Silicosis and Pulmonary Functions Among Residents Exposed to Dust in Saraburi Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Procedures and Outcomes
2.3. Statistical Analysis
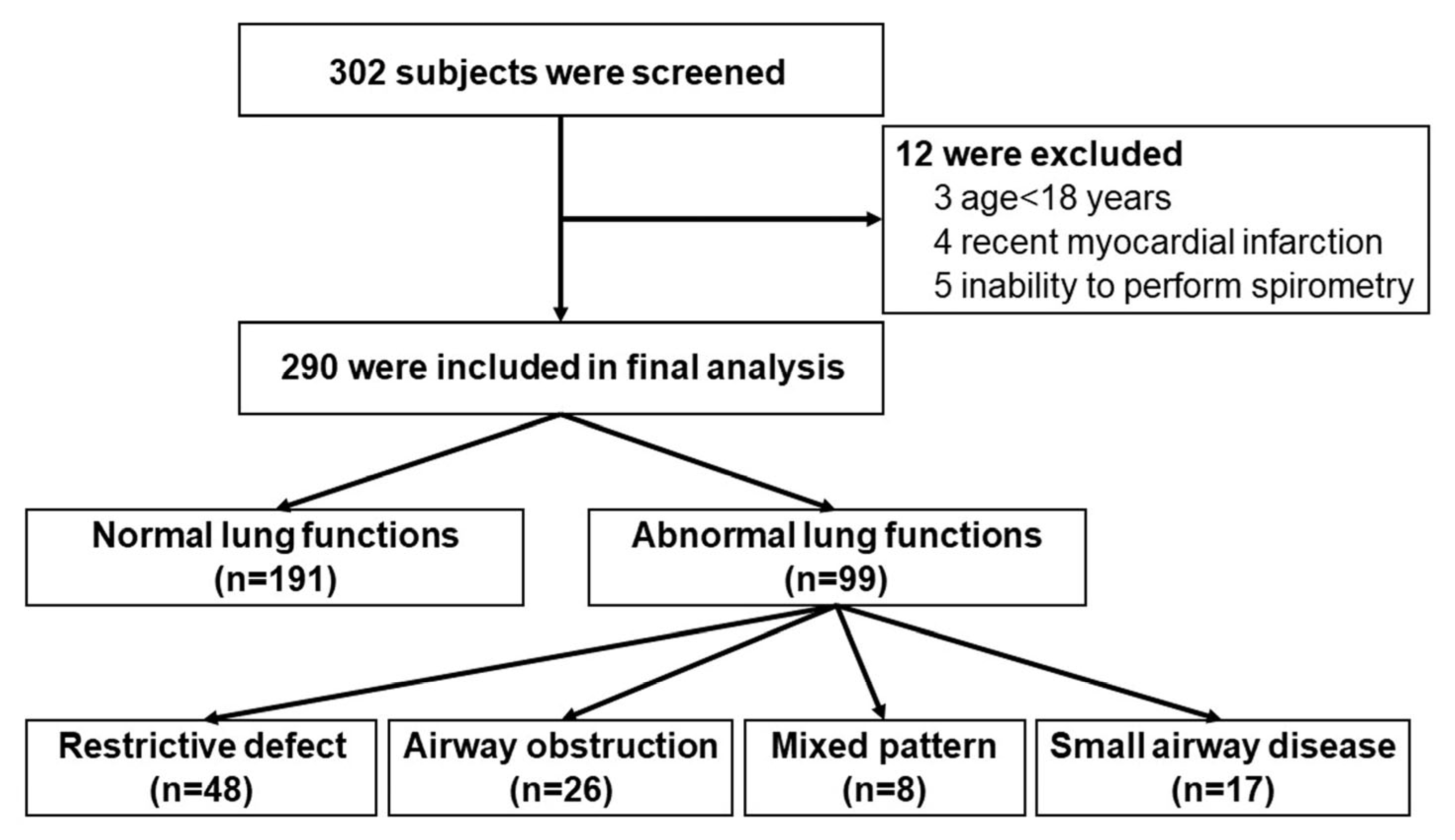
3. Results
3.1. Participants
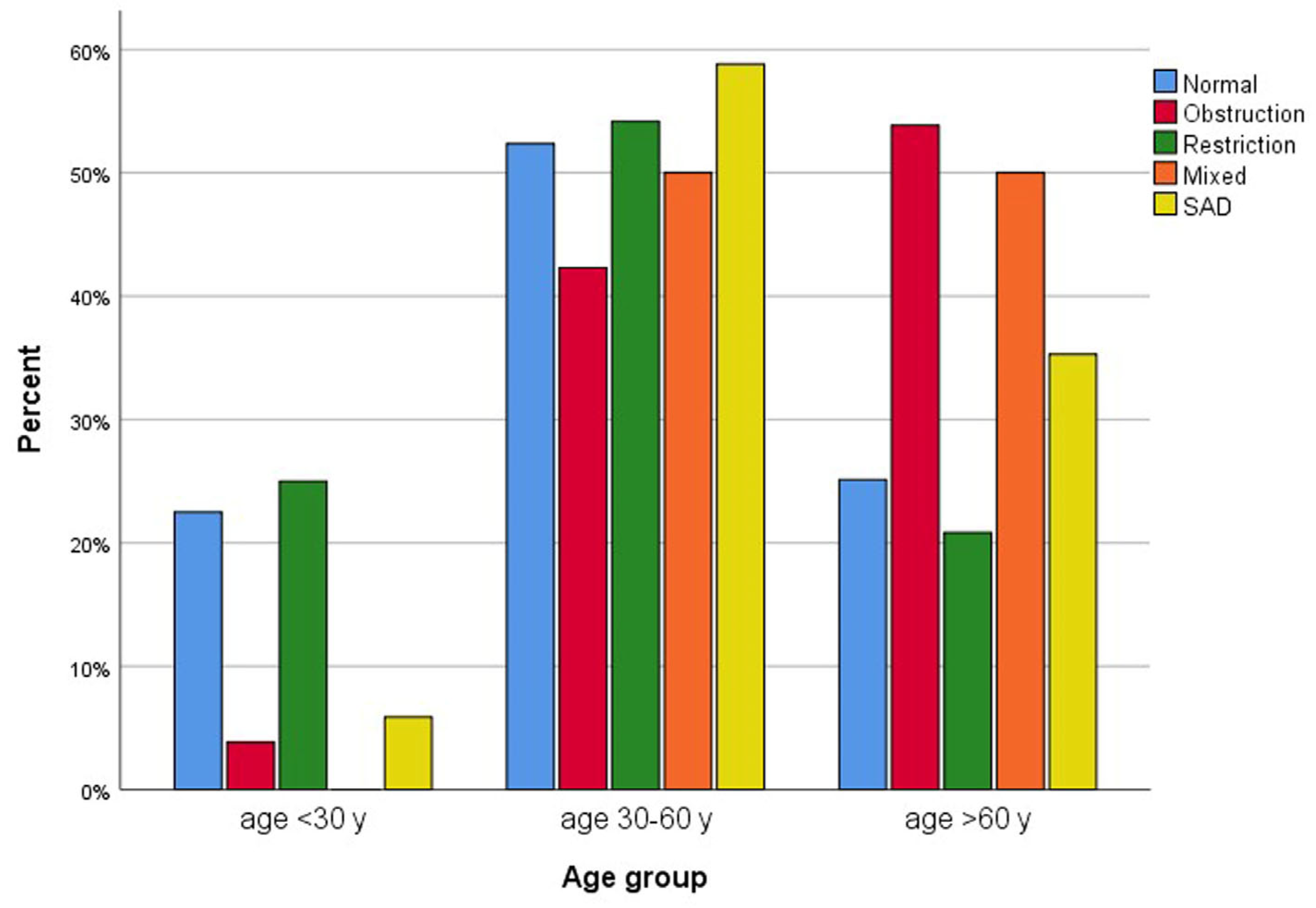
3.2. Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BDR | bronchodilator responsiveness |
| COPD | chronic obstructive pulmonary disease |
| DLCO | diffusing capacity of the lungs for carbon monoxide |
| FEF25–75 | forced expiratory flow at 25–75% of FVC |
| FEV1 | forced expiratory volume in 1 s |
| FVC | forced vital capacity |
| HRCT | high-resolution computed tomography |
| ILO | International Labour Organization |
| IOS | impulse oscillometry |
| L | liters |
| LLN | lower limit of normal |
| L/s | liters per second |
| PEF | peak expiratory flow |
| RCS | respirable crystalline silica |
References
- Hoy, R.F.; Chambers, D.C. Silica-related diseases in the modern world. Allergy 2020, 75, 2805–2817. [Google Scholar] [CrossRef]
- Leung, C.C.; Yu, I.T.; Chen, W. Silicosis. Lancet 2012, 379, 2008–2018. [Google Scholar] [CrossRef]
- Fernandez Alvarez, R.; Martinez Gonzalez, C.; Quero Martinez, A.; Blanco Perez, J.J.; Carazo Fernandez, L.; Prieto Fernandez, A. Guidelines for the diagnosis and monitoring of silicosis. Arch. Bronconeumol. 2015, 51, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chan, C.K.; Wang, M.H.; Leung, C.C.; Tai, L.B.; Tse, L.A. Association of spirometric restriction with mortality in the silicotics: A cohort study. BMC Pulm. Med. 2023, 23, 327. [Google Scholar] [CrossRef] [PubMed]
- Adverse effects of crystalline silica exposure. American Thoracic Society Committee of the Scientific Assembly on Environmental and Occupational Health. Am. J. Respir. Crit. Care Med. 1997, 155, 761–768. [Google Scholar] [CrossRef]
