Exploring Mitochondrial DNA Copy Number in Italian Children with ADHD: Implications for Neurobiological Mechanisms
Abstract
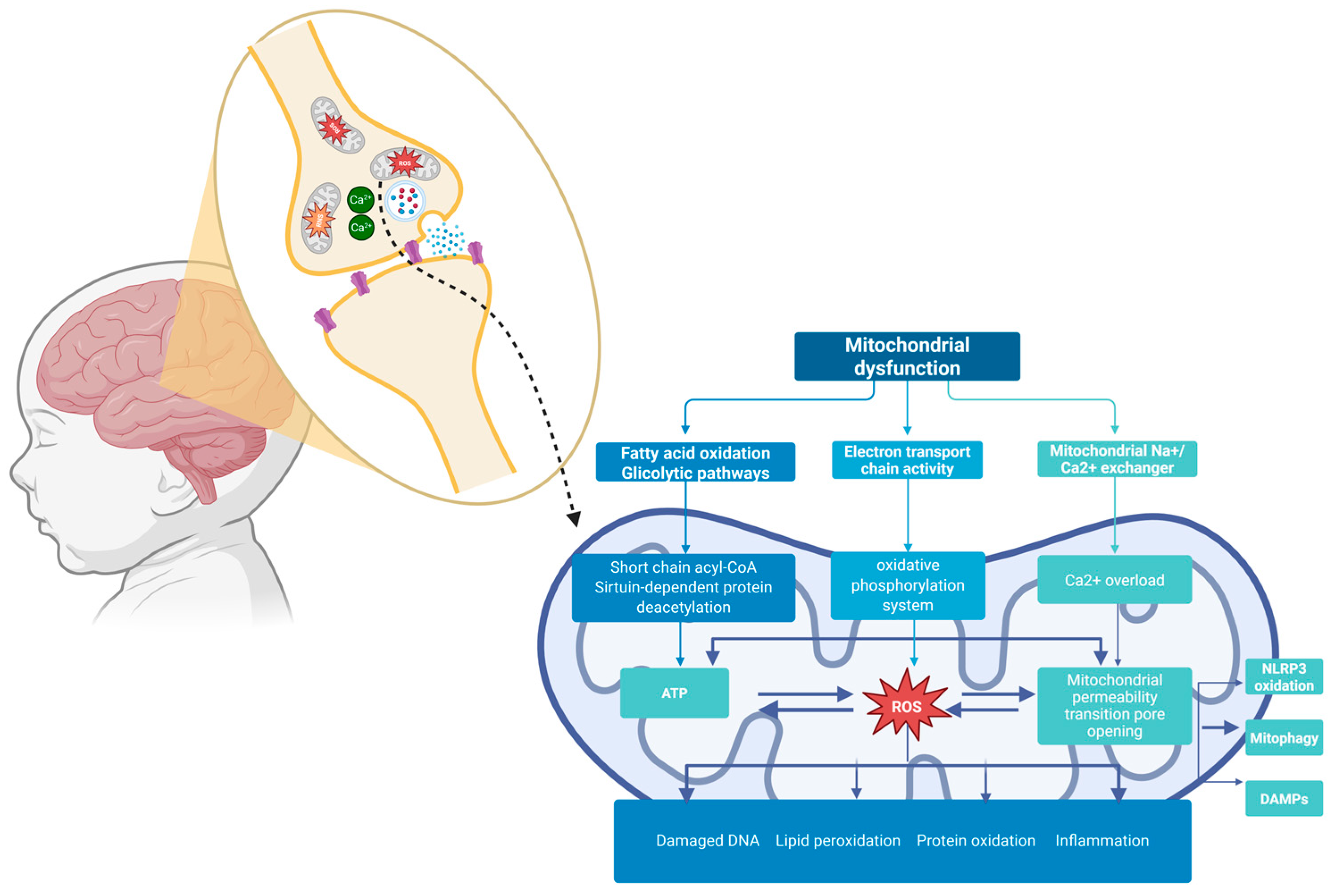
1. Introduction
2. Materials and Methods
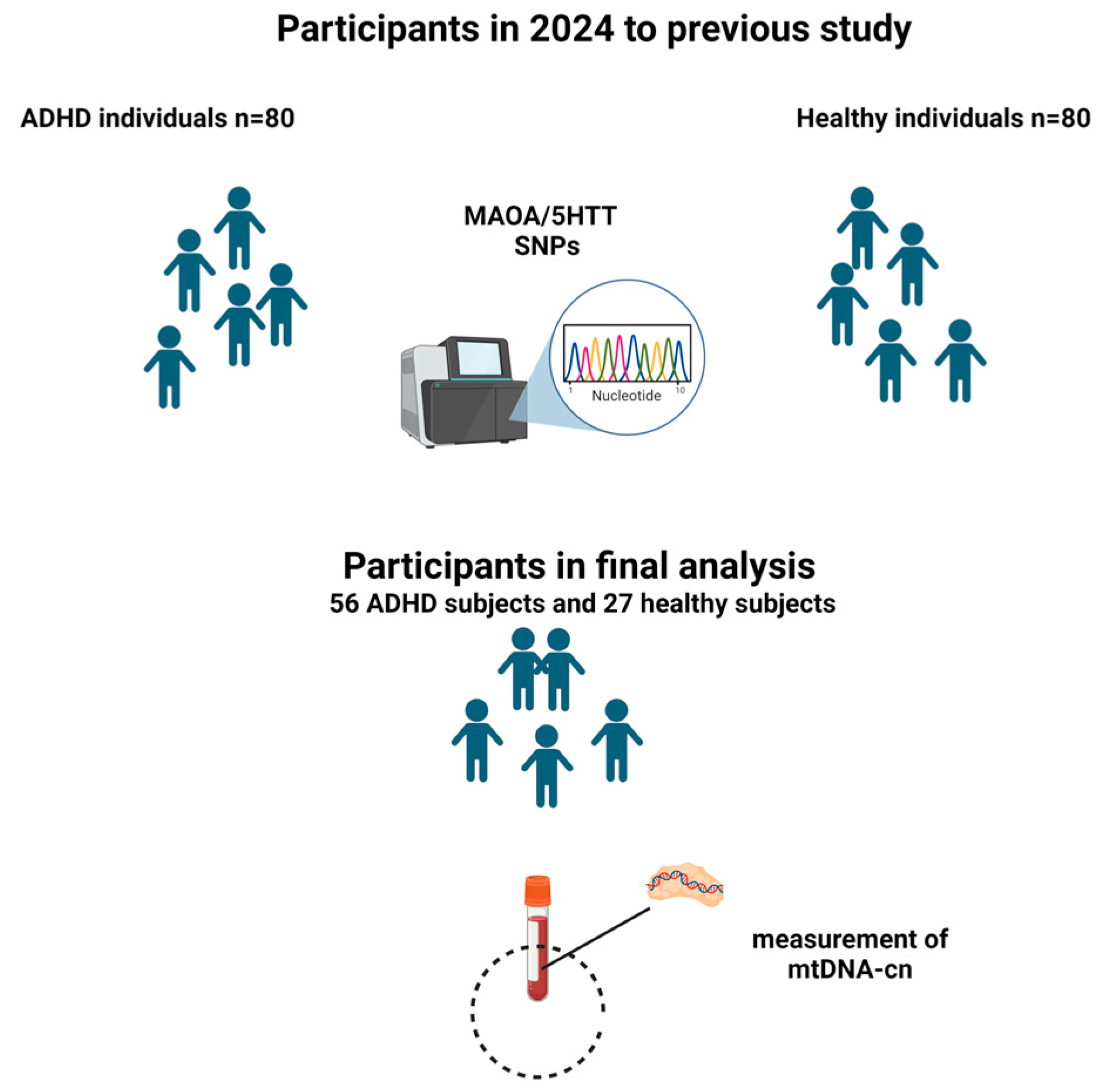
2.1. Population Sample and Diagnostic Assessment
2.2. Mitochondrial DNA Copy Number Quantification
2.3. Genotyping Analysis
2.4. Statistical Analyses
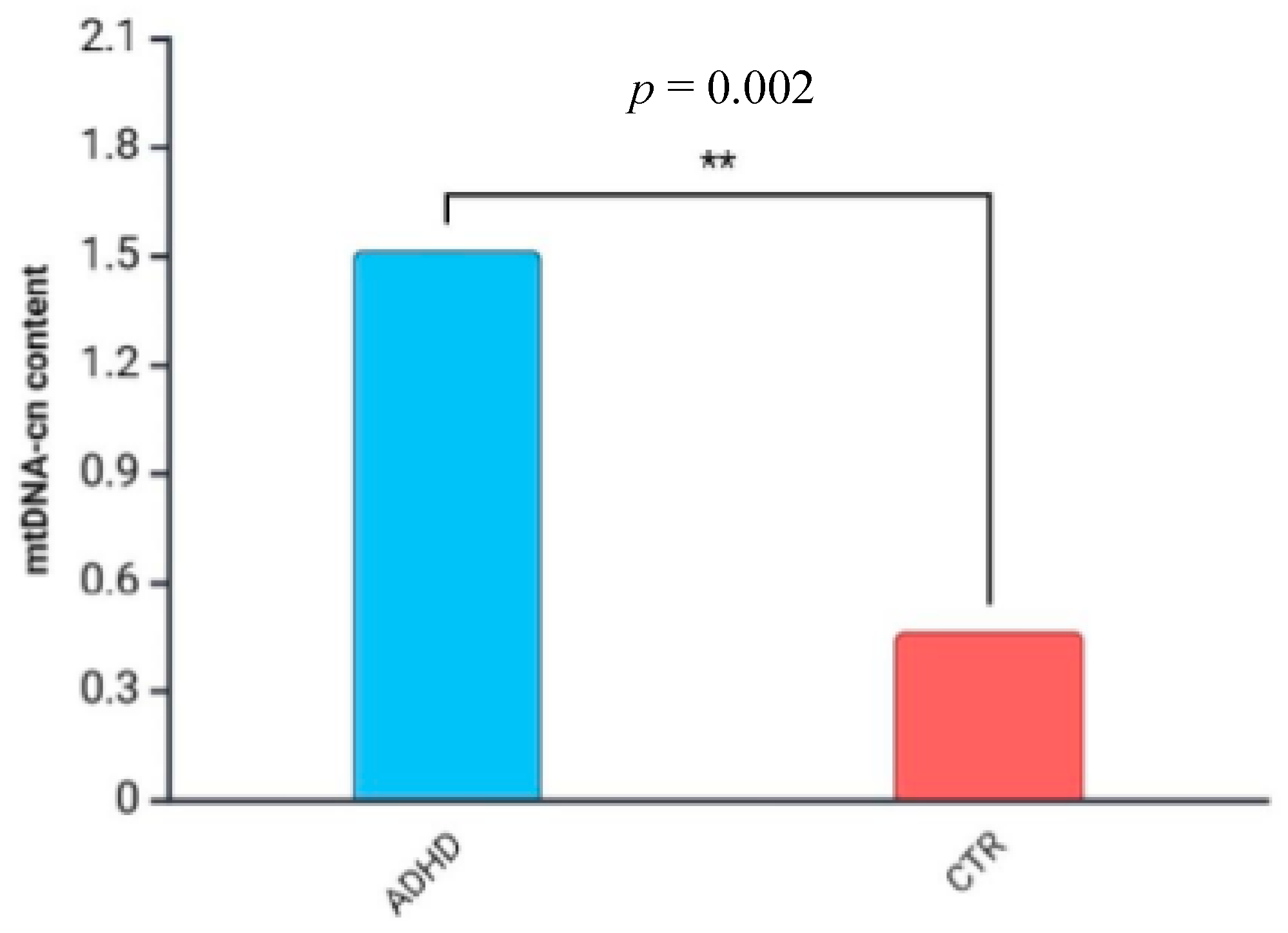
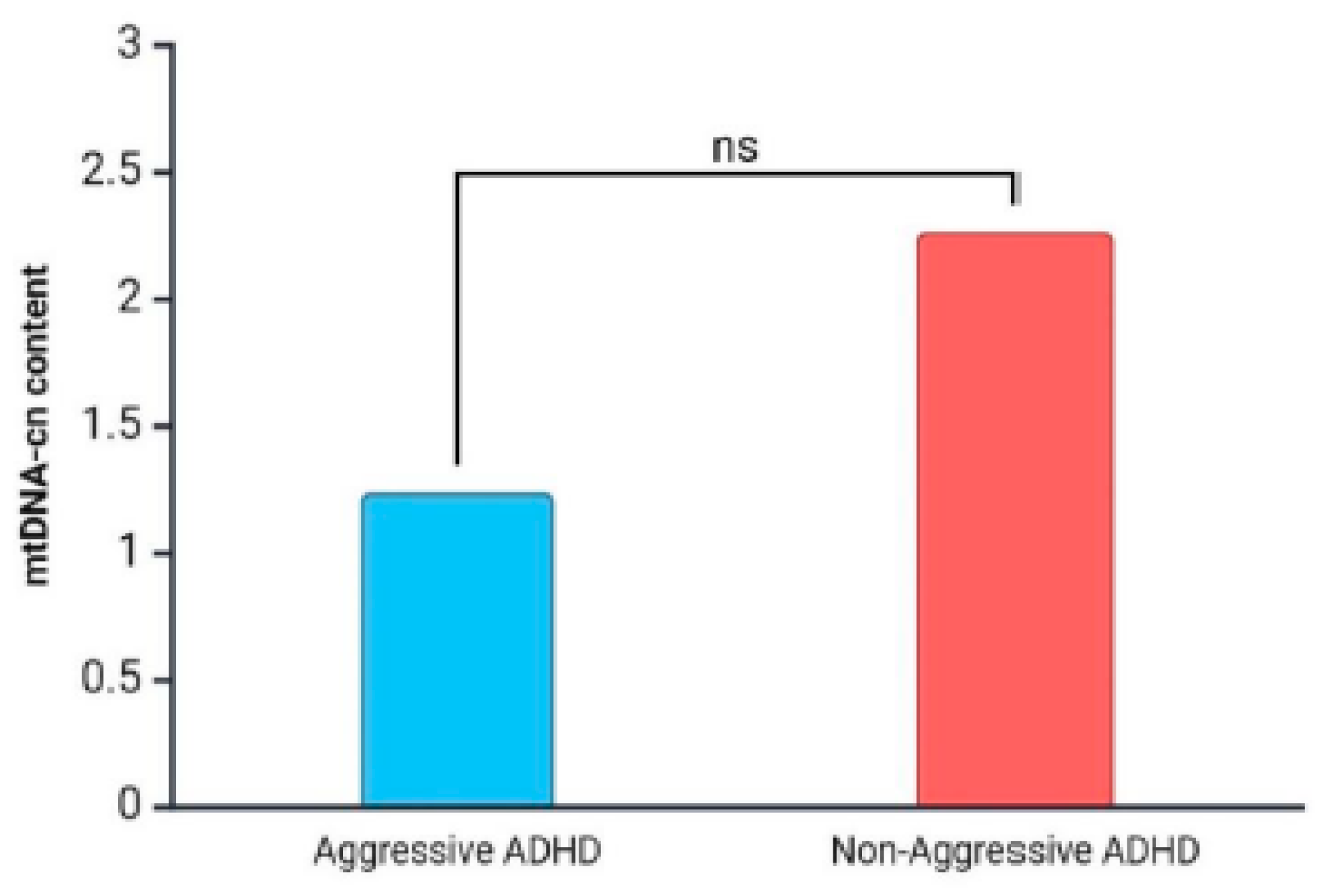
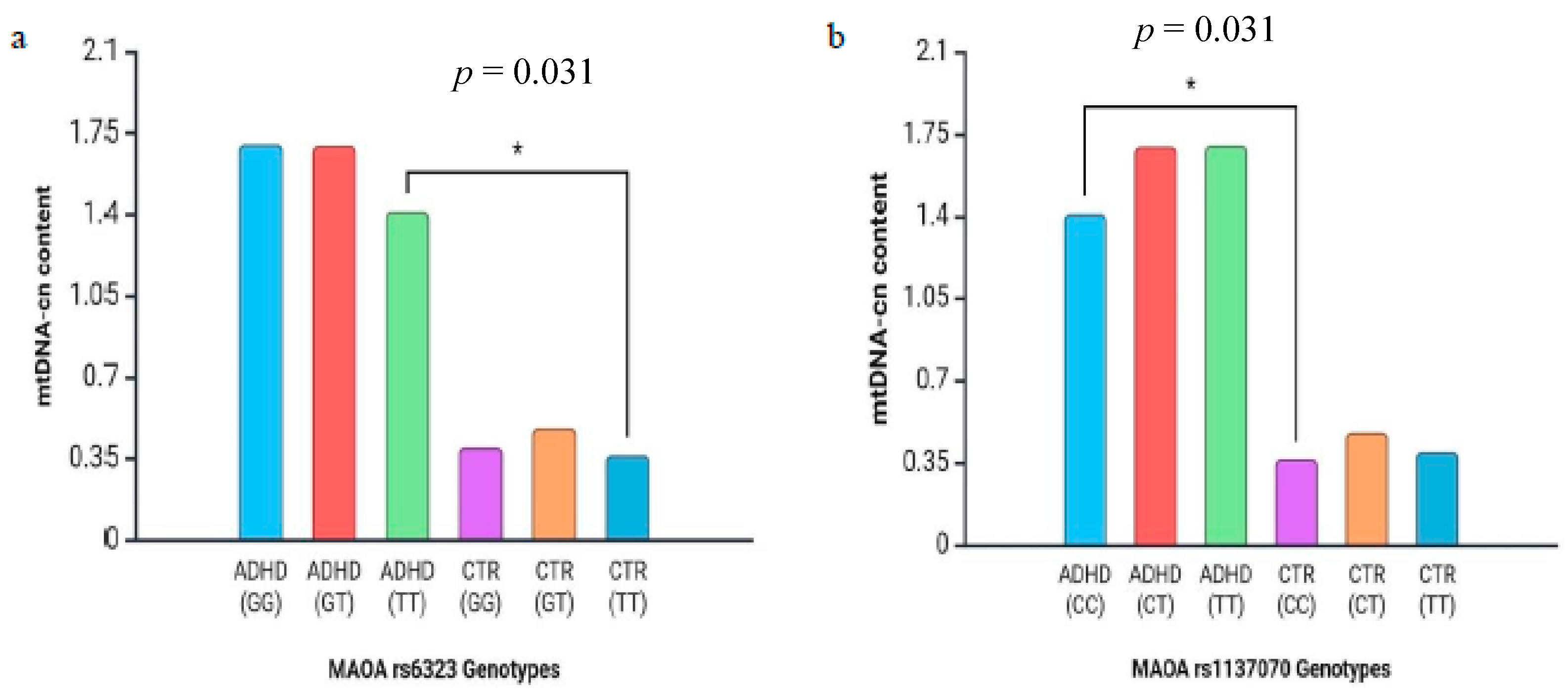
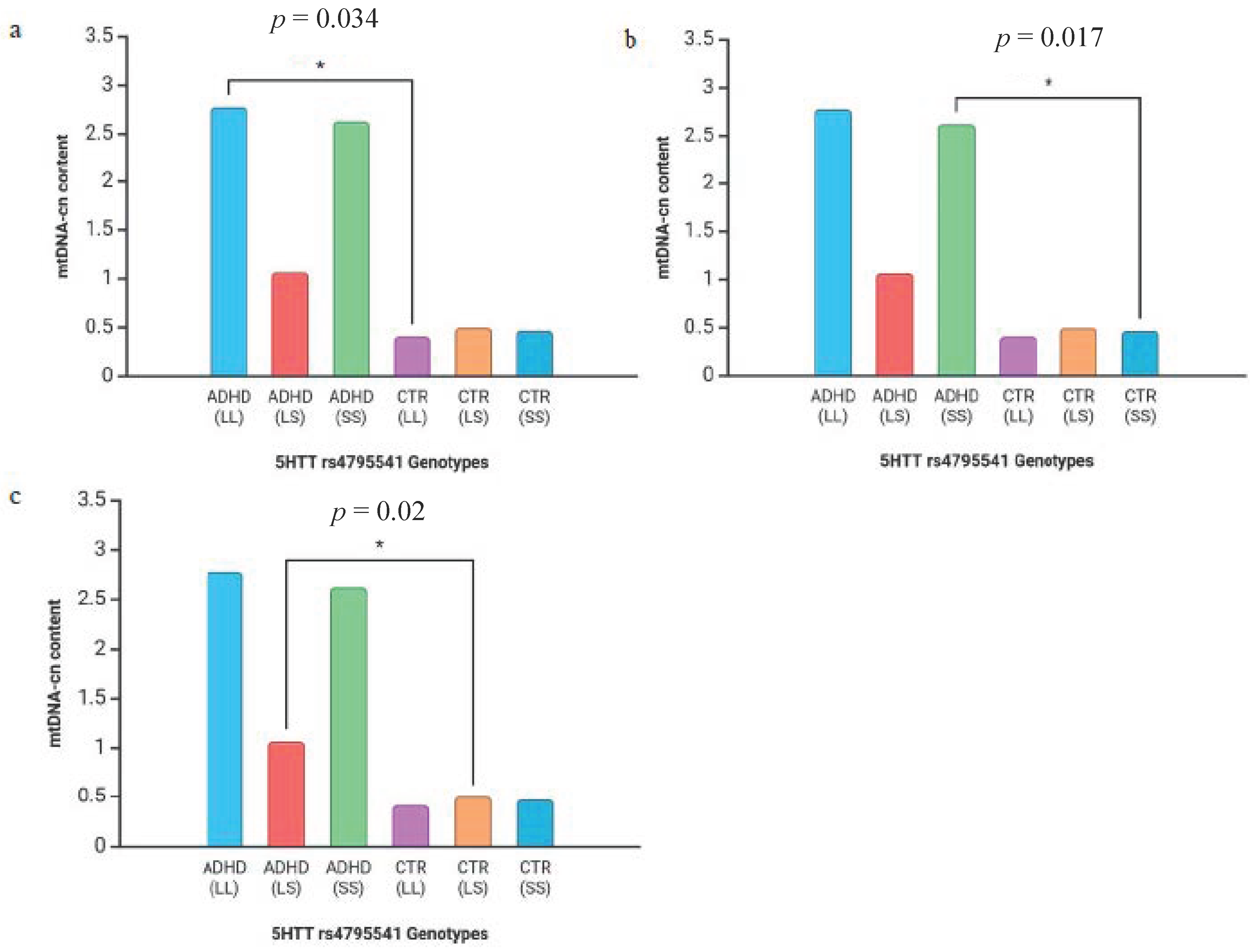
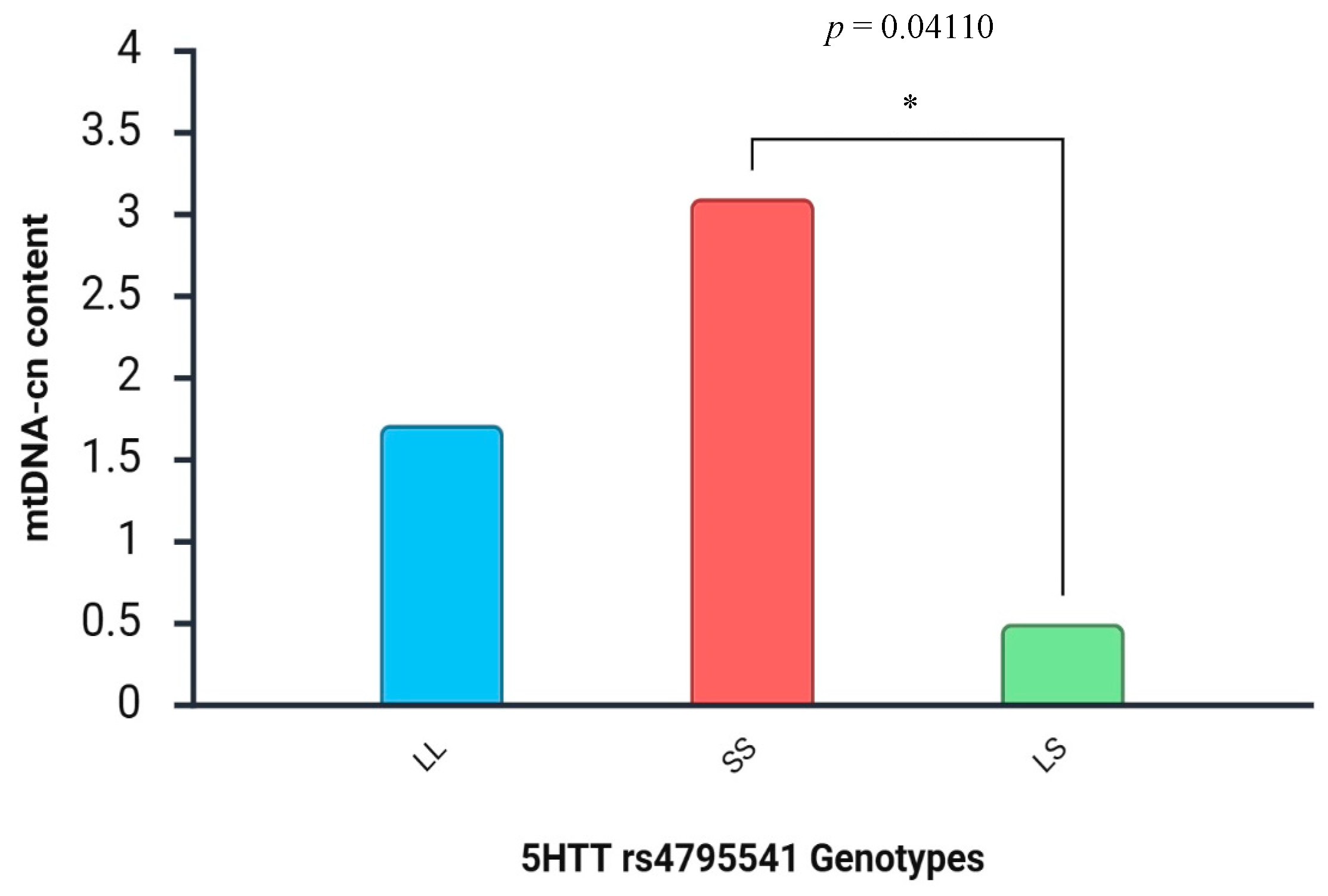
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADHD | Attention Deficit/Hyperactivity Disorder |
| ANOVA | Analysis of Variance |
| ATP | Adenosine Triphosphate |
| ASD | Autism Spectrum Disorder |
| Cq | Quantification Cycle |
| DAT1 | Dopamine Transporter 1 |
| DNA | Deoxyribonucleic Acid |
| DRD2 | Dopamine Receptor D2 |
| DRD4 | Dopamine Receptor D4 |
| DSM-5/DSM-V | Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition |
| ETC | Electron Transport Chain |
| MAOA | Monoamine Oxidase A |
| MAPK | Mitogen Activated Protein Kinase |
| mtDNA | Mitochondrial DNA |
| mtDNA cn | Mitochondrial DNA Copy Number |
| mtDAMPs | Mitochondrial Damage Associated Molecular Patterns |
| OXPHOS | Oxidative Phosphorylation |
| PB | Peripheral Blood |
| PCR | Polymerase Chain Reaction |
| PGC-1α | Peroxisome Proliferator Activated Receptor Gamma Coactivator 1 Alpha |
| qPCR | Quantitative Polymerase Chain Reaction (Real-Time PCR) |
| ROS | Reactive Oxygen Species |
| SCR | Single Copy Reference |
| SERT | Serotonin Transporter |
| SIRT1 | Sirtuin 1 |
| SLC6A4 | Solute Carrier Family 6 Member 4 (gene encoding 5-HTT) |
| VNTR | Variable Number Tandem Repeat |
References
