Type 3 Diabetes: Linking Insulin Resistance to Cognitive Decline
Abstract
1. Introduction
2. Shared Pathophysiology of Alzheimer’s Disease and Type 2 Diabetes
2.1. Major Hallmarks of Alzheimer’s Disease
2.2. Role of Glutamate and GABA
2.3. Role of Acetylcholine
2.4. Role of Mitochondria
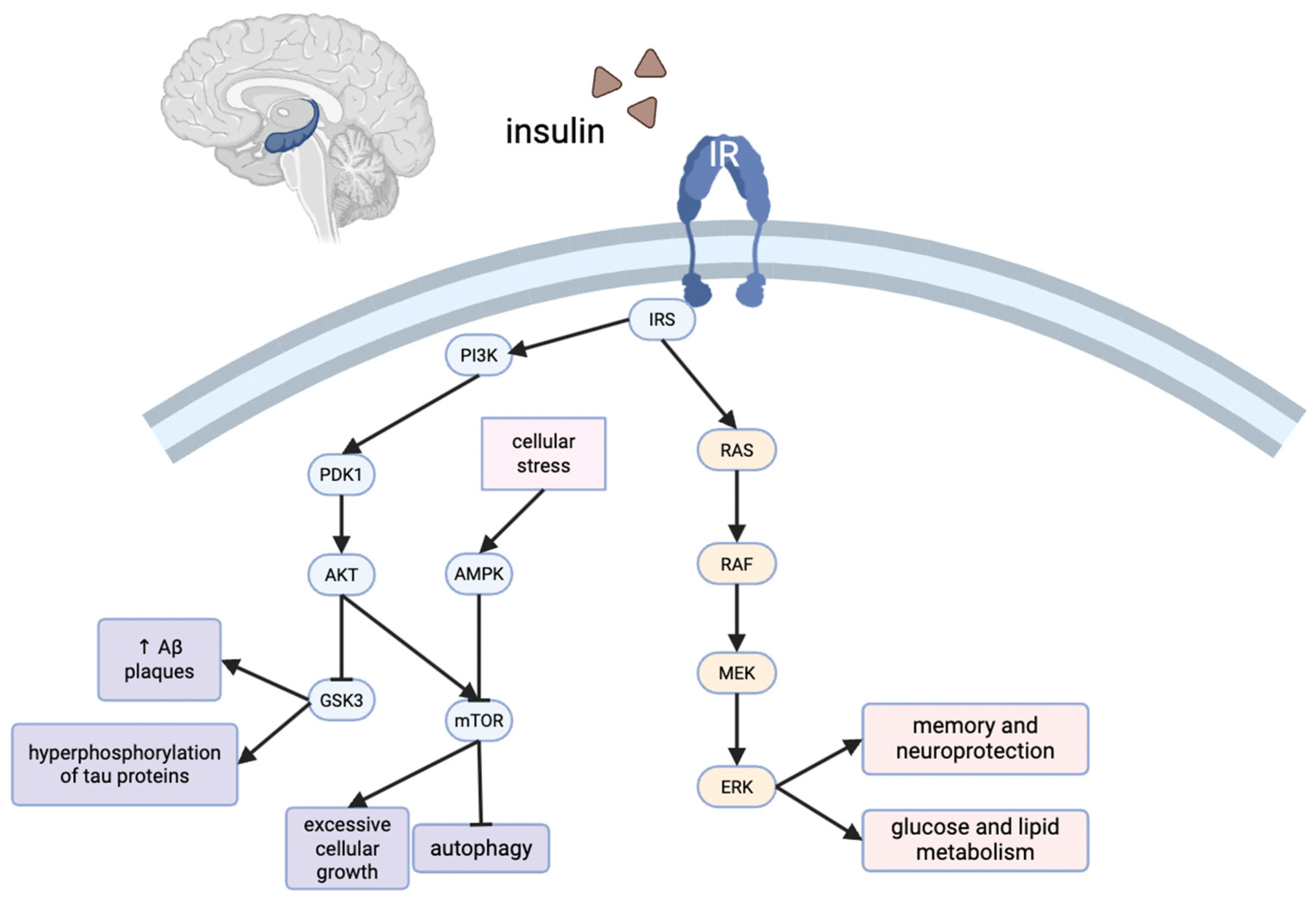
2.5. Relationship to Insulin Signaling
3. Insulin Resistance in the Brain
3.1. Central Insulin Resistance as the Defining Feature of T3D
3.2. Neuroimaging and Molecular Evidence
3.3. Peripheral–Central Interactions and the Pathogenic Feedback Loop
3.4. Mechanistic Contributions to Amyloid and Tau Pathology
3.5. Evidence from Preclinical Models
3.6. Clinical Trials and Translational Opportunities
3.7. Future Directions
4. The Cardiovascular Link
5. Hormonal Connection
5.1. Estrogens
5.2. Cortisol
5.3. Leptin
6. The Gut–Liver–Brain Connection
Major Hallmarks of Alzheimer’s Disease
7. Clinical and Behavioral Interventions
7.1. Exercise
7.2. Diet
7.3. Sleep
8. Future Research
8.1. Cellular Mechanisms
8.2. Medical Interventions
9. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| T3D | Type 3 diabetes |
| T2D | Type 2 diabetes |
| AD | Alzheimer’s disease |
| Aβ | beta amyloid Aβ |
| GABA | γ-aminobutyric acid |
| NMDA | N-methyl-D-aspartate |
| AMPA | α-amino-5-methyl-3-hydroxy-4-isoxazole propionic acid |
| Akt | protein kinase B |
| mTOR | mammalian target of rapamycin |
| IL- | Interleukin |
| TNF-α | Tumor necrosis factor alpha |
| nSMase2 | Neutral sphingomyelinase-2 |
| TLR-4 | Toll-like receptor 4 |
| Myd88 | Myeloid differentiation primary response 88 |
| IKK | (IκB) kinase |
| NLRP3 | NOD-like receptor protein 3 |
| ASC | Apoptosis-associated speck-like protein |
| GSK-3 | Glycogen synthase kinase 3 |
| IDE | Insulin-degrading enzyme |
| PP2 | Protein phosphatase 2 |
| PKC | Protein kinase C |
| MAMs | Mitochondria-associated membranes |
| AMPK | AMP-activated protein kinase |
| INSR | Insulin receptor |
| PI3K | Phosphoinositide 3 kinase |
| ERK | Extracellular signal-regulated kinases |
| MAPK | Mitogen-activated protein kinase |
| mTORC1 | Mechanistic target of rapamycin complex |
| IGF | Insulin-like growth factor |
| LDL | Low-density lipoprotein |
| ACTH | adrenocorticotropic hormone |
| MR | Mineralocorticoid receptor |
| GR | Glucocorticoid receptors |
| JAK | Janus kinase |
| STAT3 | Signal transducer and activator of transcription |
| SOC3 | Suppressor of cytokine signaling |
| GLP-1 | Glucagon-like peptide-1 |
| GIP | Gastric inhibitory polypeptide |
| SCFAs | Short-chain fatty acids |
| NF-κB | Nuclear factor kappa B |
| Nrf2/HO-1 | Nuclear factor erythroid 2-related factor 2/Heme oxygenase-1 |
| CREB | cAMP response element binding protein |
| PSD | Postsynaptic density protein |
| ROS | Reactive oxygen species |
References
- Corrada, M.M.; Brookmeyer, R.; Paganini-Hill, A.; Berlau, D.; Kawas, C.H. Dementia Incidence Continues to Increase with Age in the Oldest Old: The 90+ Study. Ann. Neurol. 2010, 67, 114–121. [Google Scholar] [CrossRef] [PubMed]
- de la Monte, S.M. Insulin Resistance and Neurodegeneration: Progress Towards the Development of New Therapeutics for Alzheimer’s Disease. Drugs 2017, 77, 47–65. [Google Scholar] [CrossRef]
- Michailidis, M.; Moraitou, D.; Tata, D.A.; Kalinderi, K.; Papamitsou, T.; Papaliagkas, V. Alzheimer’s Disease as Type 3 Diabetes: Common Pathophysiological Mechanisms between Alzheimer’s Disease and Type 2 Diabetes. Int. J. Mol. Sci. 2022, 23, 2687. [Google Scholar] [CrossRef]
- Masters, M.C.; Morris, J.C.; Roe, C.M. “Noncognitive” Symptoms of Early Alzheimer Disease: A Longitudinal Analysis. Neurology 2015, 84, 617–622. [Google Scholar] [CrossRef]
- Ikezaki, H.; Hashimoto, M.; Ishikawa, T.; Fukuhara, R.; Tanaka, H.; Yuki, S.; Kuribayashi, K.; Hotta, M.; Koyama, A.; Ikeda, M.; et al. Relationship between Executive Dysfunction and Neuropsychiatric Symptoms and Impaired Instrumental Activities of Daily Living among Patients with Very Mild Alzheimer’s Disease. Int. J. Geriatr. Psychiatry 2020, 35, 877–887. [Google Scholar] [CrossRef]
- Jack, C.R.; Andrews, J.S.; Beach, T.G.; Buracchio, T.; Dunn, B.; Graf, A.; Hansson, O.; Ho, C.; Jagust, W.; McDade, E.; et al. Revised Criteria for Diagnosis and Staging of Alzheimer’s Disease: Alzheimer’s Association Workgroup. Alzheimers Dement. 2024, 20, 5143–5169. [Google Scholar] [CrossRef]
- Hammoud, H.; Netsyk, O.; Tafreshiha, A.S.; Korol, S.V.; Jin, Z.; Li, J.-P.; Birnir, B. Insulin Differentially Modulates GABA Signalling in Hippocampal Neurons and, in an Age-dependent Manner, Normalizes GABA-activated Currents in the tg-APPSwe Mouse Model of Alzheimer’s Disease. Acta Physiol. 2021, 232, e13623. [Google Scholar] [CrossRef]
- Jones, M.L.; Liao, G.-Y.; Malecki, R.; Li, M.; Salazar, N.M.; Leonard, J.P. PI 3-Kinase and PKCζ Mediate Insulin-Induced Potentiation of NMDA Receptor Currents in Xenopus Oocytes. Brain Res. 2012, 1432, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Wang, P. Role of Insulin Receptor Substance-1 Modulating PI3K/Akt Insulin Signaling Pathway in Alzheimer’s Disease. 3 Biotech 2021, 11, 179. [Google Scholar] [CrossRef]
- Bowman, G.L.; Dayon, L.; Kirkland, R.; Wojcik, J.; Peyratout, G.; Severin, I.C.; Henry, H.; Oikonomidi, A.; Migliavacca, E.; Bacher, M.; et al. Blood-Brain Barrier Breakdown, Neuroinflammation, and Cognitive Decline in Older Adults. Alzheimers Dement. 2018, 14, 1640–1650. [Google Scholar] [CrossRef]
- Aqeel, A.; Akram, A.; Ali, M.; Iqbal, M.; Aslam, M.; Rukhma; Shah, F.I. Mechanistic Insights into Impaired β-Oxidation and Its Role in Mitochondrial Dysfunction: A Comprehensive Review. Diabetes Res. Clin. Pract. 2025, 223, 112129. [Google Scholar] [CrossRef] [PubMed]
- Wicks, S.E.; Vandanmagsar, B.; Haynie, K.R.; Fuller, S.E.; Warfel, J.D.; Stephens, J.M.; Wang, M.; Han, X.; Zhang, J.; Noland, R.C.; et al. Impaired Mitochondrial Fat Oxidation Induces Adaptive Remodeling of Muscle Metabolism. Proc. Natl. Acad. Sci. USA 2015, 112, E3300–E3309. [Google Scholar] [CrossRef] [PubMed]
