Abstract
Background: Patients with chronic kidney disease (CKD) exhibit changes in leukocyte dynamics, leading to altered hematological and biochemical parameters and deteriorating kidney function. In this study, we aim to investigate the correlation between leukocyte subpopulations and hematological and biochemical parameters in patients with end-stage CKD undergoing hemodialysis. Methods: This descriptive, analytical, cross-sectional study included 20 end-stage CKD patients on hemodialysis. Leukocyte subpopulations, including classical monocytes (CD14++/CD16−), intermediate monocytes (CD14++/CD16+), non-classical monocytes (CD14+/CD16++), CD4 T lymphocytes (CD3+/CD4+), CD8 T lymphocytes (CD3+/CD8+), B lymphocytes (CD3−/CD19+), NK cells (CD56+/CD16+), and iNKT cells (CD3+/CD56+), were analyzed using flow cytometry. Results: Patients with end-stage CKD on hemodialysis have decreased classical monocytes and increased non-classical monocytes frequency. A positive correlation was observed between non-classical monocytes and total lymphocytes (Rho-Spearman: R = 0.495, p = 0.027) as well as B lymphocytes (R = 0.567, p < 0.05). We discerned the immunological characteristics of diabetic kidney disease (DKD) and CKD due to other causes in this balanced cohort: B lymphocytes negatively correlate with alkaline phosphatase (R = −0.764, p < 0.05), parathyroid hormone (R = −0.929, p < 0.05), and ferritin (R = −0.893, p < 0.05). Additionally, in DKD, non-classical monocytes positively correlate with eosinophils (R = +0.691; p = 0.019) and classic monocytes with neutrophils (R = +0.627, p = 0.039). Meanwhile, a correlation between either total T lymphocytes or helper T lymphocytes and serum albumin was detected on patients with nephropathy due to other causes. Conclusions: CKD alters classical and non-classical monocyte frequency, whilst T and B lymphocyte frequency positively correlates to the proinflammatory non-classical monocytes. In DKD patients, the uremic environment increases classic monocytes, CD16+ inflammatory monocytes, neutrophils, eosinophils, and B lymphocytes. The described leukocyte dynamic correlates with alkaline phosphatase, parathyroid hormone, iron, and serum albumin serological concentration.
1. Introduction
Chronic kidney disease (CKD) affects approximately 10% to 14% of the general population [1]. The causes of CKD are diverse, including type 2 diabetes mellitus (DM2). Indeed, the primary cause of end-stage CKD is diabetes (diabetic kidney disease, DKD), when renal replacement therapies such as peritoneal dialysis, hemodialysis, or kidney transplantation become necessary [2]. In Peru, up to 25% of patients with DM2 develop DKD [3].
Inflammation is a prominent factor of DKD, and its progression leads to end-stage CKD [4]. Immune function is compromised by the retention of uremic toxins, which activate innate immune cells and lead to the continuous production of cytokines and reactive oxygen species, resulting in tissue damage [5]. Dysfunctions and a reduction in the number of lymphocytes have been observed, contributing to immunosuppression and an increased risk of infection in patients [6,7]. Additionally, endothelial dysfunction, vascular calcification, oxidative stress, and inflammation are exacerbated, collectively accelerating the progression and severity of nephropathy [8,9].
End-stage CKD induces senescent T cells and monocytes, with an increased expression of cytotoxic CD8 T lymphocytes and inflammatory-profile monocytes. Intermediate monocytes (CD14++/CD16+) and non-classical monocytes (CD14+CD16++) contribute to kidney dysfunction in diabetic patients [10,11]. Classical monocytes (CD14++CD16−) are associated with phagocytic function and the initial inflammatory response, characterized by the expression of IL-10 and low levels of TNF-α, while CD16+ monocytes (both non-classical and intermediate) are involved in inflammatory and infectious processes, expressing higher levels of pro-inflammatory molecules. Non-classical monocytes stimulate the production of TNF-α and IL-1β [12].
