Cardiovascular Complications of COVID-19 Disease: A Narrative Review
Abstract
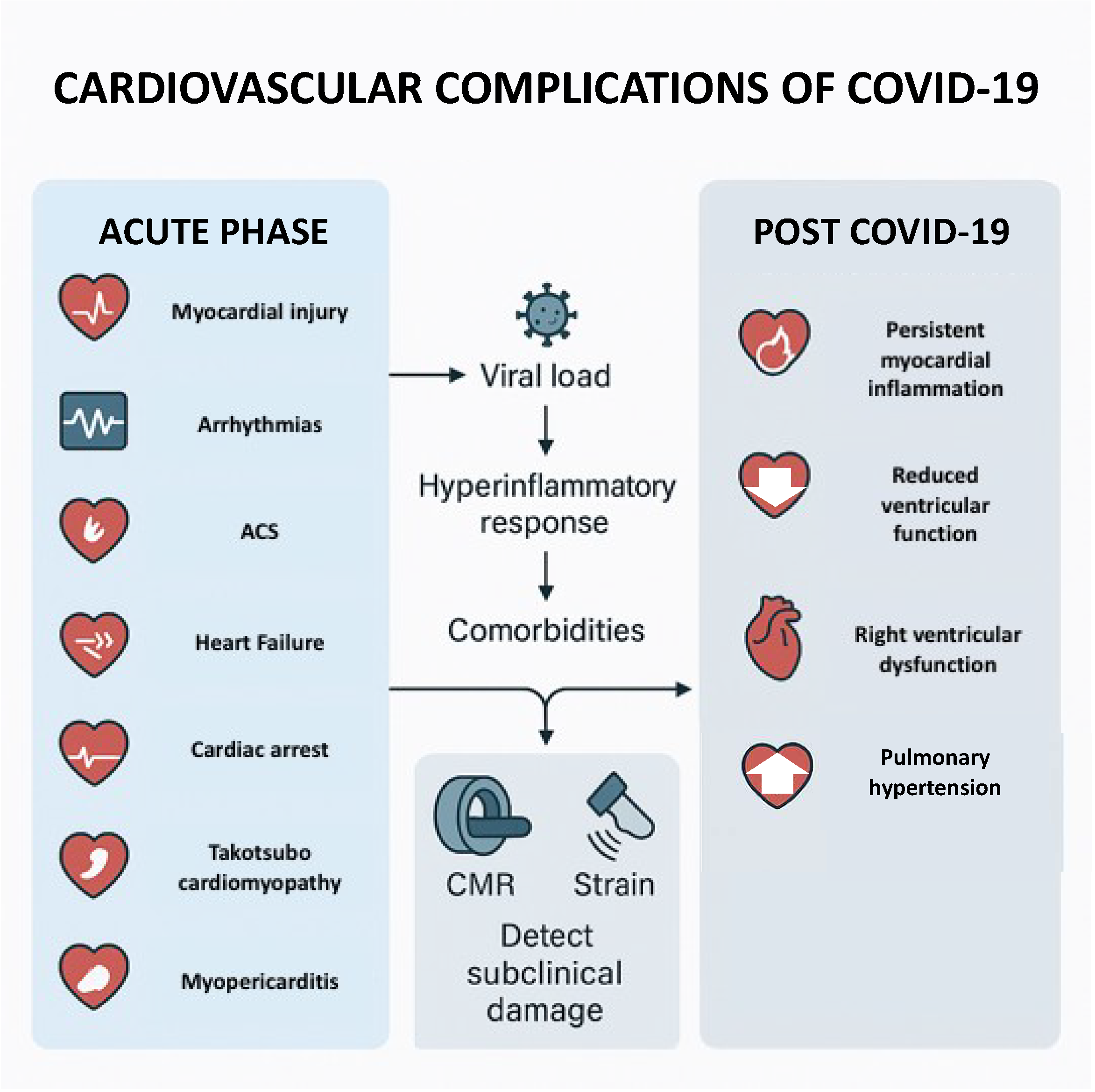
1. Introduction
2. Cardiovascular Manifestations of COVID-19
2.1. Arrhythmias
2.2. Acute Coronary Syndrome
2.3. Heart Failure
2.4. Right Ventricular Dysfunction and Pulmonary Hypertension
2.5. Takotsubo Cardiomyopathy
2.6. Myopericarditis
2.7. Cardiac Arrest
3. Post-COVID-19 Cardiovascular Complications
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dzieciatkowski, T.; Szarpak, L.; Filipiak, K.J.; Jaguszewski, M.; Ladny, J.R.; Smereka, J. COVID-19 challenge for modern medicine. Cardiol. J. 2020, 27, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Blagova, O.; Varionchik, N.; Zaidenov, V.; Savina, P.; Sarkisova, N. Anti-heart antibodies levels and their correlation with clinical symptoms and outcomes in patients with confirmed or suspected diagnosis COVID-19. Eur. J. Immunol. 2021, 51, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Pollack, A.; Kontorovich, A.R.; Fuster, V.; Dec, G.W. Viral myocarditis—Diagnosis, treatment options, and current controversies. Nat. Rev. Cardiol. 2015, 12, 670–680. [Google Scholar] [CrossRef]
- Giustino, G.; Pinney, S.P.; Lala, A.; Reddy, V.Y.; Johnston-Cox, H.A.; Mechanick, J.I.; Halperin, J.L.; Fuster, V. Coronavirus and Cardiovascular Disease, Myocardial Injury, and Arrhythmia: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 76, 2011–2023. [Google Scholar] [CrossRef]
- Wang, C.; Xie, J.; Zhao, L.; Fei, X.; Zhang, H.; Tan, Y.; Nie, X.; Zhou, L.; Liu, Z.; Ren, Y.; et al. Alveolar macrophage dysfunction and cytokine storm in the pathogenesis of two severe COVID-19 patients. EBioMedicine 2020, 57, 102833. [Google Scholar] [CrossRef]
- Denegri, A.; Morelli, M.; Pezzuto, G.; Malavasi, V.L.; Boriani, G. Atrial fibrillation is related to higher mortality in COVID-19/SARS-CoV-2 pneumonia infection. Cardiol. J. 2021, 28, 973–975. [Google Scholar] [CrossRef]
- Shah, W.; Hillman, T.; Playford, E.D.; Hishmeh, L. Managing the long term effects of COVID-19: Summary of NICE, SIGN, and RCGP rapid guideline. BMJ 2021, 372, n136. [Google Scholar] [CrossRef]
- Gasecka, A.; Pruc, M.; Kukula, K.; Gilis-Malinowska, N.; Filipiak, K.J.; Jaguszewski, M.J.; Szarpak, L. Post-COVID-19 heart syndrome. Cardiol. J. 2021, 28, 353–354. [Google Scholar] [CrossRef]
- Xu, H.; Hou, K.; Xu, R.; Li, Z.; Fu, H.; Wen, L.; Xie, L.; Liu, H.; Selvanayagam, J.B.; Zhang, N.; et al. Clinical Characteristics and Risk Factors of Cardiac Involvement in COVID-19. J. Am. Heart Assoc. 2020, 9, e016807. [Google Scholar] [CrossRef]
- Alrawashdeh, S.; Alrabadi, N.; Obeidat, O.; Alnabahneh, N.; Zanouneh, F.; Almomani, Z.; Aldalahmeh, M.; Ibdah, R.; Khassawneh, B. The Value and Applicability of the Electrocardiography in Revealing the Cardiac Involvement of COVID-19 Patients. Acta Inform. Med. 2021, 29, 253. [Google Scholar] [CrossRef]
