Phytochemicals as Chemo-Preventive and Therapeutic Agents Against Bladder Cancer: A Comprehensive Review
Abstract
1. Introduction
- i.
- What are the main signaling pathways involved in BC development?
- ii.
- What are the main phytochemicals exerting protective actions in BC?
- iii.
- What trials are being conducted in humans with BC, regarding the main phytochemicals?
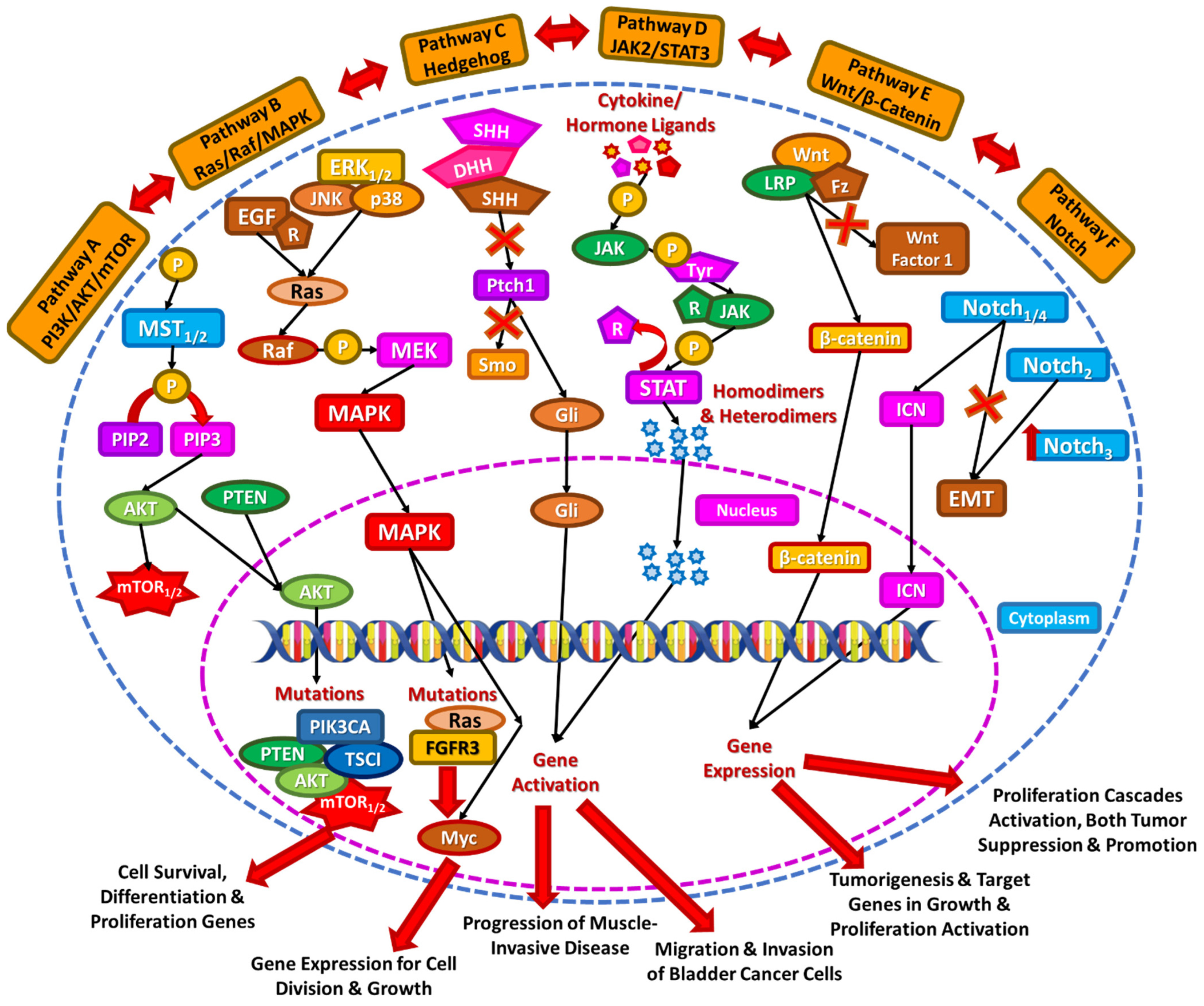
2. Signaling Pathways in Bladder Cancer
2.1. PI3K/AKT/mTOR
2.2. Ras/Raf/MAPK
2.3. Hedgehog Pathway
2.4. JAK2/STAT3 Pathway
2.5. Wnt/β-Catenin
2.6. Notch Pathway
2.7. Hippo Pathway
2.8. Platelet Activating Factor (PAF)
2.9. Other Signaling Pathways and Important Receptors in Bladder Cancer Pathogenesis
3. Phytochemicals Targeting Bladder Cancer
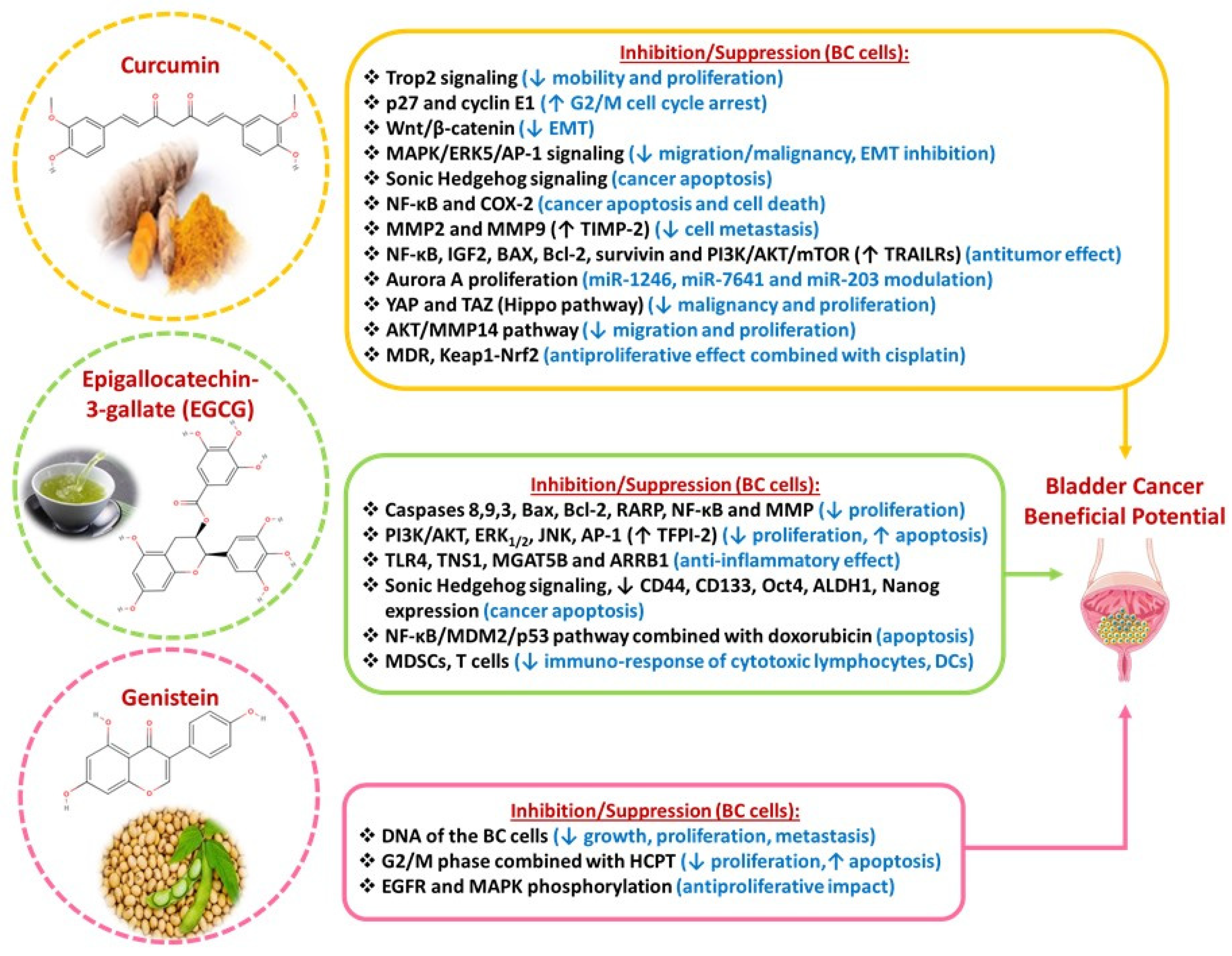
3.1. Curcumin
3.2. Epigallocatechin-3-Gallate (EGCG)
3.3. Genistein
3.4. Resveratrol
3.5. Sulforaphane-Erucin
3.6. Extra Virgin Olive Oil (EVOO) Phenols
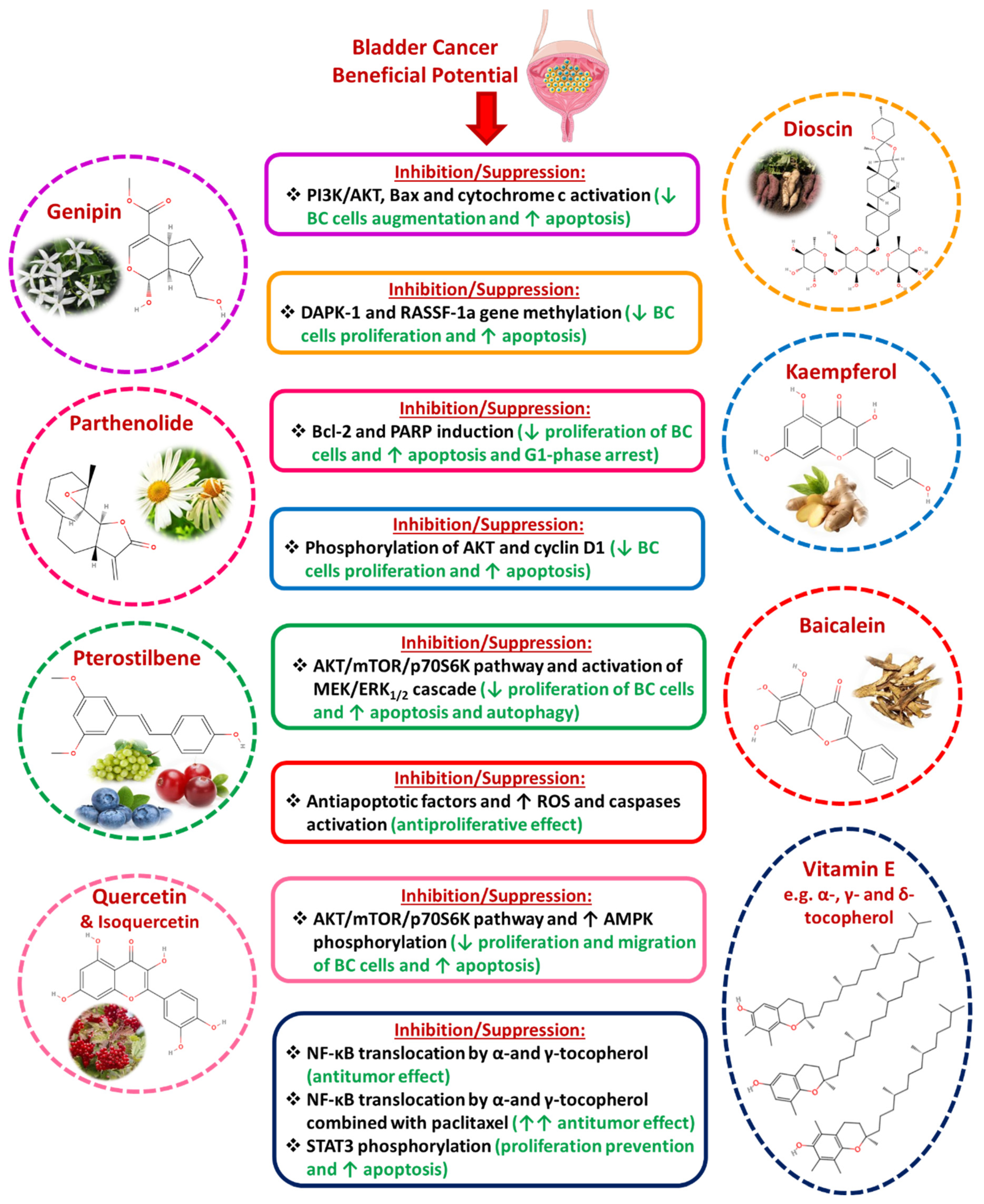
3.7. Other Phytochemicals
4. Ongoing Trials with the Use of Phytochemicals
5. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- European Association of Urology EAU Guidelines. Edn. In Proceedings of the EAU Annual Congress Paris 2024, Paris, France, 5–8 April 2024.