In patients undergoing hemodialysis, CD16+ monocyte subpopulations have shown signs of altered maturation due to dialysis or uremia [13]. Although studies have documented that dysregulation of monocyte maturation and function is associated with DKD and its complications, it is not well characterized [4]. These patients exhibit persistent and progressive dysregulation in leukocyte subpopulations, with an increased tendency toward monocyte expansion, cytokine release, and free radical production, which exacerbate oxidative stress and endothelial dysfunction [14]. This dysregulation leads to impaired monocyte function in phagocytosis and antigen presentation. Alterations in T and B lymphocyte function, significant reductions in CD4+ T lymphocytes, and low CD4+/CD8+ ratios (with variations in the CD8+ population) are linked to an increased risk of infections [15,16].
The variability of leukocyte subpopulations and their association with oxidative stress and inflammation earn significant interest, particularly concerning their prognostic value and its correlation with hematological and biochemical parameters [5,6]. Identifying risk factors for disease progression and novel monitoring strategies for patients with chronic-degenerative diseases, such as end-stage CKD, remain critical priorities. This study aims to determine the correlation between leukocyte subpopulations and hematological and biochemical parameters in CKD patients, either diabetic or not, undergoing hemodialysis.
2. Materials and Methods
2.1. Study Description
This descriptive, analytical, and cross-sectional study was conducted on a population of patients with end-stage CKD receiving outpatient hemodialysis at the Private Nephrological Center (CENESA S.A.). All participants were referred from the Edgardo Rebagliati Martins National Hospital and were admitted to CENESA with a diagnosis, treatment plan, and the need for outpatient hemodialysis.
This study was conducted in two phases. The first phase involved collecting sociodemographic and clinical data from patients’ medical records, along with the results of various biochemical parameter assessments. The second phase focused on collecting blood samples for hematological assays. This study was conducted in accordance with the protocol approved by the Institutional Research Ethics Committee of the Faculty of Human Medicine at the University of San Martin de Porres (Official Letter No. 0104-2024-CIEI-FMH-USMP).
2.2. Patients
Considering an estimated population of 2.48 million elderly individuals in Peru with CKD, of which approximately 1.75% are in advanced stages and opt for hemodialysis [17], there is a population of 43,000 Peruvian patients over the age of 55 with end-stage CKD in hemodialysis. Using this value and a confidence level of 80%, the OpenEpi program (openepi.com) indicates that a sample of 17 individuals is required to achieve a power of 90%. Twenty patients aimed to participate and signed their informed consent to be included in the study. The etiology of end-stage CKD was registered based on referral reports from the reference hospital, which included their diagnosis, comorbidities, current treatment, and clinical history. Patients with hematological, oncological, autoimmune diseases, or acute infections at the time of blood sample collection were excluded from this study. All patients underwent hemodialysis using the Helixone® polysulfone membrane.
2.3. Sample Collection and Processing
Peripheral blood was collected in tubes with ethylenediaminetetraacetic acid (EDTA) (Becton-Dickinson, San José, CA, USA). To isolate peripheral blood mononuclear cells (PBMCs), blood samples were diluted in phosphate-buffered saline (1× PBS; 1:1 v:v), added to 3 mL of Ficoll-Paque PLUS (Histopaque®-1077, Sigma-Aldrich, St. Louis, MO, USA), and centrifuged at 2000 rpm (Thermo Scientific SL 8 Centrifuge, Thermo Fisher Scientific, Waltham, MA, USA), for 20 min, at room temperature. The PBMCs were then collected and washed twice with 1x PBS solution. PBMCs were counted in a Neubauer chamber.
2.4. Flow Cytometry
PBMCs (~6 × 106 per patient) were resuspended in a PBS solution with 10% fetal bovine serum and 0.1% sodium azide (FACS solution, catalog number 349202, BD FACSTM). To perform an immunophenotypic analysis of cell subpopulations, 5 µL/million PBMCs of fluorophore-conjugated monoclonal antibodies were used. T lymphocyte detection: PE-Cy7-CD3, APC-CD4, PerCP-Cy5.5-CD8, PE-CD45RA, FITC-CCR7; B lymphocyte detection: PercP-Cy5.5-CD19; monocyte detection: PE-Cy7-CD3, FITC-CD14, APC-CD16; NK and NKT lymphocyte detection: APC-CD16, PE-CD56, PE-Cy7-CD3 (catalog IDs and suppliers are reported in Table 1). The cells were incubated with the antibodies for 30 min at 4 °C, then washed 3 times by centrifugation at 2000 rpm for 5 min, and finally resuspended in 200 µL of solution.

