Abstract
Introduction: While not considered a genuine tumorigenic pathogen, the human cytomegalovirus (CMV) has been associated with a wide assortment of malignancies, including breast cancer (BC). In recent years, increasing evidence has been detailing the potential anti-oncogenic capabilities of CMV. Works in the literature addressing the issue are scarce, and a global approach elucidating the role of CMV in breast cancer is lacking. Aim: We inquired into the association between CMV and BC on a global level and surveyed the related literature. Material and Methods: Virus–tumor interaction was examined by correlating country-specific CMV seroprevalence and the age-standardized BC incidence rates for 73 countries, as provided by the International Agency for Research on Cancer (IARC). Statistical analysis was conducted using Spearman’s correlation, along with univariate and multivariate linear regression analysis. The literature review included works available in the PubMed® database until and including February 2025. Results: The worldwide incidence of BC correlated strongly and inversely with CMV prevalence the world over (p < 0.001, Spearman ρ = −0.553). This association was upheld after univariate and multivariate linear regression, extending to other tumors such as skin melanoma and kidney cancer (p < 0.001). Conclusions: In this study, we draw attention to a previously unexplored global inverse relationship between the prevalence of CMV and the incidence of BC, which suggests a potential oncoprotective role for this pathogen. Although the association itself does not imply causality, these data provide an intriguing possibility of observing CMV as a tentative factor of protection against this malignancy.
1. Introduction
The human cytomegalovirus (CMV; Cytomegalovirus humanbeta5 or human betaherpesvirus 5 [1]) is a ubiquitous pathogen belonging to the Orthoherpesviridae family. The virus carries double-stranded DNA within an icosahedral capsid surrounded by a cell-derived phospholipid bilayer and exhibits a high level of intraspecies diversity [1]. It is a frequent cause of human infection, with seroprevalence ranging between 45% and 95%, depending on the population studied [2,3]. Patterns of exposure differ among countries, depending on various factors such as lifestyle, age, geographical area, and social and economic status. While most often causing benign disease in healthy subjects, CMV is a notorious agent of disease in the immunocompromised. In recent years, mounting evidence supports a compelling correlation between CMV and malignant diseases, implying an oncomodulatory role of the pathogen [4,5,6].
The debate regarding CMV oncogenicity is a polarized one. Although CMV is not considered an oncogenic pathogen, it has been associated with a broad swathe of tumors, such as hematopoietic malignancies, glioblastoma, neuroblastoma, prostate cancer, colon cancer, and salivary gland cancer, as well as breast cancer [6,7,8]. CMV can disrupt cellular pathways and processes, potentially increasing cancer susceptibility by affecting the cell cycle, apoptosis, angiogenesis, cell invasion, and the immune response [9,10]. CMV-induced behavior that may well promote cancer survival is also reflected in its ability to impinge upon the immune system. CMV’s ability to evade immunity may promote neoplastic transformation and metastasis [11,12]. Viral inhibition of the apoptosis of tumor cells by several CMV proteins was also noted, along with impaired p53 function, highlighting the virus’s tumorigenic potential.
A growing body of proof, however, speaks in favor of a protective effect that CMV might impart against various forms of neoplasia [13,14,15]. A possible means by which CMV orchestrates an anti-cancer response is by galvanizing a tumoricidal T-cell response, which precludes the formation of a full-blown malignancy [16,17]. Some of the oncoprotective mechanisms by which CMV may bestow a protective effect on its host include the potential inhibition of the migration of specific breast cancer cells by the virus [18], the potential to redirect the T cell immune response specific to CMV toward tumor cells instead, consequently impacting tumor regression [19], the hypothesized use of the CMV gB protein as a means of tumor suppression [18], and viral reactivation as a relapse reduction factor for a variety of hematological malignancies [20,21,22,23], to name just some of them.
Like with other neoplastic dyscrasia, the connection between CMV and breast cancer is yet to be fully elucidated. Studies investigating the role of CMV in this malignancy do not abound, and the ones that do exist are solely single-center experiences. Currently, there is a lack of comprehensive studies that would span various populations and geographical expanses, thereby giving a global perspective on the subject.
We inquired into the association between CMV and breast neoplasia on a worldwide level and surveyed the published literature. This study draws attention to a hitherto unreported inverse correlation between viral pervasiveness and breast tumor incidences the world over. These intriguing and novel findings further advance the idea of CMV as a potential agent of oncoprevention. Expanding on our prior study in this research domain [13], as well as other recent clinical investigations [14,15], we offer a perspective on the potential role of CMV as a viable factor in averting the initiation of cancer across diverse demographic, geopolitical, and socio-economic contexts.
2. Materials and Methods
To explore the potential anti-oncogenic effects of CMV, we examined the association between country-specific age-standardized cancer incidence rates and the corresponding CMV seroprevalences.
The age-adjusted annual incidence rates (per 100,000 individuals) for 6 cancer categories have been recorded across 185 countries for the year 2020, sourced from the Global Cancer Observatory (GLOBOCAN), an online database and project of the International Agency for Research on Cancer (IARC), providing global cancer statistics under the aegis of the World Health Organization (WHO) [24]. These incidences were observed collectively for a total of 34 tumors in both males and females, spanning the entire listed age range (0–85+ years). The list of scrutinized malignancies, as well as their association with CMV pervasiveness, is presented in Table 1 and Table 2.

Table 1.
The WHO’s GLOBOCAN tumor data as regards global CMV prevalence, analyzed with correlation and regression, suggest that CMV may offer oncoprotection, as higher CMV prevalence correlates with lower tumor incidence in significant cases.

Table 2.
Univariate linear regression analysis with model characteristics.
The prevalence of CMV was represented using country-specific seroprevalence data from 73 countries derived from data collected by Zuhair et al. [2], who systematically reviewed the published literature to assess global CMV IgG antibody prevalence.
For each tumor type or localization, univariate and multivariate linear regression analyses were conducted. In the univariate analysis, CMV was included as an independent predictor, based on both the correlation results and expert judgment. Multivariate analyses were adjusted for confounding variables, which were selected based on their documented association with CMV prevalence: human development index (HDI, which is used as a stand-in for socioeconomic status, or SES); average population age; smoking; breastfeeding; and with HDI being used as a parallel of socioeconomic status, encompassing three major factors: life expectancy, education (mean years of schooling completed and expected years of schooling upon entering the education system), and per capita income.
As regards the literature search, the PubMed® engine was used in order to obtain all relevant references. Studies listed in this manner were further interrogated for relevant publications, i.e., those that pertain to the underlying issue. Furthermore, works referenced within the acquired studies were additionally scoured for pertinent information and included herein. Original papers and reviews in the English language were utilized, along with papers where abstracts were the only available section of the work. All the search was completed in March 2023.
