Diabetic Ketoacidosis in Young Adults with Type 1 Diabetes: The Impact of the Ketogenic Diet—A Narrative Literature Review
Abstract
1. Introduction
1.1. Definition and Clinical Significance of the Topic
1.2. Pathophysiology, Physiology and Biochemistry of Diabetic Ketoacidosis
1.3. Purpose of the Study
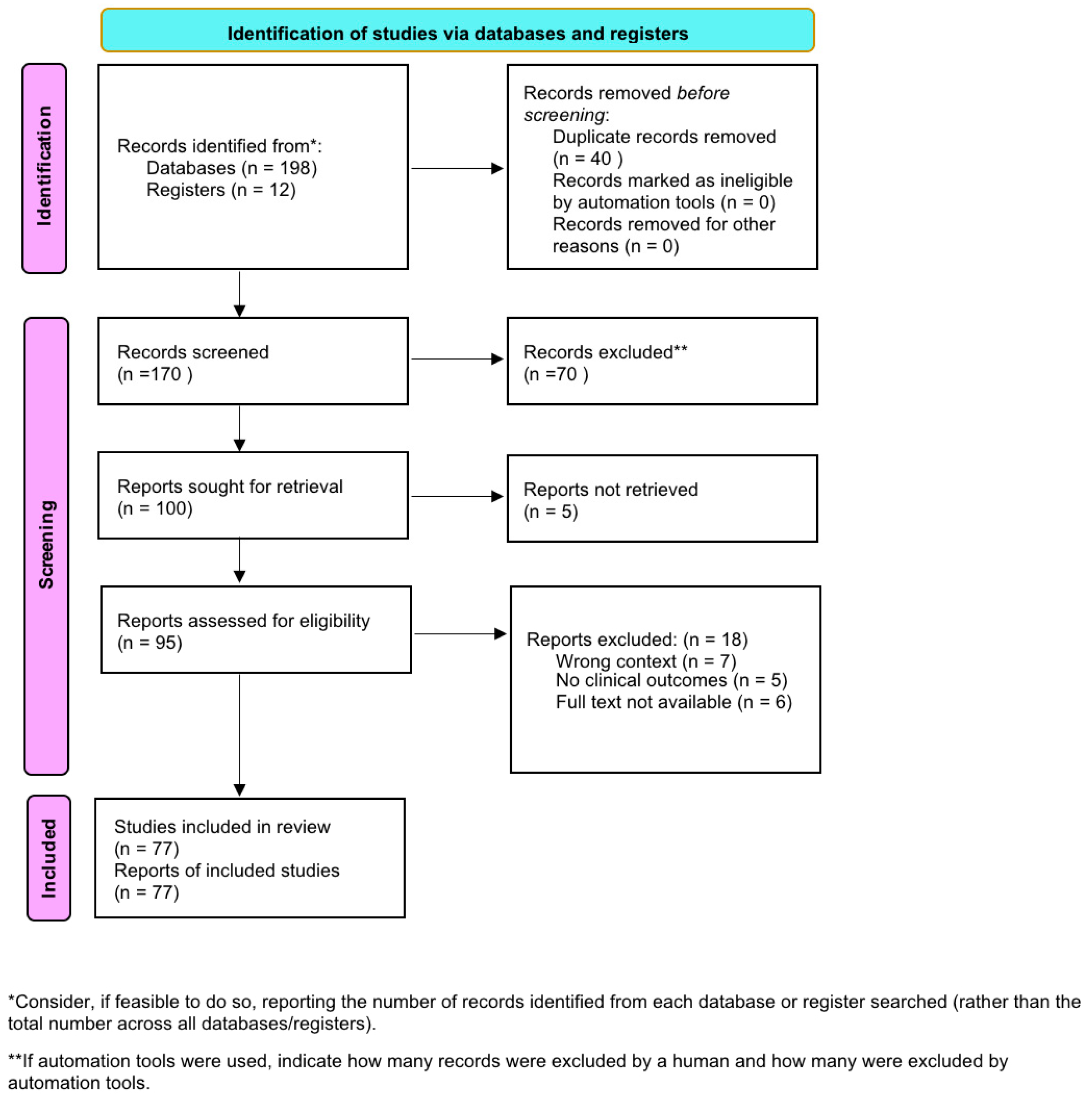
2. Materials and Methods
3. Results
3.1. Regulation of Acid-Base Balance in the Body
3.2. Triggering Factors and Risk Groups
3.3. Clinical Picture
3.4. Diagnostics
3.4.1. Laboratory Evaluation
3.4.2. Blood Glucose Concentration
3.4.3. Acid-Base Balance
3.4.4. Ketone Detection in Serum and Urine
3.4.5. Anion Gap and Electrolyte Disturbances
3.4.6. Plasma Osmolality
3.4.7. Additional Testing
3.4.8. Electrolyte Disturbances (Phosphate and Magnesium)
3.4.9. Effects of Dehydration on Renal Perfusion
3.5. Complications and Consequences
3.5.1. Cerebral Edema
3.5.2. Electrolyte Imbalances
3.5.3. Cardiovascular Complications
3.5.4. Mortality and Long-Term Consequences
3.6. The Ketogenic Diet and Diabetic Ketoacidosis in Type 1 Diabetes—A Clinical Perspective for Adolescents and Young Adults
3.7. Treatment and Therapeutic Strategies for Diabetic Ketoacidosis in Young Adults with Type 1 Diabetes
3.7.1. Intensive Insulin Therapy
3.7.2. Fluid Management Strategies
3.7.3. Correction of Electrolyte Imbalances
3.7.4. Patient Monitoring and Hospitalization Criteria
3.7.5. Prevention Strategies
3.8. Patient Education and Preventive Strategies
3.8.1. The Importance of Self-Monitoring of Blood Glucose
3.8.2. Ketone Monitoring and Education on Early Warning Signs
3.8.3. Educational Programs for Patients and Their Families
3.8.4. The Importance of Modern Technologies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Search Strategy
References
- Roberts, C.G.P.; Athinarayanan, S.J.; Ratner, R.E.; Umpierrez, G.E. Illnesses associated with ketosis including diabetic ketoacidosis during very low carbohydrate and ketogenic diets. Diabetes Obes. Metab. 2025, 27, 2531–2539. [Google Scholar] [CrossRef]
- Kostopoulou, E.; Sinopidis, X.; Fouzas, S.; Gkentzi, D.; Dassios, T.; Roupakias, S.; Dimitriou, G. Diabetic Ketoacidosis in Children and Adolescents; Diagnostic and Therapeutic Pitfalls. Diagnostics 2023, 13, 2602. [Google Scholar] [CrossRef]
- Natalia, Ś.; Paulina, T.; Ewelina, B.; Izabella, C.; Beata, J.-P. Patient with diabetic ketoacidosis. J. Educ. Health Sport 2019, 9, 96–105. [Google Scholar]
- Babiker, A.; Aljahdali, G.L.; Alsaeed, M.K.; Almunif, A.F.; Mohamud, M.S.; Mutair, A.A.; Al Juraibah, F.; Al Alwan, I. Frequency and Risk Factors of Diabetic Ketoacidosis in a Specialized Children’s Hospital, Riyadh: A Cross-sectional Study. Oman Med. J. 2022, 37, e341. [Google Scholar] [CrossRef] [PubMed]
- Monteiro LEda, R.C.; Garcia, S.P.; Bottino, L.G.; Custodio, J.L.; Telo, G.H.; Schaan, B.D. Precipitating factors of diabetic ketoacidosis in type 1 diabetes patients at a tertiary hospital: A cross-sectional study with a two-time-period comparison. Arch. Endocrinol. Metab. 2022, 66, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.K.; Huang, C.Y.; Lin, C.H.; Cheng, B.W.; Chiang, Y.T.; Lee, Y.C.; Yeh, S.-N.; Chan, C.-I.; Chua, W.-K.; Lee, Y.-J.; et al. Acute kidney injury is a common complication in children and adolescents hospitalized for diabetic ketoacidosis. PLoS ONE 2020, 15, e0239160. [Google Scholar] [CrossRef]