Table 1.
Fluorophore-conjugated monoclonal antibodies for flow cytometry assays.
Differentiation of cell subpopulations was carried out in a FACSLyricTM flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) using the Infinicyt™ analysis software v.2.0. Leukocyte subpopulations were detected based on the following markers: classical monocytes (CD14++/CD16−), intermediate monocytes (CD14++/CD16+), non-classical monocytes (CD14+/CD16++), T helper cells (CD3+/CD4+), cytotoxic T cells (CD3+/CD8+), B cells (CD3−/CD19+), NK cells (CD56+/CD16+), and iNKT cells (CD3+/CD56+). Unlabeled cells or cells stained with fluorophore-conjugated irrelevant antibodies were used as negative controls.
2.5. Statistical Analysis
A comparative analysis of qualitative variables was conducted using the Chi-square test or Fisher’s exact test. For numerical variables, Student’s t-test or the Mann–Whitney U test was used, depending on the data distribution (normal or non-normal), as determined by graphical methods and normality tests (Shapiro–Wilk test and analysis of skewness and kurtosis). The correlations between leukocyte subpopulations and hematological and biochemical parameters were analyzed using the Spearman test. A 95% confidence level was considered. All the analyses were performed using STATA v.15 software.
3. Results
3.1. Sociodemographic and Etiopathogenic Characteristics of the Patients
As shown in Table 2, the median age of end-stage CKD patients is 66.5 years. More than 50% of patients have long-lasting uremia, are diabetic and hypertensive, and have normal weight based on BMI.

Table 2.
Sociodemographic and etiopathogenic characteristics.
3.2. Analysis of Hematological and Biochemical Parameters
Hematological and biochemical markers, determined after hemodialysis, evidence higher concentrations of phosphorus, CRP, creatinine, and urea than the reference values. However, reduced levels of hemoglobin, hematocrit, transferrin, and iron were detected (Table 3). Of note, Kt/v associated with urea excretion/retention almost duplicated the reference value.

Table 3.
Analysis of hematological and biochemical parameters.
3.3. Leukocyte Subpopulations in End-Stage CKD Patients
The quantification of leukocytes (Table 4) and monocytes (Table 5) were within the reference range for end-stage CKD patients. However, the evaluation of monocyte subpopulations evidences a decreased frequency of classical monocytes while the frequency of non-classical monocytes increased in comparison to the reference values.

Table 4.
Quantification of leukocyte subpopulations in end-stage CKD patients.

Table 5.
Distribution of monocyte subpopulations in end-stage CKD patients.
3.4. Correlation of Leukocyte Subpopulations with Hematological and Biochemical Parameters
Considering the relevance of DM in end-stage CKD onset and progression, we explored whether leukocyte subpopulations have any correlation with hematological and biochemical parameters based on this comorbidity. In patients with DKD, a positive correlation was detected between total monocytes and CD4+ T cells, while B lymphocytes negatively correlate with alkaline phosphatase, parathyroid hormone, and ferritin. Additionally, negative correlations were detected between CD4+ T lymphocytes and parathyroid hormone and between iNKT cells and serum iron concentration (Table 6).

Table 6.
Correlation between leukocyte subpopulations and biochemical parameters.
On the other hand, patients with nephropathy due to other causes have a significant positive correlation between T lymphocytes and CD4+ T cells with serum albumin concentration. A significant negative correlation exists between T lymphocytes or CD4+ T lymphocytes with neutrophils count (Table 6).
Unveiled correlations of T and B lymphocytes with serum albumin, parathyroid hormone, neutrophils, and monocytes are shown in Figure 1, either in DKD or no-diabetic end-stage CKD patients.
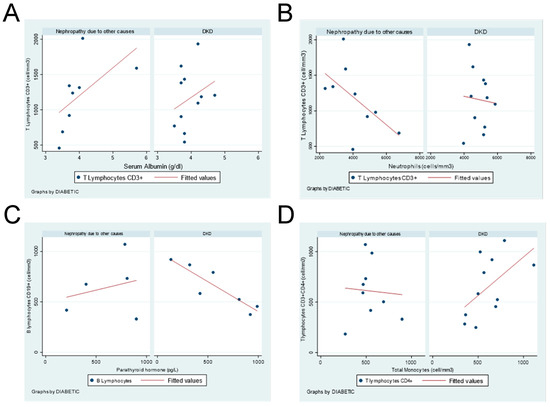
Figure 1.
