Low-Carbohydrate (Ketogenic) Diet in Children with Obesity: Part 1—Diet Impact on Anthropometric Indicators and Indicators of Metabolic Syndrome and Insulin Resistance
Abstract
1. Introduction
2. Materials and Methods
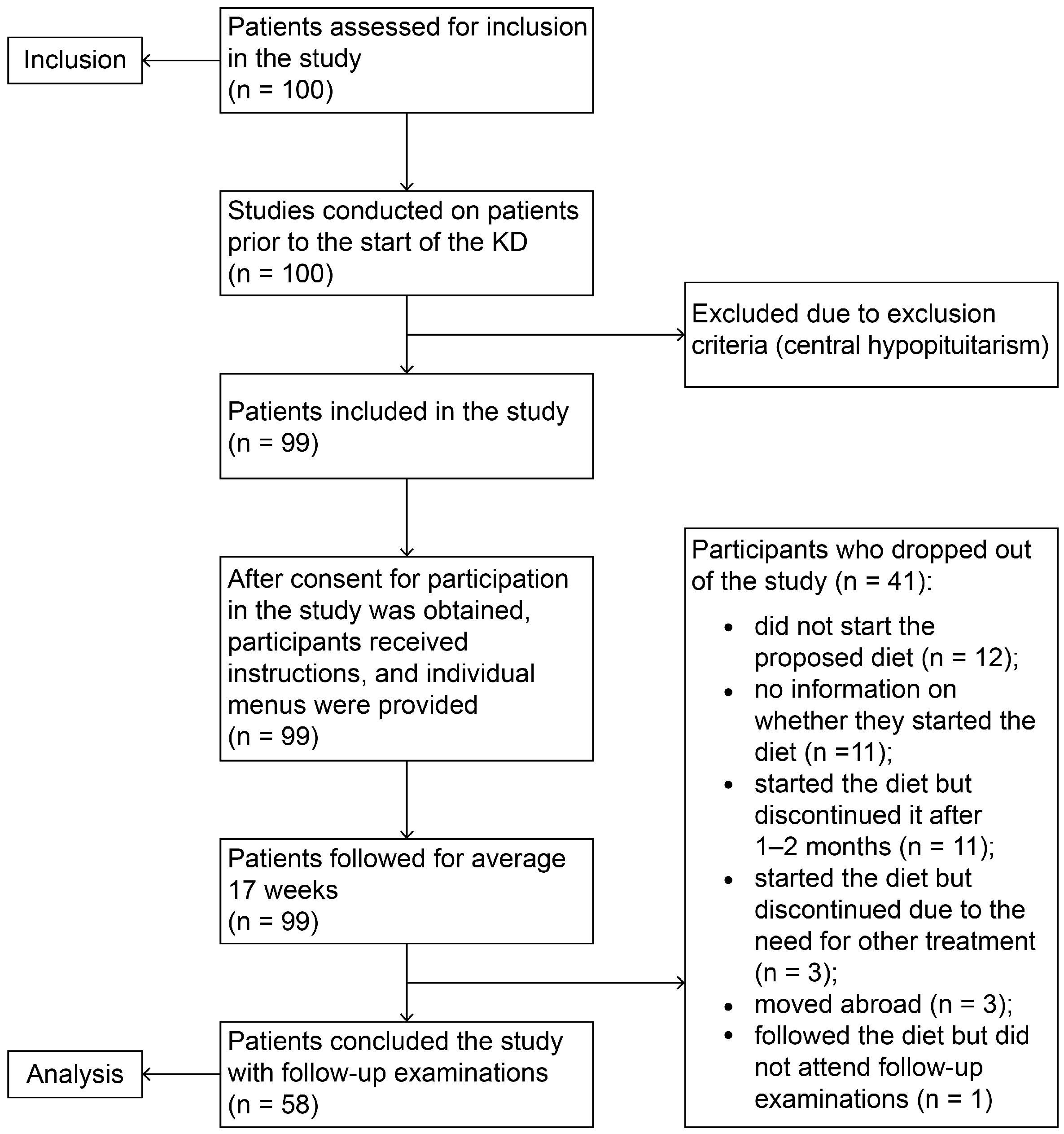
2.1. Participants Selection and Study Design
2.2. Characteristics of the Group of 58 Patients Who Completed the Study
2.3. Ketogenic Diet
- Carbohydrate intake: up to 40 g per day, divided into 10–13 g per meal.
- Protein intake: 1–1.5 g per kilogram of ideal body weight per day.
- Fat intake: enough to induce satiety without excessive consumption.
- Three to four meals per day: breakfast, lunch, dinner, and a small afternoon snack if hungry.
