Abstract
Background: Hashimoto’s thyroiditis (HT) is characterized by the loss of tolerance to thyroid autoantigens [thyroid peroxidase (TPO) and thyroglobulin (Tg)], usually identifying circulating antibodies (Abs) against these thyroid autoantigens (TPOAb and/or TgAb), together with a significant lymphocytic infiltration, causing an increased risk of hypothyroidism. Among the multiple mechanisms described for the development of HT is the nutritional status of several micronutrients, including iodine. Iodine deficiency or excess is associated with thyroid function disorders and, likely, thyroid autoimmunity. Thus, iodized salt intake [especially through universal salt iodization (USI) programs] may be influencing the prevalence of HT. The objectives of this systematic review are to describe and analyze changes over time in the prevalence of HT following the implementation of USI programs. Methods and results: The following databases were consulted for articles published from January 1965 to January 2025: Pubmed/Medline; ProQuest; Scopus; Biosis; Web of Science; and Google Scholar. The search terms were as follows: “iodine”, “salt”, “intake”, “prevalence”, AND Hashimoto’s thyroiditis. Only English language articles were taken into account, and each of them was scrutinized according to the JBI Critical Appraisal Checklist. Only those studies in which the design, study population, number of participants, country, evaluation post-USI (years), and the prevalence of thyroid Abs positivity were described were included. In total, 74 studies were identified, of which 31 evaluated thyroid Abs values post-USI. Conclusions: Excess iodine intake, mediated by USI programs without an adequate follow-up and monitoring plan, may explain (at least in part) the prevalence and distribution of HT; therefore, it is a real challenge to establish a balance between healthy salt intake, USI program strategies, and possible functional outcomes and thyroid autoimmunity in the population. Registration number: INPLASY202540074.
1. Introduction
Autoimmune thyroid diseases (AITDs) are a group of diseases characterized by the breakdown of tolerance to thyroid autoantigens [primarily thyroid peroxidase (TPO), thyroglobulin (Tg), and the thyrotropin receptor (TR)]. This induces both humoral and cellular autoimmune responses, ranging from the presence of circulating antibodies (Abs) to thyroid autoantigens (TPOAb, TgAb, and TRAb) and lymphocytic infiltration [1,2].
The two extremes of the clinical presentation of AITDs are hyperthyroidism [Graves–Basedow disease (GBD)] and hypothyroidism [Hashimoto’s thyroiditis (HT)]. HT is the leading cause of hypothyroidism in iodine-sufficient areas, with its widely variable prevalence being dependent on multiple factors. The estimated global prevalence is between 5 and 10%, with areas showing prevalences as high as >20% and as low as <0.5%. It is significantly higher in women than in men [3,4].
Numerous nutritional, genetic, environmental, and socioeconomic factors, inter alia, can affect the global prevalence of HT. Among nutritional factors, multiple studies have suggested that micronutrient deficiencies (especially those relating to iodine, selenium, zinc, iron, vitamin D, and vitamin B12, among others) are associated with increased thyroid Ab positivity.
Likewise, iodine is a micronutrient that is not only involved in thyroid function, but also in the pathogenesis of AITDs [5,6]. Iodine, as a component of thyroid hormones, represents 65% and 58% of the weight of thyroxine (T4) and triiodothyronine (T3), respectively; therefore, iodine status is directly associated with thyroid function, expressing a “U”-shaped relationship between iodine intake and the risk of thyroid diseases, e.g., an insufficient intake is associated with goiter, hypothyroidism, cretinism, adverse obstetric outcomes, and growth and intellectual development disorders, inter alia (iodine deficiency disorders—IDDs) [6,7,8].
In the presence of excessive iodine intake or exposure, hypothyroidism or hyperthyroidism may occur (when the intrinsic regulatory mechanisms of protection against excess iodine fail). Furthermore, sustained exposure to excess iodine has also been linked to multiple other outcomes; however, studies have not been able to reproduce these outcomes, including diabetes, hypertension, cardiovascular mortality, and papillary thyroid carcinoma, among others [6,7,8,9,10].
IDDs arise when iodine intake falls below the recommended levels. This term refers to each of the consequences of iodine deficiency in a given population that can be prevented, as long as an adequate intake of iodine is ensured, and remain a persistent public health problem worldwide, since they have a high clinical, social, and economic impact. It has been established that the most cost-effective strategy for their eradication and elimination is through the implementation of universal salt iodization (USI) programs [8,11,12].
This strategy has considerably reduced the health effects of IDDs in all countries where it has been implemented; however, the lack of monitoring and implementation of such programs has led to excess iodine consumption from salt (and other iodine-fortified foods) or a resurgence of IDDs in some geographic areas [11,12,13,14].
However, some studies have found that the prevalence of HT has increased in recent decades. It has been suggested that the lack of monitoring and control of USI (together with the increase in the consumption of iodized salt) are partially responsible for this discovery. On the contrary, other studies have shown that its prevalence has decreased slightly [10,13,14].
The objectives of this systematic review are to describe and analyze the change in the prevalence of HT, through the positivity of thyroid Abs (TPOAb and/or TgAb), in those areas where USI programs have been implemented.
2. Materials and Methods
This systematic review is part of a research project that determines and analyzes the distribution, associated factors, and behavior of AITDs in Colombia. This project was developed in accordance with the principles of the Declaration of Helsinki and has been approved by the Research Ethics Committee of the University of Cauca, Colombia (ID: 4656, January 2018). However, due to the nature of this study, Institutional Review Board approval was not required.
2.1. Literature Search and Selection Criteria
Using modified versions of the Population, Interventions, Comparators, and Outcomes (PICO) framework, we formulated the research question and selected the eligibility criteria for the systematic review (Table 1).

Table 1.
Inclusion criteria adopted in the systematic review.
Subsequently, a structured literature search was carried out in PubMed/Medline, ProQuest, Scopus, Biosis, Web of Science, and Google Scholar for articles published from January 1965 to January 2025 (human trials, clinical trials, meta-analyses, reviews, scoping reviews, and systematic reviews). The search criteria were as follows: Iodine [Title/Abstract] OR Salt [Title/Abstract] OR Intake [Title/Abstract] OR Prevalence [Title/Abstract] AND Hashimoto’s Disease [Title/Abstract] OR Hashimoto’s Thyroiditis [Title/Abstract] OR Chronic Lymphocytic Thyroiditis [Title/Abstract] OR Autoimmune thyroiditis [Title/Abstract].