Statistical investigation, i.e., a comparison between age-standardized annual cancer incidence rates and country-specific CMV seroprevalence, was performed by means of Spearman’s rank correlation test, which was complemented by univariate and multivariate regression analyses. The analyses were conducted using SPSS (IBM Corp. v.20.0, Armonk, NY, USA) statistical software, and p-values were used to denote corresponding levels of statistical significance.
3. Results
An inverse correlation was apparent between the prevalence of CMV and the following tumor categories: “Melanoma (skin), “Kidney”, “All cancers”, “All cancers (excluding non-melanoma skin cancer)“, and “Breast cancer”. Each of the associations demonstrated a high degree of statistical significance (p < 0.001).
The strongest rank correlation coefficient was observed for incidences of melanoma of the skin (Table 1). Although declining in number, the rest of the rho numerical values were quite similar, again indicating the similar strength and same direction of the monotonic relationship between CMV pervasiveness and tumor incidence.
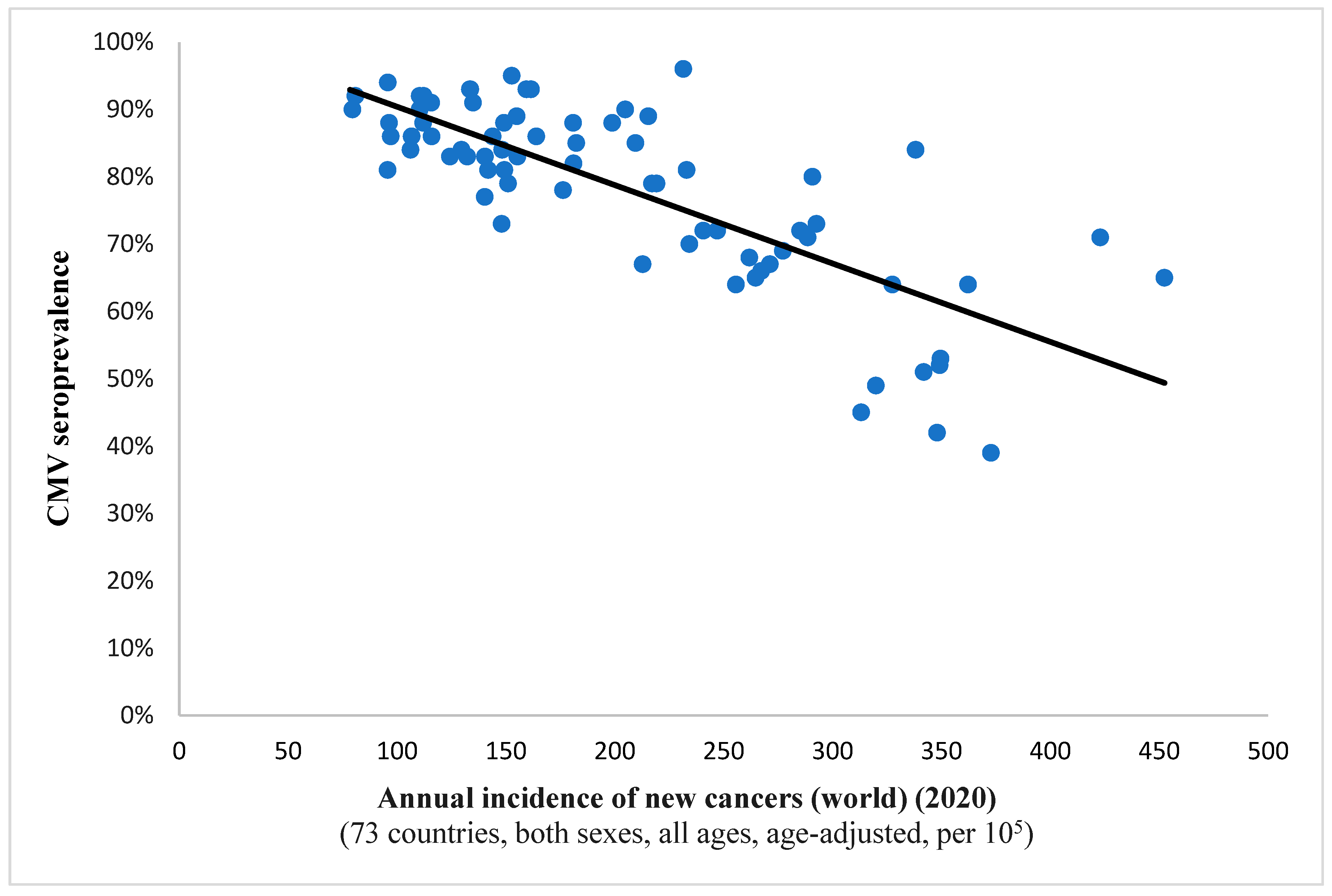
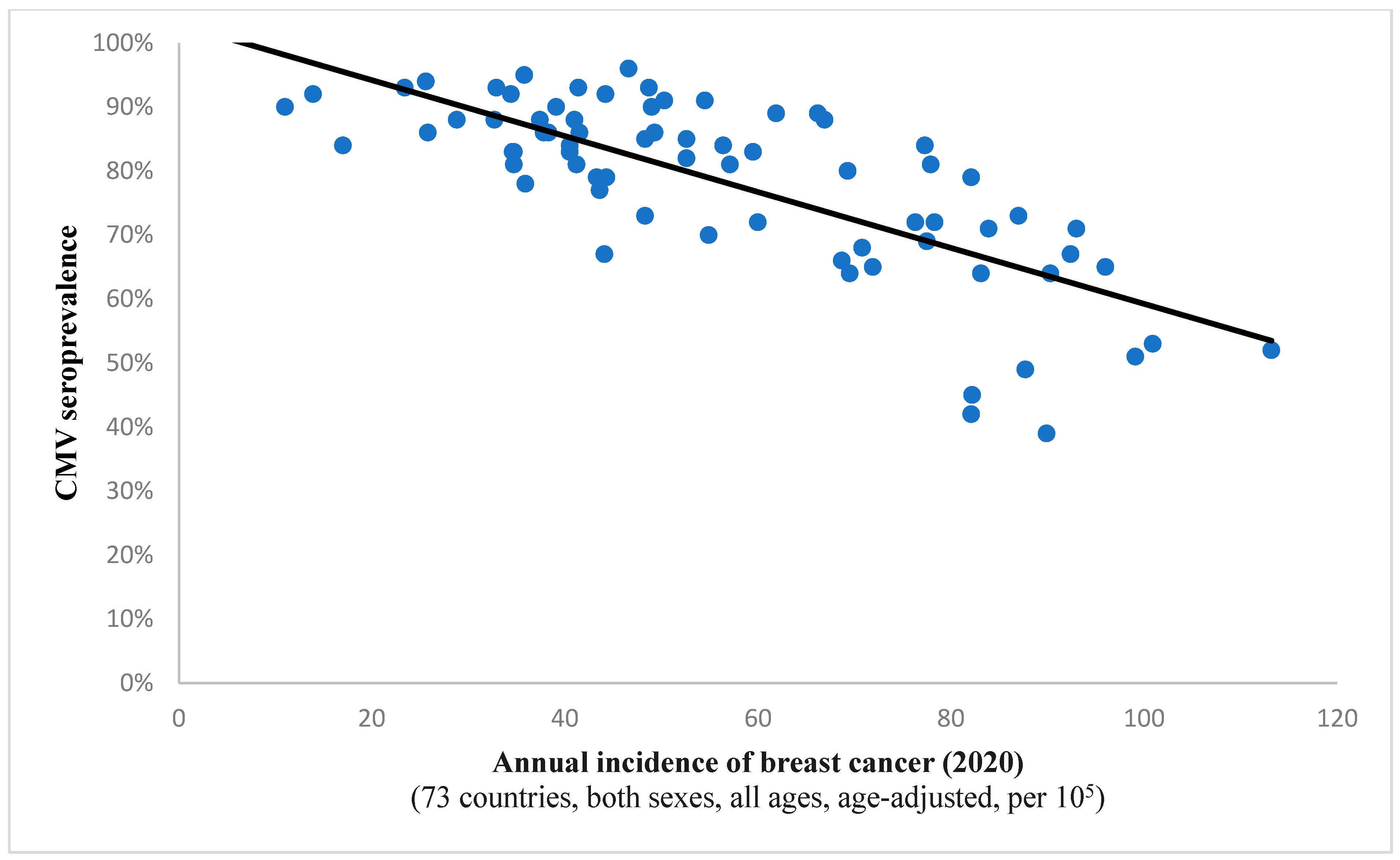
It is interesting to note the continued existence of this statistical correlation when examining incidence rates for all cancers combined (Spearman’s ρ = −0.732, p < 0.001; depicted in Figure 1). This finding implies a potential protective effect of the virus against all included neoplastic conditions on a global level. A comparable observation was noted in the case of breast cancer, suggesting a potential anti-tumor characteristic of the pathogen within this histological context as well (Figure 2).
