Increased Degenerative Biomarkers in Females with Patellofemoral Pain: A Cross-Sectional Analysis with 6-Month Progression
Abstract
1. Introduction
2. Materials and Methods
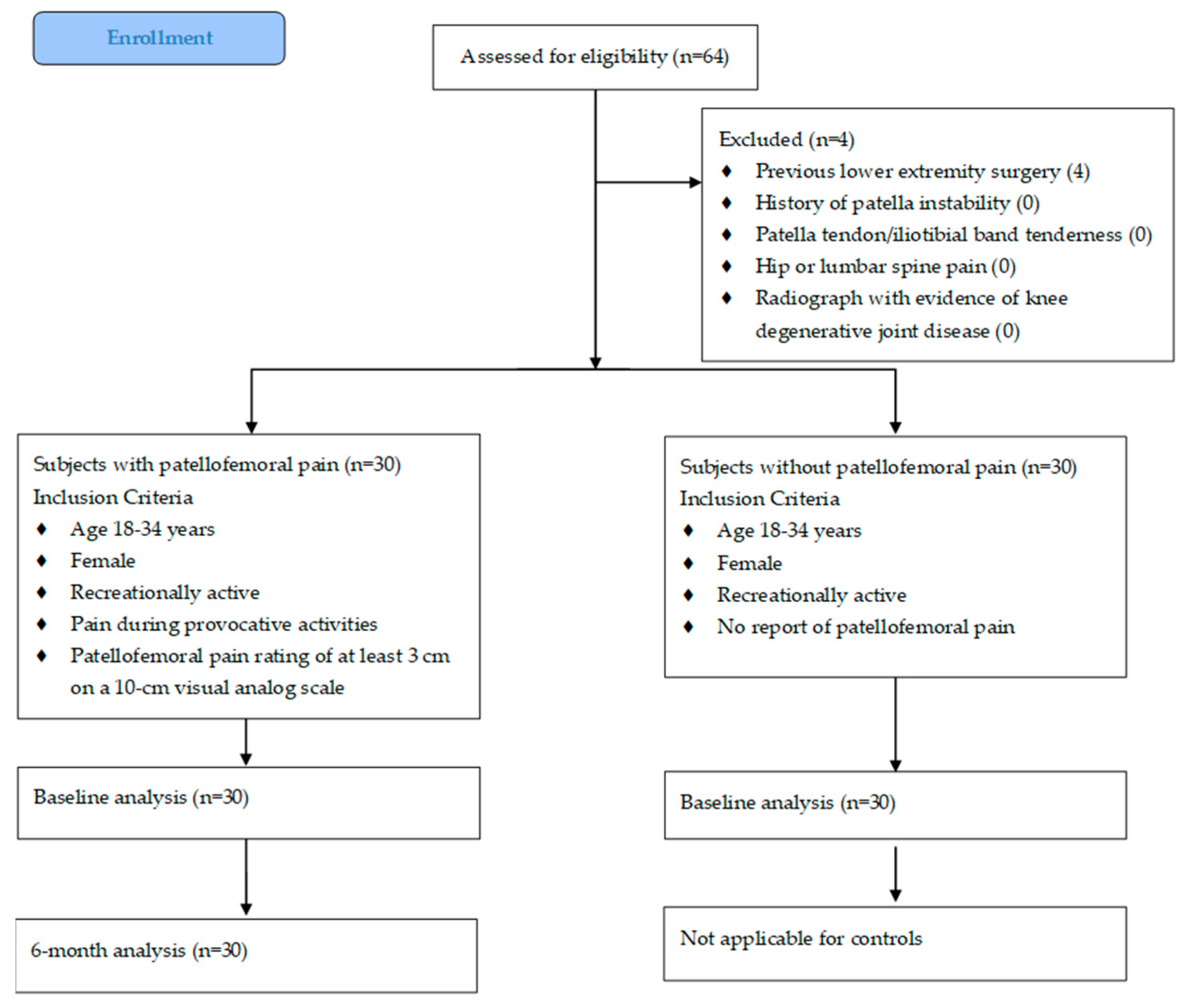
2.1. Subjects
2.2. Cartilage Biomarker Sample Collection
2.2.1. Urine Analysis
2.2.2. Serum Analysis
2.2.3. Data Reduction
2.3. Pain and Function/Quality-of-Life Measures
2.3.1. Pain Measures
2.3.2. Function/Quality-of-Life Measures
2.4. Statistical Analysis
3. Results
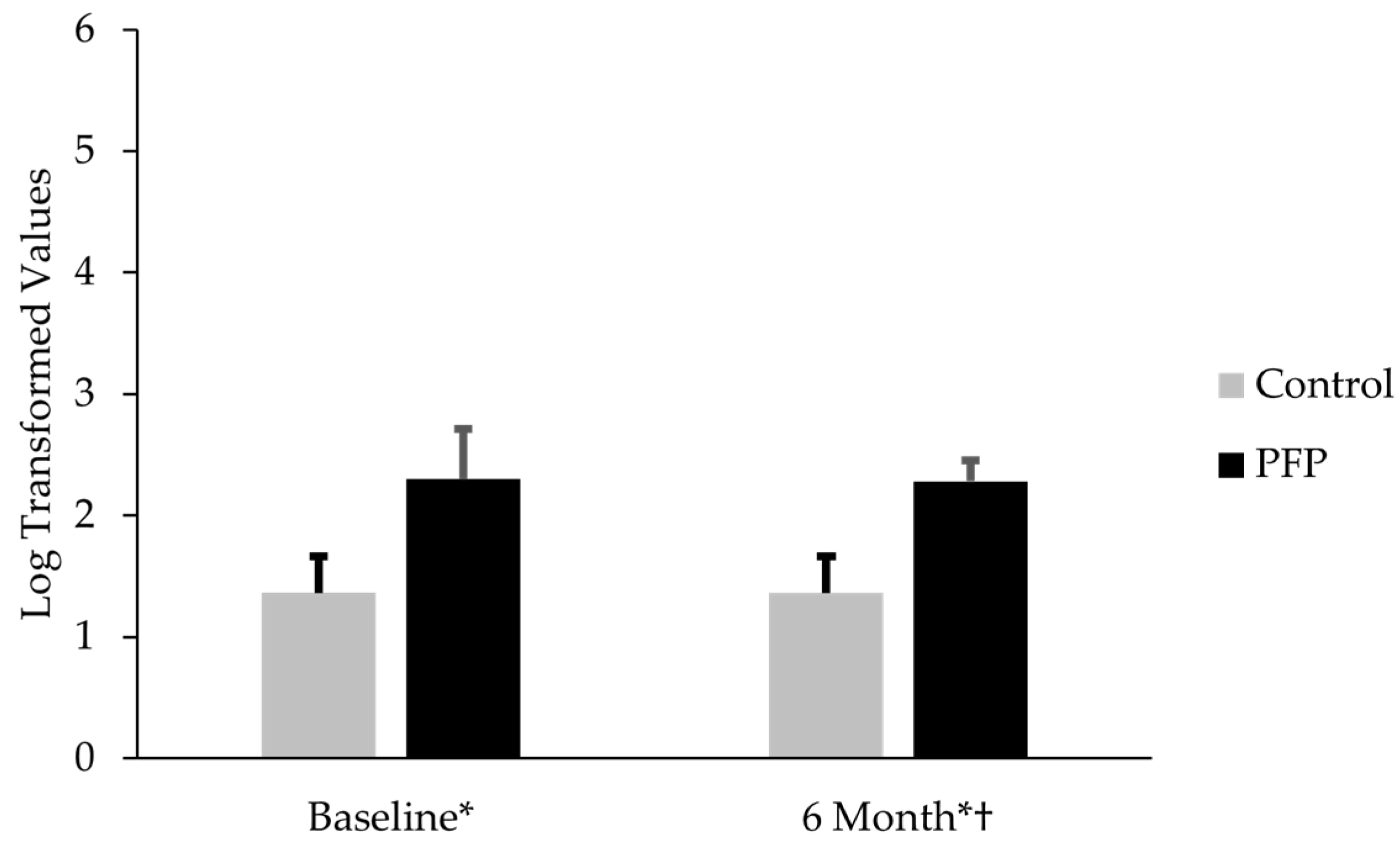
3.1. Comparison of CTX-II, CP-II, and CTX-II:CP-II Levels in Females with PFP and Controls
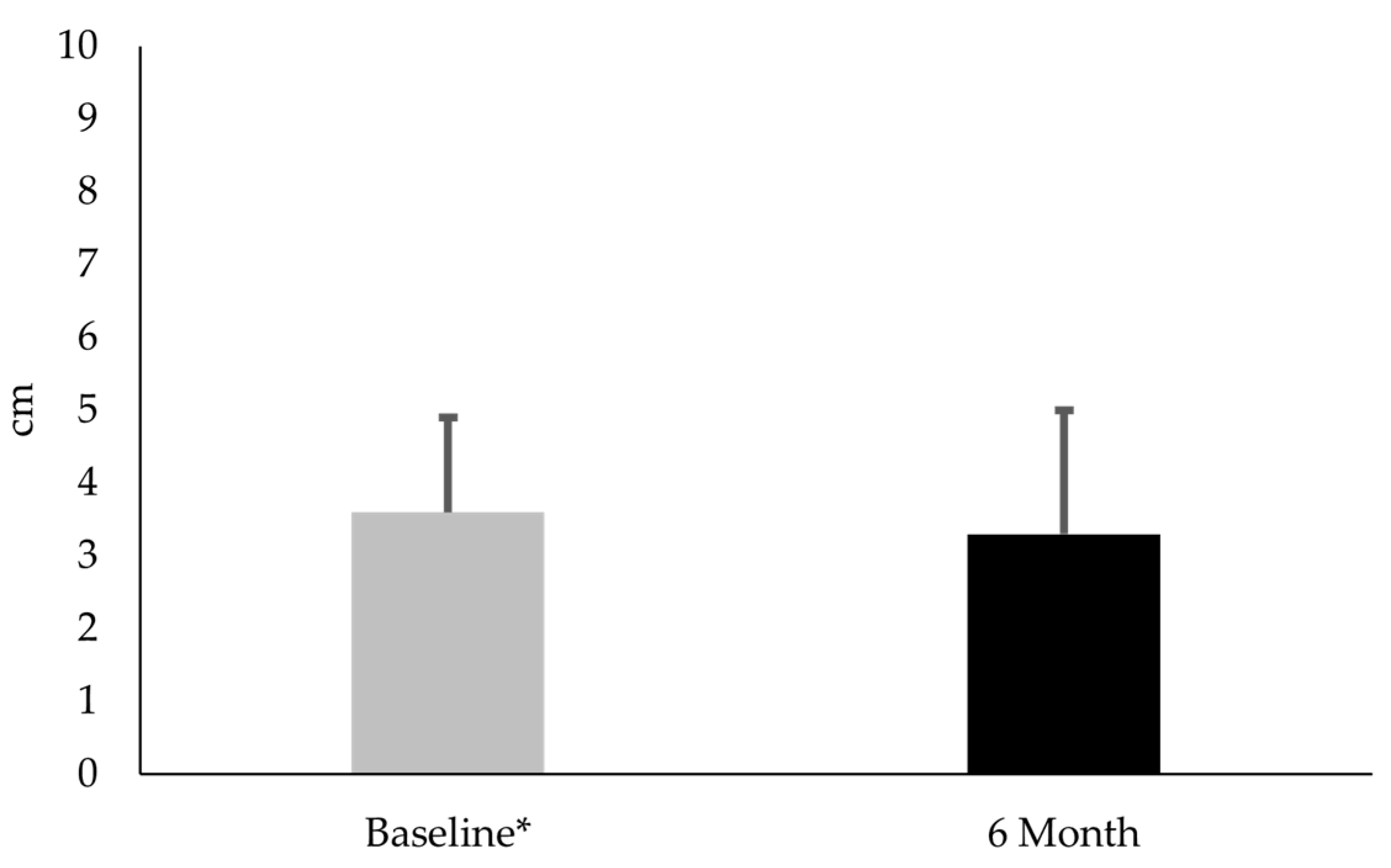
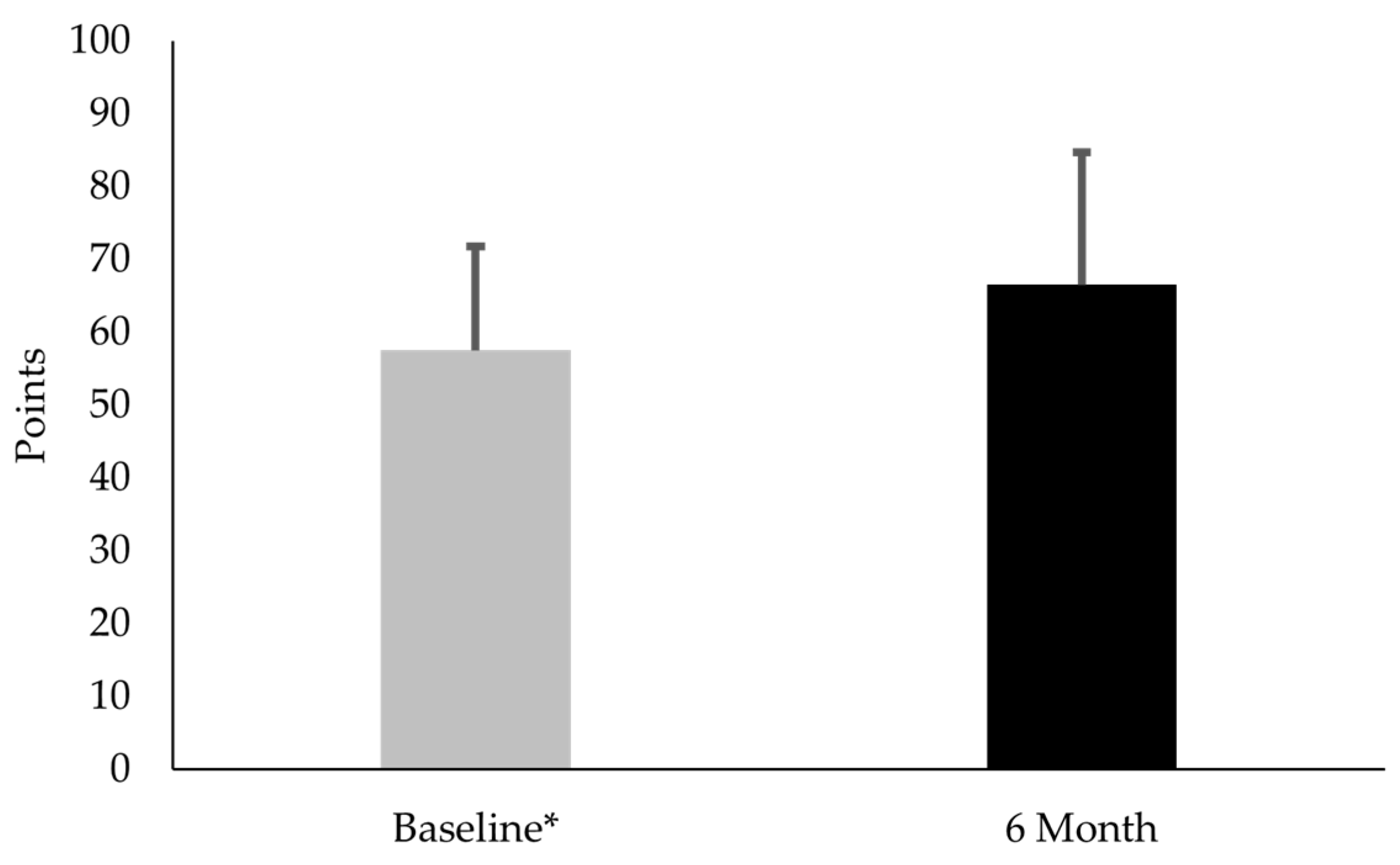
3.2. Comparison of CTX-II:CP-II, VAS, and KOOS-PF Values for Females with PFP at Baseline and 6-Month Follow-Up
4. Discussion
4.1. Comparison of CTX-II, CP-II, and CTX-II:CP-II Values for Females with and Without PFP at Baseline
4.2. Comparison of CTX-II:CP-II, VAS, and Koos-PF Values for Females with PFP at Baseline and 6 Months
4.3. Clinical Implications
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rothermich, M.A.; Glaviano, N.R.; Li, J.; Hart, J.M. Patellofemoral pain: Epidemiology, pathophysiology, and treatment options. Clin. Sports Med. 2015, 34, 313–327. [Google Scholar] [CrossRef]
- Willy, R.W.; Hoglund, L.T.; Barton, C.J.; Bolgla, L.A.; Scalzitti, D.A.; Logerstedt, D.S.; Lynch, A.D.; Snyder-Mackler, L.; McDonough, C.M. Patellofemoral pain. J. Orthop. Sports Phys. Ther. 2019, 49, CPG1–CPG95. [Google Scholar] [CrossRef] [PubMed]
