A Vitiligo-like Cutaneous Reaction Induced by Ribociclib in Advanced Breast Cancer: An Unusual Case Report from Colombia
Abstract
1. Introduction
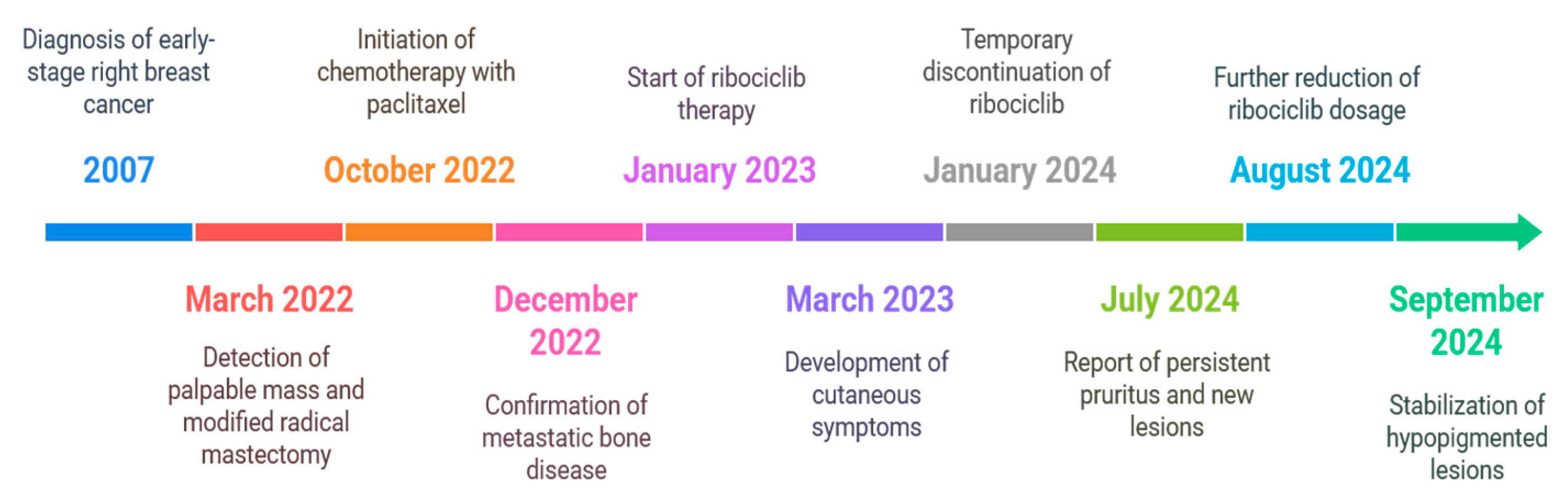
2. Case Presentation
2.1. Initial Diagnosis and Early Management
2.2. Recurrence and Progression
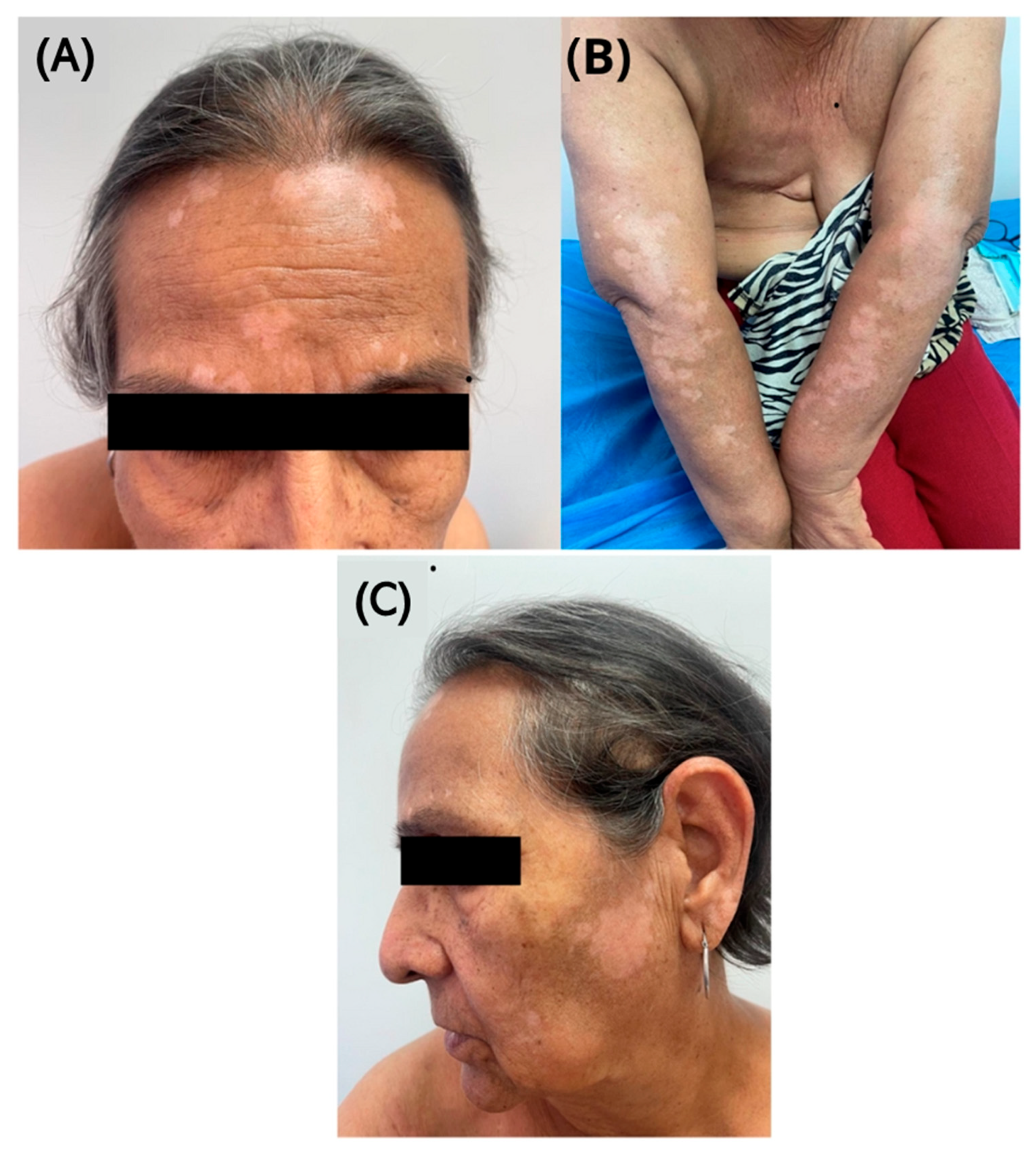
2.3. Development of Cutaneous Symptoms
2.4. Further Adjustments and Monitoring
2.5. Progression and Current Management
3. Discussion
3.1. Challenges in Managing HR+/HER2− Metastatic Breast Cancer
3.2. Dermatological Toxicities of CDK4/6 Inhibitors
3.3. Strengths of the Case Report
3.4. Review of Similar Cases in the Literature
3.5. Multidisciplinary Management Approach
3.6. Limitations and Future Research
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Speeckaert, R.; Van Geel, N. Vitiligo: An Update on Pathophysiology and Treatment Options. Am. J. Clin. Dermatol. 2017, 18, 733–744. [Google Scholar] [CrossRef]
- Grimes, P.E. New Insights and New Therapies in Vitiligo. JAMA 2005, 293, 730–735. [Google Scholar] [CrossRef]
- Premkumar, M.; Kalarani, I.B.; Mohammed, V.; Veerabathiran, R. An Extensive Review of Vitiligo-Associated Conditions. Int. J. Dermatol. Venereol. 2023, 7, 44–51. [Google Scholar] [CrossRef]
- Lotti, T.M.; Berti, S.; Hercogova, J.T.; Huggins, R.H.; Lee, B.W.; Janniger, C.K.; Schwartz, R.A. Vitiligo: Recent Insights and New Therapeutic Approaches. G. Ital. Di Dermatol. E Venereol. Organo Uff. Soc. Ital. Di Dermatol. E Sifilogr. 2012, 147, 637–647. [Google Scholar]
- Sollena, P.; Nikolaou, V.; Soupos, N.; Kotteas, E.; Voudouri, D.; Stratigos, A.J.; Fattore, D.; Annunziata, M.C.; Orlandi, A.; Di Nardo, L.; et al. Vitiligo-like Lesions in Patients with Advanced Breast Cancer Treated with Cycline-Dependent Kinases 4 and 6 Inhibitors. Breast Cancer Res. Treat. 2021, 185, 247–253. [Google Scholar] [CrossRef]
