Post-Liver Transplant Kidney Dysfunction: Incidence of Acute Kidney Injury and Chronic Kidney Disease and Risk Factors Related to Chronic Kidney Disease Development
Abstract
1. Introduction
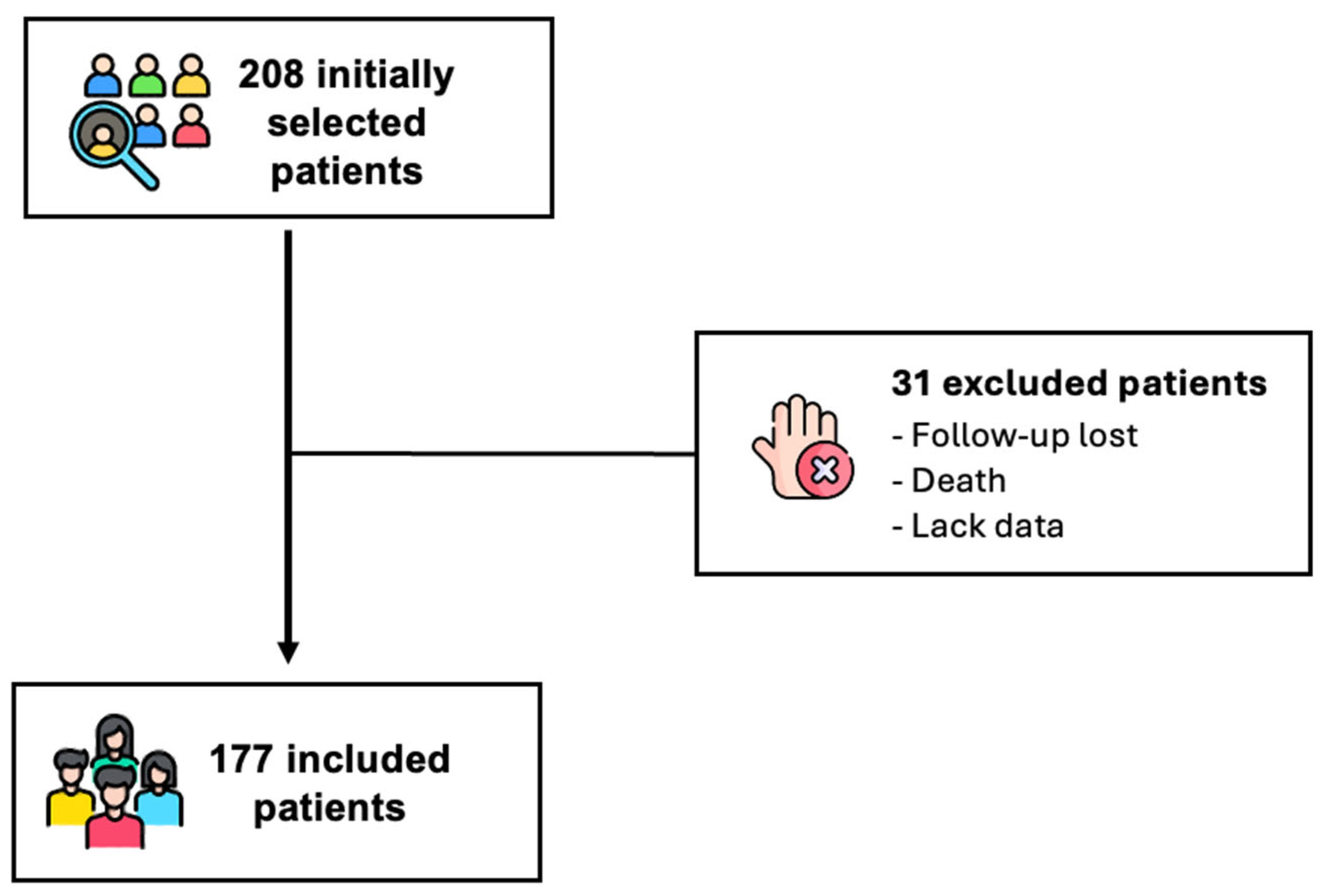
2. Methods
- Part 1: Retrospective Observational Study
- Part 2: Prospective Observational Study
2.1. Variables of Interest
2.2. Outcome Definition Criteria
2.3. Statistical Analysis
2.4. Ethical Considerations
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, L.; Jang, E.J.; Jo, J.W.; Shin, J.; Lee, H.; Lee, H.; Oh, S.-Y.; Im, H.; Ryu, H.G. Acute kidney injury after liver transplantation: Risk factors, management, and prognosis. Transplant. Proc. 2023, 55, 1230–1237. [Google Scholar] [CrossRef]
- Kassel, C.A.; Wilke, T.J.; Fremming, B.A.; Brown, B.A. 2021 Clinical Update in Liver Transplantation. J. Cardiothorac. Vasc. Anesth. 2022, 36, 4183–4191. [Google Scholar] [CrossRef]
- King, K.L.; Husain, S.A.; Cohen, D.J.; Schold, J.D.; Mohan, S. Incidence and impact of acute kidney injury after liver transplantation: A cohort study. Am. J. Transplant. 2022, 22, 2978–2987. [Google Scholar] [CrossRef]
- Hizomi, A.R.; Abbasi, M.R.; Mansournia, M.A.; Nassiri Toosi, M.; Jafarian, A.; Moosaie, F.; Karimi, E.; Moazzeni, S.S.; Abbasi, Z.; Shojamoradi, M.H. Acute Kidney Injury After Liver Transplant: Incidence, Risk Factors, and Impact on Patient Outcomes. Exp. Clin. Transplant. 2021, 19, 1277–1285. [Google Scholar] [CrossRef]
- Angeli, P.; Bezinover, D.; Biancofiore, G.; Bienholz, A.; Findlay, J.; Burtz, C.P.; Reyntjens, K.; Sakai, T.; Saner, F.H.; Tomescu, D.; et al. Acute kidney injury in liver transplant candidates: A position paper on behalf of the Liver Intensive Care Group of Europe. Minerva Anestesiol. 2017, 83, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, K.; Jahanzeb, Z.; Hafeez-ud-din; Ahmed, M.; Ilyas, A.; Ikram, S. Frequency of acute kidney injury in post-liver transplantation and associated factors: A systematic review. Bras. J. Nephrol. 2005; submitted for publication. [Google Scholar]
