Health-Related Quality of Life and Symptom Burden in Patients with Diffuse Large B-Cell Lymphoma Before Treatment with Tafasitamab and Lenalidomide: An Ad Hoc Analysis of Italian Real-World Data from the PRO-MIND Study
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Assessments
2.3. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Prior Treatments
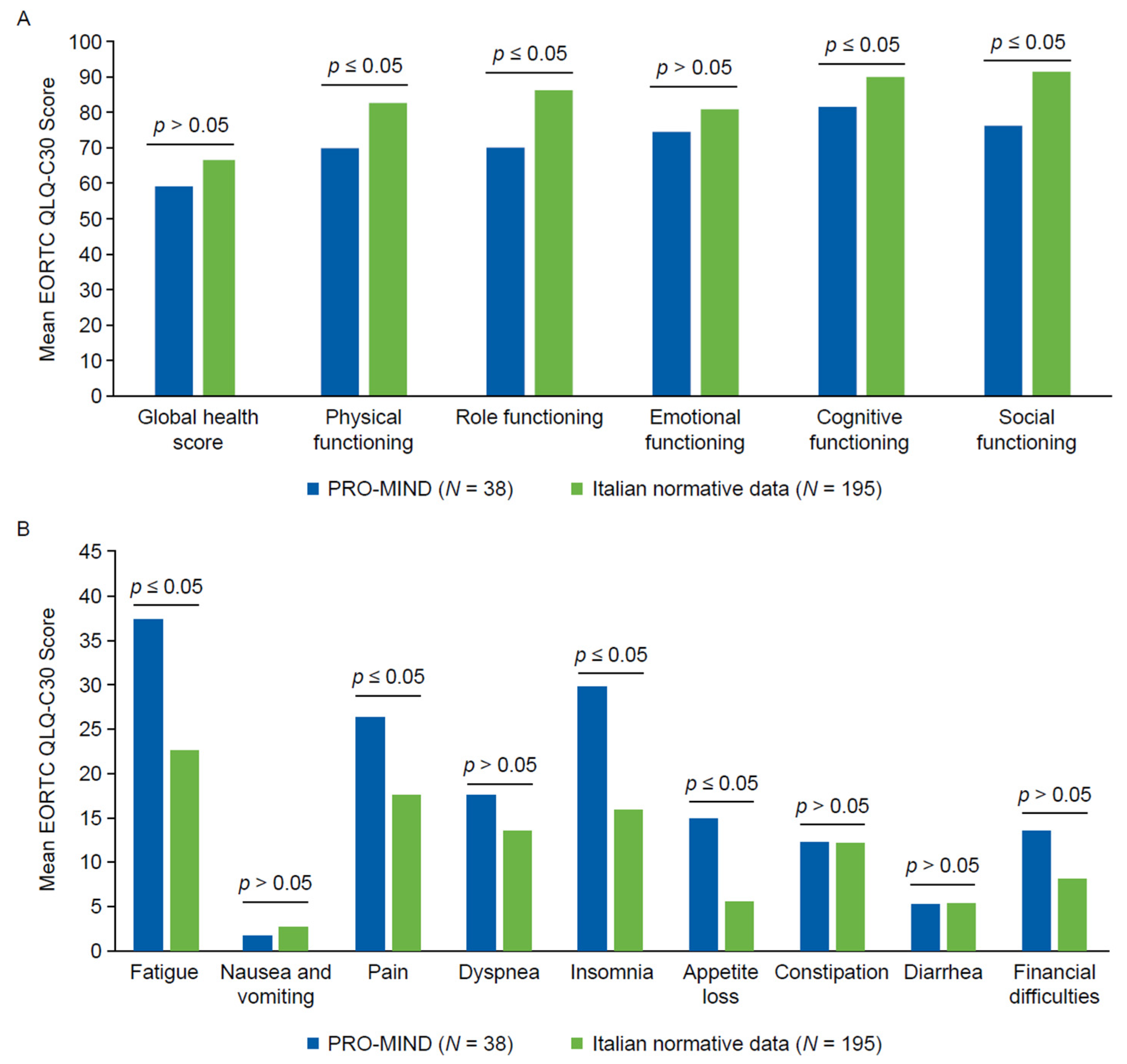
3.3. HRQoL and Symptom Burden
4. Discussion
Clinical Implications and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sehn, L.H.; Salles, G. Diffuse large B-cell lymphoma. N. Engl. J. Med. 2021, 384, 842–858. [Google Scholar] [CrossRef] [PubMed]
- Crump, M.; Neelapu, S.S.; Farooq, U.; Van Den Neste, E.; Kuruvilla, J.; Westin, J.; Link, B.K.; Hay, A.; Cerhan, J.R.; Zhu, L.; et al. Outcomes in refractory diffuse large B-cell lymphoma: Results from the international SCHOLAR-1 study. Blood 2017, 130, 1800–1808. [Google Scholar] [CrossRef]
- Brisou, G.; Paillassa, J.; Bernard, S.; Tournilhac, O.; Fornecker, L.M.; Maloisel, F.; Gastaud, L.; Durot, E.; Tempescul, A.; Braun, T.; et al. Clinical benefit of tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma: Real-world data from the EarlyMIND study. Haematologica 2025, 110, 2400–2412. [Google Scholar] [CrossRef]
- Gutierrez, A.; Zeberio, I.; Penalver, F.J.; Martinez-Barranco, P.; Perez, S.; Morillo, D.; Martin, X.; Nicolas, C.; Ferrero, A.; Jimenez-Ubieto, A.; et al. Tafasitamab plus lenalidomide as salvage therapy in diffuse large B-cell lymphoma: Real-world experience from GELTAMO. Blood Adv. 2025, 9, 4924–4935. [Google Scholar] [CrossRef]
- Salles, G.; Duell, J.; Gonzalez Barca, E.; Tournilhac, O.; Jurczak, W.; Liberati, A.M.; Nagy, Z.; Obr, A.; Gaidano, G.; Andre, M.; et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): A multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020, 21, 978–988. [Google Scholar] [CrossRef]
- Johnson, P.C.; Bailey, A.; Ma, Q.; Milloy, N.; Butcher, J.; Sanderson, I.; Weatherby, S.; Meadows, R.; Quek, R.G.W. Real-world evaluation of health-related quality of life in patients with diffuse large B-cell lymphoma based on a multinational survey. Front. Oncol. 2024, 14, 1402992. [Google Scholar] [CrossRef]
- Elsawy, M.; Chavez, J.C.; Avivi, I.; Larouche, J.-F.; Wannesson, L.; Cwynarski, K.; Osman, K.; Davison, K.; Rudzki, J.D.; Dahiya, S.; et al. Patient-reported outcomes in ZUMA-7, a phase 3 study of axicabtagene ciloleucel in second-line large B-cell lymphoma. Blood 2022, 140, 2248–2260. [Google Scholar] [CrossRef] [PubMed]
