Labdane Diterpenoids from Leonotis ocymifolia with Selective Cytotoxic Activity Against HCC70 Breast Cancer Cell Line
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedure
2.2. Plant Material
2.3. Extraction and Isolation
2.4. Physico-Chemical Properties of Isolated Compounds 1-6
2.5. Cytotoxic Assay
2.6. Physiochemical Properties (ADME)
3. Results and Discussions
3.1. Isolated Compounds
3.2. Cytotoxic Activities
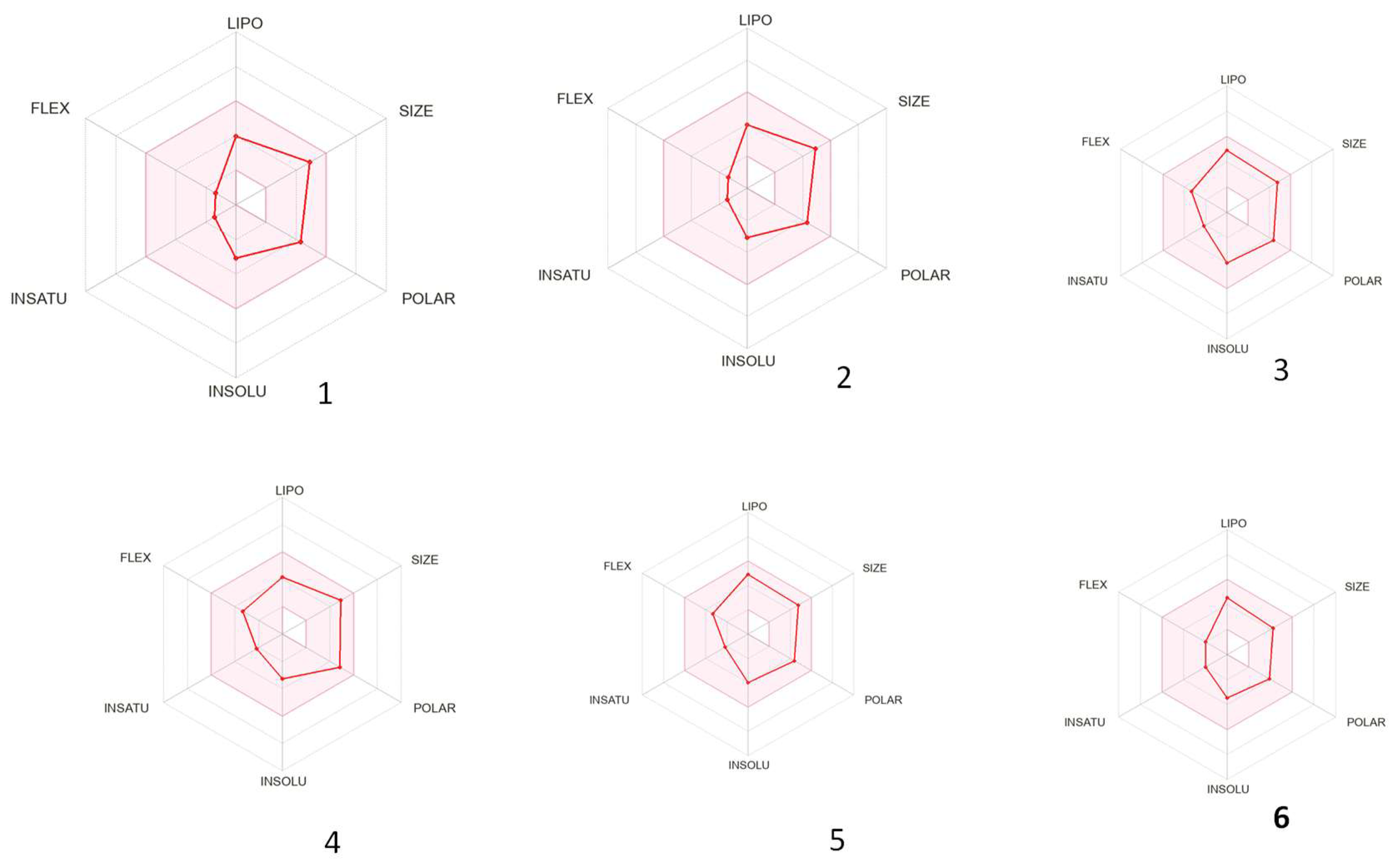
3.3. ADME Properties of the Isolated Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Howard, F.M.; Olopade, O.I. Epidemiology of triple-negative breast cancer: A review. Cancer J. 2021, 27, 8–16. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast cancer treatment: A review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Basmadjian, R.B.; Chow, K.; Kim, D.; Kenney, M.; Lukmanji, A.; O’Sullivan, D.E.; Xu, Y.; Quan, M.L.; Cheung, W.Y.; Lupichuk, S.; et al. The association between early-onset diagnosis and clinical outcomes in triple--negative breast cancer: A systematic review and meta-analysis. Cancers 2023, 15, 1923. [Google Scholar] [CrossRef] [PubMed]
- Godone, R.; Leitão, G.; Araújo, N.; Castelletti, C.; Lima-Filho, J.; Martins, D. Clinical and molecular aspects of breast cancer: Targets and therapies. Biomed. Pharmacother. 2018, 106, 14–34. [Google Scholar] [CrossRef]
- Iacopetta, D.; Ceramella, J.; Baldino, N.; Sinicropi, M.S.; Catalano, A. Targeting breast cancer: An overlook on current strategies. Int. J. Mol. Sci. 2023, 24, 3643. [Google Scholar] [CrossRef]
- Obidiro, O.; Battogtokh, G.; Akala, E.O. Triple- negative breast cancer treatment options and limitations: Future outlook. Pharmaceutics 2023, 15, 1796. [Google Scholar] [CrossRef]
- Habtemariam, S.; Gray, A.I.; Waterman, P.G. Diterpenes from the leaves of Leonotis ocymifolia var. raineriana. J. Nat. Prod. 1994, 57, 1570–1574. [Google Scholar] [CrossRef]
- Damtie, D. Review of Medicinal Plants Traditionally Used to Treat Diarrhea by the People in the Amhara Region of Ethiopia. Evid. Based Complement. Altern. Med. 2023, 2023, 8173543. [Google Scholar] [CrossRef]
