Curcumin Reverses Antibiotic Resistance and Downregulates Shiga Toxin Expression in Enterohemorrhagic E. coli
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain
2.2. Maintenance and Preservation of Microorganisms
2.3. CUR
2.4. Antibacterial Activity of CUR
2.5. EHEC Resistance Assay
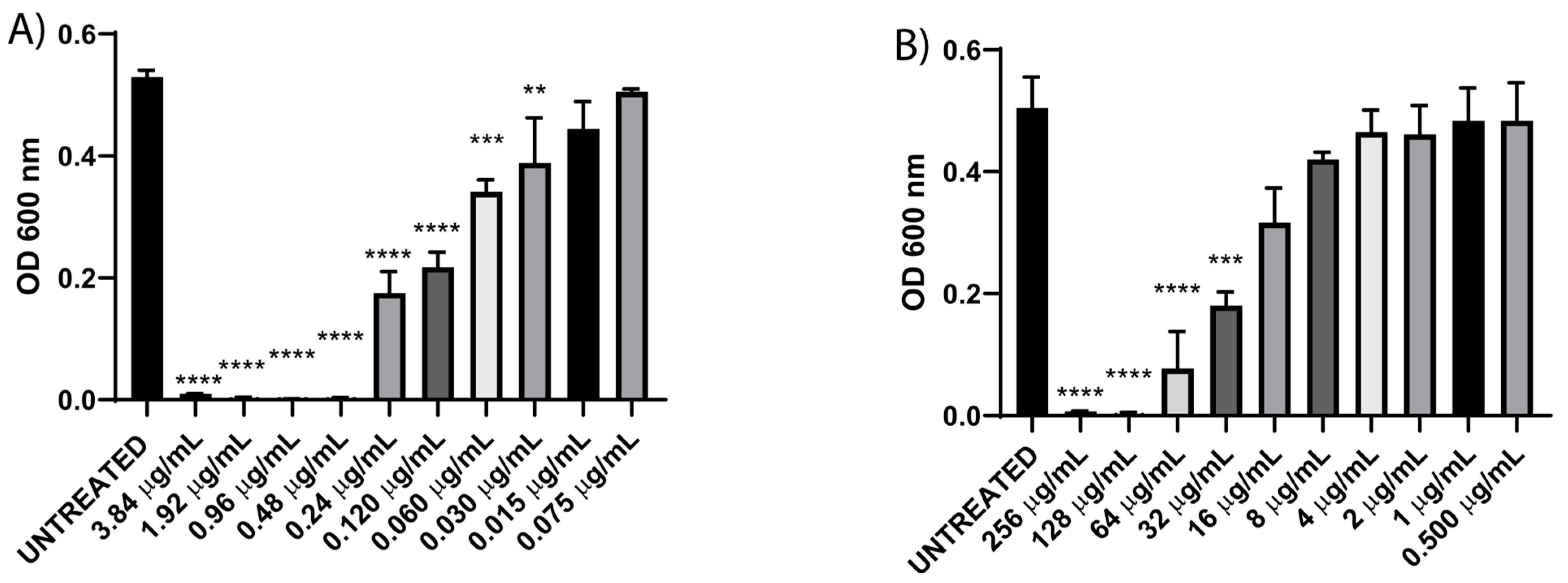
2.6. Determination of Minimum Inhibitory Concentration
2.7. Sensitizing and Synergistic Antibacterial Effects of CUR and Antibiotics
2.8. Effect of Curcumin on Gene Expression of Resistant EHEC
2.9. Effect of CUR on Shiga Toxin Expression
2.10. Statistical Analysis
3. Results
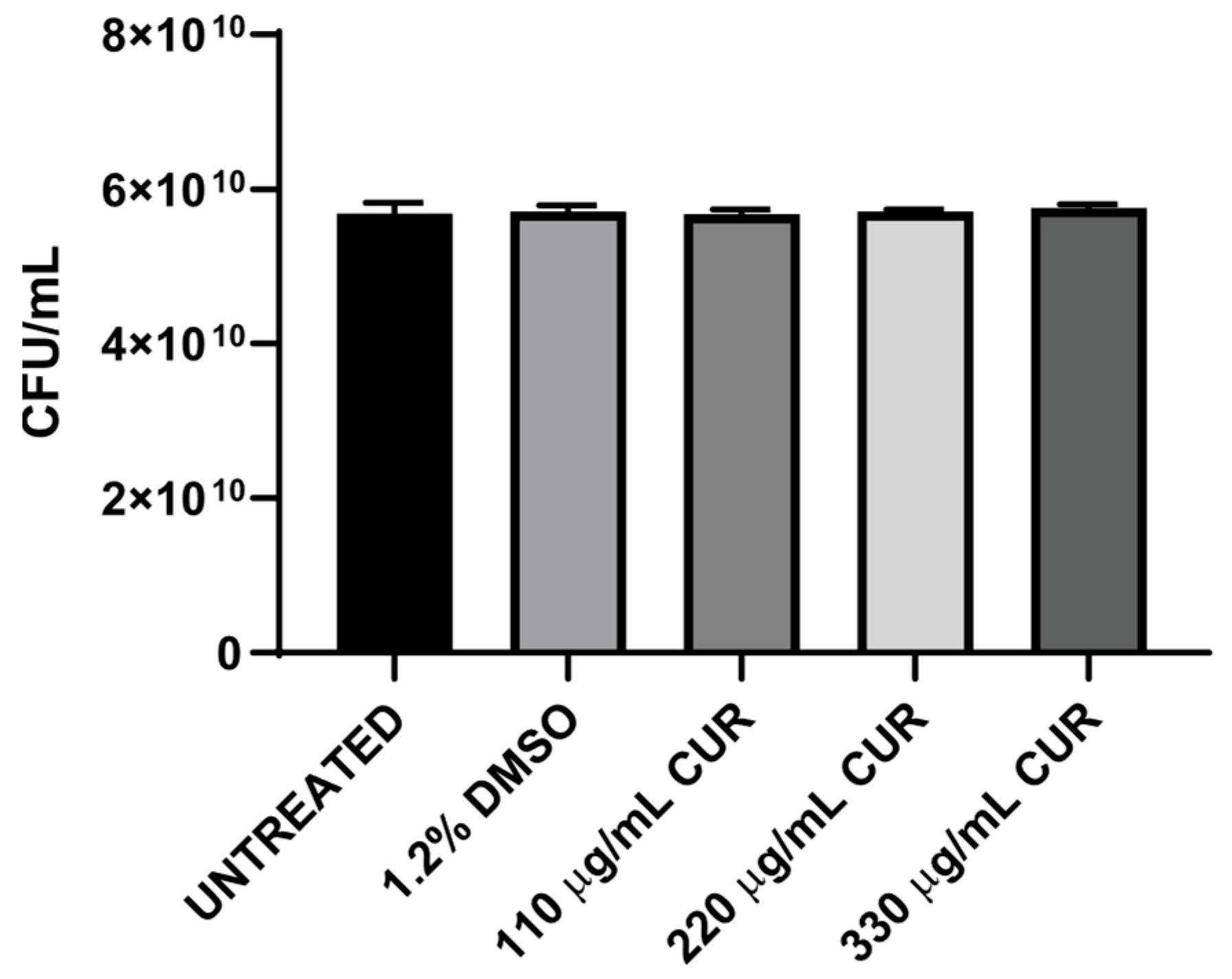
3.1. CUR Did Not Show Antibacterial Effect
3.2. Resistance Profile and Minimum Inhibitory Concentration (MIC) of Enterohemorrhagic E. coli
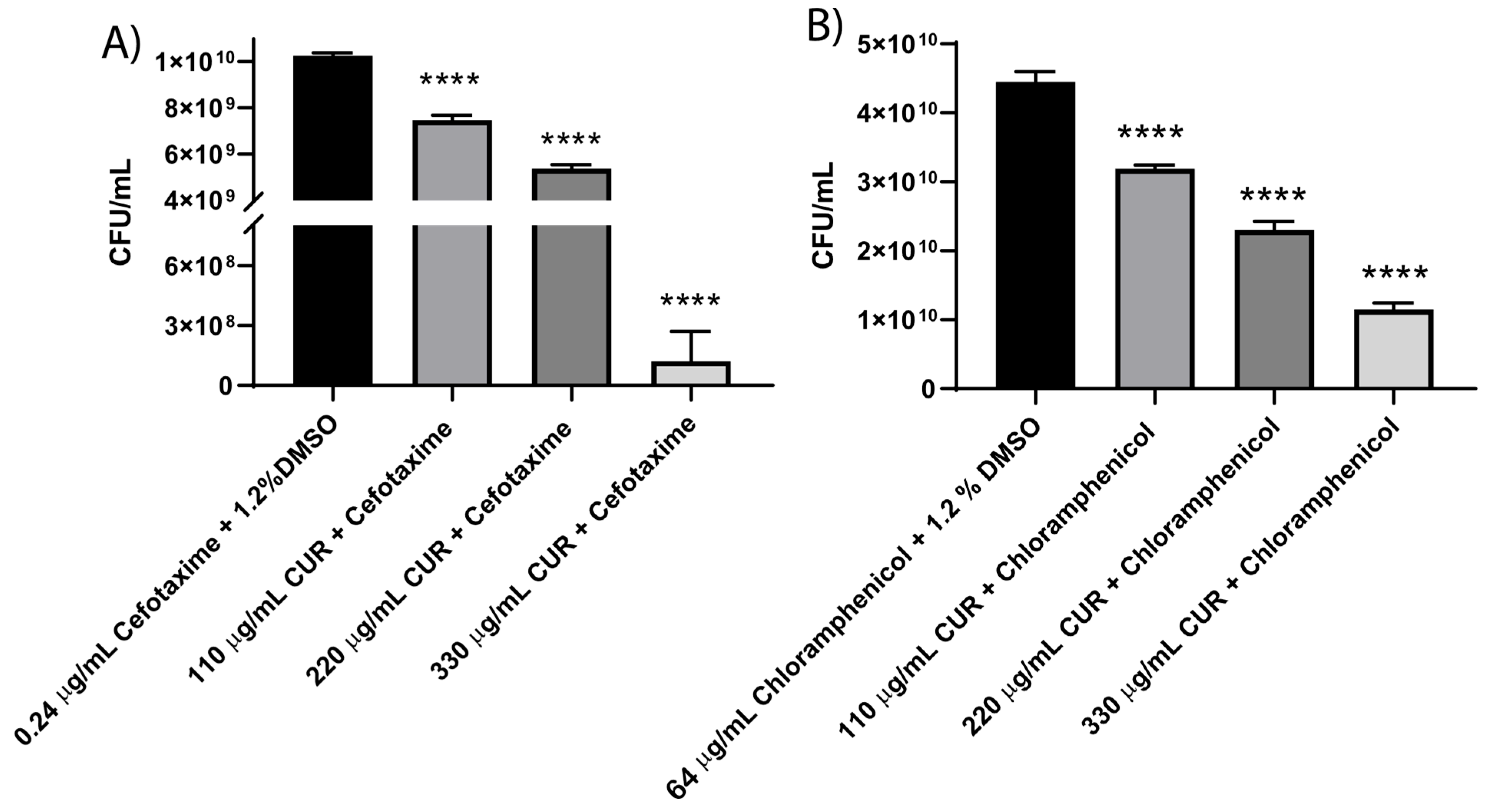
3.3. CUR Sensitizes EHEC to Different Antibiotics
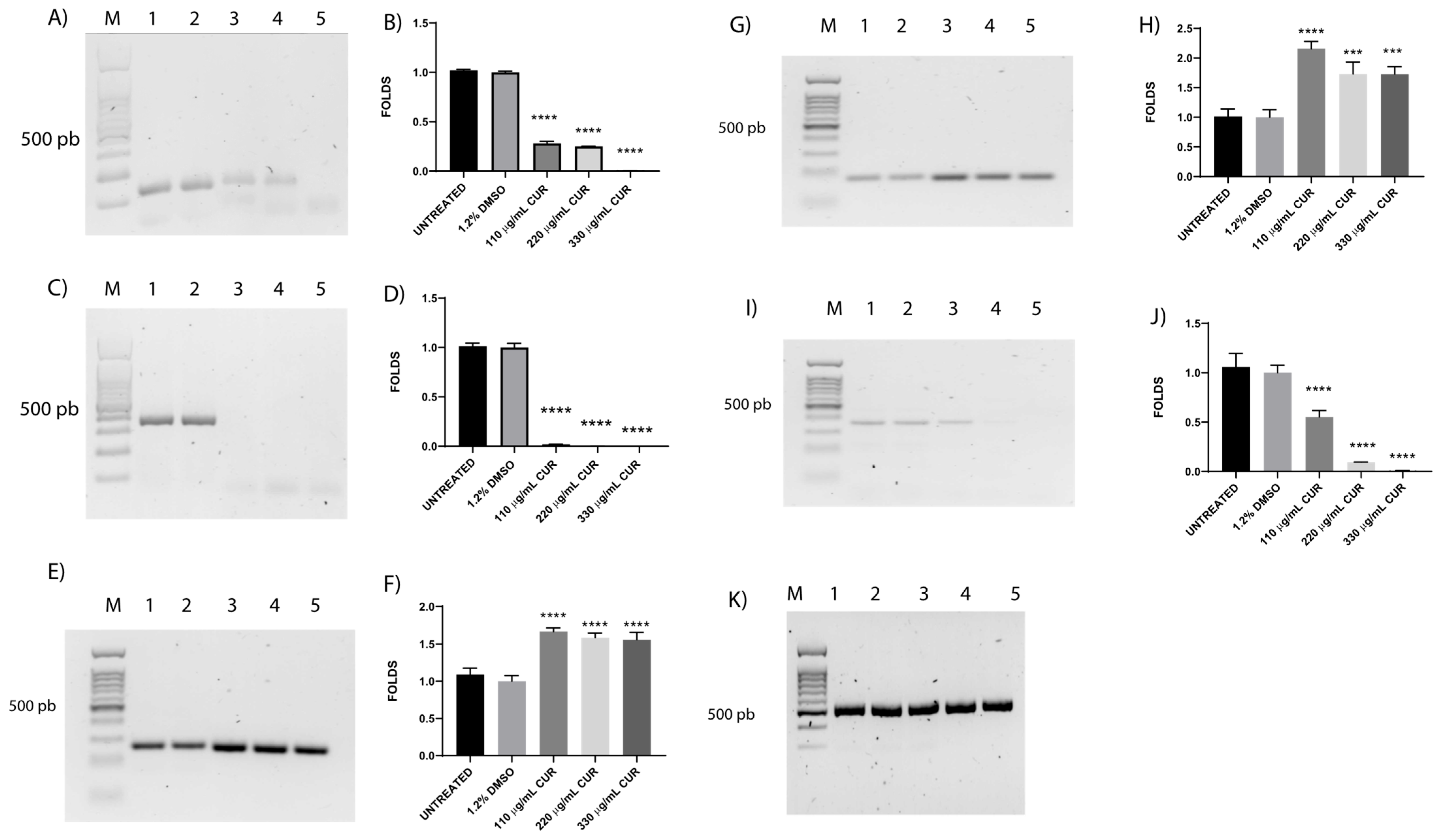
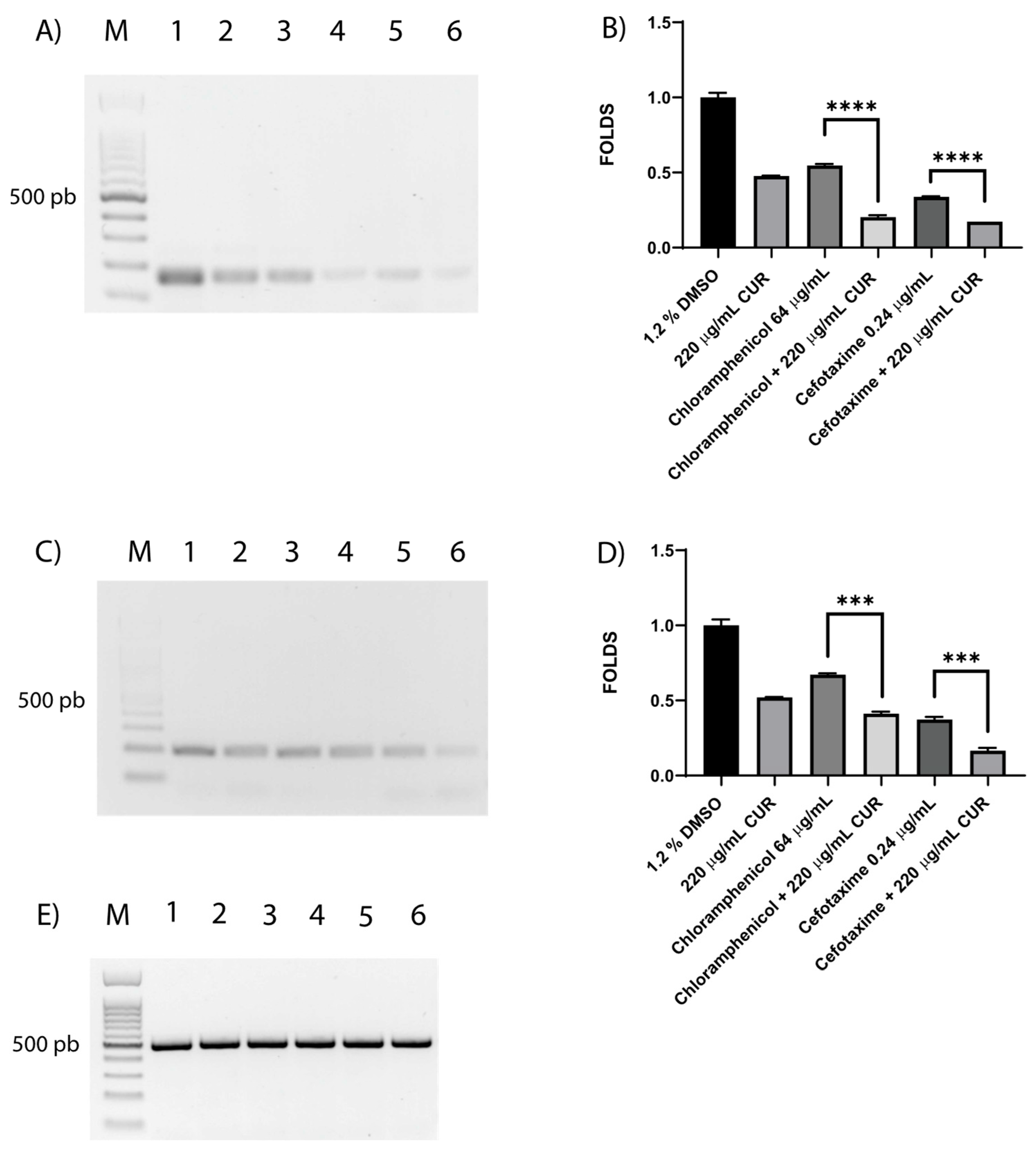
3.4. CUR Affects the Relative Expression of Antimicrobial Resistance Genes
3.5. CUR Broke Antibiotic Resistance of EHEC
3.6. Shiga Toxin Is Downregulated by CUR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, Y.; Sperandio, V. Enterohemorrhagic E. coli (EHEC) pathogenesis. Front. Cell Infect. Microbiol. 2012, 2, 90. [Google Scholar] [CrossRef] [PubMed]
- Karpman, D.; Loos, S.; Tati, R.; Arvidsson, I. Haemolytic uraemic syndrome. J. Intern. Med. 2017, 281, 123–148. [Google Scholar] [CrossRef] [PubMed]
- Karpman, D.; Stahl, A.L. Enterohemorrhagic Escherichia coli Pathogenesis and the Host Response. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.L.; Leibowitz, C.S.; Kurosawa, S.; Stearns-Kurosawa, D.J. Shiga toxins and the pathophysiology of hemolytic uremic syndrome in humans and animals. Toxins 2012, 4, 1261–1287. [Google Scholar] [CrossRef]
- Ramstad, S.N.; Taxt, A.M.; Naseer, U.; Wasteson, Y.; Bjornholt, J.V.; Brandal, L.T. Effects of antimicrobials on Shiga toxin production in high-virulent Shiga toxin-producing Escherichia coli. Microb. Pathog. 2021, 152, 104636. [Google Scholar] [CrossRef]