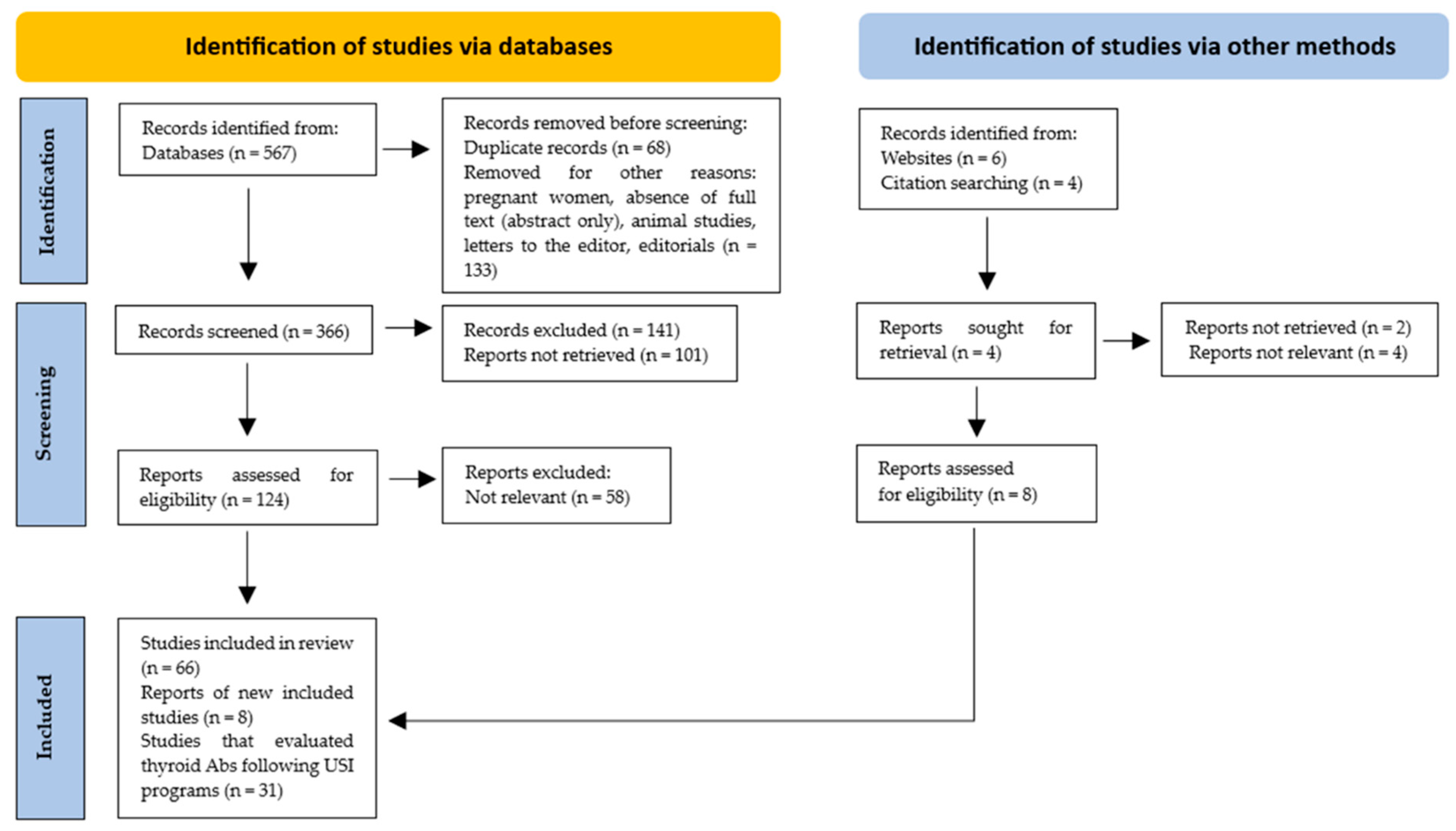
Only those studies in which the design, study population, number of participants, country and evaluation post-USI (years), and the prevalence of thyroid Ab (TPOAb and/or TgAb) positivity were described were included. In total, 74 studies were included, of which 31 evaluated thyroid Ab values after USI programs (Figure 1).
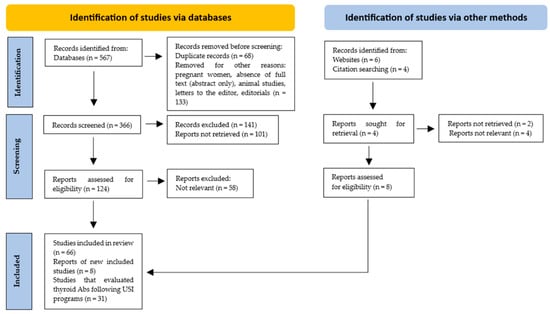
Figure 1.
PRISMA flow diagram. Method for the selection of articles.
2.2. Data Extraction
The titles and abstracts of all studies were independently reviewed by three investigators (H.V.-U., I.A.M.-C., and M.V.P.-F.) using the Rayyan web tool (this further helped reduce selection bias). Full texts for the studies that met the initial inclusion criteria were obtained and reviewed, and the data were extracted using a standardized template using a predefined data form created in Excel. In cases where discrepancies arose in the extracted data, the investigators collaboratively conducted a second round of extraction to validate the accuracy of the information. Each article was scrutinized according to the JBI Critical Appraisal Checklist. Letters, commentaries, preprints, letters to the editor, and non-peer-reviewed articles, as well as conference abstracts, were also excluded. Only articles written in English were considered (Table 2).

Table 2.
Inclusion and exclusion criteria for studies in the systematic review.
2.3. Data Analysis
The following data were collected: design, study population, number of participants, country, post-USI evaluation (years), and prevalence of TPOAb and/or TgAb positivity (95% CI). No statistical analysis or meta-analysis was performed due to the high heterogeneity observed among the studies included in this review. However, we developed a descriptive analysis to summarize and synthesize the most important characteristics of the selected studies (choosing to adopt a narrative approach).
This systematic review was registered on the “International Platform of Registered Systematic Review and Meta-Analysis Protocols INPLASY” (Registration number: INPLASY202540074; DOI: 10.37766/inplasy2025.4.0074) and followed the “Reporting Checklist for Systematic Reviews Based on the PRISMA guidelines” (Table S1).
3. Results
3.1. Global Prevalence of HT
A total of 25 countries were found where the prevalence of HT had been assessed [Australia, Bosnia and Herzegovina, Brazil, China, Colombia, Croatia, Denmark, Finland, England (United Kingdom), Germany, Ghana, Iran, Italy, Japan, Jordan, South Korea, Mexico, Nigeria, Norway, Poland, Russia, Spain, Sri Lanka, Tunisia, and the USA] [3,4,15] (Table 3).

Table 3.
Countries where prevalence studies on Hashimoto’s thyroiditis have been conducted refs. [3,4,15,16,17].
In Europe, prevalence rates as low as 0.42% and 0.43% have been reported in Russia and Bosnia and Herzegovina, respectively, and very high prevalence rates have been reported in Italy (35.5%) and Denmark (39.7%). Prevalence in Africa also varies, with the highest prevalence reported in Tunisia (22.8%) and the lowest in Nigeria (6.7%). In Oceania (Australia), prevalence ranges from 8.5% to 13%. In Asia, prevalence rates as low as 0.1% in South Korea and 0.3% in China have been reported. However, prevalence rates as high as 16.1% have also been reported in China, as well as in Japan (18%) and Jordan (15.1%). In the Americas, prevalences as low as 0.4% and 0.1% are found in the USA and Brazil, respectively, and prevalences as high as 22.4% and 22.3% have been reported in the USA and Colombia, respectively, which denotes the wide variability in the prevalence of HT depending on the geographic area studied.