- Calorie counting is not necessary. Patients should eat until satisfied while adhering to the guidelines given in the individual menu. Skipping a meal is permissible if not hungry, but prolonged and intentional starvation is not recommended.
- Preference for natural foods: meat, fish, full-fat dairy products, eggs, low-carbohydrate vegetables, and a small number of low-carb fruits.
- Avoidance of processed, packaged foods, soft drinks, sweetened juices, and liquids.
- Fluid intake without sugar: 30–40 mL per kilogram per day.
2.4. Clinical Investigations
- Skin and its appendages (acanthosis nigricans, striae, acne, and the presence of increased hair in androgen-dependent areas in pubertal girls).
- Distribution of increased adipose tissue in different parts of the body.
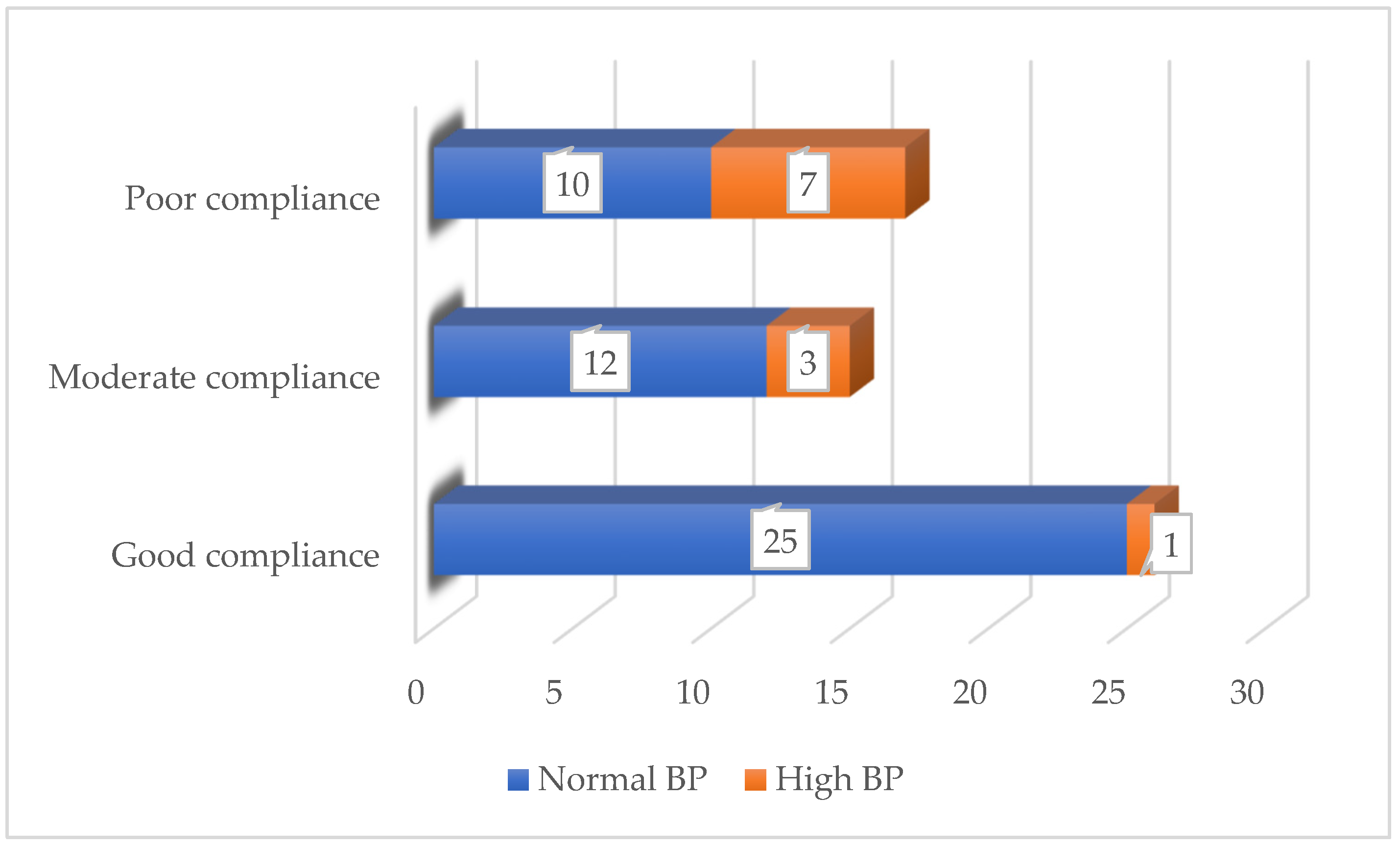
- Cardiovascular system: measurement of heart rate and rhythm by auscultation; measurement of blood pressure (BP) with an age-appropriate sphygmomanometer under standard conditions. AH was diagnosed and classified according to the criteria of the European Society of Hypertension [25] based on historical data or BP measurements by parents and/or the research team.