- Tao, L.C.; Shu, H.; Wang, Y.; Hou, Q.; Li, J.J.; Huang, X.L.; Hua, F. Inflammatory biomarkers predict higher risk of hyperglycemic crises but not outcomes in diabetic patients with COVID-19. Front. Endocrinol. 2024, 15, 1287795. [Google Scholar] [CrossRef]
- Hasan, R.A.; Hesen, J.Z.; Millican, N.; Pederson, J.M.; Agus, M.S.D. Serum Phosphorus and Hypophosphatemia During Therapy of Diabetic Ketoacidosis in Children: Single-Center, Retrospective Cohort 2016–2022. Pediatr. Crit. Care Med. 2024, 26, e77–e85. [Google Scholar] [CrossRef]
- Trainor, J.L.; Glaser, N.S.; Tzimenatos, L.; Stoner, M.J.; Brown, K.M.; McManemy, J.K.; Schunk, J.E.; Quayle, K.S.; Nigrovic, L.E.; Rewers, A.; et al. Clinical and Laboratory Predictors of Dehydration Severity in Children with Diabetic Ketoacidosis. Ann. Emerg. Med. 2023, 82, 167–178. [Google Scholar] [CrossRef]
- Szabó, G.V.; Szigetváry, C.; Turan, C.; Engh, M.A.; Terebessy, T.; Fazekas, A.; Farkas, N.; Hegyi, P.; Molnár, Z. Fluid resuscitation with balanced electrolyte solutions results in faster resolution of diabetic ketoacidosis than with 0.9% saline in adults—A systematic review and meta-analysis. Diabetes/Metabolism Res. Rev. 2024, 40, e3831. [Google Scholar] [CrossRef]
- Aygün, D.; Aygün, F.; Nişli, K.; Baş, F.; Çıtak, A. Electrocardiographic changes in children with diabetic ketoacidosis and ketosis. Turk. Pediatri Ars. 2017, 52, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.S.; Ramaldes, L.A.L.; Dualib, P.M.; Gabbay, M.A.L.; Sá, J.R.; Dib, S.A. Increased risk of death following recurrent ketoacidosis admissions: A Brazilian cohort study of young adults with type 1 diabetes. Diabetol. Metab. Syndr. 2023, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Turton, J.L.; Brinkworth, G.D.; Parker, H.M.; Lim, D.; Lee, K.; Rush, A.; Johnson, R.; Rooney, K.B. Effects of a low-carbohydrate diet in adults with type 1 diabetes management: A single arm non-randomised clinical trial. PLoS ONE 2023, 18, e0288440. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Dhaliwal, S.; Bapat, P.; Scarr, D.; Bakhsh, A.; Budhram, D.; Verhoeff, N.J.; Weisman, A.; Fralick, M.; Ivers, N.M.; et al. Point-of-care capillary blood ketone measurements and the prediction of future ketoacidosis risk in type 1 diabetes. Diabetes Care 2023, 46, 1973–1977. [Google Scholar] [CrossRef]
- Alshurtan, K.S.; Alnizari, O.; Aldarwish, H.; Al-Tufaif, A.A. Efficacy and Safety of Intravenous Insulin in Treatment of Patient With Diabetic Ketoacidosis: A Systematic Review and Meta-Analysis. Cureus 2022, 14, e30721. [Google Scholar] [CrossRef]
- Andrade-Castellanos, C.A.; Colunga-Lozano, L.E.; Delgado-Figueroa, N.; Gonzalez-Padilla, D.A. Subcutaneous rapid-acting insulin analogues for diabetic ketoacidosis. Cochrane Database Syst. Rev. 2016, 2016, CD011281. [Google Scholar] [CrossRef]
- Tamzil, R.; Yaacob, N.; Noor, N.; Baharuddin, K. Comparing the clinical effects of balanced electrolyte solutions versus normal saline in managing diabetic ketoacidosis: A systematic review and meta-analyses. Turk. J. Emerg. Med. 2023, 23, 131–138. [Google Scholar] [CrossRef]