- Gonwa, T.A.; McBride, M.A.; Anderson, K.; Mai, M.L.; Wadei, H.; Ahsan, N. Continued influence of preoperative renal function on outcome of orthotopic liver transplant (OLTX) in the US: Where will MELD lead us? Am. J. Transplant. 2006, 6, 2651–2659. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Z.; Bi, J.F.; Liu, H.; Gao, Y. Risk factors for the incidence and severity of acute kidney injury after liver transplantation. Turk. J. Gastroenterol. 2021, 32, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Dong, V.; Nadim, M.K.; Karvellas, C.J. Post–Liver Transplant Acute Kidney Injury. Liver Transplant. 2021, 27, 1653–1664. [Google Scholar] [CrossRef]
- Levitsky, J.; O’Leary, J.G.; Asrani, S.; Sharma, P.; Fung, J.; Wiseman, A.; Niemann, C.U. Protecting the Kidney in Liver Transplant Recipients: Practice-Based Recommendations From the American Society of Transplantation Liver and Intestine Community of Practice. Am. J. Transplant. 2016, 16, 2532–2544. [Google Scholar] [CrossRef]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef]
- Gojowy, D.; Kubis, P.; Gorecka, M.; Karkoszka, H.; Wiecek, A.; Adamczak, M. Chronic Kidney Disease in Patients After Liver Transplantation: A Long-Term Retrospective Analysis From 1 Transplantation Center. Transplant. Proc. 2020, 52, 2492–2496. [Google Scholar] [CrossRef]
- Cullaro, G.; Verna, E.C.; Lee, B.P.; Lai, J.C. Chronic Kidney Disease in Liver Transplant Candidates: A Rising Burden Impacting Post-Liver Transplant Outcomes. Liver Transpl. 2020, 26, 498–506. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Associação Brasileira de Transplante de Órgãos (ABTO). RBT 2023—População de Pacientes em Lista de Espera para Transplante e Transplantes Realizados no Brasil. Available online: https://site.abto.org.br/wpcontent/uploads/2024/03/RBT_2023-Populacao_Site.pdf (accessed on 27 December 2024).
