Circulating Interleukin-6 as a Prognostic Biomarker for Mortality in Melioidosis: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of Research Design and Registration
2.2. Search Strategy and Data Sources
2.3. Study Selection and Eligibility Criteria
2.4. Data Extraction and Management
2.4.1. Systematic Data Collection Process
2.4.2. Study and Patient Characteristics
2.4.3. IL-6 Measurement and Outcome Data
2.4.4. Identification and Management of Overlapping Patient Cohorts
2.5. Quality Assessment
2.6. Statistical Analysis
2.6.1. Meta-Analytic Approach and Data Synthesis
2.6.2. Heterogeneity Assessment and Exploration
2.6.3. Sensitivity Analyses
2.6.4. Publication Bias Assessment
2.6.5. Statistical Software and Reporting Standards
3. Results
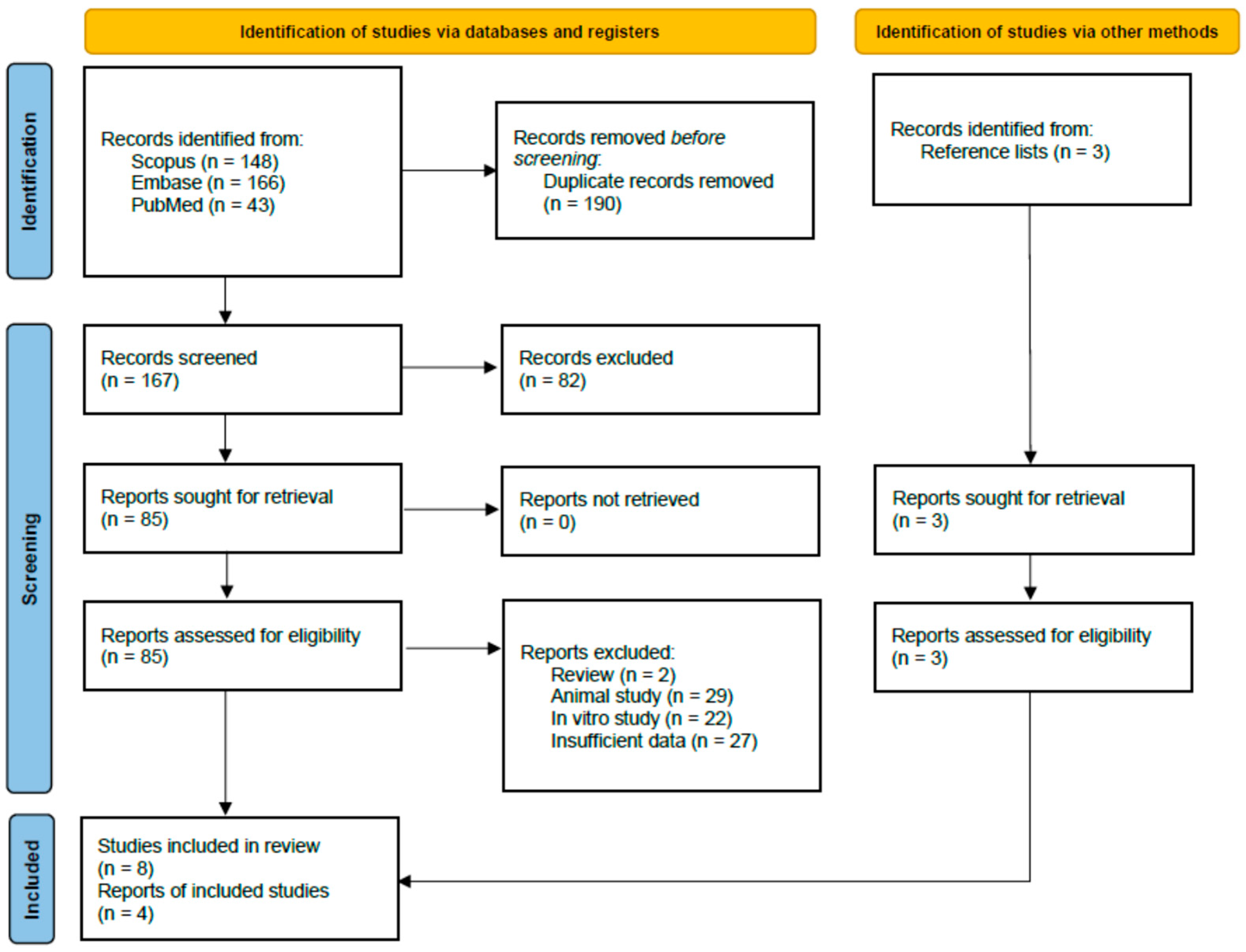
3.1. Study Selection and Identification
3.2. Characteristics of Included Studies
3.3. Quality of Included Studies
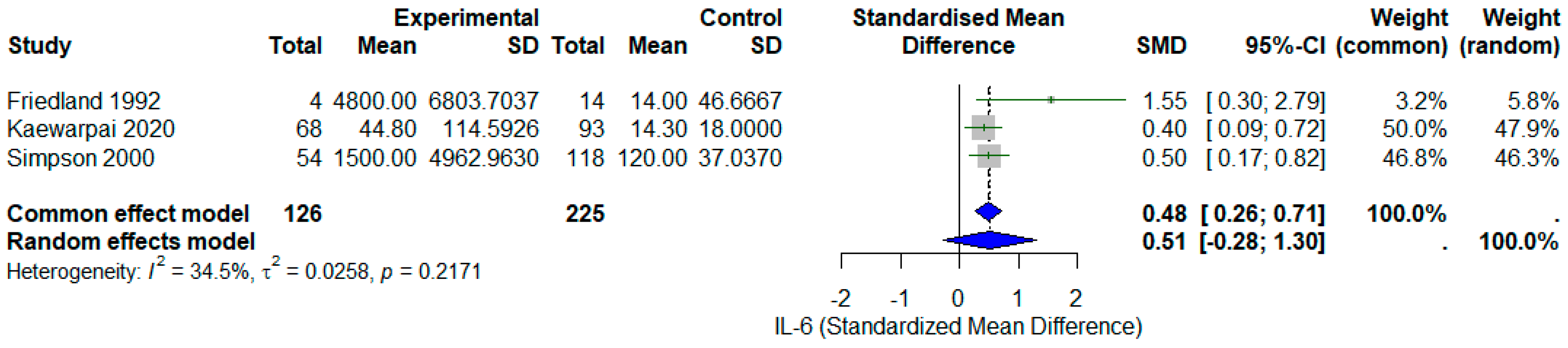
3.4. Meta-Analysis of Circulating IL-6 and Mortality
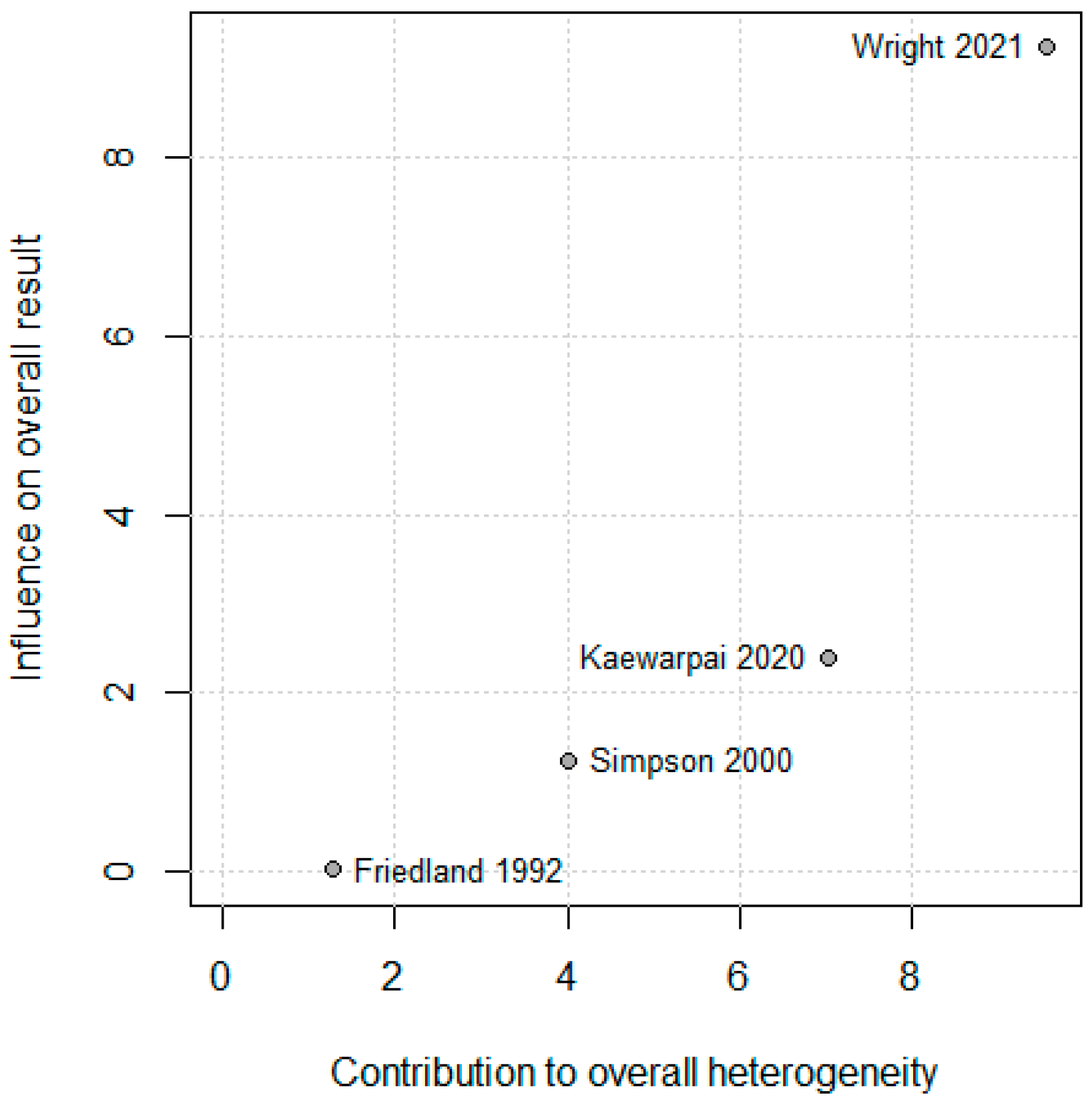
3.5. Leave-One-Out and Sensitivity Analysis
3.6. Assessment of Publication Bias and Meta-Regression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IL-6 | Interleukin 6 |
| ELISA | Enzyme-linked immunosorbent assay |
| ELC | Electrochemiluminescence |
| SMD | Standardized mean differences |
| ROM | Ratio of means |
References
- Meumann, E.M.; Limmathurotsakul, D.; Dunachie, S.J.; Wiersinga, W.J.; Currie, B.J. Burkholderia pseudomallei and melioidosis. Nate Rev. Microb. 2024, 22, 155–169. [Google Scholar] [CrossRef]
- Phillips, E.D.; Garcia, E.C. Burkholderia pseudomallei. Trends Microb. 2024, 32, 105–106. [Google Scholar] [CrossRef] [PubMed]
- Cullinane, M.; Gong, L.; Li, X.; Lazar-Adler, N.; Tra, T.; Wolvetang, E.; Prescott, M.; Boyce, J.D.; Devenish, R.J.; Adler, B. Stimulation of autophagy suppresses the intracellular survival of Burkholderia pseudomallei in mammalian cell lines. Autophagy 2008, 4, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.Y.; Monack, D.M.; Nathan, S. Genome wide transcriptome profiling of a murine acute melioidosis model reveals new insights into how Burkholderia pseudomallei overcomes host innate immunity. BMC Genom. 2010, 11, 672. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Virk, H.S.; Torres, A.G.; Currie, B.J.; Peacock, S.J.; Dance, D.A.B.; Limmathurotsakul, D. Melioidosis. Nat. Rev.—Dis. Primers 2018, 4, 17107. [Google Scholar] [CrossRef]