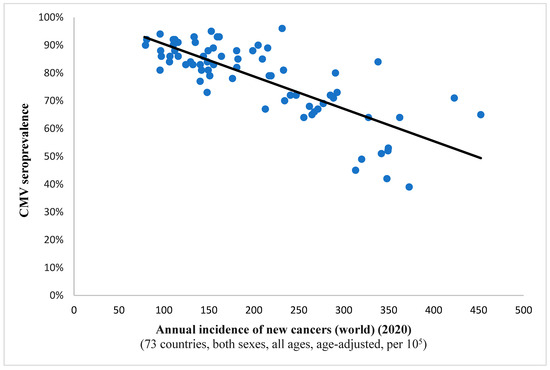
Figure 1.
This scatter plot represents a significant relation (Spearman’s ρ = −0.732; p < 0.001) between CMV prevalence and the annual incidence of newly detected malignancies the world over for a complement of 73 countries. Namely, as the viral pervasiveness rises, the cancer incidence diminishes, hinting at an antitumor effect of CMV.
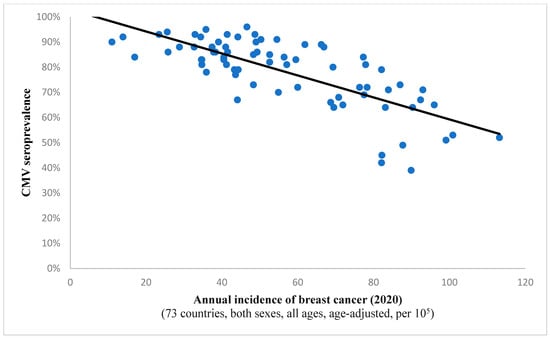
Figure 2.
The graph showcases the relationship between breast cancer frequency and CMV prevalence in 73 states across the globe. The strong and inverse correlation (Spearman’s ρ = −0.719; p < 0.001) suggests a potential protective effect of the virus against oncogenesis in this case.
No correlation was evident between CMV seroprevalence and the frequency of Kaposi’s sarcoma (Spearman’s ρ = −0.007, p = 0.953; shown in Table 1). The mentioned tumor was used in this study as a control variable against which other associations may be compared. This is in accordance with the idea of CMV-stimulated T cell tumoricidal activity [16,17,25]; namely, that individuals affected by HIV/AIDS exhibit marked impairment of the T cell immune response and are particularly affected by the prominent occurrence of Kaposi’s sarcoma.
An analysis of univariate linear regression (ULR) was conducted, with CMV being used as an independent variable. Upon implementing univariate linear regression analysis, the association (previously observed by Spearman’s correlation) still maintained both significance (p < 0.001) and direction (Cf. standardized β coefficient) for breast cancer and melanoma, as well as kidney malignancies. Notably, the correlation was also preserved for all tumors worldwide.
The prevalence of CMV is a substantial and independent predictor of tumor incidence. This means that a rise in CMV prevalence is strongly and highly correlated with a reduction in the occurrence of mentioned cancers. When exploring the effect of HDI as a potential confounder in multiple linear regression analysis, CMV remained a strong predictor for the reduced frequency of tested tumors, displaying favorable overall MLR model characteristics. As was expected, the univariate linear regression uncovered a lack of association with Kaposi’s sarcoma (p = 0.255). The MLR analysis revealed no significant changes in the results after adjusting for confounding factors such as age, smoking, and breastfeeding. Consequently, these variables were excluded from the final model.

Table 3.
Multivariate linear regression analysis with model characteristics.
4. Discussion
The interplay between CMV and tumors is the subject of ongoing discussion. Despite numerous instances demonstrating CMV’s anti-tumor properties, the broader public perception appears to focus on its potential for oncomodulation (making malignant tumors even more aggressive) and its suspected pro-oncogenic influence. In this study, we explored the association of CMV seroprevalence with breast cancer, a malignant disorder that stood as the most prevalent cancer at the conclusion of 2020.
4.1. Arguments in Favor of CMV-Mediated Carcinogenesis and Oncomodulation
Up until now, the potential association of CMV with malignancies has remained the subject of ongoing discussion. Its potential to induce cancer has been suggested on multiple occasions [26,27], and a number of researchers advocate for the tumorigenic effect of CMV [7,8,28,29,30], with some studies proposing its involvement in more than 90% of commonly occurring tumors [31]. The pathogen has been associated with a wide panoply of malignancies, such as hematopoietic malignancies, glioblastoma, neuroblastoma, prostate cancer, colon cancer, and salivary gland cancer, as well as breast cancer [6,7,8,32]. It is suggested that CMV has the capacity to impact cellular processes and pathways, potentially heightening the vulnerability of cells to cancer development by disrupting the pathways linked to the cell cycle, apoptosis, angiogenesis, cell invasion, and the host’s immune response [9,10].