- Chen, M.L.; Hao, Z.; Tian, Y.; Zhang, Q.Y.; Gao, P.J.; Jin, J.L. Different Effects of Six Antibiotics and Ten Traditional Chinese Medicines on Shiga Toxin Expression by Escherichia coli O157:H7. Evid. Based Complement. Alternat Med. 2013, 2013, 121407. [Google Scholar] [CrossRef]
- McGannon, C.M.; Fuller, C.A.; Weiss, A.A. Different classes of antibiotics differentially influence shiga toxin production. Antimicrob. Agents Chemother. 2010, 54, 3790–3798. [Google Scholar] [CrossRef]
- Venturini, C.; Beatson, S.A.; Djordjevic, S.P.; Walker, M.J. Multiple antibiotic resistance gene recruitment onto the enterohemorrhagic Escherichia coli virulence plasmid. FASEB J. 2010, 24, 1160–1166. [Google Scholar] [CrossRef]
- Bielaszewska, M.; Idelevich, E.A.; Zhang, W.; Bauwens, A.; Schaumburg, F.; Mellmann, A.; Peters, G.; Karch, H. Effects of antibiotics on Shiga toxin 2 production and bacteriophage induction by epidemic Escherichia coli O104:H4 strain. Antimicrob. Agents Chemother. 2012, 56, 3277–3282. [Google Scholar] [CrossRef]
- Surendran Nair, M.; Ma, F.; Lau, P.; Upadhyaya, I.; Venkitanarayanan, K. Inactivation of Escherichia coli O157:H7 in apple cider by resveratrol and naringenin. Food Microbiol. 2020, 86, 103327. [Google Scholar] [CrossRef]
- Karczewska, M.; Wang, A.Y.; Narajczyk, M.; Slominski, B.; Szalewska-Palasz, A.; Nowicki, D. Antibacterial activity of t-cinnamaldehyde: An approach to its mechanistic principle towards enterohemorrhagic Escherichia coli (EHEC). Phytomedicine 2024, 132, 155845. [Google Scholar] [CrossRef] [PubMed]
- Praditya, D.; Kirchhoff, L.; Bruning, J.; Rachmawati, H.; Steinmann, J.; Steinmann, E. Anti-infective Properties of the Golden Spice Curcumin. Front. Microbiol. 2019, 10, 912. [Google Scholar] [CrossRef]
