Abstract
Background: Delirium onset is associated with increased comorbidity and mortality. Identifying reliable delirium biomarkers remains challenging. Regional cerebral oxygen saturation (rSO2) offers an objective, easily obtainable measure suitable for hospital monitoring. Objective: We aimed to analyse the relationship between regional cerebral oxygen saturation (rSO2) values obtained by near-infrared spectroscopy (NIRS) and the subsequent development of delirium. Methods: Studies eligible for inclusion in our systematic review evaluated rSO2 values obtained by NIRS or a used a similar method to study hospitalised patients aged 18 years or older, some of whom subsequently developed delirium. We searched MEDLINE, Scopus and Web of Science without restrictions to 24 March 2024. Two review authors independently assessed the methodological quality of the included studies using Joanna Briggs Institute Critical Appraisal tools. Using a random-effects model in RevMan v 5.4.0 (Cochrane Collaboration, Oxford, UK), we analysed baseline and minimum rSO2 values. Results were presented as means and mean differences (MDs) with their 95% confidence intervals (CIs). We followed PRISMA guidelines and registered our review protocol in PROSPERO (CRD42024523573). Results (or Findings): We included 22 studies (20 in the meta-analysis) published between 2009 and 2024 and involving 5757 participants. The delirium group had a lower mean baseline rSO2 value (62.47%, 95% CI 58.40 to 66.55) compared with the non-delirium group (64.24%, 95% CI 61.33 to 67.15). Meta-analysis of effect estimates confirmed this result (MD −2.92%, 95% CI −4.38 to −1.47). The MD between the delirium and non-delirium group was larger among patients assessed with the INVOS device and patients who underwent cardiac surgery. Studies that analysed baseline values according to sensor location showed a larger MD in rSO2 values obtained via a right-sided sensor. Conclusions: Our results show lower baseline and minimum rSO2 in hospitalised patients who subsequently developed delirium. The difference varies according to the type of surgery and type of NIRS monitor.
1. Introduction
Since the 1990s, near-infrared spectroscopy (NIRS) has been used as a non-invasive method for continuously monitoring the balance between oxygen delivery and consumption in the brain [1]. This technology functions by measuring the absorption of near-infrared (NIR) light (wavelength range 700–950 nm) by molecules called chromophores, which have specific light absorption characteristics in the NIR wavelength [2,3]. NIRS monitors display regional cerebral oxygen saturation (rSO2) as a percentage [4].
Currently, NIRS is widely used in surgery and critical care units [5]. Surgical specialties that use the technique to monitor cerebral oxygenation and improve patients’ intraoperative condition include neurological, cardiac, maxillofacial, breast, thoracic, digestive, orthopaedic, and gynaecological surgery [6]. Most studies on the application of NIRS in surgery focus on cardiac procedures such as cardiopulmonary bypass, because they carry a substantial risk of acute intraoperative events that may affect rSO2 [7].
In critical care units, NIRS can determine haemodynamic status by measuring the perfusion and oxygenation of the brain, kidney, liver, intestine or muscle, depending on where the sensor is placed. The organ best suited to this technology is the brain, as the tissue is highly dependent on aerobic metabolism, and its function is compromised by both hypoxia and hyperoxia. An alteration in oxygen saturation, the restoration of blood pressure after hypotension or alterations to heart rate can all lead to a severe haemodynamic change, causing encephalic lesions with short- or long-term sequelae [8]. Events that can increase the risk of cognitive dysfunction after surgery or critical illness include increased inflammatory response, hypoperfusion, hypoxia and embolism [9].
Delirium is a neuropsychiatric syndrome frequently seen in acute conditions, particularly in older people. It is characterised by acute onset, a fluctuating course and alterations in consciousness, orientation, memory, thinking, perception and behaviour. The development of delirium is associated with adverse outcomes such as institutionalisation, cognitive decline and death [10].
Delirium incidence in hospitalised people varies according to the setting, ranging from 19% to 64% in medical areas, up to 50% in surgical areas and up to 75% in critical care units. In addition, older people and people with greater comorbidity are at higher risk [11,12]. The underlying aetiology can be classified according to the presence of predisposing factors, which increase an individual’s risk of developing delirium, and participating factors, which trigger the onset of delirium [13].
Poor cerebral perfusion has been implicated in the development of delirium [14]; however, studies examining the association between cerebral perfusion and delirium in high-risk populations (e.g., people undergoing cardiac or abdominal surgery, or ventilated or septic patients in the intensive care unit [ICU]) have shown inconsistent results [15].
Delirium is a severe and very common neuropsychiatric syndrome that increases an individual’s risk of disability and cognitive impairment [16]. Studies have reported a higher incidence of postoperative delirium among people with low rSO2 levels before and during surgery [17,18,19]. To our knowledge, only one meta-analysis has quantified the difference in preoperative cerebral SO2 values between people who did and did not develop postoperative delirium. It included six studies and found lower values in the delirium group [20]. We aimed to update the pooled evidence on this question because several relevant studies have been published in recent years. Specifically, we aimed to evaluate the relationship between cerebral SO2 values obtained by NIRS and the subsequent development of delirium.
2. Materials and Methods
This systematic review and meta-analysis included studies comparing rSO2 values obtained by NIRS during surgery in people who subsequently developed delirium versus those who did not. Our review protocol is registered in PROSPERO (ID: CRD42024523573).
2.1. Inclusion and Exclusion Criteria
Eligible participants were aged 18 years and older. We only included studies that evaluated the association of rSO2 values (determined by NIRS or a similar method) with onset of delirium (as assessed by a validated diagnostic method).
Studies in animals, case reports, qualitative studies, letters to the editor, abstracts from conferences, books, systematic reviews and doctoral theses were excluded. In addition, we excluded randomised control trials (RCTs) of delirium prevention interventions, studies that did not quantify rSO2 values according to group (delirium and non-delirium) and studies involving patients with previous episodes of emerging delirium or post-traumatic stress disorder and studies without a pure control group due to intervention bias.
2.2. Literature Search
Our research question was defined in PECO format:
- Population: adults aged over 18 years
- Exposure: rSO2 values obtained using NIRS
- Control: patients with no delirium
- Outcome: delirium
Using this framework, we developed the following search strategy: NIRS OR Near-infrared spectroscopy OR cerebral oximetr* AND delirium OR confusion.
We searched MEDLINE, Scopus and Web of Science from inception to 24 March 2024, using Medical Subject Headings (MeSH) terms in combination with the Boolean operators AND/OR.
2.3. Study Selection and Data Collection
All records returned by the literature search were uploaded to a web-based system, which we used to manage the screening process and remove duplicate citations. Two members of the review team (PPR and BRR) independently screened the title and abstract of each unique record to eliminate all records that were clearly irrelevant. The same two review authors then retrieved the full-text articles of the remaining records and assessed them against our inclusion and exclusion criteria. They recorded the study selection process in sufficient detail to create a PRISMA flow chart (Figure 1). To ensure rigour and transparency during data extraction, we employed digital tools that facilitated organisation and reproducibility. We used a customised spreadsheet to systematically record study characteristics, numerical values and statistical estimates, minimising potential errors during synthesis. In parallel, we used the reference management software Zotero 7to organise bibliographic sources. The same two review authors independently extracted the following data from each eligible article:
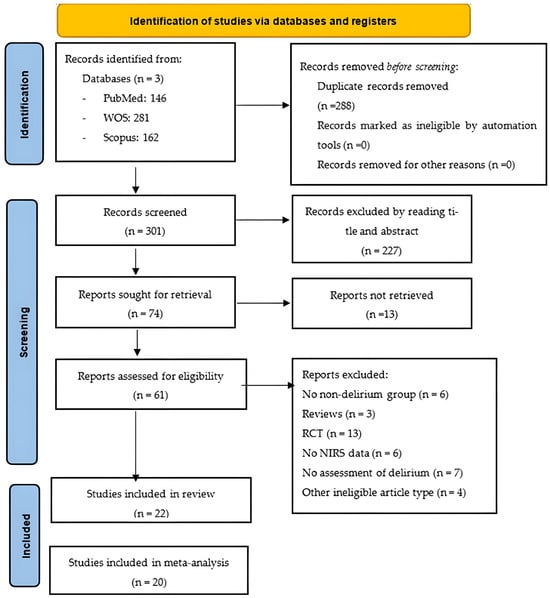
Figure 1.
PRISMA flow chart. Abbreviations: NIRS: near-infrared spectroscopy; RCT, randomised controlled trial.
- Study details: date of publication, country where study took place, setting, number of patients;
- Patient characteristics: age, sex, comorbidity;
- Type of surgery;
- Details of NIRS: type of NIRS monitor, measurement interval, timing of measurement, sensor location;
- Baseline NIRS: before induction of anaesthesia with the patient breathing ambient air and 1 min after placement of the measurement sensor;
- Minimum NIRS: the lowest recorded value during assessment;
- Maximum NIRS: the highest recorded value during assessment;
- Outcome: incidence of delirium, method of diagnosis, person who made the diagnosis.
Throughout the study selection and data collection process, disagreements were resolved by consensus on a case-by-case basis. If necessary, a third reviewer was consulted (FMMA).
2.4. Study Quality
Two review authors (PPR and BRR) independently assessed the methodological quality of the included studies using the Joanna Briggs Institute (JBI) 10-item checklist for critical appraisal [21]. Each ‘yes’ response scored 1 point, while ‘no’ and ‘unclear’ scored 0 points. A total score of 7 or more points denoted ‘high quality’, 5 to 6 points denoted ‘moderate quality’, and 4 points or fewer denoted ‘low quality’ [22]. The two review authors resolved any disagreements by consensus on a case-by-case basis, consulting a third reviewer (FMMA) if necessary.
2.5. Statistical Analysis
After data cleaning in Excel, we conducted statistical analysis using RevMan v5.4.0 (The Cochrane Collaboration, Oxford, UK). Specifically, we pooled the means and standard deviations (SDs) of baseline and minimum rSO2 values in the delirium and non-delirium groups according to device and type of surgery. We then combined the mean differences (MDs) between the groups according to NIRS monitoring device, type of surgery, and sensor placement. Where studies did not report MDs or percentages, we calculated them if there were sufficient data available. To quantify heterogeneity, we used the I2 statistic.
