Abstract
Background/Objectives: Sub-Saharan Africa (SSA) is experiencing a rising burden of non-communicable diseases (NCDs), projected to surpass infectious diseases as the leading cause of mortality. This shift reflects a complex public health challenge driven by changing dietary patterns and persistent social and gender inequities. Women of reproductive age are particularly vulnerable due to biological and sociocultural factors, with diet playing a central role in NCD development and maternal health. This scoping review explores dietary determinants of NCDs in this population and identifies evidence gaps to support context-specific, gender-responsive interventions. Methods: The review followed the Joanna Briggs Institute methodology and PRISMA-ScR guidelines. A comprehensive search was conducted across PubMed, Scopus, and Google Scholar for studies published between January 2010 and May 2025. After removing duplicates, 577 articles were screened, and 19 met the inclusion criteria. Data were synthesized using descriptive statistics and thematic analysis. An adapted conceptual framework informed by the ecological model was developed to illustrate the multilevel pathways linking dietary determinants to NCD outcomes. Results: Frequent consumption of ultra-processed foods, salty snacks, processed meats, and sugar-sweetened beverages was consistently associated with increased NCD risk. Central obesity was prevalent among nearly half of the women studied, and a high intake of sugary drinks was common across diverse populations. Among pregnant women, overweight was observed in approximately one-quarter of participants, despite the inadequate intake of protein and iron. Vitamin A deficiency was moderately prevalent, and urban residence was linked to a twofold increase in the coexistence of obesity and micronutrient deficiencies. These patterns were shaped by poverty, food insecurity, and the cultural norms influencing dietary behaviors and access to nutritious foods. Conclusion: Dietary determinants significantly contribute to the rising NCD burden among women of reproductive age in SSA, yet adolescent women remain underrepresented in research. Addressing these gaps through culturally sensitive, multisectoral interventions and biomarker-informed longitudinal studies is essential for guiding inclusive policies and sustainable health strategies for this vulnerable population.
1. Introduction
Non-communicable diseases (NCDs) are now the leading cause of mortality globally, and sub-Saharan Africa (SSA) faces a dual burden of NCD and infectious diseases [1,2,3,4]. Women of reproductive age (15–49 years) are particularly vulnerable due to intersecting metabolic and reproductive health risks [5,6]. Rising rates of obesity, hypertension, and type 2 diabetes in this group are closely linked to poor dietary quality and metabolic dysfunction, with prevalence varying across SSA countries [7,8,9,10,11]. This growing burden is driven by a regional nutrition transition, from traditional, nutrient-rich diets to energy-dense, ultra-processed foods, often accompanied by reduced physical activity [12,13,14,15,16,17]. Urbanization, food insecurity, and limited access to healthcare and nutrition education further exacerbate these risks [8,9,18,19,20]. Micronutrient deficiencies, especially in vitamin A, iron, and zinc, frequently coexist with overweight and obesity, contributing to adverse maternal and fetal outcomes [21,22,23,24].
Despite increasing research on dietary risk factors for NCDs in SSA, the evidence remains fragmented and methodologically inconsistent, particularly for women of reproductive age, who are central to intergenerational health outcomes. In addition to other dietary determinants, such as high consumption of ultra-processed foods, low dietary diversity, inadequate intake of fruits and vegetables, and reliance on refined carbohydrates, more than two-thirds of women of reproductive age in Africa are estimated to be micronutrient deficient, largely due to widespread poor dietary quality and inadequate intake of nutrient-dense foods to meet their heightened nutritional needs [25].
Dietary determinants are contextual and behavioral factors that influence both food choices and dietary patterns. These encompass individual-level factors such as affordability, nutrition knowledge, and personal preferences; environmental influences including urbanization, food availability, and food insecurity; and sociocultural norms shaped by beliefs, traditions, and gender roles that guide food practices within communities [26,27,28].