- Bzdyl, N.M.; Moran, C.L.; Bendo, J.; Sarkar-Tyson, M. Pathogenicity and virulence of Burkholderia pseudomallei. Virulence 2022, 13, 2139063. [Google Scholar] [CrossRef]
- Chousterman, B.G.; Swirski, F.K.; Weber, G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017, 39, 517–528. [Google Scholar] [CrossRef]
- Wright, S.W.; Ekchariyawat, P.; Sengyee, S.; Phunpang, R.; Dulsuk, A.; Saiprom, N.; Thiansukhon, E.; Pattanapanyasat, K.; Korbsrisate, S.; West, T.E.; et al. Dysfunctional host cellular immune responses are associated with mortality in melioidosis. Emerg. Microbes Infect. 2024, 13, 2380822. [Google Scholar] [CrossRef] [PubMed]
- Varga, N.-I.; Bagiu, I.C.; Vulcanescu, D.D.; Lazureanu, V.; Turaiche, M.; Rosca, O.; Bota, A.V.; Horhat, F.G. IL-6 Baseline Values and Dynamic Changes in Predicting Sepsis Mortality: A Systematic Review and Meta-Analysis. Biomolecules 2025, 15, 407. [Google Scholar] [CrossRef]
- Friedland, J.S.; Suputtamongkol, Y.; Remick, D.G.; Chaowagul, W.; Strieter, R.M.; Kunkel, S.L.; White, N.J.; Griffin, G.E. Prolonged elevation of interleukin-8 and interleukin-6 concentrations in plasma and of leukocyte interleukin-8 mRNA levels during septicemic and localized Pseudomonas pseudomallei infection. Infect. Immun. 1992, 60, 2402–2408. [Google Scholar] [CrossRef]
- Simpson, A.J.H.; Smith, M.D.; Weverling, G.J.; Suputtamongkol, Y.; Angus, B.J.; Chaowagul, W.; White, N.J.; van Deventer, S.J.H.; Prins, J.M. Prognostic Value of Cytokine Concentrations (Tumor Necrosis Factor–α, Interleukin-6, and Interleukin-10) and Clinical Parameters in Severe Melioidosis. J. Infect. Dis. 2000, 181, 621–625. [Google Scholar] [CrossRef]
- Kaewarpai, T.; Ekchariyawat, P.; Phunpang, R.; Wright, S.W.; Dulsuk, A.; Moonmueangsan, B.; Morakot, C.; Thiansukhon, E.; Day, N.P.J.; Lertmemongkolchai, G.; et al. Longitudinal profiling of plasma cytokines in melioidosis and their association with mortality: A prospective cohort study. Clin. Microbiol. Infect. 2020, 26, e1–e783. [Google Scholar] [CrossRef]
- Wright, S.W.; Kaewarpai, T.; Lovelace-Macon, L.; Ducken, D.; Hantrakun, V.; Rudd, K.E.; Teparrukkul, P.; Phunpang, R.; Ekchariyawat, P.; Dulsuk, A.; et al. A 2-Biomarker Model Augments Clinical Prediction of Mortality in Melioidosis. Clin. Infect. Dis. 2021, 72, 821–828. [Google Scholar] [CrossRef]