3. Results
3.1. Characteristics of Studies
The search returned 589 records, of which 288 were duplicates, and 227 were excluded during the title and abstract screen. Of the remaining 74 records, 65 were available as full-text articles. During the full-text review, 22 articles were deemed eligible for inclusion. Of the articles excluded at this stage, six had no non-delirium group [5,15,23,24,25,26], three were reviews [27,28,29], 13 were RCTs [30,31,32,33,34,35,36,37,38,39,40,41,42], seven included no assessment of delirium [43,44,45,46,47,48,49], six provided no rSO2 data [11,50,51,52,53,54], and four were other ineligible document types [55,56,57,58].
After the selection process, we included 22 studies in the systematic review and 20 in the meta-analysis. The included studies were published between 2009 and 2024 and involved a total of 5757 participants. The mean participant age ranged from 52.62 years [59] to 83 years [60], and there were more men than women in most studies. Table 1 provides further details.
One study was set in the ICU [19], and all other studies were conducted in a surgical setting. Thirteen studies (59%) involved cardiac and vascular surgeries [17,59,61,62,63,64,65,66,67,68,69,70,71], four (18%) involved abdominal surgery [72,73,74,75], one (4.5%) involved thoracic surgery [76], two (9%) involved orthopaedic surgery [60,77], and one (4.5%) included other surgical procedures (general surgery) [18].
To detect and diagnose delirium, 15 studies (68%) used the Confusion Assessment Methods (CAM) scale [18,59,60,61,70,71,72,75,76] and/or its variants: the CAM-ICU [17,19,61,64,65,67,70,71,76,77,78], Intensive Care Delirium Screening Checklist (ICDSC) [77], and the chart-based method [69]. Three studies used Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria [24,73,74]. In the remaining three studies, the diagnosis was based on symptoms such as confusion, agitation or change in mental state [62,66,69]. The professionals responsible for diagnosing delirium were physicians and neuropsychiatrists [24,67,74], nurses [59,64,66] and trained research personnel [17,18,19,60,61,65,71,72,76]. This information was missing from seven studies [62,69,70,73,75,77,78].
The proportion of participants who experienced delirium ranged from 7.1% [75] to 78.4% [19]. To measure rSO2, 15 studies (68%) used the INVOS monitor [17,18,60,61,62,64,66,67,69,70,71,72,73,75,77], four used FORESIGHT Elite [19,63,65,76], one used INT-100 (Hefei ENO Electronics) [74], and one used C2030C (CAS Medical Systems) [59]. One study did not specify monitor type [68].
Fourteen studies (63.6%) monitored rSO2 only during surgery [59,60,61,64,66,67,69,71,72,73,74,75,76,77], four continued monitoring in the hours following surgery [62,63,65,70], and three also monitored rSO2 the previous day [17,18,68]. Finally, in the study conducted in the ICU, monitoring took place over 24 h [19]. Monitoring began from the first minute after sensor placement, this first value being considered the baseline. Seven studies used continuous monitoring [17,60,62,69,71,75,77], and 10 studies recorded values at 2 s, 2 min or 5 min intervals [19,59,61,65,66,67,70,72,73,76]. One study measured rSO2 over 90 min each day of ICU admission [63], while two studies performed the measurement at pre-established time intervals [64,74]. Two studies provided no information on the time interval of monitoring [18,68].

Table 1.
Characteristics of included studies.
Table 1.
Characteristics of included studies.
| Study ID | Country | Surgery | No. of Patients/Age (Years)/% Male | Morbidity | % Delirium | Diagnostic Tool/ Professional | NIRS Monitor | Measurement Interval | Moment | Forehead Sensor Placement | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Delirium | No Delirium | ||||||||||
| Ahn 2021 [61] | Korea | Cardiac | n = 230 M 62.6 (SD 13.7) 51.7% | n = 460 M 62 (SD 14.6) 51.3% | NA | 33.3% | CAM and CAM-ICU/Trained or experienced personnel | INVOS | 5 min intervals after 1 min post sensor | During surgery | Bilateral |
| Bennett 2021 [62] | Saudi Arabia | Cardiac | n = 14 | n = 152 | NA | 8.4% | Documented in medical notes and prescribed haloperidol/NA | INVOS 5100 | Continuous | Before induction to ICU | Bilateral |
| M 8.4 a 73% a | |||||||||||
| Chan 2019 [63] | Australia | Cardiac | n = 24 Mdn 69 (IQR 64–77) 79.2% | n = 84 Mdn 66 (IQR 57–71) 71.4% | EuroSCORE, Mdn (IQR): 2.8 (1.9–4.2) vs. 2.2 (1.4–3.1) APACHE 3, Mdn (IQR): 58 (49–67) vs. 47 (39–53) *** SAPS II, Mdn (IQR): 51 (45–54) vs. 46 (42–51) | 22.2% | CAM-ICU/NA | FORESIGHT Elite | 90 min each day | ICU | Bilateral |
| Chen 2024 [64] | China | Cardiac | n = 47 M 70 (SD 5) 51.1% | n = 85 M 70 (SD 4) 50.5% | NA | 35.6% | CAM-ICU/CCU nurse specialist | INVOS 5100C | 4 intraoperative time points b | During surgery | Bilateral |
| Clemmesen 2018 [60] | Denmark | Trauma | n = 10 | n = 30 | ASA I: n = 3 (7.5%) ASA II: n = 30 (75.0%) ASA III: n = 5 (12.5%) ASA IV: n = 2 (5%) a | 25% | MDAS and CAM/Trained research personnel | INVOS 5100 | Continuous | During surgery | Right |
| Mdn 83 (IQR 78–89) a 10% a | |||||||||||
| Cui 2021 [76] | China | Thoracic | n = 35 | n = 140 | ASA I: n = 1 (0.6%) ASA II: n = 136 (77.7%) ASA III: n = 38 (21.7%) a | 20% | CAM and CAM-ICU/Trained research personnel | FORESIGHT ELITE | 2 sec intervals | During surgery | Bilateral |
| M 64.5 (SD 6.4) a 52% a | |||||||||||
| Eertmans 2020 [65] | Belgium | Cardiac | n = 29 Mdn 79 (IQR 75–83) 19% | n = 67 Mdn 75 (IQR 73–79) 45% | EuroSCORE II, Mdn (IQR): 2.61 (1.75–4.68) vs. 1.86 (1.02–3.37) * | 30% | CAM ICU/Trained research personnel | FORESIGHT Elite | 2 sec intervals | After induction to 72 h after surgery | Bilateral |
| Fischer 2022 [72] | Germany | Abdominal | n = 13 | n = 80 | NA | 16.3% | 3D-CAM/Research personnel | INVOS 5100C | 1 min intervals | During surgery | NA |
| M 66.31 (SD 10.55) a 58.7% a | |||||||||||
| Hori 2014 [66] | Japan | Cardiac | n = 45 M 69.6 (SD 9.9) 82% | n = 446 M 65.8 (SD 11. 4) 72.2% | NA | 9.2% | Presence of confusion, agitation or change in mental status/Nurses | INVOS | 10-sec intervals | Before induction of anaesthesia | Bilateral |
| Lim 2020 [67] | Korea | Cardiac | n = 105 M 71.9 (SD 8.2) 70.5% | n = 710 M 65.2 (SD 9.6) 78.3% | ASA I: n = 1 (1%) vs. n = 19 (2.7%) ASA II: n = 25 (23.8%) vs. n = 200 (28.2%) ASA III: n = 75 (71.4%) vs. n = 478 (67.3%) ASA IV: n = 4 (3.8%) vs. n = 13 (1.8%) | 14.8% | CAM-ICU/Neuropsychiatrist | INVOS | 5 min intervals | During surgery | Bilateral |
| Mailhot 2019 [68] | Canada | Cardiac | n = 173 Mdn 74 (IQR 68–74) 73.4% | n = 173 Mdn 69 (IQR 61–75) 76.3% | EuroSCORE, Mdn (IQR): 3.49 (1.96–5.38) vs. 2.20 (0.84–3.43) * NYHA ≥ 3: n = 8 (4.6%) vs. n = 15 (8.7%) | 50% | DSM-V/Physician | NA | NA | Pre-operative | NA |
| Momeni 2019 [69] | Belgium | Cardiac | n = 303 Mdn 75 (IQR 64–80) | n = 1201 Mdn 67 (IQR 57–75) | EuroSCORE II, Mdn (IQR): 2.64 (1.42–4.92) vs. 2.25 (1.19–3.86) ** | 20.14% | Validated chart review method searching in the medical record/NA | INVOS 5100 | Continuous | During surgery | Bilateral |
| 71% a | |||||||||||
| Morimoto 2009 [73] | Japan | Abdominal | n = 5 M 76 (SD 4) 75% | n = 15 M 68 (SD 3) 66% | NA | 25% | DSM-IV/NA | INVOS 3100 | 1 min intervals | During surgery | Left |
| Nakano 2021 [70] | Japan | Cardiac and ICU | n = 22 | n = 112 | EuroSCORE, Mdn (IQR): 3.45 (1.72–6.09) a | 16.4% | 3D-CAM and CAM-ICU daily/NA | INVOS | 10-sec intervals | During surgery and the day after | Bilateral |
| Mdn 65 (IQR 58–71) a 74.6% a | |||||||||||
| Schoen 2011 [17] | Germany | Cardiac | n = 62 M 73.1 (SD 6.7)/54.8% | n = 169 M 64.9 (IQR 13.3)/68% | EuroSCORE, M (SD): 7.9 (3.7) vs. 5.9 (3.5) | 26.8% | CAM-ICU/Trained research personnel | INVOS | Continuous | During surgery | Bilateral |
| Soh 2016 [77] | Korea | Trauma | n = 9 Mdn 73 (IQR 70–77) 44% | n = 100 Mdn 75 (IQR 72–77) 52% | NA | 8% | ICDSC and CAM-ICU/NA | INVOS | Continuous | During surgery | Bilateral |
| Soh 2020 [71] | Korea | Cardiac | n = 16 M 71 (SD 5) 69% | n = 97 M 70 (SD 6) 62% | EuroSCORE, Mdn (IQR): 7 (5–9) vs. 6 (4–8) | 14.16% | CAM-ICU and CAM/Trained research personnel | INVOS | Continuous | During surgery | Bilateral |
| Song 2022 [74] | China | Major abdominal | n = 16 Mdn 75 (IQR 72–80.5) 56.3% | n = 85 Mdn 72 (IQR 65–77) 70.6% | CIRS, Mdn (IQR): 11.5 (7.5–14) vs. 7 (7–10) ** | 15.8% | DSM-IV/Physician | INT-100, Hefei ENO Electronics | 6 intraoperative time points c | During surgery | Bilateral |
| Susano 2021 [18] | Portugal | Elective surgical procedures | n = 53 Mdn 76 (IQR 71–80) 60% | n = 185 Mdn 72 (IQR 68–77) 68% | ASA ≥ III: n = 35 (66%) vs. n =67 (36%) | 22.2% | CAM/Trained research personnel | INVOS 5100C | 1 min post sensor | Pre-operative | Bilateral |
| Tobar 2018 [75] | Chile | Abdominal | n = 2 | n = 26 | ASA I: 35.7% ASA II: 64.3% | 7.1% | CAM/NA | INVOS 5100 | Continuous | During surgery | Bilateral |
| M 73 (SD 7) a 39.3% a | |||||||||||
| Wang 2019 [59] | China | Cardiac | n = 14 Mdn 54.1 (IQR 46.2–62) 17.9% | n = 25 Mdn 52.6 (IQR 47.7–57.5) 46.2% | ASA, M (95% CI): 2.9 (2.1–3.1) vs. 2.8 (2.7–3.5) | 35.8% | CAM/Trained nurse | C2030C, CAS Medical Systems | 1 min intervals | During surgery | Bilateral |
| Wood 2017 [19] | Canada | General medical/surgical and trauma ICU | n = 19 Mdn 71 (IQR 67–76) 79% | n = 69 Mdn 68 (IQR 54–77) 59% | APACHE, Mdn (IQR): 21 (15–27) vs. 20 (18–23) | 78.4% | CAM-ICU/Trained researchers | FORESIGHT Elite | 2 sec intervals | 24 h | Bilateral |
a: all sample data; b: start of anaesthesia (T1), start of cardiopulmonary bypass (T2), start of rewarming (T3), and end of cardiopulmonary bypass (T4); c Before induction of anaesthesia (T0), 5 min after induction of anaesthesia (T1), 5 min after PaCO2 (partial pressure of arterial carbon dioxide) reached 25–30 mmHg (T2), 5 min after PaCO2 reached 45–50 mmHg (T3), at the end of surgery (T4), 5 min after PaCO2 reached 25–30 mmHg again (T5), and 5 min after PaCO2 reached 45–50 mmHg again (T6). Abbreviations: APACHE: Acute Physiology and Chronic Health Evaluation; ASA: American Society of Anesthesiologists; CAM: Confusion Assessment Method; CCU: critical care unit; CI: confidence interval; CIRS, cumulative illness rating scale; DSM-IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; DSM-V: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; EuroSCORE: European System for Cardiac Operative Risk Evaluation; ICDSC: Intensive Care Delirium Screening Checklist; ICU: intensive care unit; IQR: interquartile range; M: mean; Mdn: median; MDAS: Memorial Delirium Assessment Scale; NA: not available; rSO2: regional oxygen saturation; SAPS II: Simplified Acute Physiology Score II; 3D-CAM; 3 min diagnostic interview for confusion assessment method; * p < 0.05; ** p < 0.01; *** p < 0.001.