Correlation between leukocyte subpopulations and hematological parameters in DKD or nephropathy due to other causes in end-stage CKD patients. (A) CD3+ T Lymphocytes vs. serum albumin. (B) CD3+ T lymphocytes vs. neutrophils. (C) B lymphocytes vs. parathyroid hormone. (D) CD4+ T lymphocytes vs. total monocytes.
Table 7 shows that despite the ethology of end-stage CKD, non-classical monocytes significantly correlate with total lymphocytes and B lymphocytes. However, if DKD and nephropathy due to other causes in end-stage CKD patients are discerned, classical monocytes have a significant positive correlation with neutrophils, and the non-classical monocyte subpopulation significantly correlates to eosinophils. Otherwise, in non-diabetic patients, a positive correlation was detected between classical monocytes and neutrophils, as well as non-classical monocytes and total lymphocytes (Table 8).

Table 7.
Correlation between non-classical monocytes and lymphocytes in end-stage CKD patients.

Table 8.
Correlation between monocyte and leukocyte populations in DKD and nephropathy due to other causes in end-stage CKD patients.
4. Discussion
Detailed characterization of hematological and biochemical status in end-stage CKD patients is fundamental for disease prognosis and success treatment prediction. Evidence about statistical and biological correlation among these biomarkers is scarce, particularly considering that end-stage CKD etiology adds a layer of complexity to any evaluation. Regarding the relevance of DKD on progression to end-stage CKD, we explored the overall values and correlations among hematological and biochemical parameters on Peruvian patients under hemodialysis.
The end-stage CKD patients included in this study have increased values in phosphorus and CRP serological concentration. After hemodialysis, their creatinine and urea concentrations increase. Indeed, the urea excretion/retention Kt/v variable almost duplicated the reference value. Hemoglobin, hematocrit, transferrin, and iron are reduced irrespectively of the disease etiology. Regarding immunity cells, leukocytes are within the reference range, but the frequency of classical monocytes is lower, while the frequency of non-classical monocytes is higher than the reference values. Interestingly, in end-stage CKD patients, the amount of T and B lymphocytes correlates with serum albumin and parathyroid hormone concentration, as well as neutrophils and monocytes cells. Also, non-classical monocytes, total lymphocytes, and B lymphocytes are positively correlated.
In nephropathic diabetic end-stage CKD patients, helper CD4+ T cells positively correlate with total monocytes while negatively correlating with parathyroid hormone. B lymphocytes negatively correlate with alkaline phosphatase, parathyroid hormone, and ferritin. Additionally, classical monocytes correlate with neutrophils, while non-classical monocytes significantly correlate with eosinophils. Interestingly, the scarce iNKT cell population correlates with serum iron concentration.
When nephropathy due to other causes in end-stage CKD origin is considered, serum albumin concentration positively correlates with T lymphocytes and helper CD4+ T cells. However, neutrophil count negatively correlates with these lymphocytes. Otherwise, a positive correlation was detected between classical monocytes and neutrophils, as well as non-classical monocytes and total lymphocytes.
Despite the overall biochemical parameters showing no significant alterations in evaluated end-stage CKD patients, serum phosphorus concentration, alkaline phosphatase, and serum transferrin are slightly out of the reference ranges. The association between high phosphorus levels and the progression of DKD, cardiovascular calcification, and increased mortality have been described [18,19]. Alkaline phosphatase is a marker of secondary hyperparathyroidism in CKD, linked to vascular calcification and cardiovascular morbidity. The reduction in serum transferrin suggests a decreased capacity for iron transportation due to kidney damage [20].