- Pease, J.E.; Sabroe, I. The Role of Interleukin-8 and its Receptors in Inflammatory Lung Disease: Implications for Therapy. Am. J. Respir. Crit. Care Med. 2002, 1, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Zulpa, A.K.; Barathan, M.; Iyadorai, T.; Chandramathi, S.; Vellasamy, K.M.; Vadivelu, J.; Gan, G.G.; Anuar, N.A. Release of pro-inflammatory cytokines TNF-α, IFN-γ and IL-6 by Burkholderia pseudomallei- stimulated peripheral blood mononucleocytes of acute myeloid leukemia patients. Trop. Biomed. 2021, 38, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. BBA Mol. Cell Res. 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Simpson, A.J.H.; Opal, S.M.; Angus, B.J.; Prins, J.M.; Palardy, J.E.; Parejo, N.A.; Chaowagul, W.; White, N.J. Differential Antibiotic-Induced Endotoxin Release in Severe Melioidosis. J. Infect. Dis. 2000, 181, 1014–1019. [Google Scholar] [CrossRef]
- LaRosa, S.P.; Opal, S.M.; Utterback, B.; Yan, S.C.B.; Helterbrand, J.; Simpson, A.J.H.; Chaowagul, W.; White, N.J.; Fisher, C.J. Decreased protein C, protein S, and antithrombin levels are predictive of poor outcome in Gram-negative sepsis caused by Burkholderia pseudomallei. Int. J. Infect. Dis. 2006, 10, 25–31. [Google Scholar] [CrossRef]
- Saikh, K.U.; Ranji, C.M.; Ulrich, R.G.; Corea, E.; De Silva, A.D.; Natesan, M. An increase in p62/NBR1 levels in melioidosis patients of Sri Lanka exhibit a characteristic of potential host biomarker. J. Med. Microbiol. 2020, 69, 1240–1248. [Google Scholar] [CrossRef]
- Wan, K.; Liu, Y.; Chen, Y.; Sun, S.; Liang, H. Hemophagocytic Lymphohistiocytosis Secondary to Melioidosis in Paediatric Patients: A Case Series. Infect. Drug Resist. 2025, 18, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C. Understanding the Basics of Meta-Analysis and How to Read a Forest Plot: As Simple as It Gets. J. Clin. Psychiatry 2020, 81, 20f13698. [Google Scholar] [CrossRef] [PubMed]
- Esterhuizen, T.M.; Thabane, L. Con: Meta-analysis: Some key limitations and potential solutions. Nephrol. Dial. Transplant. 2016, 31, 882–885. [Google Scholar] [CrossRef] [PubMed]
- Rosolowski, M.; Oberle, V.; Ahnert, P.; Creutz, P.; Witzenrath, M.; Kiehntopf, M.; Loeffler, M.; Suttorp, N.; Scholz, M. Dynamics of cytokines, immune cell counts and disease severity in patients with community-acquired pneumonia—Unravelling potential causal relationships. Cytokine 2020, 136, 155263. [Google Scholar] [CrossRef]