- Chen, W.; Zhuang, Z.; Attfield, M.D.; Chen, B.T.; Gao, P.; Harrison, J.C.; Fu, C.; Chen, J.Q.; Wallace, W.E. Exposure to silica and silicosis among tin miners in China: Exposure-response analyses and risk assessment. Occup. Environ. Med. 2001, 58, 31–37. [Google Scholar] [CrossRef]
- Lee, H.S.; Phoon, W.H.; Ng, T.P. Radiological progression and its predictive risk factors in silicosis. Occup. Environ. Med. 2001, 58, 467–471. [Google Scholar] [CrossRef]
- Ng, T.P.; Chan, S.L. Lung function in relation to silicosis and silica exposure in granite workers. Eur. Respir. J. 1992, 5, 986–991. [Google Scholar] [CrossRef]
- Chen, C.H.; Tsai, P.J.; Chang, W.W.; Chen, C.Y.; Chen, C.Y.; Yates, D.; Guo, Y.L. Dose-response relationship between lung function and chest imaging response to silica exposures in artificial stone manufacturing workers. Environ. Health 2024, 23, 25. [Google Scholar] [CrossRef]
- Rushton, L. Chronic obstructive pulmonary disease and occupational exposure to silica. Rev. Environ. Health 2007, 22, 255–272. [Google Scholar] [CrossRef]
- Ulvestad, B.; Ulvestad, M.; Skaugset, N.P.; Aalokken, T.M.; Gunther, A.; Clemm, T.; Lund, M.B.; Ellingsen, D.G. Pulmonary function and high-resolution computed tomography in outdoor rock drillers exposed to crystalline silica. Occup. Environ. Med. 2020, 77, 611–616. [Google Scholar] [CrossRef]
- Gamble, J.F.; Hessel, P.A.; Nicolich, M. Relationship between silicosis and lung function. Scand. J. Work Environ. Health 2004, 30, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Hnizdo, E.; Vallyathan, V. Chronic obstructive pulmonary disease due to occupational exposure to silica dust: A review of epidemiological and pathological evidence. Occup. Environ. Med. 2003, 60, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Yan, Y.; Li, Z.; Jiang, Y.; Cai, Y. Chronic obstructive pulmonary disease caused by inhalation of dust: A meta-analysis. Medicine 2020, 99, e21908. [Google Scholar] [CrossRef] [PubMed]