- Jozwiak, M.; Hayes, M.M.; Canet, E.; Lautrette, A.; Duroyon, M.-M.; Molinari, N.; Jung, B. Management of diabetic keto-acidosis in adult patients admitted to intensive care unit: An ESICM-endorsed international survey. Crit. Care 2024, 28, 1–13. [Google Scholar] [CrossRef]
- Wolf, R.A.; Haw, J.S.; Paul, S.; Spezia Faulkner, M.; Cha, E.S.; Findley, M.K.; Khan, F.; Markley Webster, S.; Alexopoulos, A.S.; Mehta, K.; et al. Hospital admissions for hyperglycemic emergencies in young adults at an inner-city hospital. Diabetes Res. Clin. Pract. 2019, 157, 107869. [Google Scholar] [CrossRef]
- Toschi, E.; Atakov-Castillo, A.; Clift, A.; Bennetti, M.; Gabbay, R.A. Continuous Glucose Monitoring and Glycemic Control in Young Adults with Type 1 Diabetes: Benefit for even the Simplest Insulin Administration Methods. Diabetes Technol. Ther. 2021, 23, 586–589. [Google Scholar] [CrossRef]
- Vitale, R.J.; Card, C.E.; Lichtman, J.H.; Weyman, K.; Michaud, C.; Sikes, K.; Tamborlane, W.V.; Weinzimer, S.A. An Effective Diabetic Ketoacidosis Prevention Intervention in Children With Type 1 Diabetes. SAGE Open Nurs. 2018, 4, 2377960818804742. [Google Scholar] [CrossRef]
- Karges, B.; Schwandt, A.; Heidtmann, B.; Kordonouri, O.; Binder, E.; Schierloh, U.; Boettcher, C.; Kapellen, T.; Rosenbauer, J.; Holl, R.W. Association of insulin pump therapy vs. insulin injection therapy with severe hypoglycemia, ketoacidosis, and glycemic control among children, adolescents, and young adults with type 1 diabetes. JAMA J. Am. Med. Assoc. 2017, 318, 1358–1366. [Google Scholar] [CrossRef]
- Van Den Boom, L.; Karges, B.; Auzanneau, M.; Rami-Merhar, B.; Lilienthal, E.; Von Sengbusch, S.; Datz, N.; Schröder, C.; Kapellen, T.; Laimer, M.; et al. Temporal trends and contemporary use of insulin pump therapy and glucose monitoring among children, adolescents, and adults with type 1 diabetes between 1995 and 2017. Diabetes Care 2019, 42, 2050–2056. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, S.E.A.B.; Gonçalves, T.J.M.; Guarnieri, A.; Risegato, R.C.; Guimarães, M.P.; de Freitas, D.C. Association between thiamine deficiency and hyperlactatemia among critically ill patients with diabetes infected by SARS-CoV-2. J. Diabetes 2021, 13, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Levran, N.; Levek, N.; Sher, B.; Gruber, N.; Afek, A.; Monsonego-Ornan, E.; Pinhas-Hamiel, O. The Impact of a Low-Carbohydrate Diet on Micronutrient Intake and Status in Adolescents with Type 1 Diabetes. Nutrients 2023, 15, 1418. [Google Scholar] [CrossRef] [PubMed]
- Kitabchi, A.E.; Umpierrez, G.E.; Miles, J.M.; Fisher, J.N. Hyperglycemic crises in adult patients with diabetes. Diabetes Care 2009, 32, 1335–1343. [Google Scholar] [CrossRef]
- Family, A.; Westerberg, D.P. Diabetic Ketoacidosis: Evaluation and Treatment [Internet]. Vol. 87. 2013. Available online: www.aafp.org/afp (accessed on 14 October 2025).
- Sri, G.A.; Swandewi, A.; Windiyanto, R. Recurrent Diabetic Ketoacidosis in Adolescents with Type 1 Diabetes Mellitus. Int. J. Pharm. Bio-Med. Sci. 2024, 4, 881–888. [Google Scholar]
- Gassiep, I.; Bachmeier, C.; Hendry, S.; Emeto, T.I.; Armstrong, M.; Sangla, K.; Heather, C.S. Antimicrobial stewardship in diabetic ketoacidosis: A single-centre experience. Intern. Med. J. 2019, 50, 173–177. [Google Scholar] [CrossRef]