2.5. Anthropometric Measurements Included
- Height, weight, and waist circumference.
- Calculation of BMI and waist-to-height ratio.
2.6. Laboratory Investigations
- Oral glucose tolerance test (OGTT) with measurement of blood glucose and insulin at 0, 30, 60, and 120 min.
- Complete blood count, glycated hemoglobin, analyzed on an automated hematological analyzer Advita 2120, Siemens Healthcare Diagnostics INC., Erlangen, Germany.
- Biochemical parameters: Lipid profile (total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides), transaminases (ALT, AST), gamma-glutamyl transferase (GGT), urea, creatinine, uric acid, analyzed using original turbidimetric and immunoturbidimetric programs on a biochemical analyzer AU 480, Olympus; Beckman Coulter, Inc., Co Clare, Ireland.
- Hormones: Insulin, thyroid hormones (TSH, T3, T4), cortisol, testosterone, LH, FSH, analyzed using chemiluminescent immunoassay (CLIA) on an automated immunochemical analyzer Access 2, Beckman Coulter, Inc., Ireland.
- HOMA-IR and QUICKI indices were calculated (using online calculators) [26].
- Fatty liver index (FLI) was calculated [27].
- Urine analysis for semi-quantitative assessment of sugar and acetone; urine calcium/creatinine ratio (calculated UCa/UCr in mmol/L).
- Adiponectin level was measured using the BioVendor Human Adiponectin ELISA test.
2.7. Ultrasonographic Investigations
- Echocardiogram.
- Ultrasound examination of the urinary tract system and liver.
2.8. Statistical Analysis
3. Results
3.1. Anthropometric Indicators
3.2. Indicators of Glucose Metabolism, Insulin Resistance, Impaired Metabolic Health, and Metabolic Syndrome
3.3. Side Effects and Complaints
3.4. Sensitivity Analysis
3.5. Effect Sizes
4. Discussion
Limitations and Issues of Concern
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lobstein, T.; Jackson-Leach, R.; Powis, J.; Brinsden, H.; Gray, M. World Obesity Atlas 2023; World Obesity Federation: London, UK, 2023; Available online: https://policycommons.net/artifacts/3454894/untitled/4255209/ (accessed on 5 April 2024).
- Word Health Organization. Available online: https://www.who.int/europe/publications/i/item/9789289057738 (accessed on 5 April 2024).
- Boutari, C.; Mantzoros, C.S. A 2022 update on the epidemiology of obesity and a call to action: As its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism 2022, 133, 155217. [Google Scholar] [CrossRef] [PubMed]
- World Obesity Federation. COVID-19 and Obesity: The 2021 Atlas; World Obesity Federation: London, UK; Available online: https://www.worldobesity.org/resources/resource-library/covid-19-and-obesity-the-2021-atlas (accessed on 5 April 2024).
- Moschonis, G.; Siopis, G.; Anastasiou, C.; Iotova, V.; Stefanova, T.; Dimova, R.; Rurik, I.; Radó, A.S.; Cardon, G.; De Craemer, M.; et al. On Behalf Of The Feel Diabetes-Study Group. Prevalence of Childhood Obesity by Country, Family Socio-Demographics, and Parental Obesity in Europe: The Feel4Diabetes Study. Nutrients 2022, 14, 1830. [Google Scholar] [CrossRef]
- Gonzalez, D.O.; Michalsky, M.P. Update on pediatric metabolic and bariatric surgery. Pediatr. Obes. 2021, 16, e12794. [Google Scholar] [CrossRef]
- Mazur, A.; Zachurzok, A.; Baran, J.; Dereń, K.; Łuszczki, E.; Weres, A.; Wyszyńska, J.; Dylczyk, J.; Szczudlik, E.; Drożdż, D.; et al. Childhood Obesity: Position Statement of Polish Society of Pediatrics, Polish Society for Pediatric Obesity, Polish Society of Pediatric Endocrinology and Diabetes, the College of Family Physicians in Poland and Polish Association for Study on Obesity. Nutrients 2022, 14, 3806. [Google Scholar] [CrossRef] [PubMed]
- Valerio, G.; Maffeis, C.; Saggese, G.; Ambruzzi, M.A.; Balsamo, A.; Bellone, S.; Bergamini, M.; Bernasconi, S.; Bona, G.; Calcaterra, V.; et al. Diagnosis, treatment and prevention of pediatric obesity: Consensus position statement of the Italian Society for Pediatric Endocrinology and Diabetology and the Italian Society of Pediatrics. Ital. J. Pediatr. 2018, 44, 88. [Google Scholar] [CrossRef]