- Yu, D.; Liu, J.; Song, X.; Ao, Y.; Li, X.; Han, Y. Analysis of the inflammatory storm response and heparin binding protein levels for the diagnosis and prognosis of sepsis-associated encephalopathy. Eur. J. Med. Res. 2025, 30, 116. [Google Scholar] [CrossRef]
- Ulett, G.C.; Ketheesan, N.; Hirst, R.G. Proinflammatory cytokine mRNA responses in experimental Burkholderia pseudomallei infection in mice. Acta Trop. 2000, 74, 229–234. [Google Scholar] [CrossRef]

| Author, Year [Ref] | Country | Study Design | Sample Size | Population Details | IL6 Measurement Method | IL6 Levels Reported | Outcome Measures | Mortality Rate | Association IL6 Outcome | Effect Estimates | Notes on Bias or Confounders | Include in Meta-Analysis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Friedland et al., 1992 [10] | Thailand | Prospective observational cytokine study | 18 | Patients with septicemic (n = 8) and localized (n = 8) Pseudomonas pseudomallei infection; 2 E. coli septicemia for comparison. | Plasma IL-6 measured by B9 cell proliferation assay (sensitivity 1 pg/mL); serial samples up to 30 days. | Median admission IL-6: non-survivors 4800 pg/mL (range 60–9245), survivors much lower (p = 0.007). Elevated IL-6 (>1000 pg/mL) predicted 75% mortality. | Mortality prediction; longitudinal IL-6 and IL-8 dynamics | 22% (4/18 overall; 4/10 septicemic died, all within 24 h) | High IL-6 at admission strongly predictive of mortality. IL-6 remained persistently elevated in all patients during hospitalization. | Threshold effect: IL-6 > 1000 pg/mL associated with 75% mortality (p = 0.011). | Small sample size; older cytokine assays; single hospital; some patients died before serial samples could be obtained. | Yes |
| Kaewarpai et al., 2020 [12] | Thailand | Prospective longitudinal cohort | 161 | Adults with culture-confirmed melioidosis (81% bacteremic, 74% diabetic). Controls: 13 healthy, 11 diabetics. | Plasma cytokines (IL-6, IL-8, IL-10, IFN-γ, TNF-α, IL-17A, IL-23, etc.) measured by bead-based multiplex (Luminex) at days 0, 5, 12, 28. | Median IL-6 higher in non-survivors at day 0 (p < 0.001). Non-survivors had rising IL-6 over time, survivors declining. AUROC IL-6 = 0.75 (95% CI 0.67–0.82). | 28-day mortality prediction, longitudinal IL-6 dynamics | 42% (68/161) | IL-6 significantly higher in non-survivors vs. survivors. Persistent/increasing IL-6 trajectory predicted death (joint modeling p < 0.004). | Effect estimates: AUROC IL-6 = 0.75 (95% CI 0.67–0.82). Independent temporal predictor of survival. | Single-country, hospitalized adults; cytokine sampling only after culture confirmation (delayed in some); no external validation. | Yes |