Sensor placement for rSO2 measurement was bilateral in 17 studies (77.2%) [17,18,19,59,61,62,63,64,65,66,67,69,70,71,74,75,76,77], right-sided in one study [60] and left-sided in one study [73]. Two studies did not specify where the sensors were placed [68,72].
3.2. Oximetry Values
Fifteen studies reported baseline rSO2 values and were included in our primary analysis (Table 2).

Table 2.
Baseline, minimum and maximum cerebral oxygen saturation values in people with and without subsequent delirium.
We calculated pooled baseline values for both groups, finding a lower value for the delirium group (62.47%, 95% CI 58.40 to 66.55; Tau2 = 57.98; Chi2 = 832.82, df = 14, p < 0.001; I2 = 98%; 15 studies, 842 participants, Figure S1a) compared with the non-delirium group (64.24%, 95% CI 61.33 to 67.15; Tau2 = 30.42; Chi2 = 886.98, df = 14, p < 0.001; I2 = 98%; 15 studies, 2598 participants; Figure S1b). The lowest values were reported in studies that used the INVOS monitor (delirium: 59.91%, 95% CI 53.96 to 65.87; non-delirium: 61.89%; 95% CI 59.02 to 69.76; Figure S1).
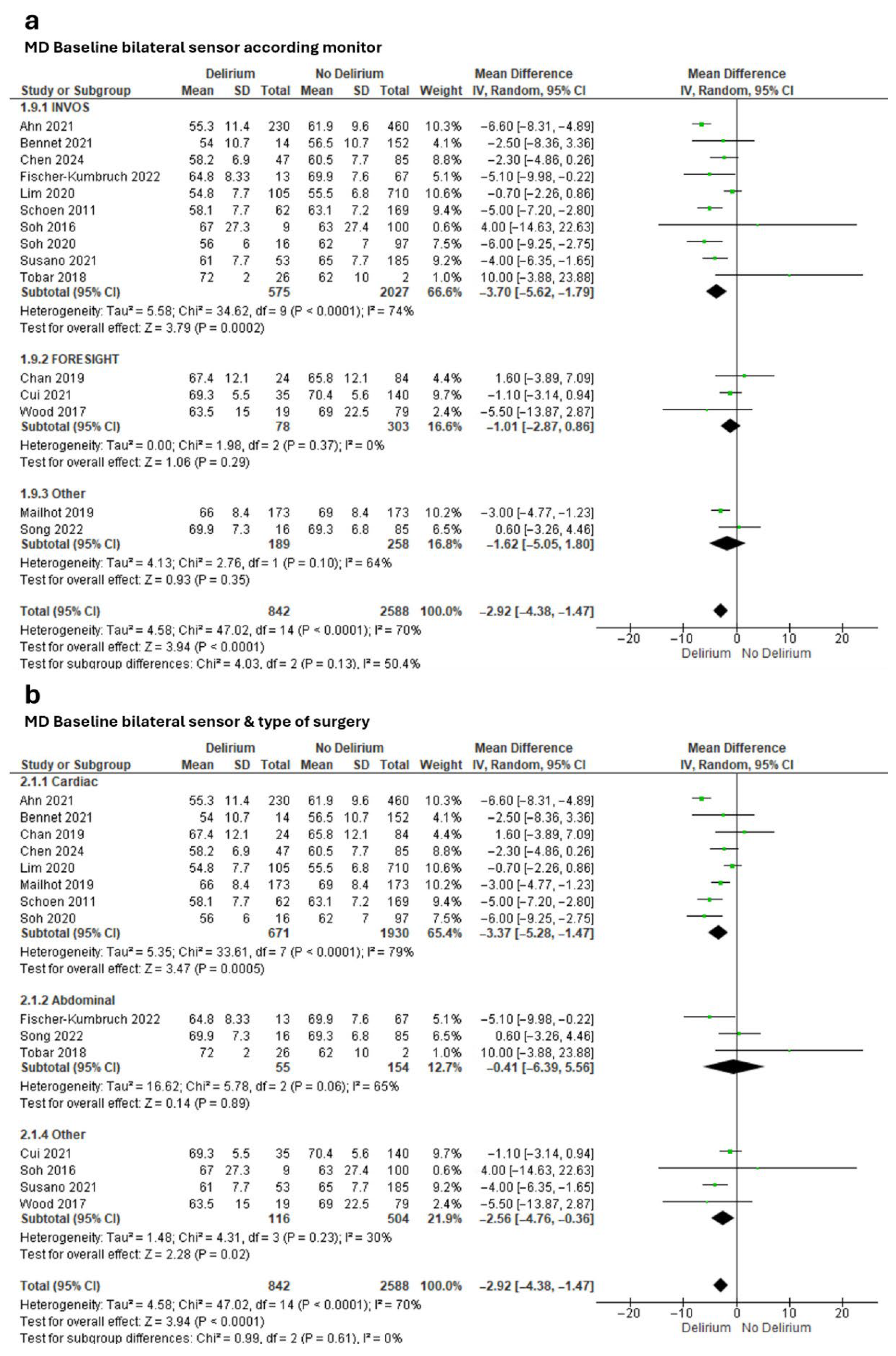
Meta-analysis of effect estimates confirmed a lower baseline rSO2 in the delirium group versus the non-delirium group (MD −2.92%, 95% CI −4.38 to −1.47; Tau2 = 4.58; Chi2 = 47.02, df = 14, p < 0.0001; I2 = 70%; 15 studies, 3430 participants; Figure 2). We found a greater difference when we included only studies that had used the INVOS monitor (MD −3.70%, 95% CI −5.62 to −1.79; Tau2 = 5.58; Chi2 = 24.62, df = 9, p < 0.0001; I2 = 74%; 10 studies, 2602 participants; Figure 2a) and only studies that had involved cardiac surgeries (MD −3.37%, 95% CI −5.28 to −1.47; Tau2 = 5.35; Chi2 = 47.02, df = 14, p < 0.0001; I2 = 79%; 8 studies, 2601 participants; Figure 2b).
We also analysed MD according to the hemisphere of sensor location in five studies that had used the INVOS device. The delirium group had lower rSO2 values as measured both in the right hemisphere (MD −4.61%, 95% CI −7.47 to −1.74; Tau2 = 5.31; Chi2 = 12.19, df = 3, p < 0.007; I2 = 75%; 4 studies, 2725 participants; Figure S3a) and the left hemisphere (MD −3.80%, 95% CI −6.40 to −1.21; Tau2 = 5.01; Chi2 = 15.31, df = 3, p < 0.002; I2 = 80%; 4 studies, 2710 participants; Figure S3b), through the difference was greater in the right hemisphere measurements. The subgroup analysis by surgical specialty showed lower rSO2 values (as measured in both the right and left hemispheres) in the delirium group in studies that involved cardiac, abdominal and orthopaedic surgery, through the difference was greater in the right hemisphere (Figure S3c,d).
When we analysed the minimum reported rSO2 values, we found a lower pooled mean minimum in the delirium group (50.67%, 95% CI 46.82 to 54.53; Tau2 = 24.92; Chi2 = 4153.78, df = 7, p < 0.00001; I2 = 95%; 8 studies, 630 participants, Figure S4) than in the non-delirium group (54.84%, 95% CI 51.91 to 57.78; Tau2 = 15.15; Chi2 = 273.44, df = 7, p < 0.00001; I2 = 97%; 8 studies, 1806 participants). The lowest values were reported in studies that used the INVOS device, for both the delirium group (47.25%, 95% CI 45.48 to 49.02; Figure S4a) and the non-delirium group (53.19%, 95% CI 49.92% to 56.46%; Figure S4b). When we analysed the minimum values according to type of surgery, we found lower values from studies that involved cardiac surgery, for both the delirium group (50.32%, 95% CI 46.19 to 54.45; Figure S4c) and the non-delirium group (54.36%, 95% CI 51.05 to 57.67; Figure S4d).