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Ghorbani, A. Cancer therapy with phytochemicals: Evidence from clinical studies. Avicenna J. Phytomedicine 2015, 5, 84–97. [Google Scholar]
- Xia, Y.; Chen, R.; Lu, G.; Li, C.; Lian, S.; Kang, T.-W.; Jung, Y.D. Natural Phytochemicals in Bladder Cancer Prevention and Therapy. Front. Oncol. 2021, 11, 652033. [Google Scholar] [CrossRef]
- Chestnut, C.; Subramaniam, D.; Dandawate, P.; Padhye, S.; Taylor, J.; Weir, S.; Anant, S. Targeting Major Signaling Pathways of Bladder Cancer with Phytochemicals: A Review. Nutr. Cancer 2020, 73, 2249–2271. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Yaribeygi, H.; Sahebkar, A. Therapeutic Effects of Curcumin against Bladder Cancer: A Review of Possible Molecular Pathways. Anti-Cancer Agents Med. Chem. 2020, 20, 667–677. [Google Scholar] [CrossRef]
- Zuo, M.; Chen, H.; Liao, Y.; He, P.; Xu, T.; Tang, J.; Zhang, N. Sulforaphane and bladder cancer: A potential novel antitumor compound. Front. Pharmacol. 2023, 14, 1254236. [Google Scholar] [CrossRef]
- Kennelley, G.E.; Amaye-Obu, T.; Foster, B.A.; Tang, L.; Paragh, G.; Huss, W.J. Mechanistic Review of Sulforaphane as a Chemoprotective Agent in Bladder Cancer. Am. J. Clin. Exp. Urol. 2023, 11, 103–120. [Google Scholar] [PubMed]
- Rasheed, S.; Rehman, K.; Shahid, M.; Suhail, S.; Akash, M.S.H. Therapeutic potentials of genistein: New insights and perspectives. J. Food Biochem. 2022, 46, e14228. [Google Scholar] [CrossRef]
- Tsao, A.S.; Kim, E.S.; Hong, W.K. Chemoprevention of Cancer. CA Cancer J. Clin. 2004, 54, 150–180. [Google Scholar] [CrossRef]
- Golemis, E.A.; Scheet, P.; Beck, T.N.; Scolnick, E.M.; Hunter, D.J.; Hawk, E.; Hopkins, N. Molecular mechanisms of the preventable causes of cancer in the United States. Genes Dev. 2018, 32, 868–902. [Google Scholar] [CrossRef]
- Khan, K.; Quispe, C.; Javed, Z.; Iqbal, M.J.; Sadia, H.; Raza, S.; Irshad, A.; Salehi, B.; Reiner, Ž.; Sharifi-Rad, J. Resveratrol, curcumin, paclitaxel and miRNAs mediated regulation of PI3K/Akt/mTOR pathway: Go four better to treat bladder cancer. Cancer Cell Int. 2020, 20, 1–19. [Google Scholar] [CrossRef]
- Yu, J.S.L.; Cui, W. Proliferation, survival and metabolism: The role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development 2016, 143, 3050–3060. [Google Scholar] [CrossRef] [PubMed]
- Ching, C.B.; Hansel, D.E. Expanding therapeutic targets in bladder cancer: The PI3K/Akt/mTOR pathway. Mod. Pathol. 2010, 90, 1406–1414. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y. Bladder Cancer and Genetic Mutations. Cell Biochem. Biophys. 2015, 73, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 1–37. [Google Scholar] [CrossRef]
- Chen, M.; Gu, J.; Delclos, G.L.; Killary, A.M.; Fan, Z.; Hildebrandt, M.A.T.; Chamberlain, R.M.; Grossman, H.B.; Dinney, C.P.; Wu, X. Genetic variations of the PI3K-AKT-mTOR pathway and clinical outcome in muscle invasive and metastatic bladder cancer patients. Carcinogenesis 2010, 31, 1387–1391. [Google Scholar] [CrossRef]
- Santarpia, L.; Lippman, S.M.; El-Naggar, A.K. Targeting the MAPK–RAS–RAF signaling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 103–119. [Google Scholar] [CrossRef]
- Jebar, A.H.; Hurst, C.D.; Tomlinson, D.C.; Johnston, C.; Taylor, C.F.; A Knowles, M. FGFR3 and Ras gene mutations are mutually exclusive genetic events in urothelial cell carcinoma. Oncogene 2005, 24, 5218–5225. [Google Scholar] [CrossRef]
- Bekele, R.T.; Samant, A.S.; Nassar, A.H.; So, J.; Garcia, E.P.; Curran, C.R.; Hwang, J.H.; Mayhew, D.L.; Nag, A.; Thorner, A.R.; et al. RAF1 amplification drives a subset of bladder tumors and confers sensitivity to MAPK-directed therapeutics. J. Clin. Investig. 2021, 131, e147849. [Google Scholar] [CrossRef]
- Degirmenci, U.; Wang, M.; Hu, J. Targeting Aberrant RAS/RAF/MEK/ERK Signaling for Cancer Therapy. Cells 2020, 9, 198. [Google Scholar] [CrossRef]
- Briscoe, J.; Thérond, P.P. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 2013, 14, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Lim, A.; Zhao, C.; Sahoo, D.; Pan, Y.; Spiekerkoetter, E.; Liao, J.C.; Beachy, P.A. Hedgehog Signaling Restrains Bladder Cancer Progression by Eliciting Stromal Production of Urothelial Differentiation Factors. Cancer Cell 2014, 26, 521–533. [Google Scholar] [CrossRef]
- Pignot, G.; Vieillefond, A.; Vacher, S.; Zerbib, M.; Debre, B.; Lidereau, R.; Amsellem-Ouazana, D.; Bieche, I. Hedgehog pathway activation in human transitional cell carcinoma of the bladder. Br. J. Cancer 2012, 106, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Syed, I.S.; Pedram, A.; Farhat, W.A. Role of Sonic Hedgehog (Shh) Signaling in Bladder Cancer Stemness and Tumorigenesis. Curr. Urol. Rep. 2016, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, J.S.; Rosler, K.M.; Harrison, D.A. The JAK/STAT signaling pathway. J. Cell Sci. 2004, 117, 1281–1283. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, W.; Wang, L.; Kazobinka, G.; Han, X.; Li, B.; Hou, T. Musashi-2 promotes migration and invasion in bladder cancer via activation of the JAK2/STAT3 pathway. Mod. Pathol. 2016, 96, 950–958. [Google Scholar] [CrossRef]
- Ho, P.L.; Lay, E.J.; Jian, W.; Parra, D.; Chan, K.S. Stat3 Activation in Urothelial Stem Cells Leads to Direct Progression to Invasive Bladder Cancer. Cancer Res. 2012, 72, 3135–3142. [Google Scholar] [CrossRef]
- Hindupur, S.V.; Schmid, S.C.; Koch, J.A.; Youssef, A.; Baur, E.-M.; Wang, D.; Horn, T.; Slotta-Huspenina, J.; Gschwend, J.E.; Holm, P.S.; et al. STAT3/5 Inhibitors Suppress Proliferation in Bladder Cancer and Enhance Oncolytic Adenovirus Therapy. Int. J. Mol. Sci. 2020, 21, 1106. [Google Scholar] [CrossRef]
- Komiya, Y.; Habas, R. Wnt signal transduction pathways. Organogenesis 2008, 4, 68–75. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014, 507, 315–322. [Google Scholar] [CrossRef]
- Urakami, S.; Shiina, H.; Enokida, H.; Kawakami, T.; Tokizane, T.; Ogishima, T.; Tanaka, Y.; Li, L.-C.; Ribeiro-Filho, L.A.; Terashima, M.; et al. Epigenetic Inactivation of Wnt Inhibitory Factor-1 Plays an Important Role in Bladder Cancer through Aberrant Canonical Wnt/β-Catenin Signaling Pathway. Clin. Cancer Res. 2006, 12, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I. The role of WNT signalling in urothelial cell carcinoma. Ind. Mark. Manag. 2015, 97, 481–486. [Google Scholar] [CrossRef][Green Version]
- Sun, S.; Wang, Y.; Wang, J.; Bi, J. Wnt pathway-related three-mRNA clinical outcome signature in bladder urothelial carcinoma: Computational biology and experimental analyses. J. Transl. Med. 2021, 19, 1–14. [Google Scholar] [CrossRef]
- Chehrazi-Raffle, A.; Dorff, T.B.; Pal, S.K.; Lyou, Y. Wnt/β-Catenin Signaling and Immunotherapy Resistance: Lessons for the Treatment of Urothelial Carcinoma. Cancers 2021, 13, 889. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wu, H.; Xu, H.; Xiong, H.; Chu, Q.; Yu, S.; Wu, G.S.; Wu, K. Notch signaling: An emerging therapeutic target for cancer treatment. Cancer Lett. 2015, 369, 20–27. [Google Scholar] [CrossRef]
- Goriki, A.; Seiler, R.; Wyatt, A.W.; Contreras-Sanz, A.; Bhat, A.; Matsubara, A.; Hayashi, T.; Black, P.C. Unravelling disparate roles of NOTCH in bladder cancer. Nat. Rev. Urol. 2018, 15, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, L.; Liu, C.; Pan, J.; Lu, G.; Zhou, Z.; Chen, Z.; Qian, C. Notch3 overexpression enhances progression and chemoresistance of urothelial carcinoma. Oncotarget 2017, 8, 34362–34373. [Google Scholar] [CrossRef]
- Zygulska, A.L.; Krzemieniecki, K.; Pierzchalski, P. Hippo Pathway—Brief Overview of Its Relevance in Cancer. J. Physiol. Pharmacol. 2017, 68, 311–335. [Google Scholar]
- Xia, J.; Zeng, M.; Zhu, H.; Chen, X.; Weng, Z.; Li, S. Emerging role of Hippo signalling pathway in bladder cancer. J. Cell. Mol. Med. 2017, 22, 4–15. [Google Scholar] [CrossRef]
- Sadhukhan, P.; Feng, M.; Illingworth, E.J.; Sloma, I.; Ooki, A.; Matoso, A.; Sidransky, D.; Johnson, B.A.; Marchionni, L.; Sillé, F.C.; et al. YAP1 induces bladder cancer progression and promotes immune evasion through IL-6/STAT3 pathway and CXCL deregulation. J. Clin. Investig. 2025, 135, e171164. [Google Scholar] [CrossRef]