- Bachy, E.; Trask, P.; Morschhauser, F.; Corradini, P.; Wu, S.-J.; Bartlett, N.L.; Mulvihill, E.; Bene Tchaleu, F.; Lundberg, L.; Carlo-Stella, C. Primary Results of the Health-Related Quality of Life (HRQoL) and Tolerability Assessments from the Phase I/II NP30179 Study of Glofitamab Monotherapy in Patients with Relapsed/Refractory (R/R) Large B-Cell Lymphoma (LBCL). Blood 2024, 144, 1726. [Google Scholar] [CrossRef]
- Phillips, T.; Lugtenburg, P.; Kalsekar, A.; Mutebi, A.; Wang, A.; Blaedel, J.; Kosa, K.; Martin, S.; Sacchi, M.; Kilavuz, N.; et al. Improvements in patient-reported outcomes in relapsed or refractory large B-cell lymphoma patients treated with epcoritamab. Clin. Lymphoma Myeloma Leuk. 2024, 24, e78–e87. [Google Scholar] [CrossRef]
- Huang, H.; Datye, A.; Fan, M.; Knapp, A.; Nielsen, T.; Bottos, A.; Paulson, J.N.; Trask, P.C.; Efficace, F. Patient-reported outcomes provide prognostic information for survival in patients with diffuse large B-cell lymphoma: Analysis of 1239 patients from the GOYA study. Cancer Med. 2022, 11, 3312–3322. [Google Scholar] [CrossRef]
- Lim, L.; Machingura, A.; Taye, M.; Pe, M.; Coens, C.; Martinelli, F.; Alanya, A.; Antunes, S.; Tu, D.; Basch, E.; et al. Prognostic value of baseline EORTC QLQ-C30 scores for overall survival across 46 clinical trials covering 17 cancer types: A validation study. eClinicalMedicine 2025, 82, 103153. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Oerlemans, S.; Efficace, F.; Kyriakou, C.; Freitas, A.C.; Shamieh, O.; Creutzberg, C.L.; Lehmann, J.; Petranovic, D.; Nagele, E.; Bredart, A.; et al. International validation of two EORTC questionnaires for assessment of health-related quality of life for patients with high-grade non-Hodgkin lymphoma (QLQ-NHL-HG29) and low-grade non-Hodgkin lymphoma (QLQ-NHL-LG20). Cancer 2023, 129, 2727–2740. [Google Scholar] [CrossRef]
- Delong, P.S.; Humler, D.; Haag, T.; Yeomans, A.; Andrus, J.; Eremenco, S.; Finan, A.; Gable, J.; Gilfillan, D.; Howry, C.; et al. Best Practice Recommendations for Electronic Clinical Outcome Assessment Data Changes. J. Soc. Clin. Data Manag. 2024, 4. [Google Scholar] [CrossRef]
- Jensen, R.E.; Moinpour, C.M.; Fairclough, D.L. Assessing health-related quality of life in cancer trials. Clin. Investig. 2012, 2, 563–577. [Google Scholar] [CrossRef]