- Crossley, K.M.; van Middelkoop, M.; Barton, C.J.; Culvenor, A.G. Rethinking patellofemoral pain: Prevention, management and long-term consequences. Best. Pract. Res. Clin. Rheumatol. 2019, 33, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Eijkenboom, J.F.A.; Waarsing, J.H.; Oei, E.H.G.; Bierma-Zeinstra, S.M.A.; van Middelkoop, M. Is patellofemoral pain a precursor to osteoarthritis?: Patellofemoral osteoarthritis and patellofemoral pain patients share aberrant patellar shape compared with healthy controls. Bone Joint Res. 2018, 7, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Collins, N.J.; Oei, E.H.G.; de Kanter, J.L.; Vicenzino, B.; Crossley, K.M. Prevalence of radiographic and magnetic resonance imaging features of patellofemoral osteoarthritis in young and middle-aged adults with persistent patellofemoral pain. Arthritis Care Res. 2019, 71, 1068–1073. [Google Scholar] [CrossRef]
- Ferre, I.M.; Roof, M.A.; Anoushiravani, A.A.; Wasterlain, A.S.; Lajam, C.M. Understanding the observed sex discrepancy in the prevalence of osteoarthritis. JBJS Rev. 2019, 7, e8. [Google Scholar] [CrossRef]
- Vitaloni, M.; Botto-van Bemden, A.; Sciortino Contreras, R.M.; Scotton, D.; Bibas, M.; Quintero, M.; Monfort, J.; Carné, X.; de Abajo, F.; Oswald, E.; et al. Global management of patients with knee osteoarthritis begins with quality of life assessment: A systematic review. BMC Musculoskelet. Disord. 2019, 20, 493. [Google Scholar] [CrossRef]
- Pazzinatto, M.F.; Silva, D.O.; Willy, R.W.; Azevedo, F.M.; Barton, C.J. Fear of movement and (re)injury is associated with condition specific outcomes and health-related quality of life in women with patellofemoral pain. Physiother. Theory Pract. 2022, 38, 1254–1263. [Google Scholar] [CrossRef]
- Glaviano, N.R.; Holden, S.; Bazett-Jones, D.M.; Singe, S.M.; Rathleff, M.S. Living well (or not) with patellofemoral pain: A qualitative study. Phys. Ther. Sport. 2022, 56, 1–7. [Google Scholar] [CrossRef]