Monocyte subpopulations include 80–90% of classical monocytes and 10–20% of CD16+ non-classical monocytes in healthy individuals. Our study shows an increase in non-classical monocytes and a decrease in classical monocytes in end-stage CKD patients, suggesting the progression of classical monocytes into non-classical forms, a process potentially moderated by hemodialysis [21,22,23,24,25]. Inflammatory monocytes increase with age, particularly in CKD patients, with classical monocytes decreasing and non-classical monocytes rising, a trend intensified by the uremic environment [26]. Classic monocytes are reduced in the overall patient population and correlates with neutrophils, either in diabetic or non-diabetic patients. In diabetic patients, CD16+ monocytes show increased pro-inflammatory activity, particularly in DKD [9,27]. Recent transcriptome and proteome studies highlight the functional differences between monocyte subpopulations, such as the role of CD14+CD16+ monocytes in gene expression regulation during phagocytosis and CD14+CD16− monocytes in antimicrobial functions. Additionally, CD16+ Slan+ monocytes, elevated in kidney lesions, contribute to inflammation in conditions like lupus nephritis, underscoring the importance of further research into monocyte subpopulations and immunity [28,29].
The negative correlation between ferritin, parathyroid hormone, and alkaline phosphatase levels can be explained due to the role of these biomarkers in B cell function [30,31,32]. Parathyroid hormone binds to the CD3+CD4+ T lymphocyte receptor influencing helper T cells, a reasonable support for negative correlation between both variables [33]. The activation of iNKT cells together with α-galactosylceramide, in vivo, triggers a substantial effect on immune cells and changes in iron homeostasis, increasing its demand [34]. It may explain the negative correlation detected between iNKT lymphocytes and serum iron levels in end-stage CKD patients.
The co-culture of T lymphocytes and neutrophils demonstrated that neutrophils suppress proliferation of early but not late activated CD4+ and CD8+ cells. In our study, we found a negative correlation between neutrophils and T cells in DKD and nephropathy due to other causes in CKD patients [35]. T cells and serum albumin concentration positively correlate in end-stage CKD patients irrespective of disease etiology. Indeed, higher concentrations of serum albumin have been described at sites of infection, which activates a glycerol monolaurate suppressive effect on human T cells [36].
This study emphasizes the need to establish reference values for hematological and biochemical parameters to characterize the influence of etiological factors in CKD patients’ evolution to the end stage. Indeed, our group recently explored the differentiation bias of helper T cells in end-stage CKD patients, evidencing a reduced frequency of naïve T cells and FOXP3+ regulatory T cells, while effector memory T cells, Th1 cells, and Th17 cells are increased [37]. End-stage CKD patients with and without diabetes have no significant differences regarding the genetic expression of the transcription factors FOXP3, GATA3, RORC, and T-bet. However, T-bet and FOXP3 are significantly correlated in diabetic patients, while RORC correlates with FOXP3 in non-diabetics. Monitoring the relationship among leukocyte subpopulations and hematological and biochemical parameters in CKD patients can substantially enrich the information input into kidney failure risk platforms [38].
5. Conclusions
The flow cytometric characterization of leukocyte subpopulations in end-stage CKD patients unveils an increased frequency of inflammatory non-classical monocytes that positively correlates with total lymphocytes and B lymphocytes. When diabetes is the etiology of CKD, a significantly positive correlation occurs between pro-inflammatory monocytes and eosinophils, classical monocytes and neutrophils, and helper T cells and total monocytes. In DKD patients, B cells show a negative correlation with biochemical parameters such as alkaline phosphatase, parathyroid hormone, and ferritin, while iNKT cells negatively correlate with serum iron. However, in non-diabetic CKD patients, non-classical monocytes and total lymphocytes are significantly correlated, as well as T lymphocytes and serum albumin. Uremic environment differentially modifies leukocyte subpopulation, the serological concentration of biochemical parameters, and the correlation among these biomarkers in diabetic and non-diabetic end-stage CKD patients.
Author Contributions
Conceptualization, G.G.L. and J.d.L.D.; methodology, V.A.C., A.G.A., M.G.C.T. and D.Z.D.-O.; validation, G.G.L., A.G.A. and K.S.R.G.; formal analysis, G.G.L., M.G.C.T., A.G.M.C. and V.A.C.; investigation, G.G.L., M.G.C.T., A.G.A., D.Z.D.-O. and M.B.C.N.; resources, D.Z.D.-O., M.B.C.N., A.G.A., M.G.C.T. and K.S.R.G.; data curation, A.G.M.C.; writing—original draft preparation, G.G.L. and J.d.L.D.; writing—review and editing, J.d.L.D., G.G.L., A.G.A. and A.G.M.C.; visualization, K.S.R.G.; supervision, J.d.L.D.; project administration, C.L.L. and D.Z.D.-O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted according to the Declaration of Helsinki and approved by the Ethics Committee of the Faculty of Human Medicine at San Martin de Porres University, as indicated by Official Letter No. 0104-2024-CIEI-FMH-USMP/approved date 23 January 2024.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The original contributions presented in this study are included in this article; further inquiries can be directed to the corresponding author/s.
