Occurrence of Metabolic Disorders in Bilateral Primary Aldosteronism Compared to Unilateral Primary Aldosteronism
Abstract
1. Introduction
2. Materials and Procedures
2.1. Patients
2.2. PA Recognition
2.3. Subtype Diagnosis
2.4. Definition of MetS
2.5. Measures
2.6. Dyslipidemia and Diabetes Diagnosis
2.7. Analysis Procedures
2.8. Analytical Methods
3. Results
4. Discussion
| Number of Patients | Prevalence of MetS | |||||
|---|---|---|---|---|---|---|
| Published Series | Cases | Controls | BAH% | Cases | Controls | p |
| Fallo 2006 [52] | 85 PA patients | 381 EH patients | 65.8% | 41.1% | 29.6% | <0.05 |
| Somlòovà 2010 [39] | 100 PA patients | 90 EH patients | 50% | 39% | 32.2% | not mentioned |
| Iacobellis 2010 [35] | 75 PA patients | 192 EH patients | 50.7% | 25.3% | 20.8% | not mentioned |
| Ronconi 2010 [53] | 89 PA patients | 164 EH patients | 68.5% | 45% | 30% | <0.05 |
| Turchi 2014 [51] | 66 PA patients | 132 EH patients | 60.8% | 47% | 32% | <0.05 |
| Hanslik 2015 [56] | 183 PA patients | 183 controls, matched for age, sex and BP | not mentioned | 56.8% | 44.8% | =0.02 |
| Monticone 2017 [57] | 99 PA patients | 1573 non-PA hypertensive patients | 70.3% | 45.4% | 29.8% | <0.001 |
| Zhang 2020 [40] | 169 PA patients | 169 non-PA hypertensive patients, matched for age and sex | 49.7% | 72.2% | 65.7% | <0.05 |
| Present study | 160 PA patients | 80 EH patients, matched for age and sex | 48.8% | 35.75% | 18.8% | <0.05 |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Conn, J.W.; Louis, L.H. Primary aldosteronism, a new clinical entity. Ann. Intern. Med. 1956, 44, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Funder, J.W.; Carey, R.M.; Mantero, F.; Hassan Murad, M.; Reincke, M.; Shibata, H.; Stowasser, M.; Young, W.F., Jr. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 1889–1916. [Google Scholar] [CrossRef]
- Calhoun, D.A. Is There an Unrecognized Epidemic of Primary Aldosteronism? Pro. Hypertens. 2007, 50, 447–453. [Google Scholar] [CrossRef]
- Milliez, P.; Girerd, X.; Plouin, P.F.; Blacher, J.; Safar, M.E.; Mourad, J.J. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J. Am. Coll. Cardiol. 2005, 45, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Fuller, P.J.; Lim-Tio, S.S.; Brennan, F.E. Specificity in mineralocorticoid versus glucocorticoid action. Kidney Int. 2000, 57, 1256–1264. [Google Scholar] [CrossRef]
- Kayes-Wandover, K.M.; White, P.C. Steroidogenic enzyme gene expression in the human heart. J. Clin. Endocrinol. Metab. 2000, 85, 2519–2525. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, J.S.; Robert, V.; Heymes, C.; Aupetit-Faisant, B.; Mouas, C.; Moalic, J.M.; Swynghedauw, B.; Delcayre, C. Myocardial production of aldosterone and corticosterone in the rat. Physiological regulation. J. Biol. Chem. 1998, 273, 4883–4891. [Google Scholar] [CrossRef]