- Lordan, R.; Tsoupras, A.; Zabetakis, I.; Demopoulos, C.A. Forty Years Since the Structural Elucidation of Platelet-Activating Factor (PAF): Historical, Current, and Future Research Perspectives. Molecules 2019, 24, 4414. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Mu, X.-D.; Song, J.-R.; Zhai, P.-T.; Cheng, Y.; Le, Y.; Li, Z.-B. PAF enhances cancer stem cell properties via β-catenin signaling in hepatocellular carcinoma. Cell Cycle 2021, 20, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, E.; Al-Dogmi, A.M.; Al-Hazani, T.M.I.; Alwaili, M.A.; Safhi, F.A.; Alneghery, L.M.; Jalal, A.S.; Alanazi, I.S.; AlQassim, F.A.; Alotaibi, M.A.; et al. Patterns of mutations in nine cancer-related genes and PAF development among smoking male patients diagnosed with bladder cancer. Tumor Biol. 2023, 45, 1–14. [Google Scholar] [CrossRef]
- Tsoupras, A.; Adamantidi, T.; Finos, M.A.; Philippopoulos, A.; Detopoulou, P.; Tsopoki, I.; Kynatidou, M.; Demopoulos, C.A. Re-Assessing the Role of Platelet Activating Factor and Its Inflammatory Signaling and Inhibitors in Cancer and Anti-Cancer Strategies. Front. Biosci. 2024, 29, 345. [Google Scholar] [CrossRef]
- Kispert, S.; Marentette, J.; McHowat, J. Cigarette smoking promotes bladder cancer via increased platelet-activating factor. Physiol. Rep. 2019, 7, e13981. [Google Scholar] [CrossRef]
- Detopoulou, P.; Nomikos, T.; Fragopoulou, E.; Chrysohoou, C.; Antonopoulou, S. Platelet Activating Factor in Heart Failure: Potential Role in Disease Progression and Novel Target for Therapy. Curr. Heart Fail. Rep. 2013, 10, 122–129. [Google Scholar] [CrossRef]
- Kelesidis, T.; Papakonstantinou, V.; Detopoulou, P.; Fragopoulou, E.; Chini, M.; Lazanas, M.C.; Antonopoulou, S. The Role of Platelet-Activating Factor in Chronic Inflammation, Immune Activation, and Comorbidities Associated with HIV Infection. AIDS Rev. 2015, 17, 191–201. [Google Scholar]
- Detopoulou, P.; Demopoulos, C.A.; Antonopoulou, S. Micronutrients, Phytochemicals and Mediterranean Diet: A Potential Protective Role against COVID-19 through Modulation of PAF Actions and Metabolism. Nutrients 2021, 13, 462. [Google Scholar] [CrossRef]
- Guan, T.; Su, M.; Luo, Z.; Peng, W.; Zhou, R.; Lu, Z.; Feng, M.; Li, W.; Teng, Y.; Jiang, Y.; et al. Long-Term Cardiovascular Mortality among 80,042 Older Patients with Bladder Cancer. Cancers 2022, 14, 4572. [Google Scholar] [CrossRef]
- Chawki, S.; Ploussard, G.; Montlahuc, C.; Verine, J.; Mongiat-Artus, P.; Desgrandchamps, F.; Molina, J.-M. Bladder Cancer in HIV-infected Adults: An Emerging Issue? Case-Reports and Systematic Review. PLoS ONE 2015, 10, e0144237. [Google Scholar] [CrossRef]
- Detopoulou, P.; Fragopoulou, E.; Nomikos, T.; Antonopoulou, S. Associations of phase angle with platelet-activating factor metabolism and related dietary factors in healthy volunteers. Front. Nutr. 2023, 10, 1237086. [Google Scholar] [CrossRef]
- Detopoulou, P.; Voulgaridou, G.; Papadopoulou, S. Cancer, Phase Angle and Sarcopenia: The Role of Diet in Connection with Lung Cancer Prognosis. Lung 2022, 200, 347–379. [Google Scholar] [CrossRef]
- Gupta, S.; Hau, A.M.; Al-Ahmadie, H.A.; Harwalkar, J.; Shoskes, A.C.; Elson, P.; Beach, J.R.; Hussey, G.S.; Schiemann, W.P.; Egelhoff, T.T.; et al. Transforming Growth Factor-β Is an Upstream Regulator of Mammalian Target of Rapamycin Complex 2–Dependent Bladder Cancer Cell Migration and Invasion. Am. J. Pathol. 2016, 186, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Kamat, A.M.; Aldousari, S.; Ye, Y.; Huang, M.; Dinney, C.P.; Wu, X. Genetic Variations in the Transforming Growth Factor Beta Pathway as Predictors of Bladder Cancer Risk. PLoS ONE 2012, 7, e51758. [Google Scholar] [CrossRef]
- Carlsson, J.; Wester, K.; De La Torre, M.; Malmström, P.-U.; Gårdmark, T. EGFR-expression in primary urinary bladder cancer and corresponding metastases and the relation to HER2-expression. On the possibility to target these receptors with radionuclides. Radiol. Oncol. 2015, 49, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Bryan, R.T.; Regan, H.L.; Pirrie, S.J.; Devall, A.J.; Cheng, K.K.; Zeegers, M.P.; James, N.D.; A Knowles, M.; Ward, D.G. Protein shedding in urothelial bladder cancer: Prognostic implications of soluble urinary EGFR and EpCAM. Br. J. Cancer 2015, 112, 1052–1058. [Google Scholar] [CrossRef]
- Mansour, A.M.; Abdelrahim, M.; Laymon, M.; Elsherbeeny, M.; Sultan, M.; Shokeir, A.; Mosbah, A.; Abol-Enein, H.; Awadalla, A.; Cho, E.; et al. Epidermal growth factor expression as a predictor of chemotherapeutic resistance in muscle-invasive bladder cancer. BMC Urol. 2018, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nedjadi, T.; Al-Maghrabi, J.; Assidi, M.; Dallol, A.; Al-Kattabi, H.; Chaudhary, A.; Al-Sayyad, A.; Al-Ammari, A.; Abuzenadah, A.; Buhmeida, A.; et al. Prognostic value of HER2 status in bladder transitional cell carcinoma revealed by both IHC and BDISH techniques. BMC Cancer 2016, 16, 653. [Google Scholar] [CrossRef]
- Zhou, L.; Shao, Z.; Liu, Y.; Yan, X.; Li, J.; Wu, X.; Tang, B.; Li, S.; Cui, C.; Chi, Z.; et al. HER2 Expression Associated with Clinical Characteristics and Prognosis of Urothelial Carcinoma in a Chinese Population. Oncologist 2023, 28, e617–e624. [Google Scholar] [CrossRef]
- Touat, M.; Ileana, E.; Postel-Vinay, S.; André, F.; Soria, J.-C. Targeting FGFR Signaling in Cancer. Clin. Cancer Res. 2015, 21, 2684–2694. [Google Scholar] [CrossRef]
- van Oers, J.M.; Zwarthoff, E.C.; Rehman, I.; Azzouzi, A.-R.; Cussenot, O.; Meuth, M.; Hamdy, F.C.; Catto, J.W. FGFR3 Mutations Indicate Better Survival in Invasive Upper Urinary Tract and Bladder Tumours. Eur. Urol. 2009, 55, 650–658. [Google Scholar] [CrossRef]
- Li, R.; Linscott, J.; Catto, J.W.; Daneshmand, S.; Faltas, B.M.; Kamat, A.M.; Meeks, J.J.; Necchi, A.; Pradere, B.; Ross, J.S.; et al. FGFR Inhibition in Urothelial Carcinoma. Eur. Urol. 2024, 87, 110–122. [Google Scholar] [CrossRef]
- Kwon, W.-A. FGFR Inhibitors in Urothelial Cancer: From Scientific Rationale to Clinical Development. J. Korean Med. Sci. 2024, 39, e320. [Google Scholar] [CrossRef] [PubMed]
- Kopparapu, P.K.; Boorjian, S.A.; Robinson, B.D.; Downes, M.; Gudas, L.J.; Mongan, N.P.; Persson, J.L. Expression of VEGF and Its Receptors VEGFR1/VEGFR2 Is Associated with Invasiveness of Bladder Cancer. Anticancer Res. 2013, 33, 2381–2390. [Google Scholar] [PubMed]
- Narayanan, S.; Srinivas, S. Incorporating VEGF-targeted therapy in advanced urothelial cancer. Ther. Adv. Med. Oncol. 2016, 9, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, N.; Houston, T.J.; Cardenas, E.; Ghosh, R. To be an ally or an adversary in bladder cancer: The NF-κB story has not unfolded. Carcinogenesis 2014, 36, 299–306. [Google Scholar] [CrossRef]
- Faba, O.R.; Palou-Redorta, J.; Fernández-Gómez, J.M.; Algaba, F.; Eiró, N.; Villavicencio, H.; Vizoso, F.J. Matrix Metalloproteinases and Bladder Cancer: What is New? ISRN Urol. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Cui, X.; Shen, D.; Kong, C.; Zhang, Z.; Zeng, Y.; Lin, X.; Liu, X. NF-κB suppresses apoptosis and promotes bladder cancer cell proliferation by upregulating survivin expression in vitro and in vivo. Sci. Rep. 2017, 7, 40723. [Google Scholar] [CrossRef]
- Kang, S.; Kim, Y.B.; Kim, M.-H.; Yoon, K.-S.; Kim, J.W.; Park, N.H.; Song, Y.S.; Kang, D.; Yoo, K.-Y.; Kang, S.B.; et al. Polymorphism in the nuclear factor kappa-B binding promoter region of cyclooxygenase-2 is associated with an increased risk of bladder cancer. Cancer Lett. 2005, 217, 11–16. [Google Scholar] [CrossRef]
- Ito, Y.; Kikuchi, E.; Tanaka, N.; Kosaka, T.; Suzuki, E.; Mizuno, R.; Shinojima, T.; Miyajima, A.; Umezawa, K.; Oya, M. Down-regulation of NF kappa B activation is an effective therapeutic modality in acquired platinum-resistant bladder cancer. BMC Cancer 2015, 15, 1–11. [Google Scholar] [CrossRef]