- Kigen, G.; Some, F.; Kibosia, J.; Rono, H.; Kiprop, E.; Wanjohi, B.; Kigen, P.; Kipkore, W. Ethnomedicinal plants traditionally used by the keiyo community in Elgeyo Marakwet County, Kenya. J. Biodivers. Biopros. Dev. 2014, 1, 11. [Google Scholar] [CrossRef]
- Afolayan, A.; Mbaebie, B. Ethnobotanical study of medicinal plants used as anti-obesity remedies in Nkonkobe Municipality of South Africa. Phcog. J. 2010, 2, 368–373. [Google Scholar] [CrossRef]
- Scott, G.; Springfield, E.; Coldrey, N. A pharmacognostical study of 26 South African plant species used as traditional medicines. Pharm. Biol. 2004, 42, 186–213. [Google Scholar] [CrossRef]
- Mufti, A.; Feriani, A.; Ouchari, W.; Mandour, Y.M.; Tlili, N.; Ibrahim, M.A.; Mahmoud, M.F.; Sobeh, M. Leonotis ocymifolia (Burm. f.) Iwarsson aerial parts aqueous extract mitigates cisplatin-induced nephrotoxicity via attenuation of inflammation, and DNA damage. Front. Pharmacol. 2023, 14, 1221486. [Google Scholar] [CrossRef]
- Alemu, A.; Tamiru, W.; Nedi, T.; Shibeshi, W. Analgesic and anti-inflammatory effects of 80% methanol extract of Leonotis ocymifolia (burm. F.) Iwarsson leaves in rodent models. eCAM 2018, 2018, 1614793. [Google Scholar] [CrossRef] [PubMed]
- Eloff, J. The major differences in chemical composition and antibacterial activity of two closely related Leonotis species (Lamiaceae) may have taxonomic value. Suid-Afrik. Tydskr. Nat. Wet. Tegnol. 2010, 29, 30–38. [Google Scholar] [CrossRef]
- Mokoka, T.A. The Discovery and Characterization of Antiprotozoal Compounds from South African Medicinal Plants by a HPLC-Based Activity Profiling Technique. Ph.D. Thesis, University of KwaZulu-Natal, Durban, South Africa, 2013. [Google Scholar]
- Tafesse, G.; Mekonnen, Y.; Makonnen, E. In vivo and in vitro anti-fertility and anti-implantation properties of Leonotis ocymifolia in rats. Afr. J. Tradit. Complement. Altern. Med. 2005, 2, 103–112. Available online: https://www.journals.athmsi.org/index.php/ajtcam/article/view/43 (accessed on 28 June 2022). [CrossRef]