- Takeda, Y.; Yoneda, T.; Demura, M.; Miyamori, I.; Mabuchi, H. Cardiac aldosterone production in genetically hypertensive rats. Hypertension 2000, 36, 495–500. [Google Scholar] [CrossRef]
- Gomez-Sanchez, E.P.; Gomez-Sanchez, C.E. First there was one, then two...why more 11beta-hydroxysteroid dehydrogenases? Endocrinology 1997, 138, 5087–5088. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, S.M.; Lai, M.; Clark, C.J.; Fraser, R.; Gomez-Sanchez, C.E.; Seckl, J.R.; Connell, J.M.C.; Davies, E. 11beta-hydroxylase and aldosterone synthase expression in fetal rat hippocampal neurons. J. Mol. Endocrinol. 2002, 29, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Kenyon, C.J.; MacKenzie, S.M.; Seckl, J.R.; Fraser, R.; Connell, J.M.C.; Davies, E. Regulation of aldosterone synthase gene expression in the rat adrenal gland and central nervous system by sodium and angiotensin II. Endocrinology 2003, 144, 321–328. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takeda, Y.; Miyamori, I.; Yoneda, M.; Hatakeyama, H.; Inaba, S.; Furukawa, K.; Mabuchi, H.; Takeda, R. Regulation of aldosterone synthase in human vascular endothelial cells by angiotensin II and adrenocorticotropin. J. Clin. Endocrinol. Metab. 1996, 81, 2797–2800. [Google Scholar] [PubMed]
- Rocha, R.; Stier, C.T., Jr.; Kifor, I.; Ochoa-Maya, M.R.; Williams, H.G.; Adler, G.K. Aldosterone: A mediator of myocardial necrosis and renal arteriopathy. Endocrinology 2000, 141, 3871–3878. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.P.; Di Bello, V.; Ganzaroli, C.; Sacchetto, A.; Cesari, M.; Bertini, A.; Giorgi, D.; Scognamiglio, R.; Mariani, M.; Pessina, A.C. Excess aldosterone is associated with alterations of myocardial texture in primary aldosteronism. Hypertension 2002, 40, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.P.; Sacchetto, A.; Visentin, P.; Canali, C.; Graniero, G.R.; Palatini, P.; Pessina, A.C. Changes in left ventricular anatomy and function in hypertension and primary aldosteronism. Hypertension 1996, 27, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Kozakova, M.; Buralli, S.; Palombo, C.; Bernini, G.; Moretti, A.; Favilla, S.; Taddei, S.; Salvetti, A. Myocardial ultrasonic backscatter in hypertension: Relation to aldosterone and endothelin. Hypertension 2003, 41, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; Di Gregorio, F.; Leccese, M.; Abete, P.; Ambrosio, G.; Giusti, R.; Casini, A.; Ferrara, N.; De Matteis, C.; Sibilio, G.; et al. Evidence of exercise induced myocardial ischemia in patients with primary aldosteronism: The Cross-sectional Primary Aldosteronism and Heart Italian Multicenter Study. J. Investig. Med. 1999, 47, 212–221. [Google Scholar]
- Farquharson, C.A.; Struthers, A.D. Spironolactone increases nitric oxide bioactivity, improves endothelial vasodilator dysfunction, and suppresses vascular angiotensin I/angiotensin II conversion in patients with chronic heart failure. Circulation 2000, 101, 594–597. [Google Scholar] [CrossRef]