Acknowledgments
We thank all the CENESA staff for providing us with all the necessary facilities to develop this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vaidya, S.R.; Aeddula, N.R. Chronic Kidney Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK535404/ (accessed on 10 February 2024).
- León-Figueroa, D.A.; Aguirre-Milachay, E.; Barboza, J.J.; Valladares-Garrido, M.J. Prevalence of hypertension and diabetes mellitus in Peruvian patients with chronic kidney disease: A systematic review and meta-analysis. BMC Nephrol. 2024, 25, 160. [Google Scholar] [CrossRef] [PubMed]
- Peruvian Society of Nephrology. Situation of Chronic Kidney Disease in Peru and Analysis of Mortality Due to Renal Failure During the COVID-19 Pandemic. Available online: https://www.spn.pe/archivos/SITUACION-DE-LA-ENFEREMEDAD-RENAL-CRONICA-EN-EL-PERU-2020-2021.pdf (accessed on 10 February 2024).
- Yang, M.; Zhang, C. The role of innate immunity in diabetic nephropathy and their therapeutic consequences. J. Pharm. Anal. 2024, 14, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Naicker, S.D.; Cormican, S.; Griffin, T.P.; Maretto, S.; Martin, W.P.; Ferguson, J.P.; Cotter, D.; Connaughton, E.P.; Dennedy, M.C.; Griffin, M.D. Chronic Kidney Disease Severity Is Associated with Selective Expansion of a Distinctive Intermediate Monocyte Subpopulation. Front. Immunol. 2018, 9, 2845. [Google Scholar] [CrossRef]
- Tecklenborg, J.; Clayton, D.; Siebert, S.; Coley, S.M. The Role of the Immune System in Kidney Disease. Clin. Exp. Immunol. 2018, 192, 142–150. [Google Scholar] [CrossRef]
- Zheng, Z.; Zheng, F. Immune Cells and Inflammation in Diabetic Nephropathy. J. Diabetes Res. 2016, 2016, 1841690. [Google Scholar] [CrossRef]
- Ozcicek, A.; Ozcicek, F.; Yildiz, G.; Timuroglu, A.; Demirtas, L.; Buyuklu, M.; Kuyrukluyildiz, U.; Akbas, E.M.; Topal, E.; Turkmen, K. Neutrophil-to-Lymphocyte Ratio as a Possible Indicator of Epicardial Adipose Tissue in Patients Undergoing Hemodialysis. Arch. Med. Sci. 2017, 13, 118–123. [Google Scholar] [CrossRef]
- Tucker, P.S.; Scanlan, A.T.; Dalbo, V.J. Chronic Kidney Disease Influences Multiple Systems: Describing the Relationship Between Oxidative Stress, Inflammation, Kidney Damage, and Concomitant Disease. Oxid. Med. Cell. Longev. 2015, 2015, 806358. [Google Scholar] [CrossRef] [PubMed]
- Kanter, J.E.; Hsu, C.C.; Bornfeldt, K.E. Monocytes and Macrophages as Protagonists in Vascular Complications of Diabetes. Front. Cardiovasc. Med. 2020, 7, 10. [Google Scholar] [CrossRef]
- Chiu, Y.L.; Shu, K.H.; Yang, F.J.; Chou, T.Y.; Chen, P.M.; Lay, F.Y.; Pan, S.-Y.; Lin, C.-J.; Litjens, N.H.R.; Betjes, M.G.H.; et al. A Comprehensive Characterization of Aggravated Aging-Related Changes in T Lymphocytes and Monocytes in End-Stage Renal Disease: The iESRD Study. Immun. Aging 2018, 15, 27. [Google Scholar] [CrossRef]
- Narasimhan, P.B.; Marcovecchio, P.; Hamers, A.A.J.; Hedrick, C.C. Nonclassical Monocytes in Health and Disease. Annu. Rev. Immunol. 2019, 37, 439–456. [Google Scholar] [CrossRef]