| LaRosa et al., 2006 [19] | Thailand | Prospective biomarker analysis (subset of RCT) | 30 | Acute severe melioidosis, 40% diabetic, 47% bacteremia, 30% lung involvement | Plasma IL-6 (ELISA, previously published dataset) | Median 240.2 pg/mL (range 14.6–745,000; IQR 64.9–1646.5) | Mortality, shock, renal failure, liver dysfunction | 40% (12/30) | Higher IL-6 inversely correlated with protein C (ρ = –0.74) and antithrombin (ρ = –0.68); prognostic value for poor outcomes | Not directly reported for IL-6 vs. mortality; IL-6 correlated with severity biomarkers | Subset analysis, small sample size, possible selection bias | No (Same samples with Simson et al., 2000) [11] |
| Saikh et al., 2020 [20] | Sri Lanka | Observational biomarker study | 116 | Confirmed melioidosis patients (B. pseudomallei culture positive), with additional probable, relapsed, and convalescent cases included for comparison. | Serum cytokines measured by Meso Scale Discovery (MSD) ultrasensitive assay; IL-6 levels compared across groups | IL-6 markedly elevated in confirmed and probable melioidosis: IQR ~2.66–197.62 pg/mL (confirmed) vs. 0.30–0.50 pg/mL (controls). ROC AUC for IL-6 = 0.967, cut-off 0.70 pg/mL. | Diagnosis of melioidosis vs. controls; differentiation from other infections (sepsis, leptospirosis) | Not directly reported | IL-6 consistently higher in melioidosis cases; strong discriminatory power with AUC 0.967; better than TNF-α, IL-1β, IFN-γ. | Effect estimates: AUC 0.967, sensitivity/specificity high at diagnostic cut-off (Youden index 0.849). | Cross-sectional, limited to Sri Lankan cohort, potential confounders from co-infections; IL-6 not tested as mortality predictor in this study. | No (diagnostic biomarker study; no mortality outcomes) |
| Simpson et al., 2000 [11] | Thailand | Prospective cohort (subset of RCT comparing imipenem vs. ceftazidime) | 172 | Adult Thai patients with severe melioidosis, admitted to Sappasitprasong Hospital (1994–1997). | Plasma IL-6 measured by ELISA (detection limit 5–10 pg/mL) | Median baseline IL-6: 227.2 pg/mL (range <5–745,000; IQR 82.6–708.2). Higher in non-survivors vs. survivors (p < 0.001). | Mortality, septicemia, APACHE II score, plasma lactate | 31.4% (54/172); 48% mortality in bacteremic subgroup | IL-6 strongly associated with mortality (OR 3.60 per log increase; 95% CI 