- Allen, A.M.; Kim, W.R.; Therneau, T.M.; Larson, J.J.; Heimbach, J.K.; Rule, A.D. Chronic kidney disease and associated mortality after liver transplantation—A time-dependent analysis using measured glomerular filtration rate. J. Hepatol. 2014, 61, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Stilley, C.S.; DiMartini, A.F.; Tarter, R.E.; DeVera, M.; Sereika, S.; Dew, M.A.; King, J.; Flynn, W.B. Liver transplant recipients: Individual, social, and environmental resources. Prog. Transplant. 2010, 20, 68–74. [Google Scholar] [CrossRef]
- Caragata, R.; Wyssusek, K.H.; Kruger, P. Acute kidney injury following liver transplantation: A systematic review of published predictive models. Anaesth. Intensive Care. 2016, 44, 251–261. [Google Scholar] [CrossRef] [PubMed]
- von Zur-Mühlen, B.; Wintzell, V.; Levine, A.; Rosenlund, M.; Kilany, S.; Nordling, S.; Wadström, J. Healthcare resource use, cost, and sick leave following kidney transplantation in Sweden: A population-based, 5-year, retrospective study of outcomes: COIN. Ann. Transplant. 2018, 23, 852–866. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.W.; Lee, I.H.; Ahn, K.S.; Kim, J.D.; Kwak, S.G.; Choi, D.L. One-year follow-up of the changes in renal function after liver transplantation in patients without chronic kidney disease. Transplant. Proc. 2016, 48, 1190e3. [Google Scholar] [CrossRef]
- Niel, O.R.P.; Berthoux, F.; Albano, L.; Dahan, P.; Aoudia, R.; Gugenheim, J.; Cassuto, E. Long-Term Glomerular Filtration Rate in Liver Allograft Recipients According to the Type of CaL.C.ineurin Inhibitors. Transplant. Proc. 2009, 41, 3329–3932. [Google Scholar] [CrossRef]
- Duan, Y.; Li, Z.; Wang, X.; Cui, L.; Gao, Z.; Zhang, H. Risk Factors and Prognosis of New-Onset Chronic Kidney Disease Following Orthotopic Liver Transplantation: A Retrospective Case-Control Study. Med. Sci. Monit. 2021, 27, e931834. [Google Scholar] [CrossRef]
- Lim, S.Y.; Wang, R.; Tan, D.J.H.; Ng, C.H.; Lim, W.H.; Quek, J.; Syn, N.; Nah, B.K.Y.; Wong, E.T.; Huang, D.Q.; et al. A meta-analysis of the cumulative incidence, risk factors, and clinical outcomes associated with chronic kidney disease after liver transplantation. Transpl. Int. 2021, 34, 2524–2533. [Google Scholar] [CrossRef] [PubMed]
- Brasília (DF): Ministério da Saúde. CONITEC. Monitoramento da Incorporação de Tecnologias em Saúde: Monitoramento do Transplante Hepático no Brasil: 2000 a 2015. 2015. Available online: https://www.gov.br/conitec/pt-br/midias/radar/mt/monitoramento_transplante_hepatico_brasil_2000-2015.pdf (accessed on 31 January 2025).