- Lair, B.; Laurens, C.; Bosch, B.V.D.; Moro, C. Novel Insights and Mechanisms of Lipotoxicity-Driven Insulin Resistance. Int. J. Mol. Sci. 2020, 21, 6358. [Google Scholar] [CrossRef]
- Salem, M.A.; Budzyńska, B.; Kowalczyk, J.; Sayed, N.S.E.; Mansour, S.M. Tadalafil and Bergapten Mitigate Streptozotocin-Induced Sporadic Alzheimer’s Disease in Mice via Modulating Neuroinflammation, PI3K/Akt, Wnt/β-Catenin, AMPK/mTOR Signaling Pathways. Toxicol. Appl. Pharmacol. 2021, 429, 115697. [Google Scholar] [CrossRef]
- Martinez, E.C.; Saji, S.Z.; Ore, J.V.S.; Borges-Sosa, O.A.; Srinivas, S.; Mareddy, N.S.R.; Manzoor, T.; Vanna, M.D.; Shanableh, Y.A.; Taneja, R.; et al. The Effects of Omega-3, DHA, EPA, Souvenaid® in Alzheimer’s Disease: A Systematic Review and Meta-analysis. Neuropsychopharmacol. Rep. 2024, 44, 545–556. [Google Scholar] [CrossRef]
- Kullmann, S.; Goj, T.; Veit, R.; Fritsche, L.; Wagner, L.; Schneeweiss, P.; Hoene, M.; Hoffmann, C.; Machann, J.; Niess, A.; et al. Exercise Restores Brain Insulin Sensitivity in Sedentary Adults Who Are Overweight and Obese. JCI Insight 2022, 7, e161498. [Google Scholar] [CrossRef]
- Kocahan, S.; Doğan, Z. Mechanisms of Alzheimer’s Disease Pathogenesis and Prevention: The Brain, Neural Pathology, N-Methyl-D-Aspartate Receptors, Tau Protein and Other Risk Factors. Clin. Psychopharmacol. Neurosci. 2017, 15, 1–8. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Wang, J.; Xia, Y.; Zhang, J.; Chen, L. Recent Advances in Alzheimer’s Disease: Mechanisms, Clinical Trials and New Drug Development Strategies. Signal. Transduct. Target. Ther. 2024, 9, 211–235. [Google Scholar] [CrossRef]
- Rodríguez-Martín, T.; Cuchillo-Ibáñez, I.; Noble, W.; Nyenya, F.; Anderton, B.H.; Hanger, D.P. Tau Phosphorylation Affects Its Axonal Transport and Degradation. Neurobiol. Aging 2013, 34, 2146–2157. [Google Scholar] [CrossRef]
- Citri, A.; Malenka, R.C. Synaptic Plasticity: Multiple Forms, Functions, and Mechanisms. Neuropsychopharmacology 2008, 33, 18–41. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, N.; Giovenzana, M.; Misztak, P.; Mingardi, J.; Musazzi, L. Glutamate-Mediated Excitotoxicity in the Pathogenesis and Treatment of Neurodevelopmental and Adult Mental Disorders. Int. J. Mol. Sci. 2024, 25, 6521. [Google Scholar] [CrossRef]
- Palazzo, E.; Marabese, I.; Ricciardi, F.; Guida, F.; Luongo, L.; Maione, S. The Influence of Glutamate Receptors on Insulin Release and Diabetic Neuropathy. Pharmacol. Ther. 2024, 263, 108724. [Google Scholar] [CrossRef] [PubMed]
- Dingledine, R.; Borges, K.; Bowie, D.; Traynelis, S.F. The Glutamate Receptor Ion Channels. Pharmacol. Rev. 1999, 51, 7–61. [Google Scholar] [CrossRef]
- Reiner, A.; Levitz, J. Glutamatergic Signaling in the Central Nervous System: Ionotropic and Metabotropic Receptors in Concert. Neuron 2018, 98, 1080–1098. [Google Scholar] [CrossRef]
- Wu, W.; Gong, X.; Qin, Z.; Wang, Y. Molecular Mechanisms of Excitotoxicity and Their Relevance to the Pathogenesis of Neurodegenerative Diseases—An Update. Acta Pharmacol. Sin. 2025. [Google Scholar] [CrossRef]
- Zhao, W.-Q.; De Felice, F.G.; Fernandez, S.; Chen, H.; Lambert, M.P.; Quon, M.J.; Krafft, G.A.; Klein, W.L. Amyloid Beta Oligomers Induce Impairment of Neuronal Insulin Receptors. FASEB J. 2008, 22, 246–260. [Google Scholar] [CrossRef]
- Pomytkin, I.; Krasil’nikova, I.; Bakaeva, Z.; Surin, A.; Pinelis, V. Excitotoxic Glutamate Causes Neuronal Insulin Resistance by Inhibiting Insulin Receptor/Akt/mTOR Pathway. Mol. Brain 2019, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Rezazadeh, H.; Sharifi, M.R.; Soltani, N. Insulin Resistance and the Role of Gamma-Aminobutyric Acid. J. Res. Med. Sci. 2021, 26, 39. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Sharma, S. GABA Receptor. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Sears, S.M.; Hewett, S.J. Influence of Glutamate and GABA Transport on Brain Excitatory/Inhibitory Balance. Exp. Biol. Med. 2021, 246, 1069–1083. [Google Scholar] [CrossRef]
- Rezazadeh, H.; Sharifi, M.R.; Sharifi, M.; Soltani, N. Gamma-Aminobutyric Acid Attenuates Insulin Resistance in Type 2 Diabetic Patients and Reduces the Risk of Insulin Resistance in Their Offspring. Biomed. Pharmacother. 2021, 138, 111440. [Google Scholar] [CrossRef]
- Sohrabipour, S.; Sharifi, M.R.; Talebi, A.; Sharifi, M.; Soltani, N. GABA Dramatically Improves Glucose Tolerance in Streptozotocin-Induced Diabetic Rats Fed with High-Fat Diet. Eur. J. Pharmacol. 2018, 826, 75–84. [Google Scholar] [CrossRef]
- Thielen, J.; Gancheva, S.; Hong, D.; Rohani Rankouhi, S.; Chen, B.; Apostolopoulou, M.; Anadol-Schmitz, E.; Roden, M.; Norris, D.G.; Tendolkar, I. Higher GABA Concentration in the Medial Prefrontal Cortex of Type 2 Diabetes Patients Is Associated with Episodic Memory Dysfunction. Hum. Brain Mapp. 2019, 40, 4287–4295. [Google Scholar] [CrossRef]
- van Bussel, F.C.G.; Backes, W.H.; Hofman, P.A.M.; Puts, N.A.J.; Edden, R.A.E.; van Boxtel, M.P.J.; Schram, M.T.; Stehouwer, C.D.A.; Wildberger, J.E.; Jansen, J.F.A. Increased GABA Concentrations in Type 2 Diabetes Mellitus Are Related to Lower Cognitive Functioning. Medicine 2016, 95, e4803. [Google Scholar] [CrossRef] [PubMed]
- Taneera, J.; Jin, Z.; Jin, Y.; Muhammed, S.J.; Zhang, E.; Lang, S.; Salehi, A.; Korsgren, O.; Renström, E.; Groop, L.; et al. γ-Aminobutyric Acid (GABA) Signalling in Human Pancreatic Islets Is Altered in Type 2 Diabetes. Diabetologia 2012, 55, 1985–1994. [Google Scholar] [CrossRef]
- d’Almeida, O.C.; Violante, I.R.; Quendera, B.; Moreno, C.; Gomes, L.; Castelo-Branco, M. The Neurometabolic Profiles of GABA and Glutamate as Revealed by Proton Magnetic Resonance Spectroscopy in Type 1 and Type 2 Diabetes. PLoS ONE 2020, 15, e0240907. [Google Scholar] [CrossRef]