- Iqbal, A.; Hussain, A.; Iqbal, A.; Kumar, V. Correlation Between Vitamin D Deficiency and Diabetic Ketoacidosis. Cureus 2019, 11, e4497. [Google Scholar] [CrossRef]
- Vine, J.; Mehta, S.; Balaji, L.; Berg, K.M.; Berlin, N.; Liu, X.; Ngo, L.; Shea, M.; Moskowitz, A.; Donnino, M.W.; et al. Thiamine as adjunctive therapy for diabetic ketoacidosis (DKAT) trial protocol and statistical analysis plan: A prospective, single-centre, double-blind, randomised, placebo-controlled clinical trial in the USA. BMJ Open 2024, 14, e077586. [Google Scholar] [CrossRef]
- Usman, A. Initial potassium replacement in diabetic ketoacidosis: The unnoticed area of gap. Front. Endocrinol. 2018, 9, 109. [Google Scholar] [CrossRef]
- Fazeli Farsani, S.; Brodovicz, K.; Soleymanlou, N. Correction: Incidence and prevalence of diabetic ketoacidosis (DKA) among adults with type 1 diabetes mellitus (T1D): A systematic literature review. BMJ Open 2017, 7, e016587. [Google Scholar] [CrossRef]
- Olga, L.; Hector, S.; Amando, G.; Rayan, Q.; Leopoldo, C. Euglycemic Diabetic Ketoacidosis in a Diabetic Patient Treated with SGLT2 Inhibitor. Clin. Diabetes Res. 2021, 5, 63–67. [Google Scholar] [CrossRef]
- Peters, A.L.; Buschur, E.O.; Buse, J.B.; Cohan, P.; Diner, J.C.; Hirsch, I.B. Euglycemic diabetic ketoacidosis: A potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care 2015, 38, 1687–1693. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, P.R.E. Euglycemic diabetic ketoacidosis: A potential pitfall for the emergency physician. Clin. Exp. Emerg. Med. Korean Soc. Emerg. Med. 2023, 10, 110–113. [Google Scholar]
- American Diabetes Association Professional Practice Committee. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2025. Diabetes Care 2024, 48 (Suppl. 1), S27–S49. Available online: https://diabetesjournals.org/care/article/48/Supplement_1/S27/157566/2-Diagnosis-and-Classification-of-Diabetes (accessed on 14 October 2025). [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. Summary of Revisions: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48 (Suppl. 1), S6–S13. Available online: https://diabetesjournals.org/care/article/48/Supplement_1/S6/157564/Summary-of-Revisions-Standards-of-Care-in-Diabetes (accessed on 14 October 2025). [CrossRef]
- American Diabetes Association Professional Practice Committee. Children and Adolescents: Standards of Care in Diabetes—2025. Diabetes Care 2024, 48 (Suppl. 1), S283–S305. Available online: https://diabetesjournals.org/care/article/48/Supplement_1/S283/157559/14-Children-and-Adolescents-Standards-of-Care-in (accessed on 14 October 2025). [CrossRef]
- Huang, J.; Yeung, A.M.; Bergenstal, R.M.; Castorino, K.; Cengiz, E.; Dhatariya, K.; Niu, I.; Sherr, J.L.; Umpierrez, G.E.; Klonoff, D.C. Update on Measuring Ketones. J. Diabetes Sci. Technol. 2024, 18, 714–726. [Google Scholar] [CrossRef]
- Umpierrez, G.E.; Davis, G.M.; Elsayed, N.A.; Fadini, G.P.; Galindo, R.J.; Hirsch, I.B.; Klonoff, D.C.; McCoy, R.G.; Misra, S.; Gabbay, R.A.; et al. Hyperglycemic Crises in Adults With Diabetes: A Consensus Report. Diabetes Care 2024, 47, 1257–1275. [Google Scholar] [CrossRef]
- Alharbi, S.A.; Meshari, A.M.; Henawi, Z.A.; Alsaadi, R.M.; Alqarni, S.S.; Hani, A.F.; Alhamrani, O.H.; Saptan, L.M.; Al Hamoud, Z.A.; Alshanbri, N.K.; et al. Management of diabetic ketoacidosis in internal medicine: Insulin protocols, electrolyte balance, and clinical outcomes. Int. J. Community Med. Public Health 2023, 11, 408–413. [Google Scholar] [CrossRef]