3.2. Global Iodine Population Status
In a 2003 analysis, the WHO estimated that the Americas and Western Pacific regions had the lowest proportions of the population with insufficient iodine intake (9.8% and 24%, respectively). In the other regions, the figures were 56.9% (Europe), 54.1% (Eastern Mediterranean), 42.6% (Africa), and 39.8% (Southeast Asia). Additionally, it was found that between 1990 and 2003, the proportion of households consuming iodized salt increased from 10% to 66%. Consequently, the number of countries with IDDs decreased from 110 to 54 [18,19].
In 2006, 15 countries had reached the target of eliminating IDDs, and in 2008, the prevalence of iodine deficiency was calculated (excluding Western European countries and the USA); the highest prevalence was found in Europe (52%), followed by the Eastern Mediterranean (47.2%) and Africa (41.5%), with the lowest prevalence in descending order being in Southeast Asia, the Western Pacific, and the Americas (30%, 21.2%, and 11%, respectively) [19,20].
Between 2004 and 2023, the number of countries with excessive iodine intake increased (from 5 countries in 2004 to 15 countries in 2023). In 2023, 26 countries were still identified as reporting insufficient iodine intake (in the general population based on school-age children) [21,22].
Very recently, it was found that over the past 30 years, the prevalence rate of iodine deficiency among adolescents has decreased significantly, from 3082.43 (95% CI: 2473.01 to 3855.86) per 100,000 of the population to 2190.84 (95% CI: 1729.18 to 2776.16) per 100,000 of the population. Globally, the iodine deficiency prevalence among adolescents and young adults shows an oscillating pattern, decreasing between 1990 and 2000, with a subsequent increase between 2000 and 2009, and a continued decrease between 2010 and 2019 [23].
3.3. Population Iodine Status in Countries Where the Prevalence of HT Has Been Assessed
In each of the 25 countries in which the prevalence of HT has been described (Table 2), the iodine status of the population has also been assessed. Studies have also been conducted to investigate countries via an assessment of their median urinary iodine level (mUIC). It was found that four countries (Finland, Germany, Norway, and Russia) had insufficient iodine intake (mUIC < 100 μg/L); two had an excessive intake (Colombia and South Korea, with mUIC values ≥ 300 μg/L); and nineteen countries reported an adequate intake (mUIC values between 100 and 299 μg/L). The vast majority of these studies were conducted in school-age children (Table 4).

Table 4.
Population iodine status in countries where Hashimoto’s thyroiditis prevalence studies have been conducted, adapted from refs. [8,13,21,24].
3.4. USI Programs, Population Iodine Status, and Thyroid Autoimmunity
A total of 31 studies (from 14 countries) have investigated the possible association between the implementation (mandatory) of USI programs and the prevalence of TPOAb and TgAb positivity. These studies evaluated the prevalence of TPOAb and TgAb positivity (at least during the first year from the time of implementation of the USI programs) (Table 5).

Table 5.
Studies that have evaluated changes in TPOAb and/or TgAb levels following the mandatory implementation of USI programs. Refs. [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46].
Following the implementation of the USI programs, the prevalence of TPOAb and/or TgAb positivity increased (or remained high) in eight countries (Sri Lanka, Greece, Denmark, Poland, Turkey, India, Italy, and Colombia); in four countries (Iran, Morocco, Brazil, and Germany), the prevalence decreased (or remained low); and in one country (Tasmania), the prevalence did not change (although the estimated baseline prevalence was high).
In China, the results were variable, with some areas where positivity increased and others where positivity decreased or remained unchanged over time [4,15].
4. Discussion
Although USI programs have significantly modified the prevalence of IDDs, the wide variability in relation to the prevalence of HT and other thyroid dysfunctions remains, both in areas where IDDs persist and in those with iodine excess [57].
In those areas that are iodine deficient, USI is the best strategy for the prevention and control of IDDs, since salt for household consumption is an ingredient that is used globally and in relatively constant quantities throughout the year. Additionally, the process of salt iodization is simple and efficient, which is why most countries decided to adopt USI programs (on a mandatory basis) [57,58].
However, the indicators used to monitor these programs are not always carried out permanently or systematically, which may be the reason for (at least in part) the global distribution of disorders associated with iodine intake [40,44].
Although iodine-induced hyperthyroidism and hypothyroidism can occur in people with normal thyroid function, both conditions are more frequently identified in individuals with HT, GBD, autonomous thyroid nodular disease, or in those who are under treatment with medications, such as amiodarone, lithium, and interferon-alpha, as well as in areas previously identified with long-term iodine deficiency (and which are exposed to a sudden ingestion of iodine) although such dysfunction can occur regardless of underlying thyroid autoimmunity [8,10,59].
Despite the existing association between excess iodine and the risk of hyperthyroidism or hypothyroidism, its relationship with the increase in the prevalence of TgAb and/or TPOAb positivity is less known, especially when referring to USI programs.
The studies described in Table 4 showed (in the short- to medium-term) a significant change in the increase in thyroid autoimmunity (determined by the presence of TPOAb and/or TgAb) in those areas with prolonged iodine deficiency (and which implemented USI programs); however, studies that have carried out longer-term follow-ups suggest that antibody positivity stabilizes or may even decrease. In fact, some studies have shown an increase in the prevalence of thyroid Abs before completing the first 10 years following the implementation of the USI programs. Meanwhile, other studies have found that when followed for a longer period (>15 years), the prevalence of thyroid Abs tends to decrease, reaching a frequency similar to that which existed before the USI programs were implemented [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,60].
Countries, such as China, have variable results in relation to the frequency of TPOAb and/or TgAb positivity depending on time. For instance, since the implementation of the USI program (more than 20 years ago), the population has been exposed to multiple iodine exposures, ranging from excessive intake (between 1996 and 2001) to more-than-adequate intake (between 2002 and 2011), and finally to adequate intake (between 2012 and 2016). Consequently, different geographic areas have intriguingly shown that the prevalence of TPOAb and/or TgAb positivity has been different following the implementation of USI programs [61].
On the other hand, in other countries, like the USA, salt fortification with iodine is voluntary (not mandatory), and the FDA does not require the iodine content to be listed on food packaging [62]. However, GBD and HT are the fourth and fifth most common autoimmune diseases in the American population, at an estimated rate (per 100,000) of 512.6 and 373.7 for GBD and HT, respectively (the median urinary iodine level in the USA is 190 µg/L) [63].
Given the distribution of HT frequency (in countries where USI programs have been established), it could be hypothesized that the prevalence of TPOAb and/or TgAb positivity (as biochemical markers of HT) varies over time depending on how USI programs are implemented, structured, maintained, and systematically evaluated.
This hypothesis can be supported by the following:
- The role of iodine in the induction of thyroid autoimmunity is robustly supported in animal models, e.g., in non-obese diabetic (NOD) mice, after the administration of 0.05% NaI in regular drinking water, the incidence of autoimmune thyroiditis increases to almost 100%. With the observation of chronic inflammation in the short term (3–4 weeks), along with the increase in TgAb synthesis (at the beginning) and then in TPOAb, concomitantly, the secretion of proinflammatory cytokines and cellular infiltration [mediated by T lymphocytes (TLs), B lymphocytes (BLs), and antigen-presenting cells, inter alia] are stimulated. Consequently, greater antigenicity towards Tg and TPO is produced. The depletion of regulatory TL (TReg) and the increased activity of autoreactive TL increases and amplifies the magnitude of the immune response, inducing the loss of immune tolerance and an increased risk of AITDs [2,64].