Studies have identified CMV as a potential risk factor for breast cancer; notably, in individuals with this malignancy, those who tested positive for CMV or had CMV DNA in the tumor tissue were more predisposed to develop stage IV metastatic tumors, implying a role of the virus in promoting metastases [33], while a link between the clinical stage and CMV infection was also observed elsewhere [34]. Additionally, the expression of viral proteins was linked to reduced overall survival in breast cancer patients [35], and the expression of the CMV IE2 gene was also correlated with this type of tumor [36]. A relationship between CMV infection and breast cancer prognosis has also been noted in a study by Touma et al. [35].
CMV-induced behavior that may well promote cancer survival is also reflected in its impinging on the immune system. In the study by Geisler et al., it was concluded that CMV-infected cells possess the capability to escape recognition and clearance by the immune system through strategic manipulation, impeding antigen presentation by expressing molecules that inhibit T cells and also regulating immune-suppressive type II macrophages [37]. Other investigations are also congruent with the abovementioned studies, suggesting that CMV infection in macrophages promotes a phenotype resembling TAM (tumor-associated macrophages), aiding in neoplastic transformation by evading the immune system, which could potentially lead to metastatic events [11]. Alsamarai and colleagues have demonstrated that CMV IE1, IE2, UL36, UL37, and UL38 proteins effectively inhibit the apoptosis of tumor cells by avoiding immune elimination by NK and cytotoxic T cells [12]. Apparently, cytomegalovirus also has negative effects on the function of p53, which contributes significantly to our comprehension of the virus’s tumorigenic characteristics [38]. Curiously enough, although tested in a cohort of women with benign breast disease and carcinoma, no connection was noted between CMV IgG and diagnosis; the authors note that viral infection influences cytokine production and contributes to changed cytokine profiles in this tumor type [39].
Our current findings suggest that CMV infection is more prevalent in breast carcinomas than in non-tumor tissues. Furthermore, the proposed pathogen-driven breast carcinogenesis model indicates that a number of oncogenic pathways may be activated by viral oncoproteins, promoting cell proliferation, survival, and the development of tumors [40].
At the very least, CMV seems to play a part in oncomodulation [4,41,42,43], supporting cancer proliferation and longevity [5,44] and enhancing its malignant potential by inducing the transition to malignant phenotypes. Haidar Ahmad et al. demonstrated that the virus prompts the heightened expression of specific oncogenes and genes associated with cell survival and cell proliferation (e.g., the Ki67 gene), as well as DNA reparation and the EMT (epithelial-mesenchymal transition) [45]. Other authors have explored the influence of CMV infection on the development of tumor metastases, suggesting that it facilitates cell invasion through interleukin-induced cellular alterations [46,47]. According to Yang and colleagues, the detection of glycoprotein B in a tumor is associated with the occurrence of metastases and the severity of the clinical condition, which could potentially assist in providing adequate therapy for the given case [33]. There seem to be other ways in which the virus creates a pro-oncogenic cellular environment, such as reduced expression of the p53 gene, enhancing the phosphorylation of the Rb gene, increasing telomerase activity, and more [48,49].
It is worth noting, however, that not all oncomodulatory effects necessarily harm the host. Specifically, there have been observations that CMV may inhibit the migration of specific breast cancer cells [18].
The CMV genome contains numerous proteins that promote cancer cell activity. The HCMV IE1 protein, as reported by Yurochko et al. [50], has been shown to induce NF-kB expression and plays a role in activating cell survival pathways in tumor cells. Additionally, Mitchell et al. [51] found that the pp65 protein was expressed in over 90% of glioblastoma multiforme tissues but was not detected in normal brain tissue, suggesting a potential association with the tumor.
Certain high-risk CMV strains have even been implicated in the active transformation of primary cells [6,37,44,52]. Notably, Herbein and Cobbs noted that CMV not only has the ability to modify epithelial cells but also participates in the transformation of epithelial to mesenchymal (EMT) cells in tumor cells and vice versa [6,52]. Furthermore, it has been suggested that high-risk HCMV strains exhibit a direct oncogenic effect that is characterized by enhanced stemness, epithelial-to-mesenchymal transition, cellular stress, and polyploid giant cancer cells (PGCCs), an effect that is eventually conducive to aggressive cancer phenotypes [53]. It is important to recognize that EMT has been suggested as the primary cause of a loss of cell-to-cell adhesion, disrupted cellular polarity, and reconfiguration of the cytoskeleton [54], thereby playing a crucial role in tumor progression and serving as a key target of interest in anti-cancer therapy [55]. Cytomegalovirus might serve as a causative agent in GBM through ARG2 upregulation [56] and STAT3 signaling [9], which is often used as an early tumor biomarker. The chemokine receptor US28 binds and triggers a proliferative response that can promote tumorigenesis [57]. CMV proteins and DNA have been identified in breast ductal carcinoma in situ, as well as infiltrating ductal carcinoma tissues [58]. Finally, a meta-analysis by Richardson and colleagues [59], along with their clinical data, did not establish a definitive etiological link between CMV and breast cancer. However, it did suggest several possibilities: (a) ‘hit and run’ oncogenesis may give rise to varying results, (b) CMV, EBV, or both may contribute to later stages of breast cancer development, (c) limitations in the molecular tests may have influenced the discoveries, (d) co-infection with multiple viruses might increase the peril of breast cancer, or (e) neither pathogen contributes.
All the evidence mentioned here, when taken together, hints at CMV being involved in oncomodulation and, possibly, in frank tumorigenesis for at least certain histologic types of breast cancer [60], although its role cannot be categorically stated as such at this time.
4.2. Arguments in Favor of CMV Oncoprevention
Although the literature has, for the most part, supported a potentially tumorigenic effect of CMV, recent years have seen rising evidence of an oncoprotective faculty that the virus might bestow upon its host [13,18,19].
In our investigation, we have described a significant and inverse correlation between the age-standardized incidence of breast cancer and CMV prevalence across 73 countries worldwide. Intriguingly, the data indicate that breast cancer incidences tend to become significantly lower as CMV pervasiveness grows higher. This association hints at a potential oncoprotective effect that CMV infection may proffer against this malignancy. While it is crucial to emphasize that correlation does not imply causation, this association is supported by various other research findings. While evidence for an anti-tumor effect of CMV in breast cancer was hitherto virtually non-existent, it was noted elsewhere that the virus may inhibit the migration of specific breast cancer cells [18].
This study builds upon our previous research on B-cell cancers, wherein we have also demonstrated the same inverse correlation occurring between these malignant dyscrasia and CMV infection [13]. A similar result was observed in a murine CMV experimental model, wherein the virus had an adverse impact on the advancement of B-cell lymphoma [61].
Çuburu et al. illustrated the potential for redirecting the T cell immune response specific to CMV toward tumor cells, consequently impacting tumor regression [19]. Some other studies have described the immune-mediated oncoprotective effect of the virus using antibodies. These effects have also been observed elsewhere, and those surveys indicate that identifying CMV gB as a TGF-β/Smad signaling inhibitor may be beneficial in developing effective therapeutic strategies using anti-TGF-β agents to promote tumor suppression [18].