- Teklu, T.; Engidawork, E.; Nedi, T.; Teklehaymanot, T.; Gebremeskel, L. Evaluation of the antimalarial activity of the hydroalcoholic extract of leaf of Leonotis ocymifolia (Burm. f.) Iwarsson (Lamiaceae) against plasmodium berghei in mice. eCAM 2020, 2020, 5384804. [Google Scholar] [CrossRef]
- Nibret, E.; Wink, M. Trypanocidal and antileukaemic effects of the essential oils of Hagenia abyssinica, Leonotis ocymifolia, Moringa stenopetala, and their main individual constituents. Phytomedicine 2010, 17, 911–920. [Google Scholar] [CrossRef]
- Kinuthia, E.; Langat, M.; Mwangi, E.; Cheplogoi, P.; Njue, W. Terpenoids from Kenyan Leonotis mollissima. J. Pharmacogn. Phytochem. 2018, 7, 918–921. [Google Scholar]
- Hussein, A.A.; Meyer, M.J.; Rodríguez, B. Complete 1H and 13C NMR assignments of three labdane diterpenoids isolated from Leonotis ocymifolia and six other related compounds. Magn. Reson. Chem. 2003, 41, 147–151. [Google Scholar] [CrossRef]
- Satoh, M.; Satoh, Y.; Isobe, K.; Fujimoto, Y. Studies on the Constituents of Leonurus sibiricus L. Chem. Pharm. Bull. 2003, 51, 341–342. [Google Scholar] [CrossRef] [PubMed]
- De la Mare, J.-A.; Lawson, J.C.; Chiwakata, M.T.; Beukes, D.R.; Edkins, A.L.; Blatch, G.L. Quinones and halogenated monoterpenes of algal origin show anti-proliferative effects against breast cancer cells in vitro. Investig. New Drugs 2012, 30, 2187–2200. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. iLOGP: A simple, robust, and efficient description of n-octanol/water partition coefficient for drug design using the GB/SA approach. J. Chem. Inf. Model. 2014, 54, 3284–3301. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Brüstle, M.; Beck, B.; Schindler, T.; King, W.; Mitchell, T.; Clark, T. Descriptors, physical properties, and drug-likeness. J. Med. Chem. 2002, 45, 3345–3355. [Google Scholar] [CrossRef] [PubMed]
- Laonigro, G.; Lanzetta, R.; Parrilli, M.; Adinolfi, M.; Mangoni, L. The configuration of the diterpene spiroethers from Marrubium vulgare and from Leonotis leonurus. Gazz. Chim. Ital. 1979, 109, 145–150. Available online: http://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=PASCAL8050124930 (accessed on 1 June 2022).