- Tustin, A.W.; Kundu-Orwa, S.; Lodwick, J.; Cannon, D.L.; McCarthy, R.B. An outbreak of work-related asthma and silicosis at a US countertop manufacturing and fabrication facility. Am. J. Ind. Med. 2022, 65, 12–19. [Google Scholar] [CrossRef]
- Rumchev, K.; Hoang, D.V.; Lee, A. Case report: Exposure to respirable crystalline silica and respiratory health among Australian mine workers. Front. Public Health 2022, 10, 798472. [Google Scholar] [CrossRef]
- Chen, Z.; Shang, Y.; Wasti, B.; Gong, S.; Xiang, X. Clinical characteristics and risk factors in pneumoconiosis patients with asthma. Sci. Rep. 2025, 15, 7000. [Google Scholar] [CrossRef]
- Mundt, K.A.; Thompson, W.J.; Dhawan, G.; Checkoway, H.; Boffetta, P. Systematic review of the epidemiological evidence of associations between quantified occupational exposure to respirable crystalline silica and the risk of silicosis and lung cancer. Front. Public Health 2025, 13, 1554006. [Google Scholar] [CrossRef]
- Hoy, R.F.; Jeebhay, M.F.; Cavalin, C.; Chen, W.; Cohen, R.A.; Fireman, E.; Go, L.H.T.; Leon-Jimenez, A.; Menendez-Navarro, A.; Ribeiro, M.; et al. Current global perspectives on silicosis-Convergence of old and newly emergent hazards. Respirology 2022, 27, 387–398. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, M.; Liu, Y.; Ma, J.; Yang, M.; Shi, T.; Chen, W. Comparison of risk of silicosis in metal mines and pottery factories: A 44-year cohort study. Chest 2020, 158, 1050–1059. [Google Scholar] [CrossRef]
- He, W.; Jin, N.; Deng, H.; Zhao, Q.; Yuan, F.; Chen, F.; Zhang, H.; Zhong, X. Workers’ occupational dust exposure and pulmonary function assessment: Cross-sectional study in China. Int. J. Environ. Res. Public Health 2022, 19, 11065. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, R.F.; de Paula Santos, U.; Arbex, R.F.; Arbex, M.A.; Terra-Filho, M. An evaluation of the impact of air pollution on the lung functions of high school students living in a ceramic industrial park zone. Int. J. Environ. Res. Public Health 2023, 20, 6964. [Google Scholar] [CrossRef] [PubMed]