There is other evidence that points to an oncopreventive faculty of CMV. The virus has shown an anti-leukemia effect in patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT); in this setting, viral reactivation was linked to a noticeable reduction in the risk of disease relapse [20]. The mitigation of risk for disease relapse by CMV reactivation was also observed in the case of non-Hodgkin lymphomas [21], acute myeloid leukemia [22], and pediatric acute leukemia in the wake of HSCT [23]. Viral reactivation after HSCT was associated with a minor decrease in early relapse risk in a group with myeloproliferative disorders [62]. Moreover, the status of CMV did not exhibit a correlation with the risk of developing most cancers in the recipients of solid organ transplants in another study [63].
Cytomegalovirus seropositivity may be influenced by a number of factors: age [64,65,66], sex [64,67,68], childcare practices [65], varied cultural norms or practices associated with breastfeeding [65], level of education [69], number of sexual partners [69], SES [69,70,71], and current smoking [69], to list only some of them. Ethnic or racial background is also linked to SES [72,73]. Certain elements among these examples could be regarded as confounding variables in this examination. However, smoking can be disregarded as it is a pro-oncogenic practice. Also, upon exploring the role of HDI (used as an alternative for SES) as a confounding variable in multiple linear regression analysis, it was noted that CMV continued to be a robust predictor for a decreased occurrence of the examined malignancies, demonstrating favorable characteristics in the overall MLR model. Recently, we have also demonstrated an inverse correlation between a number of tumors, including lung cancer, and CMV seroprevalence, hinting at the potential oncoprevention that CMV infection might impart against malignancies of the lungs [74]. This should be interpreted cautiously, however, as there are many types of lung cancer, and analysis has not been performed on specific tumors per se. Additionally, correlation should not be interpreted unequivocally as causation.
An interesting and very recent finding emerged in the clinical studies published by Rashid et al. [14] and Nagel et al. [15], where anti-tumor effects that were attributed to viral infection were described in persons with bronchogenic and colorectal carcinoma, respectively. Although not a de facto argument in favor of oncoprotection, it should also be noted that a number of research articles mention a notable lack of CMV DNA in a variety of tumors [75,76].
Finally, other preliminary results would seem to suggest that the potential oncopreventive effect of CMV seems to hold true across a spectrum of races/ethnicities on a global scale (Preprint [77]). Namely, the inverse relationship between cancer incidence and CMV seropositivity, both globally and in the U.S. specifically, suggests that CMV may play a protective role against tumorigenesis. This phenomenon maintained a consistent pattern across various racial and ethnic groups within the United States and was observed across a diverse variety of neoplasia. This preliminary research supports CMV’s potential in anti-cancer vaccinology and highlights its ability to inhibit tumor development across diverse populations and cancer types.
All argumentation in favor of a potential CMV oncopreventive faculty may be seen in Table 4.

Table 4.
Observations of the potential CMV oncopreventive effect against various tumors.
5. Conclusions
A continuous debate surrounds the precise impact of CMV on tumor tissue, with arguments ranging from direct oncogenesis to oncomodulation and oncoprevention. However, the temporal aspect must also be considered, as there is a possibility that the virus exerts a tumorigenic effect at a specific age while providing protection at another. This aspect remains to be thoroughly investigated. Nevertheless, it is indisputable that a connection between CMV and tumors exists, as demonstrated in many studies. Our investigation, along with a very small number of others, has established a link between the anti-cancer effect and breast malignancies. However, this serological evidence requires validation through comprehensive molecular studies, including CMV detection in tumor tissues, and we are still a considerable distance away from fully understanding the true role of CMV in oncogenesis.
Author Contributions
Study concept and design: M.J., S.G., A.K. and I.L.; methodology: M.J. and A.T.; data collection: D.K., I.C. and S.U.; analysis and interpretation of results: A.T., D.M., A.B. and D.K.; draft manuscript preparation: M.J., S.G., A.K., J.C., D.M. and A.B.: writing—review and editing: A.T., I.C., S.U. and I.L.; M.J. and S.G. have contributed equally in their respective roles as first listed authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ministry of Science, Technological Development, and Innovation of the Republic of Serbia, project no. 451-03-66/2024-03/200110. The funder did not play a role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript, and the decision to submit this manuscript for publication.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the use of publicly available, de-identified data from online repositories, with no direct involvement of human participants.
Informed Consent Statement
Patient consent was waived due to the use of publicly available, de-identified data from online repositories, with no direct involvement of human participants.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We express our gratitude to the personnel at the IARC and the WHO (GLOBOCAN) for making publicly accessible data available, which was crucial for our analyses.
Conflicts of Interest
The authors declare that they have no conflicts of interest to report regarding the present study.
References
- Subfamily: Betaherpesvirinae. International Committee on Taxonomy of Viruses. 2024. Available online: https://ictv.global/report/chapter/orthoherpesviridae/orthoherpesviridae/cytomegalovirus (accessed on 16 March 2024).
- Zuhair, M.; Smit, G.S.A.; Wallis, G.; Jabbar, F.; Smith, C.; Devleesschauwer, B.; Griffiths, P. Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta-analysis. Rev. Med. Virol. 2019, 29, e2034. [Google Scholar] [CrossRef]
- Fowler, K.; Mucha, J.; Neumann, M.; Lewandowski, W.; Kaczanowska, M.; Grys, M.; Schmidt, E.; Natenshon, A.; Talarico, C.; Buck, P.O.; et al. A systematic literature review of the global seroprevalence of cytomegalovirus: Possible implications for treatment, screening, and vaccine development. BMC Public Health 2022, 22, 1659. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, M.; Doerr, H.W.; Cinatl, J., Jr. Oncomodulation by human cytomegalovirus: Evidence becomes stronger. Med. Microbiol. Immunol. 2009, 198, 79–81. [Google Scholar] [CrossRef]
- Cinatl, J., Jr.; Vogel, J.-U.; Kotchetkov, R.; Doerr, H. Oncomodulatory signals by regulatory proteins encoded by human cytomegalovirus: A novel role for viral infection in tumor progression. FEMS Microbiol. Rev. 2004, 28, 59–77. [Google Scholar] [CrossRef] [PubMed]
- Herbein, G. The Human Cytomegalovirus, from Oncomodulation to Oncogenesis. Viruses 2018, 10, 408. [Google Scholar] [CrossRef]
- Taher, C.; de Boniface, J.; Mohammad, A.-A.; Religa, P.; Hartman, J.; Yaiw, K.-C.; Frisell, J.; Rahbar, A.; Söderberg-Naucler, C. High prevalence of human cytomegalovirus proteins and nucleic acids in primary breast cancer and metastatic sentinel lymph nodes. PLoS ONE 2013, 8, e56795. [Google Scholar] [CrossRef] [PubMed]
- Cox, B.; Richardson, A.; Graham, P.; Gislefoss, R.E.; Jellum, E.; Rollag, H. Breast cancer, cytomegalovirus and Epstein–Barr virus: A nested case–control study. Br. J. Cancer 2010, 102, 1665–1669. [Google Scholar] [CrossRef][Green Version]
- Cobbs, C.S. Cytomegalovirus and brain tumor: Epidemiology, biology and therapeutic aspects. Curr. Opin. Oncol. 2013, 25, 682–688. [Google Scholar] [CrossRef]
- Barami, K. Oncomodulatory mechanisms of human cytomegalovirus in gliomas. J. Clin. Neurosci. 2010, 17, 819–823. [Google Scholar] [CrossRef]
- Pasquereau, S.; Al Moussawi, F.; Karam, W.; Assaf, M.D.; Kumar, A.; Herbein, G. Cytomegalovirus, Macrophages and Breast Cancer. Open Virol. J. 2017, 11, 15–27. [Google Scholar] [CrossRef]
- Alsamarai, A.; Abdulla, S.S.; Aljumaili, Z. Epstein- Bar Virus and Cytomegalovirus Infection Association with Breast Cancer: EBV and CMV. Int. J. Med. Sci. 2022, 4, 8–36. Available online: https://isnra.net/index.php/ijms/article/view/357 (accessed on 17 February 2024).