Food fortification is a cost-effective and highly recommended food-based approach for addressing these micronutrient deficiencies in low-income settings [25]. Large-scale food fortification programs have shown benefits but are underreported [29,30,31,32]. Studies have recommended targeted fortification strategies for high-risk groups and advancements in fortification technologies, such as microencapsulation to enhance nutrient stability and monitoring systems to improve program coverage and ensure nutrient bioavailability [33,34].
This scoping review applies to the Joanna Briggs Institute methodology [35] and PRISMA-ScR guidelines [36] to map existing evidence and identify gaps. An adapted socio-ecological framework is used to illustrate the multilevel pathways linking dietary determinants to NCD outcomes in this population [16].
2. Materials and Methods
2.1. Methodological Approach
This scoping review was conducted in accordance with the Joanna Briggs Institute (JBI) methodological guidance for scoping reviews [35] and reported following the PRISMA-ScR (Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews) checklist (Supplementary File S1) [36]. A scoping review was selected to accommodate the broad and exploratory nature of the research question and to map the extent, range, and nature of evidence on dietary determinants of NCDs among women of reproductive age in SSA, where the existing literature is diverse and fragmented. Unlike systematic reviews, which address narrowly defined questions, a scoping review facilitates the mapping of key concepts, evidence types, and research gaps across a complex and heterogeneous field [37,38]. Although the review was not registered with PROSPERO, a preliminary search of the Cochrane Database of Systematic Reviews was conducted to ensure originality and transparency, confirming that no similar reviews had been published. The review process followed the five-stage framework developed by Arksey and O’Malley (2005) [37], with refinements from subsequent methodological advancements:
- Identifying the research question;
- Identifying relevant studies;
- Study selection;
- Charting the data;
- Collating, summarizing, and reporting the results.
To ensure conceptual clarity and relevance to the target population and context, the Population–Concept–Context (PCC) framework was used to guide the formulation of eligibility criteria and the scope of the review.
2.2. Search Strategy
A comprehensive and systematic literature search strategy (Supplementary File S2) was conducted across three major electronic databases: PubMed, Scopus, and Google Scholar. The search targeted peer-reviewed articles published between January 2010 and April 2025 and was limited to English-language studies with full-text availability. The strategy combined Medical Subject Headings (MeSHs) and free-text terms related to dietary intake, nutritional status, non-communicable diseases, and women of reproductive age in SSA.
To enhance relevance, terms were organized into four thematic categories: population, NCD, dietary determinants, and geographical context. For a study to be considered eligible, it had to include at least one term or phrase from each category. Initially, the search was restricted to titles and abstracts; however, this yielded limited results. The strategy was refined to include full-text searches, which significantly increased the number of eligible studies.
2.3. Study Selection
All retrieved citations were imported into EndNote reference management software, where duplicate records were identified and removed. Screening was conducted in two stages:
- Titles and abstracts were reviewed for relevance;
- Full-text articles were evaluated against inclusion and exclusion criteria.
Studies were excluded if they focused on infectious diseases, male populations, or were conducted outside SSA. Editorials, reviews, protocols, case reports, and unpublished materials were also excluded.
Two reviewers (N.T. and M.D.S.) independently screened titles, abstracts, and full texts using predefined criteria. Discrepancies were resolved through discussion, and a third reviewer (P.M.) was consulted when necessary to ensure consistency and minimize bias.
Eligibility Criteria
Studies were eligible if they fulfilled the following criteria:
- Focused on women aged 15 to 49 years (women of reproductive age);
- Examined dietary factors or nutritional status in relation to NCD outcomes;
- Were conducted in SSA;
- Used original research designs (cross-sectional, cohort, RCTs, or mixed methods).
- The MEDLINE search string included combinations of the following terms:
- Population terms: “Reproductive-Aged Women”, “Women 15–49 Years”, “WRA”, and “female”;
- NCD terms: “Non-communicable Diseases”, “diabetes”, “hypertension”, “obesity”, and “cardiovascular diseases”;
- Dietary terms: “Dietary Determinants”, “Nutritional Status”, “Dietary Patterns”, and “Food Security”;
- Context terms: “Sub-Saharan Africa”, “Southern Africa”, and “South Africa”.