- Duprez, D.A.; De Buyzere, M.L.; Rietzschel, E.R.; Taes, Y.; Clement, D.L.; Morgan, D.; Cohn, J.N. Inverse relationship between aldosterone and large artery compliance in chronically treated heart failure patients. Eur. Heart J. 1998, 19, 1371–1376. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.J.; Kim, K.S.; Chen, Y.Q.; Blevins, L.S.; Nadeau, J.H.; Meranze, S.G.; Vaughan, D.E. Synergistic effect of adrenal steroids and angiotensin II on plasminogen activator inhibitor-1 production. J. Clin. Endocrinol. Metab. 2000, 85, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Chander, P.N.; Rocha, R.; Ranaudo, J.; Singh, G.; Zuckerman, A.; Stier, C.T., Jr. Aldosterone Plays a Pivotal Role in the Pathogenesis of Thrombotic Microangiopathy in SHRSP. J. Am. Soc. Nephrol. 2003, 14, 1990–1997. [Google Scholar] [CrossRef] [PubMed]
- Scott-Burden, T.; Resink, T.J.; Hahn, A.P.; Buhler, F.R. Differential stimulation of growth related metabolism in cultured smooth muscle cells from SHR and WKY rats by combinations of EGF and LDL. Biochem. Biophys. Res. Commun. 1989, 159, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Florian, J.A.; Watts, S.W. Epidermal growth factor: A potent vasoconstrictor in experimental hypertension. Am. J. Physiol. 1999, 276, H976–H983. [Google Scholar] [CrossRef] [PubMed]
- Pucci, G.; Alcidi, R.; Tap, L.; Battista, F.; Mattace-Raso, F.; Schillaci, G. Sex- and gender-related prevalence, cardiovascular risk and therapeutic approach in metabolic syndrome: A review of the literature. Pharmacol. Res. 2017, 120, 34–42. [Google Scholar] [CrossRef]
- Basaran, E.; Actas, G. Waist-to-height ratio as a novel marker of metabolic syndrome in patients with type 2 diabetes mellitus. Explor. Endocr. Metab. Dis. 2025, 2, 101421. [Google Scholar] [CrossRef]
- Bioletto, F.; Bollati, M.; Lopez, C.; Arata, S.; Procopio, M.; Ponzetto, F.; Ghigo, E.; Maccario, M.; Parasiliti-Caprino, M. Primary Aldosteronism and Resistant Hypertension: A Pathophysiological Insight. Int. J. Mol. Sci. 2022, 23, 4803. [Google Scholar] [CrossRef] [PubMed]
- Ehrhart-Bornstein, M.; Lamounier-Zepter, V.; Schraven, A.; Langenbach, J.; Willenberg, H.S.; Barthel, A.; Hauner, H.; McCann, S.M.; Scherbaum, W.A.; Bornstein, S.R. Human adipocytes secrete mineralocorticoid-releasing factors. Proc. Natl. Acad. Sci. USA 2003, 100, 14211–14216. [Google Scholar] [CrossRef] [PubMed]