- In humans, there is a clear “U”-shaped relationship between iodine intake and the risk of thyroid dysfunction, whereby the body’s response to iodine deficiency is usually gradual, while in relation to excess iodine, the response can be acute or also gradual, depending on the duration and magnitude of exposure. Although acute exposure to a high iodine intake is well tolerated, it can eventually result in the presence of iodine-induced hyperthyroidism (the Jod–Basedow phenomenon) or hypothyroidism (when there is a failure to escape the Wolff–Chaikoff effect) [10,20,59].
- However, unlike animal models of autoimmune thyroiditis, it is actually unclear whether the immunological alterations (in patients with HT) are due to a direct effect of the iodine on the cells of the immune system (effector cells) or if, on the contrary, it is the reflection of a secondary response to the cytotoxic and/or metabolic effects on the thyroid follicular cells. In fact, sudden exposure to high amounts of iodine (in clinical scenarios where there is a known deficiency) has been associated with significant damage to thyroid tissue (mediated by the presence of free radicals) [1,2,59,65].
- In this regard, it has been proposed that an excess of iodine induces a greater expression of critical epitopes in Tg, increasing the probability of stimulating an autoimmune response towards the thyroid, which explains (at least in part) the increase in the prevalence of TgAb in areas where there is an excess of iodine or in those areas where USI programs have been implemented and which were exposed to a long-term iodine deficiency. In fact, it is suggested that the more severe the IDDs, the greater the incidence of thyroid autoimmunity after iodine supplementation. This concept gains more credibility if one takes into account that Tg is an autoantigen that is capable of presenting post-translational modifications as a consequence of the supply of iodine, exposing epitopes that were previously hidden [6,10,66,67].
- Additionally, excess iodine can have a direct impact on immune system cells that are capable of initiating and propagating the autoimmune response to the thyroid; thus, there is an increase in lymphocyte infiltration into the thyroid tissue, with a higher expression of MHC class II in thyrocytes, and an increase in the synthesis and secretion of cytokines and thyroid Abs [7,10,68].
USI Programs, Thyroid Function, and Thyroid Autoimmunity
The results of the studies described in this review suggest that the implementation of USI programs (as an intervention strategy for IDDs) should be systematically evaluated and monitored regularly, because in those scenarios where such evaluations and monitoring are not systematically performed, the probability of IDD relapses and/or population iodine excess increases (with a higher risk of thyroid autoimmunity, which is established by an increase in the prevalence of TPOAb and/or TgAb positivity).
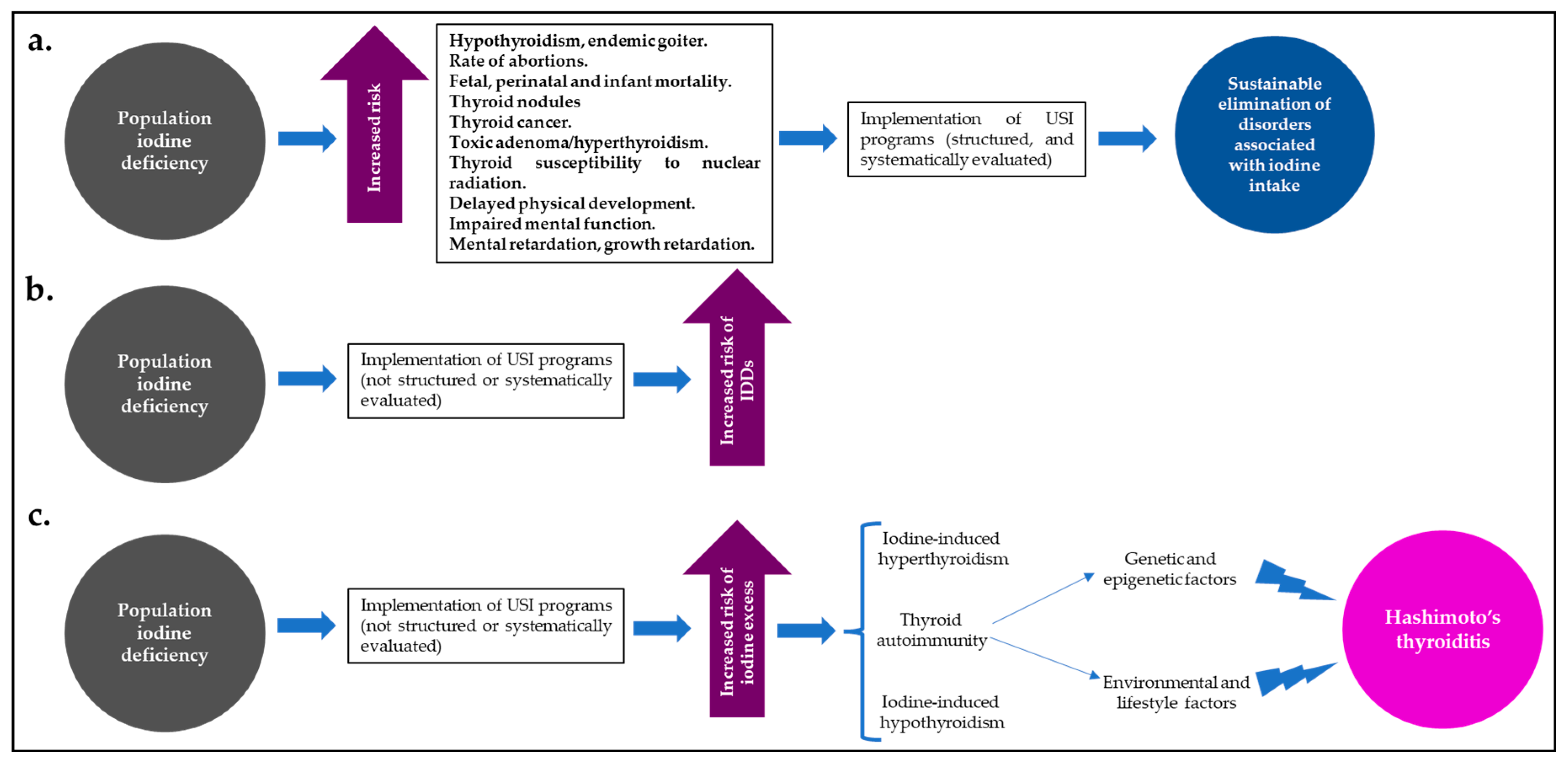
Despite the effects of iodine excess on thyroid function and the risk of HT, USI programs have widely demonstrated that they are the best (and most cost-effective) strategy for reducing IDDs and their global burden; however, for the implementation of these programs to be successful and to maintain the balance between a population state of “iodine sufficiency” and a low risk of IDDs or disorders associated with iodine excess and thyroid autoimmunity, they must be supported not only by close long-term supervision and monitoring, but also by the will of government entities committed to this public health problem [40,69,70] (Figure 2).
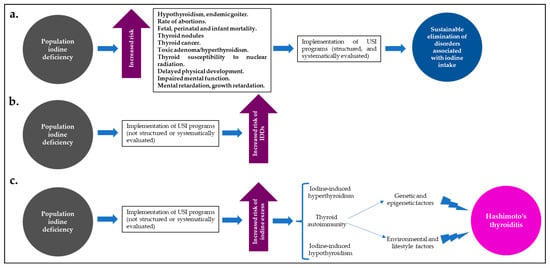
Figure 2.