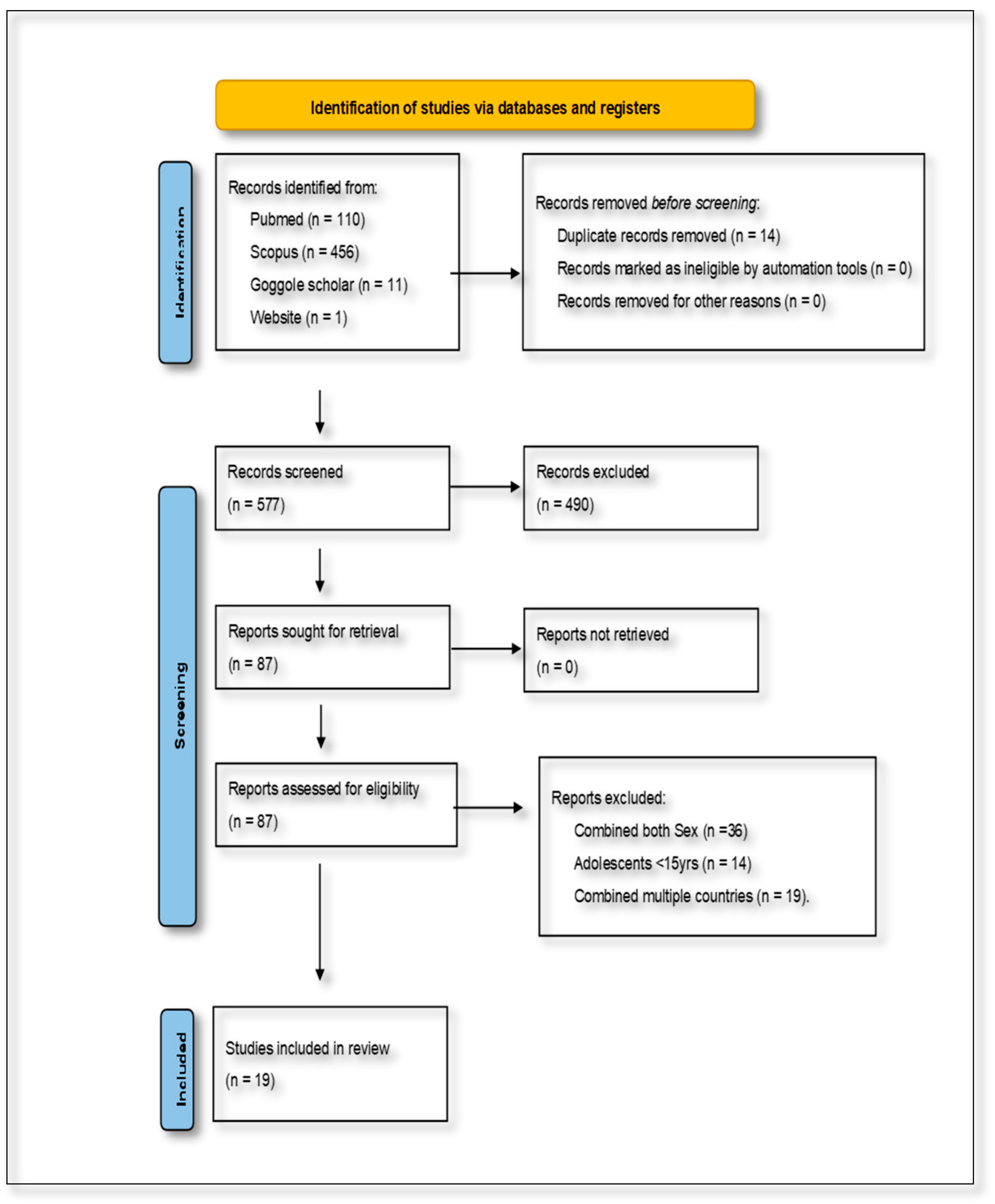
The combined database searches initially yielded 577 articles. After removing duplicates and screening for relevance based on the inclusion and exclusion criteria, 19 studies were identified as eligible for inclusion in the final review. These studies specifically addressed the dietary determinants of NCDs among women of reproductive age in SSA. The characteristics of the included studies were analyzed in terms of their rationale, participant demographics, dietary and health outcomes, study duration, research methodology, and reported impacts. This information was synthesized narratively to maintain a cohesive and contextually grounded presentation of the findings. The study selection process is visually summarized in Figure 1, which presents the PRISMA flow diagram outlining the identification, screening, eligibility, and inclusion stages of the review.