- Ohsaki, A.; Kishimoto, Y.; Isobe, T.; Fukuyama, Y. New labdane diterpenoids from Hyptis fasciculata. Chem. Pharm. Bull. 2005, 53, 1577–1579. [Google Scholar] [CrossRef]
- White, J.D.; Manchand, P.S. Structure of nepetaefolin, a prefuranoid diterpene. J. Am. Chem. Soc. 1970, 92, 5527–5528. [Google Scholar] [CrossRef]
- Von Dreele, R.B.; Pettit, G.R.; Ode, R.H.; Perdue, R.E., Jr.; White, J.D.; Manchand, P.S. Crystal and molecular structure of the unusual spiro dihydrofuran diterpene nepetaefolin. J. Am. Chem. Soc. 1975, 97, 6236–6240. [Google Scholar] [CrossRef]
- Eagle, G.A.; Rivett, D.E. Diterpenoids of Leonotis species. Part IV. Dubiin, a furanoid labdane derivative from L. dubia E. Mey. J. Chem. Soc. Perkin Trans. 1973, 1, 1701–1704. [Google Scholar] [CrossRef]
- White, J.D.; Manchand, P.S. Structures of nepetaefolin, nepetaefuran, and nepetaefuranol. J. Org. Chem. 1973, 38, 720–728. [Google Scholar] [CrossRef]
- White, J.; Manchand, P.; Whalley, W. The structure of leonotin, a novel furanoid diterpene. J. Chem. Soc. D Chem. Commun. 1969, 1315–1316. [Google Scholar] [CrossRef]
- Li, J.; Fronczek, F.R.; Ferreira, D.; Burandt Jr, C.L.; Setola, V.; Roth, B.L.; Zjawiony, J.K. Bis-spirolabdane diterpenoids from Leonotis nepetaefolia. J. Nat. Prod. 2012, 75, 728–734. [Google Scholar] [CrossRef]
- Purushothaman, K.K.; Vasanth, S.; Connolly, J.D. Nepetaefolinol and two related diterpenoids from Leonotis nepetaefolia. J. Chem. Soc. Perkin Trans. 1974, 1, 2661–2663. [Google Scholar] [CrossRef]
- Mobbili, G.; Romaldi, B.; Sabbatini, G.; Amici, A.; Marcaccio, M.; Galeazzi, R.; Laudadio, E.; Armeni, T.; Minnelli, C. Identification of Flavone Derivative Displaying a 4′-Aminophenoxy Moiety as Potential Selective Anticancer Agent in NSCLC Tumor Cells. Molecules 2023, 28, 3239. [Google Scholar] [CrossRef] [PubMed]
- Park, K.J.; Khan, Z.; Subedi, L.; Kim, S.Y.; Lee, K.R. Antineurodegenerative labdane diterpenoid glycosides from the twigs of Pinus koraiensis. J. Nat. Prod. 2020, 83, 1794–1803. [Google Scholar] [CrossRef]
- Ueda, F.; Iizuka, K.; Tago, K.; Narukawa, Y.; Kiuchi, F.; Kasahara, T.; Tamura, H.; Funakoshi-tago, M. Nepetaefuran and leonotinin isolated from Leonotis nepetaefolia R. Br. potently inhibit the LPS signaling pathway by suppressing the transactivation of NF-κB. Int. Immunopharmacol. 2015, 28, 967–976. [Google Scholar] [CrossRef]