- Sollena, P.; Vasiliki, N.; Kotteas, E.; Stratigos, A.J.; Fattore, D.; Orlandi, A.; Mannino, M.; Di Pumpo, M.; Fida, M.; Starace, M.; et al. Cyclin-Dependent Kinase 4/6 Inhibitors and Dermatologic Adverse Events: Results from the EADV Task Force “Dermatology for Cancer Patients” International Study. Cancers 2023, 15, 3658. [Google Scholar] [CrossRef]
- Ma, J.; Chen, K.-H.; Luan, C.G. Cutaneous Adverse Events under New Clinical Therapies: Drug-Induced Vitiligo-like Depigmentation. Int. J. Dermatol. Venereol. 2023, 7, E01–E30. [Google Scholar] [CrossRef]
- Borroni, R.G.; Bartolini, M.; Gaudio, M.; Jacobs, F.; Benvenuti, C.; Gerosa, R.; Tiberio, P.; Manara, S.A.A.M.; Solferino, A.; Santoro, A.; et al. Ribociclib-Induced Cutaneous Adverse Events in Metastatic HR+/HER2− Breast Cancer: Incidence, Multidisciplinary Management, and Prognostic Implication. Oncol. 2024, 29, 484–492. [Google Scholar] [CrossRef]
- Pasqualoni, M.; Orlandi, A.; Palazzo, A.; Garufi, G.; Cannizzaro, M.C.; Pontolillo, L.; Pannunzio, S.; Cutigni, C.; Sollena, P.; Federico, F.; et al. Case Report: Vitiligo-like Toxicity Due to Ribociclib during First-Line Treatment of Metastatic Breast Cancer: Two Cases of Premature Interruption of Therapy and Exceptional Response. Front. Oncol. 2023, 13, 1067264. [Google Scholar] [CrossRef]
- Silvestri, M.; Cristaudo, A.; Morrone, A.; Messina, C.; Bennardo, L.; Nisticò, S.P.; Mariano, M.; Cameli, N. Emerging Skin Toxicities in Patients with Breast Cancer Treated with New Cyclin-Dependent Kinase 4/6 Inhibitors: A Systematic Review. Drug Saf. 2021, 44, 725–732. [Google Scholar] [CrossRef]
- Restrepo, J.C.; Martínez Guevara, D.; Pareja López, A.; Montenegro Palacios, J.F.; Liscano, Y. Identification and Application of Emerging Biomarkers in Treatment of Non-Small-Cell Lung Cancer: Systematic Review. Cancers 2024, 16, 2338. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, D.; Daza, J.; Liscano, Y. Coinfections and Superinfections Associated with COVID-19 in Colombia: A Narrative Review. Medicina 2023, 59, 1336. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, B.; AlMasri, R.; Abdel-Razeq, N.; Salama, O.; Hamad, I.; Abunasser, M.; Abdel-Razeq, H. Vitiligo-Like Lesions in a Patient with Metastatic Breast Cancer Treated with Cyclin-Dependent Kinase (CDK) 4/6 Inhibitor: A Case Report and Literature Review. Clin. Cosmet. Investig. Dermatol. 2022, 15, 5–10. [Google Scholar] [CrossRef]