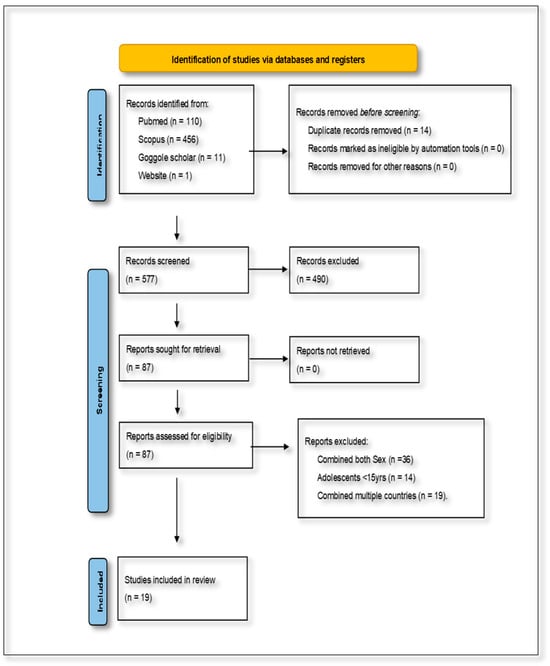
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram, showing the selection of studies for the present scoping review.
2.4. Data Charting and Analysis
Data were extracted using a structured charting form that was developed and iteratively refined by the research team to ensure consistency and relevance. The charting process captured key variables such as study characteristics (including author, year of publication, country, and study design), population demographics, dietary assessment methods, NCD outcomes, and the main findings of each study. Data extraction was conducted independently by two reviewers (N.T. and M.D.S.), with discrepancies resolved through discussion or consultation with a third reviewer (P.M). This approach ensured consistency and minimized bias in the charting process. The analytical approach combined both descriptive numerical summaries and thematic synthesis to provide a comprehensive understanding of the evidence.
Descriptive statistics were employed to quantify the distribution of studies across countries, study designs, and population characteristics, offering a broad overview of the research landscape. In parallel, thematic analysis was conducted to identify recurring patterns and contextual factors that influence dietary behaviors and NCD risk among women of reproductive age. Themes were developed inductively from the data and refined through collaborative discussions among the research team to ensure that they aligned with the objectives of the review. This dual approach enabled the synthesis of both quantitative and qualitative dimensions of the evidence base, yielding valuable insights into the dietary determinants of non-communicable diseases in the target population.
