Practical Considerations in the Management of Frail Older People with Diabetes
Abstract
1. Introduction
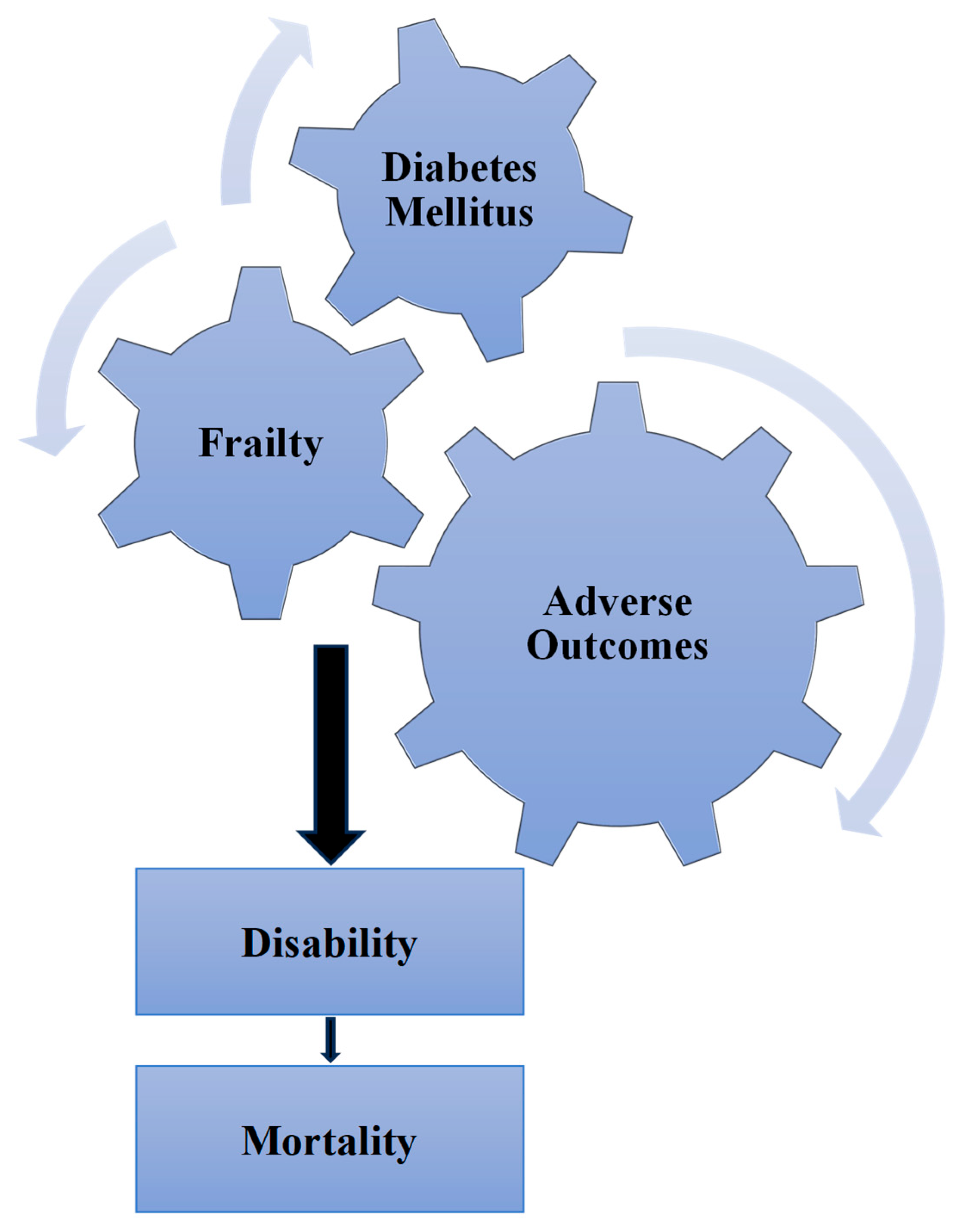
1.1. Frailty–Diabetes Relationship
1.2. Frailty and Adverse Outcomes
1.3. Frailty and Hypoglycaemia
1.4. Frailty and Dementia
2. Practical Considerations
2.1. Frailty Assessment
| A. Physical function Barthel Index to assess patient ability in performing activities of daily living by assessment of 10 different tasks; score ranges between 0 and 100: Score: ●91–100 independent; ●61–90 moderate dependence; ●21–60 severe dependence; ●0–20 total dependence. B. Mental function 1. Cognition (Mini-Cog) Ask the patients to repeat three items such as key, lemon and balloon, then provide a clock face and ask them to ●Draw the numbers of the clock face; ●Draw the hands of the clock to show time as ten to three; ●Recall the three items. Score: One mark for each task performed and for each item remembered; a score ≤3/5 defines cognitive impairment. 2. Depression (PHQ-2) Ask the patients whether they have ●Little interest in doing things? ●Been feeling down, depressed or hopeless? Score: Any positive answer triggers assessment using the detailed (PHQ-9) 3. Anxiety (GAD-2) Ask the patients whether, over last 2 weeks, they have been ●Feeling nervous, anxious or on edge? ●Unable to stop or control worrying? Score: Give 0 for not at all, 1 for several days, 2 for >half the days, 3 for nearly every day; a score of ≥3/6 defines anxiety. 4. Distress (PAID-1) ●Is the patient is worrying about the future and the possibility of serious complications? Score: A positive answer suggests underlying diabetes-related emotional distress. C. Social function Social Support Rating Scale (SSRS): A 10-item questionnaire to assess individuals’ social support. It covers three dimensions: objective support, subjective support and support utilisation.
|
2.2. Lifestyle
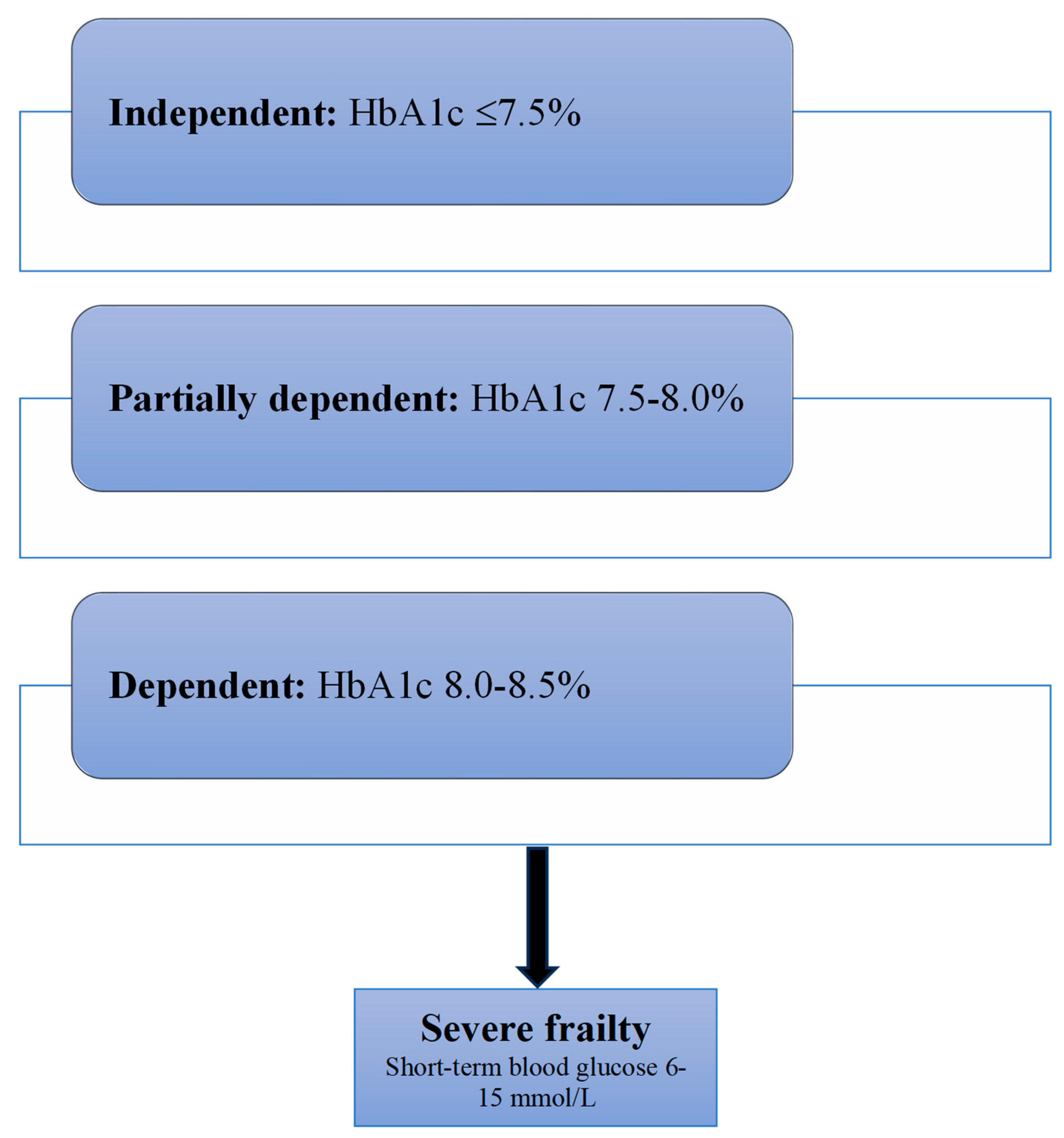
2.3. Glycaemic Control
2.4. Choice of Hypoglycaemic Therapy
2.5. Reducing Hypoglycaemia
- Hypoglycaemia risk assessment
- Yearly review of risk factors
- Polypharmacy reduction
- Optimisation of therapy
- Goals of therapy setting
- Lax glycaemic targets
- Yearlong adherence to lifestyle and meal time compliance
- Continuous glucose monitoring in appropriate patients
- Avoid drugs with high hypoglycaemic potential
- Education of patients and carers
- Monitoring of organ function
- Insulin regimen simplification
2.6. Avoiding Hospitalisation
- Hypoglycaemic medication review
- On discharge, community diabetes-specific service follow-up
- Screen for depression, dementia and fall risk
- Polypharmacy reduction
- Insulin administration ability regular review
- Tailored and individualised care plans
- Annual CGA review
- Liaison with diabetes specialist nurses
- Input from dieticians to reduce malnutrition and maintain hydration
- Specific diabetes-related training for care home staff
- Avoid RAAS inhibitors and NSAIDs in CKD patients
- Training and education for patients and carers
- Integrated primary, secondary and care home services
- Optimisation of exercise programmes to improve mobility and balance
- Need for vaccination in high-risk patients
2.7. Care Needs
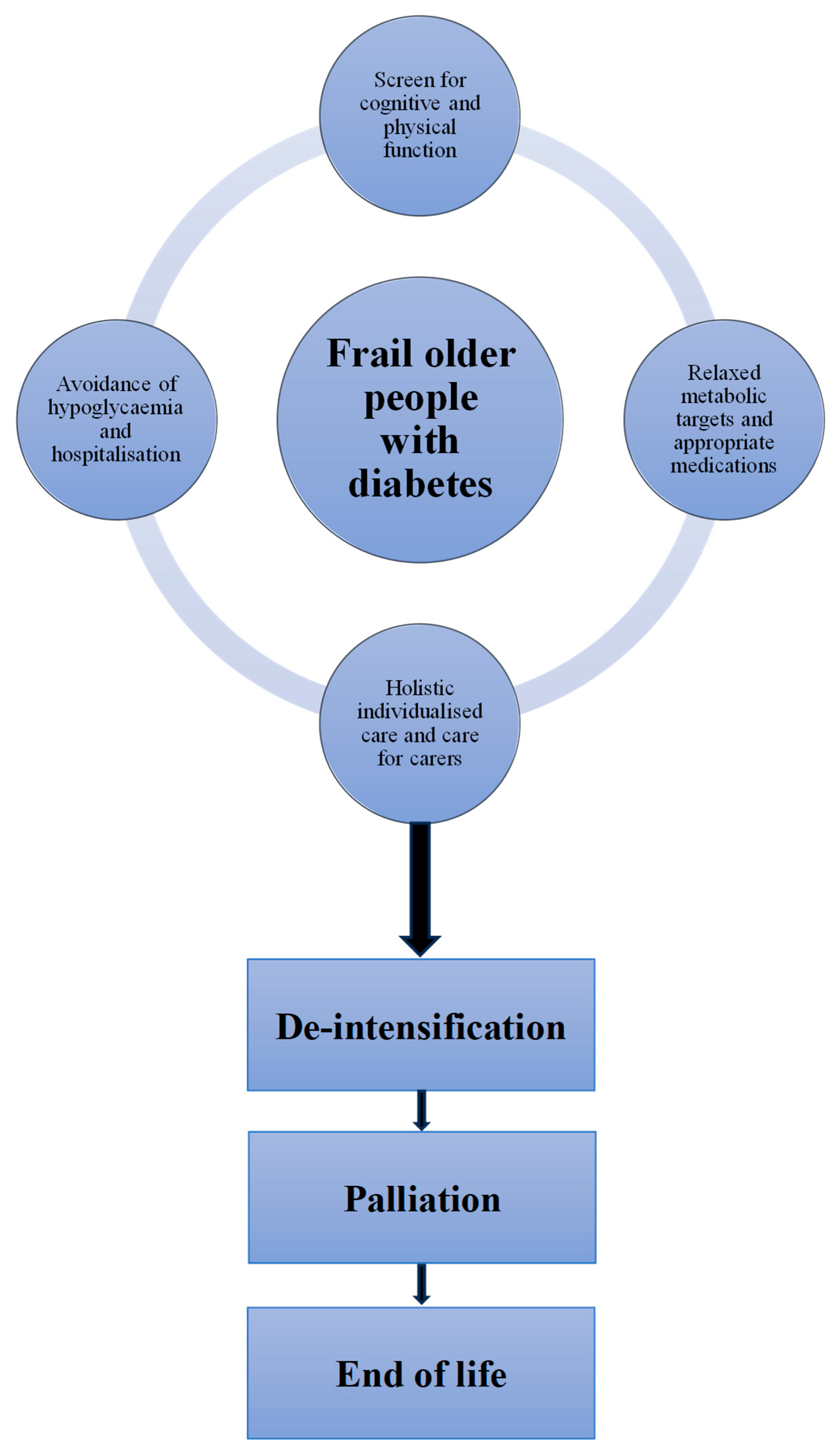
2.8. De-Intensification
2.9. Palliation and End of Life
3. Conclusions
3.1. Future Perspectives
3.2. Key Points
- With increasing life expectancy, the number of older people living with diabetes and frailty is likely to increase.
- Frailty will increase diabetes-related complications, especially hypoglycaemia, dementia and hospitalisation.
- Regular screening for frailty should be a routine part of the care plans of older people with diabetes.
- Relaxed metabolic targets and the avoidance of agents with high hypoglycaemic potential should be considered in frail older people with diabetes.
- With increasing severity of frailty, de-intensification, palliation and end-of-life plans should be in place after discussion with patients and their families.
Funding
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, A.J.; Abdelhafiz, A.H.; Rodríguez-Mañas, L. Frailty and sarcopenia-newly emerging and high impact complications of diabetes. J. Diabetes Its Complicat. 2017, 31, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Assar, M.E.; Laosa, O.; Rodríguez Mañas, L. Diabetes and frailty. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 52–57. [Google Scholar] [CrossRef]
- Bergman, H.; Ferrucci, L.; Guralnik, J.; Hogan, D.B.; Hummel, S.; Karunananthan, S.; Wolfson, C. Frailty: An Emerging Research and Clinical Paradigm—Issues and Controversies. J. Gerontol. Ser. A 2007, 62, 731–737. [Google Scholar] [CrossRef]
- Chhetri, J.K.; Zheng, Z.; Xu, X.; Ma, C.; Chan, P. The prevalence and incidence of frailty in Pre-diabetic and diabetic community-dwelling older population: Results from Beijing longitudinal study of aging II (BLSA-II). BMC Geriatr. 2017, 17, 47. [Google Scholar] [CrossRef]
- Howrey, B.T.; Al Snih, S.; Markides, K.S.; Ottenbacher, K.J. Frailty and diabetes among Mexican American older adults. Ann. Epidemiol. 2018, 28, 421–426.e1. [Google Scholar] [CrossRef]
- Castrejón-Pérez, R.C.; Aguilar-Salinas, C.A.; Gutiérrez-Robledo, L.M.; Cesari, M.; Pérez-Zepeda, M.U. Frailty, diabetes, and the convergence of chronic disease in an age-related condition: A population-based nationwide cross-sectional analysis of the Mexican nutrition and health survey. Aging Clin. Exp. Res. 2017, 30, 935–941. [Google Scholar] [CrossRef]
- Kojima, G. Frailty as a Predictor of Future Falls Among Community-Dwelling Older People: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2015, 16, 1027–1033. [Google Scholar] [CrossRef]
- Kojima, G. Frailty as a predictor of fractures among community-dwelling older people: A systematic review and meta-analysis. Bone 2016, 90, 116–122. [Google Scholar] [CrossRef]
- Kojima, G.; Taniguchi, Y.; Iliffe, S.; Walters, K. Frailty as a Predictor of Alzheimer Disease, Vascular Dementia, and All Dementia Among Community-Dwelling Older People: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2016, 17, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G. Frailty as a predictor of disabilities among community-dwelling older people: A systematic review and meta-analysis. Disabil. Rehabil. 2017, 39, 1897–1908. [Google Scholar] [CrossRef]
- Kojima, G.; Iliffe, S.; Jivraj, S.; Walters, K. Association between frailty and quality of life among community-dwelling older people: A systematic review and meta-analysis. J. Epidemiol. Community Health 2016, 70, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G.; Iliffe, S.; Walters, K. Frailty index as a predictor of mortality: A systematic review and meta-analysis. Age Ageing 2017, 47, 193–200. [Google Scholar] [CrossRef]