Meta-analysis of effect estimates confirmed the lower minimum rSO2 values in the delirium group compared with the non-delirium group (MD −4.22%, 95% CI −7.08 to −1.36; Tau2 = 10.57; Chi2 = 40.19, df = 7, p < 0.00001; I2 = 83%; 8 studies, 2440 participants; Figure S5a). We found a greater difference in the studies that used the INVOS device (MD −5.33%, 95% CI −8.10 to −2.57; Tau2 = 7.06; Chi2 = 23.47, df = 5, p < 0.0003; I2 = 79%; 6 studies, 1998 participants; Figure S5a) and the studies that involved cardiac surgeries (MD −4.27, 95% CI −7.32 to −1.23; Tau2 = 11.24; Chi2 = 39.71, df = 5, p < 0.00001; I2 = 87%; 6 studies, 2291 participants; Figure S5b).
Finally, we conducted the same analyses according to the type of measurement (continuous or intermittent). There was a higher MD in rSO2 values with continuous assessment (MD −4.15, 95% CI −6.95 to −1.35; Tau2 = 3.37; Chi2 = 6.37, df = 4, p < 0.00001; I2 = 37%; 5 studies, 647 participants) compared to intermittent assessment (MD −2.54, 95% CI −4.51 to −0.57; Tau2 = 6.11; Chi2 = 36.36, df = 8, p < 0.00001; I2 = 78%; 9 studies, 2437 participants), also reflecting the heterogeneity of the types of observation measures (Figure S6). The results were similar in the remaining analyses for baseline and minimum values (Figures S7–S9).
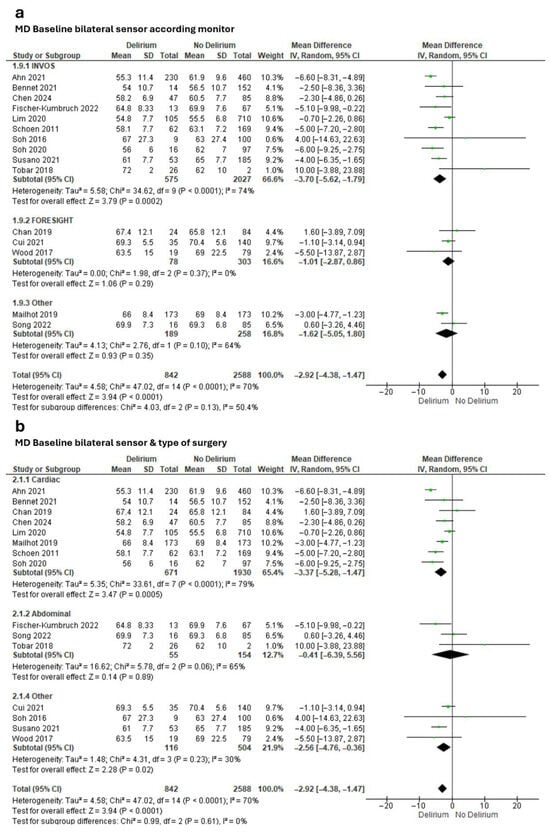
Figure 2.
Meta-analysis of mean baseline cerebral oxygen saturation values obtained through bilateral sensors in people with and without subsequent delirium according to (a) monitor and (b) type of surgery [17,18,19,61,62,63,64,67,68,71,72,74,75,76,77].
Figure 2.
Meta-analysis of mean baseline cerebral oxygen saturation values obtained through bilateral sensors in people with and without subsequent delirium according to (a) monitor and (b) type of surgery [17,18,19,61,62,63,64,67,68,71,72,74,75,76,77].
3.3. Methodological Quality of Included Studies
Overall, we rated the methodological quality of the included studies as moderate to high using the JBI Critical Appraisal Checklist for Cohort Studies. Some studies failed to identify confounding factors, to deal with confounding factors and/or to address incomplete follow-up. Table 3 presents the ratings for all studies.

Table 3.
Methodological quality of included studies according to JBI Critical Appraisal Checklist for Cohort Studies.
When we created funnel plots for our subgroup analyses, we found a few studies were outside the funnel, possibly due to small sample sizes. Most studies were distributed at the top of the funnel, which indicates greater precision (Figure S10).
4. Discussion
The pathophysiology of delirium is complex and multifactorial, involving alterations in neurotransmission, neuroinflammation, oxidative stress and blood–brain barrier dysfunction [15]. In this context, cerebral hypoxia emerges as a key precipitating factor, particularly in patients with systemic or cerebral oxygenation compromise. Cerebral oxygenation depends on a delicate balance between oxygen supply and demand, primarily regulated by cerebral blood flow and cerebral perfusion pressure [79]. In conditions such as acute brain injury or prolonged mechanical ventilation, silent cerebral hypoxia may occur even in the absence of systemic hypoxemia or elevated intracranial pressure. This hypoxia can trigger a cascade of pathophysiological events, including mitochondrial dysfunction, free radical accumulation, pro-inflammatory cytokine release, and neurotransmitter imbalances, particularly in the cholinergic and dopaminergic systems [80]. Recent studies have demonstrated that both acute and chronic hypoxia can lead to significant cognitive impairment, affecting attention, memory, and executive function—all domains commonly disrupted in delirium. Moreover, prolonged hypoxia has been associated with hippocampal and cortical atrophy, as well as structural changes such as amyloid plaque deposition and neurofibrillary tangles, suggesting a potential link between delirium and neurodegenerative processes [80].
Some systematic reviews with meta-analyses have investigated the risk of delirium after NIRS monitoring versus no monitoring [81,82,83,84,85]. They found that NIRS monitoring reduced postoperative cognitive dysfunction but were unable to draw solid conclusions regarding the risk of postoperative delirium, stroke, cardiac dysfunction, length of stay and mortality. In contrast, some recent studies have shown that people with low cerebral SO2 levels before and during surgery have a higher incidence of postoperative delirium [15,17,18,19,55]. We aimed to update the pooled evidence on this question because several relevant studies have been published in recent years. Specifically, we aimed to evaluate the relationship between cerebral SO2 values obtained by NIRS and the subsequent development of delirium. We found lower baseline and minimum rSO2 values in the delirium group.
Almost all studies included in our review were conducted in a surgical setting, most frequently cardiac surgery. NIRS is widely used in cardiac surgery owing to the high risk of cerebral hypoxia and haemodynamic instability, which increase the vulnerability of the brain and other vital organs [4]. In the study populations, older age and male sex predominated, which is consistent with the higher prevalence of cardiac pathology in older men [86]. Most studies reported comorbidity indices, but only four found significant differences, with higher comorbidity in the delirium group. This suggests comorbidity could act as a risk factor for delirium independently of oximetry values [87]. To enable better comparisons, future studies should present separate comorbidity levels for each group.
The percentage of participants who developed delirium across the included studies ranged from 7.1% to 78.4%. According to the literature, 24% of older people develop postoperative delirium [88], although the risk may vary depending on the type of surgery [89]. The presence of predisposing risk factors prior to assessment may also influence the incidence of delirium. Although some studies propose excluding patients with predisposing risk factors to determine the true utility of cerebral oximetry [7], we argue that including these patients and stratifying the analysis by risk factor may be more useful. This approach acknowledges the multifactorial nature of delirium and the clinical reality that predisposing factors elevate risk. Surgery itself is a precipitating factor, as is the ICU setting [90]. Most included studies did not indicate the specific time point of delirium diagnosis during admission. The main method of screening for delirium was the CAM scale, in line with most similar studies [7,90], as it is easy to administer and has excellent diagnostic accuracy [91].
Cerebral SO2 values are generally assessed through two sensors placed on the forehead. The bilateral baseline values are the most crucial results from cerebral oximetry and should be acquired prior to the administration of oxygen or any drugs that may affect these values. Normal rSO2 levels are around 60% to 80%, as cerebral arterial blood has an oxygen saturation of 98% to 100%, venous blood has an oxygen saturation of around 60%, and the ratio of arterial to venous blood is between 70:30 and 75:25. It is also important to measure the percentage variation from baseline throughout the surgical procedure, as values that fall outside of this range may be considered normal [4]. In our analysis, the pooled baseline rSO2 values were 62.47% in the delirium group and 64.24% in the non-delirium group. The baseline value reported by Chan and colleagues in their systematic review was 66.4% (95% CI 65.0 to 67.7); based on this result, the authors proposed a normal baseline preoperative range of 51.0% to 81.8% [92].
Cerebral oximetry values are related to age as well as the presence of cardiovascular disease, cerebral atrophy, smoking and other factors [93]. In our subgroup analysis by type of surgery, the lowest pooled baseline rSO2 value was observed in cardiac surgery patients (58.89% in delirium vs. 61.76% in non-delirium). This finding is in line with the literature, which associates cardiovascular disease with decreased cerebral oxygenation [93]. Lower rSO2 values are considered an indicator of cognitive frailty or poor brain reserve, which predisposes individuals to a greater risk of later cognitive impairment [94]. In addition, our subgroup analysis by type of NIRS monitor showed lower baseline rSO2 in people evaluated with the INVOS monitor in both groups. (59.91% in delirium vs. 61.89% in non-delirium). Most studies used the INVOS monitor because it has been on the market for more than 20 years. Differences with other monitors include the duration of sensor placement and type of light [93]. Therefore, analyses should be stratified according to manufacturer to avoid biases in the assessment method [93,95].
The forest plots reveal considerable heterogeneity among the included studies, as indicated by I2 values of 99% and 98% and highly significant Chi2 tests (p < 0.00001). This suggests that the variability in effect sizes is not due to chance but reflects true differences across studies. Potential sources of heterogeneity include differences in patient populations (age, comorbidities, baseline cognitive status), variability in monitoring devices (INVOS vs. FORE-SIGHT) with distinct calibration and sensitivity profiles, and differences in clinical contexts such as surgical type and perioperative management. Additionally, inconsistencies in baseline measurement timing and protocols may have contributed to the wide confidence intervals observed. Although subgroup analysis by device type partially explains this variability (p = 0.05), residual heterogeneity remains substantial, indicating that unmeasured confounders or methodological differences persist.