- Iorio, A.; Lombardi, C.M.; Specchia, C.; Merlo, M.; Nuzzi, V.; Ferraro, I.; Peveri, G.; Oriecuia, C.; Pozzi, A.; Inciardi, R.M.; et al. Combined Role of Troponin and Natriuretic Peptides Measurements in Patients with COVID-19 (from the Cardio-COVID-Italy Multicenter Study). Am. J. Cardiol. 2022, 167, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Szekely, Y.; Lichter, Y.; Taieb, P.; Banai, A.; Hochstadt, A.; Merdler, I.; Gal Oz, A.; Rothschild, E.; Baruch, G.; Peri, Y.; et al. Spectrum of Cardiac Manifestations in COVID-19: A Systematic Echocardiographic Study. Circulation 2020, 142, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Bieber, S.; Kraechan, A.; Hellmuth, J.C.; Muenchhoff, M.; Scherer, C.; Schroeder, I.; Irlbeck, M.; Kaeaeb, S.; Massberg, S.; Hausleiter, J.; et al. Left and right ventricular dysfunction in patients with COVID-19-associated myocardial injury. Infection 2021, 49, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Dai, N.; Tang, X.; Hu, Y.; Lu, H.; Chen, Z.; Duan, S.; Guo, W.; Edavi, P.P.; Yu, Y.; Huang, D.; et al. SARS-CoV-2 Infection Association with Atherosclerotic Plaque Progression at Coronary CT Angiography and Adverse Cardiovascular Events. Radiology 2025, 314, e240876. [Google Scholar] [CrossRef]
- Moser, L.J.; Lisi, C.; Gutberlet, M.; Boccalini, S.; Budde, R.P.J.; Francone, M.; Paar, M.H.; Loewe, C.; Muscogiuri, G.; Natale, L.; et al. Impact of the COVID-19 pandemic on cardiac magnetic resonance imaging practices: Insights from the MRCT registry. Eur. Radiol. 2025, 35, 4805–4813. [Google Scholar] [CrossRef]
- Minhas, A.S.; Shade, J.K.; Cho, S.M.; Michos, E.D.; Metkus, T.; Gilotra, N.A.; Sharma, G.; Trayanova, N.; Hays, A.G. The role of sex and inflammation in cardiovascular outcomes and mortality in COVID-19. Int. J. Cardiol. 2021, 337, 127–131. [Google Scholar] [CrossRef]
- Pelà, G.; Frizzelli, A.; Pisi, R.; Calzetta, L.; Marchese, A.; Chetta, A.; Aiello, M. Post-COVID-19 exaggerated exertional tachycardia: Relationship with pulmonary and cardiac sequelae. Heart Lung 2025, 73, 228–235. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar]
- Han, K.Y.; Qiao, Q.; Zhu, Y.Q.; Chen, X.G.; Kang, X.X.; Zhang, G.-F.; Cai, X.-C.; Du, Y.; Jin, J.; Di, R.-M.; et al. Atrial Arrhythmias in Patients with Severe COVID-19. Cardiol. Res. Pract. 2021, 2021, 8874450. [Google Scholar] [CrossRef]
- Jirak, P.; Shomanova, Z.; Larbig, R.; Dankl, D.; Frank, N.; Seelmaier, C.; Butkiene, D.; Lichtenauer, M.; Mirna, M.; Strohmer, B.; et al. Higher Incidence of Stroke in Severe COVID-19 Is Not Associated with a Higher Burden of Arrhythmias: Comparison with Other Types of Severe Pneumonia. Front. Cardiovasc. Med. 2021, 8, 763827. [Google Scholar] [CrossRef]
- Jehangir, Q.; Lee, Y.; Latack, K.; Poisson, L.; Wang, D.D.; Song, S.; Apala, D.R.; Patel, K.; Halabi, A.R.; Krishnamoorthy, G.; et al. Incidence, Mortality, and Imaging Outcomes of Atrial Arrhythmias in COVID-19. Am. J. Cardiol. 2022, 173, 64–72. [Google Scholar] [CrossRef]
- Musikantow, D.R.; Turagam, M.K.; Sartori, S.; Chu, E.; Kawamura, I.; Shivamurthy, P.; Bokhari, M.; Oates, C.; Zhang, C.; Pumill, C.; et al. Atrial Fibrillation in Patients Hospitalized with COVID-19: Incidence, Predictors, Outcomes, and Comparison to Influenza. JACC Clin. Electrophysiol. 2021, 7, 1120–1130. [Google Scholar] [CrossRef]