- Roe, L.; Normand, C.; Wren, M.-A.; Browne, J.; O’hAlloran, A.M. The impact of frailty on healthcare utilisation in Ireland: Evidence from the Irish longitudinal study on ageing. BMC Geriatr. 2017, 17, 203. [Google Scholar] [CrossRef]
- Salinas-Rodríguez, A.; Manrique-Espinoza, B.; Heredia-Pi, I.; Rivera-Almaraz, A.; Ávila-Funes, J.A. Healthcare Costs of Frailty: Implications for Long-term Care. J. Am. Med. Dir. Assoc. 2019, 20, 102–103.e2. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Almaraz, A.; Manrique-Espinoza, B.; Ávila-Funes, J.A.; Chatterji, S.; Naidoo, N.; Kowal, P.; Salinas-Rodríguez, A. Disability, quality of life and all-cause mortality in older Mexican adults: Association with multimorbidity and frailty. BMC Geriatr. 2018, 18, 236. [Google Scholar] [CrossRef] [PubMed]
- Nagi, S.Z. Disability concepts revisited: Implications for prevention. In Disability in America: Toward a National Agenda for Prevention; Chan, F., da Silva Cardoso, E., Chronister, J.A., Eds.; National Academy Press: Washington, DC, USA, 1991; pp. 309–327. [Google Scholar]
- Lee, S.; Lee, S.; Harada, K.; Bae, S.; Makizako, H.; Doi, T.; Tsutsumimoto, K.; Hotta, R.; Nakakubo, S.; Park, H.; et al. Relationship between chronic kidney disease with diabetes or hypertension and frailty in community-dwelling Japanese older adults. Geriatr. Gerontol. Int. 2016, 17, 1527–1533. [Google Scholar] [CrossRef]
- Castrejón-Pérez, R.C.; Gutiérrez-Robledo, L.M.; Cesari, M.; Pérez-Zepeda, M.U. Diabetes mellitus, hypertension and frailty: A population-based, cross-sectional study of Mexican older adults. Geriatr. Gerontol. Int. 2017, 17, 925–930. [Google Scholar] [CrossRef]
- Lu, F.P.; Chang, W.C.; Wu, S.C. Geriatric conditions, rather than multimorbidity, as predictors of disability and mortality among octogenarians: A population-based cohort study. Geriatr. Gerontol. Int. 2016, 16, 345–351. [Google Scholar] [CrossRef]
- Gonzalez-Freire, M.; de Cabo, R.; Studenski, S.A.; Ferrucci, L. The Neuromuscular Junction: Aging at the Crossroad between Nerves and Muscle. Front. Aging Neurosci. 2014, 6, 208. [Google Scholar] [CrossRef]
- Kim, K.; Park, K.; Kim, M.; Kim, S.; Cho, Y.; Park, S.W. Type 2 diabetes is associated with low muscle mass in older adults. Geriatr. Gerontol. Int. 2014, 14, 115–121. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Tra, Y.; Yeh, H.; Egan, J.M.; Ferrucci, L.; Brancati, F.L. Quadriceps Strength, Quadriceps Power, and Gait Speed in Older U.S. Adults with Diabetes Mellitus: Results from the National Health and Nutrition Examination Survey, 1999–2002. J. Am. Geriatr. Soc. 2013, 61, 769–775. [Google Scholar] [CrossRef]
- Chao, C.-T.; Wang, J.; Huang, J.-W.; Chan, D.-C.; Chien, K.-L. Hypoglycemic episodes are associated with an increased risk of incident frailty among new onset diabetic patients. J. Diabetes Its Complicat. 2020, 34, 107492. [Google Scholar] [CrossRef]
- Funes, J.A.Á.; Aguilar-Navarro, S.G.; Amieva, H.; Gutiérrez-Robledo, L.M. Frailty among Mexican community-dwelling elderly: A story told 11 years later. The Mexican Health and Aging Study. Salud Publica Mex. 2015, 57, 62–69. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. A Ser. Biol. Sci. Med. Sci. 2014, 69 (Suppl. 1), S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, K.; Kivimäki, M.; Hamer, M.; Shipley, M.J.; Akbaraly, T.N.; Tabak, A.; Singh-Manoux, A.; Batty, G.D. Diabetes Risk Factors, Diabetes Risk Algorithms, and the Prediction of Future Frailty: The Whitehall II Prospective Cohort Study. J. Am. Med. Dir. Assoc. 2013, 14, 851.e1–851.e6. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, P.; Jani, B.D.; Butterly, E.; Nicholl, B.; Lewsey, J.; McAllister, D.A.; Mair, F.S. An analysis of frailty and multimorbidity in 20,566 UK Biobank participants with type 2 diabetes. Commun. Med. 2021, 1, 28. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Harris, K.; Woodward, M.; Chalmers, J.; Cooper, M.; Hamet, P.; Harrap, S.; Heller, S.; MacMahon, S.; Mancia, G.; et al. The Impact of Frailty on the Effectiveness and Safety of Intensive Glucose Control and Blood Pressure–Lowering Therapy for People with Type 2 Diabetes: Results from the ADVANCE Trial. Diabetes Care 2021, 44, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Espeland, M.A.; Justice, J.N.; Bahnson, J.; Evans, J.K.; Munshi, M.; Hayden, K.M.; Simpson, F.R.; Johnson, K.C.; Johnston, C.; Kritchevsky, S.R.; et al. Eight-Year Changes in Multimorbidity and Frailty in Adults with Type 2 Diabetes Mellitus: Associations with Cognitive and Physical Function and Mortality. J. Gerontol. Ser. A 2021, 77, 1691–1698. [Google Scholar] [CrossRef]
- Sable-Morita, S.; Tanikawa, T.; Satake, S.; Okura, M.; Tokuda, H.; Arai, H. Microvascular complications and frailty can predict adverse outcomes in older patients with diabetes. Geriatr. Gerontol. Int. 2021, 21, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-T.; Wang, J.; Huang, J.-W.; Chan, D.-C.; Chien, K.-L. Frailty Predicts an Increased Risk of End-Stage Renal Disease with Risk Competition by Mortality among 165,461 Diabetic Kidney Disease Patients. Aging Dis. 2019, 10, 1270–1281. [Google Scholar] [CrossRef]
- The LONGEVO-SCA registry investigators; Gual, M.; Formiga, F.; Ariza-Solé, A.; López-Palop, R.; Sanchís, J.; Marín, F.; Vidán, M.T.; Martínez-Sellés, M.; Sionis, A.; et al. Diabetes mellitus, frailty and prognosis in very elderly patients with acute coronary syndromes. Aging Clin. Exp. Res. 2019, 31, 1635–1643. [Google Scholar] [CrossRef]
- COhort of GEriatric Nephrology in NTUH (COGENT) study group; Chao, C.-T.; Wang, J.; Chien, K.-L. Both pre-frailty and frailty increase healthcare utilization and adverse health outcomes in patients with type 2 diabetes mellitus. Cardiovasc. Diabetol. 2018, 17, 130. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-L.; Stanaway, F.F.; Lin, J.-D.; Chang, H.-Y. Frailty and health care use among community-dwelling older adults with diabetes: A population-based study. Clin. Interv. Aging 2018, ume 13, 2295–2300. [Google Scholar] [CrossRef]
- Thein, F.S.; Li, Y.; Nyunt, M.S.Z.; Gao, Q.; Wee, S.L.; Ng, T.P. Physical frailty and cognitive impairment is associated with diabetes and adversely impact functional status and mortality. Postgrad. Med. 2018, 130, 561–567. [Google Scholar] [CrossRef]
- Liang, H.; Li, X.; Lin, X.; Ju, Y.; Leng, J. The correlation between nutrition and frailty and the receiver operating characteristic curve of different nutritional indexes for frailty. BMC Geriatr. 2021, 21, 619. [Google Scholar] [CrossRef]
- Sanford, A.M. Anorexia of aging and its role for frailty. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 54–60. [Google Scholar] [CrossRef]