- Fayers, P.M.; Aaronson, N.K.; Bjordal, K.; Groenvold, M.; Curran, D.; Bottomley, A.; on behalf of the EORTC Quality of Life Group. The EORTC QLQ-C30 Scoring Manual, 3rd ed.; European Organisation for Research and Treatment of Cancer: Brussels, Belgium, 2001. [Google Scholar]
- van de Poll-Franse, L.; Oerlemans, S.; Bredart, A.; Kyriakou, C.; Sztankay, M.; Pallua, S.; Daniëls, L.; Creutzberg, C.L.; Cocks, K.; Malak, S.; et al. International development of four EORTC disease-specific quality of life questionnaires for patients with Hodgkin lymphoma, high- and low-grade non-Hodgkin lymphoma and chronic lymphocytic leukaemia. Qual. Life Res. 2018, 27, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Pilz, M.J.; Gamper, E.M.; Efficace, F.; Arraras, J.I.; Nolte, S.; Liegl, G.; Rose, M.; Giesinger, J.M.; EORTC Quality of Life Group. EORTC QLQ-C30 general population normative data for Italy by sex, age and health condition: An analysis of 1036 individuals. BMC Public Health 2022, 22, 1040. [Google Scholar] [CrossRef]
- Osoba, D.; Rodrigues, G.; Myles, J.; Zee, B.; Pater, J. Interpreting the significance of changes in health-related quality-of-life scores. J. Clin. Oncol. 2023, 41, 5345–5350. [Google Scholar] [CrossRef]
- Olsson, C.; Larsson, M.; Josse Eklund, A.; Ringnér, A. Associations between sexuality, body image and health-related quality of life in patients treated for diffuse large B-cell lymphoma: A cross-sectional study. Eur. J. Oncol. Nurs. 2024, 73, 102729. [Google Scholar] [CrossRef]
- van der Poel, M.W.; Oerlemans, S.; Schouten, H.C.; Mols, F.; Pruijt, J.F.; Maas, H.; van de Poll-Franse, L.V. Quality of life more impaired in younger than in older diffuse large B cell lymphoma survivors compared to a normative population: A study from the population-based PROFILES registry. Ann. Hematol. 2014, 93, 811–819. [Google Scholar] [CrossRef]
- Wasse, S.K.; Mounier, M.; Assogba, E.; Rossi, C.; Adnet, J.; Gauthier, S.; Girard, S.; Atsou, K.M.; Dabakuyo-Yonli, T.S.; Maynadie, M. Factors Affecting Health-Related Quality of Life among Survivors of Non-Hodgkin Lymphoma: A Population-Based Study. Cancers 2023, 15, 3885. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.P.; Chaganti, S.; McIlroy, G.; Barrington, S.F.; Burton, C.; Cwynarski, K.; Eyre, T.A.; Illidge, T.; Kalakonda, N.; Kuhnl, A.; et al. The management of newly diagnosed large B-cell lymphoma: A British Society for Haematology guideline. Br. J. Haematol. 2024, 204, 1178–1192. [Google Scholar] [CrossRef]