- Maclachlan, L.R.; Collins, N.J.; Hodges, P.W.; Vicenzino, B. Psychological and pain profiles in persons with patellofemoral pain as the primary symptom. Eur. J. Pain. 2020, 24, 1182–1196. [Google Scholar] [CrossRef]
- GBD 2021 Osteoarthritis Collaborators. Global, regional, and national burden of osteoarthritis, 1990-2020 and projections to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e508–e522. [Google Scholar] [CrossRef]
- van der Heijden, R.A.; de Kanter, J.L.; Bierma-Zeinstra, S.M.; Verhaar, J.A.; van Veldhoven, P.L.; Krestin, G.P.; Oei, E.H.; van Middelkoop, M. Structural abnormalities on magnetic resonance imaging in patients with patellofemoral pain: A cross-sectional case-control study. Am. J. Sports Med. 2016, 44, 2339–2346. [Google Scholar] [CrossRef]
- van der Heijden, R.A.; Oei, E.H.; Bron, E.E.; van Tiel, J.; van Veldhoven, P.L.; Klein, S.; Verhaar, J.A.; Krestin, G.P.; Bierma-Zeinstra, S.M.; van Middelkoop, M. No difference on quantitative magnetic resonance imaging in patellofemoral cartilage composition between patients with patellofemoral pain and healthy controls. Am. J. Sports Med. 2016, 44, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Poole, A.R. Can serum biomarker assays measure the progression of cartilage degeneration in osteoarthritis? Arthritis Rheum. 2002, 46, 2549–2552. [Google Scholar] [CrossRef]
- Kraus, V.B.; Karsdal, M.A. Clinical monitoring in osteoarthritis: Biomarkers. Osteoarthr. Cartil. 2022, 30, 1159–1173. [Google Scholar] [CrossRef]
- Bedson, J.; Croft, P.R. The discordance between clinical and radiographic knee osteoarthritis: A systematic search and summary of the literature. BMC Musculoskelet. Disord. 2008, 9, 116. [Google Scholar] [CrossRef]