- Shoaib, A.; Tabish, M.; Ali, S.; Arafah, A.; Wahab, S.; Almarshad, F.M.; Rashid, S.; Rehman, M.U. Dietary Phytochemicals in Cancer Signalling Pathways: Role of miRNA Targeting. Curr. Med. Chem. 2021, 28, 8036–8067. [Google Scholar] [CrossRef] [PubMed]
- Liskova, A.; Kubatka, P.; Samec, M.; Zubor, P.; Mlyncek, M.; Bielik, T.; Samuel, S.M.; Zulli, A.; Kwon, T.K.; Büsselberg, D. Dietary Phytochemicals Targeting Cancer Stem Cells. Molecules 2019, 24, 899. [Google Scholar] [CrossRef]
- Pop-Bica, C.; Gulei, D.; Cojocneanu-Petric, R.; Braicu, C.; Petrut, B.; Berindan-Neagoe, I. Understanding the Role of Non-Coding RNAs in Bladder Cancer: From Dark Matter to Valuable Therapeutic Targets. Int. J. Mol. Sci. 2017, 18, 1514. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M.; Bhalerao, M.; Cruz-Martins, N.; Kumar, D. Curcumin and cancer biology: Focusing regulatory effects in different signalling pathways. Phytotherapy Res. 2021, 35, 4913–4929. [Google Scholar] [CrossRef]
- Tsuda, T. Curcumin as a functional food-derived factor: Degradation products, metabolites, bioactivity, and future perspectives. Food Funct. 2017, 9, 705–714. [Google Scholar] [CrossRef]
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef]
- Hani, U.; Shivakumar, H. Solubility Enhancement and Delivery Systems of Curcumin a Herbal Medicine: A Review. Curr. Drug Deliv. 2014, 11, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Shvartsur, A.; Bonavida, B. Trop2 and its overexpression in cancers: Regulation and clinical/ therapeutic implications. Genes Cancer 2014, 6, 84–105. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, G.; Zhang, R.; Dong, L.; Chen, H.; Bo, J.; Xue, W.; Huang, Y. Curcumin inhibits cell proliferation and motility via suppression of TROP2 in bladder cancer cells. Int. J. Oncol. 2018, 53, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Lu, L.; Mao, J.; Li, X.; Qian, H.; Xu, W. Curcumin reversed chronic tobacco smoke exposure induced urocystic EMT and acquisition of cancer stem cells properties via Wnt/β-catenin. Cell Death Dis. 2017, 8, e3066. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, J.; Zhao, L.; Geng, H.; Ma, J.; Zhang, Z.; Yu, D.; Zhong, C. Curcumin reverses benzidine-induced epithelial-mesenchymal transition via suppression of ERK5/AP-1 in SV-40 immortalized human urothelial cells. Int. J. Oncol. 2017, 50, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Kong, X.; Li, Y.; Qian, W.; Ma, J.; Wang, D.; Yu, D.; Zhong, C. Curcumin inhibits bladder cancer stem cells by suppressing Sonic Hedgehog pathway. Biochem. Biophys. Res. Commun. 2017, 493, 521–527. [Google Scholar] [CrossRef]
- Shrestha, S.; Zhu, J.; Wang, Q.; Du, X.; Liu, F.; Jiang, J.; Song, J.; Xing, J.; Sun, D.; Hou, Q.; et al. Melatonin potentiates the antitumor effect of curcumin by inhibiting IKKβ/NF-κB/COX-2 signaling pathway. Int. J. Oncol. 2017, 51, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhang, X.; Shi, T.; Li, H. Antitumor effects of curcumin in human bladder cancer in vitro. Oncol. Lett. 2017, 14, 1157–1161. [Google Scholar] [CrossRef]
- Kamat, A.M.; Tharakan, S.T.; Sung, B.; Aggarwal, B.B. Curcumin Potentiates the Antitumor Effects of Bacillus Calmette-Guerin against Bladder Cancer through the Downregulation of NF-κB and Upregulation of TRAIL Receptors. Cancer Res. 2009, 69, 8958–8966. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Arora, S.; Majid, S.; Shahryari, V.; Chen, Y.; Deng, G.; Yamamura, S.; Ueno, K.; Dahiya, R. Curcumin Modulates MicroRNA-203–Mediated Regulation of the Src-Akt Axis in Bladder Cancer. Cancer Prev. Res. 2011, 4, 1698–1709. [Google Scholar] [CrossRef]
- Wang, K.; Tan, S.-L.; Lu, Q.; Xu, R.; Cao, J.; Wu, S.-Q.; Wang, Y.-H.; Zhao, X.-K.; Zhong, Z.-H. Curcumin Suppresses microRNA-7641-Mediated Regulation of p16 Expression in Bladder Cancer. Am. J. Chin. Med. 2018, 46, 1357–1368. [Google Scholar] [CrossRef]
- Liu, H.-S.; Ke, C.-S.; Cheng, H.-C.; Huang, C.-Y.F.; Su, C.-L. Curcumin-Induced Mitotic Spindle Defect and Cell Cycle Arrest in Human Bladder Cancer Cells Occurs Partly through Inhibition of Aurora A. Mol. Pharmacol. 2011, 80, 638–646. [Google Scholar] [CrossRef]
- Tian, B.; Zhao, Y.; Liang, T.; Ye, X.; Li, Z.; Yan, D.; Fu, Q.; Li, Y. Curcumin inhibits urothelial tumor development by suppressing IGF2 and IGF2-mediated PI3K/AKT/mTOR signaling pathway. J. Drug Target. 2017, 25, 626–636. [Google Scholar] [CrossRef]
- Pan, Z.-J.; Deng, N.; Zou, Z.-H.; Chen, G.-X. The effect of curcumin on bladder tumor in rat model. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 884–889. [Google Scholar]
- Gao, Y.; Shi, Q.; Xu, S.; Du, C.; Liang, L.; Wu, K.; Wang, K.; Wang, X.; Chang, L.S.; He, D.; et al. Curcumin Promotes KLF5 Proteasome Degradation through Downregulating YAP/TAZ in Bladder Cancer Cells. Int. J. Mol. Sci. 2014, 15, 15173–15187. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Xiao, W.; Zeng, Q. Curcumin Inhibits Bladder Cancer by Inhibiting Invasion via AKT/MMP14 Pathway. Discov. Med. 2024, 36, 71–81. [Google Scholar] [CrossRef]
- Afsharmoghadam, N.; Haghighatian, Z.; Mazdak, H.; Mirkheshti, N.; Koushki, R.M.; Alavi, S.A. Concentration- Dependent Effects of Curcumin on 5-Fluorouracil Efficacy in Bladder Cancer Cells. Asian Pac. J. Cancer Prev. 2017, 18, 3225–3230. [Google Scholar] [CrossRef]
- Wei, Y.; Pu, X.; Zhao, L. Preclinical studies for the combination of paclitaxel and curcumin in cancer therapy. Oncol. Rep. 2017, 37, 3159–3166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-N.; Yong, Q.; Wu, X.-L.; Liu, X.-P. [Synergism inhibition of curcumin combined with cisplatin on T24 bladder carcinoma cells and its related mechanism]. Zhong Yao Cai 2014, 37, 2043–2046. [Google Scholar] [PubMed]
- Baird, L.; Yamamoto, M. The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Mansouri, K.; Rasoulpoor, S.; Daneshkhah, A.; Abolfathi, S.; Salari, N.; Mohammadi, M.; Shabani, S. Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer 2020, 20, 791. [Google Scholar] [CrossRef]
- Moon, D.-O. Curcumin in Cancer and Inflammation: An In-Depth Exploration of Molecular Interactions, Therapeutic Potentials, and the Role in Disease Management. Int. J. Mol. Sci. 2024, 25, 2911. [Google Scholar] [CrossRef]
- Capasso, L.; De Masi, L.; Sirignano, C.; Maresca, V.; Basile, A.; Nebbioso, A.; Rigano, D.; Bontempo, P. Epigallocatechin Gallate (EGCG): Pharmacological Properties, Biological Activities and Therapeutic Potential. Molecules 2025, 30, 654. [Google Scholar] [CrossRef]
- Mereles, D.; Hunstein, W. Epigallocatechin-3-gallate (EGCG) for Clinical Trials: More Pitfalls than Promises? Int. J. Mol. Sci. 2011, 12, 5592–5603. [Google Scholar] [CrossRef]
- Granja, A.; Frias, I.; Neves, A.R.; Pinheiro, M.; Reis, S. Therapeutic Potential of Epigallocatechin Gallate Nanodelivery Systems. BioMed Res. Int. 2017, 2017, 1–15. [Google Scholar] [CrossRef]
- Luo, K.-W.; Chen, W.; Lung, W.-Y.; Wei, X.-Y.; Cheng, B.-H.; Cai, Z.-M.; Huang, W.-R. EGCG inhibited bladder cancer SW780 cell proliferation and migration both in vitro and in vivo via down-regulation of NF-κB and MMP-9. J. Nutr. Biochem. 2017, 41, 56–64. [Google Scholar] [CrossRef]
- Luo, K.-W.; Lung, W.-Y.; Xie, C.; Luo, X.-L.; Huang, W.-R. EGCG inhibited bladder cancer T24 and 5637 cell proliferation and migration via PI3K/AKT pathway. Oncotarget 2018, 9, 12261–12272. [Google Scholar] [CrossRef] [PubMed]
- Sah, D.K.; Khoi, P.N.; Li, S.; Arjunan, A.; Jeong, J.-U.; Jung, Y.D. (-)-Epigallocatechin-3-Gallate Prevents IL-1β-Induced uPAR Expression and Invasiveness via the Suppression of NF-κB and AP-1 in Human Bladder Cancer Cells. Int. J. Mol. Sci. 2022, 23, 14008. [Google Scholar] [CrossRef]
- Feng, C.; Ho, Y.; Sun, C.; Xia, G.; Ding, Q.; Gu, B. Epigallocatechin gallate inhibits the growth and promotes the apoptosis of bladder cancer cells. Exp. Ther. Med. 2017, 14, 3513–3518. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-Y.; Chen, Y.-J.; Chang, W.-A.; Li, W.-M.; Ke, H.-L.; Wu, W.-J.; Kuo, P.-L. Effects of Epigallocatechin Gallate (EGCG) on Urinary Bladder Urothelial Carcinoma―Next-Generation Sequencing and Bioinformatics Approaches. Medicina 2019, 55, 768. [Google Scholar] [CrossRef]