- Efficace, F.; Zinzani, P.; Bonifazi, F.; Clerico, M.; Chiappella, A.; Russo, D.; Saccardi, R.; Botto, B.; Baldi, T.; Tartaglia, F.; et al. Health-related quality of life profile and symptom burden of patients with aggressive B-cell lymphomas just before CAR-T-cell therapy: A real-world study by the Gimema. Blood 2023, 142, 7384. [Google Scholar] [CrossRef]
- Paunescu, A.-C.; Copie, C.B.; Malak, S.; Gouill, S.L.; Ribrag, V.; Bouabdallah, K.; Sibon, D.; Rumpold, G.; Preau, M.; Mounier, N.; et al. Quality of life of survivors 1 year after the diagnosis of diffuse large B-cell lymphoma: A LYSA study. Ann. Hematol. 2022, 101, 317–332. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Patients (N = 38) |
|---|---|
| Age, years, median (Q1–Q3) | 77.0 (73.0–82.0) |
| Female sex, n (%) | 23 (60.5) |
| Ethnicity, n (%) | |
| White | 37 (97.4) |
| Other | 1 (2.6) |
| Patients with ≥1 comorbidity, n (%) | 35 (92.1) |
| Total comorbid events | 172 |
| Comorbidities, n (%) | |
| Hypertension | 23 (60.5) |
| Dyslipidemia/hypercholesterolemia | 6 (15.8) |
| Diabetes mellitus | 5 (13.2) |
| Depression | 5 (13.2) |
| Thyroid disorder | 4 (10.5) |
| Atrial fibrillation | 4 (10.5) |
| Osteoporosis | 2 (5.3) |
| Ann Arbor stage, n (%) | |
| Stage II | 7/37 (18.9) |
| Stage III | 8/37 (21.6) |
| Stage IV | 22/37 (59.5) |
| Stage III–IV | 30/37 (81.1) |
| Histology, n (%) | |
| DLBCL NOS | 31/37 (83.8) |
| THRLBCL | 1/37 (2.7) |
| Follicular grade 3b | 1/37 (2.7) |
| Composite lymphoma | 4/37 (10.8) |
| Bulky disease, n (%) | 8/37 (21.6) |
| Transplant-ineligible, n (%) | 37/37 (100) |
| Best prior response, n (%) | |
| Complete response | 15/37 (40.5) |
| Partial response | 17/37 (45.9) |
| Stable disease | 5/37 (13.5) |
| ECOG PS, n (%) | |
| 0 | 14/30 (46.7) |
| 1 | 15/30 (50.0) |
| 2 | 1/30 (3.3) |
| ≥1 prior systemic therapy, n (%) | 37/38 (97.4) |
| Prior lines of systemic therapy, n (%) | |
| 1 | 22/37 (59.5) |
| 2 | 12/37 (32.4) |
| 3 | 3/37 (8.1) |
| Received anti-CD20 therapy, n (%) | 34/37 (91.9) |
| Anti-CD20 cycles, n (%) | |
| 2 | 1/37 (2.7) |
| 3 | 2/37 (5.4) |
| 4 | 2/37 (5.4) |
| 6 | 26/37 (70.3) |
| 8 | 6/37 (16.2) |
| Completed planned cycles, n (%) | 35/37 (94.6) |
| Discontinued due to refractory disease, n (%) | 2/37 (5.4) |
| First-line regimen, n (%) | |