- Guan, H.; Liu, J.; Ding, J.; Liu, W.; Feng, Y.; Bao, Y.; Li, H.; Wang, X.; Zhou, Z.; Chen, Z. Arrhythmias in patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: Incidences and implications. J. Electrocardiol. 2021, 65, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Yarmohammadi, H.; Morrow, J.P.; Dizon, J.; Biviano, A.; Ehlert, F.; Saluja, D.; Waase, M.; Elias, P.; Poterucha, T.J.; Berman, J.; et al. Frequency of Atrial Arrhythmia in Hospitalized Patients with COVID-19. Am. J. Cardiol. 2021, 147, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, V.; Kumar, A.; Sadaf, M.I.; Lu, E.; Al’Aref, S.J.; Tarun, T.; Galiatsatos, P.; Gulati, M.; Blumenthal, R.S.; Leucker, T.M.; et al. COVID-19 in the Initiation and Progression of Atherosclerosis: Pathophysiology During and Beyond the Acute Phase. JACC Adv. 2024, 3, 101107. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, T.F.; Bradley, C.J.; Abbas, A.E.; Williamson, B.D.; Rusia, A.; Tawney, A.M.; Gaines, R.; Schott, J.; Dmitrienko, A.; Haines, D.E. Hydroxychloroquine/Azithromycin Therapy and QT Prolongation in Hospitalized Patients with COVID-19. JACC Clin. Electrophysiol. 2021, 7, 16–25. [Google Scholar] [CrossRef]
- Yousafzai, A.D.K.; Bangash, A.H.; Asghar, S.Y.; Abbas, S.M.M.; Khawaja, H.F.; Zehra, S.; Khan, A.U.; Kamil, M.; Ayesha, N.; Khan, A.K.; et al. Clinical efficacy of Azithromycin for COVID-19 management: A systematic meta-analysis of meta-analyses. Heart Lung 2023, 60, 127–132. [Google Scholar] [CrossRef]
- Zylla, M.M.; Merle, U.; Vey, J.A.; Korosoglou, G.; Hofmann, E.; Müller, M.; Herth, F.; Schmidt, W.; Blessing, E.; Göggelmann, C.; et al. Predictors and Prognostic Implications of Cardiac Arrhythmias in Patients Hospitalized for COVID-19. J. Clin. Med. 2021, 10, 133. [Google Scholar] [CrossRef]
- Dagher, L.; Shi, H.; Zhao, Y.; Wetherbie, A.; Johnsen, E.; Sangani, D.; Nedunchezhian, S.; Brown, M.; Miller, P.; Denson, J.; et al. New-onset atrial arrhythmias associated with mortality in black and white patients hospitalized with COVID-19. Pacing Clin. Electrophysiol. 2021, 44, 856–864. [Google Scholar] [CrossRef]
- Denegri, A.; Sola, M.; Morelli, M.; Farioli, F.; Alberto, T.; D’Arienzo, M.; Savorani, F.; Pezzuto, G.S.; Boriani, G.; Szarpak, L.; et al. Arrhythmias in COVID-19/SARS-CoV-2 Pneumonia Infection: Prevalence and Implication for Outcomes. J. Clin. Med. 2022, 11, 1463. [Google Scholar] [CrossRef]
- Peltzer, B.; Manocha, K.K.; Ying, X.; Kirzner, J.; Ip, J.E.; Thomas, G.; Liu, C.F.; Markowitz, S.M.; Lerman, B.B.; Safford, M.M.; et al. Arrhythmic Complications of Patients Hospitalized with COVID-19: Incidence, Risk Factors, and Outcomes. Circ. Arrhythm. Electrophysiol. 2020, 13, e009121. [Google Scholar] [CrossRef]
- Reynbakh, O.; Braunstein, E.D.; Hsu, M.; Ellis, J.; Crosson, L.; Lenane, J.; Krumerman, A.; Di Biase, L.; Ferrick, K.J. Arrhythmia patterns during and after hospitalization for COVID-19 infection detected via patch-based mobile cardiac telemetry. Am. Heart J. Plus. 2022, 13, 100084. [Google Scholar] [CrossRef]
- Parwani, A.S.; Haug, M.; Keller, T.; Guthof, T.; Blaschke, F.; Tscholl, V.; Biewener, S.; Kamieniarz, P.; Zieckler, D.; Kruse, J.; et al. Cardiac arrhythmias in patients with COVID-19: Lessons from 2300 telemetric monitoring days on the intensive care unit. J. Electrocardiol. 2021, 66, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Turagam, M.K.; Musikantow, D.; Goldman, M.E.; Bassily-Marcus, A.; Chu, E.; Shivamurthy, P.; Lampert, J.; Kawamura, I.; Bokhari, M.; Whang, W.; et al. Malignant Arrhythmias in Patients with COVID-19: Incidence, Mechanisms, and Outcomes. Circ. Arrhythm. Electrophysiol. 2020, 13, e008920. [Google Scholar] [CrossRef] [PubMed]