- Türkel, A.; Karaçin, C.; Öner, İ.; Şeyran, E.; Öksüzoğlu, B. Vitiligo-like Lesions Associated with Ribociclib in a Woman with Metastatic Breast Cancer. J. Oncol. Pharm. Pract. 2023, 10781552231156521. [Google Scholar] [CrossRef]
- Sammons, S.L.; Topping, D.L.; Blackwell, K.L. HR+, HER2– Advanced Breast Cancer and CDK4/6 Inhibitors: Mode of Action, Clinical Activity, and Safety Profiles. Curr. Cancer Drug Targets 2017, 17. [Google Scholar] [CrossRef]
- Henning, S.W.; Jaishankar, D.; Barse, L.W.; Dellacecca, E.R.; Lancki, N.; Webb, K.; Janusek, L.; Mathews, H.L.; Price, R.N.; Le Poole, I.C. The Relationship between Stress and Vitiligo: Evaluating Perceived Stress and Electronic Medical Record Data. PLoS ONE 2020, 15, e0227909. [Google Scholar] [CrossRef] [PubMed]
- Anders, C.K.; LeBoeuf, N.R.; Bashoura, L.; Faiz, S.A.; Shariff, A.I.; Thomas, A. What’s the Price? Toxicities of Targeted Therapies in Breast Cancer Care. Am. Soc. Clin. Oncol. Educ. Book 2020, 55–70. [Google Scholar] [CrossRef]
- Silvestre Torner, N.; Aguilar Martínez, A.; Echarri González, M.J.; Tabbara Carrascosa, S.; Román Sainz, J.; Gruber Velasco, F. Ribociclib-Induced Vitiligo: A Case Report. Dermatol. Pract. Concept. 2022, e20220045. [Google Scholar] [CrossRef]
- Chawla, S.; Hill, A.; Fearfield, L.; Johnston, S.; Parton, M.; Heelan, K. Cutaneous Toxicities Occurring during Palbociclib (CDK4/6 Inhibitor) and Endocrine Therapy in Patients with Advanced Breast Cancer: A Single-Centre Experience. Breast Cancer Res. Treat. 2021, 188, 535–545. [Google Scholar] [CrossRef]
- Tripathy, D.; Bardia, A.; Sellers, W.R. Ribociclib (LEE011): Mechanism of Action and Clinical Impact of This Selective Cyclin-Dependent Kinase 4/6 Inhibitor in Various Solid Tumors. Clin. Cancer Res. 2017, 23, 3251–3262. [Google Scholar] [CrossRef]
