Indole derivatives can activate the AhR. | [118] |
| 5-HT | Enterochromaffin cells produce 5-HT influenced by gut microbiota | 5-hydroxytryptaminecan regulates glucose homeostasis. | [119] |
| PGN | All species of bacteria | Peptidoglycan can activate host immunity. | [120] |
| 2-octagenoate | Blautia bacterium | 2-octagenoate can lead to liver hypertrophy, steatosis, inflammation of liver cells, and fibrosis. | [121] |
| DAT | Clostridium orbiscindens | DAT can trigger tonic IFN signaling and regulate the phagocytic activity of macrophages. | [111] |
| TMA | Gut microbiota | Trimethylamine is converted to trimethylamine-N-oxide (TMAO) in the liver. TMAO regulates glucose metabolism and causes adipose tissue inflammation. | [122] |
| Bile acids | Clostridium scindens | BAs activate virus-induced NF-κB. | [110] |
| 18-HEPE | Clostridium strain C. butyricum | 18-HEPE activates the production of tonic IFN-λ by lung epithelial cells via GPR120, leading to enhanced resistance to influenza infection. | [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, X.; Sun, J. Impact of Obesity on Immunity to the Influenza Virus: Gut Microbiota, Mechanisms, and Novel Therapeutic Strategies. Diseases 2025, 13, 267. https://doi.org/10.3390/diseases13080267
Ji X, Sun J. Impact of Obesity on Immunity to the Influenza Virus: Gut Microbiota, Mechanisms, and Novel Therapeutic Strategies. Diseases. 2025; 13(8):267. https://doi.org/10.3390/diseases13080267
Chicago/Turabian StyleJi, Xiaoyue, and Jing Sun. 2025. "Impact of Obesity on Immunity to the Influenza Virus: Gut Microbiota, Mechanisms, and Novel Therapeutic Strategies" Diseases 13, no. 8: 267. https://doi.org/10.3390/diseases13080267
APA StyleJi, X., & Sun, J. (2025). Impact of Obesity on Immunity to the Influenza Virus: Gut Microbiota, Mechanisms, and Novel Therapeutic Strategies. Diseases, 13(8), 267. https://doi.org/10.3390/diseases13080267