Meta-analysis of 15 studies showed an MD between the groups of nearly three percentage points in baseline rSO2. This differed according to monitor type, with larger MDs observed in studies that used the INVOS monitor and in studies that involved cardiac surgery. Clinically, a difference of nearly 3% in NIRS values may seem modest, but in the context of cerebral autoregulation, this variation may reflect a critical decrease in cerebral perfusion [92]. Because the brain has high metabolic demand and low tolerance to hypoxia, even small sustained reductions in oxygenation could trigger neuronal dysfunction and contribute to the development of delirium [94]. Furthermore, as ours is the first meta-analysis to report this difference, it serves as a starting point for quantifying significantly lower values in patients who develop delirium, encouraging further prospective studies to report more complete data in a larger sample.
We also observed a greater difference in continuous assessment compared to intermittent assessment, with the latter showing greater heterogeneity. This may be due to the different protocols used, capturing intervals of 2 s, 5 min, or at different times during the intervention. Continuous assessment is more sensitive, detecting small changes with greater reliability, which could explain the values obtained in the meta-analysis [96].
When we analysed studies that had reported baseline values by sensor location (right or left forehead), we found a slightly larger MD in rSO2 obtained via the right forehead sensor. Because the right hemisphere is responsible for attention, lesions in this side of the brain are more likely to lead to delirium [97]. Although rSO2 under normal conditions is mainly symmetrical, differences of around 10% are considered physiological [98]. Furthermore, studies indicate no differences in rSO2 obtained through one sensor (regardless of hemisphere) or two, unless there is in situ pathology [92,99].
Several included studies reported the minimum rSO2 value obtained during assessment. The pooled mean minimum value was lower in patients who developed delirium (50.67%) versus those who did not (54.84%), with an MD of −4.22% (95% CI −7.08 to −1.36). While we performed subgroup analyses of minimum values according to NIRS monitor and type of surgery, substantial between-study heterogeneity limited meaningful comparison of the results. It is more complex to detect minimum values through intermittent assessment compared with continuous assessment. In addition, beyond the minimum value, it is important to assess the reduction from baseline, as the current literature defines cerebral desaturation through two criteria: absolute values below 50% (range 40% to 60%) or relative reductions exceeding 20% (range 10% to 30%) with respect to the baseline [94]. We were unable to perform a meta-analysis according to desaturation cut-off value due to the lack of unified criteria to compare them. Some studies provided an absolute value and others a percentage of desaturation. Some even used different cut-off values for the right and left sensor. Although surgery, especially cardiac surgery, is a hemodynamic risk factor, patients can maintain near-baseline hemodynamic conditions if there are no stressors during anaesthesia [89,100]. However, in our review, lack of data precluded analysis of the surgical protocol, medication administered, management of fraction of inspired oxygen (FiO2) and blood pressure or duration of surgery. Future RCTs and other longitudinal studies should take these factors into account in their analyses [81].
Despite methodological challenges in cross-study comparisons, we found lower baseline and minimum rSO2 values in people who developed delirium. These findings provide a basis for future studies to quantitatively characterise cerebral desaturation during surgery and at delirium onset.
5. Limitations
The first limitation of this review is that our search was restricted to the predefined terms and selected databases, which means we may have missed some relevant studies published in other sources or using different terminology. We acknowledge that the included studies may not constitute an exhaustive representation of all relevant research. The different tools of detecting delirium could also represent a bias in identification. Second, it was not possible to include all the articles in the subgroup analyses because some provided insufficient data. We contacted the study authors to request these data but received no reply. Third, there was a lack of information on some confounding factors, such as comorbidities (e.g., diabetes and hypertension, which can lead to narrowed cerebral arteries through glycosylation and vasculitis), predisposing and precipitating risk factors, and time from NIRS assessment to delirium onset. It was not possible to perform a systematic check for rSO2 symmetry or apply corrections for individual anatomical factors due to the design and scope of the study. Likewise, we could not rule out the potential influence of local hemodynamic or technical artefacts (e.g., oedema, internal carotid artery atherosclerosis or variations in sensor pressure on the skin), which may have affected the interpretation of the observed asymmetry. Fourth, there was substantial between-study heterogeneity in the method of cerebral oximetry assessment, stemming from variation in the monitor used, the data collection protocol (continuous or intermittent), and other factors. Finally, the funnel plot assessment indicated possible publication bias.
We recommend that future studies provide detailed participant characteristics for both groups, including demographic data (age and sex), a validated comorbidity index, predisposing risk factors (e.g., diabetes, dementia, stroke history) and medication profile. It is important to describe how the oximetry data is collected (continuous or intermittent), specify the NIRS monitor used and provide mean values for both groups at baseline with SDs. In the surgical setting, researchers should report the minimum value observed, quantifying percentage desaturation or decline from baseline and indicating the time of the observation together with total surgery time. Complete reporting should also include the postoperative care setting, delirium assessment methodology (instrument, assessment frequency, duration of follow-up) and timing of delirium diagnosis. Finally, reporting complications and duration of admission enables adjustment for confounding factors.
6. Conclusions
Baseline and minimum regional cerebral oxygen saturation (rSO2) values obtained via near-infrared spectroscopy (NIRS) were lower among people who subsequently developed delirium compared with those who did not. We found that rSO2 values varied according to the type of surgery and type of NIRS monitor, with the lowest values among people who underwent cardiac surgeries and those assessed with INVOS monitors. The studies that analysed baseline values according to sensor location showed a greater mean difference in rSO2 obtained with the right sensor compared with the left. This is a starting point for studies aiming to investigate the exact nature of cerebral oxygen decline in surgical patients and its relationship to delirium onset. Given that rSO2 is an objective and easily obtainable measurement, its routine use in hospital settings could improve early detection of delirium risk and optimise clinical decision-making. Studies in other community institutional settings are also warranted.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diseases13120383/s1, Figure S1: Mean baseline cerebral oxygen saturation values for (a) delirium and (b) non-delirium according to type of monitor; Figure S2: Mean baseline cerebral oxygen saturation values for (a) delirium and (b) non-delirium according to type of surgery; Figure S3: Meta-analysis of mean baseline cerebral oxygen saturation values—obtained via (a) right sensor and (b) left sensor with the INVOS device, and via (c) right sensor and (d) left sensor according to type of surgery—in people with and without subsequent delirium; Figure S4: Mean minimum cerebral oxygen saturation values for (a) delirium and (b) non-delirium according to monitor, and (c) delirium and (d) non-delirium according to surgery; Figure S5: Meta-analysis of mean minimum cerebral oxygen saturation values obtained via bilateral sensors in people with and without subsequent delirium according to (a) monitor and (b) type of surgery; Figure S6: Meta-analysis of mean baseline cerebral oxygen saturation values obtained through bilateral sensors in people with and without subsequent delirium according to type of measurement. Figure S7: Mean baseline cerebral oxygen saturation values for (a) delirium and (b) non-delirium according to type of surgery. Figure S8: Mean minimum cerebral oxygen saturation values for: (a) delirium and (b) non-delirium according to type of measurement. Figure S9: Meta-analysis of mean minimum cerebral oxygen saturation values obtained via bilateral sensors in people with and without subsequent delirium according to type of measurement. Figure S10: Funnel plot of (a) subgroup baseline mean difference according to device baseline, (b) mean difference according to device right sensor, (c) baseline mean difference according to device left sensor and (d) minimum mean difference according to device.
Author Contributions
B.R.-R.: Conceptualisation, Data curation, Formal analysis, Methodology, Supervision, Validation, Writing—original draft, Writing—review and editing. F.M.M.-A.: Data curation, Validation, Writing—original draft, Writing—review and editing. P.P.-R.: Conceptualisation, Data curation, Formal analysis, Methodology, Supervision, Validation, Writing—original draft, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available within the article or its Supplementary Materials.
Acknowledgments
The authors thank all included studies.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this manuscript.