- van Middelkoop, M.; Macri, E.M.; Eijkenboom, J.F.; van der Heijden, R.A.; Crossley, K.M.; Bierma-Zeinstra, S.M.A.; de Kanter, J.L.; Oei, E.H.; Collins, N.J. Are patellofemoral joint alignment and shape associated with structural magnetic resonance imaging abnormalities and symptoms among people with patellofemoral pain? Am. J. Sports Med. 2018, 46, 3217–3226. [Google Scholar] [CrossRef]
- Lotz, M.; Martel-Pelletier, J.; Christiansen, C.; Brandi, M.L.; Bruyere, O.; Chapurlat, R.; Collette, J.; Cooper, C.; Giacovelli, G.; Kanis, J.A.; et al. Value of biomarkers in osteoarthritis: Current status and perspectives. Ann. Rheum. Dis. 2013, 72, 1756–1763. [Google Scholar] [CrossRef]
- Young, J.L.; Snodgrass, S.J.; Cleland, J.A.; Rhon, D.I. Timing of physical therapy for individuals with patellofemoral pain and the influence on healthcare use, costs and recurrence rates: An observational study. BMC Health Serv. Res. 2021, 21, 751. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; FitzGerald, O.; Saxne, T.; Bresnihan, B. Increased serum cartilage oligomeric matrix protein levels and decreased patellar bone mineral density in patients with chondromalacia patellae. Ann. Rheum. Dis. 2002, 61, 981–985. [Google Scholar] [CrossRef]
- Bolgla, L.A.; Gordon, R.; Sloan, G.; Pretlow, L.G.; Lyon, M.; Fulzele, S. Comparison of patella alignment and cartilage biomarkers in young adult females with and without patellofemoral pain: A pilot study. Int. J. Sports Phys. Ther. 2019, 14, 46–54. [Google Scholar] [CrossRef]
- Cibere, J.; Zhang, H.; Garnero, P.; Poole, A.R.; Lobanok, T.; Saxne, T.; Kraus, V.B.; Way, A.; Thorne, A.; Wong, H.; et al. Association of biomarkers with pre-radiographically defined and radiographically defined knee osteoarthritis in a population-based study. Arthritis Rheum. 2009, 60, 1372–1380. [Google Scholar] [CrossRef]