- Hogg, R.C.; Raggenbass, M.; Bertrand, D. Nicotinic Acetylcholine Receptors: From Structure to Brain Function. In Reviews of Physiology, Biochemistry and Pharmacology; Springer: Berlin/Heidelberg, Germany, 2003; pp. 1–46. ISBN 978-3-540-36622-5. [Google Scholar]
- Xu, Y.; Cao, K.; Guo, B.; Xiang, J.; Dong, Y.-T.; Qi, X.-L.; Yu, W.-F.; Xiao, Y.; Guan, Z.-Z. Lowered Levels of Nicotinic Acetylcholine Receptors and Elevated Apoptosis in the Hippocampus of Brains from Patients with Type 2 Diabetes Mellitus and Db/Db Mice. Aging 2020, 12, 14205–14218. [Google Scholar] [CrossRef]
- Umegaki, H. Insulin Resistance in the Brain: A New Therapeutic Target for Alzheimer’s Disease. J. Diabetes Investig. 2013, 4, 150–151. [Google Scholar] [CrossRef] [PubMed]
- Sripetchwandee, J.; Chattipakorn, N.; Chattipakorn, S.C. Links Between Obesity-Induced Brain Insulin Resistance, Brain Mitochondrial Dysfunction, and Dementia. Front. Endocrinol. 2018, 9, 496. [Google Scholar] [CrossRef]
- Kim, J.; Wei, Y.; Sowers, J.R. Role of Mitochondrial Dysfunction in Insulin Resistance. Circ. Res. 2008, 102, 401–414. [Google Scholar] [CrossRef]
- Potenza, M.A.; Sgarra, L.; Desantis, V.; Nacci, C.; Montagnani, M. Diabetes and Alzheimer’s Disease: Might Mitochondrial Dysfunction Help Deciphering the Common Path? Antioxidants 2021, 10, 1257. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.G.; Ferreira, S.T. Inflammation, Defective Insulin Signaling, and Mitochondrial Dysfunction as Common Molecular Denominators Connecting Type 2 Diabetes to Alzheimer Disease. Diabetes 2014, 63, 2262–2272. [Google Scholar] [CrossRef]
- Lutz, T.A.; Meyer, U. Amylin at the Interface between Metabolic and Neurodegenerative Disorders. Front. Neurosci. 2015, 9, 216. [Google Scholar] [CrossRef]
- Sheppard, O.; Coleman, M. Alzheimer’s Disease: Etiology, Neuropathology and Pathogenesis. In Alzheimer’s Disease: Drug Discovery; Exon Publications: Brisbane, Australia, 2020. [Google Scholar]
- Ly, H.; Despa, F. Diabetes-Related Amylin Dyshomeostasis: A Contributing Factor to Cerebrovascular Pathology and Dementia. J. Lipid Atheroscler. 2019, 8, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, M.; Bourassa, P.; Vandal, M.; Caron, V.; Virgili, J.; Tremblay, C.; Emond, V.; Bennett, D.A.; Calon, F. Interactions between Insulin, the Blood-brain Barrier, and Beta-amyloid in Alzheimer’s Disease. Alzheimers Dement. 2020, 16, e039510. [Google Scholar] [CrossRef]
- Xie, L.; Helmerhorst, E.; Taddei, K.; Plewright, B.; van Bronswijk, W.; Martins, R. Alzheimer’s Beta -Amyloid Peptides Compete for Insulin Binding to the Insulin Receptor. J. Neurosci. 2002, 22, RC221. [Google Scholar] [CrossRef]
- Sun, X.J.; Crimmins, D.L.; Myers, M.G.; Miralpeix, M.; White, M.F. Pleiotropic Insulin Signals Are Engaged by Multisite Phosphorylation of IRS-1. Mol. Cell. Biol. 1993, 13, 7418–7428. [Google Scholar] [CrossRef]
- Boura-Halfon, S.; Zick, Y. Phosphorylation of IRS Proteins, Insulin Action, and Insulin Resistance. Am. J. Physiol. Endocrinol. Metab. 2009, 296, 581. [Google Scholar] [CrossRef]
- Sánchez-Alegría, K.; Flores-León, M.; Avila-Muñoz, E.; Rodríguez-Corona, N.; Arias, C. PI3K Signaling in Neurons: A Central Node for the Control of Multiple Functions. Int. J. Mol. Sci. 2018, 19, 3725. [Google Scholar] [CrossRef]
- Metz, M.; O’Hare, J.; Cheng, B.; Puchowicz, M.; Buettner, C.; Scherer, T. Brain Insulin Signaling Suppresses Lipolysis in the Absence of Peripheral Insulin Receptors and Requires the MAPK Pathway. Mol. Metab. 2023, 73, 101723. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Lee, H.; Raina, A.K.; Perry, G.; Smith, M.A. The Role of Mitogen-Activated Protein Kinase Pathways in Alzheimer’s Disease. Neurosignals 2002, 11, 270–281. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Y.; Gharavi, R.; Park, H.R.; Lee, J.; Siddiqui, S.; Telljohann, R.; Nassar, M.R.; Cutler, R.G.; Becker, K.G.; et al. The Mitochondrial Uncoupler DNP Triggers Brain Cell mTOR Signaling Network Reprogramming and CREB Pathway Up-regulation. J. Neurochem. 2015, 134, 677–692. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Carling, D.; Prentki, M.; Cacicedo, J.M. AMPK, Insulin Resistance, and the Metabolic Syndrome. J. Clin. Investig. 2013, 123, 2764–2772. [Google Scholar] [CrossRef]
- Ronnett, G.V.; Ramamurthy, S.; Kleman, A.M.; Landree, L.E.; Aja, S. AMPK in the Brain: Its Roles in Energy Balance and Neuroprotection. J. Neurochem. 2009, 109, 17–23. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Ta, Q.T.H.; Nguyen, T.K.O.; Nguyen, T.T.D.; Giau, V.V. Type 3 Diabetes and Its Role Implications in Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 3165. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.J.O.; de Oliveira, L.M.; Bittencourt, A.M.V.; Lourenço, L.G.C.; de Oliveira, G.C.M. Brain Insulin Resistance and Alzheimer’s Disease: A Systematic Review. Dement. Neuropsychol. 2024, 18, e20230032. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.E.; Arvanitakis, Z.; Macauley-Rambach, S.L.; Koenig, A.M.; Wang, H.-Y.; Ahima, R.S.; Craft, S.; Gandy, S.; Buettner, C.; Stoeckel, L.E.; et al. Brain Insulin Resistance in Type 2 Diabetes and Alzheimer Disease: Concepts and Conundrums. Nat. Rev. Neurol. 2018, 14, 168–181. [Google Scholar] [CrossRef]
- Hong, S.; Baek, S.-H.; Lai, M.K.P.; Arumugam, T.V.; Jo, D.-G. Aging-Associated Sensory Decline and Alzheimer’s Disease. Mol. Neurodegener. 2024, 19, 93. [Google Scholar] [CrossRef]
- Milstein, J.L.; Ferris, H.A. The Brain as an Insulin-Sensitive Metabolic Organ The Brain as an Insulin-Sensitive Metabolic Organ. Mol. Metab. 2021, 52, 101234. [Google Scholar] [CrossRef]
- Widden, H.; Placzek, W.J. The Multiple Mechanisms of MCL1 in the Regulation of Cell Fate. Commun. Biol. 2021, 4, 1029. [Google Scholar] [CrossRef]