- de Oliveira, E.F.; Tosati, J.V.; Tikekar, R.V.; Monteiro, A.R.; Nitin, N. Antimicrobial activity of curcumin in combination with light against Escherichia coli O157:H7 and Listeria innocua: Applications for fresh produce sanitation. Postharvest Biol. Technol. 2018, 137, 86–94. [Google Scholar] [CrossRef]
- Marini, E.; Di Giulio, M.; Magi, G.; Di Lodovico, S.; Cimarelli, M.E.; Brenciani, A.; Nostro, A.; Cellini, L.; Facinelli, B. Curcumin, an antibiotic resistance breaker against a multiresistant clinical isolate of Mycobacterium abscessus. Phytother. Res. 2018, 32, 488–495. [Google Scholar] [CrossRef]
- Itzia Azucena, R.C.; Jose Roberto, C.L.; Martin, Z.R.; Rafael, C.Z.; Leonardo, H.H.; Gabriela, T.P.; Araceli, C.R. Drug Susceptibility Testing and Synergistic Antibacterial Activity of Curcumin with Antibiotics against Enterotoxigenic Escherichia coli. Antibiotics 2019, 8, 43. [Google Scholar] [CrossRef]
- Zheng, M.; Wu, X.; Xu, Y.; Ma, S.; Shen, J.; Li, T.; Zhai, Y.; Yuan, L.; Hu, G.; Pan, Y.; et al. Curcumin reverses high-level tigecycline resistance mediated by different mechanisms in Gram-negative bacteria. Phytomedicine 2025, 136, 156319. [Google Scholar] [CrossRef]
- CLSI. M100 Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2023; Volume 416. [Google Scholar]
- van den Bijllaardt, W.; Voermans, P.C.M.; Buiting, A.G.; Mouton, J.W.; Muller, A.E. Shortening the incubation time for the combination disk diffusion extended-spectrum beta-lactamase (ESBL) confirmation test: How far can we go? Int. J. Antimicrob. Agents 2017, 50, 473–476. [Google Scholar] [CrossRef]
- Masadeh, M.M.; Mhaidat, N.M.; Al-Azzam, S.I.; Alzoubi, K.H. Investigation of the antibacterial activity of pioglitazone. Drug Des. Devel Ther. 2011, 5, 421–425. [Google Scholar] [CrossRef]
- Belley, A.; Huband, M.D.; Fedler, K.A.; Watters, A.A.; Flamm, R.K.; Shapiro, S.; Knechtle, P. Development of Broth Microdilution MIC and Disk Diffusion Antimicrobial Susceptibility Test Quality Control Ranges for the Combination of Cefepime and the Novel β-Lactamase Inhibitor Enmetazobactam. J. Clin. Microbiol. 2019, 57, e00607-19. [Google Scholar] [CrossRef]