- Stefanini, G.G.; Montorfano, M.; Trabattoni, D.; Andreini, D.; Ferrante, G.; Ancona, M.; Metra, M.; Curello, S.; Maffeo, D.; Pero, G.; et al. ST-Elevation myocardial infarction in patients with COVID-19: Clinical and angiographic outcomes. Circulation 2020, 141, 2113–2116. [Google Scholar] [CrossRef] [PubMed]
- Milovančev, A.; Petrović, M.; Popadić, V.; Miljković, T.; Klašnja, S.; Djuran, P.; Ilić, A.; Kovačević, M.; Milosavljević, A.S.; Brajković, M.; et al. Characteristics and Outcomes of Patients with Acute Coronary Syndrome and COVID-19. J. Clin. Med. 2022, 11, 1791. [Google Scholar] [CrossRef]
- De Filippo, O.; D’Ascenzo, F.; Angelini, F.; Bocchino, P.P.; Conrotto, F.; Saglietto, A.; Secco, G.G.; Campo, G.; Gallone, G.; Verardi, R.; et al. Reduced Rate of Hospital Admissions for ACS during COVID-19 Outbreak in Northern Italy. N. Engl. J. Med. 2020, 383, 88–89. [Google Scholar] [CrossRef]
- Hamadeh, A.; Aldujeli, A.; Briedis, K.; Tecson, K.M.; Sanz-Sánchez, J.; Al Dujeili, M.; Al-Obeidi, A.; Diez, J.L.; Žaliūnas, R.; Stoler, R.C.; et al. Characteristics and Outcomes in Patients Presenting with COVID-19 and ST-Segment Elevation Myocardial Infarction. Am. J. Cardiol. 2020, 131, 1–6. [Google Scholar] [CrossRef]
- Abizaid, A.; Campos, C.M.; Guimarães, P.O.; Costa, J.R., Jr.; Falcão, B.A.A.; Mangione, F.; Caixeta, A.; Lemos, P.A.; de Brito, F.S., Jr.; Cavalcante, R.; et al. Patients with COVID-19 who experience a myocardial infarction have complex coronary morphology and high in-hospital mortality: Primary results of a nationwide angiographic study. Catheter. Cardiovasc. Interv. 2021, 98, E370–E378. [Google Scholar] [CrossRef]
- Çınar, T.; Şaylık, F.; Akbulut, T.; Asal, S.; Selçuk, M.; Çiçek, V.; Kılıç, Ş.; Orhan, A.L. One-year outcomes of invasively managed acute coronary syndrome patients with COVID-19. Heart Lung 2022, 52, 159–164. [Google Scholar] [CrossRef]
- Primessnig, U.; Pieske, B.M.; Sherif, M. Increased mortality and worse cardiac outcome of acute myocardial infarction during the early COVID-19 pandemic. ESC Heart Fail. 2021, 8, 333–343. [Google Scholar] [CrossRef]
- Chan, K.; Conroy, A.; Khosla, A.; Rubens, M.; Saxena, A.; Ramamoorthy, V.; Roy, M.; Appunni, S.; Doke, M.; Ahmed, A.; et al. Prevalence and effects of acute myocardial infarction on hospital outcomes among COVID-19 patients. Coron. Artery Dis. 2024, 35, 38–43. [Google Scholar] [CrossRef]
- Rey, J.R.; Caro-Codón, J.; Rosillo, S.O.; Iniesta, Á.M.; Castrejón-Castrejón, S.; Marco-Clement, I.; Martín-Polo, L.; Merino-Argos, C.; Rodríguez-Sotelo, L.; García-Veas, J.M.; et al. Heart failure in COVID-19 patients: Prevalence, incidence and prognostic implications. Eur. J. Heart Fail. 2020, 22, 2205–2215. [Google Scholar] [CrossRef]