- Morikawa, A.; Henry, N.L. Palbociclib for the Treatment of Estrogen Receptor–Positive, HER2-Negative Metastatic Breast Cancer. Clin. Cancer Res. 2015, 21, 3591–3596. [Google Scholar] [CrossRef] [PubMed]
- Alikhan, A.; Felsten, L.M.; Daly, M.; Petronic-Rosic, V. Vitiligo: A Comprehensive Overview. J. Am. Acad. Dermatol. 2011, 65. [Google Scholar] [CrossRef] [PubMed]
- Bang, A.S.; Fay, C.J.; LeBoeuf, N.R.; Etaee, F.; Leventhal, J.S.; Sibaud, V.; Arbesman, J.; Wang, J.Y.; Kwong, B.Y. Multi-Center Retrospective Review of Vitiligo-like Lesions in Breast Cancer Patients Treated with Cyclin-Dependent Kinase 4 and 6 Inhibitors. Breast Cancer Res. Treat. 2024, 204, 643–647. [Google Scholar] [CrossRef] [PubMed]

| Author | Age and Gender | Clinical Background | Clinical Manifestation | Drug Administered | Treatment |
|---|---|---|---|---|---|
| Sharaf et al. (2022) [13] | 71, female | HR+ HER2− breast cancer | Vitiligo, pruritus, alopecia | Aromatase inhibitor, ribociclib | Topical corticosteroids |
| Pasqualoni et al. (2023) [9] | 46, female | HR+ HER2− breast cancer | Erythematous lesions, hypopigmentation, pruritus | Aromatase inhibitor, ribociclib | Dose reduction, short steroid course |
| Türkel et al. (2023) [14] | 56, female | HR+ HER2− breast cancer with metastases | Vitiligo-like hypopigmentation | Aromatase inhibitor, ribociclib | Continued ribociclib, topical/oral corticosteroids |
| Pasqualoni et al. (2023) [9] | 80, female | HR+ HER2− breast cancer | Vesicular rashes, persistent hypopigmentation | Aromatase inhibitor, ribociclib | Therapy discontinuation |
| Nicolás Silvestre Torner et al. (España, 2022) [18] | 70, woman | Hormone receptor-positive, HER2-negative | Asymptomatic hypopigmented macules on face and neck; Wood lamp: bright white sharply delineated lesions | Ribociclib + letrozole | Dermatology referral; topical immunosuppressants + oral corticosteroids (partial response); avoid systemic immunosuppressants |