- Luther, J.M. Aldosterone in vascular and metabolic dysfunction. Curr. Opin. Nephrol. Hypertens. 2016, 25, 16–21. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huby, A.C.; Otvos, L., Jr.; Belin de Chantemele, E.J. Leptin induces hypertension and endothelial dysfunction via aldosteronedependent mechanisms in obese female mice. Hypertension 2016, 67, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, J.L.; Bruder-Nascimento, T.; Belin de Chantemèle, E.J. The regulation of aldosterone secretion by leptin: Implications in obesity-related cardiovascular disease. Curr. Opin. Nephrol. Hypertens. 2018, 27, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Giacchetti, G.; Faloia, E.; Mariniello, B.; Sardu, C.; Gatti, C.; Camilloni, M.A.; Guerrieri, M.; Mantero, F. Overexpression of the renin-angiotensin system in human visceral adipose tissue in normal and overweight subjects. Am. J. Hypertens. 2002, 15, 381–388. [Google Scholar] [CrossRef]
- Engeli, S.; Bohnke, J.; Gorzelniak, K.; Janke, J.; Schling, P.; Bader, M.; Luft, F.C.; Sharma, A.M. Weight loss and the renin-angiotensin-aldosterone system. Hypertension 2005, 45, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Tuck, M.L.; Sowers, J.; Dornfeld, L.; Kledzik, G.; Maxwell, M. The effect of weight reduction on blood pressure, plasma renin activity, and plasma aldosterone levels in obese patients. N. Engl. J. Med. 1981, 304, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Min, S.H.; Kim, S.-H.; Jeong, I.-K.; Cho, H.C.; Jeong, J.-O.; Lee, J.-H.; Kang, H.-J.; Kim, H.S.; Park, K.S.; Lim, S. Independent Association of Serum Aldosterone Level with Metabolic Syndrome and Insulin Resistance in Korean Adults. Korean Circ. J. 2018, 48, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Petramala, L.; Cotesta, D.; Pergolini, M.; Zinnamosca, L.; Cianci, R.; De Toma, G.; Sciomer, S.; Letizia, C. Adipokines and Cardiometabolic Profile in Primary Hyperaldosteronism. J. Clin. Endocrinol. Metab. 2010, 95, 2391–2398. [Google Scholar] [CrossRef]
- Giacchetti, G.; Ronconi, V.; Turchi, F.; Agostinelli, L.; Mantero, F.; Rilli, S.; Boscaro, M. Aldosterone as a key mediator of the cardiometabolic syndrome in primary aldosteronism: An observational study. J. Hypertens. 2007, 25, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.A.; Mulatero, P.; Bidlingmaier, M.; Beuschlein, F.; Reincke, M. Genetic and Potential Autoimmune Triggers of Primary Aldosteronism. Hypertension 2015, 66, 248–253. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Young, W.F. Diagnosis and treatment of primary aldosteronism: Practical clinical perspectives. J. Intern. Med. 2018, 285, 126–148. [Google Scholar] [CrossRef]
- Šomlóová, Z.; Widimský, J.; Rosa, J.; Wichterle, D.; Štrauch, B.; Petrák, O.; Zelinka, T.; Vlková, J.; Mašek, M.; Dvořáková, J.; et al. The prevalence of metabolic syndrome and its components in two main types of primary aldosteronism. J. Hum. Hypertens. 2010, 24, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Luo, Q.; Tuersun, T.; Wang, G.; Wu, T.; Zhang, D.; Wang, M.; Zhou, K.; Sun, L.; Yue, N.; et al. Higher prevalence of metabolic disorders in patients with bilateral primary aldosteronism than unilateral primary aldosteronism. Clin. Endocrinol. (Oxf) 2021, 94, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Sone, M.; Inagaki, N.; Yamasaki, T.; Ogawa, O.; Takeda, Y.; Kurihara, I.; Umakoshi, H.; Ichijo, T.; Katabami, T.; et al. Obesity as a Key Factor Underlying Idiopathic Hyperaldosteronism. J. Clin. Endocrinol. Metab. 2018, 103, 4456–4464. [Google Scholar] [CrossRef]