- Poma, P.; Labbozzetta, M.; D’Alessandro, N.; Notarbartolo, M. NF-κB is a potential molecular drug target in triple-negative breast cancers. OMICS 2017, 21, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.C.; Chou, Y.J.; Lin, C.C.; Liu, S.H.; Oswita, A.; Huang, Y.L.; Wang, Y.L.; Syu, J.L.; Sun, C.M.; Leu, C.M.; et al. Andrographolide and its potent derivative exhibit anticancer effects against imatinib-resistant chronic myeloid leukemia cells by downregulating the Bcr-Abl oncoprotein. Biochem. Pharmacol. 2019, 163, 308–320. [Google Scholar] [CrossRef]
- Tran, Q.T.N.; Wong, W.S.F.; Chai, C.L.L. Labdane diterpenoids as potential anti-inflammatory agents. Pharmacol. Res. 2017, 124, 43–63. [Google Scholar] [CrossRef]
- Franco, Y.E.M.; Okubo, M.Y.; Torre, A.M.; Paiva, P.; Rosa, P.N.M.; Silva, V.A.O.; Reis, R.M.; Ruiz, T.G.A.L.; Imamura, P.M.; de Carvalho, J.E.; et al. Coronarin D induces apoptotic cell death and cell cycle arrest in human glioblastoma cell line. Molecules 2019, 24, 4498. [Google Scholar] [CrossRef]
- Jia, C.Y.; Li, J.Y.; Hao, G.F.; Yang, G.F. A drug-likeness toolbox facilitates ADMET study in drug discovery. Drug Discov. Today 2020, 25, 248–258. [Google Scholar] [CrossRef]
- Durán-Iturbide, A.N.; Bárbara, I.D.; Medina-Franco, J.L. In Silico ADME/Tox Profiling of Natural Products: A Focus on BIOFACQUIM. ACS Omega 2020, 5, 16076–16084. [Google Scholar] [CrossRef]
- Liu, X.; Testa, B.; Fahr, A. Lipophilicity and its relationship with passive drug permeation. Pharm. Res. 2011, 28, 962–977. [Google Scholar] [CrossRef] [PubMed]
- Waring, M.J. Defining optimum lipophilicity and molecular weight ranges for drug candidates-molecular weight dependent lower logD limits based on permeability. Bioorg Med. Chem. Lett. 2009, 19, 2844–2851. [Google Scholar] [CrossRef]
- Gleeson, M.P.; Hersey, A.; Montanari, D.; Overington, J. Probing the links between in vitro potency, ADMET and physicochemical parameters. Nat. Rev. Drug Discov. 2011, 10, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.P. Generation of a set of simple, interpretable ADMET rules of thumb. J. Med. Chem. 2008, 51, 817–834. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]

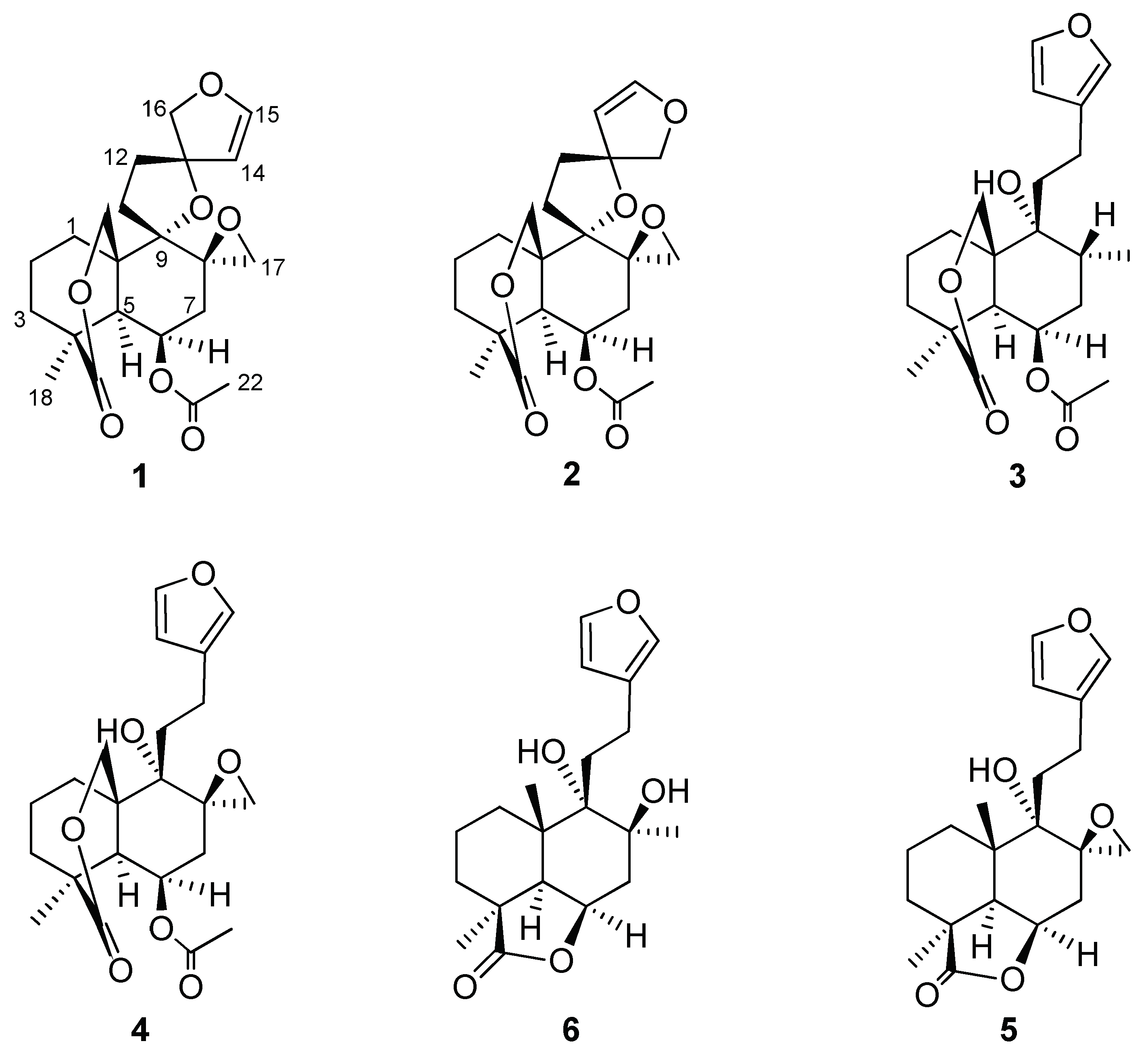

| Compound 1 | |||||
|---|---|---|---|---|---|
| Carbon No. | δ 1H NMR (J in Hz) | δ 13C NMR (J in Hz) | COSY | HMBC | NOESY |