- Liamis, G. Diabetes mellitus and electrolyte disorders. World J. Clin. Cases 2014, 2, 488. [Google Scholar] [CrossRef] [PubMed]
- Hasona, N.; Elasbali, A. Evaluation of Electrolytes Imbalance and Dyslipidemia in Diabetic Patients. Med. Sci. 2016, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Wolfsdorf, J.I.; Glaser, N.; Agus, M.; Fritsch, M.; Hanas, R.; Rewers, A.; Sperling, M.A.; Codner, E. ISPAD Clinical Practice Consensus Guidelines 2018: Diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr. Diabetes 2018, 19, 155–177. [Google Scholar] [CrossRef]
- El-Naggar, A.; Sameer, G.; Sadek, A. Impact of Hypophosphatemia and Hypomagnesaemia on Diabetic Ketoacidosis patient’s Outcome in Medical Intensive Care Unit. Zagazig Univ. Med. J. 2021, 28, 45–53. [Google Scholar] [CrossRef]
- Halstead, M.R.; Geocadin, R.G. The Medical Management of Cerebral Edema: Past, Present, and Future Therapies. Neurotherapeutics 2019, 16, 1133–1148. [Google Scholar] [CrossRef]
- Natarajan, S.; Kulkarni, R.; Tangri, A. Fatal Cerebral Edema in a Young Adult with Diabetic Ketoacidosis: Blame the Bicarbonate? Case Rep. Crit. Care 2020, 2020, 5917459. [Google Scholar] [CrossRef]
- Azova, S.; Rapaport, R.; Wolfsdorf, J. Brain injury in children with diabetic ketoacidosis: Review of the literature and a proposed pathophysiologic pathway for the development of cerebral edema. Pediatr. Diabetes 2021, 22, 148–160. [Google Scholar] [CrossRef]
- Kaefer, K.; Botta, I.; Mugisha, A.; Berdaoui, B.; De Bels, D.; Attou, R.; Honoré, P.M.; Redant, S. Acute coronary syndrome and diabetic keto acidosis: The chicken or the egg? Ann. Transl. Med. 2019, 7, 397. [Google Scholar] [CrossRef]
- Dyńka, D.; Rodzeń, Ł.; Rodzeń, M.; Pacholak-Klimas, A.; Ede, G.; Sethi, S.; Łojko, D.; Bartoń, K.; Berry, K.; Deptuła, A.; et al. Ketogenic Diets for Body Weight Loss: A Comparison with Other Diets. Nutrients 2025, 17, 965. [Google Scholar] [CrossRef]
- Gardemann, C.; Knowles, S.; Marquardt, T. Managing type 1 diabetes mellitus with a ketogenic diet. Endocrinol. Diabetes Metab. Case Rep. 2023, 2023, 23-0008. [Google Scholar] [CrossRef] [PubMed]
- Buehler, L.A.; Noe, D.; Knapp, S.; Diana, I.P.D.; Pantalone, K.M. Ketogenic diets in the management of type 1 diabetes: Safe or safety concern? Clevel. Clin. J. Med. 2021, 88, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Helvaci, B.C.; Erdogan, B.T.; Ozdemir, D.; Topaloglu, O.; Cakir, B. Euglycemic diabetic ketoacidosis in a patient with new-onset type 1 diabetes following a ketogenic diet: A potential risk of a dangerous dietary trend. Arch. Endocrinol. Metab. 2024, 68, e230229. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.E.; Zimmerman, R. Classic diabetic ketoacidosis and the euglycemic variant: Something old, something new. Clevel. Clin. J. Med. 2025, 92, 33–39. [Google Scholar] [CrossRef]
- Elendu, C.; David, J.A.; Udoyen, A.O.; Egbunu, E.O.; Ogbuiyi-Chima, I.C.; Unakalamba, L.O.; Temitope, A.I.M.; Ibhiedu, J.O.M.; Ibhiedu, A.O.M.; Nwosu, P.U.M.; et al. Comprehensive review of diabetic ketoacidosis: An update. Ann. Med. Surg. 2023, 85, 2802–2807. [Google Scholar] [CrossRef]