- Janković, M.; Knežević, A.; Todorović, M.; Đunić, I.; Mihaljević, B.; Soldatović, I.; Protić, J.; Miković, N.; Stoiljković, V.; Jovanović, T. Cytomegalovirus infection may be oncoprotective against neoplasms of B-lymphocyte lineage: Single-institution experience and survey of global evidence. Virol. J. 2022, 19, 155. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Ardeljan, A.; Frankel, L.R.; Cardeiro, M.; Kim, E.; Nagel, B.M.; Takabe, K.; Rashid, O. Human Cytomegalovirus (CMV) Infection Associated with Decreased Risk of Bronchogenic Carcinoma: Understanding How a Previous CMV Infection Leads to an Enhanced Immune Response Against Malignancy. Cureus 2023, 15, e37265. [Google Scholar] [CrossRef]
- Nagel, B.; Frankel, L.; Ardeljan, A.; Cardeiro, M.; Rashid, S.; Takabe, K.; Rashid, O.M. The Association of Human Cytomegalovirus Infection and Colorectal Cancer: A Clinical Analysis. World J. Oncol. 2023, 14, 119–124. [Google Scholar] [CrossRef]
- Britsch, I.; van Wijngaarden, A.P.; Helfrich, W. Applications of Anti-Cytomegalovirus T Cells for Cancer (Immuno)Therapy. Cancers 2023, 15, 3767. [Google Scholar] [CrossRef]
- Luo, X.-H.; Meng, Q.; Liu, Z.; Paraschoudi, G. Generation of high-affinity CMV-specific T cells for adoptive immunotherapy using IL-2, IL-15, and IL-21. Clin. Immunol. 2020, 217, 108456. [Google Scholar] [CrossRef]
- Yang, R.; Liang, J.; Xu, G.X.; Ding, L.M.; Huang, H.M.; Su, Q.Z.; Yan, J.; Li, Y.C. Human cytomegalovirus glycoprotein B inhibits migration of breast cancer MDA-MB-231 cells and impairs TGF-β/Smad2/3 expression. Oncol. Lett. 2018, 15, 7730–7738. [Google Scholar] [CrossRef]
- Çuburu, N.; Bialkowski, L.; Pontejo, S.M.; Sethi, S.K.; Bell, A.T.F.; Kim, R.; Thompson, C.D.; Lowy, D.R.; Schiller, J.T. Harnessing anti-cytomegalovirus immunity for local immunotherapy against solid tumors. Proc. Natl. Acad. Sci. USA 2022, 119, e2116738119. [Google Scholar] [CrossRef] [PubMed]
- Bigley, A.B.; Baker, F.L.; Simpson, R.J. Cytomegalovirus: An unlikely ally in the fight against blood cancers? Clin. Exp. Immunol. 2018, 193, 265–274. [Google Scholar] [CrossRef]
- Koldehoff, M.; Ross, S.R.; Dührsen, U.; Beelen, D.W.; Elmaagacli, A.H. Early CMV-replication after allogeneic stem cell transplantation is associated with a reduced relapse risk in lymphoma. Leuk. Lymphoma 2016, 58, 822–833. [Google Scholar] [CrossRef]
- Elmaagacli, A.H.; Steckel, N.K.; Koldehoff, M.; Hegerfeldt, Y.; Trenschel, R.; Ditschkowski, M.; Christoph, S.; Gromke, T.; Kordelas, L.; Ottinger, H.D.; et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: Evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood 2011, 118, 1402–1412. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, J.; Noguchi, M.; Kurauchi, K.; Tanioka, S.; Fukano, R.; Okamura, J. Effect of Cytomegalovirus Reactivation on Relapse after Allogeneic Hematopoietic Stem Cell Transplantation in Pediatric Acute Leukemia. Biol. Blood Marrow Transplant. 2016, 22, 300–306. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). GLOBOCAN 2020: Global Cancer Observatory; World Health Organization: Geneva, Switzerland, 2020; Available online: https://gco.iarc.fr/ (accessed on 27 October 2022).