| 1 α | 1.80 (1H, m) | 33.07 (CH2) | H-1 β | C-5 | |
| 1 β | 1.73 (1H, m) | H-1 α | C-5 | ||
| 2 α | 1.80 (1H, m) | 20.32 (CH2) | H-2 β | C-10, C-4 | |
| 2 β | 1.80 (1H, m) | H-2 α | C-10, C-4 | ||
| 3 α | 1.54 (1H, m) | 39.69 (CH2) | H-3 β | C-4 | |
| 3 β | 1.84 (1H, m) | H-3 α | C-4 | ||
| 4 | - | 40.77 (C) | - | - | - |
| 5 | 2.01 (1H, d, J = 3.6 Hz) | 46.98 (CH) | H-6 | C-20, C-10, C-19 | H-6 |
| 6 | 5.18 (1H, d, J = 3.4 Hz) | 67.97 (CH) | H-5 | H-18, H-7α, H-5 | |
| 7 α | 2.63 (1H, dd, J = 15.4, 3.4 Hz) | 32.43 (CH2) | H-7 β, H-6 | C-6, C-8, C-9, C-17 | H-6 |
| 7 β | 1.59 (1H, dd, J = 15.4, 3.4 Hz) | H-7α, H-6 | C-6, C-8, C-9, C-17 | ||
| 8 | - | 56.44 (C) | - | - | - |
| 9 | - | 85.92 (C) | - | - | - |
| 10 | - | 41.02 (C) | - | - | - |
| 11 α | 1.45 (1H, ddd, J = 14.0, 9.6, 4.9 Hz) | 23.63 (CH2) | H-11 β | C-13, C-9, C-12 | |
| 11 β | 1.76 (1H, m) | H-11 α | C-13, C-9, C-12 | ||
| 12 α | 1.95 (1H, m) | 37.46 (CH2) | H-12 β | C-9, C-15, C-13, C-16 | |
| 12 β | 2.18 (1H, m) | H-12 α | C-9, C-13, C-16 | ||
| 13 | - | 92.41 (C) | - | - | - |
| 14 | 4.88 (1H, d, J = 2.7 Hz) | 107.01 (CH) | H-15 | C16, C13, C15 | H-15, H-17β |
| 15 | 6.48 (1H, d, J = 2.7 Hz) | 149.43 (CH) | H-14 | C13, C16 | H-14 |
| 16 α | 4.03 (1H, d, J = 10.6 Hz) | 80.07 (CH2) | H-16 β | C13, C12 | |
| 16 β | 4.43 (1H, d, J = 10.6 Hz) | H-16 α | C15, C13 | ||
| 17 α | 2.34 (1H, d, J = 3.9 Hz) | 47.53 (CH2) | H-17 β | C-8 | |
| 17 β | 2.67 (1H, d, J = 3.9 Hz) | H-17 α | C-8 | H-14 | |
| 18 | 1.07 (3H, s) | 22.21 (CH3) | - | C-19, C-3, C5, C4 | H-6 |
| 19 | - | 175.91 (C) | - | - | - |
| 20 α | 3.97 (1H, d, J = 11.7 Hz) | 73.9 (CH2) | H-20 β | C1, C10, C5, C19 | |
| 20 β | 5.10 (1H, d, J = 11.7 Hz) | H-20 α | C9, C5, C1 | ||
| 21 | - | 170.33 (C) | - | - | - |
| 22 | 1.98 (3H, s) | 20.95 (CH3) | - | C-21 | |
| Compound | HCC70 (IC50 and SD) R2 | MCF-7 (IC50 and SD) R2 | MCF-12A (IC50 and SD) R2 | Selectivity Index (SI) MCF-7 | Selectivity Index (SI) HCC70 |
|---|---|---|---|---|---|
| 13S-Nepetaefolin (1) | 24.65 ± 1.18 0.9656 | NT | 26.55 ± 1.32 0.9968 | <0.132 | 1.08 |
| 13R-Nepetaefolin (2) | NT | NT | NT | N/A | N/A |
| Dubiin (3) | 127.90 ± 1.23 0.8173 | NT | NT | N/A | 1.56 |
| Nepetaefuran (4) | 73.66 ± 1.10 0.9689 | NT | NT | N/A | 2.72 |
| Leonotinin (6) | 94.89 ± 1.10 0.9417 | NT | NT | N/A | 2.11 |