- Sokolski, M.; Trenson, S.; Sokolska, J.M.; D’Amario, D.; Meyer, P.; Poku, N.K.; Biering-Sørensen, T.; Højbjerg Lassen, M.C.; Skaarup, K.G.; Barge-Caballero, E.; et al. Heart failure in COVID-19: The multicentre, multinational PCHF-COVICAV registry. ESC Heart Fail. 2021, 8, 4955–4967. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.D.; Alviar, C.L.; Bhatt, A.S.; Baird-Zars, V.M.; Barnett, C.F.; Daniels, L.B.; Defilippis, A.P.; Fagundes, A.; Katrapati, P.; Kenigsberg, B.B.; et al. Epidemiology of Acute Heart Failure in Critically Ill Patients with COVID-19: An Analysis from the Critical Care Cardiology Trials Network. J. Card. Fail. 2022, 28, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Rubens, M.; Ramamoorthy, V.; Saxena, A.; Khosla, A.A.; Doke, M.; McGranaghan, P.; Appunni, S.; Zhang, Y.; Körfer, D.; Chaparro, S.; et al. Trends and outcomes of heart failure hospitalizations during COVID-19 pandemic. BMC Public Health 2025, 25, 864. [Google Scholar] [CrossRef]
- Mishra, T.; Patel, D.A.; Awadelkarim, A.; Sharma, A.; Patel, N.; Yadav, N.; Almas, T.; Sattar, Y.; Alraies, M.C. A National Perspective on the Impact of the COVID-19 Pandemic on Heart Failure Hospitalizations in the United States. Curr. Probl. Cardiol. 2023, 48, 101749. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, A.; Liao, H.; Liu, Z.; Yang, F. Pathological Interplay and Clinical Complications between COVID-19 and Cardiovascular Diseases: An Overview in 2023. Cardiology 2024, 149, 60–70. [Google Scholar] [CrossRef]
- Bleakley, C.; Singh, S.; Garfield, B.; Morosin, M.; Surkova, E.; Mandalia, S.; Dias, B.; Androulakis, E.; Price, L.C.; McCabe, C.; et al. Right ventricular dysfunction in critically ill COVID-19 ARDS. Int. J. Cardiol. 2021, 327, 251–258. [Google Scholar] [CrossRef]
- Khokhlov, L.; Khokhlov, R.; Lipovka, S.; Yarmonova, M.; Tribunzeva, L.; Prozorova, G.; Dubrovina, M.; Lobas, I. Cardiac injury described by contrast-enhanced cardiac magnetic resonance imaging in patients recovered from COVID-19. J. Am. Coll. Cardiol. 2022, 79, 2100. [Google Scholar] [CrossRef]
- Li, Y.L.; Zheng, J.B.; Jin, Y.; Tang, R.; Li, M.; Xiu, C.H.; Dai, Q.-Q.; Zuo, S.; Wang, H.-Q.; Wang, H.-L.; et al. Acute right ventricular dysfunction in severe COVID-19 pneumonia. Rev. Cardiovasc. Med. 2020, 21, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Scudiero, F.; Silverio, A.; Muraca, I.; Russo, V.; Di Maio, M.; Silvestro, A.; Personeni, D.; Citro, R.; Canonico, M.E.; Galasso, G.; et al. Long-Term Prognostic Impact of Right Ventricular Dysfunction in Patients with COVID-19. J. Pers. Med. 2022, 12, 162. [Google Scholar] [CrossRef] [PubMed]
- Tsolaki, V.; Zakynthinos, G.E.; Karavidas, N.; Vazgiourakis, V.; Papanikolaou, J.; Parisi, K.; Zygoulis, P.; Makris, D.; Zakynthinos, E. Comprehensive temporal analysis of right ventricular function and pulmonary haemodynamics in mechanically ventilated COVID-19 ARDS patients. Ann. Intensive Care 2024, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Tudoran, C.; Tudoran, M.; Lazureanu, V.E.; Marinescu, A.R.; Cut, T.G.; Oancea, C.; Pescariu, S.A.; Pop, G.N. Factors Influencing the Evolution of Pulmonary Hypertension in Previously Healthy Subjects Recovering from a SARS-CoV-2 Infection. J. Clin. Med. 2021, 10, 5272. [Google Scholar] [CrossRef]
- Kanelidis, A.J.; Miller, P.J.; Singh, A.; Addetia, K.; Lang, R.M. Takotsubo Syndrome from Coronavirus Disease 2019. J. Am. Soc. Echocardiogr. 2022, 35, 883–885. [Google Scholar] [CrossRef]