In iodine-deficient populations, well-structured and systematically evaluated USI programs (a) lead to a significant and sustained reduction in disorders associated with iodine intake. However, those programs that have not been well-structured or systematically evaluated may increase the risk of IDDs (b) or the risk of population iodine excess (c), with a greater likelihood of iodine-induced hyperthyroidism or hypothyroidism, or thyroid autoimmunity (HT). Abbreviations: HT: Hashimoto’s thyroiditis; USI: universal salt iodization.
Therefore, the strategies, action plans, and various legislation of USI programs should be reviewed (and the amount of iodine in salt for human consumption should be adjusted, if necessary).
Additionally, nationally representative surveys (and sentinel studies) should be conducted periodically, addressing potential factors that may affect the normal development of USI programs, e.g., supply chain management systems, poor salt iodization practices at the population level, political will, government support, monitoring systems, continuing medical education, and public awareness of this health issue [40,57,69,70].
Furthermore, education for the population must be planned very carefully, in the sense that the consumption of iodized salt must be promoted (but in small quantities), with the aim of achieving a balance between the health benefits of consuming iodized salt and the potential risk of functional disorders, thyroid autoimmunity, high blood pressure, and the increased risk of cardiovascular outcomes, mortality, cancer, chronic kidney disease, and osteoporosis, among other undesirable outcomes [70,71,72,73].
Despite the biological plausibility between iodine excess and the presence of thyroid autoimmunity, data related to USI programs and HT are mostly incomplete, and records on baseline thyroid function and TPOAb/TgAb positivity status are not always available before USI programs are implemented (making it difficult to establish a cause–effect relationship).
It should also be noted that AITDs (and in this case, HT) are complex diseases, with multiple causes (genetic, epigenetic, environmental, and lifestyle) and triggers; therefore, iodine status would only contribute part of the total predisposing factors for its development [2,6,8,10,74]. Therefore, excess iodine (e.g., as a result of improperly implemented USI programs) can be considered a trigger for HT.
Additionally, it should be noted that a significant number of the reviewed studies focused on younger populations (schoolchildren and adolescents); therefore, and due to the fact that iodine requirements vary with age and due to different studies that have shown that AITD is more prevalent during the development of secondary sex characteristics and at puberty, these factors could have influenced the positivity rates of thyroid Abs in this population group [75,76].
5. Strengths and Weaknesses
This systematic review demonstrated some strengths, e.g., the comprehensive scoping review and the sensitive and robust collection of relevant data and studies from six major databases (PubMed/Medline, ProQuest, Scopus, Biosis, Web of Science, and Google Scholar), providing a broad overview of the topic.
However, several weaknesses can also be identified, e.g., the language limitation in our study selection, as well as the fact that studies evaluating other outcomes of interest (such as hypothyroidism, hyperthyroidism, goiter, and thyroid nodular disease, inter alia) were not considered. Furthermore, the methods and ways in which the diagnosis of HT was made were not the same in the studies evaluated.
Moreover, it is important to highlight that the prevalence of thyroid Ab positivity may also have changed due to the increased sensitivity and specificity of the laboratory kits used in the last two decades (in addition to other possible factors, such as overdiagnosis, changes in dietary patterns, seasonality, and diagnostic criteria and disease definition, inter alia) [15].
Therefore, the results described should only be extrapolated to populations with similar characteristics and clinical settings to those of the studies developed.
6. Future Implications
Long-term, multicenter, observational studies are needed in different populations and in different clinical settings, assessing not only the prevalence of thyroid Abs, but also thyroid function, goiter frequency (and glandular imaging features), and the population’s status in terms of other micronutrients (e.g., selenium, vitamin D, zinc, and iron, inter alia) and other possible factors (genetic, epigenetic, environmental, and lifestyle). Such studies are needed in order to establish the role that iodine (after the implementation of USI programs) may have on thyroid function and the risk of AITDs. Also, other sources of iodine from both food and non-food sources should be taken into account.
7. Conclusions
Excess iodine, mediated by poorly designed USI programs or those lacking adequate follow-up and monitoring strategies, could explain (at least in part) the prevalence and distribution of AITDs, specifically HT. Therefore, it is necessary not only to implement these programs but also to monitor them continuously to maintain low rates of IDDs and excess iodine in the population. Only a balance and collaboration between the policies and initiatives of the USI programs, together with well-designed educational campaigns and recommendations focused on reducing salt consumption, will allow for the maintenance of a low rate (or the eradication) of IDDs and a low risk of HT.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diseases13060166/s1, Table S1: PRISMA 2020 Main Checklist and PRISMA Abstract Checklist.
Author Contributions
Conceptualization, H.V.-U., I.A.M.-C., M.V.P.-F., K.U.-N. and H.V.-S.; methodology, H.V.-U., M.V.P.-F. and I.A.M.-C.; investigation, H.V.-U., I.A.M.-C., A.C.-P. and M.V.P.-F.; resources, H.V.-U., I.A.M.-C., K.U.-N. and M.V.P.-F.; data curation, H.V.-U. and I.A.M.-C., K.U.-N., H.V.-S., A.C.-P. and M.V.P.-F.; writing—original draft preparation, H.V.-U., I.A.M.-C., K.U.-N., A.C.-P., H.V.-S. and M.V.P.-F.; writing—review and editing, H.V.-U., A.C.-P., I.A.M.-C. and H.V.-S.; visualization, M.V.P.-F., K.U.-N., I.A.M.-C. and H.V.-S.; supervision, H.V.-U.; project administration, H.V.-U. and M.V.P.-F.; funding acquisition, H.V.-U., A.C.-P. and M.V.P.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This study received funding from the Colombian Association of Endocrinology, Diabetes, and Metabolism (003/26 February 2025).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pyzik, A.; Grywalska, E.; Matyjaszek-Matuszek, B.; Roliński, J. Immune disorders in Hashimoto’s thyroiditis: What do we know so far? J. Immunl. Res. 2015, 2015, 979167. [Google Scholar] [CrossRef] [PubMed]