- Luo, Q.; Li, N.-F.; Yao, X.-G.; Zhang, D.-L.; Abulikemu, S.-F.; Chang, G.-J.; Zhou, K.-M.; Wang, G.-L.; Wang, M.-H.; Ouyang, W.-J.; et al. Potential effects of age on screening for primary aldosteronism. J. Hum. Hypertens. 2015, 30, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.P.; Bisogni, V.; Bacca, A.V.; Belfiore, A.; Cesari, M.; Concistrè, A.; Del Pinto, R.; Fabris, B.; Fallo, F.; Fava, C.; et al. The 2020 Italian Society of Arterial Hypertension (SIIA) practical guidelines for the management of primary aldosteronism. Int. J. Cardiol. Hypertens. 2020, 5, 100029. [Google Scholar] [CrossRef] [PubMed]
- Mulatero, P.; Morello, F.; Veglio, F. Genetics of primary aldosteronism. J. Hypertens. 2004, 22, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Mulatero, P.; Bertello, C.; Rossato, D.; Mengozzi, G.; Milan, A.; Garrone, C.; Giraudo, G.; Passarino, G.; Garabello, D.; Verhovez, A.; et al. Roles of Clinical Criteria, Computed Tomography Scan, and Adrenal Vein Sampling in Differential Diagnosis of Primary Aldosteronism Subtypes. J. Clin. Endocrinol. Metab. 2008, 93, 1366–1371. [Google Scholar] [CrossRef] [PubMed]
- Stowasser, M.; Gordon, R.D.; Gunasekera, T.G.; Cowley, D.C.; Ward, G.; Archibald, C.; Smithers, B.M. High rate of detection of primary aldosteronism, including surgically treatable forms, after ‘non-selective’ screening of hypertensive patients. J. Hypertens. 2003, 21, 2149–2157. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Executive Summary of the Third Report of The National Cholesterol Education Program (NCEP). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Swinburn, B.A.; Kraak, V.I.; Allender, S.; Atkins, V.J.; Baker, P.I.; Bogard, J.R.; Brinsden, H.; Calvillo, A.; De Schutter, O.; Devarajan, R.; et al. The Global Syndemic of Obesity, Undernutrition, and Climate Change: The Lancet Commission report. Lancet 2019, 393, 791–846. [Google Scholar] [CrossRef] [PubMed]
- Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, J.M.; Drexel, H.; Hoes, A.V.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS Guidelines for the management of dyslipidaemias. Rev. Esp. Cardiol. 2017, 70, 115. [Google Scholar]
- World Health Orgnization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation; WHO Document Production Services: Geneva, Switzerland, 2006.
- Turchi, F.; Ronconi, V.; di Tizio, V.; Ceccoli, L.; Boscaro, M.; Giacchetti, G. Primary aldosteronism and essential hypertension: Assessment of cardiovascular risk at diagnosis and after treatment. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 476–482. [Google Scholar] [CrossRef]