- Zuin, M.; Mugnai, G.; Anselmi, M.; Bonapace, S.; Bozzini, P.; Chirillo, F.; Cutolo, A.; Grassi, G.; Mancuso, D.; Meneghin, S.; et al. Takotsubo Syndrome during COVID-19 Pandemic in the Veneto Region, Italy. Viruses 2022, 14, 1971. [Google Scholar] [CrossRef]
- Pogran, E.; Zweiker, D.; Gargiulo, L.; El-Razek, A.A.; Lechner, I.; Vosko, I.; Rechberger, S.; Bugger, H.; Christ, G.; Bonderman, D.; et al. Takotsubo syndrome before and during the COVID-19 pandemic in Austria: A retrospective cohort study (TOSCA-19). ESC Heart Fail. 2023, 10, 3667–3676. [Google Scholar] [CrossRef]
- Titus, A.; Sattar, Y.; Patel, N.; Taha, A.; Sandhyavenu, H.; Gonuguntla, K.; Thyagaturu, H.; Almas, T.; Balla, S. In-Hospital Outcomes of Takotsubo Cardiomyopathy During the COVID-19 Pandemic: Propensity Matched National Cohort. Curr. Probl. Cardiol. 2023, 48, 101598. [Google Scholar] [CrossRef]
- Ammirati, E.; Lupi, L.; Palazzini, M.; Hendren, N.S.; Grodin, J.L.; Cannistraci, C.V.; Schmidt, M.; Hekimian, G.; Peretto, G.; Bochaton, T.; et al. Prevalence, Characteristics, and Outcomes of COVID-19-Associated Acute Myocarditis. Circulation 2022, 145, 1123–1139. [Google Scholar] [CrossRef]
- Annie, F.H.; Alkhaimy, H.; Nanjundappa, A.; Elashery, A. Association Between Myocarditis and Mortality in COVID-19 Patients in a Large Registry. Mayo Clin. Proc. Innov. Qual. Outcomes 2022, 6, 114–119. [Google Scholar] [CrossRef]
- Daniels, C.J.; Rajpal, S.; Greenshields, J.T.; Rosenthal, G.L.; Chung, E.H.; Terrin, M.; Jeudy, J.; Mattson, S.E.; Law, I.H.; Borchers, J.; et al. Big Ten COVID-19 Cardiac Registry Investigators. Prevalence of Clinical and Subclinical Myocarditis in Competitive Athletes with Recent SARS-CoV-2 Infection: Results from the Big Ten COVID-19 Cardiac Registry. JAMA Cardiol. 2021, 6, 1078–1087. [Google Scholar] [CrossRef]
- Ferro, M.D.; Bussani, R.; Paldino, A.; Nuzzi, V.; Collesi, C.; Zentilin, L.; Schneider, E.; Correa, R.; Silvestri, F.; Zacchigna, S.; et al. SARS-CoV-2, myocardial injury and inflammation: Insights from a large clinical and autopsy study. Clin. Res. Cardiol. 2021, 110, 1822–1831. [Google Scholar] [CrossRef]
- Mirò, Ò.; Sabaté, M.; Jiménez, S.; Mebazaa, A.; Martínez-Nadal, G.; Piñera, P.; Burillo-Putze, G.; Martín, A.; Martín-Sánchez, F.J.; Jacob, J.; et al. Spanish Investigators on Emergency Situations TeAm (SIESTA) network; SIESTA network. A case-control, multicentre study of consecutive patients with COVID-19 and acute (myo)pericarditis: Incidence, risk factors, clinical characteristics and outcomes. Emerg. Med. J. 2022, 39, 402–410. [Google Scholar] [CrossRef]
- Buckley, B.J.R.; Harrison, S.L.; Fazio-Eynullayeva, E.; Underhill, P.; Lane, D.A.; Lip, G.Y.H. Prevalence and clinical outcomes of myocarditis and pericarditis in 718,365 COVID-19 patients. Eur. J. Clin. Investig. 2021, 51, e13679. [Google Scholar] [CrossRef]
- Thenpandiyan, A.A.; Ling, R.R.; Grignani, R.; Ling, M.R.; Tai, B.C.; Somani, J.; Ramanathan, K.; Quek, S.C. Myopericarditis following COVID-19 vaccination in children: A systematic review and meta-analysis. Singapore Med. J. 2024. [Google Scholar] [CrossRef] [PubMed]