- Alman, K.L.; Lister, N.B.; Garnett, S.P.; Gow, M.L.; Aldwell, K.; Jebeile, H. Dietetic management of obesity and severe obesity in children and adolescents: A scoping review of guidelines. Obes. Rev. 2021, 22, e13132. [Google Scholar] [CrossRef]
- Hill, J.O.; Commerford, R. Physical activity, fat balance, and energy balance. Int. J. Sport Nutr. 1996, 6, 80–92. [Google Scholar] [CrossRef]
- Hill, J.O.; Levine, J.S.; Saris, W.H.M. Energy expenditure and physical activity. In Handbook of Obesity, 2nd ed.; Bray, G., Bouchard, C., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2003; pp. 631–654. [Google Scholar]
- Ludwig, D.S.; Ebbeling, C.B.; Bikman, B.T.; Johnson, J.D. Testing the carbohydrate-insulin model in mice: The importance of distinguishing primary hyperinsulinemia from insulin resistance and metabolic dysfunction. Mol. Metab. 2020, 35, 100960. [Google Scholar] [CrossRef]
- Ludwig, D.S.; Aronne, L.J.; Astrup, A.; de Cabo, R.; Cantley, L.C.; Friedman, M.I.; Heymsfield, S.B.; Johnson, J.D.; King, J.C.; Krauss, R.M.; et al. The carbohydrate-insulin model: A physiological perspective on the obesity pandemic. Am. J. Clin. Nutr. 2021, 114, 1873–1885. [Google Scholar] [CrossRef]
- Ludwig, D.S.; Ebbeling, C.B. The Carbohydrate-Insulin Model of Obesity: Beyond “Calories In, Calories Out”. JAMA Intern. Med. 2018, 178, 1098–1103. [Google Scholar] [CrossRef]
- Soto-Mota, A.; Pereira, M.A.; Ebbeling, C.B.; Aronica, L.; Ludwig, D.S. Evidence for the carbohydrate-insulin model in a reanalysis of the Diet Intervention Examining The Factors Interacting with Treatment Success (DIETFITS) trial. Am. J. Clin. Nutr. 2023, 117, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Kossoff, E.H.; Turner, Z.R.; Cervenka, M.C.; Barron, B.J. Ketogenic Diet Therapies for Epilepsy and Other Conditions, 7th ed.; Springer Publishing Company: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Brehm, B.J.; Seeley, R.J.; Daniels, S.R.; D’Alessio, D.A. A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J. Clin. Endocrinol. Metab. 2003, 88, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, O.; Tasabehji, M.W.; Elseaidy, T.; Tomah, S.; Ashrafzadeh, S.; Mottalib, A. Fat Versus Carbohydrate-Based Energy-Restricted Diets for Weight Loss in Patients with Type 2 Diabetes. Curr. Diab. Rep. 2018, 18, 128. [Google Scholar] [CrossRef] [PubMed]
- Bueno, N.B.; de Melo, I.S.; de Oliveira, S.L.; da Rocha, A.T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2013, 110, 1178–1187. [Google Scholar] [CrossRef]
- Sackner-Bernstein, J.; Kanter, D.; Kaul, S. Dietary Intervention for Overweight and Obese Adults: Comparison of Low-Carbohydrate and Low-Fat Diets. A Meta-Analysis. PLoS ONE 2015, 10, e0139817. [Google Scholar] [CrossRef]
- Shai, I.; Schwarzfuchs, D.; Henkin, Y.; Shahar, D.R.; Witkow, S.; Greenberg, I.; Golan, R.; Fraser, D.; Bolotin, A.; Vardi, H.; et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N. Engl. J. Med. 2008, 359, 229–241. [Google Scholar] [CrossRef]
- O’Neill, B.J. Effect of low-carbohydrate diets on cardiometabolic risk, insulin resistance, and metabolic syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 301–307. [Google Scholar] [CrossRef]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef]
- Volek, J.S.; Phinney, S.D. The Art and Science of Low Carbohydrate Living, 1st ed.; Beyond Obesity LLC: Miami, FL, USA, 2011. [Google Scholar]
- Lurbe, E.; Agabiti-Rosei, E.; Cruickshank, J.K.; Dominiczak, A.; Erdine, S.; Hirth, A.; Invitti, C.; Litwin, M.; Mancia, G.; Pall, D.; et al. European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J. Hypertens. 2016, 34, 1887–1920. [Google Scholar] [CrossRef]
- Omni Calculator sp. z o.o. Available online: https://www.omnicalculator.com/health/homa-ir (accessed on 5 April 2024).