- Liakopoulos, V.; Jeron, A.; Shah, A.; Bruder, D.; Mertens, P.R.; Gorny, X. Hemodialysis-Related Changes in Phenotypical Fea-tures of Monocytes. Sci. Rep. 2018, 8, 13964. [Google Scholar] [CrossRef]
- Francis, E.R.; Kuo, C.C.; Bernabe-Ortiz, A.; Nessel, L.; Gilman, R.H.; Checkley, W.; Miranda, J.J.; Feldman, H.I.; CRONICAS Cohort Study Group. Burden of Chronic Kidney Disease in Resource-Limited Settings from Peru: A Population-Based Study. BMC Nephrol. 2015, 16, 114. [Google Scholar] [CrossRef]
- Xiong, J.; Qiao, Y.; Yu, Z.; Huang, Y.; Yang, K.; He, T.; Zhao, J. T-Lymphocyte Subsets Alteration, Infection and Renal Outcome in Advanced Chronic Kidney Disease. Front. Med. 2021, 8, 742419. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Rao, X.; Zhong, J. Role of T Lymphocytes in Type 2 Diabetes and Diabetes-Associated Inflammation. J. Diabetes Res. 2017, 2017, 6494795. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Zúñiga, J.; Saldarriaga, E.M.; Chávez-Gómez, R.; Gálvez-Inga, J.; Valdivia-Vega, R.; Villavicencio-Carranza, M.; Espejo-Sotelo, J.; Medina-Sal y Rosas, C.; Suarez-Moreno, V.; Hurtado-Roca, Y. Effectiveness of Adherence to a Renal Health Program in a Health Network in Peru. Rev. Saúde Pública 2020, 54, 80. [Google Scholar] [CrossRef]
- Shah, S.; Mehta, H. Reproducibility of the Neutrophil-Lymphocyte Ratio Measurement: An Analysis. J. Clin. Lab. Anal. 2021, 35, e23878. [Google Scholar] [CrossRef]
- Shaman, A.M.; Kowalski, S.R. Hyperphosphatemia Management in Patients with Chronic Kidney Disease. Saudi Pharm. J. 2016, 24, 494–505. [Google Scholar] [CrossRef]
- Flores, A.R.; Melchor-López, A.; Huerta-Ramírez, S.; Cerda-Téllez, F.; Elizalde-Hernández, P.D.; González-Antuvo, A.; Valdés-Solís, E. Use of Alkaline Phosphatase as an Alternative Marker to Parathyroid Hormone in the Diagnosis of Secondary Hyperparathyroidism in Chronic Kidney Disease. Internal Med. Mex. 2015, 31, 650–659. [Google Scholar]
- Becerra Ortiz, M.L.; Rodríguez López, E.R. Assessment of the Nutritional Status of Patients on Hemodialysis at the SERSALUD Amazonia Hemodialysis Center EIRL Iquitos, 2016. Sci. J. Health Sci. 2017, 9, 54–62. [Google Scholar] [CrossRef]
- Patel, A.A.; Zhang, Y.; Fullerton, J.N.; Boelen, L.; Rongvaux, A.; Maini, A.A.; Bigley, V.; Flavell, R.A.; Gilroy, D.W.; Asquith, B.; et al. The Fate and Lifespan of Human Monocyte Subsets in Steady State and Systemic Inflammation. J. Exp. Med. 2017, 214, 1913–1923. [Google Scholar] [CrossRef]
- Jeng, Y.; Lim, P.S.; Wu, M.Y.; Tseng, T.Y.; Chen, C.H.; Chen, H.P.; Wu, T.K. Proportions of Proinflammatory Monocytes Are Important Predictors of Mortality Risk in Hemodialysis Patients. Mediat. Inflamm. 2017, 2017, 1070959. [Google Scholar] [CrossRef]
- Sampath, P.; Moideen, K.; Ranganathan, U.D.; Bethunaickan, R. Monocyte Subsets: Phenotypes and Function in Tuberculosis Infection. Front. Immunol. 2018, 9, 1726. [Google Scholar] [CrossRef]
- Fan, Y.; Yi, Z.; D’Agati, V.D.; Sun, Z.; Zhong, F.; Zhang, W.; Wen, J.; Zhou, T.; Li, Z.; He, L.; et al. Comparison of Kidney Transcriptomic Profiles of Early and Advanced Diabetic Nephropathy Reveals Potential New Mechanisms for Disease Progression. Diabetes 2019, 68, 2301–2314. [Google Scholar] [CrossRef] [PubMed]