- Ni, W.; Shao, X.; Di, X.; Cui, J.; Wang, R.; Liu, Y. In vitro synergy of polymyxins with other antibiotics for Acinetobacter baumannii: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2015, 45, 8–18. [Google Scholar] [CrossRef]
- Aranda, P.S.; LaJoie, D.M.; Jorcyk, C.L. Bleach gel: A simple agarose gel for analyzing RNA quality. Electrophoresis 2012, 33, 366–369. [Google Scholar] [CrossRef]
- Quackenbush, J. Microarray data normalization and transformation. Nat. Genet. 2002, 32, 496–501. [Google Scholar] [CrossRef]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Rout, L.; Jena, M.; Efferth, T.; Bhutia, S.K. Chemotherapeutic efficacy of curcumin and resveratrol against cancer: Chemoprevention, chemoprotection, drug synergism and clinical pharmacokinetics. Semin. Cancer Biol. 2021, 73, 310–320. [Google Scholar] [CrossRef]
- Batra, H.; Pawar, S.; Bahl, D. Curcumin in combination with anti-cancer drugs: A nanomedicine review. Pharmacol. Res. 2019, 139, 91–105. [Google Scholar] [CrossRef]
- Sasidharan, N.K.; Sreekala, S.R.; Jacob, J.; Nambisan, B. In vitro synergistic effect of curcumin in combination with third generation cephalosporins against bacteria associated with infectious diarrhea. Biomed. Res. Int. 2014, 2014, 561456. [Google Scholar] [CrossRef]
- Zdravko Podlesek, D.Ž.B. The Escherichia coli SOS Response: Much More than DNA Damage Repair. In Escherichia coli–Old and New Insights; IntechOpen: London, UK, 2021. [Google Scholar]
- Baharoglu, Z.; Mazel, D. Vibrio cholerae triggers SOS and mutagenesis in response to a wide range of antibiotics: A route towards multiresistance. Antimicrob. Agents Chemother. 2011, 55, 2438–2441. [Google Scholar] [CrossRef]
- Zheng, D.; Huang, C.; Huang, H.; Zhao, Y.; Khan, M.R.U.; Zhao, H.; Huang, L. Antibacterial Mechanism of Curcumin: A Review. Chem. Biodivers. 2020, 17, e2000171. [Google Scholar] [CrossRef]