- MDCalc. Available online: https://www.mdcalc.com/calc/10001/fatty-liver-index (accessed on 5 April 2024).
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B.; et al. Waist circumference as a vital sign in clinical practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar] [CrossRef]
- Neeland, I.J.; Ross, R.; Després, J.P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Tang, H.; Huang, P.; Wang, J.; Deng, P.; Li, Y.; Zheng, J.; Weng, L. Assessment of causal effects of visceral adipose tissue on risk of cancers: A Mendelian randomization study. Int. J. Epidemiol. 2022, 51, 1204–1218. [Google Scholar] [CrossRef] [PubMed]
- Lasbleiz, A.; Gaborit, B.; Soghomonian, A.; Bartoli, A.; Ancel, P.; Jacquier, A.; Dutour, A. COVID-19 and Obesity: Role of Ectopic Visceral and Epicardial Adipose Tissues in Myocardial Injury. Front. Endocrinol. 2021, 12, 726967. [Google Scholar] [CrossRef] [PubMed]
- Cerhan, J.R.; Moore, S.C.; Jacobs, E.J.; Kitahara, C.M.; Rosenberg, P.S.; Adami, H.O.; Ebbert, J.O.; English, D.R.; Gapstur, S.M.; Giles, G.G.; et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin. Proc. 2014, 89, 335–345. [Google Scholar] [CrossRef]
- Zhang, C.; Rexrode, K.M.; van Dam, R.M.; Li, T.Y.; Hu, F.B. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: Sixteen years of follow-up in US women. Circulation 2008, 117, 1658–1667. [Google Scholar] [CrossRef]
- Schleinitz, D.; Böttcher, Y.; Blüher, M.; Kovacs, P. The genetics of fat distribution. Diabetologia 2014, 57, 1276–1286. [Google Scholar] [CrossRef]
- Pulit, S.L.; Stoneman, C.; Morris, A.P.; Wood, A.R.; Glastonbury, C.A.; Tyrrell, J.; Yengo, L.; Ferreira, T.; Marouli, E.; Ji, Y.; et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum. Mol. Genet. 2019, 28, 166–174. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, N.; Yin, N.; Liu, R.; He, Y.; Li, D.; Tong, M.; Gao, A.; Lu, P.; Zhao, Y.; et al. The rs1421085 variant within FTO promotes brown fat thermogenesis. Nat. Metab. 2023, 5, 1337–1351. [Google Scholar] [CrossRef]
- Partsalaki, I.; Karvela, A.; Spiliotis, B.E. Metabolic impact of a ketogenic diet compared to a hypocaloric diet in obese children and adolescents. J. Pediatr. Endocrinol. Metab. 2012, 25, 697–704. [Google Scholar] [CrossRef]
- Pauley, M.; Mays, C.; Bailes, J.R., Jr.; Schwartzman, M.L.; Castle, M.; McCoy, M.; Patick, C.; Preston, D.; Nudelman, M.J.R.; Denning, K.L.; et al. Carbohydrate-Restricted Diet: A Successful Strategy for Short-Term Management in Youth with Severe Obesity-An Observational Study. Metab. Syndr. Relat. Disord. 2021, 19, 281–287. [Google Scholar] [CrossRef]
- Flemming, C.; Bussler, S.; Körner, A.; Kiess, W. Definition and early diagnosis of metabolic syndrome in children. J. Pediatr. Endocrinol. Metab. 2020, 33, 821–833. [Google Scholar] [CrossRef]
- Blüher, M. The distinction of metabolically ’healthy’ from ’unhealthy’ obese individuals. Curr. Opin. Lipidol. 2010, 21, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Moran, A.; Jacobs, D.R., Jr.; Steinberger, J.; Hong, C.P.; Prineas, R.; Luepker, R.; Sinaiko, A.R. Insulin resistance during puberty: Results from clamp studies in 357 children. Diabetes 1999, 48, 2039–2044. [Google Scholar] [CrossRef] [PubMed]
- Page, M.M.; Johnson, J.D. Mild Suppression of Hyperinsulinemia to Treat Obesity and Insulin Resistance. Trends Endocrinol. Metab. 2018, 29, 389–399. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.; DeFronzo, R.A. Insulin Resistance and Hyperinsulinemia: The Egg and the Chicken. J. Clin. Endocrinol. Metab. 2021, 106, e1897–e1899. [Google Scholar] [CrossRef]