- Shorr, R.I.; Ray, W.A.; Daugherty, J.R.; Griffin, M.R. Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas. Arch. Intern. Med. 1997, 157, 1681–1686. [Google Scholar] [CrossRef]
- Bonds, D.E.; Miller, M.E.; Bergenstal, R.M.; Buse, J.B.; Byington, R.P.; Cutler, J.A.; Dudl, R.J.; Ismail-Beigi, F.; Kimel, A.R.; Hoogwerf, B.; et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: Retrospective epidemiological analysis of the ACCORD study. BMJ 2010, 340, b4909. [Google Scholar] [CrossRef] [PubMed]
- Zoungas, S.; Patel, A.; Chalmers, J.; de Galan, B.E.; Li, Q.; Billot, L.; Woodward, M.; Ninomiya, T.; Neal, B.; MacMahon, S.; et al. Severe Hypoglycemia and Risks of Vascular Events and Death. N. Engl. J. Med. 2010, 363, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafiz, A.H.; Sinclair, A.J. Low HbA1c and Increased Mortality Risk-is Frailty a Confounding Factor? Aging Dis. 2015, 6, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafiz, A.H.; Koay, L.; Sinclair, A.J. The Emergence of Frailty May Lead to a State of Burnt Out Type 2 Diabetes. J. Frailty Aging 2016, 5, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Searle, S.D.; Rockwood, K. Frailty and the risk of cognitive impairment. Alzheimer’s Res. Ther. 2015, 7, 54. [Google Scholar] [CrossRef]
- Lafortune, L.; Martin, S.; Kelly, S.; Kuhn, I.; Remes, O.; Cowan, A.; Brayne, C. Behavioural risk factors in midlife associated with successful ageing, disability, dementia and frailty in later life: A rapid systematic review. PLoS ONE 2016, 11, e0144405. [Google Scholar] [CrossRef]
- Buchman, A.S.; Yu, L.; Wilson, R.S.; Schneider, J.A.; Bennett, D.A. Association of brain pathology with the progression of frailty in older adults. Neurology 2013, 80, 2055–2061. [Google Scholar] [CrossRef]
- Fukazawa, R.; Hanyu, H.; Sato, T.; Shimizu, S.; Koyama, S.; Kanetaka, H.; Sakurai, H.; Iwamoto, T. Subgroups of Alzheimer’s Disease Associated with Diabetes Mellitus Based on Brain Imaging. Dement. Geriatr. Cogn. Disord. 2013, 35, 280–290. [Google Scholar] [CrossRef]
- Hirose, D.; Hanyu, H.; Fukasawa, R.; Hatanaka, H.; Namioka, N.; Sakurai, H. Frailty in diabetes-related dementia. Geriatr. Gerontol. Int. 2016, 16, 653–655. [Google Scholar] [CrossRef]
- Kelaiditi, E.; Cesari, M.; Canevelli, M.; van Kan, G.A.; Ousset, P.-J.; Gillette-Guyonnet, S.; Ritz, P.; Duveau, F.; Soto, M.E.; Provencher, V.; et al. Cognitive frailty: Rational and definition from an (I.A.N.A./I.A.G.G.) International Consensus Group. J. Nutr. Health Aging 2013, 17, 726–734. [Google Scholar] [CrossRef]
- de Vries, N.M.; Staal, J.B.; van Ravensberg, C.D.; Hobbelen, J.S.M.; Rikkert, M.O.; Nijhuis-van der Sanden, M.W.G. Outcome instruments to measure frailty: A systematic review. Ageing Res. Rev. 2011, 10, 104–114. [Google Scholar] [CrossRef]
- Abdelhafiz, A.H.; Sinclair, A.J. Cognitive Frailty in Older People with Type 2 Diabetes Mellitus: The Central Role of Hypoglycaemia and the Need for Prevention. Curr. Diabetes Rep. 2019, 19, 15. [Google Scholar] [CrossRef] [PubMed]
- Gorska-Ciebiada, M.; Saryusz-Wolska, M.; Ciebiada, M.; Loba, J. Mild Cognitive Impairment and Depressive Symptoms in Elderly Patients with Diabetes: Prevalence, Risk Factors, and Comorbidity. J. Diabetes Res. 2014, 2014, 179648. [Google Scholar] [CrossRef]
- Nicolucci, A.; Pintaudi, B.; Rossi, M.C.; Messina, R.; Dotta, F.; Frontoni, S.; Caputo, S.; Lauro, R. The social burden of hypoglycemia in the elderly. Acta Diabetol. 2015, 52, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Junius-Walker, U.; Onder, G.; Soleymani, D.; Wiese, B.; Albaina, O.; Bernabei, R.; Marzetti, E.; ADVANTAGE JA WP4 group. The essence of frailty: A systematic review and qualitative synthesis on frailty concepts and definitions. Eur. J. Intern. Med. 2018, 56, 3–10. [Google Scholar] [CrossRef]
- Sezgin, D.; O’dOnovan, M.; Cornally, N.; Liew, A.; O’cAoimh, R. Defining frailty for healthcare practice and research: A qualitative systematic review with thematic analysis. Int. J. Nurs. Stud. 2019, 92, 16–26. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older adults: Evidence for a phenotype. J. Gerontol. Ser. A 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef]
- Mitnitski, A.B.; Mogilner, A.J.; Rockwood, K. Accumulation of Deficits as a Proxy Measure of Aging. Sci. World J. 2001, 1, 323–336. [Google Scholar] [CrossRef]
- Morley, J.E. Developing Novel Therapeutic Approaches to Frailty. Curr. Pharm. Des. 2009, 15, 3384–3395. [Google Scholar] [CrossRef] [PubMed]
- Rolfson, D.B.; Majumdar, S.R.; Tsuyuki, R.T.; Tahir, A.; Rockwood, K. Validity and reliability of the Edmonton Frail Scale. Age Ageing 2006, 35, 526–529. [Google Scholar] [CrossRef]
- Clegg, A.; Bates, C.; Young, J.; Ryan, R.; Nichols, L.; Teale, E.A.; Mohammed, M.A.; Parry, J.; Marshall, T. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing 2016, 45, 353–360. [Google Scholar] [CrossRef] [PubMed]
- García-García, F.J.; Carcaillon, L.; Fernandez-Tresguerres, J.; Alfaro, A.; Larrion, J.L.; Castillo, C.; Rodriguez-Mañas, L. A New Operational Definition of Frailty: The Frailty Trait Scale. J. Am. Med. Dir. Assoc. 2014, 15, 371.e7–371.e13. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Geldhof, G.J.; Xue, Q.-L.; Kim, D.H.; Newman, A.B.; Odden, M.C. Development, Construct Validity, and Predictive Validity of a Continuous Frailty Scale: Results From 2 Large US Cohorts. Am. J. Epidemiol. 2018, 187, 1752–1762. [Google Scholar] [CrossRef] [PubMed]
- Romera-Liebana, L.; Urbina-Juez, A.; Micó-Pérez, R.; Bravo, J.M.; Martinez, A.M.; Gómez-Peralta, F.; Cubo-Romano, P.; Formiga, F. Assessment of frailty in the person with type 2 diabetes mellitus: Expert analysis. Rev. Clin. Esp. 2023, 223, 552–561. [Google Scholar] [CrossRef]
- Guevara, E.; Simó-Servat, A.; Perea, V.; Quirós, C.; Puig-Jové, C.; Formiga, F.; Barahona, M.-J. Frailty Detection in Older Adults with Diabetes: A Scoping Review of Assessment Tools and Their Link to Key Clinical Outcomes. J. Clin. Med. 2024, 13, 5325. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Sinclair, A.; Gadsby, R.; Hillson, R.; Forbes, A.; Bayer, A. Brief report: Use of the Mini-Cog as a screening tool for cognitive impairment in diabetes in primary care. Diabetes Res. Clin. Pract. 2013, 100, e23–e25. [Google Scholar] [CrossRef] [PubMed]
- Maurer, D.M. Screening for depression. Am. Fam. Physician 2012, 85, 139–144. [Google Scholar]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B.; Monahan, P.O.; Löwe, B. Anxiety Disorders in Primary Care: Prevalence, Impairment, Comorbidity, and Detection. Ann. Intern. Med. 2007, 146, 317–325. [Google Scholar] [CrossRef]