- Fallo, F.; Veglio, F.; Bertello, C.; Sonino, N.; Della Mea, P.; Ermani, M.; Rabbia, F.; Federspil, G.; Mulatero, P. Prevalence and Characteristics of the Metabolic Syndrome in Primary Aldosteronism. J. Clin. Endocrinol. Metab. 2006, 91, 454–459. [Google Scholar] [CrossRef]
- Ronconi, V.; Turchi, F.; Rilli, S.; Di Mattia, D.; Agostinelli, L.; Boscaro, M.; Giacchetti, G. Metabolic syndrome in primary aldosteronism and essential hypertension: Relationship to adiponectin gene variants. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Matrozova, J.; Steichen, O.; Amar, L.; Zacharieva, S.; Jeunemaitre, X.; Plouin, P.F. Fasting plasma glucose and serum lipids in patients with primary aldosteronism: A controlled cross-sectional study. Hypertension 2009, 53, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Zhou, C.; Gong, M.; Zhang, Y.; Jiang, Y.; Song, W. The prevalence of metabolic syndrome in primary aldosteronism and essential hypertension: A systematic review and meta-analysis. J. Clin. Hypertens. 2024, 26, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Hanslik, G.; Wallaschofski, H.; Dietz, A.; Riester, A.; Reincke, M.; Allolio, B.; Lang, K.; Quack, I.; Rump, L.C.; Willenberg, H.S.; et al. Increased prevalence of diabetes mellitus and the metabolic syndrome in patients with primary aldosteronism of the German Conn’s Registry. Eur. J. Endocrinol. 2015, 173, 665–675. [Google Scholar] [CrossRef]
- Monticone, S.; Burrello, J.; Tizzani, D.; Bertello, C.; Viola, A.; Buffolo, F.; Gabetti, L.; Mengozzi, G.; Williams, T.A.; Rabbia, F.; et al. Prevalence and Clinical Manifestations of Primary Aldosteronism Encountered in Primary Care Practice. J. Am. Coll. Cardiol. 2017, 69, 1811–1820. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sanchez, C.E.; Kuppusamy, M.; Reincke, M.; Williams, T.A. Disordered CYP11B2 Expression in Primary Aldosteronism. Horm. Metab. Res. 2017, 49, 957–962. [Google Scholar] [CrossRef]
- Omata, K.; Satoh, F.; Morimoto, R.; Ito, S.; Yamazaki, Y.; Nakamura, Y.; Anand, S.K.; Guo, Z.; Stowasser, M.; Sasano, H.; et al. Cellular and Genetic Causes of Idiopathic Hyperaldosteronism. Hypertension 2018, 72, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Shibayama, Y.; Wada, N.; Baba, S.; Miyano, Y.; Obara, S.; Iwasaki, R.; Nakajima, H.; Sakai, H.; Usubuchi, H.; Terae, S.; et al. Relationship Between Visceral Fat and Plasma Aldosterone Concentration in Patients with Primary Aldosteronism. J. Endocr. Soc. 2018, 2, 1236–1245. [Google Scholar] [CrossRef]