- Herbein, G.; Nehme, Z. Tumor Control by Cytomegalovirus: A Door Open for Oncolytic Virotherapy? Mol. Ther.-Oncolyt. 2020, 17, 1–8. [Google Scholar] [CrossRef]
- Geder, L.; Sanford, E.J.; Rohner, T.J.; Rapp, F. Cytomegalovirus and cancer of the prostate: In vitro transformation of human cells. Cancer Treat. Rep. 1977, 61, 139–146. [Google Scholar]
- Herbein, G. Tumors and Cytomegalovirus: An Intimate Interplay. Viruses 2022, 14, 812. [Google Scholar] [CrossRef]
- Oseguera, C.A.V.; Spencer, J.V. cmvIL-10 stimulates the invasive potential of MDA-MB-231 breast cancer cells. PLoS ONE 2014, 9, e88708. [Google Scholar] [CrossRef]
- Söderberg-Nauclér, C. New mechanistic insights of the pathogenicity of high-risk cytomegalovirus (CMV) strains derived from breast cancer: Hope for new cancer therapy options. EBioMedicine 2022, 81, 104103. [Google Scholar] [CrossRef]
- Nakhaie, M.; Charostad, J.; Azaran, A.; Arabzadeh, S.A.M.; Motamedfar, A.; Iranparast, S.; Ahmadpour, F.; Talaeizadeh, A.; Makvandi, M. Molecular and Serological Prevalence of HCMV in Iranian Patients with Breast Cancer. Asian Pac. J. Cancer Prev. 2021, 22, 2011–2016. [Google Scholar] [CrossRef] [PubMed]
- Nauclér, C.S.; Geisler, J.; Vetvik, K. The emerging role of human cytomegalovirus infection in human carcinogenesis: A review of current evidence and potential therapeutic implications. Oncotarget 2019, 10, 4333–4347. [Google Scholar] [CrossRef]
- Herbein, G.; Kumar, A. The oncogenic potential of human cytomegalovirus and breast cancer. Front. Oncol. 2014, 4, 230. [Google Scholar] [CrossRef]
- Yang, Z.; Tang, X.; Hasing, M.E.; Pang, X.; Ghosh, S.; McMullen, T.P.W.; Brindley, D.N.; Hemmings, D.G. Human Cytomegalovirus Seropositivity and Viral DNA in Breast Tumors Are Associated with Poor Patient Prognosis. Cancers 2022, 14, 1148. [Google Scholar] [CrossRef]
- Khasawneh, A.I.; Himsawi, N.; Sammour, A.; Al Shboul, S.; Alorjani, M.; Al-Momani, H.; Shahin, U.; Al-Momani, H.; Alotaibi, M.R.; Saleh, T. Association of Human Papilloma Virus, Cytomegalovirus, and Epstein–Barr Virus with Breast Cancer in Jordanian Women. Medicina 2024, 60, 699. [Google Scholar] [CrossRef] [PubMed]
- Touma, J.; Pantalone, M.R.; Rahbar, A.; Liu, Y.; Vetvik, K.; Sauer, T.; Söderberg-Naucler, C.; Geisler, J. Human Cytomegalovirus Protein Expression Is Correlated with Shorter Overall Survival in Breast Cancer Patients: A Cohort Study. Viruses 2023, 15, 732. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Wang, Q.; Wang, H.-B.; Wang, B.; Li, L. Protein and DNA evidences of HCMV infection in primary breast cancer tissues and metastatic sentinel lymph nodes. Cancer Biomark. 2018, 21, 769–780. [Google Scholar] [CrossRef]
- Geisler, J.; Touma, J.; Rahbar, A.; Söderberg-Nauclér, C.; Vetvik, K. A Review of the Potential Role of Human Cytomegalovirus (HCMV) Infections in Breast Cancer Carcinogenesis and Abnormal Immunity. Cancers 2019, 11, 1842. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Belcher, J.D.; Marker, P.H.; Wilcken, D.E.; Vercellotti, G.M.; Wang, X.L. Cytomegalovirus inhibits p53 nuclear localization signal function. J. Mol. Med. 2000, 78, 642–647. [Google Scholar] [CrossRef]
- Spencer, J.V.; Liu, J.; Deyarmin, B.; Hu, H.; Shriver, C.D.; Somiari, S. Cytokine levels in breast cancer are highly dependent on cytomegalovirus (CMV) status. Breast Cancer Res. Treat. 2024, 208, 631–641. [Google Scholar] [CrossRef]
- Blanco, R.; Muñoz, J.P. Human Cytomegalovirus Infection and Breast Cancer: A Literature Review of Clinical and Experimental Data. Biology 2025, 14, 174. [Google Scholar] [CrossRef]
- Oberstein, A.; Shenk, T. Cellular responses to human cytomegalovirus infection: Induction of a mesenchymal-to-epithelial transition (MET) phenotype. Proc. Natl. Acad. Sci. USA 2017, 114, E8244–E8253. [Google Scholar] [CrossRef]
- Pandey, J.P.; Namboodiri, A.M.; Mohan, S.; Nietert, P.J.; Peterson, L. Genetic markers of immunoglobulin G and immunity to cytomegalovirus in patients with breast cancer. Cell. Immunol. 2017, 312, 67–70. [Google Scholar] [CrossRef]
- El Baba, R.; Pasquereau, S.; Ahmad, S.H.; Diab-Assaf, M.; Herbein, G. Oncogenic and Stemness Signatures of the High-Risk HCMV Strains in Breast Cancer Progression. Cancers 2022, 14, 4271. [Google Scholar] [CrossRef] [PubMed]
- Herbein, G. High-Risk Oncogenic Human Cytomegalovirus. Viruses 2022, 14, 2462. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.H.; Pasquereau, S.; El Baba, R.; Nehme, Z.; Lewandowski, C.; Herbein, G. Distinct Oncogenic Transcriptomes in Human Mammary Epithelial Cells Infected with Cytomegalovirus. Front. Immunol. 2021, 12, 772160. [Google Scholar] [CrossRef]
- Oseguera, C.A.V.; Spencer, J.V. Human cytomegalovirus interleukin-10 enhances matrigel invasion of MDA-MB-231 breast cancer cells. Cancer Cell Int. 2017, 17, 24. [Google Scholar] [CrossRef]
- Bishop, R.K.; Oseguera, C.A.V.; Spencer, J. Human Cytomegalovirus interleukin-10 promotes proliferation and migration of MCF-7 breast cancer cells. Cancer Cell Microenviron. 2015, 2, e678. [Google Scholar] [CrossRef]
- Kumar, A.; Tripathy, M.K.; Pasquereau, S.; Al Moussawi, F.; Abbas, W.; Coquard, L.; Khan, K.A.; Russo, L.; Algros, M.-P.; Valmary-Degano, S.; et al. The Human Cytomegalovirus Strain DB Activates Oncogenic Pathways in Mammary Epithelial Cells. EBioMedicine 2018, 30, 167–183. [Google Scholar] [CrossRef]
- Moussawi, F.A.; Kumar, A.; Pasquereau, S.; Tripathy, M.K.; Karam, W.; Diab-Assaf, M.; Herbein, G. The transcriptome of human mammary epithelial cells infected with the HCMV-DB strain displays oncogenic traits. Sci. Rep. 2018, 8, 12574. [Google Scholar] [CrossRef]
- Yurochko, A.D.; Kowalik, T.F.; Huong, S.M.; Huang, E.S. Human cytomegalovirus upregulates NF-kappa B activity by transactivating the NF-kappa B p105/p50 and p65 promoters. J. Virol. 1995, 69, 5391–5400. [Google Scholar] [CrossRef]
- Mitchell, D.A.; Xie, W.; Schmittling, R.; Learn, C.; Friedman, A.; McLendon, R.E.; Sampson, J.H. Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro-Oncology 2008, 10, 10–18. [Google Scholar] [CrossRef]
- Cobbs, C.S. Cytomegalovirus is a tumor-associated virus: Armed and dangerous. Curr. Opin. Virol. 2019, 39, 49–59. [Google Scholar] [CrossRef]