- McGuire, B.E.; Morrison, T.G.; Hermanns, N.; Skovlund, S.; Eldrup, E.; Gagliardino, J.; Kokoszka, A.; Matthews, D.; Pibernik-Okanović, M.; Rodríguez-Saldaña, J.; et al. Short-form measures of diabetes-related emotional distress: The Problem Areas in Diabetes Scale (PAID)-5 and PAID-1. Diabetologia 2009, 53, 66–69. [Google Scholar] [CrossRef]
- Xiao, S.Y. The Theoretical Basis and Research Application of Social Support Rating Scale. J. Clin. Psychiatry 1994, 4, 98–100. [Google Scholar]
- Look AHEADResearch Group Wing, R.R. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: Four-year results of the Look AHEAD trial. Arch. Intern. Med. 2010, 170, 1566–1575. [Google Scholar]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levälahti, E.; Ahtiluoto, S.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 2015, 385, 2255–2263. [Google Scholar] [CrossRef]
- Kirkman, M.S.; Briscoe, V.J.; Clark, N.; Florez, H.; Haas, L.B.; Halter, J.B.; Huang, E.S.; Korytkowski, M.T.; Munshi, M.N.; Odegard, P.S.; et al. Diabetes in Older Adults: A Consensus Report. J. Am. Geriatr. Soc. 2012, 60, 2342–2356. [Google Scholar] [CrossRef]
- Umegaki, H. Sarcopenia and frailty in older patients with diabetes mellitus. Geriatr. Gerontol. Int. 2016, 16, 293–299. [Google Scholar] [CrossRef]
- Rahi, B.; Morais, J.A.; Gaudreau, P.; Payette, H.; Shatenstein, B. Energy and protein intakes and their association with a decline in functional capacity among diabetic older adults from the NuAge cohort. Eur. J. Nutr. 2015, 55, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Garcia, E.; Hagan, K.A.; Fung, T.T.; Hu, F.B.; Rodríguez-Artalejo, F. Mediterranean diet and risk of frailty syndrome among women with type 2 diabetes. Am. J. Clin. Nutr. 2018, 107, 763–771. [Google Scholar] [CrossRef]
- Yang, Y.; Breen, L.; Burd, N.A.; Hector, A.J.; Churchward-Venne, T.A.; Josse, A.R.; Tarnopolsky, M.A.; Phillips, S.M. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br. J. Nutr. 2012, 108, 1780–1788. [Google Scholar] [CrossRef]
- Bauer, J.M.; Verlaan, S.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; McMurdo, M.E.; Mets, T.; Seal, C.; Wijers, S.L.; et al. Effects of a vitamin D and leucine enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: A randomized, double-blind, placeobo-controlled trial. J. Am. Med. Dir. Assoc. 2015, 16, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-Based Recommendations for Optimal Dietary Protein Intake in Older People: A Position Paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Brooks, N.; Layne, J.E.; Gordon, P.L.; Roubenoff, R.; Nelson, M.E.; Castaneda-Sceppa, C. Strength training improves muscle quality and insulin sensitivity in Hispanic older adults with type 2 diabetes. Int. J. Med. Sci. 2007, 4, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Tieland, M.; Dirks, M.L.; Van Der Zwaluw, N.; Verdijk, L.B.; Van De Rest, O.; de Groot, L.C.; van Loon, L.J. Protein Supplementation Increases Muscle Mass Gain During Prolonged Resistance-Type Exercise Training in Frail Elderly People: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Med. Dir. Assoc. 2012, 13, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Rahi, B.; Morais, J.A.; Dionne, I.J.; Gaudreau, P.; Payette, H.; Shatenstein, B. The combined effects of diet quality and physical activity on maintenance of muscle strength among diabetic older adults from the NuAge cohort. Exp. Gerontol. 2014, 49, 40–46. [Google Scholar] [CrossRef]
- Tshiananga, J.K.T.; Kocher, S.; Weber, C.; Erny-Albrecht, K.; Berndt, K.; Neeser, K. The Effect of Nurse-led Diabetes Self-management Education on Glycosylated Hemoglobin and Cardiovascular Risk Factors. Diabetes Educ. 2011, 38, 108–123. [Google Scholar] [CrossRef]
- García-Esquinas, E.; Graciani, A.; Guallar-Castillón, P.; López-García, E.; Rodríguez-Mañas, L.; Rodríguez-Artalejo, F. Diabetes and Risk of Frailty and Its Potential Mechanisms: A Prospective Cohort Study of Older Adults. J. Am. Med. Dir. Assoc. 2015, 16, 748–754. [Google Scholar] [CrossRef]
- Zaslavsky, O.; Walker, R.L.; Crane, P.K.; Gray, S.L.; Larson, E.B. Glucose Levels and Risk of Frailty. J. Gerontol. Ser. A 2016, 71, 1223–1229. [Google Scholar] [CrossRef]
- Morita, T.; Okuno, T.; Himeno, T.; Watanabe, K.; Nakajima, K.; Koizumi, Y.; Yano, H.; Iritani, O.; Okuro, M.; Morimoto, S. Glycemic control and disability-free survival in hypoglycemic agent-treated community-dwelling older patients with type 2 diabetes mellitus. Geriatr. Gerontol. Int. 2017, 17, 1858–1865. [Google Scholar] [CrossRef] [PubMed]
- Yanagita, I.; Fujihara, Y.; Eda, T.; Tajima, M.; Yonemura, K.; Kawajiri, T.; Yamaguchi, N.; Asakawa, H.; Nei, Y.; Kayashima, Y.; et al. Low glycated hemoglobin level is associated with severity of frailty in Japanese elderly diabetes patients. J. Diabetes Investig. 2017, 9, 419–425. [Google Scholar] [CrossRef]
- Aguayo, G.A.; Hulman, A.; Vaillant, M.T.; Donneau, A.-F.; Schritz, A.; Stranges, S.; Malisoux, L.; Huiart, L.; Guillaume, M.; Sabia, S.; et al. Prospective Association Among Diabetes Diagnosis, HbA1c, Glycemia, and Frailty Trajectories in an Elderly Population. Diabetes Care 2019, 42, 1903–1911. [Google Scholar] [CrossRef]
- Hyde, Z.; Smith, K.; Flicker, L.; Atkinson, D.; Fenner, S.; Skeaf, L.; Malay, R.; Giudice, D.L. HbA1c is Associated with Frailty in a Group of Aboriginal Australians. J. Frailty Aging 2019, 8, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Bilgin, S.; Aktas, G.; Kurtkulagi, O.; Atak, B.M.; Duman, T.T. Edmonton frail score is associated with diabetic control in elderly type 2 diabetic subjects. J. Diabetes Metab. Disord. 2020, 19, 511–514. [Google Scholar] [CrossRef]
- MacKenzie, H.T.; Tugwell, B.; Rockwood, K.; Theou, O. Frailty and Diabetes in Older Hospitalized Adults: The Case for Routine Frailty Assessment. Can. J. Diabetes 2020, 44, 241–245.e1. [Google Scholar] [CrossRef]
- Fung, E.; Lui, L.-T.; Huang, L.; Cheng, K.F.; Lau, G.H.W.; Chung, Y.T.; Ahmadabadi, B.N.; Xie, S.; Lee, J.S.W.; Hui, E.; et al. Characterising frailty, metrics of continuous glucose monitoring, and mortality hazards in older adults with type 2 diabetes on insulin therapy (HARE): A prospective, observational cohort study. Am. J. Med. Sci. 2021, 2, e724–e735. [Google Scholar] [CrossRef]
- Kong, L.; Zhao, H.; Fan, J.; Wang, Q.; Li, J.; Bai, J.; Mao, J. Predictors of frailty among Chinese community-dwelling older adults with type 2 diabetes: A cross-sectional survey. BMJ Open 2021, 11, e041578. [Google Scholar] [CrossRef]
- Lin, C.; Yu, N.; Wu, H.; Liu, Y. Risk factors associated with frailty in older adults with type 2 diabetes: A cross-sectional study. J. Clin. Nurs. 2021, 31, 967–974. [Google Scholar] [CrossRef]