| Variables * | n (%) |
|---|---|
| Age (years) | 53.61 ± 12.65 |
| MELD score at admission | 19.87 ± 8.16 |
| Creatinine at admission (mg/dL) | 0.83 ± 0.30 |
| eGFR at admission (mL/min/1.73 m2) | 99.90 ± 15.42 |
| Gender | |
| Female | 48 (27.1) |
| Male | 129 (72.9) |
| Diabetes Mellitus | |
| No | 85 (48.9) |
| Yes | 89 (51.1) |
| Hypertension | |
| No | 82 (47.1) |
| Yes | 92 (52.9) |
| Dyslipidemia | |
| No | 132 (76.3) |
| Yes | 41 (23.7) |
| Overweight/Obesity | |
| No | 116 (67.4) |
| Yes | 56 (32.6) |
| Indications for liver transplantation | |
| Cryptogenic cirrhosis | 13 (7.3) |
| Autoimune hepatitis | 11 (6.2) |
| NASH | 10 (5.6) |
| Other causes | 71 (40.1) |
| ALD | 45 (25.4) |
| HBV | 9 (5.1) |
| HCV | 63 (35.6) |
| Variables * | N (%) |
|---|---|
| AKI < 7 days PO (n = 177) | |
| No | 86 (48.6) |
| Yes | 91 (51.4) |
| AKI stage < 7 days PO (n = 91) | |
| KDIGO 1 | 57 (62.6) |
| KDIGO 2 | 17 (18.7) |
| KDIGO 3 | 17 (18.7) |
| RRT < 7 days PO (n = 91) | |
| No | 81 (89) |
| Yes | 10 (11) |
| CKD after 1 year PO (n = 177) | |
| No | 124 (70) |
| Yes | 53 (30) |
| CKD stage after 1 year PO (n = 53) | |
| G1 | 7 (13.2) |
| G2 | 12 (22.6) |
| G3A | 13 (24.5) |
| G3B | 17 (32.1) |
| G4 | 2 (3.8) |
| G5 | 2 (3.8) |
| CKD after 5 years PO (n = 177) | |
| No | 101 (57.1) |
| Yes | 76 (42.9) |
| CKD stage after 5 years PO (n = 76) | |
| G1 | 7 (9.2) |
| G2 | 8 (10.5) |
| G3A | 39 (38.1) |
| G3B | 20 (26.3) |
| G4 | 5 (6.6) |
| G5 | 7 (9.3) |
| Variables * | N (%) |
|---|---|
| AKI < 7 days PO (n = 177) | |
| No | 86 (48.6) |
| Yes | 91 (51.4) |
| CKD in the first year PO (n = 91) | |
| No | 67 (73.6) |
| Yes | 24 (26.4) |
| CKD after 5 years PO (n = 91) | |
| No | 50 (55) |
| Yes | 41 (45) |
| Variables | N (%) |
|---|---|
| Death | 29 (16.4) |
| Without AKI < 7 days or CKD < 5 years PO | 10 (34.5) |
| AKI < 7 days PO | 16 (55.2) |
| CKD < 5 years PO | 13 (44.8) |
| CKD and RRT < 5 years PO | 6 (20.7) |
| Variables * | Without CKD n (%) | With CKD n (%) | p-Value ** |
|---|---|---|---|
| Age (years) | 51.47 ± 13.75 | 58.16 ± 5.33 | 0.003 |
| MELD score at admission | 20.46 ± 8.85 | 18.79 ± 6.70 | 0.314 |
| Creatinine at admission (mg/dL) | 0.76 ± 0.26 | 0.97 ± 0.34 | 0.000 |
| eGFR at admission (mL/min/1.73 m2) | 100.96 ± 14.90 | 90.79 ± 14.91 | 0.000 |
| Gender | 0.247 | ||
| Female | 36 (29.8) | 12 (21.4) | |
| Male | 85 (70.2) | 44 (78.6) | |
| Diabetes Mellitus | 0.276 | ||
| No | 61 (51.7) | 24 (42.99) | |
| Yes | 57 (48.3) | 32 (57.1) | |
| Hypertension | 0.038 | ||
| No | 62 (52.5) | 20 (35.7) | |
| Yes | 56 (47.5) | 36 (64.3) | |
| Dyslipidemia | 0.255 | ||
| No | 93 (78.8) | 39 (70.9) | |
| Yes | 25 (21.2) | 16 (29.1) | |