- Luo, K.-W.; Zhu, X.-H.; Zhao, T.; Zhong, J.; Gao, H.-C.; Luo, X.-L.; Huang, W.-R. EGCG Enhanced the Anti-tumor Effect of Doxorubicine in Bladder Cancer via NF-κB/MDM2/p53 Pathway. Front. Cell Dev. Biol. 2020, 8, 606123. [Google Scholar] [CrossRef]
- Li, D.; Cao, D.; Sun, Y.; Cui, Y.; Zhang, Y.; Jiang, J.; Cao, X. The roles of epigallocatechin gallate in the tumor microenvironment, metabolic reprogramming, and immunotherapy. Front. Immunol. 2024, 15, 1331641. [Google Scholar] [CrossRef]
- Hu, J.; Webster, D.; Cao, J.; Shao, A. The safety of green tea and green tea extract consumption in adults—Results of a systematic review. Regul. Toxicol. Pharmacol. 2018, 95, 412–433. [Google Scholar] [CrossRef]
- Yang, Z.; Kulkarni, K.; Zhu, W.; Hu, M. Bioavailability and Pharmacokinetics of Genistein: Mechanistic Studies on its ADME. Anti-Cancer Agents Med. Chem. 2012, 12, 1264–1280. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Q.; An, Q.; Gong, L.; Yang, S.; Zhang, B.; Su, B.; Yang, D.; Zhang, L.; Lu, Y.; et al. Optimized solubility and bioavailability of genistein based on cocrystal engineering. Nat. Prod. Bioprospecting 2023, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Zhang, W.; Shao, C.; Xu, P.; Shi, C.H.; Shi, J.G.; Li, Y.M.; Fu, Q.; Xue, W.; et al. Genistein Sensitizes Bladder Cancer Cells to HCPT Treatment In Vitro and In Vivo via ATM/NF-κB/IKK Pathway-Induced Apoptosis. PLoS ONE 2013, 8, e50175. [Google Scholar] [CrossRef]
- Messing, E.; Gee, J.R.; Saltzstein, D.R.; Kim, K.; Disant’Agnese, A.; Kolesar, J.; Harris, L.; Faerber, A.; Havighurst, T.; Young, J.M.; et al. A Phase 2 Cancer Chemoprevention Biomarker Trial of Isoflavone G-2535 (Genistein) in Presurgical Bladder Cancer Patients. Cancer Prev. Res. 2012, 5, 621–630. [Google Scholar] [CrossRef]
- Singh, A.V.; Franke, A.A.; Blackburn, G.L.; Zhou, J.-R. Soy Phytochemicals Prevent Orthotopic Growth and Metastasis of Bladder Cancer in Mice by Alterations of Cancer Cell Proliferation and Apoptosis and Tumor Angiogenesis. Cancer Res. 2006, 66, 1851–1858. [Google Scholar] [CrossRef]
- Tuli, H.S.; Tuorkey, M.J.; Thakral, F.; Sak, K.; Kumar, M.; Sharma, A.K.; Sharma, U.; Jain, A.; Aggarwal, V.; Bishayee, A. Molecular Mechanisms of Action of Genistein in Cancer: Recent Advances. Front. Pharmacol. 2019, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- Yammine, A.; Zarrouk, A.; Nury, T.; Vejux, A.; Latruffe, N.; Vervandier-Fasseur, D.; Samadi, M.; Mackrill, J.J.; Greige-Gerges, H.; Auezova, L.; et al. Prevention by Dietary Polyphenols (Resveratrol, Quercetin, Apigenin) Against 7-Ketocholesterol-Induced Oxiapoptophagy in Neuronal N2a Cells: Potential Interest for the Treatment of Neurodegenerative and Age-Related Diseases. Cells 2020, 9, 2346. [Google Scholar] [CrossRef]
- Ali, M.; Benfante, V.; Di Raimondo, D.; Salvaggio, G.; Tuttolomondo, A.; Comelli, A. Recent Developments in Nanoparticle Formulations for Resveratrol Encapsulation as an Anticancer Agent. Pharmaceuticals 2024, 17, 126. [Google Scholar] [CrossRef] [PubMed]
- Smoliga, J.M.; Blanchard, O. Enhancing the Delivery of Resveratrol in Humans: If Low Bioavailability is the Problem, What is the Solution? Molecules 2014, 19, 17154–17172. [Google Scholar] [CrossRef]
- Bai, Y.; Mao, Q.; Qin, J.; Zheng, X.; Wang, Y.; Yang, K.; Shen, H.; Xie, L. Resveratrol induces apoptosis and cell cycle arrest of human T24 bladder cancer cells in vitro and inhibits tumor growth in vivo. Cancer Sci. 2010, 101, 488–493. [Google Scholar] [CrossRef]
- Lin, X.; Wu, G.; Huo, W.; Zhang, Y.; Jin, F. Resveratrol induces apoptosis associated with mitochondrial dysfunction in bladder carcinoma cells. Int. J. Urol. 2012, 19, 757–764. [Google Scholar] [CrossRef]
- Stocco, B.; Toledo, K.; Salvador, M.; Paulo, M.; Koyama, N.; Toloi, M.R.T. Dose-dependent effect of Resveratrol on bladder cancer cells: Chemoprevention and oxidative stress. Maturitas 2012, 72, 72–78. [Google Scholar] [CrossRef]
- Zhou, C.; Ding, J.; Wu, Y. Resveratrol induces apoptosis of bladder cancer cells via miR-21 regulation of the Akt/Bcl-2 signaling pathway. Mol. Med. Rep. 2014, 9, 1467–1473. [Google Scholar] [CrossRef]
- Wu, M.-L.; Li, H.; Yu, L.-J.; Chen, X.-Y.; Kong, Q.-Y.; Song, X.; Shu, X.-H.; Liu, J. Short-Term Resveratrol Exposure Causes In Vitro and In Vivo Growth Inhibition and Apoptosis of Bladder Cancer Cells. PLoS ONE 2014, 9, e89806. [Google Scholar] [CrossRef]
- Bai, Y.; Yang, H.; Zhang, G.; Hu, L.; Lei, Y.; Qin, Y.; Yang, Y.; Wang, Q.; Li, R.; Mao, Q. Inhibitory effects of resveratrol on the adhesion, migration and invasion of human bladder cancer cells. Mol. Med. Rep. 2016, 15, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Meng, Q.; Xie, Q.; Zhang, M. Effect and mechanism of resveratrol on drug resistance in human bladder cancer cells. Mol. Med. Rep. 2017, 15, 1179–1187. [Google Scholar] [CrossRef]
- Shen, M.; Cai, Y.; Yang, Y.; Yan, X.; Liu, X.; Zhou, T. Centrosomal protein FOR20 is essential for S-phase progression by recruiting Plk1 to centrosomes. Cell Res. 2013, 23, 1284–1295. [Google Scholar] [CrossRef]
- Baylin, S.B.; Herman, J.G. DNA hypermethylation in tumorigenesis: Epigenetics joins genetics. Trends Genet. 2000, 16, 168–174. [Google Scholar] [CrossRef]
- Almeida, T.C.; Guerra, C.C.C.; De Assis, B.L.G.; Soares, R.D.d.O.A.; Garcia, C.C.M.; Lima, A.A.; da Silva, G.N. Antiproliferative and toxicogenomic effects of resveratrol in bladder cancer cells with different TP53 status. Environ. Mol. Mutagen. 2019, 60, 740–751. [Google Scholar] [CrossRef]
- Almeida, T.C.; Melo, A.S.; Lima, A.P.B.; Branquinho, R.T.; da Silva, G.N. Resveratrol induces the production of reactive oxygen species, interferes with the cell cycle, and inhibits the cell migration of bladder tumour cells with different TP53 status. Nat. Prod. Res. 2022, 37, 3838–3843. [Google Scholar] [CrossRef]
- Zucchi, A.; Claps, F.; Pastore, A.L.; Perotti, A.; Biagini, A.; Sallicandro, L.; Gentile, R.; Caglioti, C.; Palazzetti, F.; Fioretti, B. Focus on the Use of Resveratrol in Bladder Cancer. Int. J. Mol. Sci. 2023, 24, 4562. [Google Scholar] [CrossRef]
- Alayev, A.; Salamon, R.S.; Schwartz, N.S.; Berman, A.Y.; Wiener, S.L.; Holz, M.K. Combination of Rapamycin and Resveratrol for Treatment of Bladder Cancer. J. Cell. Physiol. 2016, 232, 436–446. [Google Scholar] [CrossRef]
- Cho, C.; Yu, C.; Wu, C.; Ho, J.; Yang, C.; Yu, D. Decreased drug resistance of bladder cancer using phytochemicals treatment. Kaohsiung J. Med Sci. 2020, 37, 128–135. [Google Scholar] [CrossRef]
- Soares, L.B.M.; Lima, A.P.B.; Melo, A.S.; Almeida, T.C.; Teixeira, L.F.d.M.; da Silva, G.N. Additive effects of resveratrol and doxorubicin on bladder cancer cells. Anti-Cancer Drugs 2021, 33, e389–e397. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef]
- Clarke, J.D.; Hsu, A.; Riedl, K.; Bella, D.; Schwartz, S.J.; Stevens, J.F.; Ho, E. Bioavailability and inter-conversion of sulforaphane and erucin in human subjects consuming broccoli sprouts or broccoli supplement in a cross-over study design. Pharmacol. Res. 2011, 64, 456–463. [Google Scholar] [CrossRef]
- Michaud, D.S.; Spiegelman, D.; Clinton, S.K.; Rimm, E.B.; Willett, W.C.; Giovannucci, E.L. Fruit and Vegetable Intake and Incidence of Bladder Cancer in a Male Prospective Cohort. JNCI J. Natl. Cancer Inst. 1999, 91, 605–613. [Google Scholar] [CrossRef]
- Munday, R.; Mhawech-Fauceglia, P.; Munday, C.M.; Paonessa, J.D.; Tang, L.; Munday, J.S.; Lister, C.; Wilson, P.; Fahey, J.W.; Davis, W.; et al. Inhibition of Urinary Bladder Carcinogenesis by Broccoli Sprouts. Cancer Res. 2008, 68, 1593–1600. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, Y.; Jobson, H.E.; Li, J.; Stephenson, K.K.; Wade, K.L.; Fahey, J.W. Potent activation of mitochondria-mediated apoptosis and arrest in S and M phases of cancer cells by a broccoli sprout extract. Mol. Cancer Ther. 2006, 5, 935–944. [Google Scholar] [CrossRef]