- Sinclair, A.J.; Abdelhafiz, A.; Dunning, T.; Izquierdo, M.; Rodriguez Manas, L.; Bourdel-Marchasson, I.; Morley, J.E.; Munshi, M.; Woo, J.; Vellas, B. An international position statement on the management of frailty in diabetes mellitus: Summary of recommendations 2017. J. Frailty Aging 2018, 7, 10–20. [Google Scholar] [CrossRef]
- Huang, E.S.; Zhang, Q.; Gandra, N.; Chin, M.H.; Meltzer, D.O. The effect of comorbid illness and functional status on the expected benefits of intensive glucose control in older patients with type 2 diabetes: A decision analysis. Ann. Intern. Med. 2008, 149, 11–19. [Google Scholar] [CrossRef]
- Cox, D.J.; Kovatchev, B.P.; Gonder-Frederick, L.A.; Summers, K.H.; McCall, A.; Grimm, K.J.; Clarke, W.L. Relationships between hyperglycemia and cognitive performance among adults with type 1 and type 2 diabetes. Diabetes Care 2005, 28, 71–77. [Google Scholar] [CrossRef]
- Huang, C.-F.; Shiao, M.-S.; Mao, T.-Y. Effects of low-dose metformin on pre-frailty among middle-aged and elderly pre-diabetic people. JCSM Rapid. Commun. 2022, 5, 33–39. [Google Scholar] [CrossRef]
- Baskaran, D.; Aparicio-Ugarriza, R.; Ferri-Guerra, J.; Milyani, R.; Florez, H.; Ruiz, J.G. Is There an Association Between Metformin Exposure and Frailty? Gerontol. Geriatr. Med. 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-P.; Lorenzo, C.; Habib, S.L.; Jo, B.; Espinoza, S.E. Differential effects of metformin on age related comorbidities in older men with type 2 diabetes. J. Diabetes Its Complicat. 2017, 31, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Schwartz, A.V.; Yaffe, K.; Hillier, T.A.; LeBlanc, E.S.; Cawthon, P.M. The Study of Osteoporotic Fractures Research Group Changes in Physical Performance in Older Women According to Presence and Treatment of Diabetes Mellitus. J. Am. Geriatr. Soc. 2013, 61, 1872–1878. [Google Scholar] [CrossRef]
- Wang, C.P.; Lorenzo, C.; Espinoza, S.E. Frailty attenuates the impact of metformin on reducing mortality in older adults with type 2diabetes. J. Endocrinol. Diabetes Obes. 2014, 2, 1031. [Google Scholar]
- Mele, A.; Calzolaro, S.; Cannone, G.; Cetrone, M.; Conte, D.; Tricarico, D. Database search of spontaneous reports and pharmacological investigations on the sulfonylureas and glinides-induced atrophy in skeletal muscle. Pharmacol. Res. Perspect. 2014, 2, e00028. [Google Scholar] [CrossRef] [PubMed]
- Aghili, R.; Malek, M.; Valojerdi, A.E.; Banazadeh, Z.; Najafi, L.; Khamseh, M.E. Body composition in adults with newly diagnosed type 2 diabetes: Effects of met-formin. J. Diabetes Metab. Disord. 2014, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, H.; Phillips, B.; Smith, K.; Wilkinson, D.; Atherton, P.J.; Idris, I. Physiological Mechanisms of Action of Incretin and Insulin in Regulating Skeletal Muscle Metabolism. Curr. Diabetes Rev. 2014, 10, 327–335. [Google Scholar] [CrossRef]
- Sasaki, T.; Sugawara, M.; Fukuda, M. Sodium–glucose cotransporter 2 inhibitor-induced changes in body composition and simultaneous changes in metabolic profile: 52-week prospective LIGHT (Luseogliflozin: The Components of Weight Loss in Japanese Patients with Type 2 Diabetes Mellitus) Study. J. Diabetes Investig. 2018, 10, 108–117. [Google Scholar] [CrossRef]
- Bouchi, R.; Terashima, M.; Sasahara, Y.; Asakawa, M.; Fukuda, T.; Takeuchi, T.; Nakano, Y.; Murakami, M.; Minami, I.; Izumiyama, H.; et al. Luseogliflozin reduces epicardial fat accumulation in patients with type 2 diabetes: A pilot study. Cardiovasc. Diabetol. 2017, 16, 32. [Google Scholar] [CrossRef]
- Cefalu, W.T.; Leiter, L.A.; Yoon, K.H.; Arias, P.; Niskanen, L.; Xie, J.; Balis, D.A.; Canovatchel, W.; Meininger, G. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet 2013, 382, 941–950. [Google Scholar] [CrossRef]
- Sugiyama, S.; Jinnouchi, H.; Kurinami, N.; Hieshima, K.; Yoshida, A.; Jinnouchi, K.; Nishimura, H.; Suzuki, T.; Miyamoto, F.; Kajiwara, K.; et al. Dapagliflozin Reduces Fat Mass without Affecting Muscle Mass in Type 2 Diabetes. J. Atheroscler. Thromb. 2018, 25, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Meguro, S.; Kawai, T.; Suzuki, Y. Increased grip strength with sodium–glucose cotransporter 2. J. Diabetes 2016, 8, 736–737. [Google Scholar] [CrossRef]
- Pan, R.; Zhang, Y.; Wang, R.; Xu, Y.; Ji, H.; Zhao, Y.; Aktas, G. Effect of SGLT-2 inhibitors on body composition in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. PLoS ONE 2022, 17, e0279889. [Google Scholar] [CrossRef]
- Yabe, D.; Shiki, K.; Homma, G.; Meinicke, T.; Ogura, Y.; Seino, Y.; EMPA-ELDERLY Investigators. Efficacy and safety of the sodium-glucose co-transporter-2 inhibitor empagliflozin in elderly Japanese adults (≥65 years) with type 2 diabetes: A ran-domized, double-blind, placebo-controlled, 52-week clinical trial (EMPA-ELDERLY). Diabetes Obes. Metab. 2023, 25, 3538–3548. [Google Scholar] [CrossRef] [PubMed]
- Ikejima, S.; Kondo, S.; Sakai, T.; Taniai, H.; Takahashi, T.; Umezu, J.; Iseka, M.; Inoue, M.; Nishihara, H.; Murata, K.; et al. Novel Approach to Sarcopenia in Diabetic Patients Treated with GLP-1 Receptor Agonists (GLP-1RA). Diabetes 2018, 67, 673-P. [Google Scholar] [CrossRef]
- Karakasis, P.; Patoulias, D.; Fragakis, N.; Mantzoros, C.S. Effect of glucagon-like peptide-1 receptor agonists and co-agonists on body composition: Systematic review and network meta-analysis. Metabolism 2024, 164, 156113. [Google Scholar] [CrossRef] [PubMed]
- Kawamori, D.; Shirakawa, J.; Liew, C.W.; Hu, J.; Morioka, T.; Duttaroy, A.; Burkey, B.; Kulkarni, R.N. GLP-1 signalling compensates for impaired insulin signalling in regulating beta cell proliferation in βIRKO mice. Diabetologia 2017, 60, 1442–1453. [Google Scholar] [CrossRef]
- Hao, T.; Zhang, H.; Li, S.; Tian, H. Glucagon-like peptide 1 receptor agonist ameliorates the insulin resistance function of islet β cells via the activation of PDX-1/JAK signaling transduction in C57/BL6 mice with high-fat diet-induced diabetes. Int. J. Mol. Med. 2017, 39, 1029–1036. [Google Scholar] [CrossRef]
- Mone, P.; Varzideh, F.; Jankauskas, S.S.; Pansini, A.; Lombardi, A.; Frullone, S.; Santulli, G. SGLT2 Inhibition via Empagliflozin Improves Endothelial Function and Reduces Mitochondrial Oxidative Stress: Insights from Frail Hypertensive and Diabetic Patients. Hypertension 2022, 79, 1633–1643. [Google Scholar] [CrossRef]
- Abdelhafiz, A.; Bisht, S.; Kovacevic, I.; Pennells, D.; Sinclair, A. Insulin in Frail, Older People with Type 2 Diabetes—Low Threshold for Therapy. Diabetology 2022, 3, 369–383. [Google Scholar] [CrossRef]
- Elgendy, I.Y.; Mahmoud, A.N.; Barakat, A.F.; Elgendy, A.Y.; Saad, M.; Abuzaid, A.; Wayangankar, S.A.; Bavry, A.A. Cardiovascular Safety of Dipeptidyl-Peptidase IV Inhibitors: A Meta-Analysis of Placebo-Controlled Randomized Trials. Am. J. Cardiovasc. Drugs 2016, 17, 143–155. [Google Scholar] [CrossRef]