- Vargas–Uricoechea, H. Molecular Mechanisms in Autoimmune Thyroid Disease. Cells 2023, 12, 918. [Google Scholar] [CrossRef]
- Taylor, P.N.; Albrecht, D.; Scholz, A.; Gutierrez–Buey, G.; Lazarus, J.H.; Dayan, C.M.; Okosieme, O.E. Global epidemiology of hyperthyroidism and hypothyroidism. Nat. Rev. Endocrinol. 2018, 14, 301–316. [Google Scholar] [CrossRef]
- Hu, X.; Chen, Y.; Shen, Y.; Tian, R.; Sheng, Y.; Que, H. Global prevalence and epidemiological trends of Hashimoto’s thyroiditis in adults: A systematic review and meta–analysis. Front. Public Health 2022, 10, 1020709. [Google Scholar] [CrossRef]
- Shulhai, A.M.; Rotondo, R.; Petraroli, M.; Patianna, V.; Predieri, B.; Iughetti, L.; Esposito, S.; Street, M.E. The Role of Nutrition on Thyroid Function. Nutrients 2024, 16, 2496. [Google Scholar] [CrossRef]
- Zhou, Q.; Xue, S.; Zhang, L.; Chen, G. Trace elements and the thyroid. Front. Endocrinol. 2022, 13, 904889. [Google Scholar] [CrossRef]
- Sorrenti, S.; Baldini, E.; Pironi, D.; Lauro, A.; D’Orazi, V.; Tartaglia, F.; Tripodi, D.; Lori, E.; Gagliardi, F.; Praticò, M.; et al. Iodine: Its Role in Thyroid Hormone Biosynthesis and Beyond. Nutrients 2021, 13, 4469. [Google Scholar] [CrossRef]
- Bath, S.C. Thyroid function and iodine intake: Global recommendations and relevant dietary trends. Nat. Rev. Endocrinol. 2024, 20, 474–486. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, F.; Li, Q.; Feng, C.; Teng, W. Iodine nutrition and papillary thyroid cancer. Front. Nutr. 2022, 9, 1022650. [Google Scholar] [CrossRef]
- Sohn, S.Y.; Inoue, K.; Rhee, C.M.; Leung, A.M. Risks of Iodine Excess. Endocr. Rev. 2024, 45, 858–879. [Google Scholar] [CrossRef]
- Lisco, G.; De Tullio, A.; Triggiani, D.; Zupo, R.; Giagulli, V.A.; De Pergola, G.; Piazzolla, G.; Guastamacchia, E.; Sabbà, C.; Triggiani, V. Iodine Deficiency and Iodine Prophylaxis: An Overview and Update. Nutrients 2023, 15, 1004. [Google Scholar] [CrossRef] [PubMed]
- Bouga, M.; Lean, M.E.J.; Combet, E. Contemporary challenges to iodine status and nutrition: The role of foods, dietary recommendations, fortification and supplementation. Proc. Nutr. Soc. 2018, 77, 302–313. [Google Scholar] [CrossRef]
- Hatch–McChesney, A.; Lieberman, H.R. Iodine and Iodine Deficiency: A Comprehensive Review of a Re–Emerging Issue. Nutrients 2022, 14, 3474. [Google Scholar] [CrossRef]
- Rigutto–Farebrother, J.; Zimmermann, M.B. Salt Reduction and Iodine Fortification Policies Are Compatible: Perspectives for Public Health Advocacy. Nutrients 2024, 16, 2517. [Google Scholar] [CrossRef]
- Vargas–Uricoechea, H.; Castellanos–Pinedo, A.; Urrego Noguera, K.; Pinzón–Fernández, M.V.; Meza–Cabrera, I.A.; Vargas–Sierra, H. A Scoping Review on the Prevalence of Hashimoto’s Thyroiditis and the Possible Associated Factors. Med. Sci. 2025, 13, 43. [Google Scholar]
- McLeod, D.S.; Cooper, D.S. The incidence and prevalence of thyroid autoimmunity. Endocrine 2012, 42, 252–265. [Google Scholar] [CrossRef]
- Merrill, S.J.; Mu, Y. Thyroid autoimmunity as a window to autoimmunity: An explanation for sex differences in the prevalence of thyroid autoimmunity. J. Theor. Biol. 2015, 375, 95–100. [Google Scholar] [CrossRef]
- Andersson, M.; de Benoist, B.; Rogers, L. Epidemiology of iodine deficiency: Salt iodisation and iodine status. Best Pr. Res. Clin. Endocrinol. Metab. 2010, 24, 1–11. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Andersson, M. Update on iodine status worldwide. Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 382–387. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Boelaert, K. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol. 2015, 3, 286–295. [Google Scholar] [CrossRef]
- The Iodine Global Network. Global Scorecard of Iodine Nutrition in 2022 in the General Population Based on School–Age Children (SAC); IGN: Ottawa, ON, Canada, 2022. [Google Scholar]
- Berger, M.M.; Amrein, K.; Barazzoni, R.; Bindels, L.; Bretón, I.; Calder, P.C.; Cappa, S.; Cuerda, C.; D’Amelio, P.; de Man, A.; et al. The science of micronutrients in clinical practice—Report on the ESPEN symposium. Clin. Nutr. 2024, 43, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; Wang, C.; Yang, W.; Shan, Z. Changing trends in the global, regional, and national burden of iodine deficiency among adolescents and young adults: Population–based study. Eur. J. Pediatr. 2024, 183, 2855–2863. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Andersson, M. GLOBAL ENDOCRINOLOGY: Global perspectives in endocrinology: Coverage of iodized salt programs and iodine status in 2020. Eur. J. Endocrinol. 2021, 185, R13–R21. [Google Scholar] [CrossRef]
- Heydarian, P.; Ordookhani, A.; Azizi, F. Goiter rate, serum thyrotropin, thyroid autoantibodies and urinary iodine concentration in Tehranian adults before and after national salt iodization. J. Endocrinol. Investig. 2007, 30, 404–410. [Google Scholar] [CrossRef]
- Gołkowski, F.; Buziak–Bereza, M.; Trofimiuk, M.; Bałdys–Waligórska, A.; Szybiński, Z.; Huszno, B. Increased prevalence of hyperthyroidism as an early and transient side–effect of implementing iodine prophylaxis. Public Health Nutr. 2007, 10, 799–802. [Google Scholar] [CrossRef]
- Li, Y.; Teng, D.; Shan, Z.; Teng, X.; Guan, H.; Yu, X.; Fan, C.; Chong, W.; Yang, F.; Dai, H.; et al. Antithyroperoxidase and antithyroglobulin antibodies in a five–year follow–up survey of populations with different iodine intakes. J. Clin. Endocrinol. Metab. 2008, 93, 1751–1757. [Google Scholar] [CrossRef]
- Aminorroaya, A.; Amini, M.; Hovsepian, S. Prevalence of goitre in Isfahan, Iran, fifteen years after initiation of universal salt iodization. J. Health Popul. Nutr. 2010, 28, 351–358. [Google Scholar] [CrossRef]