- Le Stunff, C.; Bougnères, P. Early changes in postprandial insulin secretion, not in insulin sensitivity, characterize juvenile obesity. Diabetes 1994, 43, 696–702. [Google Scholar] [CrossRef]
- McAuley, K.A.; Williams, S.M.; Mann, J.I.; Walker, R.J.; Lewis-Barned, N.J.; Temple, L.A.; Duncan, A.W. Diagnosing insulin resistance in the general population. Diabetes Care 2001, 24, 460–464. [Google Scholar] [CrossRef]
- Staverosky, T. Ketogenic Weight Loss: The Lowering of Insulin Levels Is the Sleeping Giant in Patient Care. J. Med. Pract. Manag. 2016, 32, 63–66. [Google Scholar] [PubMed]
- Paoli, A.; Mancin, L.; Giacona, M.C.; Bianco, A.; Caprio, M. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J. Transl. Med. 2020, 18, 104. [Google Scholar] [CrossRef]
- Volek, J.S.; Sharman, M.J.; Gómez, A.L.; DiPasquale, C.; Roti, M.; Pumerantz, A.; Kraemer, W.J. Comparison of a very low-carbohydrate and low-fat diet on fasting lipids, LDL subclasses, insulin resistance, and postprandial lipemic responses in overweight women. J. Am. Coll. Nutr. 2004, 23, 177–184. [Google Scholar] [CrossRef]
- Krebs, N.F.; Gao, D.; Gralla, J.; Collins, J.S.; Johnson, S.L. Efficacy and safety of a high protein, low carbohydrate diet for weight loss in severely obese adolescents. J. Pediatr. 2010, 157, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Sondike, S.B.; Copperman, N.; Jacobson, M.S. Effects of a low-carbohydrate diet on weight loss and cardiovascular risk factor in overweight adolescents. J. Pediatr. 2003, 142, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Santoleri, D.; Titchenell, P.M. Resolving the Paradox of Hepatic Insulin Resistance. Cell Mol. Gastroenterol. Hepatol. 2019, 7, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Campbell-Sargent, C.; Mirshahi, F.; Rizzo, W.B.; Contos, M.J.; Sterling, R.K.; Luketic, V.A.; Shiffman, M.L.; Clore, J.N. Nonalcoholic steatohepatitis: Association of insulin resistance and mitochondrial abnormalities. Gastroenterology 2001, 120, 1183–1192. [Google Scholar] [CrossRef]
- Tendler, D.; Lin, S.; Yancy, W.S., Jr.; Mavropoulos, J.; Sylvestre, P.; Rockey, D.C.; Westman, E.C. The effect of a low-carbohydrate, ketogenic diet on nonalcoholic fatty liver disease: A pilot study. Dig. Dis. Sci. 2007, 52, 589–593. [Google Scholar] [CrossRef]
- Luukkonen, P.K.; Dufour, S.; Lyu, K.; Zhang, X.M.; Hakkarainen, A.; Lehtimäki, T.E.; Cline, G.W.; Petersen, K.F.; Shulman, G.I.; Yki-Järvinen, H. Effect of a ketogenic diet on hepatic steatosis and hepatic mitochondrial metabolism in nonalcoholic fatty liver disease. Proc. Natl. Acad. Sci. USA 2020, 117, 7347–7354. [Google Scholar] [CrossRef]
- D’Abbondanza, M.; Ministrini, S.; Pucci, G.; Nulli Migliola, E.; Martorelli, E.E.; Gandolfo, V.; Siepi, D.; Lupattelli, G.; Vaudo, G. Very Low-Carbohydrate Ketogenic Diet for the Treatment of Severe Obesity and Associated Non-Alcoholic Fatty Liver Disease: The Role of Sex Differences. Nutrients 2020, 12, 2748. [Google Scholar] [CrossRef]
- Goss, A.M.; Dowla, S.; Pendergrass, M.; Ashraf, A.; Bolding, M.; Morrison, S.; Amerson, A.; Soleymani, T.; Gower, B. Effects of a carbohydrate-restricted diet on hepatic lipid content in adolescents with non-alcoholic fatty liver disease: A pilot, randomized trial. Pediatr. Obes. 2020, 15, e12630. [Google Scholar] [CrossRef]