- Urbanet, R.; Cat, A.N.D.; Feraco, A.; Venteclef, N.; El Mogrhabi, S.; Sierra-Ramos, C.; de la Rosa, D.A.; Adler, G.K.; Quilliot, D.; Rossignol, P.; et al. Adipocyte Mineralocorticoid Receptor Activation Leads to Metabolic Syndrome and Induction of Prostaglandin D2 Synthase. Hypertension 2015, 66, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Krug, A.W.; Vleugels, K.; Schinner, S.; Lamounier-Zepter, V.; Ziegler, C.G.; Bornstein, S.R.; Ehrhart-Bornstein, M. Human adipocytes induce an ERK1/2 MAP kinases-mediated upregulation of steroidogenic acute regulatory protein (StAR) and an angiotensin II-sensitization in human adrenocortical cells. Int. J. Obes. 2007, 31, 1605–1616. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fallo, F.; Dellamea, P.; Sonino, N.; Bertello, C.; Ermani, M.; Vettor, R.; Veglio, F.; Mulatero, P. Adiponectin and Insulin Sensitivity in Primary Aldosteronism. Am. J. Hypertens. 2007, 20, 855–861. [Google Scholar] [CrossRef]
- Arlt, W.; Lang, K.; Sitch, A.J.; Dietz, A.S.; Rhayem, Y.; Bancos, I.; Feuchtinger, A.; Chortis, V.; Gilligan, L.C.; Ludwig, P.; et al. Steroid metabolome analysis reveals prevalent glucocorticoid excess in primary aldosteronism. JCI Insight 2017, 2, e93136. [Google Scholar] [CrossRef]
- Rossi, E.; Foroni, M.; Regolisti, G.; Perazzoli, F.; Negro, A.; Santi, R.; Grasselli, C.; Galli, P.; Gardini, G. Combined Conn’s Syndrome and Subclinical Hypercortisolism from an Adrenal Adenoma Associated with Homolateral Renal Carcinoma. Am. J. Hypertens. 2008, 21, 1269–1272. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Akehi, Y.; Yanase, T.; Motonaga, R.; Umakoshi, H.; Tsuiki, M.; Takeda, Y.; Yoneda, T.; Kurihara, I.; Itoh, H.; Katabami, T.; et al. High Prevalence of Diabetes in Patients with Primary Aldosteronism (PA) Associated with Subclinical Hypercortisolism and Prediabetes More Prevalent in Bilateral Than Unilateral PA: A Large, Multicenter Cohort Study in Japan. Diabetes Care 2019, 42, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Ferraù, F.; Korbonits, M. Metabolic Syndrome in Cushing’s Syndrome patients. Front. Horm. Res. 2018, 49, 85–103. [Google Scholar] [PubMed]
| EH (n = 80) | APA (n = 82) | BAH (n = 78) | p Value APA vs. BAH | p Value EH vs. APA | p Value EH vs. BAH | |
|---|---|---|---|---|---|---|
| Age (years) | 55 ± 0.66 | 49 ± 0.70 | 51 ± 0.69 | 0.126 | 0.342 | 0.774 |
| Sex (male), n (%) | 38 (47.5%) | 37 (45.1%) | 38 (48.7%) | 0.385 | 0.874 | 0.878 |
| SBP (mmHg) | 139 ± 0.42 | 180 ± 0.36 | 180 ± 0.36 | 0.37 | 0.001 | 0.001 |
| DBP (mmHg) | 90 ± 0.52 | 105 ± 0.48 | 110 ± 0.47 | 0.07 | 0.001 | 0.001 |
| K+ (mmol/L) | 4.1 ± 2.45 | 3.8 ± 2.83 | 4 ± 2.45 | 0.006 | 0.006 | 0.007 |
| PAC (ng/dL) | 99 ± 0.49 | 22.3 ± 0.33 | 21.8 ± 0.33 | 0.203 | 0.001 | 0.001 |
| ARR | 11 ± 1.48 | 122 ± 0.44 | 65.8 ± 0.60 | 0.002 | 0.001 | 0.001 |
| BMI (kg/m2) | 25 ± 0.98 | 28.6 ± 0.92 | 27.8 ± 0.94 | 0.201 | 0.099 | 0.8 |
| Tryglicerides (mg/dL) | 98 ± 0.49 | 96 ± 0.50 | 104 ± 0.48 | 0.046 | 0.136 | 0.515 |
| HDL-C (mg/dL) | 51.5 ± 0.69 | 48 ± 0.70 | 48 ± 0.70 | 0.282 | 0.627 | 0.219 |
| LDL-C (mg/dL) | 117 ± 0.45 | 95.6 ± 0.50 | 112 ± 0.46 | 0.046 | 0.041 | 0.184 |