- Liao, H.-W.; Saver, J.L.; Wu, Y.-L.; Chen, T.-H.; Lee, M.; Ovbiagele, B. Pioglitazone and cardiovascular outcomes in patients with insulin resistance, pre-diabetes and type 2 diabetes: A systematic review and meta-analysis. BMJ Open 2017, 7, e013927. [Google Scholar] [CrossRef]
- Abdelhafiz, A.; Emmerton, D.; Sinclair, A. New hypoglycaemic therapy in frail older people with diabetes mellitus-phenotypic status likely to be more important than functional status. Diabetes Res. Clin. Pract. 2020, 169, 108438. [Google Scholar] [CrossRef]
- Sinclair, A.J.; Abdelhafiz, A.H. The Use of SGLT-2 Inhibitors and GLP-1RA in Frail Older People with Diabetes: A Personalised Approach Is Required. Metabolites 2025, 15, 49. [Google Scholar] [CrossRef]
- Abdelhafiz, A.H.; Rodríguez-Mañas, L.; Morley, J.E.; Sinclair, A.J. Hypoglycemia in Older People - A Less Well Recognized Risk Factor for Frailty. Aging Dis. 2015, 6, 156–167. [Google Scholar] [CrossRef]
- Hope, S.V.; Taylor, P.J.; Shields, B.M.; Hattersley, A.T.; Hamilton, W. Are we missing hypoglycaemia? Elderly patients with insulin-treated diabetes present to primary care frequently with non-specific symptoms associated with hypoglycaemia. Prim. Care Diabetes 2017, 12, 139–146. [Google Scholar] [CrossRef]
- Bonds, D.E.; Miller, M.E.; Dudl, J.; Feinglos, M.; Ismail-Beigi, F.; Malozowski, S.; Seaquist, E.; Simmons, D.L.; Sood, A. Severe hypoglycaemia symptoms, antecedent behaviors, immediate consequences and association with glycemia medication usage: Secondary analysis of theACCORD clinical trial data. BMC Endocr. Disord. 2012, 12, 5. [Google Scholar] [CrossRef]
- Abdelhafiz, A.H.; McNicholas, E.; Sinclair, A.J. Hypoglycemia, frailty and dementia in older people with diabetes: Reciprocal relations and clinical implications. J. Diabetes Its Complicat. 2016, 30, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Greco, D.; Pisciotta, M.; Gambina, F.; Maggio, F. Severe Hypoglycaemia Leading to Hospital Admission in Type 2 Diabetic Patients Aged 80 Years or Older. Exp. Clin. Endocrinol. Diabetes 2010, 118, 215–219. [Google Scholar] [CrossRef] [PubMed]
- McCoy, R.G.; Lipska, K.J.; Yao, X.; Ross, J.S.; Montori, V.M.; Shah, N.D. Intensive Treatment and Severe Hypoglycemia Among Adults with Type 2 Diabetes. JAMA Intern. Med. 2016, 176, 969–978. [Google Scholar] [CrossRef]
- Munshi, M.D.; Slyne, C.; Segal, A.R.; Saul, N.; Lyons, C.; Weinger, K. Liberating A1C goals in older adults may not protect against the risk of hypoglycaemia. J. Diabetes Its Complicat. 2017, 31, 1197–1199. [Google Scholar] [CrossRef] [PubMed]
- Malanda, U.L.; Welschen, L.M.; Riphagen, I.I.; Dekker, J.M.; Nijpels, G.; Bot, S.D.; Cochrane Metabolic and Endocrine Disorders Group. Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst. Rev. 2012, 2012, CD005060. [Google Scholar] [CrossRef]
- Ahmed, A.K.; Kamath, N.S.; El Kossi, M.; El Nahas, A.M. The impact of stopping inhibitors of the renin-angiotensin system in patients with advanced chronic kidney disease. Nephrol. Dial. Transplant. 2009, 25, 3977–3982. [Google Scholar] [CrossRef] [PubMed]
- Gadsby, R.; Galloway, M.; Barker, P.; Sinclair, A. Prescribed medicines for elderly frail people with diabetes resident in nursing homes—Issues of polypharmacy and medication costs. Diabet. Med. 2011, 29, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Allet, L.; Armand, S.; de Bie, R.A.; Golay, A.; Monnin, D.; Aminian, K.; Staal, J.B.; de Bruin, E.D. The gait and balance of patients with diabetes can be improved: A randomised controlled trial. Diabetologia 2010, 53, 458–466. [Google Scholar] [CrossRef]
- Finkelstein, E.A.; Bray, J.W.; Chen, H.; Larson, M.J.; Miller, K.; Tompkins, C.; Keme, A.; Manderscheid, R. Prevalence and Costs of Major Depression Among Elderly Claimants with Diabetes. Diabetes Care 2003, 26, 415–420. [Google Scholar] [CrossRef]
- Feil, D.G.; Zhu, C.W.; Sultzer, D.L. The relationship between cognitive impairment and diabetes self-management in a population-based community sample of older adults with Type 2 diabetes. J. Behav. Med. 2011, 35, 190–199. [Google Scholar] [CrossRef]
- Colquhoun, A.J.; Nicholson, K.G.; Botha, J.L.; Raymond, N.T. Effectiveness of influenza vaccine in reducing hospital admissions in people with diabetes. Epidemiol. Infect. 1997, 119, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Tattersall, R.; Simon, P. Managing diabetes in residential and nursing homes: Presents a complex set of problems with no one solution. BMJ 1998, 316, 89. [Google Scholar] [CrossRef][Green Version]
- Wilson, C.; Curtis, J.; Lipke, S.; Bochenski, C.; Gilliland, S. Nurse case manager effectiveness and case load in a large clinical practice: Implications for workforce development. Diabet. Med. 2005, 22, 1116–1120. [Google Scholar] [CrossRef]
- Courtney, M.; Edwards, H.; Chang, A.; Parker, A.; Finlayson, K.; Hamilton, K. Fewer Emergency Readmissions and Better Quality of Life for Older Adults at Risk of Hospital Readmission: A Randomized Controlled Trial to Determine the Effectiveness of a 24-Week Exercise and Telephone Follow-Up Program. J. Am. Geriatr. Soc. 2009, 57, 395–402. [Google Scholar] [CrossRef]
- McAiney, C.A.; Haughton, D.; Jennings, J.; Farr, D.; Hillier, L.; Morden, P. A unique practice model for Nurse Practitioners in long-term care homes. J. Adv. Nurs. 2008, 62, 562–571. [Google Scholar] [CrossRef]
- Baig, A.A.; Benitez, A.; Quinn, M.T.; Burnet, D.L. Family interventions to improve diabetes outcomes for adults. Ann. New York Acad. Sci. 2015, 1353, 89–112. [Google Scholar] [CrossRef] [PubMed]
- Seng, J.J.B.; Gwee, M.F.R.; Yong, M.H.A.; Kwan, Y.H.; Thumboo, J.; Low, L.L. Role of Caregivers in Remote Management of Patients with Type 2 Diabetes Mellitus: Systematic Review of Literature. J. Med. Internet Res. 2023, 25, e46988. [Google Scholar] [CrossRef]
- Longacre, M.L.; Wong, Y.-N.; Fang, C.Y. Caregiver Psychological Health and Hospitalization Characteristics of Older Adult Care Recipients: An Integrative Review of U.S. Studies. Res. Gerontol. Nurs. 2014, 7, 139–147. [Google Scholar] [CrossRef]
- Jayani, R.; Hurria, A. Caregivers of older adults with cancer. Semin. Oncol. Nurs. 2012, 28, 221–225. [Google Scholar] [CrossRef]
- Ripoll, J.M.S.; Llinares, V.J.S.; Pérez, M.J.R.; Cervera, C.G.; Cruz, J.M.N.; Atiénzar, P.E.; Escandell, S.B.; Serrano, A.L.; Ríos, M.D.J.; Mora, J.M.; et al. Caregiver Burden in the Management of Frail Elderly Patients with Diabetes in Internal Medicine. Health 2018, 10, 1383–1391. [Google Scholar] [CrossRef]
- Kristianingrum, N.D.; Ramadhani, D.A.; Hayati, Y.S.; Setyoadi, S. Correlation between the Burden of Family Caregivers and Health Status of People with Diabetes Mellitus. J. Public Health Res. 2021, 10. [Google Scholar] [CrossRef]