- Castellana, M.; Conte, E.; Cignarelli, A.; Perrini, S.; Giustina, A.; Giovanella, L.; Giorgino, F.; Trimboli, P. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2020, 21, 5–16. [Google Scholar] [CrossRef]
- Hession, M.; Rolland, C.; Kulkarni, U.; Wise, A.; Broom, J. Systematic review of randomized controlled trials of low-carbohydrate vs. low-fat/low-calorie diets in the management of obesity and its comorbidities. Obes. Rev. 2009, 10, 36–50. [Google Scholar] [CrossRef]
- Cicero, A.F.; Benelli, M.; Brancaleoni, M.; Dainelli, G.; Merlini, D.; Negri, R. Middle and Long-Term Impact of a Very Low-Carbohydrate Ketogenic Diet on Cardiometabolic Factors: A Multi-Center, Cross-Sectional, Clinical Study. High Blood Press. Cardiovasc. Prev. 2015, 22, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Verde, L.; Santangeli, P.; Lucà, S.; Docimo, A.; Savastano, S.; Colao, A.; Muscogiuri, G. Very low-calorie ketogenic diet (VLCKD): An antihypertensive nutritional approach. J. Transl. Med. 2023, 21, 128. [Google Scholar] [CrossRef] [PubMed]
- Civantos Modino, S.; Guijarro de Armas, M.G.; Monereo Mejías, S.; Montaño Martínez, J.M.; Iglesias Bolaños, P.; Merino Viveros, M.; Ladero Quesada, J.M. Hyperuricemia and metabolic syndrome in children with overweight and obesity. Endocrinol. Nutr. 2012, 59, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Hyde, P.N.; Sapper, T.N.; Crabtree, C.D.; LaFountain, R.A.; Bowling, M.L.; Buga, A.; Fell, B.; McSwiney, F.T.; Dickerson, R.M.; Miller, V.J.; et al. Dietary carbohydrate restriction improves metabolic syndrome independent of weight loss. JCI Insight 2019, 4, e128308. [Google Scholar] [CrossRef]
- Moreno-Sepúlveda, J.; Capponi, M. Dieta baja en carbohidratos y dieta cetogénica: Impacto en enfermedades metabólicas y reproductivas [The impact on metabolic and reproductive diseases of low-carbohydrate and ketogenic diets]. Rev. Med. Chil. 2020, 148, 1630–1639. [Google Scholar] [CrossRef]
- Gershuni, V.M.; Yan, S.L.; Medici, V. Nutritional Ketosis for Weight Management and Reversal of Metabolic Syndrome. Curr. Nutr. Rep. 2018, 7, 97–106. [Google Scholar] [CrossRef]
- Watson, F.; Taylor, A.; Rayner, M.; Lobstein, T.; Hinks, R. Priority actions for addressing the obesity epidemic in England. Public Health Nutr. 2018, 21, 1002–1010. [Google Scholar] [CrossRef]
- Kossoff, E.H.; Zupec-Kania, B.A.; Auvin, S.; Ballaban-Gil, K.R.; Christina Bergqvist, A.G.; Blackford, R.; Buchhalter, J.R.; Caraballo, R.H.; Cross, J.H.; Dahlin, M.G.; et al. Practice Committee of the Child Neurology Society. Optimal clinical management of children receiving dietary therapies for epilepsy: Updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open 2018, 3, 175–192. [Google Scholar] [CrossRef]
- Varesio, C.; Pasca, L.; Parravicini, S.; Zanaboni, M.P.; Ballante, E.; Masnada, S.; Ferraris, C.; Bertoli, S.; Tagliabue, A.; Veggiotti, P.; et al. Quality of Life in Chronic Ketogenic Diet Treatment: The GLUT1DS Population Perspective. Nutrients 2019, 11, 1650. [Google Scholar] [CrossRef]
| Variable | N = 58 |
|---|---|
| Age, years (x ± SD) | (13.79 ± 2.63) |
| Age groups, n (%) | |
| 8–10 years | 8 (13.79) |
| 11–15 years | 25 (43.10) |
| 16–18 years | 25 (43.10) |
| Gender, n (%) | |
| Female | 23 (39.66) |
| Male | 35 (60.34) |
| Comorbidities, n (%) | |
| Metabolic syndrome | 33 (56.89%) |
| One or more criteria for impaired metabolic health | 25 (43.10%) |
| Polycystic ovary syndrome | 8 (34.78%) |