| TC (mg/dL) | 187 ± 0.36 | 173 ± 0.37 | 184 ± 0.36 | 0.07 | 0.328 | 0.381 |
| glycemia (mg/dL) | 91 ± 0.51 | 89 ± 0.51 | 91 ± 0.51 | 0.371 | 0.841 | 0.249 |
| DM, n (%) | 9 (11.3%) | 6 (7.3%) | 5 (6.4%) | 0.118 | 0.388 | 0.284 |
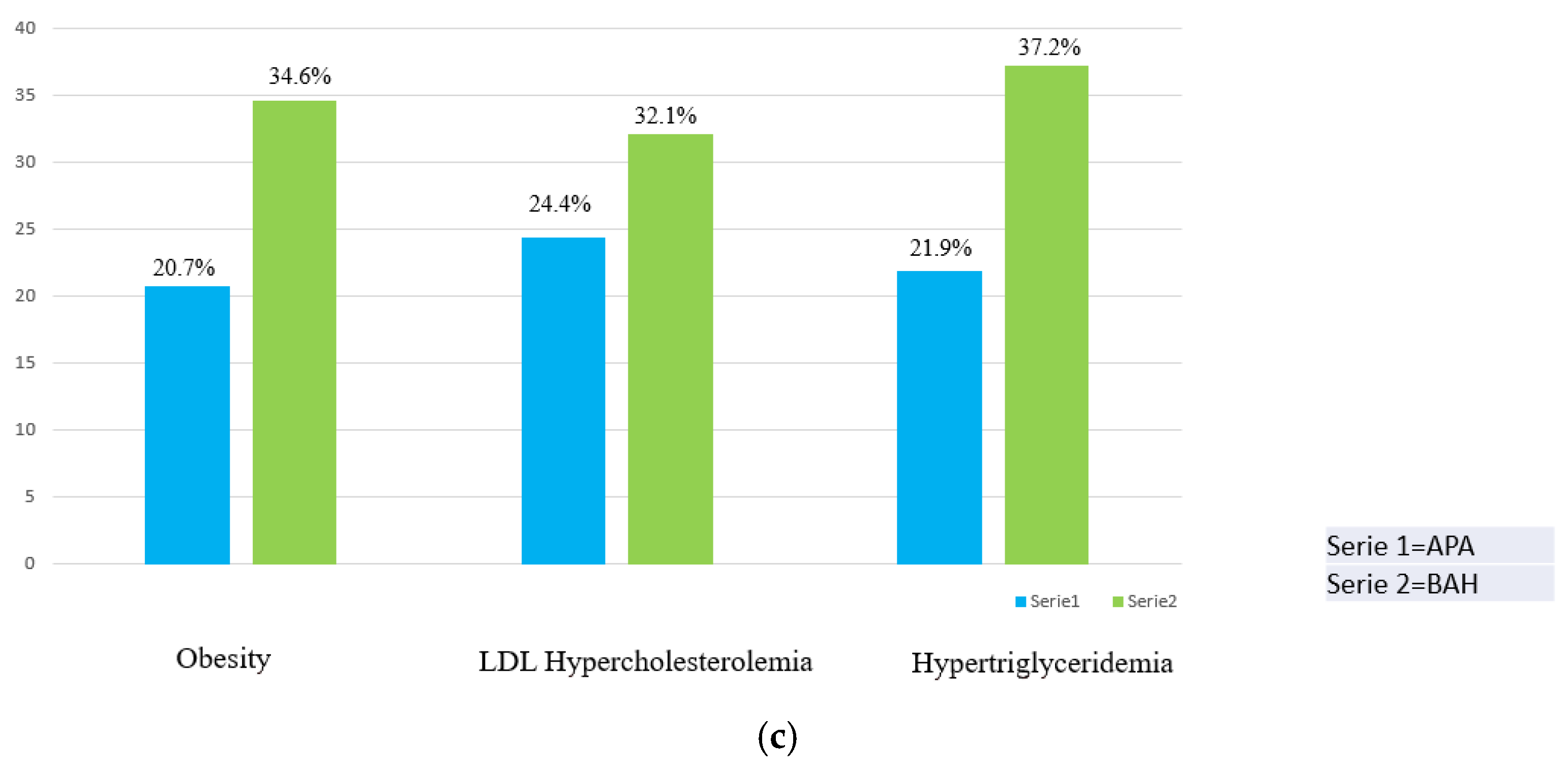
| Obesity, n (%) | 20 (25%) | 17 (20.7%) | 27 (34.6%) | 0.001 | 0.518 | 0.046 |
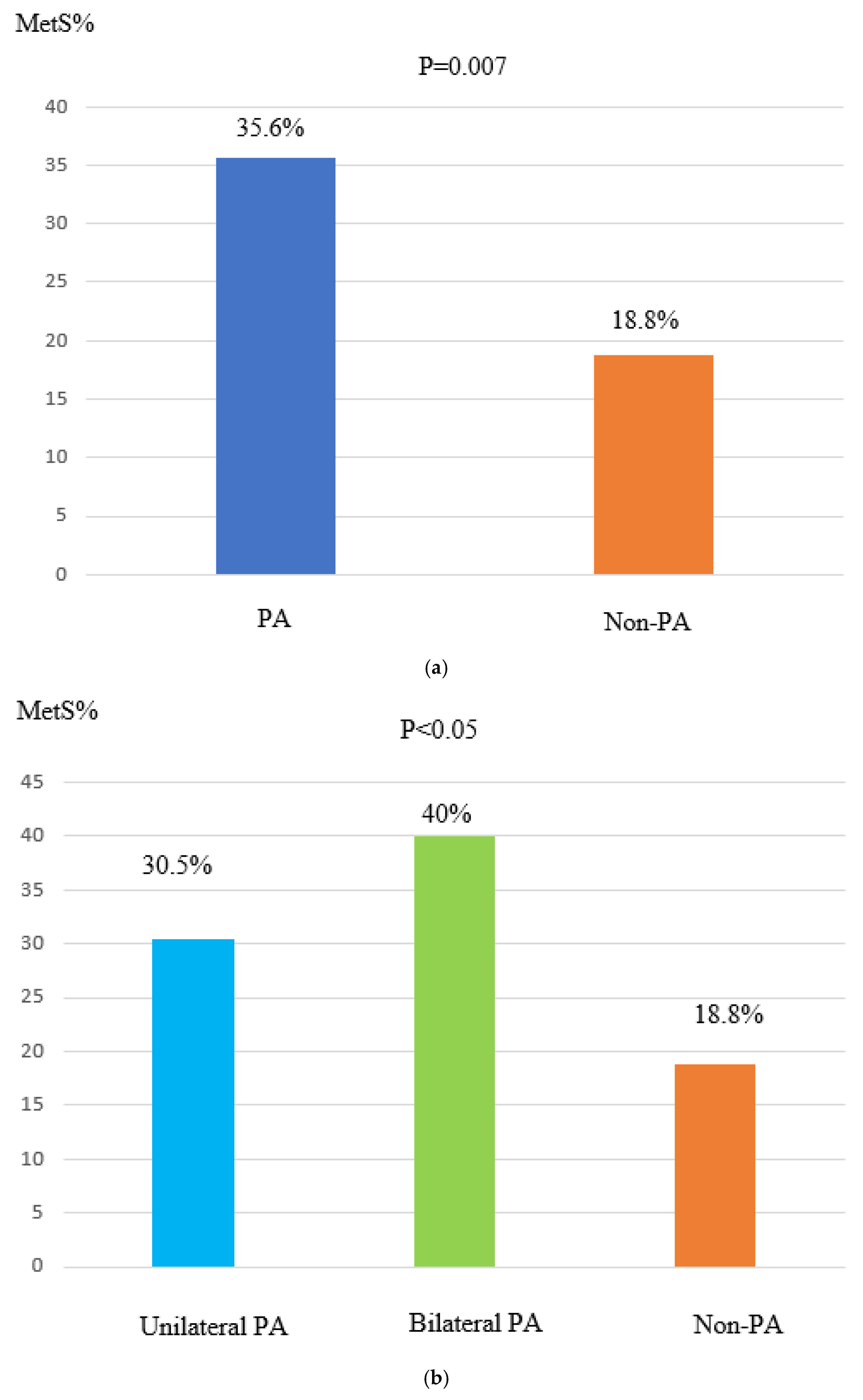
| MetS, n (%) | 15 (18.8%) | 25 (30.5%) | 32 (41%) | 0.001 | 0.083 | 0.002 |
| Subtype (BAH), n (%) | Without MetS (n = 46) | With MetS (n = 32) | p | Without Obesity (n = 51) | With Obesity (n = 27) | p |
|---|---|---|---|---|---|---|
| Age (years) | 50 ± 0.69 | 50 ± 0.69 | 0.435 | 50 ± 0.69 | 42 ± 0.76 | 0.046 |
| Sex (males), n (%) | 33 (72%) | 16 (50%) | 0.008 | 26 (51%) | 17 (63%) | 0.093 |
| BMI (kg/m2) | 25.3 ± 0.98 | 29.65 ± 0.90 | 0.036 | 25.45 ± 0.98 | 31.3 ± 0.88 | 0.952 |
| K+ (mmol/L) | 4 ± 2.45 | 4 ± 2.45 | 0.62 | 4 ± 2.45 | 4 ± 2.45 | 0.465 |
| PAC (ng/dL) | 186 ± 0.36 | 199 ± 0.35 | 0.715 | 197 ± 0.35 | 168.5 ± 0.38 | 0.622 |
| ARR | 82.7 ± 0.54 | 57.5 ± 0.65 | 0.822 | 72 ± 0.58 | 53.75 ± 0.67 | 0.301 |
| MetS | Obesity | |||
|---|---|---|---|---|
| Adjusted variables | OR (95% CI) | p Value | OR (95% CI) | p Value |
| Model 1 * | 2.398 (1.2544–4.5844) | 0.008 | 3.667 (1.8745–7.1721) | 0.001 |
| Model 1 * + age | 2.397 (1.2544–4.5844) | 0.008 | 3.688 (1.8739–7.1825) | 0.003 |
| Model 1 * + sex | 2.410 (1.2662–4.5990) | 0.013 | 3.544 (1.8673–7.1514) | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grasselli, C.; Baldini, M.; Salvi, L.; Vestita, G.; Zizzo, M.; Felaco, D.; Balli, M.C.; Besutti, G.; Negro, A.; Ghirarduzzi, A. Occurrence of Metabolic Disorders in Bilateral Primary Aldosteronism Compared to Unilateral Primary Aldosteronism. Diseases 2025, 13, 52. https://doi.org/10.3390/diseases13020052
Grasselli C, Baldini M, Salvi L, Vestita G, Zizzo M, Felaco D, Balli MC, Besutti G, Negro A, Ghirarduzzi A. Occurrence of Metabolic Disorders in Bilateral Primary Aldosteronism Compared to Unilateral Primary Aldosteronism. Diseases. 2025; 13(2):52. https://doi.org/10.3390/diseases13020052
Chicago/Turabian StyleGrasselli, Chiara, Maicol Baldini, Lucia Salvi, Grazia Vestita, Maurizio Zizzo, Davide Felaco, Maria Carolina Balli, Giulia Besutti, Aurelio Negro, and Angelo Ghirarduzzi. 2025. "Occurrence of Metabolic Disorders in Bilateral Primary Aldosteronism Compared to Unilateral Primary Aldosteronism" Diseases 13, no. 2: 52. https://doi.org/10.3390/diseases13020052
APA StyleGrasselli, C., Baldini, M., Salvi, L., Vestita, G., Zizzo, M., Felaco, D., Balli, M. C., Besutti, G., Negro, A., & Ghirarduzzi, A. (2025). Occurrence of Metabolic Disorders in Bilateral Primary Aldosteronism Compared to Unilateral Primary Aldosteronism. Diseases, 13(2), 52. https://doi.org/10.3390/diseases13020052