- Park, H.S.; Han, M.H.; Kim, G.-Y.; Moon, S.-K.; Kim, W.-J.; Hwang, H.J.; Park, K.Y.; Choi, Y.H. Sulforaphane induces reactive oxygen species-mediated mitotic arrest and subsequent apoptosis in human bladder cancer 5637 cells. Food Chem. Toxicol. 2014, 64, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Jo, G.H.; Kim, G.-Y.; Kim, W.-J.; Park, K.Y.; Choi, Y.H. Sulforaphane induces apoptosis in T24 human urinary bladder cancer cells through a reactive oxygen species-mediated mitochondrial pathway: The involvement of endoplasmic reticulum stress and the Nrf2 signaling pathway. Int. J. Oncol. 2014, 45, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Juengel, E.; Erb, H.H.; Haferkamp, A.; Rutz, J.; Chun, F.K.-H.; Blaheta, R.A. Relevance of the natural HDAC inhibitor sulforaphane as a chemopreventive agent in urologic tumors. Cancer Lett. 2018, 435, 121–126. [Google Scholar] [CrossRef]
- Abbaoui, B.; Riedl, K.M.; Ralston, R.A.; Thomas-Ahner, J.M.; Schwartz, S.J.; Clinton, S.K.; Mortazavi, A. Inhibition of bladder cancer by broccoli isothiocyanates sulforaphane and erucin: Characterization, metabolism, and interconversion. Mol. Nutr. Food Res. 2012, 56, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Bertl, E.; Bartsch, H.; Gerhäuser, C. Inhibition of angiogenesis and endothelial cell functions are novel sulforaphane-mediated mechanisms in chemoprevention. Mol. Cancer Ther. 2006, 5, 575–585. [Google Scholar] [CrossRef]
- Shan, Y.; Wu, K.; Wang, W.; Wang, S.; Lin, N.; Zhao, R.; Cassidy, A.; Bao, Y. Sulforaphane down-regulates COX-2 expression by activating p38 and inhibiting NF-κB-DNA-binding activity in human bladder T24 cells. Int. J. Oncol. 2009, 34, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Paonessa, J.D.; Randall, K.L.; Argoti, D.; Chen, L.; Vouros, P.; Zhang, Y. Sulforaphane inhibits 4-aminobiphenyl-induced DNA damage in bladder cells and tissues. Carcinogenesis 2010, 31, 1999–2003. [Google Scholar] [CrossRef]
- Xie, H.; Rutz, J.; Maxeiner, S.; Grein, T.; Thomas, A.; Juengel, E.; Chun, F.K.-H.; Cinatl, J.; Haferkamp, A.; Tsaur, I.; et al. Sulforaphane Inhibits Adhesion and Migration of Cisplatin- and Gemcitabine-Resistant Bladder Cancer Cells In Vitro. Nutrients 2024, 16, 623. [Google Scholar] [CrossRef]
- Xie, H.; Rutz, J.; Maxeiner, S.; Grein, T.; Thomas, A.; Juengel, E.; Chun, F.K.-H.; Cinatl, J.; Haferkamp, A.; Tsaur, I.; et al. Plant-Derived Sulforaphane Suppresses Growth and Proliferation of Drug-Sensitive and Drug-Resistant Bladder Cancer Cell Lines In Vitro. Cancers 2022, 14, 4682. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, J.; Wang, X.; Zheng, S.; Liang, K.; Kang, Y.E.; Chang, J.W.; Koo, B.S.; Liu, L.; Gal, A.; et al. Sulforaphane suppresses bladder cancer metastasis via blocking actin nucleation-mediated pseudopodia formation. Cancer Lett. 2024, 601, 217145. [Google Scholar] [CrossRef]
- Huang, L.; He, C.; Zheng, S.; Wu, C.; Ren, M.; Shan, Y. AKT1/HK2 Axis-mediated Glucose Metabolism: A Novel Therapeutic Target of Sulforaphane in Bladder Cancer. Mol. Nutr. Food Res. 2021, 66, 2100738. [Google Scholar] [CrossRef]
- Islam, S.S.; Mokhtari, R.B.; Akbari, P.; Hatina, J.; Yeger, H.; A Farhat, W. Simultaneous Targeting of Bladder Tumor Growth, Survival, and Epithelial-to-Mesenchymal Transition with a Novel Therapeutic Combination of Acetazolamide (AZ) and Sulforaphane (SFN). Target. Oncol. 2015, 11, 209–227. [Google Scholar] [CrossRef]
- Sailo, B.L.; Liu, L.; Chauhan, S.; Girisa, S.; Hegde, M.; Liang, L.; Alqahtani, M.S.; Abbas, M.; Sethi, G.; Kunnumakkara, A.B. Harnessing Sulforaphane Potential as a Chemosensitizing Agent: A Comprehensive Review. Cancers 2024, 16, 244. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, C.; Moccia, S.; Tedesco, I.; Crescente, G.; Volpe, M.G.; Russo, M.; Russo, G.L. Phenolic Extract from Extra Virgin Olive Oil Induces Different Anti-Proliferative Pathways in Human Bladder Cancer Cell Lines. Nutrients 2022, 15, 182. [Google Scholar] [CrossRef]
- Fabiani, R. Anti-cancer properties of olive oil secoiridoid phenols: A systematic review of in vivo studies. Food Funct. 2016, 7, 4145–4159. [Google Scholar] [CrossRef]
- Casaburi, I.; Puoci, F.; Chimento, A.; Sirianni, R.; Ruggiero, C.; Avena, P.; Pezzi, V. Potential of olive oil phenols as chemopreventive and therapeutic agents against cancer: A review of in vitro studies. Mol. Nutr. Food Res. 2012, 57, 71–83. [Google Scholar] [CrossRef]
- Gil, A.P.R.; Kodonis, I.; Ioannidis, A.; Nomikos, T.; Dimopoulos, I.; Kosmidis, G.; Katsa, M.E.; Melliou, E.; Magiatis, P. The Effect of Dietary Intervention With High-Oleocanthal and Oleacein Olive Oil in Patients With Early-Stage Chronic Lymphocytic Leukemia: A Pilot Randomized Trial. Front. Oncol. 2022, 11, 810249. [Google Scholar] [CrossRef]
- Brinkman, M.T.; Buntinx, F.; Kellen, E.; Van Dongen, M.C.; Dagnelie, P.C.; Muls, E.; Zeegers, M.P. Consumption of animal products, olive oil and dietary fat and results from the Belgian case–control study on bladder cancer risk. Eur. J. Cancer 2011, 47, 436–442. [Google Scholar] [CrossRef]
- Coccia, A.; Bastianelli, D.; Mosca, L.; Monticolo, R.; Panuccio, I.; Carbone, A.; Calogero, A.; Lendaro, E. Extra Virgin Olive Oil Phenols Suppress Migration and Invasion of T24 Human Bladder Cancer Cells Through Modulation of Matrix Metalloproteinase-2. Nutr. Cancer 2014, 66, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Coccia, A.; Mosca, L.; Puca, R.; Mangino, G.; Rossi, A.; Lendaro, E. Extra-virgin olive oil phenols block cell cycle progression and modulate chemotherapeutic toxicity in bladder cancer cells. Oncol. Rep. 2016, 36, 3095–3104. [Google Scholar] [CrossRef]
- Rutz, J.; Maxeiner, S.; Juengel, E.; Chun, F.K.-H.; Tsaur, I.; Blaheta, R.A. Olive Mill Wastewater Inhibits Growth and Proliferation of Cisplatin- and Gemcitabine-Resistant Bladder Cancer Cells In Vitro by Down-Regulating the Akt/mTOR-Signaling Pathway. Nutrients 2022, 14, 369. [Google Scholar] [CrossRef]
- Cheng, G.; Xie, L. Parthenolide Induces Apoptosis and Cell Cycle Arrest of Human 5637 Bladder Cancer Cells In Vitro. Molecules 2011, 16, 6758–6768. [Google Scholar] [CrossRef]
- Gil da Costa, R.M.; Levesque, C.; Bianchi-Frias, D.; Chatterjee, P.; Lam, H.; Santos, C.; Coleman, I.M.; Ferreirinha, P.; Vilanova, M.; da Cunha, N.P.; et al. Pharmacological NF-κB inhibition decreases cisplatin chemoresistance in muscle-invasive bladder cancer and reduces cisplatin-induced toxicities. Mol. Oncol. 2023, 17, 2709–2727. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Song, W.; Xiao, W. Dioscin induces demethylation of DAPK-1 and RASSF-1alpha genes via the antioxidant capacity, resulting in apoptosis of bladder cancer T24 cells. EXCLI J. 2017, 16, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Meng, X.; Zheng, H.; Zeng, Q.; Chen, T.; Wang, W.; Zhang, X.; Su, J. Kaempferol Attenuates ROS-Induced Hemolysis and the Molecular Mechanism of Its Induction of Apoptosis on Bladder Cancer. Molecules 2018, 23, 2592. [Google Scholar] [CrossRef]
- Chen, R.; Ho, C.; Wang, Y. Pterostilbene induces autophagy and apoptosis in sensitive and chemoresistant human bladder cancer cells. Mol. Nutr. Food Res. 2010, 54, 1819–1832. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, K.; Yang, L.; Zhang, G. Bladder cancer cell viability inhibition and apoptosis induction by baicalein through targeting the expression of anti-apoptotic genes. Saudi J. Biol. Sci. 2017, 25, 1478–1482. [Google Scholar] [CrossRef]
- Choi, E.-O.; Park, C.; Hwang, H.-J.; Hong, S.H.; Kim, G.-Y.; Cho, E.-J.; Kim, W.-J.; Choi, Y.H. Baicalein induces apoptosis via ROS-dependent activation of caspases in human bladder cancer 5637 cells. Int. J. Oncol. 2016, 49, 1009–1018. [Google Scholar] [CrossRef]
- Su, Q.; Peng, M.; Zhang, Y.; Xu, W.; Darko, K.O.; Tao, T.; Huang, Y.; Tao, X.; Yang, X. Quercetin induces bladder cancer cells apoptosis by activation of AMPK signaling pathway. Am. J. Cancer Res. 2016, 6, 498–508. [Google Scholar]