- Polanczyk, G.; de Lima, M.S.; Horta, B.L.; Biederman, J.; Rohde, L.A. The worldwide prevalence of ADHD: A systematic review and meta regression analysis. Am. J. Psychiatry 2007, 164, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Tuvblad, C.; Baker, L.A. Human aggression across the lifespan: Genetic propensities and environmental moderators. Adv. Genet. 2011, 75, 171–214. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shelton, T.L.; Barkley, R.A.; Crosswait, C.; Moorehouse, M.; Fletcher, K.; Barrett, S.; Jenkins, L.; Metevia, L. Psychiatric and psychological morbidity as a function of adaptive disability in preschool children with aggressive and hyperactive-impulsive-inattentive behavior. J. Abnorm. Child. Psychol. 1998, 26, 475–494. [Google Scholar] [CrossRef] [PubMed]
- McKay, K.E.; Halperin, J.M. ADHD, aggression, and antisocial behavior across the lifespan. Interactions with neurochemical and cognitive function. Ann. N. Y. Acad. Sci. 2001, 931, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Willcutt, E.G. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: A meta-analytic review. Neurotherapeutics 2012, 9, 490–499. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Young, S.; Adamo, N.; Ásgeirsdóttir, B.B.; Branney, P.; Beckett, M.; Colley, W.; Cubbin, S.; Deeley, Q.; Farrag, E.; Gudjonsson, G.; et al. Females with ADHD: An expert consensus statement taking a lifespan approach providing guidance for the identification and treatment of attention-deficit/hyperactivity disorder in girls and women. BMC Psychiatry 2020, 20, 404. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gershon, J. A meta-analytic review of gender differences in ADHD. J. Atten. Disord. 2002, 5, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Ghasemi, H.; Abdoli, N.; Rahmani, A.; Shiri, M.H.; Hashemian, A.H.; Akbari, H.; Mohammadi, M. The global prevalence of ADHD in children and adolescents: A systematic review and meta-analysis. Ital. J. Pediatr. 2023, 49, 48. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- American Psychiatric Association, DSM-5 Task Force. Diagnostic and Statistical Manual of Mental Disorders: DSM-5™, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- Faraone, S.V.; Mick, E. Molecular genetics of attention deficit hyperactivity disorder. Psychiatr. Clin. N. Am. 2010, 33, 159–180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Faraone, S.V.; Larsson, H. Genetics of attention deficit hyperactivity disorder. Mol. Psychiatry 2019, 24, 562–575. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Demontis, D.; Walters, R.K.; Rajagopal, V.M.; Waldman, I.D.; Grove, J.; Als, T.D.; Dalsgaard, S.; Ribasés, M.; Bybjerg-Grauholm, J.; Bækvad-Hansen, M.; et al. Risk variants and polygenic architecture of disruptive behavior disorders in the context of attention-deficit/hyperactivity disorder. Nat. Commun. 2021, 12, 576, Erratum in Nat. Commun. 2021, 12, 1166. https://doi.org/10.1038/s41467-021-21566-w. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thapar, A.; Harrington, R.; McGuffin, P. Examining the comorbidity of ADHD-related behaviors and conduct problems using a twin study design. Br. J. Psychiatry 2001, 179, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Bhat, A.A.; Hashem, S.; Nisar, S.; Kamal, M.; Syed, N.; Temanni, M.-R.; Gupta, R.K.; Kamran, S.; Azeem, M.W.; et al. Genetic variations influence brain changes in patients with attention-deficit hyperactivity disorder. Transl. Psychiatry 2021, 11, 349. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Manuck, S.B.; Flory, J.D.; Ferrell, R.E.; Mann, J.J.; Muldoon, M.F. A regulatory polymorphism of the monoamine oxidase-A gene may be associated with variability in aggression, impulsivity, and central nervous system serotonergic responsivity. Psychiatry Res. 2000, 95, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Gerra, G.; Garofano, L.; Santoro, G.; Bosari, S.; Pellegrini, C.; Zaimovic, A.; Moi, G.; Bussandri, M.; Moi, A.; Brambilla, F.; et al. Association between low-activity serotonin transporter genotype and heroin dependence: Behavioral and personality correlates. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2004, 126B, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Twitchell, G.R.; Hanna, G.L.; Cook, E.H.; Stoltenberg, S.F.; Fitzgerald, H.E.; Zucker, R.A. Serotonin transporter promoter polymorphism genotype is associated with behavioral disinhibition and negative affect in children of alcoholics. Alcohol. Clin. Exp. Res. 2001, 25, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Thome, J.; Ehlis, A.C.; Fallgatter, A.J.; Krauel, K.; Lange, K.W.; Riederer, P.; Romanos, M.; Taurines, R.; Tucha, O.; Uzbekov, M.; et al. Biomarkers for attention-deficit/hyperactivity disorder (ADHD). A consensus report of the WFSBP task force on biological markers and the World Federation of ADHD. World J. Biol. Psychiatry 2012, 13, 379–400. [Google Scholar] [CrossRef] [PubMed]
- Corona, J.C. Role of Oxidative Stress and Neuroinflammation in Attention-Deficit/Hyperactivity Disorder. Antioxidants 2020, 9, 1039. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Disha, B.; Mathew, R.P.; Dalal, A.B.; Mahato, A.K.; Satyamoorthy, K.; Singh, K.K.; Thangaraj, K.; Govindaraj, P. Mitochondria in biology and medicine—2023. Mitochondrion 2024, 76, 101853. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.; Jones, E.; Ramos, M.; Innis-Whitehouse, W.; Gilkerson, R. The little big genome: The organization of mitochondrial DNA. Front. Biosci. (Landmark Ed.) 2017, 22, 710–721. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clay Montier, L.L.; Deng, J.J.; Bai, Y. Number matters: Control of mammalian mitochondrial DNA copy number. J. Genet. Genom. 2009, 36, 125–131. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pello, R.; Martín, M.A.; Carelli, V.; Nijtmans, L.G.; Achilli, A.; Pala, M.; Torroni, A.; Gómez-Durán, A.; Ruiz-Pesini, E.; Martinuzzi, A.; et al. Mitochondrial DNA background modulates the assembly kinetics of OXPHOS complexes in a cellular model of mitochondrial disease. Hum. Mol. Genet. 2008, 17, 4001–4011. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosebush, P.I.; Anglin, R.E.; Rasmussen, S.; Mazurek, M.F. Mental illness in patients with inherited mitochondrial disorders. Schizophr. Res. 2017, 187, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Martin, M.V.; Watson, S.J.; Schatzberg, A.; Akil, H.; Myers, R.M.; Jones, E.G.; Bunney, W.E.; Vawter, M.P. Mitochondrial involvement in psychiatric disorders. Ann. Med. 2008, 40, 281–295, Erratum in Ann. Med. 2011, 43, 329. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nissanka, N.; Moraes, C.T. Mitochondrial DNA damage and reactive oxygen species in neurodegenerative disease. FEBS Lett. 2018, 592, 728–742. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Öğütlü, H.; Esin, İ.S.; Erdem, H.B.; Tatar, A.; Dursun, O.B. Mitochondrial DNA Copy Number is Associated with Attention Deficit Hyperactivity Disorder. Psychiatr. Danub. 2020, 32, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Pinto Payares, D.V.; Spooner, L.; Vosters, J.; Dominguez, S.; Patrick, L.; Harris, A.; Kanungo, S. A systematic review on the role of mitochondrial dysfunction/disorders in neurodevelopmental disorders and psychiatric/behavioral disorders. Front. Psychiatry 2024, 15, 1389093. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frye, R.E.; Rincon, N.; McCarty, P.J.; Brister, D.; Scheck, A.C.; Rossignol, D.A. Biomarkers of mitochondrial dysfunction in autism spectrum disorder: A systematic review and meta-analysis. Neurobiol. Dis. 2024, 197, 106520. [Google Scholar] [CrossRef] [PubMed]
- Neri, L.; Marziani, B.; Sebastiani, P.; Del Beato, T.; Colanardi, A.; Legge, M.P.; Aureli, A. Aggressiveness in Italian Children with ADHD: MAOA Gene Polymorphism Involvement. Diseases 2024, 12, 70. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giannoulis, S.V.; Müller, D.; Kennedy, J.L.; Gonçalves, V. Systematic review of mitochondrial genetic variation in attention-deficit/hyperactivity disorder. Eur. Child. Adolesc. Psychiatry 2024, 33, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Yin, P.H.; Lu, C.Y.; Chi, C.W.; Wei, Y.H. Increase of mitochondria and mitochondrial DNA in response to oxidative stress in human cells. Biochem. J. 2000, 348 Pt 2, 425–432. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- DiMauro, S.; Schon, E.A. Mitochondrial disorders in the nervous system. Annu. Rev. Neurosci. 2008, 31, 91–123. [Google Scholar] [CrossRef] [PubMed]