- Trigo-Gutierrez, J.K.; Vega-Chacon, Y.; Soares, A.B.; Mima, E.G.O. Antimicrobial Activity of Curcumin in Nanoformulations: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 7130. [Google Scholar] [CrossRef]
- Landau, E.; Shapira, R. Effects of Subinhibitory Concentrations of Menthol on Adaptation, Morphological, and Gene Expression Changes in Enterohemorrhagic Escherichia coli. Appl. Environ. Microbiol. 2012, 78, 5361–5367. [Google Scholar] [CrossRef]
- Rivas, L.; McDonnell, M.J.; Burgess, C.M.; O’Brien, M.; Navarro-Villa, A.; Fanning, S.; Duffy, G. Inhibition of verocytotoxigenic Escherichia coli in model broth and rumen systems by carvacrol and thymol. Int. J. Food Microbiol. 2010, 139, 70–78. [Google Scholar] [CrossRef]
- Jaiswal, S.; Mishra, P. Antimicrobial and antibiofilm activity of curcumin-silver nanoparticles with improved stability and selective toxicity to bacteria over mammalian cells. Med. Microbiol. Immunol. 2018, 207, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Alos, J.I. Antibiotic resistance: A global crisis. Enferm. Infecc. Microbiol. Clin. 2015, 33, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Dodds, D.R. Antibiotic resistance: A current epilogue. Biochem. Pharmacol. 2017, 134, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E. Curcumin Combination Chemotherapy: The Implication and Efficacy in Cancer. Molecules 2019, 24, 2527. [Google Scholar] [CrossRef]
- Ngo, S.T.; Li, M.S. Curcumin binds to Abeta1-40 peptides and fibrils stronger than ibuprofen and naproxen. J. Phys. Chem. B 2012, 116, 10165–10175. [Google Scholar] [CrossRef]
- Javadi, K.; Ahmadi, M.H.; Rajabnia, M.; Halaji, M. Effects of Curcumin on Biofilm Production and Associated Gene in Multidrug-Resistant Acinetobacter baumannii Isolated from Hospitalized Patients. Int. J. Mol. Cell Med. 2025, 14, 567–575. [Google Scholar] [CrossRef]