| Primary arterial hypertension Hepatic steatosis | 27 (46.55%) 39 (67.24%) |
| Hashimoto’s autoimmune thyroiditis | 7 (12.07%) |
| Compliance with the diet, n (%) | |
| Good | 26 (44,83) |
| Moderate | 15 (25,86) |
| Poor | 17 (29,31) |
| Variable | Before KD (n = 58) | After KD (n = 58) | p-Value | |
|---|---|---|---|---|
| Anthropometric indicators | Weight, kg | 89.43 ± 3.30 | 82.98 ± 3.25 | <0.0001 |
| BMI, kg/m2 | 33.35 ± 7.29 | 30.23 ± 7.14 | <0.0001 | |
| Waist circumference, cm | 103.97 ± 17.17 | 91.38 ± 15.03 | 0.003 | |
| Waist-to-hip ratio | 0.64 ± 0.09 | 0.55 ± 0.08 | <0.0001 | |
| Laboratory indicators | Fasting glucose (mmol/L) | 4.94 ± 0,45 | 4.80 ± 0,45 | 0.07 |
| Fasting insulin (mlU/L) | 20.12 ± 8.88 | 13.17 ± 5.81 | <0.0001 | |
| HOMA-IR | 4.51 ± 2.20 | 2.85 ± 1.45 | <0.0001 | |
| QUICKI | 0.31 ± 0.019 | 0.33 ± 0.024 | <0.0001 | |
| Glycated hemoglobin (HbA1c) % | 5.21 ± 0.37 | 5.30 ± 0.38 | 0.27 | |
| Total cholesterol (mmol/L) | 4.38 ± 0.76 | 4.26 ± 0.91 | 0.25 | |
| HDL (mmol/L) | 1.17 ± 0.21 | 1.15 ± 0.22 | 0.45 | |
| LDL (mmol/L) | 2.739 ± 0.68 | 2.742 ± 0.75 | 0.97 | |
| Triglycerides (mmol/L) | 1.07 ± 0.45 | 0.88 ± 0.36 | 0.001 | |
| Triglycerides/HDL | 2.22 ± 1.13 | 1.83 ± 0.87 | <0.0001 | |
| Creatinine (mmol/L) | 63.48 ± 7.53 | 63.40 ± 10.19 | 0.924 | |
| Uric acid (µmol/L) | 380.59 ± 90.91 | 373.36 ± 91.65 | 0.37 | |
| Urinary calcium/creatinine ratio (mmol/mmol) | 0.195 ± 8.88 | 0.193 ± 5.81 | 0.71 | |
| ALT (IU/L) | 28.87 ± 35.49 | 22.62 ± 16.21 | 0.001 | |
| AST (IU/L) | 25.82 ± 12.86 | 23.30 ± 8.54 | 0.006 | |
| GGT (IU/L) | 24.17 ± 12.93 | 19.62 ± 8.72 | <0.0001 | |
| FLI | 60.60 ± 29.45 | 40.67 ± 31.91 | <0.0001 | |
| Adiponectin (mcg/mL) | 8.61 ± 3.61 | 9.13 ± 3.86 | 0.04 | |
| Reported Complaints and Symptoms During the Diet | Number of Patients | % |
|---|---|---|
| Constipation | 7 | 12.04 |
| Diarrhea | 4 | 6.88 |
| Abdominal pains | 4 | 6.88 |
| Fatigue | 10 | 17.20 |
| Hunger | 5 | 8.60 |
| Headache | 14 | 24.08 |
| Longer menstrual cycles (girls) | 1 | 1.72 |
| Change in breath | 1 | 1.72 |
| Nervousness | 1 | 1.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paskaleva, I.N.; Kaleva, N.N.; Dimcheva, T.D.; Markova, P.P.; Ivanov, I.S. Low-Carbohydrate (Ketogenic) Diet in Children with Obesity: Part 1—Diet Impact on Anthropometric Indicators and Indicators of Metabolic Syndrome and Insulin Resistance. Diseases 2025, 13, 94. https://doi.org/10.3390/diseases13040094
Paskaleva IN, Kaleva NN, Dimcheva TD, Markova PP, Ivanov IS. Low-Carbohydrate (Ketogenic) Diet in Children with Obesity: Part 1—Diet Impact on Anthropometric Indicators and Indicators of Metabolic Syndrome and Insulin Resistance. Diseases. 2025; 13(4):94. https://doi.org/10.3390/diseases13040094
Chicago/Turabian StylePaskaleva, Ivanka N., Nartsis N. Kaleva, Teodora D. Dimcheva, Petya P. Markova, and Ivan S. Ivanov. 2025. "Low-Carbohydrate (Ketogenic) Diet in Children with Obesity: Part 1—Diet Impact on Anthropometric Indicators and Indicators of Metabolic Syndrome and Insulin Resistance" Diseases 13, no. 4: 94. https://doi.org/10.3390/diseases13040094
APA StylePaskaleva, I. N., Kaleva, N. N., Dimcheva, T. D., Markova, P. P., & Ivanov, I. S. (2025). Low-Carbohydrate (Ketogenic) Diet in Children with Obesity: Part 1—Diet Impact on Anthropometric Indicators and Indicators of Metabolic Syndrome and Insulin Resistance. Diseases, 13(4), 94. https://doi.org/10.3390/diseases13040094