- Ashuro, Z.; Debela, B.G.; Daba, C.; Hareru, H.E.; Abaya, S.W.; Byrne, A.L. The effect of occupational exposure to organic dust on lung function parameters among African industrial workers: A systematic review and meta-analysis. Front. Public Health 2024, 12, 1424315. [Google Scholar] [CrossRef] [PubMed]
- Silanun, K.; Chaiear, N.; Rechaipichitkul, W. Prevalence of silicosis in stone carving workers being exposed to inorganic dust at Sikhiu District Nakhonratchasima Province, Thailand; Preliminary results. J. Med. Assoc. Thail. 2017, 100, 598–602. [Google Scholar]
- Miller, M.R.; Crapo, R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. General considerations for lung function testing. Eur. Respir. J. 2005, 26, 153–161. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of spirometry 2019 update. An official American Thoracic Society and European Respiratory Society technical statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.; Zheng, J.; et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef]
- Stanojevic, S.; Kaminsky, D.A.; Miller, M.R.; Thompson, B.; Aliverti, A.; Barjaktarevic, I.; Cooper, B.G.; Culver, B.; Derom, E.; Hall, G.L.; et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur. Respir. J. 2022, 60, 2101499. [Google Scholar] [CrossRef]
- Ciprandi, G.; Capasso, M.; Tosca, M.; Salpietro, C.; Salpietro, A.; Marseglia, G.; La Rosa, M. A forced expiratory flow at 25–75% value <65% of predicted should be considered abnormal: A real-world, cross-sectional study. Allergy Asthma Proc. 2012, 33, e5–e8. [Google Scholar]
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease 2024 Report. Available online: https://goldcopd.org (accessed on 31 August 2025).
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention 2024 Update. Available online: https://ginasthma.org/ (accessed on 31 August 2025).