- Linseman, D.A.; Butts, B.D.; Precht, T.A.; Phelps, R.A.; Le, S.S.; Laessig, T.A.; Bouchard, R.J.; Florez-McClure, M.L.; Heidenreich, K.A. Glycogen Synthase Kinase-3beta Phosphorylates Bax and Promotes Its Mitochondrial Localization during Neuronal Apoptosis. J. Neurosci. 2004, 24, 9993–10002. [Google Scholar] [CrossRef]
- Jacobs, K.M.; Bhave, S.R.; Ferraro, D.J.; Jaboin, J.J.; Hallahan, D.E.; Thotala, D. GSK-3β: A Bifunctional Role in Cell Death Pathways. Int. J. Cell Biol. 2012, 2012, 1163–1173. [Google Scholar] [CrossRef]
- Potz, B.A.; Scrimgeour, L.A.; Sabe, S.A.; Clements, R.T.; Sodha, N.R.; Sellke, F.W. Glycogen Synthase Kinase 3β Inhibition Reduces Mitochondrial Oxidative Stress in Chronic Myocardial Ischemia. J. Thorac. Cardiovasc. Surg. 2018, 155, 2492–2503. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial Dysfunction and Oxidative Stress in Metabolic Disorders—A Step towards Mitochondria Based Therapeutic Strategies. Biochim. Biophys. Acta 2017, 1863, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Berlanga-Acosta, J.; Guillén-Nieto, G.; Rodríguez-Rodríguez, N.; Bringas-Vega, M.L.; García-del-Barco-Herrera, D.; Berlanga-Saez, J.O.; García-Ojalvo, A.; Valdés-Sosa, M.J.; Valdés-Sosa, P.A. Insulin Resistance at the Crossroad of Alzheimer Disease Pathology: A Review. Front. Endocrinol. 2020, 11, 560375. [Google Scholar] [CrossRef] [PubMed]
- Kullmann, S.; Heni, M.; Fritsche, A.; Preissl, H. Insulin Action in the Human Brain: Evidence from Neuroimaging Studies. J. Neuroendocrinol. 2015, 27, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.R.; Sheng, L.Q.; Pan, P.L.; Wang, G.D.; Luo, R.; Shi, H.C.; Dai, Z.Y.; Zhong, J.G. Cerebral Glucose Metabolic Prediction from Amnestic Mild Cognitive Impairment to Alzheimer’s Dementia: A Meta-Analysis. Transl. Neurodegener. 2018, 7, 9–10. [Google Scholar] [CrossRef]
- Bedse, G.; Domenico, F.D.; Serviddio, G.; Cassano, T. Aberrant Insulin Signaling in Alzheimer’s Disease: Current Knowledge. Front. Neurosci. 2015, 9, 204. [Google Scholar] [CrossRef]
- Ochiai, T.; Sano, T.; Nagayama, T.; Kubota, N.; Kadowaki, T.; Wakabayashi, T.; Iwatsubo, T. Differential Involvement of Insulin Receptor Substrate (IRS)-1 and IRS-2 in Brain Insulin Signaling Is Associated with the Effects on Amyloid Pathology in a Mouse Model of Alzheimer’s Disease Differential Involvement of Insulin Receptor Substrate (IRS)-1 and IRS-2 in Brain Insulin Signaling Is Associated with the Effects on Amyloid Pathology in a Mouse Model of Alzheimer’s Disease. Neurobiol. Dis. 2021, 159, 105510. [Google Scholar]
- Gabbouj, S.; Ryhänen, S.; Marttinen, M.; Wittrahm, R.; Takalo, M.; Kemppainen, S.; Martiskainen, H.; Tanila, H.; Haapasalo, A.; Hiltunen, M.; et al. Altered Insulin Signaling in Alzheimer’s Disease Brain—Special Emphasis on PI3K-Akt Pathway. Front. Neurosci. 2019, 13, 629. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Rhea, E.M.; Leclerc, M.; Yassine, H.N.; Capuano, A.W.; Tong, H.; Petyuk, V.A.; Macauley, S.L.; Fioramonti, X.; Carmichael, O.; Calon, F.; et al. State of the Science on Brain Insulin Resistance and Cognitive Decline Due to Alzheimer’s Disease. Aging Dis. 2024, 15, 1688–1725. [Google Scholar] [CrossRef] [PubMed]
- Dutta, B.J.; Singh, S.; Seksaria, S.; Gupta, G.D.; Singh, A. Inside the Diabetic Brain: Insulin Resistance and Molecular Mechanism Associated with Cognitive Impairment and Its Possible Therapeutic Strategies. Pharmacol. Res. 2022, 182, 106358. [Google Scholar] [CrossRef]
- Liang, F.; Wang, H.; Chen, R.; Chen, L. Mechanisms Linking Inflammation to Insulin Resistance. Int. J. Endocrinol. 2015, 2015, 508409. [Google Scholar] [CrossRef]
- de la Monte, S.M. Triangulated Mal-Signaling in Alzheimer’s Disease: Roles of Neurotoxic Ceramides, ER Stress, and Insulin Resistance Reviewed. J. Alzheimers Dis. 2012, 30, S231–S249. [Google Scholar] [CrossRef]
- Gu, L.; Huang, B.; Shen, W.; Gao, L.; Ding, Z.; Wu, H.; Guo, J. Early Activation of nSMase2/Ceramide Pathway in Astrocytes Is Involved in Ischemia-Associated Neuronal Damage via Inflammation in Rat Hippocampi. J. Neuroinflamm. 2013, 10, 879. [Google Scholar] [CrossRef]
- Olona, A.; Hateley, C.; Muralidharan, S.; Wenk, M.R.; Torta, F.; Behmoaras, J. Sphingolipid Metabolism during Toll-like Receptor 4 (TLR4)-Mediated Macrophage Activation. Br. J. Pharmacol. 2021, 178, 4575–4587. [Google Scholar] [CrossRef] [PubMed]
- Cuschieri, J.; Bulger, E.; Billgrin, J.; Garcia, I.; Maier, R.V. Acid Sphingomyelinase Is Required for Lipid Raft TLR4 Complex Formation. Surg. Infect. 2007, 8, 91–106. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Signaling to NF-κB by Toll-like Receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in Biology and Targeted Therapy: New Insights and Translational Implications. Sig. Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Bauernfeind, F.; Horvath, G.; Stutz, A.; Alnemri, E.S.; MacDonald, K.; Speert, D.; Fernandes-Alnemri, T.; Wu, J.; Monks, B.G.; Fitzgerald, K.A.; et al. NF-kB Activating Pattern Recognition and Cytokine Receptors License NLRP3 Inflammasome Activation by Regulating NLRP3 Expression. J. Immunol. 2009, 183, 787–791. [Google Scholar] [CrossRef]
- Sakai, J.; Cammarota, E.; Wright, J.A.; Cicuta, P.; Gottschalk, R.A.; Li, N.; Fraser, I.D.C.; Bryant, C.E. Lipopolysaccharide-Induced NF-κB Nuclear Translocation Is Primarily Dependent on MyD88, but TNFα Expression Requires TRIF and MyD88. Sci. Rep. 2017, 7, 1428. [Google Scholar] [CrossRef]
- Diaz-Vegas, A.; Madsen, S.; Cooke, K.C.; Carroll, L.; Khor, J.X.; Turner, N.; Lim, X.Y.; Astore, M.A.; Morris, J.C.; Don, A.S.; et al. Mitochondrial Electron Transport Chain, Ceramide, and Coenzyme Q Are Linked in a Pathway That Drives Insulin Resistance in Skeletal Muscle. eLife 2023, 12, RP87340. [Google Scholar] [CrossRef]
- Valenzuela-Ahumada, L.A.; Mercado-Gómez, O.F.; Viveros-Contreras, R.; Guevara-Guzmán, R.; Camacho-Morales, A. Fasting the Mitochondria to Prevent Neurodegeneration: The Role of Ceramides. Front. Neurosci. 2025, 19, 1602149. [Google Scholar] [CrossRef]