| Leonotis DCM crude extract | 37.76 ± 1.78 0.9401 | NT | NT | N/A | 5.30 |
| Paclitaxel (nM) | 3.920 ± 1.03 0.9920 | 2.410 ± 1.11 0.9743 | 16.16 ± 1.08 0.9877 | 6.71 | 4.12 |
| Analysis | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Water solubility | ||||||
| LogS (ESOL) | −3.10 | −3.10 | −4.00 | −3.28 | −3.53 | −3.47 |
| Solubility | 3.18 × 10−1 mg/mL; 7.87 × 10−4 mol/L | 3.18 × 10−1 mg/mL; 7.87 × 10−4 mol/L | 2.13 × 10−1 mg/mL; 5.26 × 10−4 mol/L | 3.87 × 10−2 mg/mL; 9.92 × 10−5 mol/L | 1.04 × 10−1 mg/mL; 2.98 × 10−4 mol/L | 1.16 × 10−1 mg/mL; 3.35 × 10−4 mol/L |
| Class | Soluble | Soluble | Soluble | Moderately soluble | Soluble | Soluble |
| LogS (Ali) | −2.77 | −2.77 | −4.55 | −3.49 | −3.82 | −3.60 |
| Solubility | 1.66 × 10−1 mg/mL; 4.11 × 10−4 mol/L | 1.66 × 10−1 mg/mL; 4.11 × 10−4 mol/L | 8.26 × 10−3 mg/mL; 2.04 × 10−5 mol/L | 5.43 × 10−3 mg/mL; 1.39 × 10−5 mol/L | 5.23 × 10−2 mg/mL; 1.50 × 10−4 mol/L | 8.71 × 10−2 mg/mL; 2.52 × 10−4 mol/L |
| Class | Soluble | Soluble | Moderately soluble | Moderately soluble | Moderately soluble | Soluble |
| LogS (SILICOS-IT) | −3.39 | −3.39 | −4.69 | −4.86 | −4.57 | −4.77 |
| Solubility | 1.66 × 10−1 mg/mL; 4.11 × 10−4 mol/L | 1.66 × 10−1 mg/mL; 4.11 × 10−4 mol/L | 8.26 × 10−3 mg/mL; 2.04 × 10−5 mol/L | 5.43 × 10−3 mg/mL; 1.39 × 10−5 mol/L | 9.49 × 10−3 mg/mL; 2.72 × 10−5 mol/L | 5.94 × 10−3 mg/mL; 1.71 × 10−5 mol/L |
| Class | Soluble | Soluble | Moderately soluble | Moderately soluble | Moderately soluble | Moderately soluble |
| Physiochemical properties | ||||||
| No. of heavy atoms | 29 | 29 | 28 | 29 | 25 | 25 |
| No. of aromatic heavy atoms | 0 | 0 | 5 | 5 | 5 | 5 |
| No. of rotatable bonds | 2 | 2 | 5 | 5 | 3 | 3 |
| No. of H-bonds acceptors | 7 | 7 | 6 | 7 | 5 | 5 |
| No. of H-bonds donors | 0 | 0 | 1 | 1 | 2 | 2 |
| Molar refractivity | 100.13 | 100.13 | 102.30 | 101.31 | 96.60 | 72.20 |
| Gastrointestinal absorption | High | High | High | High | High | High |
| CYPC19 inhibitor | No | No | No | No | No | No |
| CYP1A2 inhibitor | No | No | No | No | No | No |
| CYP2C9 inhibitor | No | No | No | No | No | No |
| CYP3A4 inhibitor | No | No | Yes | Yes | No | No |
| CYP2D6 inhibitor | No | No | No | Yes | Yes | Yes |
| Log Kp (skin penetration) in cm/s | −7.77 | −7.77 | −6.50 | −7.49 | −2.88 | −2.73 |
| P-gp substrate | No | No | No | No | Yes | Yes |