References
- Deschamps, A.; Hall, R.; Grocott, H.; Mazer, C.D.; Choi, P.T.; Turgeon, A.F.; De Medicis, E.; Bussières, J.S.; Hudson, C.; Syed, S.; et al. Cerebral oximetry monitoring to maintain normal cerebral oxygen saturation during high-risk cardiac surgery. Anesthesiology 2016, 124, 826–836. [Google Scholar] [CrossRef]
- Edmonds, H.L.; Isley, M.R.; Balzer, J.R. A guide to central nervous system near-infrared spectroscopic monitoring. In Monitoring the Nervous System for Anesthesiologists and Other Health Care Professionals; Springer: Cham, Switzerland, 2017; pp. 205–217. [Google Scholar] [CrossRef]
- Weigl, W.; Milej, D.; Janusek, D.; Wojtkiewicz, S.; Sawosz, P.; Kacprzak, M.; Gerega, A.; Maniewski, R.; Liebert, A. Application of optical methods in the monitoring of traumatic brain injury: A review. J. Cereb. Blood Flow Metab. 2016, 36, 1825–1843. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.; Cody, J.; Maldonado, Y.; Ramakrishna, H. Near-Infrared Spectroscopy (NIRS) for cerebral and tissue oximetry: Analysis of evolving applications. J. Cardiothorac. Vasc. Anesth. 2022, 36, 2758–2766. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.M.; Shore, A.; Lee, K.F.H.; Wood, M.D.; Maslove, D.M.; Hunt, M.; Georgescu, I.; Muscedere, J.; Boyd, J.G. Cerebral autoregulation-based mean arterial pressure targets and delirium in critically ill adults without brain injury: A retrospective cohort study. Can. J. Anaesth. 2024, 71, 107–117. [Google Scholar] [CrossRef]
- Rea-Olivar, D.A. ¿Es útil el NIRS en anestesia? ¿A quién y por qué? Rev. Mex. Anestesiol. 2019, 42, 11–15. [Google Scholar]
- Moore, C.C.; Yu, S.; Aljure, O. A comprehensive review of cerebral oximetry in cardiac surgery. J. Card. Surg. 2022, 37, 5418–5433. [Google Scholar] [CrossRef]
- Turcatti, L.K. Aplicación de tecnologías al cuidado de enfermería: Monitorización de la saturación regional de oxígeno por espectroscopia por infrarrojo cercano. Rev. Enferm. Neonatal 2017, 24, 3–10. [Google Scholar]
- Loberman, D.; Consalvi, C.; Healey, A.; Rivera, B.; Poulin, K.; Mohr, R.; Ziv-Baran, T. Adverse cerebral outcomes after coronary artery bypass surgery—More than a decade of experience in a single center. Thorac. Cardiovasc. Surg. 2018, 66, 452–456. [Google Scholar] [CrossRef]
- Fong, T.G.; Inouye, S.K. The inter-relationship between delirium and dementia: The importance of delirium prevention. Nat. Rev. Neurol. 2022, 18, 579–596. [Google Scholar] [CrossRef]
- Lee, S.; Howard, M.A.; Han, J.H. Delirium and delirium prevention in the emergency department. Clin. Geriatr. Med. 2023, 39, 535–551. [Google Scholar] [CrossRef]
- Ormseth, C.H.; LaHue, S.C.; Oldham, M.A.; Josephson, S.A.; Whitaker, E.; Douglas, V.C. Predisposing and precipitating factors associated with delirium: A systematic review. JAMA Netw. Open 2023, 6, e2249950. [Google Scholar] [CrossRef]
- Inouye, S.K.; Zhang, Y.; Jones, R.N.; Kiely, D.K.; Yang, F.; Marcantonio, E.R. Risk Factors for Delirium at Discharge: Development and Validation of a Predictive Model. Arch. Intern. Med. 2007, 167, 1406. [Google Scholar] [CrossRef] [PubMed]
- Fong, T.G.; Jones, R.N.; Marcantonio, E.R.; Tommet, D.; Gross, A.L.; Habtemariam, D.; Schmitt, E.; Yap, L.; Inouye, S.K. Adverse outcomes after hospitalization and delirium in persons with alzheimer disease. Ann. Intern. Med. 2012, 156, 848. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.M.; Wood, M.D.; Lee, K.F.H.; Maslove, D.; Muscedere, J.; English, S.W.; Ball, I.; Slessarev, M.; Boyd, J.G. Delirium, Cerebral Perfusion, and High-Frequency Vital-Sign Monitoring in the Critically Ill. The CONFOCAL-2 Feasibility Study. Ann. Am. Thorac. Soc. 2021, 18, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Welch, C.; McCluskey, L.; Wilson, D. Geriatric Medicine Research Collaborative Delirium is prevalent in older hospital inpatients and associated with adverse outcomes: Results of a prospective multi-centre study on World Delirium Awareness Day. BMC Med. 2019, 17, 229. [Google Scholar] [CrossRef]
- Schoen, J.; Meyerrose, J.; Paarmann, H.; Heringlake, M.; Hueppe, M.; Berger, K.-U. Preoperative regional cerebral oxygen saturation is a predictor of postoperative delirium in on-pump cardiac surgery patients: A prospective observational trial. Crit. Care 2011, 15, R218. [Google Scholar] [CrossRef]
- Susano, M.J.; Dias, M.; Seixas, F.S.; Vide, S.; Grasfield, R.; Abelha, F.J.; Crosby, G.; Culley, D.J.; Amorim, P. Association Among Preoperative Cognitive Performance, Regional Cerebral Oxygen Saturation, and Postoperative Delirium in Older Portuguese Patients. Anesth. Analg. 2021, 132, 846–855. [Google Scholar] [CrossRef]
- Wood, M.D.; Maslove, D.M.; Muscedere, J.G.; Day, A.G.; Boyd, J.G.; The Cerebral Oxygenation and Neurological Outcomes Following Critical Illness (CONFOCAL) Research Group. Low brain tissue oxygenation contributes to the development of delirium in critically ill patients: A prospective observational study. J. Crit. Care 2017, 41, 289–295. [Google Scholar] [CrossRef]
- He, K.; Wang, S.; Zhang, W.; Liu, Q.; Chai, X. What is the impact of perioperative cerebral oxygen desaturation on postoperative delirium in old population: A systemic review and meta-analysis. Aging Clin. Exp. Res. 2022, 34, 1761–1770. [Google Scholar] [CrossRef]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef]
- Shenkin, S.D.; Harrison, J.K.; Wilkinson, T.; Dodds, R.M.; Ioannidis, J.P.A. Systematic reviews: Guidance relevant for studies of older people. Age Ageing 2017, 46, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Funk, D.J.; Kumar, A.; Klar, G. Decreases in cerebral saturation in patients with septic shock are associated with increased risk of death: A prospective observational single center study. J. Intensive Care 2016, 4, 42. [Google Scholar] [CrossRef] [PubMed]
- Mailhot, T.; Cossette, S.; Lambert, J.; Cournoyer, A.; Denault, A.Y. Cerebral oximetry as a biomarker of postoperative delirium in cardiac surgery patients. J. Crit. Care 2016, 34, 17–23. [Google Scholar] [CrossRef]
- Rajaram, A.; Milej, D.; Suwalski, M.; Yip, L.C.M.; Guo, L.R.; Chu, M.W.A.; Chui, J.; Diop, M.; Murkin, J.M.; St Lawrence, K. Optical monitoring of cerebral perfusion and metabolism in adults during cardiac surgery with cardiopulmonary bypass. Biomed. Opt. Express 2020, 11, 5967–5981. [Google Scholar] [CrossRef]
- Schmidt, G.; Kreissl, H.; Vigelius-Rauch, U.; Schneck, E.; Edinger, F.; Nef, H.; Böning, A.; Sander, M.; Koch, C. Cerebral Tissue Oxygen Saturation Is Enhanced in Patients following Transcatheter Aortic Valve Implantation: A Retrospective Study. J. Clin. Med. 2022, 11, 1930. [Google Scholar] [CrossRef]
- Badenes, R.; Gouvea Bogossian, E.; Chisbert, V.; Robba, C.; Oddo, M.; Taccone, F.S.; Matta, B.F. The role of noninvasive brain oximetry in adult critically ill patients without primary non-anoxic brain injury. Minerva Anestesiol. 2021, 87, 1226–1238. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, P.; Liu, H.; Ge, S. Decoding Action Observation Using Complex Brain Networks from Simultaneously Recorded EEG-fNIRS Signals; Gedeon, T., Wong, K., Lee, M., Eds.; Springer: Cham, Switzerland, 2019; Volume 1142, pp. 559–569. [Google Scholar] [CrossRef]
- Li, D.; Liu, H. Cognitive function assessment should be included in preoperative evaluation. J. Biomed. Res. 2018, 32, 161–163. [Google Scholar] [CrossRef]
- Amouzegar Zavareh, S.M.; Araghizade, H.; Eskandari, N.; Lak, M. Brain oximetry is not a good monitor on reducing neurological complications after cardiac surgery. Universa Med. 2019, 38, 81–89. [Google Scholar] [CrossRef]
- Fuest, K.E.; Servatius, A.; Ulm, B.; Schaller, S.J.; Jungwirth, B.; Blobner, M.; Schmid, S. Perioperative Hemodynamic Optimization in Patients at Risk for Delirium—A Randomized-Controlled Trial. Front. Med. 2022, 9, 893459. [Google Scholar] [CrossRef]
- Kunst, G.; Gauge, N.; Salaunkey, K.; Spazzapan, M.; Amoako, D.; Ferreira, N.; Green, D.W.; Ballard, C. Intraoperative Optimization of Both Depth of Anesthesia and Cerebral Oxygenation in Elderly Patients Undergoing Coronary Artery Bypass Graft Surgery—A Randomized Controlled Pilot Trial. J. Cardiothorac. Vasc. Anesth. 2020, 34, 1172–1181. [Google Scholar] [CrossRef]
- Lei, L.; Katznelson, R.; Fedorko, L.; Carroll, J.; Poonawala, H.; Machina, M.; Styra, R.; Rao, V.; Djaiani, G. Cerebral oximetry and postoperative delirium after cardiac surgery: A randomised, controlled trial. Anaesthesia 2017, 72, 1456–1466. [Google Scholar] [CrossRef]
- Murniece, S.; Soehle, M.; Vanags, I.; Mamaja, B. Near Infrared Spectroscopy Based Clinical Algorithm Applicability During Spinal Neurosurgery and Postoperative Cognitive Disturbances. Medicina 2019, 55, 179. [Google Scholar] [CrossRef] [PubMed]
- Onur, T.; Karaca, Ü.; Ata, F.; Sayan, H.E.; Onur, A.; Yilmaz, C.; Balkaya, A.N.; Eriş, C. Intraoperative hyperoxygenation may negatively affect postoperative cognitive functions in coronary artery bypass graft operations: A randomized controlled study. J. Card. Surg. 2022, 37, 2552–2563. [Google Scholar] [CrossRef] [PubMed]
- Pfister, D.; Siegemund, M.; Dell-Kuster, S.; Smielewski, P.; Rüegg, S.; Strebel, S.P.; Marsch, S.C.U.; Pargger, H.; Steiner, L.A. Cerebral perfusion in sepsis-associated delirium. Crit. Care 2008, 12, R63. [Google Scholar] [CrossRef] [PubMed]