- Chiarini, A.; Gui, L.; Viviani, C.; Armato, U.; Dal Prà, I. NLRP3 Inflammasome’s Activation in Acute and Chronic Brain Diseases—An Update on Pathogenetic Mechanisms and Therapeutic Perspectives with Respect to Other Inflammasomes. Biomedicines 2023, 11, 999. [Google Scholar] [CrossRef]
- Mata-Martínez, E.; Díaz-Muñoz, M.; Vázquez-Cuevas, F.G. Glial Cells and Brain Diseases: Inflammasomes as Relevant Pathological Entities. Front. Cell. Neurosci. 2022, 16, 929529. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Henry, R.J.; Stoica, B.A.; Loane, D.J.; Abulwerdi, G.; Bhat, S.A.; Faden, A.I. Neutral Sphingomyelinase Inhibition Alleviates LPS-Induced Microglia Activation and Neuroinflammation after Experimental Traumatic Brain Injury. J. Pharmacol. Exp. Ther. 2019, 368, 338–352. [Google Scholar] [CrossRef]
- Chakraborty, M.; Lou, C.; Huan, C.; Kuo, M.-S.; Park, T.-S.; Cao, G.; Jiang, X.-C. Myeloid Cell–Specific Serine Palmitoyltransferase Subunit 2 Haploinsufficiency Reduces Murine Atherosclerosis. J. Clin. Investig. 2013, 123, 1784–1797. [Google Scholar] [CrossRef]
- Eguchi, K.; Mikami, D.; Sun, H.; Tsumita, T.; Takahashi, K.; Mukai, K.; Yuyama, K.; Igarashi, Y. Blood-Brain Barrier Permeability Analysis of Plant Ceramides. PLoS ONE 2020, 15, e0241640. [Google Scholar] [CrossRef]
- García-Ruiz, C.; Colell, A.; Marí, M.; Morales, A.; Fernández-Checa, J.C. Direct Effect of Ceramide on the Mitochondrial Electron Transport Chain Leads to Generation of Reactive Oxygen Species: ROLE OF MITOCHONDRIAL GLUTATHIONE. J. Biol. Chem. 1997, 272, 11369–11377. [Google Scholar] [CrossRef]
- Cavaliere, G.; Cimmino, F.; Trinchese, G.; Catapano, A.; Petrella, L.; D’Angelo, M.; Lucchin, L.; Mollica, M.P. From Obesity-Induced Low-Grade Inflammation to Lipotoxicity and Mitochondrial Dysfunction: Altered Multi-Crosstalk between Adipose Tissue and Metabolically Active Organs. Antioxidants 2023, 12, 1172. [Google Scholar] [CrossRef]
- Jembrek, M.J.; Šimić, G.; Hof, P.R. Ceramides in Alzheimer’s Disease: Key Mediators of Neuronal Apoptosis Induced by Oxidative Stress and Aβ Accumulation. Oxid. Med. Cell. Longev. 2015, 2015, 346783. [Google Scholar] [CrossRef]
- Marini, F.; Kim, N.; Schuffert, A.; Wood, R.D. POLN, a Nuclear PolA Family DNA Polymerase Homologous to the DNA Cross-Link Sensitivity Protein Mus308. J. Biol. Chem. 2003, 278, 32014–32019. [Google Scholar] [CrossRef]
- Jaworski, T.; Gralec, K.; Banach-Kasper, E. GSK-3β at the Intersection of Neuronal Plasticity and Neurodegeneration. Neural Plast. 2019, 2019, 4209475. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Hao, S.; Wosiski-Kuhn, M.; Stranahan, A.M. Glucocorticoid-Mediated Activation of GSK3β Promotes Tau Phosphorylation and Impairs Memory in Type 2 Diabetes. Neurobiol. Aging 2017, 57, 75–83. [Google Scholar] [CrossRef]
- Tittelmeier, J.; Nussbaum-Krammer, C. Broken Balance: Emerging Cross-Talk Between Proteostasis and Lipostasis in Neurodegenerative Diseases. Cells 2025, 14, 845. [Google Scholar] [CrossRef]
- Chen, T.; Dai, Y.; Hu, C.; Lin, Z.; Wang, S.; Yang, J.; Zeng, L.; Li, S.; Li, W. Cellular and Molecular Mechanisms of the Blood–Brain Barrier Dysfunction in Neurodegenerative Diseases. Fluids Barriers CNS 2024, 21, 60. [Google Scholar] [CrossRef]
- Raza, A.; Saleem, S.; Imran, S.; Rahman, S.; Haroon, M.; Razzaq, A.; Hussain, A.; Iqbal, J.; Sathian, B. From Metabolic Dysregulation to Neurodegenerative Pathology: The Role of Hyperglycemia, Oxidative Stress, and Blood-Brain Barrier Breakdown in T2D-Driven Alzheimer’s Disease. Metab. Brain Dis. 2025, 40, 276. [Google Scholar] [CrossRef]
- Sędzikowska, A.; Szablewski, L. Insulin and Insulin Resistance in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 9987. [Google Scholar] [CrossRef]
- Cerasuolo, M.; Auriemma, M.C.; Meo, I.D.; Lenti, C.; Papa, M.; Paolisso, G.; Rizzo, M.R. Understanding the Insulin-Degrading Enzyme: A New Look at Alzheimer’s Disease and Aβ Plaque Management. Int. J. Mol. Sci. 2025, 26, 6693. [Google Scholar] [CrossRef]
- Rippin, I.; Eldar-Finkelman, H. Mechanisms and Therapeutic Implications of GSK-3 in Treating Neurodegeneration. Cells 2021, 10, 262. [Google Scholar] [CrossRef]
- Sviridov, D.; Mukhamedova, N.; Miller, Y.I. Lipid Rafts as a Therapeutic Target. J. Lipid Res. 2020, 61, 687–695. [Google Scholar] [CrossRef]
- Płóciennikowska, A.; Hromada-Judycka, A.; Borzęcka, K.; Kwiatkowska, K. Co-Operation of TLR4 and Raft Proteins in LPS-Induced pro-Inflammatory Signaling. Cell. Mol. Life Sci. 2014, 72, 557–581. [Google Scholar] [CrossRef]
- Zhang, Q.-J.; Holland, W.L.; Wilson, L.; Tanner, J.M.; Kearns, D.; Cahoon, J.M.; Pettey, D.; Losee, J.; Duncan, B.; Gale, D.; et al. Ceramide Mediates Vascular Dysfunction in Diet-Induced Obesity by PP2A-Mediated Dephosphorylation of the eNOS-Akt Complex. Diabetes 2012, 61, 1848–1859. [Google Scholar] [CrossRef]
- Ghosh, N.; Patel, N.; Jiang, K.; Watson, J.E.; Cheng, J.; Chalfant, C.E.; Cooper, D.R. Ceramide-Activated Protein Phosphatase (CAPP) Involvement in Insulin Resistance via Akt, SRp40, and RNA Splicing in L6 Skeletal Muscle Cells. Endocrinology 2007, 148, 1359–1366. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. The Pathogenesis of Insulin Resistance: Integrating Signaling Pathways and Substrate Flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef]
- Kolczynska, K.; Loza-Valdes, A.; Hawro, I.; Sumara, G. Diacylglycerol-Evoked Activation of PKC and PKD Isoforms in Regulation of Glucose and Lipid Metabolism: A Review. Lipids Health Dis. 2020, 19, 113. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P.-Y. The NLRP3 Inflammasome: Molecular Activation and Regulation to Therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Pereira, A.C.; De Pascale, J.; Resende, R.; Cardoso, S.; Ferreira, I.; Neves, B.M.; Carrascal, M.A.; Zuzarte, M.; Madeira, N.; Morais, S.; et al. ER-Mitochondria Communication Is Involved in NLRP3 Inflammasome Activation under Stress Conditions in the Innate Immune System. Cell. Mol. Life Sci. 2022, 79, 213. [Google Scholar] [CrossRef]