- Charoensri, S.; Sothornwit, J.; Trirattanapikul, A.; Pongchaiyakul, C. Ketogenic Diet-Induced Diabetic Ketoacidosis in a Young Adult with Unrecognized Type 1 Diabetes. Case Rep. Endocrinol. 2021, 2021, 6620832. [Google Scholar] [CrossRef]
- White-Cotsmire, A.J.; Healy, A.M. Ketogenic diet as a trigger for diabetic ketoacidosis in a misdiagnosis of diabetes: A case report. Clin. Diabetes 2020, 38, 318–321. [Google Scholar] [CrossRef]
- Cogen, F.R. Incorporation of the ketogenic diet in a youth with type 1 diabetes. Clin. Diabetes 2020, 38, 412–415. [Google Scholar] [CrossRef]
- Kong, Y.W.; Morrison, D.; Lu, J.C.; Lee, M.H.; Jenkins, A.J.; O’Neal, D.N. Continuous ketone monitoring: Exciting implications for clinical practice. Diabetes Obes. Metab. 2024, 26, 47–58. [Google Scholar] [CrossRef]
- Neyman, A.; Hannon, T.S. Low-Carbohydrate Diets in Children and Adolescents With or at Risk for Diabetes. Pediatrics 2023, 152, e2023063755. [Google Scholar] [CrossRef]
- Crosby, L.; Davis, B.; Joshi, S.; Jardine, M.; Paul, J.; Neola, M.; Barnard, N.D. Ketogenic Diets and Chronic Disease: Weighing the Benefits Against the Risks. Front. Nutr. 2021, 8, 702802. [Google Scholar] [CrossRef] [PubMed]
- Annan, S.F.; Higgins, L.A.; Jelleryd, E.; Hannon, T.; Rose, S.; Salis, S.; Baptista, J.; Chinchilla, P.; Marcovecchio, M.L. ISPAD Clinical Practice Consensus Guidelines 2022: Nutritional management in children and adolescents with diabetes. Pediatr. Diabetes 2022, 23, 1297–1321. [Google Scholar] [CrossRef] [PubMed]
- Eledrisi, M.; Elzouki, A.N. Management of diabetic ketoacidosis in adults: A narrative review. Saudi J. Med. Med. Sci. 2020, 8, 165–173. [Google Scholar]
- Gupta, P.; Nasa, P.; Shahabdeen, S.M. Effectiveness of Balanced Electrolyte Solution vs. Normal Saline in the Resuscitation of Adult Patients with Diabetic Ketoacidosis: An Updated Systematic Review and Meta-analysis. Indian J. Crit. Care Med. 2024, 29, 65–74. [Google Scholar] [CrossRef]
- Gripp, K.E.; Trottier, E.D.; Thakore, S.; Sniderman, J.; Lawrence, S. Current recommendations for management of paediatric diabetic ketoacidosis. Paediatr. Child Health 2023, 28, 128–132. [Google Scholar] [CrossRef]
- Dunn, B.K.; Coore, H.; Bongu, N.; Brewer, K.L.; Kumar, D.; Malur, A.; Alkhalisy, H. Treatment Challenges and Controversies in the Management of Critically Ill Diabetic Ketoacidosis (DKA) Patients in Intensive Care Units. Cureus 2024, 16, e68785. [Google Scholar] [CrossRef]
- Ilkowitz, J.T.; Choi, S.; Rinke, M.L.; Vandervoot, K.; Heptulla, R.A. Pediatric type 1 diabetes: Reducing admission rates for diabetes ketoacidosis. Qual. Manag. Health Care 2016, 25, 231–237. [Google Scholar] [CrossRef]
- Foti Randazzese, S.; La Rocca, M.; Bombaci, B.; Di Pisa, A.; Giliberto, E.; Inturri, T.; Militi, D.; Lombardo, F.; Gitto, E.; Salzano, G.; et al. Severe Diabetic Ketoacidosis in Children with Type 1 Diabetes: Ongoing Challenges in Care. Children 2025, 12, 110. [Google Scholar] [CrossRef]
- Sundheim, B.; Hirani, K.; Blaschke, M.; Lemos, J.R.N.; Mittal, R. Pre-Type 1 Diabetes in Adolescents and Teens: Screening, Nutritional Interventions, Beta-Cell Preservation, and Psychosocial Impacts. J. Clin. Med. 2025, 14, 383. [Google Scholar] [CrossRef]
- Koutnik, A.P.; Klein, S.; Robinson, A.T.; Watso, J.C. Efficacy and Safety of Long-term Ketogenic Diet Therapy in a Patient With Type 1 Diabetes. JCEM Case Rep. 2024, 2, luae102. [Google Scholar] [CrossRef]