- Haidar Ahmad, S.; El Baba, R.; Herbein, G. Polyploid giant cancer cells, cytokines and cytomegalovirus in breast cancer progression. Cancer Cell Int. 2023, 23, 119. [Google Scholar] [CrossRef] [PubMed]
- Roche, J. The Epithelial-to-Mesenchymal Transition in Cancer. Cancers 2018, 10, 52, Erratum in Cancers 2018, 10, 79. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marcucci, F.; Stassi, G.; De Maria, R. Epithelial–mesenchymal transition: A new target in anticancer drug discovery. Nat. Rev. Drug Discov. 2016, 15, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Costa, H.; Xu, X.; Overbeek, G.; Vasaikar, S.; Patro, C.P.K.; Kostopoulou, O.N.; Jung, M.; Shafi, G.; Ananthaseshan, S.; Tsipras, G.; et al. Human cytomegalovirus may promote tumour progression by upregulating arginase-2. Oncotarget 2016, 7, 47221–47231. [Google Scholar] [CrossRef]
- Maussang, D.; Verzijl, D.; van Walsum, M.; Leurs, R.; Holl, J.; Pleskoff, O.; Michel, D.; van Dongen, G.A.; Smit, M.J. Human cytomegalovirus-encoded chemokine receptor US28 promotes tumorigenesis. Proc. Natl. Acad. Sci. USA 2006, 103, 13068–13073. [Google Scholar] [CrossRef]
- Branch, K.M.; Garcia, E.C.; Chen, Y.M.; McGregor, M.; Min, M.; Prosser, R.; Whitney, N.; Spencer, J.V. Productive Infection of Human Breast Cancer Cell Lines with Human Cytomegalovirus (HCMV). Pathogens 2021, 10, 641. [Google Scholar] [CrossRef]
- Richardson, A.K.; Currie, M.J.; Robinson, B.A.; Morrin, H.; Phung, Y.; Pearson, J.F.; Anderson, T.P.; Potter, J.D.; Walker, L.C. Cytomegalovirus and Epstein-Barr virus in breast cancer. PLoS ONE 2015, 10, e0118989. [Google Scholar] [CrossRef]
- Richardson, A.K.; Walker, L.C.; Cox, B.; Rollag, H.; Robinson, B.A.; Morrin, H.; Pearson, J.F.; Potter, J.D.; Paterson, M.; Surcel, H.-M.; et al. Breast cancer and cytomegalovirus. Clin. Transl. Oncol. 2019, 22, 585–602. [Google Scholar] [CrossRef]
- Erlach, K.C.; Podlech, J.; Rojan, A.; Reddehase, M.J. Tumor control in a model of bone marrow transplantation and acute liver-infiltrating B-cell lymphoma: An unpredicted novel function of cytomegalovirus. J. Virol. 2002, 76, 2857–2870. [Google Scholar] [CrossRef]
- Peric, Z.; Wilson, J.; Durakovic, N.; Ostojic, A.; Desnica, L.; Vranjes, V.R.; Marekovic, I.; Serventi-Seiwerth, R.; Vrhovac, R. Early human cytomegalovirus reactivation is associated with lower incidence of relapse of myeloproliferative disorders after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2018, 53, 1450–1456. [Google Scholar] [CrossRef]
- Geris, J.M.; Spector, L.G.; Pfeiffer, R.M.; Limaye, A.P.; Yu, K.J.; Engels, E.A. Cancer risk associated with cytomegalovirus infection among solid organ transplant recipients in the United States. Cancer 2022, 128, 3985–3994. [Google Scholar] [CrossRef] [PubMed]
- Hecker, M.; Qiu, D.; Marquardt, K.; Bein, G.; Hackstein, H. Continuous cytomegalovirus seroconversion in a large group of healthy blood donors. Vox Sang. 2004, 86, 41–44. [Google Scholar] [CrossRef]
- Staras, S.A.S.; Dollard, S.C.; Radford, K.W.; Flanders, W.D.; Pass, R.F.; Cannon, M.J. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin. Infect. Dis. 2006, 43, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Ahlfors, K. IgG antibodies to cytomegalovirus in a normal urban Swedish population. Scand. J. Infect. Dis. 1984, 16, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Varga, M.; Görög, D.; Kári, D.; Környei, E.; Kis, É.; Túryné, H.; Jankovics, I.; Péter, A.; Toronyi, É.; Sárváry, E.; et al. Cytomegalovirus seroprevalence among solid organ donors in Hungary: Correlations with age, gender, and blood group. Transplant. Proc. 2011, 43, 1233–1235. [Google Scholar] [CrossRef]
- Bate, S.L.; Dollard, S.C.; Cannon, M.J. Cytomegalovirus seroprevalence in the United States: The national health and nutrition examination surveys, 1988–2004. Clin. Infect. Dis. 2010, 50, 1439–1447. [Google Scholar] [CrossRef]
- Lachmann, R.; Loenenbach, A.; Waterboer, T.; Brenner, N.; Pawlita, M.; Michel, A.; Thamm, M.; Poethko-Müller, C.; Wichmann, O.; Wiese-Posselt, M. Cytomegalovirus (CMV) seroprevalence in the adult population of Germany. PLoS ONE 2018, 13, e0200267. [Google Scholar] [CrossRef]
- Cannon, M.J.; Schmid, D.S.; Hyde, T.B. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev. Med. Virol. 2010, 20, 202–213. [Google Scholar] [CrossRef]
- Marshall, G.S.; Rabalais, G.P.; Stewart, J.A.; Dobbins, J.G. Cytomegalovirus seroprevalence in women bearing children in Jefferson County, Kentucky. Am. J. Med. Sci. 1993, 305, 292–296. [Google Scholar] [CrossRef]
- Clarke, C.A.; Glaser, S.L.; Gomez, S.L.; Wang, S.S.; Keegan, T.H.; Yang, J.; Chang, E.T. Lymphoid malignancies in US Asians: Incidence rate differences by birthplace and acculturation. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1064–1077. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Wang, Z.; Yi, D.; Ma, S. Racial Differences in Three major NHL Subtypes: Descriptive epidemiology. Cancer Epidemiol. 2015, 39, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, M.; Knezevic, T.; Tomic, A.; Milicevic, O.; Jovanovic, T.; Djunic, I.; Mihaljevic, B.; Knezevic, A.; Todorovic-Balint, M. Human Cytomegalovirus Oncoprotection across Diverse Populations, Tumor Histologies, and Age Groups: The Relevance for Prospective Vaccinal Therapy. Int. J. Mol. Sci. 2024, 25, 3741. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.S.; Lee, J.J.; Cheng, S.P. No evidence of association between human cytomegalovirus infection and papillary thy-roid cancer. World J. Surg. Oncol. 2014, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, J.F.; van Hecke, W.; Jansen, M.K.; Spliet, W.G.; Broekhuizen, R.; Bovenschen, N. No evidence for human cytomegalovirus infection in pediatric medulloblastomas. Neuro-Oncology 2016, 18, 1461–1462. [Google Scholar] [CrossRef]
- Janković, M.; Milićević, O.; Todorović-Balint, M.; Đunić, I.; Mihaljević, B.; Jovanović, T.; Knežević, A. Cytomegalovirus seropositivity relates inversely to cancer incidences across races and ethnicities: Implications for oncoprevention. medRxiv 2023. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).