- Patergnani, S.; Bouhamida, E.; Leo, S.; Pinton, P.; Rimessi, A. Mitochondrial Oxidative Stress and “Mito-Inflammation”: Actors in the Diseases. Biomedicines 2021, 9, 216. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, T.; Xie, H.; Hu, P.P.; Li, P. Experimental Cell Models of Insulin Resistance: Overview and Appraisal. Front. Endocrionol. 2024, 15, 1469565. [Google Scholar] [CrossRef]
- Mishra, S.K.; Singh, S.; Shukla, S.; Shukla, R. Intracerebroventricular Streptozotocin Impairs Adult Neurogenesis and Cognitive Functions via Regulating Neuroinflammation and Insulin Signaling in Adult Rats. Neurochem. Int. 2018, 113, 56–68. [Google Scholar] [CrossRef]
- Erichsen, J.M.; Calva, C.B.; Reagan, L.P.; Fadel, J.R. Intranasal Insulin and Orexins to Treat Age-Related Cognitive Decline. Physiol. Behav. 2021, 234, 113370. [Google Scholar] [CrossRef]
- AboEl-Azm, Y.H.; El-Samahy, M.; Hendi, N.I.; Arar, A.; Yasen, N.S.; Ramadan, S.; Zedan, E.M.; Al-Dardery, N.M.; Khaity, A. Safety and Efficacy of Intranasal Insulin in Patients with Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Clin. Transl. Res. 2023, 9, 222–235. [Google Scholar] [CrossRef]
- Hallschmid, M. Intranasal Insulin for Alzheimer’s Disease. CNS Drugs 2021, 35, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Craft, S.; Claxton, A.; Baker, L.D.; Hanson, A.J.; Cholerton, B.; Trittschuh, E.H.; Dahl, D.; Caulder, E.; Neth, B.; Montine, T.J.; et al. Effects of Regular and Long-Acting Insulin on Cognition and Alzheimer’s Disease Biomarkers: A Pilot Clinical Trial. J. Alzheimers Dis. 2017, 57, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.K.; Retnakaran, R.; Zinman, B. Insulin and Insulin Analogs as Antidiabetic Therapy: A Perspective from Clinical Trials. Cell Metab. 2021, 33, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Long, C.; Han, X.; Yang, Y.; Li, T.; Zhou, Q.; Chen, Q. Efficacy of Intranasal Insulin in Improving Cognition in Mild Cognitive Impairment or Dementia: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2022, 14, 963933. [Google Scholar] [CrossRef]
- Kellar, D.; Lockhart, S.N.; Aisen, P.; Raman, R.; Rissman, R.A.; Brewer, J.; Craft, S. Intranasal Insulin Reduces White Matter Hyperintensity Progression in Association with Improvements in Cognition and CSF Biomarker Profiles in Mild Cognitive Impairment and Alzheimer’s Disease. J. Prev. Alzheimers Dis. 2021, 8, 240–248. [Google Scholar] [CrossRef]
- Gaddam, M.; Singh, A.; Jain, N.; Avanthika, C.; Jhaveri, S.; De la Hoz, I.; Sanka, S.; Goli, S.R. A Comprehensive Review of Intranasal Insulin and Its Effect on the Cognitive Function of Diabetics. Cureus 2021, 13, e17219. [Google Scholar] [CrossRef]
- Al-Onaizi, M.; ElAli, A.; Alzaid, F. Editorial: Neuroinflammation, Neurodegeneration and Metabolic Disease: From Molecular Mechanisms to Therapeutic Innovation. Front. Neurol. 2024, 15, 1478550. [Google Scholar] [CrossRef]
- Kosmas, C.E.; Sourlas, A.; Oikonomakis, K.; Zoumi, E.-A.; Papadimitriou, A.; Kostara, C.E. Biomarkers of Insulin Sensitivity/Resistance. J. Int. Med. Res. 2024, 52, 03000605241285550. [Google Scholar] [CrossRef]
- Kim, B.; Feldman, E.L. Insulin Resistance as a Key Link for the Increased Risk of Cognitive Impairment in the Metabolic Syndrome. Exp. Mol. Med. 2015, 47, e149. [Google Scholar] [CrossRef]
- Fluca, A.L.; Pani, B.; Janjusevic, M.; Zwas, D.R.; Abraham, Y.; Calligaris, M.; Beltrami, A.P.; Corgosinho, F.C.; Marketou, M.; D’Errico, S.; et al. Unraveling the Relationship among Insulin Resistance, IGF-1, and Amyloid-Beta 1–40: Is the Definition of Type 3 Diabetes Applicable in the Cardiovascular Field? Life Sci. 2024, 352, 122911. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T. Pathophysiology of Diabetic Dyslipidemia. J. Atheroscler. Thromb. 2018, 25, 771–782. [Google Scholar] [CrossRef]
- Boon, J.; Hoy, A.J.; Stark, R.; Brown, R.D.; Meex, R.C.; Henstridge, D.C.; Schenk, S.; Meikle, P.J.; Horowitz, J.F.; Kingwell, B.A.; et al. Ceramides Contained in LDL Are Elevated in Type 2 Diabetes and Promote Inflammation and Skeletal Muscle Insulin Resistance. Diabetes 2013, 62, 401–410. [Google Scholar] [CrossRef]
- Cignarella, A.; Kratz, M.; Bolego, C. Emerging Role of Estrogen in the Control of Cardiometabolic Disease. Trends Pharmacol. Sci. 2010, 31, 183–189. [Google Scholar] [CrossRef]
- Janicki, S.C.; Schupf, N. Hormonal Influences on Cognition and Risk for Alzheimer’s Disease. Curr. Neurol. Neurosci. Rep. 2010, 10, 359–366. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, L.; Li, N.; Dai, C.; Yin, N.; Chu, Z.; Duan, X.; Niu, X.; Yan, P.; Lv, P. Estrogen Exerts Neuroprotective Effects in Vascular Dementia Rats by Suppressing Autophagy and Activating the Wnt/β-Catenin Signaling Pathway. Neurochem. Res. 2020, 45, 2100–2112. [Google Scholar] [CrossRef]
- Govindpani, K.; McNamara, L.G.; Smith, N.R.; Vinnakota, C.; Waldvogel, H.J.; Faull, R.L.; Kwakowsky, A. Vascular Dysfunction in Alzheimer’s Disease: A Prelude to the Pathological Process or a Consequence of It? J. Clin. Med. 2019, 8, 651. [Google Scholar] [CrossRef]
- Iadecola, C. The Pathobiology of Vascular Dementia. Neuron 2013, 80, 844–866. [Google Scholar] [CrossRef] [PubMed]
- Jin, T. WNT Signalling Pathway and Diabetes Mellitus. Diabetologia 2008, 51, 1771–1780. [Google Scholar] [CrossRef]
- Yuasa, T.; Takata, Y.; Aki, N.; Kunimi, K.; Satoh, M.; Nii, M.; Izumi, Y.; Otoda, T.; Hashida, S.; Osawa, H.; et al. Insulin Receptor Cleavage Induced by Estrogen Impairs Insulin Signaling. BMJ Open Diabetes Res. Care 2021, 9, e002467. [Google Scholar] [CrossRef] [PubMed]