| Drug likeness | ||||||
| Lipinski | Yes; violation | Yes; 0 violation | Yes; 0 violation | Yes; 0 violation | Yes; 0 violation | Yes; 0 violation |
| Ghose | Yes | Yes | Yes | Yes | Yes | Yes |
| Veber | Yes | Yes | Yes | Yes | Yes | Yes |
| Egan | Yes | Yes | Yes | Yes | Yes | Yes |
| Muegge | Yes | Yes | Yes | Yes | Yes | Yes |
| Bioavailability score | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 |
| Medicinal chemistry | ||||||
| Lead likeness | No; Violation; MV < 350 | No; Violation; MV < 350 | No; Violation; MV < 350 | No; Violation; MV < 350 | Yes | Yes |
| Synthetic accessibility | 6.40 | 6.40 | 5.61 | 5.81 | 4.95 | 5.29 |
| Lipophilicity | ||||||
| Implicit logP (iLOGP) | 2.35 | 2.35 | 2.72 | 2.57 | 2.85 | 2.73 |
| LogPo/w (XLOGP3) | 1.41 | 1.41 | 1.80 | 3.08 | 2.50 | 2.44 |
| LogPo/w (WLOGP) | 2.27 | 2.27 | 2.40 | 3.26 | 2.84 | 2.85 |
| LogPo/w (MLOGP) | 1.62 | 1.62 | 1.48 | 2.28 | 1.93 | 1.93 |
| LogPo/w (SILICOS-IT) | 3.02 | 3.02 | 3.65 | 3.68 | 3.46 | 3.95 |
| Consensus logPo/w | 2.13 | 2.13 | 3.00 | 2.38 | 2.72 | 2.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ncongwane, J.B.; Tembu, V.J.; Nkambule, C.M.; Kemboi, D.; Fouche, G.; Vukea, N.; de la Mare, J.-A. Labdane Diterpenoids from Leonotis ocymifolia with Selective Cytotoxic Activity Against HCC70 Breast Cancer Cell Line. Diseases 2025, 13, 140. https://doi.org/10.3390/diseases13050140
Ncongwane JB, Tembu VJ, Nkambule CM, Kemboi D, Fouche G, Vukea N, de la Mare J-A. Labdane Diterpenoids from Leonotis ocymifolia with Selective Cytotoxic Activity Against HCC70 Breast Cancer Cell Line. Diseases. 2025; 13(5):140. https://doi.org/10.3390/diseases13050140
Chicago/Turabian StyleNcongwane, Jane Busisiwe, Vuyelwa Jacqueline Tembu, Comfort Mduduzi Nkambule, Douglas Kemboi, Gerda Fouche, Nyeleti Vukea, and Jo-Anne de la Mare. 2025. "Labdane Diterpenoids from Leonotis ocymifolia with Selective Cytotoxic Activity Against HCC70 Breast Cancer Cell Line" Diseases 13, no. 5: 140. https://doi.org/10.3390/diseases13050140
APA StyleNcongwane, J. B., Tembu, V. J., Nkambule, C. M., Kemboi, D., Fouche, G., Vukea, N., & de la Mare, J.-A. (2025). Labdane Diterpenoids from Leonotis ocymifolia with Selective Cytotoxic Activity Against HCC70 Breast Cancer Cell Line. Diseases, 13(5), 140. https://doi.org/10.3390/diseases13050140