- Chansaengpetch, S.; Dumavibhat, N.; Kaewlai, R.; Jaroonpipatkul, A.; Virojskulchai, T.; Bunman, S.; Khantharot, K.; Pholngam, A.; Thanakunchai, T. Underdiagnosis of silicosis revealed by reinterpretation of chest radiographs in Thai ceramic workers. Multidiscip. Respir. Med. 2023, 18, 910. [Google Scholar] [CrossRef]
- Hoy, R.F.; Jones, C.; Newbigin, K.; Abramson, M.J.; Barnes, H.; Dimitriadis, C.; Ellis, S.; Glass, D.C.; Gwini, S.M.; Hore-Lacy, F.; et al. Chest x-ray has low sensitivity to detect silicosis in artificial stone benchtop industry workers. Respirology 2024, 29, 785–794. [Google Scholar] [CrossRef]
- Savranlar, A.; Altin, R.; Mahmutyazicioglu, K.; Ozdemir, H.; Kart, L.; Ozer, T.; Gundogdu, S. Comparison of chest radiography and high-resolution computed tomography findings in early and low-grade coal worker’s pneumoconiosis. Eur. J. Radiol. 2004, 51, 175–180. [Google Scholar] [CrossRef]
- Li, T.; Yang, X.; Xu, H.; Liu, H. Early identification, accurate diagnosis, and treatment of silicosis. Can. Respir. J. 2022, 2022, 3769134. [Google Scholar] [CrossRef]
- Blanc, P.D.; Annesi-Maesano, I.; Balmes, J.R.; Cummings, K.J.; Fishwick, D.; Miedinger, D.; Murgia, N.; Naidoo, R.N.; Reynolds, C.J.; Sigsgaard, T.; et al. The occupational burden of nonmalignant respiratory diseases. An official American Thoracic Society and European Respiratory Society statement. Am. J. Respir. Crit. Care Med. 2019, 199, 1312–1334. [Google Scholar] [CrossRef]
- Xie, M.; Liu, X.; Cao, X.; Guo, M.; Li, X. Trends in prevalence and incidence of chronic respiratory diseases from 1990 to 2017. Respir. Res. 2020, 21, 49. [Google Scholar] [CrossRef]
- Saiphoklang, N.; Ruchiwit, P.; Kanitsap, A.; Tantiyavarong, P.; Vatcharavongvan, P.; Palungrit, S.; Leelasittikul, K.; Pugongchai, A.; Poachanukoon, O. Prevalence of chronic obstructive pulmonary disease and asthma in the community of Pathumthani, Thailand. Diseases 2025, 13, 130. [Google Scholar] [CrossRef] [PubMed]