- Golmohammadi, M.; Elmaghraby, D.A.; Ramírez-Coronel, A.A.; Rakhimov, N.; Mohammed, S.S.; Romero-Parra, R.M.; Jawad, M.A.; Zamanian, M.Y.; Soltani, A.; Taheri, N.; et al. A comprehensive view on the quercetin impact on bladder cancer: Focusing on oxidative stress, cellular, and molecular mechanisms. Fundam. Clin. Pharmacol. 2023, 37, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Kanai, K.; Kikuchi, E.; Mikami, S.; Suzuki, E.; Uchida, Y.; Kodaira, K.; Miyajima, A.; Ohigashi, T.; Nakashima, J.; Oya, M. Vitamin E succinate induced apoptosis and enhanced chemosensitivity to paclitaxel in human bladder cancer cells in vitro and in vivo. Cancer Sci. 2009, 101, 216–223. [Google Scholar] [CrossRef]
- Ye, C.; Zhao, W.; Li, M.; Zhuang, J.; Yan, X.; Lu, Q.; Chang, C.; Huang, X.; Zhou, J.; Xie, B.; et al. δ-Tocotrienol Induces Human Bladder Cancer Cell Growth Arrest, Apoptosis and Chemosensitization through Inhibition of STAT3 Pathway. PLoS ONE 2015, 10, e0122712. [Google Scholar] [CrossRef]
- Bryan, R.T.; Pirrie, S.J.; Abbotts, B.; Maycock, S.; During, V.; Lewis, C.; Grant, M.; Bird, D.; Devall, A.J.; Wallace, D.M.A.; et al. Selenium and Vitamin E for Prevention of Non–Muscle-Invasive Bladder Cancer Recurrence and Progression. JAMA Netw. Open 2023, 6, e2337494. [Google Scholar] [CrossRef]
- Ryan, J.L.; Heckler, C.E.; Ling, M.; Katz, A.; Williams, J.P.; Pentland, A.P.; Morrow, G.R. Curcumin for Radiation Dermatitis: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial of Thirty Breast Cancer Patients. Radiat. Res. 2013, 180, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Belcaro, G.; Hosoi, M.; Pellegrini, L.; Appendino, G.; Ippolito, E.; Ricci, A.; Ledda, A.; Dugall, M.; Cesarone, M.R.; Maione, C.; et al. A Controlled Study of a Lecithinized Delivery System of Curcumin (Meriva®) to Alleviate the Adverse Effects of Cancer Treatment. Phytotherapy Res. 2013, 28, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, J.; Rastmanesh, R.; Taleban, F.-A.; Molana, S.-H.; Hejazi, E.; Ehtejab, G.; Hara, N. Effect of Curcumin Supplementation During Radiotherapy on Oxidative Status of Patients with Prostate Cancer: A Double Blinded, Randomized, Placebo-Controlled Study. Nutr. Cancer 2016, 68, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Howells, L.M.; O O Iwuji, C.; Irving, G.R.B.; Barber, S.; Walter, H.; Sidat, Z.; Griffin-Teall, N.; Singh, R.; Foreman, N.; Patel, S.R.; et al. Curcumin Combined with FOLFOX Chemotherapy Is Safe and Tolerable in Patients with Metastatic Colorectal Cancer in a Randomized Phase IIa Trial. J. Nutr. 2019, 149, 1133–1139. [Google Scholar] [CrossRef]
- Hidayat, Y.M.; Wagey, F.; Suardi, D.; Susanto, H.; Laihad, B.J.; Tobing, M.D.L. Analysis of Curcumin as a Radiosensitizer in Cancer Therapy with Serum Survivin Examination: Randomised Control Trial. Asian Pac. J. Cancer Prev. 2021, 22, 139–143. [Google Scholar] [CrossRef]
- Chaiworramukkul, A.; Seetalarom, K.; Saichamchan, S.; Prasongsook, N. A Double-Blind, Placebo-Controlled Randomized Phase IIa Study: Evaluating the Effect of Curcumin for Treatment of Cancer Anorexia–Cachexia Syndrome in Solid Cancer Patients. Asian Pac. J. Cancer Prev. 2022, 23, 2333–2340. [Google Scholar] [CrossRef]
- Gunther, J.R.; Chadha, A.S.; Guha, S.; Raju, G.S.; Maru, D.M.; Munsell, M.F.; Jiang, Y.; Yang, P.; Felix, E.; Clemons, M.; et al. A phase II randomized double blinded trial evaluating the efficacy of curcumin with pre-operative chemoradiation for rectal cancer. J. Gastrointest. Oncol. 2022, 13, 2938–2950. [Google Scholar] [CrossRef]
- Ramezani, V.; Ghadirian, S.; Shabani, M.; Boroumand, M.A.; Daneshvar, R.; Saghafi, F. Efficacy of curcumin for amelioration of radiotherapy-induced oral mucositis: A preliminary randomized controlled clinical trial. BMC Cancer 2023, 23, 1–9. [Google Scholar] [CrossRef]
- Farhood, B.; Najafi, M.; Talakesh, T.; Tabatabaee, N.; Atoof, F.; Aliasgharzadeh, A.; Sarvizade, M. Effect of Nano-Curcumin on Radiotherapy-Induced Skin Reaction in Breast Cancer Patients: A Randomized, Triple-Blind, Placebo-Controlled Trial. Curr. Radiopharm. 2022, 15, 332–340. [Google Scholar] [CrossRef]
- Cruz-Correa, M.; Hylind, L.M.; Marrero, J.H.; Zahurak, M.L.; Murray-Stewart, T.; Casero, R.A.; Montgomery, E.A.; Iacobuzio-Donahue, C.; Brosens, L.A.; Offerhaus, G.J.; et al. Efficacy and Safety of Curcumin in Treatment of Intestinal Adenomas in Patients With Familial Adenomatous Polyposis. Gastroenterology 2018, 155, 668–673. [Google Scholar] [CrossRef]
- Saadipoor, A.; Razzaghdoust, A.; Simforoosh, N.; Mahdavi, A.; Bakhshandeh, M.; Moghadam, M.; Abdollahi, H.; Mofid, B. Randomized, double-blind, placebo-controlled phase II trial of nanocurcumin in prostate cancer patients undergoing radiotherapy. Phytotherapy Res. 2018, 33, 370–378. [Google Scholar] [CrossRef]
- Zhu, W.; Mei, H.; Jia, L.; Zhao, H.; Li, X.; Meng, X.; Zhao, X.; Xing, L.; Yu, J. Epigallocatechin-3-gallate mouthwash protects mucosa from radiation-induced mucositis in head and neck cancer patients: A prospective, non-randomised, phase 1 trial. Investig. New Drugs 2019, 38, 1129–1136. [Google Scholar] [CrossRef]
- van Die, M.D.; Williams, S.G.; Emery, J.; Bone, K.M.; Taylor, J.M.; Lusk, E.; Pirotta, M.V. A Placebo-Controlled Double-Blinded Randomized Pilot Study of Combination Phytotherapy in Biochemically Recurrent Prostate Cancer. Prostate 2017, 77, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.M.; Wang, P.; Lee, R.-P.; Trang, A.; Husari, G.; Yang, J.; Grojean, E.M.; Ly, A.; Hsu, M.; Heber, D.; et al. Prospective randomized trial evaluating blood and prostate tissue concentrations of green tea polyphenols and quercetin in men with prostate cancer. Food Funct. 2020, 11, 4114–4122. [Google Scholar] [CrossRef]
- Kooshyar, M.M. A Randomized Placebo- Controlled Double Blind Clinical Trial of Quercetin in the Prevention and Treatment of Chemotherapy-Induced Oral Mucositis. J. Clin. Diagn. Res. 2017, 11, ZC46–ZC50. [Google Scholar] [CrossRef] [PubMed]
- Zwicker, J.I.; Schlechter, B.L.; Stopa, J.D.; Liebman, H.A.; Aggarwal, A.; Puligandla, M.; Caughey, T.; Bauer, K.A.; Kuemmerle, N.; Wong, E.; et al. Targeting protein disulfide isomerase with the flavonoid isoquercetin to improve hypercoagulability in advanced cancer. JCI Insight 2019, 4, e125851. [Google Scholar] [CrossRef]
- Howells, L.M.; Berry, D.P.; Elliott, P.J.; Jacobson, E.W.; Hoffmann, E.; Hegarty, B.; Brown, K.; Steward, W.P.; Gescher, A.J. Phase I Randomized, Double-Blind Pilot Study of Micronized Resveratrol (SRT501) in Patients with Hepatic Metastases—Safety, Pharmacokinetics, and Pharmacodynamics. Cancer Prev. Res. 2011, 4, 1419–1425. [Google Scholar] [CrossRef]
- Zhu, W.; Qin, W.; Zhang, K.; Rottinghaus, G.E.; Chen, Y.-C.; Kliethermes, B.; Sauter, E.R. Trans-Resveratrol Alters Mammary Promoter Hypermethylation in Women at Increased Risk for Breast Cancer. Nutr. Cancer 2012, 64, 393–400. [Google Scholar] [CrossRef]
- Popat, R.; Plesner, T.; Davies, F.; Cook, G.; Cook, M.; Elliott, P.; Jacobson, E.; Gumbleton, T.; Oakervee, H.; Cavenagh, J. A phase 2 study of SRT501 (resveratrol) with bortezomib for patients with relapsed and or refractory multiple myeloma. Br. J. Haematol. 2013, 160, 714–717. [Google Scholar] [CrossRef]
- Holcombe, R.F.; Nguyen, A.V.; Martinez, M.; Stamos, M.J.; Moyer, M.P.; Planutis, K.; Hope, C. Results of a phase I pilot clinical trial examining the effect of plant-derived resveratrol and grape powder on Wnt pathway target gene expression in colonic mucosa and colon cancer. Cancer Manag. Res. 2009, 1, 25–37. [Google Scholar] [CrossRef]
- Traka, M.H.; Melchini, A.; Coode-Bate, J.; Al Kadhi, O.; Saha, S.; Defernez, M.; Troncoso-Rey, P.; Kibblewhite, H.; O’Neill, C.M.; Bernuzzi, F.; et al. Transcriptional changes in prostate of men on active surveillance after a 12-mo glucoraphanin-rich broccoli intervention—Results from the Effect of Sulforaphane on prostate CAncer PrEvention (ESCAPE) randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1133–1144. [Google Scholar] [CrossRef]