- Sewitch, M.J.; McCusker, J.; Dendukuri, N.; Yaffe, M.J. Depression in frail elders: Impact on family caregivers. Int. J. Geriatr. Psychiatry 2004, 19, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P.; Park, J.C.; Kalantar-Zadeh, K. Glycemic Control and Burnt-Out Diabetes in ESRD. Semin. Dial. 2010, 23, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Sjoblom, P.; Tengblad, A.; Lofgren, U.B.; Lannering, C.; Anderberg, N.; Rosenqvist, U.; Mölstad, S.; Ostgren, C.J. Can diabetes medication be reduced in elderly patients? An observational study of diabetes drug withdrawal in nursing home patients with tight glycaemic control. Diabetes Res. Clin. Pract. 2008, 82, 197–202. [Google Scholar]
- Abdelhafiz, A.H.; Chakravorty, P.; Gupta, S.; Haque, A.; Sinclair, A.J. Can hypoglycaemic medications be withdrawn in older people with type 2 diabetes? Int. J. Clin. Pract. 2014, 68, 790–792. [Google Scholar] [CrossRef]
- Campitelli, M.A.; Maxwell, C.J.; Maclagan, L.C.; Ko, D.T.; Bell, C.M.; Jeffs, L.; Morris, A.M.; Lapane, K.L.; Daneman, N.; Bronskill, S.E. One-year survival and admission to hospital for cardiovascular events among older residents of long-term care facilities who were prescribed intensive- and moderate-dose statins. Can. Med. Assoc. J. 2019, 191, E32–E39. [Google Scholar] [CrossRef]
- Marshall, B.; Bennett, N. PURL: How Old Is Too Old Statins? J. Fam. Pract. 2020, 69, 257–259. [Google Scholar] [PubMed]
- Sheppard, J.P.; Burt, J.; Lown, M.; Temple, E.; Lowe, R.; Fraser, R.; Allen, J.; Ford, G.A.; Heneghan, C.; Hobbs, F.D.R.; et al. OPTIMISE Investigators. Effect of Antihypertensive Medication Reduction vs Usual Care on Short-term Blood Pressure Control in Patients with Hypertension Aged 80 Years and Older: The OPTIMISE Ran-domized Clinical Trial. JAMA 2020, 323, 2039–2051. [Google Scholar] [CrossRef]
- Mossello, E.; Pieraccioli, M.; Nesti, N.; Bulgaresi, M.; Lorenzi, C.; Caleri, V.; Tonon, E.; Cavallini, M.C.; Baroncini, C.; Di Bari, M.; et al. Effects of Low Blood Pressure in Cognitively Impaired Elderly Patients Treated with Antihypertensive Drugs. JAMA Intern. Med. 2015, 175, 578–585. [Google Scholar] [CrossRef]
- Odden, M.C.; Peralta, C.A.; Haan, M.N.; Covinsky, K.E. Rethinking the association of high blood pressure with mortality in elderly adults: The impact of frailty. Arch. Intern. Med. 2012, 172, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- ASCEND Study Collaborative Group; Bowman, L.; Mafham, M.; Wallendszus, K.; Stevens, W.; Buck, G.; Barton, J.; Murphy, K.; Aung, T.; Haynes, R.; et al. Effects of Aspirin for Primary Prevention in Persons with Diabetes Mellitus. N. Engl. J. Med. 2018, 379, 1529–1539. [Google Scholar]
- Zhou, Z.; Webb, K.L.; Nelson, M.R.; Woods, R.L.; Ernst, M.E.; Murray, A.M.; Chan, A.T.; Tonkin, A.; Reid, C.M.; Orchard, S.G.; et al. Short- and long-term impact of aspirin cessation in older adults: A target trial emulation. BMC Med. 2024, 22, 306. [Google Scholar] [CrossRef]
- Glare, P.; Virik, K.; Jones, M.; Hudson, M.; Eychmuller, S.; Simes, J.; Christakis, N. A systematic review of physicians’ survival predictions in terminally ill cancer patients. BMJ 2003, 327, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Ferrell, B.R.; Twaddle, M.L.; Melnick, A.; Meier, D.E. National Consensus Project Clinical Practice Guidelines for Quality Palliative Care Guidelines, 4th Edition. J. Palliat. Med. 2018, 21, 1684–1689. [Google Scholar] [CrossRef]
- Scott, I.A.; Mitchell, G.K.; Reymond, E.J.; Daly, M.P. Difficult but necessary conversations—The case for advance care planning. Med. J. Aust. 2013, 199, 662–666. [Google Scholar] [CrossRef]
- Becker, C.; Lord, S.R.; Studenski, S.A.; Warden, S.J.; Fielding, R.A.; Recknor, C.P.; Hochberg, M.C.; Ferrari, S.L.; Blain, H.; Binder, E.F.; et al. Myostatin antibody (LY2495655) in older weak fallers: A proof-of-concept, randomised, phase 2 trial. Lancet Diabetes Endocrinol. 2015, 3, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Makimura, H.; Feldpausch, M.N.; Rope, A.M.; Hemphill, L.C.; Torriani, M.; Lee, H.; Grinspoon, S.K. Metabolic Effects of a Growth Hormone-Releasing Factor in Obese Subjects with Reduced Growth Hormone Secretion: A Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2012, 97, 4769–4779. [Google Scholar] [CrossRef] [PubMed]
- Marty, E.; Liu, Y.; Samuel, A.; Or, O.; Lane, J. A review of sarcopenia: Enhancing awareness of an increasingly prevalent disease. Bone 2017, 105, 276–286. [Google Scholar] [CrossRef] [PubMed]

| Tool | Criteria | Advantage |
|---|---|---|
| Fried frailty phenotype [56] | 5-point scale: Weight loss, weakness assessed by grip strength, self-reported exhaustion, reduced physical activity and slowness measured by gait speed | Identifies robust (score 0), pre-frail (score 1–2) and frail (score >3) individuals but requires two practical measurements |
| Clinical frailty scale [57] | 9-point scale that describes patients’ functional characteristics and categorises them from very fit to severely frail | Uses clinical description and pictographs to stratify older people according to level of function to predict mortality or institutionalisation risk |
| Frailty index [58] | Clinical items based on data from chronic conditions, disabilities in activities of daily living, cognitive function, nutrition, visual and hearing impairments | Includes holistic data as a part of comprehensive geriatric assessment |
| FRAIL scale [59] | 5-point scale: Fatigue, resistance, ambulation, illness and loss of weight | Can be self-assessed and does not require measurements by health care professionals |
| Edmonton frail scale [60] | 9 domains: General health, social support, cognition, physical function, mental state, independence, nutrition, pharmacological condition and continence | Can be completed by people without special training in geriatric medicine |
| Electronic frailty index [61] | Uses the cumulative deficit model to identify and score frailty based on routine patient–general practitioner interactions | Can be used to screen for whole practice population > 65 years old |
| Frailty trait scale [62] | Evaluates three dimensions of nutrition, physical activity and nervous system | Can predict hospitalisation and mortality |
| Continuous Frailty Scale [63] | Frailty conceptualised as continuous rather than categorical construct, assessed by 5 Fried criteria | Provides risk stratification for mortality and disability beyond Fried scale |
| Study | Population | Aim | Findings |
|---|---|---|---|
| García-Esquinas E et al., prospective, Spain, 2015 [85] | 346 subjects with DM, mean (SD) age 69.4 (6.4) Y, F/U 3.5 Y | Investigate mechanism of frailty in DM | ↑ 1% unit in HbA1c increased risk of frailty, OR 1.48, 95% CI 1.20 to 1.81 |