- Yadav, S.; Singh, A.K.; Agrahari, A.K.; Sharma, K.; Singh, A.S.; Gupta, M.K.; Tiwari, V.K.; Prakash, P. Making of water soluble curcumin to potentiate conventional antimicrobials by inducing apoptosis-like phenomena among drug-resistant bacteria. Sci. Rep. 2020, 10, 14204. [Google Scholar] [CrossRef]
- Pun, M.; Khazanov, N.; Galsurker, O.; Kerem, Z.; Senderowitz, H.; Yedidia, I. Inhibition of AcrAB-TolC enhances antimicrobial activity of phytochemicals in Pectobacterium brasiliense. Front. Plant Sci. 2023, 14, 1161702. [Google Scholar] [CrossRef]
- Ciusa, M.L.; Marshall, R.L.; Ricci, V.; Stone, J.W.; Piddock, L.J.V. Absence, loss-of-function, or inhibition of Escherichia coli AcrB does not increase expression of other efflux pump genes supporting the discovery of AcrB inhibitors as antibiotic adjuvants. J. Antimicrob. Chemother. 2022, 77, 633–640. [Google Scholar] [CrossRef]
- Isidora Nikolic, V.A.S.; Gavric, D.; Knezevic, P. Anti-Staphylococcus aureus Activity of Volatile Phytochemicals and Their Combinations with Conventional Antibiotics Against Methicillin-Susceptible S. aureus (MSSA) and Methicillin-Resistant S. aureus (MRSA) Strains. Antibiotics 2024, 13, 1030. [Google Scholar] [CrossRef]
- Siriyong, T.; Srimanote, P.; Chusri, S.; Yingyongnarongkul, B.E.; Suaisom, C.; Tipmanee, V.; Voravuthikunchai, S.P. Conessine as a novel inhibitor of multidrug efflux pump systems in Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2017, 17, 405. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Singh, M.; Kumari, H.; Kumari, A.; Mukhopadhyay, K. Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PLoS ONE 2015, 10, e0121313. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Mathew, R.; Trachtman, H. Cytoprotective effect of curcumin in human proximal tubule epithelial cells exposed to shiga toxin. Biochem. Biophys. Res. Commun. 2001, 283, 36–41. [Google Scholar] [CrossRef] [PubMed]
| Gene | Sequence (5′ to 3′) | Amplification Conditions | Size (bp) |
|---|---|---|---|