- Soflaei, S.S.; Momtazi-Borojeni, A.A.; Majeed, M.; Derosa, G.; Maffioli, P.; Sahebkar, A. Curcumin: A Natural Pan-HDAC Inhibitor in Cancer. Curr. Pharm. Des. 2018, 24, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.G.; Solsona, E.; Esteban, E.; Saez, A.; Gonzalez-Larriba, J.; Anton, A.; Hevia, M.; de la Rosa, F.; Guillem, V.; Bellmunt, J. Randomized phase III trial comparing adjuvant paclitaxel/gemcitabine/cisplatin (PGC) to observation in patients with resected invasive bladder cancer: Results of the Spanish Oncology Genitourinary Group (SOGUG) 99/01 study. J. Clin. Oncol. 2010, 28, LBA4518. [Google Scholar] [CrossRef]
- Heidar, N.A.; Bhat, T.A.; Shabir, U.; Hussein, A.A. The Urinary Microbiome and Bladder Cancer. Life 2023, 13, 812. [Google Scholar] [CrossRef] [PubMed]
- A Ingersoll, M.; Albert, M.L. From infection to immunotherapy: Host immune responses to bacteria at the bladder mucosa. Mucosal Immunol. 2013, 6, 1041–1053. [Google Scholar] [CrossRef]
- Russo, A.E.; Memon, A.; Ahmed, S. Bladder Cancer and the Urinary Microbiome—New Insights and Future Directions: A Review. Clin. Genitourin. Cancer 2024, 22, 434–444. [Google Scholar] [CrossRef]
- Ohashi, Y.; Nakai, S.; Tsukamoto, T.; Masumori, N.; Akaza, H.; Miyanaga, N.; Kitamura, T.; Kawabe, K.; Kotake, T.; Kuroda, M.; et al. Habitual Intake of Lactic Acid Bacteria and Risk Reduction of Bladder Cancer. Urol. Int. 2002, 68, 273–280. [Google Scholar] [CrossRef]
- Rathaur, P.; Sr, K.J. Metabolism and Pharmacokinetics of Phytochemicals in the Human Body. Curr. Drug Metab. 2020, 20, 1085–1102. [Google Scholar] [CrossRef]
- Lippi, G.; Del Rio, D. Nutritional habits and bladder cancer. Transl. Androl. Urol. 2018, 7, S90–S92. [Google Scholar] [CrossRef]
- Pourkerman, M.; Rashidkhani, B.; Moslehi, N. Correlating Dietary Pattern and Bladder Cancer Risk Using Principal Component and Reduced Rank Regression Analyses. Nutr. Cancer 2022, 74, 2955–2963. [Google Scholar] [CrossRef]
- Detopoulou, P.; Fragopoulou, E.; Nomikos, T.; Yannakoulia, M.; Stamatakis, G.; Panagiotakos, D.B.; Antonopoulou, S. The relation of diet with PAF and its metabolic enzymes in healthy volunteers. Eur. J. Nutr. 2014, 54, 25–34. [Google Scholar] [CrossRef]
- Tan, A.C.; Konczak, I.; Sze, D.M.-Y.; Ramzan, I. Molecular Pathways for Cancer Chemoprevention by Dietary Phytochemicals. Nutr. Cancer 2011, 63, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Psaltopoulou, T.; Panagiotakos, D.; Pitsavos, C.; Chrysochoou, C.; Detopoulou, P.; Skoumas, J.; Stefanadis, C. Dietary antioxidant capacity is inversely associated with diabetes biomarkers: The ATTICA study. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 561–567. [Google Scholar] [CrossRef]
- Detopoulou, P.; Tsiouda, T.; Pilikidou, M.; Palyvou, F.; Tsekitsidi, E.; Mantzorou, M.; Pezirkianidou, P.; Kyrka, K.; Methenitis, S.; Voulgaridou, G.; et al. Changes in Body Weight, Body Composition, Phase Angle, and Resting Metabolic Rate in Male Patients with Stage IV Non-Small-Cell Lung Cancer Undergoing Therapy. Medicina 2022, 58, 1779. [Google Scholar] [CrossRef]
- Papadopoulou, S.K.; Detopoulou, P.; Voulgaridou, G.; Tsoumana, D.; Spanoudaki, M.; Sadikou, F.; Papadopoulou, V.G.; Zidrou, C.; Chatziprodromidou, I.P.; Giaginis, C.; et al. Mediterranean Diet and Sarcopenia Features in Apparently Healthy Adults over 65 Years: A Systematic Review. Nutrients 2023, 15, 1104. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chang, A.; Tan, W.P.; Fantony, J.J.; Gopalakrishna, A.; Barton, G.J.; Wischmeyer, P.E.; Gupta, R.T.; Inman, B.A. Diet and Exercise Are not Associated with Skeletal Muscle Mass and Sarcopenia in Patients with Bladder Cancer. Eur. Urol. Oncol. 2021, 4, 237–245. [Google Scholar] [CrossRef]
- Hu, Y.; Li, C.; Li, H.; Li, M.; Shu, X. Resveratrol-Mediated Reversal of Tumor Multi-Drug Resistance. Curr. Drug Metab. 2015, 15, 703–710. [Google Scholar] [CrossRef]
- Qian, F.; Wei, D.; Zhang, Q.; Yang, S. Modulation of P-glycoprotein function and reversal of multidrug resistance by (–)-epigallocatechin gallate in human cancer cells. Biomed. Pharmacother. 2005, 59, 64–69. [Google Scholar] [CrossRef]
- Rigalli, J.P.; Ciriaci, N.; Arias, A.; Ceballos, M.P.; Villanueva, S.S.M.; Luquita, M.G.; Mottino, A.D.; Ghanem, C.I.; Catania, V.A.; Ruiz, M.L. Regulation of Multidrug Resistance Proteins by Genistein in a Hepatocarcinoma Cell Line: Impact on Sorafenib Cytotoxicity. PLoS ONE 2015, 10, e0119502. [Google Scholar] [CrossRef]
- Li, J.; Feng, S.; Wang, X.; Zhang, B.; He, Q. Exploring the Targets and Molecular Mechanisms of Curcumin for the Treatment of Bladder Cancer Based on Network Pharmacology, Molecular Docking and Molecular Dynamics. Mol. Biotechnol. 2024, 1–22. [Google Scholar] [CrossRef]
- Detopoulou, P.; Voulgaridou, G.; Moschos, P.; Levidi, D.; Anastasiou, T.; Dedes, V.; Diplari, E.M.; Fourfouri, N.; Giaginis, C.; Panoutsopoulos, G.I.; et al. Artificial intelligence, nutrition, and ethical issues: A mini-review. Clin. Nutr. Open Sci. 2023, 50, 46–56. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, S.; Shi, D.; Bi, J. Construction of endothelial cell signatures for predicting the diagnosis, prognosis and immunotherapy response of bladder cancer via machine learning. J. Cell. Mol. Med. 2024, 28, e18155. [Google Scholar] [CrossRef] [PubMed]


| Phytochemical | Number of Patients | Inclusion Criteria | Exclusion Criteria | Dose | Duration | Endpoints | Identification Number |
|---|---|---|---|---|---|---|---|
| Genistein | supplement (N = 44) or placebo (N = 44) | >18 years or older Superficial bladder cancer Programmed BCG intravesical therapy | Pregnancy, muscle-invasive bladder cancer HIV-immunocompromised Concurrent immune or chemotherapy Concurrent active second cancer | 30 mg x3 | 10 weeks | Reducing toxicity and enhancing the efficacy of intravesical therapy, urinary symptoms, recurrence | NCT01489813 |
| Curcumin | Single group | Ureteral stent Patient reports pain, spasms, or urgency symptoms after stent placement, English knowledge Willingness to participate in a follow-up visit Willingness to provide mandatory 24-h urine collection Able to swallow supplements History of cancer or active cancer Registration ≥ 7 days after placement of a new stent or ≥3 days after a stent exchange; Willingness to refrain from grapefruit juice for 7 days before and for 7 days during the study | Warfarin at registration; Active cholecystitis The following drugs: epidermal growth factor receptor inhibitor, topoisomerase 1 inhibitor, buspirone, benzodiazepines, zolpidem, calcium channel blockers; digoxin or quinidine; codeine or fentanyl; phenytoin, propranolol, rifampin, or theophylline; History of alcohol abuse | Varies (dose-response study) | 7 days, 1 month follow-up | Adverse events Maximum tolerated dose of curcumin in combination with piperine Optimal biologically active dose for curcumin in combination with piperine extract quality of life Change in prostaglandin E2 concentrations | NCT02598726 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porfyris, O.; Detopoulou, P.; Adamantidi, T.; Tsoupras, A.; Papageorgiou, D.; Ioannidis, A.; Rojas Gil, A.P. Phytochemicals as Chemo-Preventive and Therapeutic Agents Against Bladder Cancer: A Comprehensive Review. Diseases 2025, 13, 103. https://doi.org/10.3390/diseases13040103
Porfyris O, Detopoulou P, Adamantidi T, Tsoupras A, Papageorgiou D, Ioannidis A, Rojas Gil AP. Phytochemicals as Chemo-Preventive and Therapeutic Agents Against Bladder Cancer: A Comprehensive Review. Diseases. 2025; 13(4):103. https://doi.org/10.3390/diseases13040103
Chicago/Turabian StylePorfyris, Orestis, Paraskevi Detopoulou, Theodora Adamantidi, Alexandros Tsoupras, Dimitris Papageorgiou, Anastasios Ioannidis, and Andrea Paola Rojas Gil. 2025. "Phytochemicals as Chemo-Preventive and Therapeutic Agents Against Bladder Cancer: A Comprehensive Review" Diseases 13, no. 4: 103. https://doi.org/10.3390/diseases13040103
APA StylePorfyris, O., Detopoulou, P., Adamantidi, T., Tsoupras, A., Papageorgiou, D., Ioannidis, A., & Rojas Gil, A. P. (2025). Phytochemicals as Chemo-Preventive and Therapeutic Agents Against Bladder Cancer: A Comprehensive Review. Diseases, 13(4), 103. https://doi.org/10.3390/diseases13040103