- Williams, F.M. Biomarkers: In combination they may do better. Arthritis Res. Ther. 2009, 11, 130. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Mouritzen, U.; Christgau, S.; Lehmann, H.J.; Tankó, L.B.; Christiansen, C. Cartilage turnover assessed with a newly developed assay measuring collagen type II degradation products: Influence of age, sex, menopause, hormone replacement therapy, and body mass index. Ann. Rheum. Dis. 2003, 62, 332–336. [Google Scholar] [CrossRef]
- Kolhe, R.; Owens, V.; Sharma, A.; Lee, T.J.; Zhi, W.; Ghilzai, U.; Mondal, A.K.; Liu, Y.; Isales, C.M.; Hamrick, M.W.; et al. Sex-specific differences in extracellular vesicle protein cargo in synovial fluid of patients with osteoarthritis. Life 2020, 10, 337. [Google Scholar] [CrossRef]
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine 2020, 29–30, 100587. [Google Scholar] [CrossRef]
- Ferber, R.; Bolgla, L.; Earl-Boehm, J.E.; Emery, C.; Hamstra-Wright, K. Strengthening of the hip and core versus knee muscles for the treatment of patellofemoral pain: A multicenter randomized controlled trial. J. Athl. Train. 2015, 50, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Arunrukthavon, P.; Heebthamai, D.; Benchasiriluck, P.; Chaluay, S.; Chotanaphuti, T.; Khuangsirikul, S. Can urinary CTX-II be a biomarker for knee osteoarthritis? Arthroplasty 2020, 2, 6. [Google Scholar] [CrossRef]
- Chmielewski, T.L.; Trumble, T.N.; Joseph, A.M.; Shuster, J.; Indelicato, P.A.; Moser, M.W.; Cicuttini, F.M.; Leeuwenburgh, C. Urinary CTX-II concentrations are elevated and associated with knee pain and function in subjects with ACL reconstruction. Osteoarthr. Cartil. 2012, 20, 1294–1301. [Google Scholar] [CrossRef]