| Zaslavsky O et al., prospective, US, 2016 [86] | 200 subjects with and 1648 without DM | Explore association of blood glucose levels with frailty | U-shaped relation of HbA1c and frailty Reference HbA1c: 7.6% A. ↑ HbA1c (8.2): HR 1.3, 95% CI 1.1 to 1.6 A. ↓ HbA1c (6.9%): HR 1.4 (1.1 to 1.8) |
| Morita T et al., prospective, Japan, 2017 [87] | 184 patients with DM aged 65–94 Y, F/U 5Y | Examine whether low HbA1c increases risk of support/care need certification | HbA1c < 6.0% increased risk of support/care need certification: HR 3.45, 95% CI 1.02 to 11.6, p = 0.046 compared to HbA1c 6.5–7% |
| Yanagita I et al., retrospective, Japan, 2018 [88] | 132 hospitalised patients with DM, mean (SD) age 78.3 (8.0) Y | Explore risk factors of frailty including HbA1c | A. Mean (SD) HbA1c significantly lower in frail compared to non-frail patients, 7.13 (0.99) vs. 7.27 (1.04), p < 0.001 B. HbA1c inversely correlated with CFS (r = −0.31, p < 0.01) C. HbA1c independently predicted frailty (ß = −0.367, p < 0.01) |
| Aguayo GA et al., prospective, UK, 2019 [89] | 5377 subjects, median (IQR) age 70 (65, 77) Y, F/U 10 Y | Investigate if subjects with DM or high HbA1c have different frailty trajectories with ageing | A. Subjects with DM had higher frailty throughout later life B. ↑ HbA1c associated with frailty (b = 4.2, 95% CI 2.5 to 5.9) |
| Hyde Z et al., cross-sectional, Australia, 2019 [90] | 141 Aboriginal Australians, mean (SD) age 62.2 (11.1) Y | Investigate whether HbA1c is associated with frailty | A. 51.1% subjects had DM, 59.6% frail B. Mean (SD) HbA1c 7.9% (2.1) in subjects with DM and 6.1% (0.9) in those without C. Frailty greater with higher HbA1c (p = 0.025) D. HbA1c ≥ 6.5% associated with frailty (OR 2.39, 95% CI 1.17 to 4.89) |
| Bilgin S et al., cross-sectional, Turkey, 2020 [91] | 101 subjects with DM, 41 frail, mean (SD) age 64.2 (8.0) Y, 60 non-frail, mean (SD) age 62.2 (7.0) Y | Examine clinical and laboratory indices of frail and non-frail patients with DM | Edmonton frail score correlated positively with HbA1c (r = 0.44, p < 0.001) |
| Mackenzie HT et al., retrospective, Canada, 2020 [92] | 400 hospitalised subjects, mean (SD) age 81.4 (8.1) Y | Investigate association of frailty and DM with hospital outcomes | A. Mean admission glucose decreased with increased frailty (p = 0.003); mean (SD) glucose 13.0 (8.4) mmol/L for mild, 9.0 (3.4) mmol/L for moderate and 8.6 (3.4) mmol/L for severe frailty, respectively B. Nine patients had hypoglycaemia on admission, all were frail |
| Fung E et al., prospective, Hong Kong, 2021 [93] | 215 subjects with DM, median (IQR) age 74 (71–78) Y | Investigate association of blood glucose levels with frailty | Less glucose control (TIR ≤ 50% of time) associated with frailty as compared to better control, median FI 0.23 (IQR 0.17–0.30) vs. 0.18 (0.13–0.25), p = 0.0045 |
| Kong L et al., cross-sectional, China, 2021 [94] | 291 older people, median (IQR) age 69 (67–72) Y with DM | Identify predictors of frailty in community-dwelling older people with DM | Higher HbA1c was significant predictor of frailty: OR 1.434, 95% CI 1.045 to 1.968 |
| Lin CL et al., cross-sectional, Taiwan, 2022 [95] | 248 subjects with DM, mean (SD) age 73.9 (5.9) Y | Investigate risk factors associated with frailty. | ↑ HbA1c (≥8.0%) and frequent hyperglycaemia (16.7 mmol/L) associated with frailty (p = 0.038 and 0.001, respectively) |
| Agent | Advantage | Disadvantage |
|---|---|---|
| Metformin | Less risk of hypoglycaemia, cardiovascular benefit, weight-neutral, may have positive effect on frailty, depression and dementia but effect may be confounded by vitamin B12 deficiency | Not suitable for patients with significant weight loss or at risk of lactic acidosis, such as those with renal impairment, dehydration, heart failure and acute illness |
| Thiazolidinediones | Suitable for patients with renal impairment, less risk of hypoglycaemia, may have positive effect on frailty and dementia but no data on depression | Not suitable for patients with fluid retention or heart failure, concerns over use in patients with osteoporosis |
| Sulfonylureas | Suitable for patients with renal impairment and lean patients with less risk of hypoglycaemia | Not suitable for obese individuals or those at risk of recurrent hypoglycaemia, particularly those living alone; may have negative effects on frailty, depression and cognition; long-acting sulfonylureas should be avoided |
| Meglitinides | Short-acting, suitable for patients with erratic eating patterns | Risk of hypoglycaemia and weight gain, may have negative effects on frailty, depression and cognition |
| Alpha-glucosidase inhibitors | Less risk of weight gain and hypoglycaemia | Weak hypoglycaemic action, gastrointestinal side effects |
| Dipeptidyl peptidase-4 (DPP-4) inhibitors | Low risk of hypoglycaemia, weight loss, may have positive effect on depression, cognition and muscles | Gastrointestinal side effects, dose mostly needs to be adjusted with renal impairment |
| Sodium glucose cotransporter-2 (SGLT-2) inhibitors | Low risk of hypoglycaemia; weight loss in obese frail individuals; not enough data on effect on frailty, depression or cognition. | Not suitable for frail older people with weight loss and heavy glycosuria; increases risk of urinary tract infections, candidiasis, dehydration and hypotension. |
| Glucagon like-1 receptor agonists (GLP-1RAs) | Low risk of hypoglycaemia; weight loss in obese frail individuals; not enough data on effect on frailty, depression or cognition | Injectable, weight loss in frail anorexic individuals, not suitable in renal failure, nausea is common, possible risk of pancreatitis |
| Insulin | Effective, tailored rapidly to changes in need, improves quality of life; not enough data of effect on frailty, depression or cognition | High risk of hypoglycaemia and weight gain |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelhafiz, D.; Abdelhafiz, A. Practical Considerations in the Management of Frail Older People with Diabetes. Diseases 2025, 13, 249. https://doi.org/10.3390/diseases13080249
Abdelhafiz D, Abdelhafiz A. Practical Considerations in the Management of Frail Older People with Diabetes. Diseases. 2025; 13(8):249. https://doi.org/10.3390/diseases13080249
Chicago/Turabian StyleAbdelhafiz, Dima, and Ahmed Abdelhafiz. 2025. "Practical Considerations in the Management of Frail Older People with Diabetes" Diseases 13, no. 8: 249. https://doi.org/10.3390/diseases13080249
APA StyleAbdelhafiz, D., & Abdelhafiz, A. (2025). Practical Considerations in the Management of Frail Older People with Diabetes. Diseases, 13(8), 249. https://doi.org/10.3390/diseases13080249