- Stansfield, B.K.; Ingram, D.A. Clinical Significance of Monocyte Heterogeneity. Clin. Transl. Med. 2015, 4, 5. [Google Scholar] [CrossRef]
- Bonan, N.B.; Schepers, E.; Pecoits-Filho, R.; Pletinck, A.; De Somer, F.; Vanholder, R.; Van Biesen, W.; Moreno-Amaral, A.; Glorieux, G. Contribution of the Uremic Milieu to an Increased Pro-Inflammatory Monocytic Phenotype in Chronic Kidney Disease. Sci. Rep. 2019, 9, 10236. [Google Scholar] [CrossRef]
- Prieto, V.; de los Angeles, M. Effect of Aging on Calcium Signals in Human Neutrophils and Its Relationship with the Expression of CRAC and TRPM2 Channels. Master’s Thesis, Veracruz University, Xalapa Region, Mexico, 2019. Available online: https://cdigital.uv.mx/handle/1944/50318 (accessed on 21 August 2024).
- Zhao, C.; Zhang, H.; Wong, W.C.; Sem, X.; Han, H.; Ong, S.M.; Tan, Y.-C.; Yeap, W.-H.; Gan, C.-S.; Ng, K.-Q.; et al. Identification of Novel Functional Differences in Monocyte Subsets Using Proteomic and Transcriptomic Methods. J. Proteome Res. 2009, 8, 4028–4038. [Google Scholar] [CrossRef]
- Nageshwari, B.; Merugu, R. Alkaline Phosphatase Activity of Normal and Malignant Human Lymphocytes. Indian J. Clin. Biochem. 2019, 34, 272–279. [Google Scholar] [CrossRef]
- Rios-Serna, L.J.; Rosero, A.M.; Tobón, G.J.; Cañas, C.A. Biological Changes in Human B-Cell Line Ramos (RA.1) Related to Increasing Doses of Human Parathyroid Hormone. Heliyon 2024, 10, e30556. [Google Scholar] [CrossRef]
- Chong, B.F.; Mohan, C. Targeting the CXCR4/CXCL12 Axis in Systemic Lupus Erythematosus. Expert Opin. Ther. Targets 2009, 13, 1147–1153. [Google Scholar]
- Weitzmann, M.N.; Pacifici, R. Parathyroid Diseases and T Cells. Curr. Osteoporos. Rep. 2017, 15, 135–141. [Google Scholar] [CrossRef]
- Huang, H.; Zuzarte-Luis, V.; Fragoso, G.; Calvé, A.; Hoang, T.A.; Oliero, M.; Chabot-Roy, G.; Mullins-Dansereau, V.; Lesage, S. Acute invariant NKT cell activation triggers an immune response that drives prominent changes in iron homeostasis. Sci. Rep. 2020, 10, 21026. [Google Scholar] [CrossRef]
- Minns, D.; Smith, K.J.; Hardisty, G.; Rossi, A.G.; Gwyer Findlay, E. The outcome of neutrophil-T cell contact differs depending on activation status of both cell types. Front. Immunol. 2021, 12, 633486. [Google Scholar] [CrossRef]
- Zhang, M.S.; Houtman, J.C.D. Human serum albumin (HSA) suppresses the effects of glycerol monolaurate (GML) on human T cell activation and function. PLoS ONE 2016, 11, e0165083. [Google Scholar] [CrossRef]
- Granda Alacote, A.C.; Goyoneche Linares, G.; Castañeda Torrico, M.G.; Diaz-Obregón, D.Z.; Núñez, M.B.C.; Murillo Carrasco, A.G.; Liendo, C.L.; Rufasto Goche, K.S.; Correa, V.A.; de León Delgado, J. T-Cell subpopulations and differentiation bias in diabetic and non-diabetic patients with chronic kidney disease. Biomedicines 2025, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Kidney Failure Risk Calculation Platform. Available online: https://kidneyfailurerisk.com/ (accessed on 21 August 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).