- Saylor, K.E.; Amann, B.H. Impulsive Aggression as a Comorbidity of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. J. Child. Adolesc. Psychopharmacol. 2016, 26, 19–25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Avshalumov, M.V.; Rice, M.E. Activation of ATP-sensitive K+ (K(ATP)) channels by H2O2 underlies glutamate-dependent inhibition of striatal dopamine release. Proc. Natl. Acad. Sci. USA 2003, 100, 11729–11734, Erratum in Proc. Natl. Acad. Sci. USA 2003, 100, 15285. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miyazaki, I.; Asanuma, M. Dopaminergic neuron-specific oxidative stress caused by dopamine itself. Acta Med. Okayama 2008, 62, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, H.; Deater-Deckard, K.; Zhang, W. Interacting Effect of Catechol-O-Methyltransferase (COMT) and Monoamine Oxidase A (MAOA) Gene Polymorphisms, and Stressful Life Events on Aggressive Behavior in Chinese Male Adolescents. Front. Psychol. 2018, 9, 1079. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kolla, N.J.; Bortolato, M. The role of monoamine oxidase A in the neurobiology of aggressive, antisocial, and violent behavior: A tale of mice and men. Prog. Neurobiol. 2020, 194, 101875. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hotamisligil, G.S.; Breakefield, X.O. Human monoamine oxidase A gene determines levels of enzyme activity. Am. J. Hum. Genet. 1991, 49, 383–392. [Google Scholar] [PubMed] [PubMed Central]
- Beucher, L.; Gabillard-Lefort, C.; Baris, O.R.; Mialet-Perez, J. Monoamine oxidases: A missing link between mitochondria and inflammation in chronic diseases? Redox Biol. 2024, 77, 103393. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scholpa, N.E.; Lynn, M.K.; Corum, D.; Boger, H.A.; Schnellmann, R.G. 5-HT1F receptor-mediated mitochondrial biogenesis for the treatment of Parkinson’s disease. Br. J. Pharmacol. 2018, 175, 348–358. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beitchman, J.H.; Baldassarra, L.; Mik, H.; De Luca, V.; King, N.; Bender, D.; Ehtesham, S.; Kennedy, J.L. Serotonin transporter polymorphisms and persistent, pervasive childhood aggression. Am. J. Psychiatry 2006, 163, 1103–1105. [Google Scholar] [CrossRef] [PubMed]