| Overweight/obesity | 0.838 | ||
| No | 79 (66.9) | 37 (68.5) | |
| Yes | 39 (33.1) | 17 (31.5) | |
| AKI < 7 days PO | 0.003 | ||
| No | 68 (56.2) | 18 (32.1) | |
| Yes | 53 (43.8) | 38 (67.9) | |
| AKI stage < 7 days | 0.011 | ||
| No | 68 (56.2) | 18 (32.1) | |
| KDIGO 1 | 30 (24.8) | 27 (48.3) | |
| KDIGO 2 | 12 (9.9) | 5 (8.9) | |
| KDIGO 3 | 11 (9.1) | 6 (10.7) | |
| RRT < 7 days PO | 0.180 & | ||
| No | 112 (95.7) | 50 (90.9) | |
| Yes | 5 (4.3) | 5 (9.1) | |
| Cause of Liver Failure | 0.419 | ||
| Cryptogenic cirrhosis | 9 (7.4) | 4 (7.1) | |
| Autoimune Hepatitis | 8 (6.6) | 3 (5.4) | |
| NASH | 5 (4.1) | 5 (8.9) | |
| Others | 20 (16.5) | 6 (10.8) | |
| ALD | 34 (28.2) | 11 (19.6) | |
| HBV | 5 (4.1) | 4 (7.1) | |
| HCV | 40 (33.1) | 23 (41.1) |
| Preditors | Crude Odds | Adjusted Odds |
|---|---|---|
| AKI < 7 days after LT | 2.71 (1.39–5.27) | 2.72 (1.22–6.06) |
| Creatinine at admission | - | 7.74 (1.99–30.02) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moura, A.F.; Moura-Neto, J.A.; Ribeiro, B.d.M.; Oliveira, P.R.; Freitas, A.G.d.; Costa, A.L.; Moura-Landim, D.; Codes, L.; Bittencourt, P.L.; Cruz, C.M.S. Post-Liver Transplant Kidney Dysfunction: Incidence of Acute Kidney Injury and Chronic Kidney Disease and Risk Factors Related to Chronic Kidney Disease Development. Diseases 2025, 13, 144. https://doi.org/10.3390/diseases13050144
Moura AF, Moura-Neto JA, Ribeiro BdM, Oliveira PR, Freitas AGd, Costa AL, Moura-Landim D, Codes L, Bittencourt PL, Cruz CMS. Post-Liver Transplant Kidney Dysfunction: Incidence of Acute Kidney Injury and Chronic Kidney Disease and Risk Factors Related to Chronic Kidney Disease Development. Diseases. 2025; 13(5):144. https://doi.org/10.3390/diseases13050144
Chicago/Turabian StyleMoura, Ana Flavia, José A. Moura-Neto, Beatriz de Melo Ribeiro, Paula Ribeiro Oliveira, Arthur Guimarães de Freitas, Alessandra Lima Costa, Daniela Moura-Landim, Liana Codes, Paulo Lisboa Bittencourt, and Constança Margarida Sampaio Cruz. 2025. "Post-Liver Transplant Kidney Dysfunction: Incidence of Acute Kidney Injury and Chronic Kidney Disease and Risk Factors Related to Chronic Kidney Disease Development" Diseases 13, no. 5: 144. https://doi.org/10.3390/diseases13050144
APA StyleMoura, A. F., Moura-Neto, J. A., Ribeiro, B. d. M., Oliveira, P. R., Freitas, A. G. d., Costa, A. L., Moura-Landim, D., Codes, L., Bittencourt, P. L., & Cruz, C. M. S. (2025). Post-Liver Transplant Kidney Dysfunction: Incidence of Acute Kidney Injury and Chronic Kidney Disease and Risk Factors Related to Chronic Kidney Disease Development. Diseases, 13(5), 144. https://doi.org/10.3390/diseases13050144