- Rotterud, J.H.; Reinholt, F.P.; Beckstrom, K.J.; Risberg, M.A.; Aroen, A. Relationship between CTX-II and patient characteristics, patient-reported outcome, muscle strength, and rehabilitation in patients with a focal cartilage lesion of the knee: A prospective exploratory cohort study of 48 patients. BMC Musculoskelet. Disord. 2014, 15, 99. [Google Scholar] [CrossRef]
- Crossley, K.M.; Bennell, K.L.; Cowan, S.M.; Green, S. Analysis of outcome measures for persons with patellofemoral pain: Which are reliable and valid? Arch. Phys. Med. Rehabil. 2004, 85, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Crossley, K.M.; Macri, E.M.; Cowan, S.M.; Collins, N.J.; Roos, E.M. The patellofemoral pain and osteoarthritis subscale of the KOOS (KOOS-PF): Development and validation using the COSMIN checklist. Br. J. Sports Med. 2018, 52, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Hoglund, L.T.; Scalzitti, D.A.; Bolgla, L.A.; Jayaseelan, D.J.; Wainwright, S.F. Patient-reported outcome measures for adults and adolescents with patellofemoral pain: A systematic review of content validity and feasibility using the COSMIN methodology. J. Orthop. Sports Phys. Ther. 2023, 53, 23–39. [Google Scholar] [CrossRef]
- Hoglund, L.T.; Scalzitti, D.A.; Jayaseelan, D.J.; Bolgla, L.A.; Wainwright, S.F. Patient-reported outcome measures for adults and adolescents With patellofemoral pain: A systematic review of construct validity, reliability, responsiveness, and interpretability using the COSMIN methodology. J. Orthop. Sports Phys. Ther. 2023, 53, 1–20. [Google Scholar] [CrossRef]