- Brivio, P.; Gallo, M.T.; Karel, P.; Cogi, G.; Fumagalli, F.; Homberg, J.R.; Calabrese, F. Alterations of mitochondrial dynamics in serotonin transporter knockout rats: A possible role in the fear extinction recall mechanisms. Front. Behav. Neurosci. 2022, 16, 957702. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tian, J.; Du, E.; Guo, L. Mitochondrial Interaction with Serotonin in Neurobiology and Its Implication in Alzheimer’s Disease. J. Alzheimers Dis. Rep. 2023, 7, 1165–1177. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fanibunda, S.E.; Deb, S.; Maniyadath, B.; Tiwari, P.; Ghai, U.; Gupta, S.; Figueiredo, D.; Weisstaub, N.; Gingrich, J.A.; Vaidya, A.D.B.; et al. Serotonin regulates mitochondrial biogenesis and function in rodent cortical neurons via the 5-HT2A receptor and SIRT1-PGC-1α axis. Proc. Natl. Acad. Sci. USA 2019, 116, 11028–11037. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, L.; Tian, J.; Du, H. Mitochondrial Dysfunction and Synaptic Transmission Failure in Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1071–1086. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alatalo, A.; de Sousa Maciel, I.; Kucháriková, N.; Chew, S.; van Kamp, I.; Foraster, M.; Julvez, J.; Kanninen, K.M. The Interaction between Circulating Cell-Free Mitochondrial DNA and Inflammatory Cytokines in Predicting Human Mental Health Issue Risk in Adolescents: An Explorative Study. Biomedicines 2023, 11, 818. [Google Scholar] [CrossRef] [PubMed]
| ADHD Subjects n = 56 | |
|---|---|
| Age (Mean ± SD) | 10.1 ± 2.6 |
| Female | 2 |
| Male | 54 |
| ADHD subtypes | |
| hyperactive-impulsive | 3 |
| inattentive | 9 |
| combined | 44 |
| ADHD | Controls | p | OR | |||
|---|---|---|---|---|---|---|
| MAOA SNP rs6323 (G891T) | ||||||
| Genotypes | N = 56 | % | N = 27 | % | ||
| TT | 36 | 0.65 | 19 | 0.7 | ns | |
| GT | 3 | 0.05 | 6 | 0.23 | 0.02 | 0.2 |
| GG | 17 | 0.3 | 2 | 0.07 | 0.01 | 5.45 |