| Sumir Chawla et al. (2021) [19] | 70 (average age) | Advanced breast cancer (324 patients in total) | Maculopapular rash, xerosis, pruritus, alopecia, eczematous lesions | Palbociclib | Topical treatments (corticosteroids), UVA/UVB phototherapy, dose adjustments, temporary discontinuation in some cases |
| Sollena P et al. (2023) [6] | Not specified | Cancer patients treated with CDK4/6 inhibitors | Pruritus, alopecia, erythema, acneiform eruptions, eczematous lesions | CDK4/6 inhibitors (various) | Topical corticosteroids, antihistamines, dose reduction, temporary discontinuation, dermatology referral |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montenegro, J.F.; Rivas-Tafurt, G.P.; Vidal-Cañas, S.; Diaz-Diaz, M.Á.; Bermudez, C.E.; Florez, D.; Bravo-Gustin, A.F.; Liscano, Y. A Vitiligo-like Cutaneous Reaction Induced by Ribociclib in Advanced Breast Cancer: An Unusual Case Report from Colombia. Diseases 2025, 13, 158. https://doi.org/10.3390/diseases13050158
Montenegro JF, Rivas-Tafurt GP, Vidal-Cañas S, Diaz-Diaz MÁ, Bermudez CE, Florez D, Bravo-Gustin AF, Liscano Y. A Vitiligo-like Cutaneous Reaction Induced by Ribociclib in Advanced Breast Cancer: An Unusual Case Report from Colombia. Diseases. 2025; 13(5):158. https://doi.org/10.3390/diseases13050158
Chicago/Turabian StyleMontenegro, John Fernando, Giovanna Patricia Rivas-Tafurt, Sinthia Vidal-Cañas, Miguel Ángel Diaz-Diaz, Cesar Eduardo Bermudez, Daniel Florez, Andres Felipe Bravo-Gustin, and Yamil Liscano. 2025. "A Vitiligo-like Cutaneous Reaction Induced by Ribociclib in Advanced Breast Cancer: An Unusual Case Report from Colombia" Diseases 13, no. 5: 158. https://doi.org/10.3390/diseases13050158
APA StyleMontenegro, J. F., Rivas-Tafurt, G. P., Vidal-Cañas, S., Diaz-Diaz, M. Á., Bermudez, C. E., Florez, D., Bravo-Gustin, A. F., & Liscano, Y. (2025). A Vitiligo-like Cutaneous Reaction Induced by Ribociclib in Advanced Breast Cancer: An Unusual Case Report from Colombia. Diseases, 13(5), 158. https://doi.org/10.3390/diseases13050158