- Hiriart, M.; Sanchez-Soto, C.; Diaz-Garcia, C.M.; Castanares, D.T.; Avitia, M.; Velasco, M.; Mas-Oliva, J.; Macias-Silva, M.; González-Villalpando, C.; Delgado-Coello, B.; et al. Hyperinsulinemia Is Associated with Increased Soluble Insulin Receptors Release from Hepatocytes. Front. Endocrinol. 2014, 5, 95. [Google Scholar] [CrossRef]
- Ouanes, S.; Popp, J. High Cortisol and the Risk of Dementia and Alzheimer’s Disease: A Review of the Literature. Front. Aging Neurosci. 2019, 11, 43. [Google Scholar] [CrossRef] [PubMed]
- Martocchia, A.; Stefanelli, M.; Falaschi, G.M.; Toussan, L.; Ferri, C.; Falaschi, P. Recent Advances in the Role of Cortisol and Metabolic Syndrome in Age-Related Degenerative Diseases. Aging Clin. Exp. Res. 2016, 28, 17–23. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef]
- Ring, M. An Integrative Approach to HPA Axis Dysfunction: From Recognition to Recovery. Am. J. Med. 2025, 138, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.; Marwaha, K.; Sanvictores, T.; Awosika, A.O.; Ayers, D. Physiology, Stress Reaction. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Thau, L.; Gandhi, J.; Sharma, S. Physiology, Cortisol. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Chiodini, I.; Adda, G.; Scillitani, A.; Coletti, F.; Morelli, V.; Lembo, S.D.; Epaminonda, P.; Masserini, B.; Beck-Peccoz, P.; Orsi, E.; et al. Cortisol Secretion in Patients with Type 2 Diabetes: Relationship with Chronic Complications. Diabetes Care 2007, 30, 83–88. [Google Scholar] [CrossRef]
- Hinterberger, M.; Zehetmayer, S.; Jungwirth, S.; Huber, K.; Krugluger, W.; Leitha, T.; Krampla, W.; Tragl, K.-H.; Fischer, P. High Cortisol and Low Folate Are the Only Routine Blood Tests Predicting Probable Alzheimer’s Disease After Age 75-Results of the Vienna Transdanube Aging Study. J. Am. Geriatr. Soc. 2013, 61, 648–651. [Google Scholar] [CrossRef]
- Dronse, J.; Ohndorf, A.; Richter, N.; Bischof, G.N.; Fassbender, R.; Behfar, Q.; Gramespacher, H.; Dillen, K.; Jacobs, H.I.L.; Kukolja, J.; et al. Serum Cortisol Is Negatively Related to Hippocampal Volume, Brain Structure, and Memory Performance in Healthy Aging and Alzheimer’s Disease. Front. Aging Neurosci. 2023, 15, 1154112. [Google Scholar] [CrossRef]
- McEwen, B.S. Stress and Hippocampal Plasticity. Ann. Rev. Neurosci. 1999, 22, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Cameron, H.A.; Tanapat, P.; Gould, E. Adrenal Steroids and N-Methyl-D-Aspartate Receptor Activation Regulate Neurogenesis in the Dentate Gyrus of Adult Rats through a Common Pathway. Neuroscience 1997, 82, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.A.; Syty, M.D.; Wu, K.; Ge, S. Adult Hippocampal Neurogenesis and Its Impairment in Alzheimer’s Disease. Zool. Res. 2022, 43, 481–496. [Google Scholar] [CrossRef]
- Fewlass, D.C.; Noboa, K.; Pi-Sunyer, F.X.; Johnston, J.M.; Yan, S.D.; Tezapsidis, N. Obesity-related Leptin Regulates Alzheimer’s Aβ. FASEB J. 2004, 18, 1870–1878. [Google Scholar] [CrossRef]
- Schmidt, M.I.; Duncan, B.B.; Vigo, A.; Pankow, J.S.; Couper, D.; Ballantyne, C.M.; Hoogeveen, R.C.; Heiss, G. Leptin and Incident Type 2 Diabetes: Risk or Protection? Diabetologia 2006, 49, 2086–2096. [Google Scholar] [CrossRef][Green Version]
- Zhao, S.; Kusminski, C.M.; Elmquist, J.K.; Scherer, P.E. Leptin: Less Is More. Diabetes 2020, 69, 823–829. [Google Scholar] [CrossRef]
- Tezapsidis, N.; Johnston, J.M.; Smith, M.A.; Ashford, J.W.; Casadesus, G.; Robakis, N.K.; Wolozin, B.; Perry, G.; Zhu, X.; Greco, S.J.; et al. Leptin: A Novel Therapeutic Strategy for Alzheimer’s Disease. J. Alzheimers Dis. 2009, 16, 731–740. [Google Scholar] [CrossRef]
- Yan, M.; Man, S.; Sun, B.; Ma, L.; Guo, L.; Huang, L.; Gao, W. Gut Liver Brain Axis in Diseases: The Implications for Therapeutic Interventions. Signal Transduct. Target. Ther. 2023, 8, 443. [Google Scholar] [CrossRef]
- Momen, Y.S.; Mishra, J.; Kumar, N. Brain-Gut and Microbiota-Gut-Brain Communication in Type-2 Diabetes Linked Alzheimer’s Disease. Nutrients 2024, 16, 2558. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, B.; Chen, F.; Xia, R.; Zhu, D.; Chen, B.; Lin, A.; Zheng, C.; Hou, D.; Li, X.; et al. Fecal Microbiota Transplantation Reverses Insulin Resistance in Type 2 Diabetes: A Randomized, Controlled, Prospective Study. Front. Cell. Infect. Microbiol. 2023, 12, 1089991. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Jiang, Y.; Zhao, Q.; Zhu, Z.; Liang, X.; Zhang, K.; Wu, W.; Dong, Q.; An, Y.; Tang, H.; et al. Metabolomics and Incident Dementia in Older Chinese Adults: The Shanghai Aging Study. Alzheimers Dement. 2020, 16, 779–788. [Google Scholar] [CrossRef]
- Zhao, S.; Jang, C.; Liu, J.; Uehara, K.; Gilbert, M.; Izzo, L.; Zeng, X.; Trefely, S.; Fernandez, S.; Carrer, A.; et al. Dietary Fructose Feeds Hepatic Lipogenesis via Microbiota-Derived Acetate. Nature 2020, 579, 586–591. [Google Scholar] [CrossRef]
- Roumans, K.H.M.; Lindeboom, L.; Veeraiah, P.; Remie, C.M.E.; Phielix, E.; Havekes, B.; Bruls, Y.M.H.; Brouwers, M.C.G.J.; Ståhlman, M.; Alssema, M.; et al. Hepatic Saturated Fatty Acid Fraction Is Associated with de Novo Lipogenesis and Hepatic Insulin Resistance. Nat. Commun. 2020, 11, 1891. [Google Scholar] [CrossRef]
- Wachsmuth, H.R.; Weninger, S.N.; Duca, F.A. Role of the Gut–Brain Axis in Energy and Glucose Metabolism. Exp. Mol. Med. 2022, 54, 377–392. [Google Scholar] [CrossRef]
- Zeng, Y.; Wu, Y.; Zhang, Q.; Xiao, X. Crosstalk between Glucagon-like Peptide 1 and Gut Microbiota in Metabolic Diseases. mBio 2024, 15, e02032-23. [Google Scholar] [CrossRef]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-Chain Fatty Acids Stimulate Glucagon-Like Peptide-1 Secretion via the G-Protein–Coupled Receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef]
- Seino, Y.; Fukushima, M.; Yabe, D. GIP and GLP-1, the Two Incretin Hormones: Similarities and Differences. J. Diabetes Investig. 2010, 1, 8–23. [Google Scholar] [CrossRef]