- Louisias, M.; Ramadan, A.; Naja, A.S.; Phipatanakul, W. The effects of the environment on asthma disease activity. Immunol. Allergy Clin. N. Am. 2019, 39, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Etzel, R.A. How environmental exposures influence the development and exacerbation of asthma. Pediatrics 2003, 112, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Kitjakrancharoensin, P.; Yasan, K.; Hongyantarachai, K.; Ratanachokthorani, K.; Thammasarn, J.; Kuwuttiwai, D.; Ekanaprach, T.; Jittakarm, R.; Nuntapravechpun, R.; Hotarapavanon, S.; et al. Prevalence and risk factors of chronic obstructive pulmonary disease among agriculturists in a rural community, central Thailand. Int. J. Chron. Obs. Pulmon Dis. 2020, 15, 2189–2198. [Google Scholar] [CrossRef]
- Pothirat, C.; Chaiwong, W.; Phetsuk, N.; Pisalthanapuna, S.; Chetsadaphan, N.; Inchai, J. A comparative study of COPD burden between urban vs rural communities in northern Thailand. Int. J. Chron. Obs. Pulmon Dis. 2015, 10, 1035–1042. [Google Scholar] [CrossRef]
- Aungkasuvapala, N.; Juengprasert, W.; Obhasi, N. Silicosis and pulmonary tuberculosis in stone-grinding factories in Saraburi, Thailand. J. Med. Assoc. Thail. 1995, 78, 662–669. [Google Scholar]
- Xin, L.; An, T.M.; Ying, L.; Rong, D.W.; Lei, H. Prevalence and risk factors for obstructive pulmonary dysfunction caused by silica dust exposure: A multicenter cross-sectional study. BMC Pulm. Med. 2024, 24, 297. [Google Scholar] [CrossRef]
| Characteristics | Total (n = 290) | Normal Lung Function (n = 191) | Abnormal Lung Function (n = 99) | p-Value |
|---|---|---|---|---|
| Age, years | 47.6 ± 16.4 | 45.9 ± 16.6 | 50.9 ± 15.6 | 0.013 |
| Female | 162 (55.9) | 111 (58.1) | 51 (51.5) | 0.283 |
| Male | 128 (44.1) | 80 (41.9) | 48 (48.5) | 0.283 |
| Body mass index, kg/m2 | 25.2 ± 5.2 | 25.2 ± 4.7 | 25.2 ± 6.2 | 0.957 |
| Smoking | 86 (29.7) | 34 (34.3) | 52 (27.2) | 0.208 |
| Amount of smoking, pack-years | 9.7 ± 14.0 | 8.4 ± 13.6 | 11.5 ± 14.5 | 0.329 |