| blaCTX-M-15 | F: 5′-ATC CGA TTG CGG AAA AGC ACG TCA-3′ R: 5′-GGA ACG TTT CGT CTC CCA GCT-3′ | 95 °C for 60 s and 95 °C for 30 s, followed by 40 cycles at 66 °C for 30 s, 72 °C for 60 s, and 72 °C for 7 min | 165 |
| catA1 | F: 5′-GGT GAT ATG GGA TAG TGT TCA CCC-3′ R: 5′-CTG AAT CGC CAG CGG CAT CA-3′ | 95 °C for 60 s and 95 °C for 30 s, followed by 40 cycles at 62 °C for 30 s, 72 °C for 60 s, and 72 °C for 7 min | 325 |
| acrA | F: 5′-ACA GAG GGT TTA CGC CTC TGG C-3′ R: 5′-TCT GCG ATC CGG TAG GCA CT-3′ | 190 | |
| acrB | F: 5′-GCG ATC CTC AAA CTG CCG GT-3′ R: 5′-TGC GTC ATG GTG CCA TCG GT-3′ | 377 | |
| tolC | F: 5′-ATC GGC CTG AGC CTT TCT GGG-3′ R: 5′-GTT AAC GCA CGC CAT TTC GAC-3′ | 296 | |
| gapdh | F: 5′-GGT TTT GGC CGT ATC GGT CGC A-3′ R: 5′-TGA ACG GTG GTC ATC AGA CCT TCG-3′ | 506 | |
| stx2A | F: 5′-ACG GTT TCC ATG ACA ACG GAC AGC-3′ R: 5′-AGA ACT GCT CTG GAT GCA TCT CTG G-3′ | 95 °C for 60 s and 95 °C for 30 s, followed by 40 cycles at 60 °C for 30 s, 72 °C for 60 s, and 72 °C for 7 min | 164 |
| stx2B | F: 5′-TGC AAT GGC GGC GGA TTG TGC-3′ R: 5′-ACT GCA CTT CAGCAA ATC CGG AGC-3 | 95 °C for 60 s and 95 °C for 30 s, followed by 40 cycles at 65 °C for 30 s, 72 °C for 60 s, and 72 °C for 7 min | 209 |
| Antibiotic | Inhibition Diameter (mm) |
|---|---|
| EHEC | |
| Cefotaxime (CTX) | 25 ± 0 (I) |
| Chloramphenicol (C) | 11 ± 0 (R) |
| CUR-Sensitized EHEC | |||||
|---|---|---|---|---|---|
| Antibiotic | Inhibition Diameter (mm) | ||||
| Untreated | 1.2% DMSO | 110 µg/mL CUR | 220 µg/mL CUR | 330 µg/mL CUR | |
| Cefotaxime (CTX) | 25 ± 0 (I) | 25 ± 0.7 (I) | 31 ± 0 (S) | 30 ± 1.4 (S) | 33 ± 1.4 (S) |
| Chloramphenicol (C) | 11 ± 0 (R) | 12 ± 0 (R) | 20 ± 0.7 (S) | 21 ± 1.4 (S) | 21 ± 1.4 (S) |
| 0.24 μg/mL CTX + 1.2% DMSO | 0.24 μg/mL CTX + 110 µg/mL CUR | 0.24 μg/mL CTX + 220 µg/mL CUR | 0.24 μg/mL CTX + 330 µg/mL CUR |
| 25 ± 0.7 (I) | 33 ± 0.7 (S) | 34 ± 0.7 (S) | 34 ± 0.7 (S) |