- Umpierrez, G.E.; Davis, G.M.; ElSayed, N.A.; Fadini, G.P.; Galindo, R.J.; Hirsch, I.B.; Klonoff, D.C.; McCoy, R.G.; Misra, S.; Gabbay, R.A.; et al. Hyperglycaemic crises in adults with diabetes: A consensus report. Diabetologia 2024, 67, 1455–1479. [Google Scholar] [CrossRef]
- Rydin, A.A.; Spiegel, G.; Frohnert, B.I.; Kaess, A.; Oswald, L.; Owen, D.; Simmons, K.M. Medical management of children with type 1 diabetes on low-carbohydrate or ketogenic diets. Pediatr. Diabetes 2021, 22, 448–454. [Google Scholar] [CrossRef]
| Authors | Title | Journal | Type of Article | Number of Participants |
|---|---|---|---|---|
| Babiker A et al., 2022 [4] | Frequency and Risk Factors of Diabetic Ketoacidosis in a Specialized Children’s Hospital, Riyadh: A Cross-sectional Study | Oman Medical Journal | Original research | 562 |
| da Rosa Carlos Monteiro LE et al., 2022 [5] | Precipitating factors of diabetic ketoacidosis in type 1 diabetes patients at a tertiary hospital: a cross-sectional study with a two-time-period comparison. | Archives of endocrinology and Metabolism | Original research | 97 |
| Huang SK et al., 2020 [6] | Acute kidney injury is a common complication in children and adolescents hospitalized for diabetic ketoacidosis. | PLoS ONE | Original research | 223 |
| Tao LC et al., 2024 [7] | Inflammatory biomarkers predict higher risk of hyperglycemic crises but not outcomes in diabetic patients with COVID-19. | Frontiers in Endocrinology | Original research | 124 |
| Hasan RA, Hesen JZ et al., 2024 [8] | Serum Phosphorus and Hypophosphatemia During Therapy of Diabetic Ketoacidosis in Children: Single-Center, Retrospective Cohort 2016–2022. | Pediatric Critical Care Medicine | Original research | 365 |
| Trainor JL et al., 2023 [9] | Clinical and Laboratory Predictors of Dehydration Severity in Children With Diabetic Ketoacidosis. | Annals of Emergency Medicine | Original research | 753 |
| Szabó GV et al., 2024 [10] | Fluid resuscitation with balanced electrolyte solutions results in faster resolution of diabetic ketoacidosis than with 0.9% saline in adults—A systematic review and meta-analysis. | Diabetes/Metabolism Research and Reviews | Meta-analysis | 1006 |
| Aygün D, Aygün F et al., 2017 [11] | Electrocardiographic changes in children with diabetic ketoacidosis and ketosis. | Turkish Archives of Pediatrics | Original research | 40 |
| Santos SS et al., 2023 [12] | Increased risk of death following recurrent ketoacidosis admissions: a Brazilian cohort study of young adults with type 1 diabetes. | Diabetology and Metabolic Syndrome | Original research | 231 |
| Turton JL et al., 2023 [13] | Effects of a low-carbohydrate diet in adults with type 1 diabetes management: A single arm non-randomized clinical trial. | PLoS ONE | Clinical Trial | 20 |
| Song C et al., 2023 [14] | Point-of-Care Capillary Blood Ketone Measurements and the Prediction of Future Ketoacidosis Risk in Type 1 Diabetes. | Diabetes Care | Clinical Trial | 484 |
| Alshurtan KS et al., 2022 [15] | Efficacy and Safety of Intravenous Insulin in Treatment of Patient With Diabetic Ketoacidosis: A Systematic Review and Meta-Analysis. | Cureus | Meta-analysis | 3258 |