| Pre-existing comorbidities | ||||
| Hypertension | 75 (25.9) | 47 (24.6) | 28 (28.3) | 0.498 |
| Hyperlipidemia | 51 (17.6) | 32 (16.8) | 19 (19.2) | 0.605 |
| Diabetes | 24 (8.3) | 13 (6.8) | 11 (11.1) | 0.207 |
| Coronary heart disease | 7 (2.4) | 2 (1.0) | 5 (5.1) | 0.048 |
| Cerebrovascular disease | 2 (0.7) | 1 (0.5) | 1 (1.0) | 1.000 |
| Obesity | 4 (1.4) | 1 (0.5) | 3 (3.0) | 0.117 |
| Allergic rhinitis | 27 (9.3) | 19 (9.9) | 8 (8.1) | 0.604 |
| Asthma | 9 (3.1) | 1 (0.5) | 8 (8.1) | 0.001 |
| COPD | 0 (0) | 0 (0) | 0 (0) | NA |
| Respiratory symptoms | ||||
| Presence of symptom | 121 (41.7) | 71 (37.2) | 50 (50.5) | 0.029 |
| Cough | 64 (22.1) | 36 (18.8) | 28 (28.3) | 0.066 |
| Breathlessness | 40 (13.8) | 20 (10.5) | 20 (20.2) | 0.023 |
| Sputum production | 59 (20.3) | 34 (17.8) | 25 (25.3) | 0.135 |
| Wheezing | 9 (3.1) | 5 (2.6) | 4 (4.0) | 0.497 |
| Sore throat | 5 (1.7) | 3 (1.6) | 2 (2.0) | 1.000 |
| Chest tightness | 7 (2.4) | 4 (2.1) | 3 (3.0) | 0.694 |
| Nasal obstruction | 19 (6.6) | 13 (6.8) | 6 (6.1) | 0.808 |
| Runny nose | 21 (7.2) | 14 (7.3) | 7 (7.1) | 0.936 |
| Variable | Data (n = 290) |
|---|---|
| Spirometry data | |
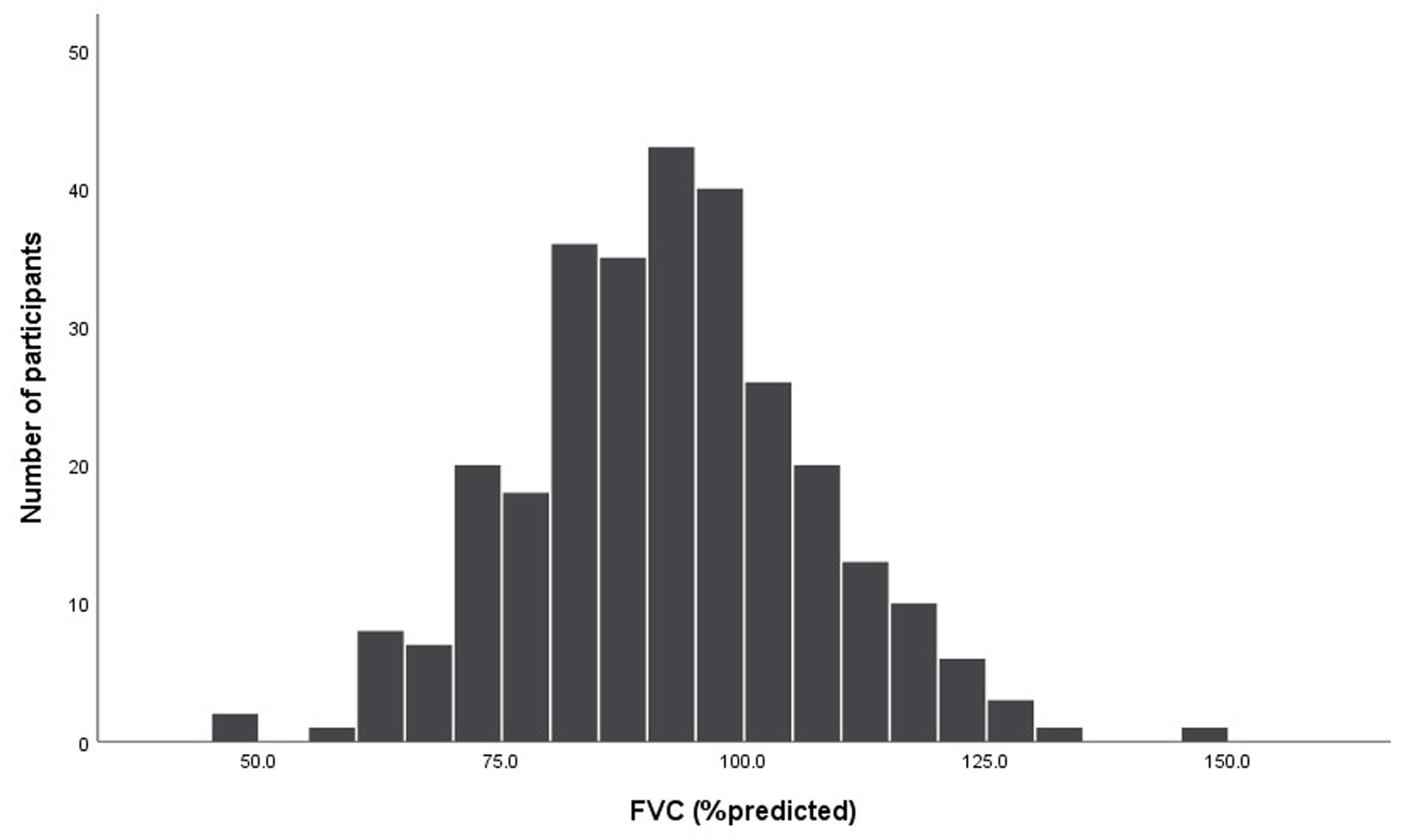
| FVC, L | 2.94 ± 0.87 |
| FVC, %predicted | 92.2 ± 14.9 |
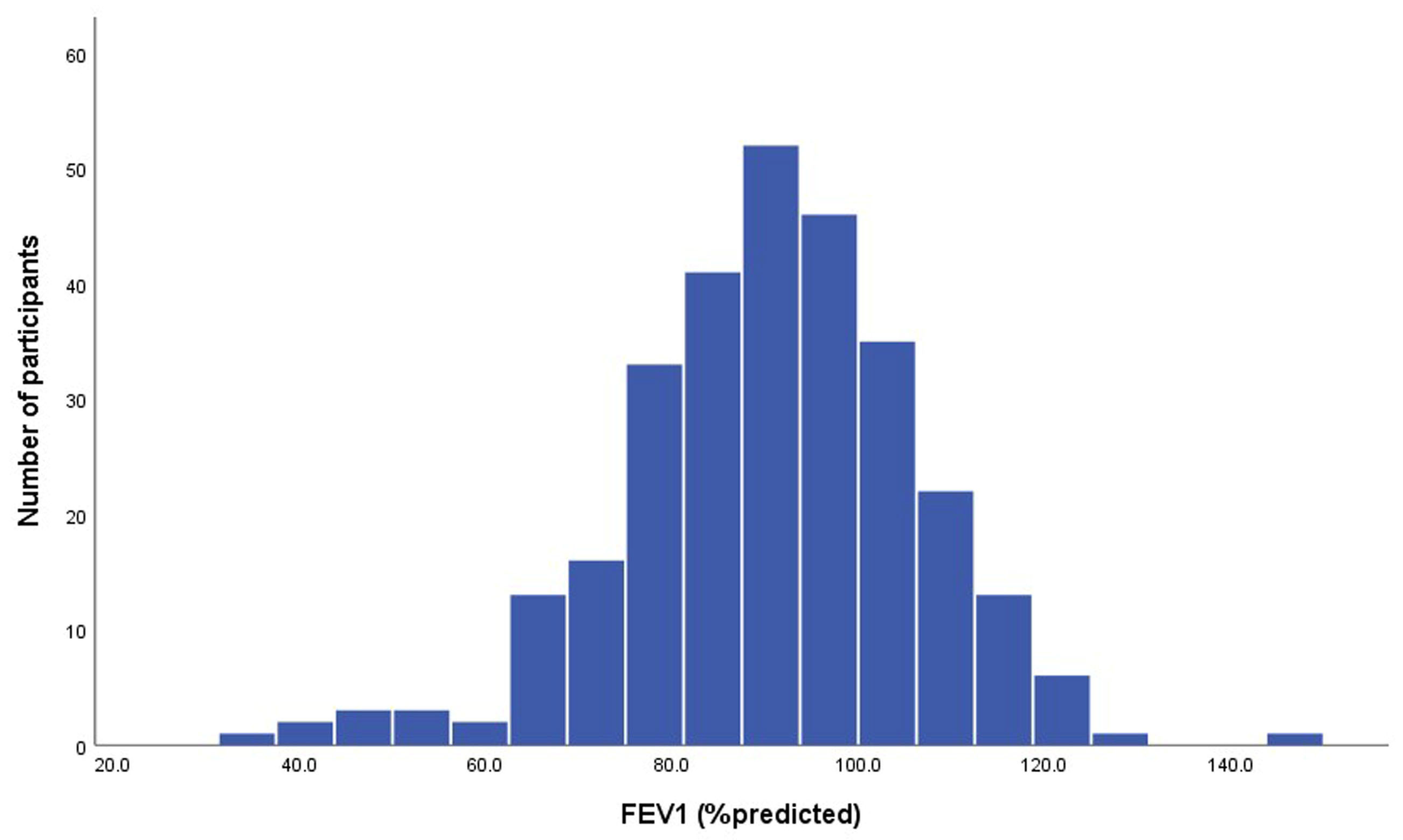
| FEV1, L | 2.41 ± 0.77 |
| FEV1, % predicted | 90.4 ± 15.9 |
| FEV1 change after BD test, % | 3.22 ± 4.61 |
| FVC change after BD test, % | 1.02 ± 5.94 |
| FEV1/FVC, % | 81.99 ± 8.40 |
| FEV1/FVC, % predicted | 97.94 ± 8.77 |
| PEF, L/s | 6.53 ± 1.97 |
| PEF, % predicted | 92.65 ± 19.40 |
| FEF25–75, L/s | 2.49 ± 1.18 |
| FEF25–75, %predicted | 85.75 ± 31.39 |
| Diagnosis of airway disease | |
| Asthma | 13 (4.5) |
| COPD | 30 (10.3) |
| Characteristics | Data (n = 290) |
|---|---|
| Abnormal pulmonary function | 99 (34.1) |
| Restrictive defect | 48 (16.6) |
| Airway obstruction | 26 (9.0) |
| Mixed obstructive and restrictive defect | 8 (2.8) |
| Small-airway disease | 17 (5.9) |