| Alleles | 2n = 112 | % | N = 54 | % | ||
| T | 75 | 0.67 | 44 | 0.81 | ||
| G | 37 | 0.33 | 10 | 0.19 | ||
| MAOA SNP rs1137070 (T1410C) | ||||||
| Genotypes | N = 56 | % | N = 27 | % | ||
| CC | 36 | 0.64 | 19 | 0.7 | ns | |
| CT | 9 | 0.16 | 6 | 0.23 | 0.0345 | 0.2 |
| TT | 17 | 0.3 | 2 | 0.07 | 0.0342 | 4.72 |
| Alleles | 2n = 112 | % | N = 54 | % | ||
| C | 81 | 0.72 | 44 | 0.81 | ||
| T | 43 | 0.31 | 10 | 0.19 | ||
| 5-HTT SNP rs4795541 (L/S) | ||||||
| Genotypes | N = 55 * | % | N = 27 | % | ||
| LL | 14 | 0.26 | 5 | 0.18 | ns | |
| LS | 32 | 0.58 | 14 | 0.52 | ns | |
| SS | 9 | 0.16 | 8 | 0.3 | ns | |
| Alleles | 2n = 110 * | % | N = 54 | % | ||
| L | 60 | 0.54 | 24 | 0.45 | ||
| S | 50 | 0.46 | 30 | 0.55 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Citrigno, L.; Cerantonio, A.; Neri, L.; Sebastiani, P.; Colanardi, A.; Turacchio, G.; Del Beato, T.; Marziani, B.; Aureli, A. Exploring Mitochondrial DNA Copy Number in Italian Children with ADHD: Implications for Neurobiological Mechanisms. Diseases 2025, 13, 378. https://doi.org/10.3390/diseases13110378
Citrigno L, Cerantonio A, Neri L, Sebastiani P, Colanardi A, Turacchio G, Del Beato T, Marziani B, Aureli A. Exploring Mitochondrial DNA Copy Number in Italian Children with ADHD: Implications for Neurobiological Mechanisms. Diseases. 2025; 13(11):378. https://doi.org/10.3390/diseases13110378
Chicago/Turabian StyleCitrigno, Luigi, Annamaria Cerantonio, Ludovico Neri, Pierluigi Sebastiani, Alessia Colanardi, Gabriele Turacchio, Tiziana Del Beato, Beatrice Marziani, and Anna Aureli. 2025. "Exploring Mitochondrial DNA Copy Number in Italian Children with ADHD: Implications for Neurobiological Mechanisms" Diseases 13, no. 11: 378. https://doi.org/10.3390/diseases13110378
APA StyleCitrigno, L., Cerantonio, A., Neri, L., Sebastiani, P., Colanardi, A., Turacchio, G., Del Beato, T., Marziani, B., & Aureli, A. (2025). Exploring Mitochondrial DNA Copy Number in Italian Children with ADHD: Implications for Neurobiological Mechanisms. Diseases, 13(11), 378. https://doi.org/10.3390/diseases13110378