- Siepe, M.; Pfeiffer, T.; Gieringer, A.; Zemann, S.; Benk, C.; Schlensak, C.; Beyersdorf, F. Increased systemic perfusion pressure during cardiopulmonary bypass is associated with less early postoperative cognitive dysfunction and delirium. Eur. J. Cardiothorac. Surg. 2011, 40, 200–207. [Google Scholar] [CrossRef]
- Trafidlo, T.; Gaszynski, T.; Gaszynski, W.; Nowakowska-Domagala, K. Intraoperative monitoring of cerebral NIRS oximetry leads to better postoperative cognitive performance: A pilot study. Int. J. Surg. 2015, 16, 23–30. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, L.; Li, Y.; Yin, C.; Hou, Z.; Wang, Q. The potential role of lung-protective ventilation in preventing postoperative delirium in elderly patients undergoing prone spinal surgery: A preliminary study. Med. Sci. Monit. 2020, 26, e926526. [Google Scholar] [CrossRef]
- Wang, D.D.; Li, Y.; Hu, X.W.; Zhang, M.C.; Xu, X.M.; Tang, J. Comparison of restrictive fluid therapy with goal-directed fluid therapy for postoperative delirium in patients undergoing spine surgery: A randomized controlled trial. Perioper. Med. 2021, 10, 48. [Google Scholar] [CrossRef]
- Xu, X.; Hu, X.; Wu, Y.; Li, Y.; Zhang, Y.; Zhang, M.; Yang, Q. Effects of different BP management strategies on postoperative delirium in elderly patients undergoing hip replacement: A single center randomized controlled trial. J. Clin. Anesth. 2020, 62, 109730. [Google Scholar] [CrossRef]
- Xu, N.; Li, L.X.; Wang, T.L.; Jiao, L.Q.; Hua, Y.; Yao, D.X.; Wu, J.; Ma, Y.H.; Tian, T.; Sun, X.L. Processed multiparameter electroencephalogram-guided general anesthesia management can reduce postoperative delirium following carotid endarterectomy: A randomized clinical trial. Front. Neurol. 2021, 12, 666814. [Google Scholar] [CrossRef]
- Baehner, T.; Perlewitz, O.; Ellerkmann, R.K.; Menzenbach, J.; Brand, G.; Thudium, M.; Velten, M. Preoperative cerebral oxygenation in high-risk noncardiac surgical patients: An observational study on postoperative mortality and complications. J. Clin. Monit. Comput. 2023, 37, 743–752. [Google Scholar] [CrossRef]
- Papadopoulos, G.; Karanikolas, M.; Liarmakopoulou, A.; Papathanakos, G.; Korre, M.; Beris, A. Cerebral oximetry and cognitive dysfunction in elderly patients undergoing surgery for hip fractures: A prospective observational study. Open Orthop. J. 2012, 6, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Sahin, S.H.; Copuroglu, E.; Delen, E.; Tutunculer, B.; Sut, N.; Colak, A.; Sagiroglu, G.; Sag, F. The Effect of Cerebral Oxygen Saturation Changes on Early Postoperative Neuropsychological Function in Patients Undergoing Cranial Surgery. Turk. Neurosurg. 2023, 33, 618–625. [Google Scholar] [CrossRef]
- Uysal, S.; Lin, H.-M.; Trinh, M.; Park, C.H.; Reich, D.L. Optimizing cerebral oxygenation in cardiac surgery: A randomized controlled trial examining neurocognitive and perioperative outcomes. J. Thorac. Cardiovasc. Surg. 2020, 159, 943–953.E3. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.D.; Jacobson, J.A.; Maslove, D.M.; Muscedere, J.G.; Boyd, J.G.; Cerebral Oxygenation and Neurological Outcomes Following Critical Illness (CONFOCAL) Research Group. The physiological determinants of near-infrared spectroscopy-derived regional cerebral oxygenation in critically ill adults. Intensive Care Med. Exp. 2019, 7, 23. [Google Scholar] [CrossRef]
- Wood, M.D.; Khan, J.; Lee, K.F.H.; Maslove, D.M.; Muscedere, J.; Hunt, M.; Scott, S.H.; Day, A.; Jacobson, J.A.; Ball, I.; et al. Assessing the relationship between near-infrared spectroscopy-derived regional cerebral oxygenation and neurological dysfunction in critically ill adults: A prospective observational multicentre protocol, on behalf of the Canadian Critical Care Trials Group. BMJ Open 2019, 9, e029189. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, J.; Li, P.; Zhang, X.; Yang, Y.; Liu, Y.; Fu, Q.; Cao, J.; Mi, W.; Zhang, H.; et al. The perioperative application of continuous cerebral autoregulation monitoring for cerebral protection in elderly patients. Ann. Palliat. Med. 2021, 10, 4582–4592. [Google Scholar] [CrossRef]
- Hori, D.; Max, L.; Laflam, A.; Brown, C.; Neufeld, K.J.; Adachi, H.; Sciortino, C.; Conte, J.V.; Cameron, D.E.; Hogue, C.W.J.; et al. Blood pressure deviations from optimal mean arterial pressure during cardiac surgery measured with a novel monitor of cerebral blood flow and risk for perioperative delirium: A pilot study. J. Cardiothorac. Vasc. Anesth. 2016, 30, 606–612. [Google Scholar] [CrossRef]
- Ordóñez-Velasco, L.M.; Hernández-Leiva, E. Factors associated with delirium after cardiac surgery: A prospective cohort study. Ann. Card. Anaesth. 2021, 24, 183–189. [Google Scholar] [CrossRef]
- Palmbergen, W.A.C.; van Sonderen, A.; Keyhan-Falsafi, A.M.; Keunen, R.W.M.; Wolterbeek, R. Improved perioperative neurological monitoring of coronary artery bypass graft patients reduces the incidence of postoperative delirium: The Haga Brain Care Strategy. Interact. Cardiovasc. Thorac. Surg. 2012, 15, 671–677. [Google Scholar] [CrossRef]
- Vlisides, P.E.; Li, D.; Maywood, M.; Zierau, M.; Lapointe, A.P.; Brooks, J.; McKinney, A.M.; Leis, A.M.; Mentz, G.; Mashour, G.A. Electroencephalographic Biomarkers, Cerebral Oximetry, and Postoperative Cognitive Function in Adult Noncardiac Surgical Patients: A Prospective Cohort Study. Anesthesiology 2023, 139, 568–579. [Google Scholar] [CrossRef]
- Yoshimura, A.; Goodson, C.; Johns, J.T.; Towe, M.M.; Irvine, E.S.; Rendradjaja, N.A.; Max, L.K.; LaFlam, A.; Ledford, E.C.; Probert, J.; et al. Altered cortical brain activity in end stage liver disease assessed by multi-channel near-infrared spectroscopy: Associations with delirium. Sci. Rep. 2017, 7, 9258. [Google Scholar] [CrossRef] [PubMed]
- Semrau, J.S. Characterizing the Association Between Regional Cerebral Oxygen Saturation and Neurological Impairment After Cardiac Surgery. Ph.D. Thesis, Queen’s University, Kingston, ON, Canada, 2021. [Google Scholar]
- Snyder, B.; Simone, S.M.; Giovannetti, T.; Floyd, T.F. Cerebral Hypoxia: Its Role in Age-Related Chronic and Acute Cognitive Dysfunction. Anesth. Analg. 2021, 132, 1502–1513. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.-J.; Yuan, S.; Zhou, C.-H.; Yan, F.-X. The Effect of Intraoperative Cerebral Oximetry Monitoring on Postoperative Cognitive Dysfunction and ICU Stay in Adult Patients Undergoing Cardiac Surgery: An Updated Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 814313. [Google Scholar] [CrossRef] [PubMed]
- Vu, E.L.; Brady, K.; Hogue, C.W. High-resolution perioperative cerebral blood flow autoregulation measurement: A practical and feasible approach for widespread clinical monitoring. Br. J. Anaesth. 2022, 128, 405–408. [Google Scholar] [CrossRef]
- Wang, X.; Feng, K.; Liu, H.; Liu, Y.; Ye, M.; Zhao, G.; Wang, T. Regional cerebral oxygen saturation and postoperative delirium in endovascular surgery: A prospective cohort study. Trials 2019, 20, 504. [Google Scholar] [CrossRef]
- Clemmesen, C.G.; Pedersen, L.M.; Hougaard, S.; Andersson, M.L.; Rosenkvist, V.; Nielsen, H.B.; Palm, H.; Foss, N.B. Cerebral oximetry during preoperative resuscitation in elderly patients with hip fracture: A prospective observational study. J. Clin. Monit. Comput. 2018, 32, 1033–1040. [Google Scholar] [CrossRef]
- Ahn, J.H.; Lee, E.K.; Kim, D.; Kang, S.; Choi, W.-J.; Byun, J.-H.; Shim, J.-G.; Lee, S.H. Effect of changes in cerebral oximeter values during cardiac surgery on the incidence of postoperative neurocognitive deficits (POND): A retrospective study based on propensity score-matched analysis. PLoS ONE 2021, 16, e0260945. [Google Scholar] [CrossRef]
- Bennett, S.R.; Abukhodair, A.W.; Alqarni, M.S.; Fernandez, J.A.; Fernandez, A.J.; Bennett, M.R. Outcomes in cardiac surgery based on preoperative, mean intraoperative and stratified cerebral oximetry values. Cureus 2021, 13, e17123. [Google Scholar] [CrossRef]
- Chan, B.; Aneman, A. A prospective, observational study of cerebrovascular autoregulation and its association with delirium following cardiac surgery. Anaesthesia 2019, 74, 33–44. [Google Scholar] [CrossRef]
- Chen, N.; Mo, Y.-C.; Xu, M.; Chen, S.-S.; Gao, W.; Zheng, Q.; Wang, J.; Wang, X.-C.; Wang, J.-L. Risk factors for postoperative delirium in elderly patients undergoing heart valve surgery with cardiopulmonary bypass. J. Cardiothorac. Surg. 2024, 19, 106. [Google Scholar] [CrossRef]
- Eertmans, W.; De Deyne, C.; Genbrugge, C.; Marcus, B.; Bouneb, S.; Beran, M.; Fret, T.; Gutermann, H.; Boer, W.; Vander Laenen, M.; et al. Association between postoperative delirium and postoperative cerebral oxygen desaturation in older patients after cardiac surgery. Br. J. Anaesth. 2020, 124, 146–153. [Google Scholar] [CrossRef]
- Hori, D.; Brown, C.; Ono, M.; Rappold, T.; Sieber, F.; Gottschalk, A.; Neufeld, K.J.; Gottesman, R.; Adachi, H.; Hogue, C.W. Arterial pressure above the upper cerebral autoregulation limit during cardiopulmonary bypass is associated with postoperative delirium. Br. J. Anaesth. 2014, 113, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.; Nam, K.; Lee, S.; Cho, Y.J.; Yeom, C.-W.; Jung, S.; Moon, J.Y.; Jeon, Y. The relationship between intraoperative cerebral oximetry and postoperative delirium in patients undergoing off-pump coronary artery bypass graft surgery: A retrospective study. BMC Anesthesiol. 2020, 20, 285. [Google Scholar] [CrossRef] [PubMed]