| R-COMP | 22/37 (59.5) |
| R-CHOP | 5/37 (13.5) |
| Others | 5/37 (13.5) |
| Polatuzumab-based salvage regimen, n (%) | 2/37 (5.4) |
| Primary refractory disease, * n (%) | 25/37 (67.6) |
| Symptom or Concern | n | (%) |
|---|---|---|
| Worries about future health | 32 | 84.2% |
| Concerns about disease recurrence | 31 | 81.6% |
| Worries about becoming dependent | 30 | 78.9% |
| Lack of energy (fatigue) | 27 | 71.1% |
| Fear of developing a chronic condition | 26 | 68.4% |
| Sleep difficulties | 24 | 63.2% |
| Pain or discomfort | 23 | 60.5% |
| Problems with memory or concentration | 22 | 57.9% |
| Difficulties carrying out daily activities | 21 | 55.3% |
| Social restrictions | 20 | 52.6% |
| Emotional distress | 19 | 50.0% |
| Problems at work/place of study | 19 | 50.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zinzani, P.L.; Battaglia, N.; Lapecorella, M.; Gini, G.; Cox, M.C.; Hohaus, S.; Pinto, A. Health-Related Quality of Life and Symptom Burden in Patients with Diffuse Large B-Cell Lymphoma Before Treatment with Tafasitamab and Lenalidomide: An Ad Hoc Analysis of Italian Real-World Data from the PRO-MIND Study. Diseases 2025, 13, 399. https://doi.org/10.3390/diseases13120399
Zinzani PL, Battaglia N, Lapecorella M, Gini G, Cox MC, Hohaus S, Pinto A. Health-Related Quality of Life and Symptom Burden in Patients with Diffuse Large B-Cell Lymphoma Before Treatment with Tafasitamab and Lenalidomide: An Ad Hoc Analysis of Italian Real-World Data from the PRO-MIND Study. Diseases. 2025; 13(12):399. https://doi.org/10.3390/diseases13120399
Chicago/Turabian StyleZinzani, Pier Luigi, Nicola Battaglia, Mario Lapecorella, Guido Gini, Maria Cristina Cox, Stefan Hohaus, and Antonio Pinto. 2025. "Health-Related Quality of Life and Symptom Burden in Patients with Diffuse Large B-Cell Lymphoma Before Treatment with Tafasitamab and Lenalidomide: An Ad Hoc Analysis of Italian Real-World Data from the PRO-MIND Study" Diseases 13, no. 12: 399. https://doi.org/10.3390/diseases13120399
APA StyleZinzani, P. L., Battaglia, N., Lapecorella, M., Gini, G., Cox, M. C., Hohaus, S., & Pinto, A. (2025). Health-Related Quality of Life and Symptom Burden in Patients with Diffuse Large B-Cell Lymphoma Before Treatment with Tafasitamab and Lenalidomide: An Ad Hoc Analysis of Italian Real-World Data from the PRO-MIND Study. Diseases, 13(12), 399. https://doi.org/10.3390/diseases13120399