- McGlothlin, A.E.; Lewis, R.J. Minimal clinically important difference: Defining what really matters to patients. JAMA 2014, 312, 1342–1343. [Google Scholar] [CrossRef]
| PFP | Controls | p-Value | |
|---|---|---|---|
| Age, y | 22.9 ± 2.9 | 22.6 ± 3.3 | 0.75 |
| Mass, kg | 71.9 ± 17.3 | 65.9 ± 16.9 | 0.19 |
| Height, cm | 168.3 ± 7.1 | 165.9 ± 7.2 | 0.20 |
| PFP | Controls | p-Value | |
|---|---|---|---|
| CTX-II | 2.9 ± 0.4 | 2.9 ± 0.3 | 0.91 |
| CP-II | 0.6 ± 0.1 | 1.5 ± 0.1 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolgla, L.A.; Curry-McCoy, T.V.; Giddens, M.; Overton, M.; Barrera, B.; Crockett, J.; Hunter, M. Increased Degenerative Biomarkers in Females with Patellofemoral Pain: A Cross-Sectional Analysis with 6-Month Progression. Diseases 2025, 13, 155. https://doi.org/10.3390/diseases13050155
Bolgla LA, Curry-McCoy TV, Giddens M, Overton M, Barrera B, Crockett J, Hunter M. Increased Degenerative Biomarkers in Females with Patellofemoral Pain: A Cross-Sectional Analysis with 6-Month Progression. Diseases. 2025; 13(5):155. https://doi.org/10.3390/diseases13050155
Chicago/Turabian StyleBolgla, Lori A., Tiana V. Curry-McCoy, Maya Giddens, Madelyn Overton, Bryaunna Barrera, Jasmine Crockett, and Monte Hunter. 2025. "Increased Degenerative Biomarkers in Females with Patellofemoral Pain: A Cross-Sectional Analysis with 6-Month Progression" Diseases 13, no. 5: 155. https://doi.org/10.3390/diseases13050155
APA StyleBolgla, L. A., Curry-McCoy, T. V., Giddens, M., Overton, M., Barrera, B., Crockett, J., & Hunter, M. (2025). Increased Degenerative Biomarkers in Females with Patellofemoral Pain: A Cross-Sectional Analysis with 6-Month Progression. Diseases, 13(5), 155. https://doi.org/10.3390/diseases13050155