- Shao, F.; Xu, S.; Ma, X.; Xu, Z.; Lyu, J.; Ng, M.; Cui, H.; Yu, C.; Zhang, Q.; Sun, P.; et al. In-hospital cardiac arrest outcomes among patients with COVID-19 pneumonia in Wuhan, China. Resuscitation 2020, 151, 18–23. [Google Scholar] [CrossRef]
- Mechineni, A.; Samuel, A.; Manickam, R. Cardiac arrest and clinical outcomes in COVID-19 patients: A single center experience. J. Am. Coll. Cardiol. 2021, 77, 3180. [Google Scholar] [CrossRef]
- Acharya, P.; Ranka, S.; Sethi, P.; Bharati, R.; Hu, J.; Noheria, A.; Nallamothu, B.K.; Hayek, S.S.; Gupta, K. Incidence, Predictors, and Outcomes of In-Hospital Cardiac Arrest in COVID-19 Patients Admitted to Intensive and Non-Intensive Care Units: Insights from the AHA COVID-19 CVD Registry. J. Am. Heart Assoc. 2021, 10, e021204. [Google Scholar] [CrossRef]
- Hayek, S.S.; Brenner, S.K.; Azam, T.U.; Shadid, H.R.; Anderson, E.; Berlin, H.; Pan, M.; Meloche, C.; Feroz, R.; O’hAyer, P.; et al. STOP-COVID Investigators. In-hospital cardiac arrest in critically ill patients with COVID-19: Multicenter cohort study. BMJ 2020, 371, m3513. [Google Scholar]
- Roedl, K.; Söffker, G.; Wichmann, D.; Boenisch, O.; de Heer, G.; Burdelski, C.; Frings, D.; Sensen, B.; Nierhaus, A.; Westermann, D.; et al. Characteristics and Risk Factors for Intensive Care Unit Cardiac Arrest in Critically Ill Patients with COVID-19-A Retrospective Study. J. Clin. Med. 2021, 10, 2195. [Google Scholar] [CrossRef]
- Yuriditsky, E.; Mitchell, O.J.; Brosnahan, S.B.; Smilowitz, N.R.; Drus, K.W.; Gonzales, A.M.; Xia, Y.; Parnia, S.; Horowitz, J.M. Clinical characteristics and outcomes of in-hospital cardiac arrest among patients with and without COVID-19. Resusc. Plus 2020, 4, 100054. [Google Scholar] [CrossRef]
- Girotra, S.; Chan, M.L.; Starks, M.A.; Churpek, M.; Chan, P.S. American Heart Association Get with the Guidelines–Resuscitation Investigators. Association of COVID-19 Infection with Survival After In-Hospital Cardiac Arrest Among US Adults. JAMA Netw. Open 2022, 5, e220752. [Google Scholar] [CrossRef]
- Andersen, L.W.; Holmberg, M.J.; Berg, K.M.; Donnino, M.W.; Granfeldt, A. In-Hospital Cardiac Arrest: A Review. JAMA 2019, 321, 1200–1210. [Google Scholar] [CrossRef]
- Shrestha, D.B.; Sedhai, Y.R.; Dawadi, S.; Dhakal, B.; Shtembari, J.; Singh, K.; Acharya, R.; Basnyat, S.; Waheed, I.; Khan, M.S.; et al. Outcome of In-Hospital Cardiac Arrest among Patients with COVID-19: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 2796. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.E.; Friedrich, M.G.; Leiner, T.; Elias, M.D.; Ferreira, V.M.; Fenski, M.; Flamm, S.D.; Fogel, M.; Garg, R.; Halushka, M.K.; et al. Cardiovascular Magnetic Resonance for Patients with COVID-19. JACC Cardiovasc. Imaging 2022, 15, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Caiado, L.D.C.; Azevedo, N.C.; Azevedo, R.R.C.; Caiado, B.R. Cardiac involvement in patients recovered from COVID-19 identified using left ventricular longitudinal strain. J. Echocardiogr. 2022, 20, 51–56. [Google Scholar] [CrossRef]
- Wang, H.; Li, R.; Zhou, Z.; Jiang, H.; Yan, Z.; Tao, X.; Li, H.; Xu, L. Cardiac involvement in COVID-19 patients: Mid-term follow up by cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2021, 23, 14. [Google Scholar] [CrossRef]