- Pedersen, I.B.; Knudsen, N.; Carlé, A.; Vejbjerg, P.; Jorgensen, T.; Perrild, H.; Ovesen, L.; Rasmussen, L.B.; Laurberg, P. A cautious iodization program bringing iodine intake to a low recommended level is associated with an increase in the prevalence of thyroid autoantibodies in the population. Clin. Endocrinol. 2011, 75, 120–126. [Google Scholar] [CrossRef]
- Marwaha, R.K.; Tandon, N.; Garg, M.K.; Desai, A.; Kanwar, R.; Sastry, A.; Narang, A.; Arora, S.; Bhadra, K. Thyroid status two decades after salt iodization: Country–wide data in school children from India. Clin. Endocrinol. 2012, 76, 905–910. [Google Scholar] [CrossRef]
- Marwaha, R.K.; Tandon, N.; Ganie, M.A.; Kanwar, R.; Sastry, A.; Garg, M.K.; Bhadra, K.; Singh, S. Status of thyroid function in Indian adults: Two decades after universal salt iodization. J. Assoc. Physicians India 2012, 60, 32–36. [Google Scholar]
- Fernando, R.F.; Chandrasinghe, P.C.; Pathmeswaran, A.A. The prevalence of autoimmune thyroiditis after universal salt iodisation in Sri Lanka. Ceylon Med. J. 2012, 57, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Aghini Lombardi, F.; Fiore, E.; Tonacchera, M.; Antonangeli, M.; Rago, T.; Frigeri, M.; Provenzale, A.M.; Montanelli, L.; Grasso, L.; Pinchera, A.; et al. The effect of voluntary iodine prophylaxis in a small rural community: The Pescopagano survey 15 years later. J. Clin. Endocrinol. Metab. 2013, 98, 1031–1039. [Google Scholar] [CrossRef]
- Miranda, D.M.; Massom, J.N.; Catarino, R.M.; Santos, R.T.; Toyoda, S.S.; Marone, M.M.; Tomimori, E.K.; Monte, O. Impact of nutritional iodine optimization on rates of thyroid hypoechogenicity and autoimmune thyroiditis: A cross–sectional, comparative study. Thyroid 2015, 25, 118–124. [Google Scholar] [CrossRef]
- Shan, Z.; Chen, L.; Lian, X.; Liu, C.; Shi, B.; Shi, L.; Tong, N.; Wang, S.; Weng, J.; Zhao, J.; et al. Iodine status and prevalence of thyroid disorders after introduction of mandatory universal salt iodization for 16 years in China: A cross–sectional study in 10 Cities. Thyroid 2016, 26, 1125–1130. [Google Scholar] [CrossRef]
- Khattak, R.M.; Ittermann, T.; Nauck, M.; Below, H.; Völzke, H. Monitoring the prevalence of thyroid disorders in the adult population of Northeast Germany. Popul. Health Metr. 2016, 14, 39. [Google Scholar] [CrossRef]
- Hong, A.; Stokes, B.; Otahal, P.; Owens, D.; Burgess, J.R. Temporal trends in thyroid–stimulating hormone (TSH) and thyroid peroxidase antibody (ATPO) testing across two phases of iodine fortification in Tasmania (1995–2013). Clin. Endocrinol. 2017, 87, 386–393. [Google Scholar] [CrossRef]
- Bonofiglio, D.; Catalano, S.; Perri, A.; Santoro, M.; Siciliano, L.; Lofaro, D.; Gallo, M.; Marsico, S.; Bruno, R.; Giordano, C.; et al. Monitoring the effects of iodine prophylaxis in the adult population of southern Italy with deficient and sufficient iodine intake levels: A cross–sectional, epidemiological study. Br. J. Nutr. 2017, 117, 170–175. [Google Scholar] [CrossRef][Green Version]
- Chen, X.; Chen, L.; Lian, X.; Liu, C.; Shan, Z.; Shi, B.; Shi, L.; Tong, N.; Weng, J.; Zhao, J.; et al. Urinary Iodine Concentration Is Inversely Associated with Thyroglobulin Antibodies. Endocr. Pract. 2019, 25, 454–460. [Google Scholar] [CrossRef]
- Vargas–Uricoechea, H.; Pinzón–Fernández, M.V.; Bastidas–Sánchez, B.E.; Jojoa–Tobar, E.; Ramírez–Bejarano, L.E.; Murillo–Palacios, J. Iodine Status in the Colombian Population and the Impact of Universal Salt Iodization: A Double–Edged Sword? J. Nutr. Metab. 2019, 2019, 6239243. [Google Scholar] [CrossRef]
- Wan, S.; Qu, M.; Wu, H.; Ren, B.; Jiang, W.; Wang, X.; Liu, L.; Shen, H. Autoimmune thyroid diseases after 25 years of universal salt iodization: An epidemiological study of Chinese adults in areas with different water iodine levels. Br. J. Nutr. 2020, 124, 853–864. [Google Scholar] [CrossRef]
- Teng, D.; Yang, W.; Shi, X.; Li, Y.; Ba, J.; Chen, B.; Du, J.; He, L.; Lai, X.; Li, Y.; et al. An Inverse Relationship Between Iodine Intake and Thyroid Antibodies: A National Cross–Sectional Survey in Mainland China. Thyroid 2020, 30, 1656–1665. [Google Scholar] [CrossRef] [PubMed]
- Jayatissa, R.; Okosieme, O.E.; Ranasinghe, S.; Carter, J.L.; Gunatunga, I.P.; Lazarus, J.H.; Premawardhana, L.D. Thyroid Autoimmunity and Dysfunction in Sri Lankan Children and Adolescents After 22 Years of Sustained Universal Salt Iodization. Thyroid 2021, 31, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Vargas–Uricoechea, H.; Mera–Mamian, A.; Bastidas–Sanchez, B.; Pinzon–Fernandez, M.; Murillo–Palacios, J.; Ramirez–Bejarano, L. Population Status of Iodine and Its Potential Effects on Thyroid Function and Autoimmunity in Southwestern Colombia. J. Clin. Med. Res. 2022, 14, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Shi, X.; Teng, D.; Teng, X.; Teng, W.; Shan, Z. Thyroid Disorders, Iodine Status and Diabetes Epidemiological Survey Group. Prevalence and risk factors of hypothyroidism after universal salt iodisation: A large cross–sectional study from 31 provinces of China. BMJ Open 2023, 13, e064613. [Google Scholar] [CrossRef]
- Fan, X.; Zhao, L.; Wang, S.; Song, K.; Wang, B.; Xie, Y.; Jiang, Y.; Lin, L.; Teng, W.; Cai, C.; et al. Relation between iodine nutrition and thyroid diseases in Qinghai, China. Front. Endocrinol. 2023, 14, 1234482. [Google Scholar] [CrossRef]
- Premawardhana, L.D.K.E.; Parker, A.R.; Mazziotti, G.; Lazarus, J.H. Autoimmune thyroiditis after elimination of iodine deficiency in Sri Lanka. Thyroid 2003, 13, 1187–1188. [Google Scholar] [CrossRef]
- Azizi, F.; Navai, L.; Fattahi, F. Goiter prevalence, urinary iodine excretion, thyroid function and anti–thyroid function and anti–thyroid antibodies after 12 years of salt iodization in Shahriar, Iran. Int. J. Vitam. Nutr. Res. 2002, 72, 291–295. [Google Scholar] [CrossRef]
- Mazziotti, G.; Premawardhana, L.D.K.E.; Parkes, A.B.; Adams, H.; Smyth, P.P.A.; Smith, D.F.; Kaluarachi, W.N.; Wijeyaratne, C.N.; Jayasinghe, A.; de Silva, D.G.H.; et al. Evolution of thyroid autoimmunity during iodine prophylaxis. The Sri Lankan experience. Eur. J. Endocrinol. 2003, 149, 103–110. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Moretti, D.; Chaouki, N.; Torresani, T. Introduction of iodized salt to severely iodine–deficient children does not provoke thyroid autoimmunity: A one–year prospective trial in northern Morocco. Thyroid 2003, 13, 199–203. [Google Scholar] [CrossRef]