| Andrade-Castellanos CA et al., 2016 [16] | Subcutaneous rapid-acting insulin analogues for diabetic ketoacidosis. | Cochrane Library | Original research | 201 |
| Tamzil R, Yaacob N et al., 2023 [17] | Comparing the clinical effects of balanced electrolyte solutions versus normal saline in managing diabetic ketoacidosis: A systematic review and meta-analyses. | Turkish Journal of Emergency Medicine | Original research | 595 |
| Jozwiak M et al., 2024 [18] | Management of diabetic keto-acidosis in adult patients admitted to intensive care unit: an ESICM-endorsed international survey. | Critical Care | Original research | 522 |
| Wolf RA et al., 2019 [19] | Hospital admissions for hyperglycemic emergencies in young adults at an inner-city hospital. | Diabetes Research and Clinical Practice | Original research | 273 |
| Toschi E et al., 2021 [20] | Continuous Glucose Monitoring and Glycemic Control in Young Adults with Type 1 Diabetes: Benefit for Even the Simplest Insulin Administration Methods | Diabetes Technology and Therapeutics | Original research | 888 |
| Vitale RJ et al., 2018 [21] | An Effective Diabetic Ketoacidosis Prevention Intervention in Children With Type 1 Diabetes. | SAGE Open Nursing | Original research | 76 |
| Karges B et al., 2017 [22] | Association of Insulin Pump Therapy vs. Insulin Injection Therapy With Severe Hypoglycemia, Ketoacidosis, and Glycemic Control Among Children, Adolescents, and Young Adults With Type 1 Diabetes. | JAMA Network | Original research | 30579 |
| van den Boom L et al., 2019 [23] | Temporal Trends and Contemporary Use of Insulin Pump Therapy and Glucose Monitoring Among Children, Adolescents, and Adults With Type 1 Diabetes Between 1995 and 2017. | American Diabetes Association | Original research | 96,547 |
| Gonçalves SEAB et al., 2021 [24] | Association between thiamine deficiency and hyperlactatemia among critically ill patients with diabetes infected by SARS-CoV-2. | Journal of Diabetes | Original research | 270 |
| Levran N et al., 2023 [25] | The Impact of a Low-Carbohydrate Diet on Micronutrient Intake and Status in Adolescents with Type 1 Diabetes. | Nutrients | Clinical Trial | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cielecka, J.; Szkamruk, Z.; Walędziak, M.; Różańska-Walędziak, A. Diabetic Ketoacidosis in Young Adults with Type 1 Diabetes: The Impact of the Ketogenic Diet—A Narrative Literature Review. Diseases 2025, 13, 347. https://doi.org/10.3390/diseases13100347
Cielecka J, Szkamruk Z, Walędziak M, Różańska-Walędziak A. Diabetic Ketoacidosis in Young Adults with Type 1 Diabetes: The Impact of the Ketogenic Diet—A Narrative Literature Review. Diseases. 2025; 13(10):347. https://doi.org/10.3390/diseases13100347
Chicago/Turabian StyleCielecka, Joanna, Zuzanna Szkamruk, Maciej Walędziak, and Anna Różańska-Walędziak. 2025. "Diabetic Ketoacidosis in Young Adults with Type 1 Diabetes: The Impact of the Ketogenic Diet—A Narrative Literature Review" Diseases 13, no. 10: 347. https://doi.org/10.3390/diseases13100347
APA StyleCielecka, J., Szkamruk, Z., Walędziak, M., & Różańska-Walędziak, A. (2025). Diabetic Ketoacidosis in Young Adults with Type 1 Diabetes: The Impact of the Ketogenic Diet—A Narrative Literature Review. Diseases, 13(10), 347. https://doi.org/10.3390/diseases13100347