- Zhou, M.; Wong, C.K.; Un, K.C.; Lau, Y.M.; Lee, J.C.; Tam, F.C.; Lau, Y.M.; Lai, W.H.; Tam, A.R.; Lam, Y.Y.; et al. Cardiovascular sequalae in uncomplicated COVID-19 survivors. PLoS ONE 2021, 16, e0246732. [Google Scholar] [CrossRef]
- Wu, X.; Deng, K.-Q.; Li, C.; Yang, Z.; Hu, H.; Cai, H.; Zhang, C.; He, T.; Zheng, F.; Wang, H.; et al. Cardiac Involvement in Recovered Patients from COVID-19: A Preliminary 6-Month Follow-Up Study. Front. Cardiovasc. Med. 2021, 8, 654405. [Google Scholar] [CrossRef]
- Kotecha, T.; Knight, D.S.; Razvi, Y.; Kumar, K.; Vimalesvaran, K.; Thornton, G.; Patel, R.; Chacko, L.; Brown, J.T.; Coyle, C.; et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur. Heart J. 2021, 42, 1866–1878. [Google Scholar] [CrossRef]

| Patients | Type | AF | VF | Mortality | |
|---|---|---|---|---|---|
| Han K.Y. et al. | 84 | Hospitalized | 4 (4.8%) | 3 (3.6%) | nd |
| Rav-Acha M. et al. | 390 | Hospitalized | 20 (5.1%) | 2 (0.51%) | nd |
| Denegri A. et al. | 637 | Hospitalized | 49 (7.7%) | nd | Increased |
| Bertini M. et al. | 432 | Critical | 95 (22%) | nd | nd |
| Merino JL et al. | 3416 | Hospitalized | 48 (3.2%) | 9 (0.6%) | Increased |
| Peltzer B. et al. | 1053 | Hospitalized | 166 (15.8%) | 27 (2.6%) | Increased |
| Jirak P. et al. | 60 | Critical | 6 (10%) | 18 (30%) | Increased |
| Jehangir Q. et al. | 6927 | Hospitalized | 626 (9%) | nd | Increased |
| Garciía-Granja P.E. et al. | 517 | Hospitalized | 54 (10.4%) | nd | Increased |
| Musikantow D. R. et al. | 3970 | Hospitalized | 146 * (3.7%) | nd | Increased |
| Guan H. et al. | 463 | Hospitalized | 17 ** (3.7%) | 1 (0.2%) | nd |
| Reynbakh O. et al. | 59 | Hospitalized | 9 (15%) | 15 (25%) | nd |
| Yarmohammadi H. et al. | 1029 | Hospitalized | 46 * (4.5%) | nd | Increased |
| Parwani A. S. et al. | 113 | Critical | 40 ** (35.4%) | 35 (31%) | nd |
| Turagam M. K. El al. | 140 | Hospitalized | nd | 7 (5%) | nd |
| Vijayabharathy K. et al. | 109 | Critical | 16 (14.6%) | nd | Increased |
| Dagher L. et al. | 310 | Hospitalized | 23 * (7.4%) | nd | Increased |
| Erguün B. et al. | 248 | Critical | 37 (14.9%) | nd | Increased |
| Sano T. et al. | 673 | Hospitalized | 28 (4.2%) | nd | Increased |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denegri, A.; Dall’Ospedale, V.; Covani, M.; Pruc, M.; Szarpak, L.; Niccoli, G. Cardiovascular Complications of COVID-19 Disease: A Narrative Review. Diseases 2025, 13, 252. https://doi.org/10.3390/diseases13080252
Denegri A, Dall’Ospedale V, Covani M, Pruc M, Szarpak L, Niccoli G. Cardiovascular Complications of COVID-19 Disease: A Narrative Review. Diseases. 2025; 13(8):252. https://doi.org/10.3390/diseases13080252
Chicago/Turabian StyleDenegri, Andrea, Valeria Dall’Ospedale, Marco Covani, Michal Pruc, Lukasz Szarpak, and Giampaolo Niccoli. 2025. "Cardiovascular Complications of COVID-19 Disease: A Narrative Review" Diseases 13, no. 8: 252. https://doi.org/10.3390/diseases13080252
APA StyleDenegri, A., Dall’Ospedale, V., Covani, M., Pruc, M., Szarpak, L., & Niccoli, G. (2025). Cardiovascular Complications of COVID-19 Disease: A Narrative Review. Diseases, 13(8), 252. https://doi.org/10.3390/diseases13080252