1.76–7.38, p < 0.001). Independent predictor of outcome alongside APACHE II score. | Multivariate: APACHE II (OR 1.17 per point) + IL-6 remained predictors; IL-10 lost significance when both included. IL-6/IL-10 ratio associated with outcome in univariate but not independent. | Large cohort, single hospital; cytokine levels measured at baseline only; antibiotic treatment arms balanced. | Yes |
| Simpson et al., 2000 (Endotoxin study) [18] | Thailand | Randomized trial substudy (imipenem vs. ceftazidime) | 68 | Adults with severe septicemic melioidosis randomized to ceftazidime or imipenem. | Baseline and post-antibiotic cytokines (IL-6, TNF-α, IL-10) measured by ELISA; endotoxin measured by turbidimetric LAL assay. | Median baseline IL-6: Survivors 116.5 pg/mL (IQR 43–245); Non-survivors 825 pg/mL (IQR 229–2829), p < 0.001. | Mortality during hospitalization; endotoxin and cytokine release after antibiotics | 35% (24/68) | IL-6 strongly predictive of death (baseline higher in non-survivors, p < 0.001). IL-6 correlated with TNF-α and IL-10, but not endotoxin levels. | Effect estimate: IL-6 median non-survivors 825 vs. survivors 116 pg/mL (p < 0.001). No difference by antibiotic group. | Small sample; cytokines measured only up to 6 h post-antibiotics; single hospital study. | No (Sub study of Simson 2000) [11] |
| Wan et al., 2025 [21] | China (Hainan Island) | Case series (3 pediatric cases in one family) | 3 | Three children (ages 4, 6, 12) with culture-confirmed B. pseudomallei septicemia complicated by HLH. | Serum cytokines (IL-2, IL-4, IL-6, IL-10, IL-17A, IFN-γ, TNF-α) measured; blood culture confirmed B. pseudomallei. | IL-6 extremely elevated: 1453–2031 pg/mL in two cases; IL-10 > 5700 pg/mL in one case; all had cytokine storm profile. | Mortality, HLH complications, multi-organ failure | 100% (3/3) | Markedly high IL-6 and IL-10 levels during cytokine storm; associated with HLH and fatal outcome. | Not quantified as OR; descriptive case series with biomarker table. | Small case series, familial cluster, no controls; findings not generalizable but highlight IL-6 role in cytokine storm with HLH. | No (case series, descriptive only) |