| Bronchodilator response | 14 (4.8) |
| Abnormal chest radiographic findings | 24 (8.3) |
| Lung fibrosis | 3 (2.8) |
| Pleural fibrosis | 6 (2.1) |
| Lung nodule | 6 (2.1) |
| Mixed pattern | 8 (2.8) |
| Silicotic pattern | 1 (0.3) |
| Variables | Adjusted Odds Ratio (95%CI) | p-Value |
|---|---|---|
| Age for every 1-year increase | 1.017 (1.001–1.033) | 0.042 |
| Smoking | 1.002 (0.687–1.213) | 0.657 |
| Coronary heart disease | 1.005 (0.564–1.126) | 0.458 |
| Pre-existing asthma | 1.012 (0.987–1.135) | 0.105 |
| Presence of respiratory symptoms | 0.798 (0.448–1.422) | 0.244 |
| Breathlessness | 0.574 (0.265–1.243) | 0.159 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saiphoklang, N.; Ruchiwit, P.; Kanitsap, A.; Tantiyavarong, P.; Vatcharavongvan, P.; Palungrit, S.; Leelasittikul, K.; Pugongchai, A.; Poachanukoon, O. Silicosis and Pulmonary Functions Among Residents Exposed to Dust in Saraburi Thailand. Diseases 2025, 13, 372. https://doi.org/10.3390/diseases13110372
Saiphoklang N, Ruchiwit P, Kanitsap A, Tantiyavarong P, Vatcharavongvan P, Palungrit S, Leelasittikul K, Pugongchai A, Poachanukoon O. Silicosis and Pulmonary Functions Among Residents Exposed to Dust in Saraburi Thailand. Diseases. 2025; 13(11):372. https://doi.org/10.3390/diseases13110372
Chicago/Turabian StyleSaiphoklang, Narongkorn, Pitchayapa Ruchiwit, Apichart Kanitsap, Pichaya Tantiyavarong, Pasitpon Vatcharavongvan, Srimuang Palungrit, Kanyada Leelasittikul, Apiwat Pugongchai, and Orapan Poachanukoon. 2025. "Silicosis and Pulmonary Functions Among Residents Exposed to Dust in Saraburi Thailand" Diseases 13, no. 11: 372. https://doi.org/10.3390/diseases13110372
APA StyleSaiphoklang, N., Ruchiwit, P., Kanitsap, A., Tantiyavarong, P., Vatcharavongvan, P., Palungrit, S., Leelasittikul, K., Pugongchai, A., & Poachanukoon, O. (2025). Silicosis and Pulmonary Functions Among Residents Exposed to Dust in Saraburi Thailand. Diseases, 13(11), 372. https://doi.org/10.3390/diseases13110372