- Mailhot, T.; Cossette, S.; Lambert, J.; Beaubien-Souligny, W.; Cournoyer, A.; O’Meara, E.; Maheu-Cadotte, M.-A.; Fontaine, G.; Bouchard, J.; Lamarche, Y.; et al. Delirium after cardiac surgery and cumulative fluid balance: A case-control cohort study. J. Cardiothorac. Vasc. Anesth. 2019, 33, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Momeni, M.; Meyer, S.; Docquier, M.-A.; Lemaire, G.; Kahn, D.; Khalifa, C.; Rosal Martins, M.; Van Dyck, M.; Jacquet, L.-M.; Peeters, A.; et al. Predicting postoperative delirium and postoperative cognitive decline with combined intraoperative electroencephalogram monitoring and cerebral near-infrared spectroscopy in patients undergoing cardiac interventions. J. Clin. Monit. Comput. 2019, 33, 999–1009. [Google Scholar] [CrossRef]
- Nakano, M.; Nomura, Y.; Whitman, G.; Sussman, M.; Schena, S.; Kilic, A.; Choi, C.W.; Akiyoshi, K.; Neufeld, K.J.; Lawton, J.; et al. Cerebral autoregulation in the operating room and intensive care unit after cardiac surgery. Br. J. Anaesth. 2021, 126, 967–974. [Google Scholar] [CrossRef]
- Soh, S.; Shim, J.-K.; Song, J.-W.; Choi, N.; Kwak, Y.-L. Preoperative transcranial Doppler and cerebral oximetry as predictors of delirium following valvular heart surgery: A case-control study. J. Clin. Monit. Comput. 2020, 34, 715–723. [Google Scholar] [CrossRef]
- Fischer-Kumbruch, M.; Jung, C.; Hinken, L.; Trübenbach, D.; Fielbrand, R.; Schenk, I.; Diegmann, O.; Krauß, T.; Scheinichen, D.; Schultz, B. Pre- and intraoperative cerebral near-infrared spectroscopy and postoperative delirium: Results of a prospective cross-sectional trial. Medicine 2022, 101, e31520. [Google Scholar] [CrossRef]
- Morimoto, Y.; Yoshimura, M.; Utada, K.; Setoyama, K.; Matsumoto, M.; Sakabe, T. Prediction of postoperative delirium after abdominal surgery in the elderly. J. Anesth. 2009, 23, 51–56. [Google Scholar] [CrossRef]
- Song, J.; Cheng, C.; Sheng, K.; Jiang, L.-L.; Li, Y.; Xia, X.-Q.; Hu, X.-W. Association between the reactivity of local cerebral oxygen saturation after hypo-to-hypercapnic tests and delirium after abdominal surgery in older adults: A prospective study. Front. Psychiatry 2022, 13, 907870. [Google Scholar] [CrossRef]
- Tobar, E.; Abedrapo, M.A.; Godoy, J.A.; Llanos, J.L.; Díaz, M.J.; Azolas, R.; Bocic, G.R.; Escobar, J.A.; Cornejo, R.A.; Romero, C.M. Impact of hypotension and global hypoperfusion in postoperative delirium: A pilot study in older adults undergoing open colon surgery. Braz. J. Anesthesiol. (Engl. Ed.) 2018, 68, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Zhao, W.; Mu, D.-L.; Zhao, X.; Li, X.-Y.; Wang, D.-X.; Jia, H.-Q.; Dai, F.; Meng, L. Association between cerebral desaturation and postoperative delirium in thoracotomy with one-lung ventilation: A prospective cohort study. Anesth. Analg. 2021, 133, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Soh, S.; Shim, J.-K.; Song, J.-W.; Kim, K.-N.; Noh, H.-Y.; Kwak, Y.-L. Postoperative delirium in elderly patients undergoing major spinal surgery: Role of cerebral oximetry. J. Neurosurg. Anesthesiol. 2016, 29, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.; Butler, E.; Frost, S.A.; Chuan, A.; Aneman, A. Cerebrovascular autoregulation monitoring and patient-centred outcomes after cardiac surgery: A systematic review. ACTA Anaesthesiol. Scand. 2018, 62, 588–599. [Google Scholar] [CrossRef]
- Robba, C.; Taccone, F.S.; Citerio, G. Monitoring cerebral oxygenation in acute brain-injured patients. Intensive Care Med. 2022, 48, 1463–1466. [Google Scholar] [CrossRef]
- Wang, X.; Cui, L.; Ji, X. Cognitive impairment caused by hypoxia: From clinical evidences to molecular mechanisms. Metab. Brain Dis. 2022, 37, 51–66. [Google Scholar] [CrossRef]
- Qiu, L.; Ma, Y.; Ge, L.; Zhou, H.; Jia, W. Efficacy of cerebral oxygen saturation monitoring for perioperative neurocognitive disorder in adult noncardiac surgical patients: A systematic review and meta-Analysis of randomized controlled trials. World Neurosurg. 2025, 194, 123570. [Google Scholar] [CrossRef]
- Sun, Q.; Wu, W. Effect of near-infrared spectroscopy on postoperative delirium in cardiac surgery with cardiopulmonary bypass: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2024, 11, 1404210. [Google Scholar] [CrossRef]
- Wong, Z.Z.; Chiong, X.H.; Chaw, S.H.; Hashim, N.H.B.M.; Abidin, M.F.B.Z.; Yunus, S.N.B.; Subramaniam, T.; Ng, K.T. The use of cerebral oximetry in surgery: A systematic review and meta-analysis of randomized controlled trials. J. Cardiothorac. Vasc. Anesth. 2022, 36, 2002–2011. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, K.; Zhang, L.; Zong, H.; Meng, L.; Han, R. Cerebral near-infrared spectroscopy (NIRS) for perioperative monitoring of brain oxygenation in children and adults. Cochrane Database Syst. Rev. 2018, 1, CD010947. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Sheinberg, R.; Yee, M.-S.; Ono, M.; Zheng, Y.; Hogue, C.W. Cerebral near-infrared spectroscopy monitoring and neurologic outcomes in adult cardiac surgery patients: A systematic review. Anesth. Analg. 2013, 116, 663–676. [Google Scholar] [CrossRef]
- Sarink, D.; Nedkoff, L.; Briffa, T.; Shaw, J.E.; Magliano, D.J.; Stevenson, C.; Mannan, H.; Knuiman, M.; Hung, J.; Hankey, G.J.; et al. Trends in age- and sex-specific prevalence and incidence of cardiovascular disease in Western Australia. Eur. J. Prev. Cardiol. 2018, 25, 1280–1290. [Google Scholar] [CrossRef]
- Mevorach, L.; Forookhi, A.; Farcomeni, A.; Romagnoli, S.; Bilotta, F. Perioperative risk factors associated with increased incidence of postoperative delirium: Systematic review, meta-analysis, and Grading of Recommendations Assessment, Development, and Evaluation system report of clinical literature. Br. J. Anaesth. 2023, 130, e254–e262. [Google Scholar] [CrossRef]
- Ho, M.; Nealon, J.; Igwe, E.; Traynor, V.; Chang, H.; Chen, K.; Montayre, J. Postoperative delirium in older patients: A systematic review of assessment and incidence of postoperative delirium. Worldviews Evid.-Based Nurs. 2021, 18, 290–301. [Google Scholar] [CrossRef]
- Igwe, E.O.; Nealon, J.; O’Shaughnessy, P.; Bowden, A.; Chang, H.; Ho, M.; Montayre, J.; Montgomery, A.; Rolls, K.; Chou, K.; et al. Incidence of postoperative delirium in older adults undergoing surgical procedures: A systematic literature review and meta-analysis. Worldviews Evid.-Based Nurs. 2023, 20, 220–237. [Google Scholar] [CrossRef]
- Liu, S.B.; Wu, H.Y.; Duan, M.L.; Yang, R.L.; Ji, C.H.; Liu, J.J.; Zhao, H. Delirium in the ICU: How much do we know? A narrative review. Ann. Med. 2024, 56, 2405072. [Google Scholar] [CrossRef]
- Wei, L.A.; Fearing, M.A.; Sternberg, E.J.; Inouye, S.K. The Confusion Assessment Method: A systematic review of current usage. J. Am. Geriatr. Soc. 2008, 56, 823–830. [Google Scholar] [CrossRef]
- Chan, M.J.; Chung, T.; Glassford, N.J.; Bellomo, R. Near-infrared spectroscopy in adult cardiac surgery patients: A systematic review and meta-analysis. J. Cardiothorac. Vasc. Anesth. 2017, 31, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Newman, L.; Nolan, H.; Carey, D.; Reilly, R.B.; Kenny, R.A. Age and sex differences in frontal lobe cerebral oxygenation in older adults—Normative values using novel, scalable technology: Findings from the Irish Longitudinal Study on Ageing (TILDA). Arch. Gerontol. Geriatr. 2020, 87, 103988. [Google Scholar] [CrossRef] [PubMed]
- Semrau, J.S.; Motamed, M.; Ross-White, A.; Boyd, J.G. Cerebral oximetry and preventing neurological complication post-cardiac surgery: A systematic review. Eur. J. Cardiothorac. Surg. 2021, 59, 1144–1154. [Google Scholar] [CrossRef]
- Schmidt, C.; Heringlake, M.; Kellner, P.; Berggreen, A.E.; Maurer, H.; Brandt, S.; Bucsky, B.; Petersen, M.; Charitos, E.I. The effects of systemic oxygenation on cerebral oxygen saturation and its relationship to mixed venous oxygen saturation: A prospective observational study comparison of the INVOS and ForeSight Elite cerebral oximeters. Can. J. Anesth./J. Can. Anesth. 2018, 65, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Takegawa, R.; Hayashida, K.; Rolston, D.M.; Li, T.; Miyara, S.J.; Ohnishi, M.; Shiozaki, T.; Becker, L.B. Near-Infrared Spectroscopy Assessments of Regional Cerebral Oxygen Saturation for the Prediction of Clinical Outcomes in Patients with Cardiac Arrest: A Review of Clinical Impact, Evolution, and Future Directions. Front. Med. 2020, 7, 587930. [Google Scholar] [CrossRef]
- Rudolph, J.L.; Marcantonio, E.R.; Culley, D.J.; Silverstein, J.H.; Rasmussen, L.S.; Crosby, G.J.; Inouye, S.K. Delirium is associated with early postoperative cognitive dysfunction. Anaesthesia 2008, 63, 941–947. [Google Scholar] [CrossRef]
- Matcan, S.; Sanabria Carretero, P.; Gómez Rojo, M.; Castro Parga, L.; Reinoso-Barbero, F. Importancia de la monitorización bilateral de la oxigenación cerebral: Caso clínico de asimetría durante el bypass cardiopulmonar secundaria a infarto cerebral previo. Rev. Esp. Anestesiol. Reanim. 2018, 65, 165–169. [Google Scholar] [CrossRef]
- Daal, S.M.; Keyhan-Falsafi, M.A.; Hoohenkerk, G.J.F.; Ayan, K.; De Vroege, R.; Van Alphen, J.; Van Kampen, P.M.; Keunen, R.W.M. Unilateral versus bilateral cerebral oximetry in delirium prevention during CABG and valve surgery. Acta Anaesthesiol. Belg. 2024, 75, 91–97. [Google Scholar] [CrossRef]
- Lin, D.; Zhou, R. Cerebral oximetry: Defining baseline value and desaturation cautiously. Ann. Thorac. Surg. 2025, 119, 926–927. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).