| Wright et al., 2021 [13] | Thailand | Prospective biomarker cohort (derivation, validation, external validation) | 352 | Hospitalized adults with culture-confirmed melioidosis; derivation set (113), internal validation (78), external validation (161). | Plasma cytokines (IL-6, IL-8, IL-10, TNF-α, IFN-γ, G-CSF, IL-17A, IL-1β) measured by electrochemiluminescence and bead-based multiplex assays. | Median IL-6 (derivation): Survivors 41 pg/mL (IQR 16–239), non-survivors 1938 pg/mL (IQR 132–1980). Validation: Survivors 44 pg/mL, non-survivors 948 pg/mL. External: Survivors 14 pg/mL, non-survivors 45 pg/mL (p < 0.001 across cohorts). | 28-day mortality prediction | Overall mortality ~42–55% across cohorts | IL-6 independent predictor of 28-day mortality (OR 3.62; 95% CI 1.97–6.66; p < 0.001). IL-6 + IL-8 biomarker model improved AUC from 0.78 to 0.86 (derivation), validated in external cohorts (AUC 0.81). | IL-6 significantly associated with mortality even after adjusting for comorbidities and SOFA score. IL-6 + IL-8 performed comparably to clinical risk models. | Robust multi-cohort validation; single-time point cytokine measurement; assay differences across cohorts. | Yes (Separate to 3 studies; derivation, validation, and external validation) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khemla, S.; Chanthot, C.; Chittamma, A.; Thanasai, J.; Phongphithakchai, A.; Chatatikun, M.; Tangpong, J.; Laklaeng, S.-n.; Klangbud, W.K. Circulating Interleukin-6 as a Prognostic Biomarker for Mortality in Melioidosis: A Systematic Review and Meta-Analysis. Diseases 2025, 13, 385. https://doi.org/10.3390/diseases13120385
Khemla S, Chanthot C, Chittamma A, Thanasai J, Phongphithakchai A, Chatatikun M, Tangpong J, Laklaeng S-n, Klangbud WK. Circulating Interleukin-6 as a Prognostic Biomarker for Mortality in Melioidosis: A Systematic Review and Meta-Analysis. Diseases. 2025; 13(12):385. https://doi.org/10.3390/diseases13120385
Chicago/Turabian StyleKhemla, Supphachoke, Chaimongkhon Chanthot, Anchalee Chittamma, Jongkonnee Thanasai, Atthaphong Phongphithakchai, Moragot Chatatikun, Jitabanjong Tangpong, Sa-ngob Laklaeng, and Wiyada Kwanhian Klangbud. 2025. "Circulating Interleukin-6 as a Prognostic Biomarker for Mortality in Melioidosis: A Systematic Review and Meta-Analysis" Diseases 13, no. 12: 385. https://doi.org/10.3390/diseases13120385
APA StyleKhemla, S., Chanthot, C., Chittamma, A., Thanasai, J., Phongphithakchai, A., Chatatikun, M., Tangpong, J., Laklaeng, S.-n., & Klangbud, W. K. (2025). Circulating Interleukin-6 as a Prognostic Biomarker for Mortality in Melioidosis: A Systematic Review and Meta-Analysis. Diseases, 13(12), 385. https://doi.org/10.3390/diseases13120385