- Zois, C.; Stavrou, I.; Kalogera, C.; Svarna, E.; Dimoliatis, I.; Seferiadis, K.; Tsatsoulis, A. High prevalence of autoimmune thyroiditis in schoolchildren after elimination of iodine deficiency in northwestern Greece. Thyroid 2003, 13, 485–489. [Google Scholar] [CrossRef]
- Fountoulakis, S.; Philippou, G.; Tsatsoulis, A. The role of iodine in the evolution of thyroid disease in Greece: From endemic goiter to thyroid autoimmunity. Hormones 2007, 6, 25–35. [Google Scholar] [PubMed]
- Pedersen, I.B.; Knudsen, N.; Jørgensen, T.; Perrild, H.; Ovesen, L.; Laurberg, P. Thyroid peroxidase and thyroglobulin autoantibodies in a large survey of populations with mild and moderate iodine deficiency. Clin. Endocrinol. 2003, 58, 36–42. [Google Scholar] [CrossRef]
- Laurberg, P.; Jorgensen, T.; Perrild, H.; Ovesen, L.; Knudsen, N.; Pedersen, I.B.; Rasmussen, L.B.; Carle, A.; Vejbjerg, P. The Danish investigation on iodine intake and thyroid disease, DanThyr: Status and perspectives. Eur. J. Endocrinol. 2006, 155, 219–228. [Google Scholar] [CrossRef]
- Teng, W.; Shan, Z.; Teng, X.; Guan, H.; Li, Y.; Teng, D.; Jin, Y.; Yu, X.; Fan, C.; Chong, W.; et al. Effect of iodine intake on thyroid diseases in China. N. Engl. J. Med. 2006, 354, 2783–2793. [Google Scholar] [CrossRef]
- Luo, Y.; Kawashima, A.; Ishido, Y.; Yoshihara, A.; Oda, K.; Hiroi, N.; Ito, T.; Ishii, N.; Suzuki, K. Iodine excess as an environmental risk factor for autoimmune thyroid disease. Int. J. Mol. Sci. 2014, 15, 12895–12912. [Google Scholar] [CrossRef]
- World Health Organization/International Council for the Control of the Iodine Deficiency Disorders/United Nations Childrens Fund (WHO/ICCIDD/UNICEF). Assessment of the Iodine Deficiency Disorders and Monitoring Their Elimination; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Eastman, C.J.; Zimmermann, M.B. The Iodine Deficiency Disorders. [Updated on 6 February 2018]. In Endotext [Internet]; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK285556/ (accessed on 19 January 2025).
- Koukkou, E.G.; Roupas, N.D.; Markou, K.B. Effect of excess iodine intake on thyroid on human health. Minerva Med. 2017, 108, 136–146. [Google Scholar] [CrossRef]
- Atapattu, N.; Jayatissa, R.; de Silva, H.; Adlan, M.A.; Obuobie, E.K.; Premawardhana, L.D. Thyroid Autoimmunity During Universal Salt Iodisation–Possible Short–Term Modulation with Longer–Term Stability. Nutrients 2024, 16, 4299. [Google Scholar] [CrossRef]
- Gong, B.; Wang, X.; Wang, C.; Yang, W.; Shan, Z.; Lai, Y. Iodine–induced thyroid dysfunction: A scientometric study and visualization analysis. Front. Endocrinol. 2023, 14, 1239038. [Google Scholar] [CrossRef]
- Available online: https://www.ars.usda.gov/ARSUSERFILES/80400535/DATA/IODINE/IODINE%20DATABASE_RELEASE_3_DOCUMENTATION.PDF (accessed on 12 March 2025).
- Abend, A.H.; He, I.; Bahroos, N.; Christianakis, S.; Crew, A.B.; Wise, L.M.; Lipori, G.P.; He, X.; Murphy, S.N.; Herrick, C.D.; et al. Estimation of prevalence of autoimmune diseases in the United States using electronic health record data. J. Clin. Investig. 2024, 135, e178722. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, P.; Zhou, L.; Han, L.; Zhao, L.; Yu, X. Research progress in the construction of animal models of autoimmune thyroiditis. Autoimmunity 2024, 57, 2317190. [Google Scholar] [CrossRef]
- Kochman, J.; Jakubczyk, K.; Bargiel, P.; Janda–Milczarek, K. The Influence of Oxidative Stress on Thyroid Diseases. Antioxidants 2021, 10, 1442. [Google Scholar] [CrossRef] [PubMed]
- Bílek, R.; Čeřovská, J.; Zamrazil, V. The relationship between iodine intake and serum thyroglobulin in the general population. Physiol Res. 2015, 64, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Bílek, R.; Dvořáková, M.; Grimmichová, T.; Jiskra, J. Iodine, thyroglobulin and thyroid gland. Physiol. Res. 2020, 69 (Suppl. S2), S225–S236. [Google Scholar] [CrossRef]
- Kalarani, I.B.; Veerabathiran, R. Impact of iodine intake on the pathogenesis of autoimmune thyroid disease in children and adults. Ann. Pediatr. Endocrinol. Metab. 2022, 27, 256–264. [Google Scholar] [CrossRef]
- Gorstein, J.L.; Bagriansky, J.; Pearce, E.N.; Kupka, R.; Zimmermann, M.B. Estimating the Health and Economic Benefits of Universal Salt Iodization Programs to Correct Iodine Deficiency Disorders. Thyroid 2020, 30, 1802–1809. [Google Scholar] [CrossRef]
- Mente, A.; O’Donnell, M.; Yusuf, S. Sodium Intake and Health: What Should We Recommend Based on the Current Evidence? Nutrients 2021, 13, 3232. [Google Scholar] [CrossRef]
- Zhang, X.; Ye, W.Q.; Xin, X.K.; Gao, Y.J.; Yang, F. Global, regional, and national burden of stroke attributable to diet high in sodium from 1990 to 2019: A systematic analysis from the global burden of disease study 2019. Front. Neurol. 2024, 15, 1437633. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, L.; Yin, W.; Wang, J.; Zuo, X. Global, regional, and national burden of chronic kidney disease attributable to high sodium intake from 1990 to 2019. Front. Nutr. 2023, 10, 1078371. [Google Scholar] [CrossRef]
- Aljuraiban, G.S.; Jose, A.P.; Gupta, P.; Shridhar, K.; Prabhakaran, D. Sodium intake, health implications, and the role of population–level strategies. Nutr. Rev. 2021, 79, 351–359. [Google Scholar] [CrossRef]
- Wrońska, K.; Hałasa, M.; Szczuko, M. The Role of the Immune System in the Course of Hashimoto’s Thyroiditis: The Current State of Knowledge. Int. J. Mol. Sci. 2024, 25, 6883. [Google Scholar] [CrossRef]
- Loviselli, A.; Velluzzi, F.; Mossa, P.; Cambosu, M.A.; Secci, G.; Atzeni, F.; Taberlet, A.; Balestrieri, A.; Martino, E.; Grasso, L.; et al. Sardinian Schoolchildren Study Group. The Sardinian Autoimmunity Study: 3. Studies on circulating antithyroid antibodies in Sardinian schoolchildren: Relationship to goiter prevalence and thyroid function. Thyroid 2001, 11, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, G.; Gallizzi, R.; Aversa, T.; Salzano, G.; Valenzise, M.; Wasniewska, M.; De Luca, F.; Zirilli, G. Thyroid function test evolution in children with Hashimoto’s thyroiditis is closely conditioned by the biochemical picture at diagnosis. Ital. J. Pediatr. 2018, 44, 22. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).