| 64 μg/mL C + 1.2% DMSO | 64 μg/mL C + 110 µg/mL CUR | 64 μg/mL C + 220 µg/mL CUR | 64 μg/mL C + 330 µg/mL CUR |
| 12 ± 0 (R) | 14 ± 0.7 (I) | 15 ± 1.4 (I) | 15 ± 1.4 (I) |
| 0.24 μg/mL CTX + 110 µg/mL CUR | 0.24 μg/mL CTX + 220 µg/mL CUR | 0.24 μg/mL CTX + 330 µg/mL CUR |
| FIC index of 0.86 | FIC index of 0.62 | FIC index of 0.01 |
| 64 μg/mL C + 110 µg/mL CUR | 64 μg/mL C + 220 µg/mL CUR | 64 μg/mL C + 330 µg/mL CUR |
| FIC index of 1.30 | FIC index of 0.93 | FIC index of 0.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zermeño-Ruiz, M.; Cobos-Vargas, M.; Saucedo-Plascencia, M.D.; Cortés-Zárate, R.; Hernandez-Hernandez, L.; Garcia-Cobian, T.A.; Estrada-Garcia, T.; Castillo-Romero, A. Curcumin Reverses Antibiotic Resistance and Downregulates Shiga Toxin Expression in Enterohemorrhagic E. coli. Diseases 2025, 13, 154. https://doi.org/10.3390/diseases13050154
Zermeño-Ruiz M, Cobos-Vargas M, Saucedo-Plascencia MD, Cortés-Zárate R, Hernandez-Hernandez L, Garcia-Cobian TA, Estrada-Garcia T, Castillo-Romero A. Curcumin Reverses Antibiotic Resistance and Downregulates Shiga Toxin Expression in Enterohemorrhagic E. coli. Diseases. 2025; 13(5):154. https://doi.org/10.3390/diseases13050154
Chicago/Turabian StyleZermeño-Ruiz, Martin, Mirian Cobos-Vargas, Mauro Donaldo Saucedo-Plascencia, Rafael Cortés-Zárate, Leonardo Hernandez-Hernandez, Teresa Arcelia Garcia-Cobian, Teresa Estrada-Garcia, and Araceli Castillo-Romero. 2025. "Curcumin Reverses Antibiotic Resistance and Downregulates Shiga Toxin Expression in Enterohemorrhagic E. coli" Diseases 13, no. 5: 154. https://doi.org/10.3390/diseases13050154
APA StyleZermeño-Ruiz, M., Cobos-Vargas, M., Saucedo-Plascencia, M. D., Cortés-Zárate, R., Hernandez-Hernandez, L., Garcia-Cobian, T. A., Estrada-Garcia, T., & Castillo-Romero, A. (2025). Curcumin Reverses Antibiotic Resistance and Downregulates Shiga Toxin Expression in Enterohemorrhagic E. coli. Diseases, 13(5), 154. https://doi.org/10.3390/diseases13050154