- Holst, J.J. The Incretin System in Healthy Humans: The Role of GIP and GLP-1. Metabolism 2019, 96, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Daily, J.W.; Kang, S.; Park, S. Protection against Alzheimer’s Disease by Luteolin: Role of Brain Glucose Regulation, Anti-inflammatory Activity, and the Gut Microbiota-liver-brain Axis. BioFactors 2021, 47, 218–231. [Google Scholar] [CrossRef]
- Coulson, E.J.; Bartlett, P.F. An Exercise Path to Preventing Alzheimer’s Disease. J. Neurochem. 2017, 142, 191–193. [Google Scholar] [CrossRef]
- Daubenmier, J.; Kristeller, J.; Hecht, F.M.; Maninger, N.; Kuwata, M.; Jhaveri, K.; Lustig, R.H.; Kemeny, M.; Karan, L.; Epel, E.S. Mindfulness Intervention for Stress Eating to Reduce Cortisol and Abdominal Fat among Overweight and Obese Women: An Exploratory Randomized Controlled Study. J. Obes. 2011, 2011, 651936. [Google Scholar] [CrossRef]
- Mielke, J.G.; Taghibiglou, C.; Liu, L.; Zhang, Y.; Jia, Z.; Adeli, K.; Wang, Y.T. A Biochemical and Functional Characterization of Diet-induced Brain Insulin Resistance. J. Neurochem. 2005, 93, 1568–1578. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Yamaguchi, K.; Matsui, K.; Sano, T.; Kubota, T.; Hashimoto, T.; Mano, A.; Yamada, K.; Matsuo, Y.; Kubota, N.; et al. Differential Effects of Diet- and Genetically-Induced Brain Insulin Resistance on Amyloid Pathology in a Mouse Model of Alzheimer’s Disease. Mol. Neurodegener. 2019, 14, 15. [Google Scholar] [CrossRef]
- Ahmadi, S.; Nagpal, R.; Wang, S.; Gagliano, J.; Kitzman, D.W.; Soleimanian-Zad, S.; Sheikh-Zeinoddin, M.; Read, R.; Yadav, H. Prebiotics from Acorn and Sago Prevent High-Fat-Diet-Induced Insulin Resistance via Microbiome–Gut–Brain Axis Modulation. J. Nutr. Biochem. 2019, 67, 1–13. [Google Scholar] [CrossRef]
- Arnold, S.E.; Lucki, I.; Brookshire, B.R.; Carlson, G.C.; Browne, C.A.; Kazi, H.; Bang, S.; Choi, B.-R.; Chen, Y.; McMullen, M.F.; et al. High Fat Diet Produces Brain Insulin Resistance, Synaptodendritic Abnormalities and Altered Behavior in Mice. Neurobiol. Dis. 2014, 67, 79–87. [Google Scholar] [CrossRef]
- Sundaram, K.; Mu, J.; Kumar, A.; Behera, J.; Lei, C.; Sriwastva, M.K.; Xu, F.; Dryden, G.W.; Zhang, L.; Chen, S.; et al. Garlic Exosome-like Nanoparticles Reverse High-Fat Diet Induced Obesity via the Gut/Brain Axis. Theranostics 2022, 12, 1220–1246. [Google Scholar] [CrossRef]
- Fang, F.; Li, H.; Qin, T.; Li, M.; Ma, S. Thymol Improves High-Fat Diet-Induced Cognitive Deficits in Mice via Ameliorating Brain Insulin Resistance and Upregulating NRF2/HO-1 Pathway. Metab. Brain Dis. 2017, 32, 385–393. [Google Scholar] [CrossRef]
- Chapple, B.; Woodfin, S.; Moore, W. The Perfect Cup? Coffee-Derived Polyphenols and Their Roles in Mitigating Factors Affecting Type 2 Diabetes Pathogenesis. Molecules 2024, 29, 751. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, A.A.; Makboul, R.M.; Al-Mokhtar, M.A.; Nicola, M.A. Polyphenol-Rich Boswellia Serrata Gum Prevents Cognitive Impairment and Insulin Resistance of Diabetic Rats through Inhibition of GSK3β Activity, Oxidative Stress and pro-Inflammatory Cytokines. Biomed. Pharmacother. 2019, 109, 281–292. [Google Scholar] [CrossRef]
- Tai, X.Y.; Chen, C.; Manohar, S.; Husain, M. Impact of Sleep Duration on Executive Function and Brain Structure. Commun. Biol. 2022, 5, 201. [Google Scholar] [CrossRef]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep Drives Metabolite Clearance from the Adult Brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef]
- Jin, D.; Choi, J.W. Sleep Disorders and Risk of Dementia in Patients with New-onset Type 2 Diabetes: A Nationwide Population-based Cohort Study. J. Diabetes 2021, 13, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Ulland, T.K.; Ewald, A.C.; Knutson, A.O.; Marino, K.M.; Smith, S.M.C.; Watters, J.J. Alzheimer’s Disease, Sleep Disordered Breathing, and Microglia: Puzzling out a Common Link. Cells 2021, 10, 2907. [Google Scholar] [CrossRef]
- Ramasubbu, K.; Ramanathan, G.; Venkatraman, G.; Rajeswari, V.D. Sleep-Associated Insulin Resistance Promotes Neurodegeneration. Mol. Biol. Rep. 2023, 50, 8665–8681. [Google Scholar] [CrossRef]
- Tasali, E.; Leproult, R.; Ehrmann, D.A.; Cauter, E.V. Slow-Wave Sleep and the Risk of Type 2 Diabetes in Humans. Proc. Natl. Acad. Sci. USA 2008, 105, 1044–1049. [Google Scholar] [CrossRef]
- Westwood, A.J.; Beiser, A.; DeCarli, C.; Harris, T.B.; Chen, T.C.; He, X.; Roubenoff, R.; Pikula, A.; Au, R.; Braverman, L.E.; et al. Insulin-like Growth Factor-1 and Risk of Alzheimer Dementia and Brain Atrophy. Neurology 2014, 82, 1613–1619. [Google Scholar] [CrossRef]
- de Matos, M.A.; Duarte, T.C.; Ottone Vde, O.; Sampaio, P.F.; Costa, K.B.; de Oliveira, M.F.; Moseley, P.L.; Schneider, S.M.; Coimbra, C.C.; Brito-Melo, G.E.; et al. The Effect of Insulin Resistance and Exercise on the Percentage of CD16+ Monocyte Subset in Obese Individuals. Cell Biochem. Funct. 2016, 34, 209–216. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chapple, B.; Bayliss, E.; Woodfin, S.; Smith, M.; Winter, J.; Moore, W. Type 3 Diabetes: Linking Insulin Resistance to Cognitive Decline. Diseases 2025, 13, 359. https://doi.org/10.3390/diseases13110359
Chapple B, Bayliss E, Woodfin S, Smith M, Winter J, Moore W. Type 3 Diabetes: Linking Insulin Resistance to Cognitive Decline. Diseases. 2025; 13(11):359. https://doi.org/10.3390/diseases13110359
Chicago/Turabian StyleChapple, Brooke, Emily Bayliss, Seth Woodfin, Merritt Smith, Jeremiah Winter, and William Moore. 2025. "Type 3 Diabetes: Linking Insulin Resistance to Cognitive Decline" Diseases 13, no. 11: 359. https://doi.org/10.3390/diseases13110359
APA StyleChapple, B., Bayliss, E., Woodfin, S., Smith, M., Winter, J., & Moore, W. (2025). Type 3 Diabetes: Linking Insulin Resistance to Cognitive Decline. Diseases, 13(11), 359. https://doi.org/10.3390/diseases13110359