3. Results
3.1. Study Characteristics
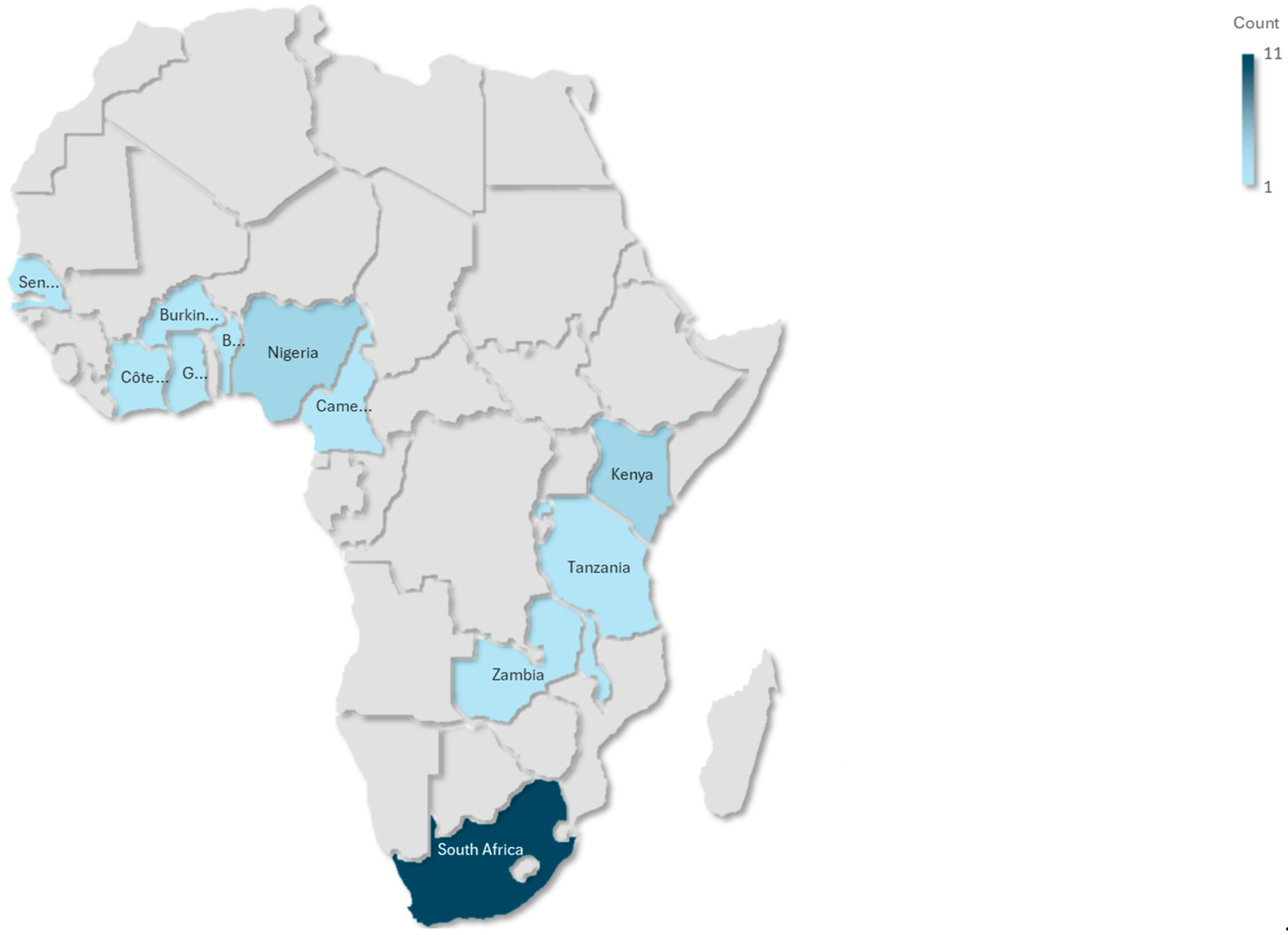
This scoping review synthesized findings from 19 studies conducted across SSA, with South Africa being the most represented country (n = 11), while a small cluster of countries have minimal coverage, including Ghana, Kenya, Burkina Faso, Zambia, Rwanda, Malawi, Nigeria, and Benin. This pattern suggests substantial research inequality across the continent, with evidence concentrated in South Africa, while most of Africa remains understudied. The studies covered a range of geographical contexts, urban, peri-urban, and rural, and diverse methodological designs. These included cross-sectional analyses (n = 15), randomized controlled trials (n = 1), mixed-method approaches (n = 1), and longitudinal cohort studies (n = 2). The study populations were predominantly composed of socioeconomically disadvantaged women, many of whom were unemployed or experiencing food insecurity. For example, in Kenya and Burkina Faso, 38% of participants were classified as severely food insecure. Several studies focused on specific subgroups, such as women living with HIV, women with obesity, pregnant women with micronutrient deficiencies, and urban women at elevated risk of type 2 diabetes. Table 1 reflects the stratification variables, including age group, geographic setting (urban/rural), HIV status, and dietary assessment method, factors that are critical in shaping dietary exposures and NCD risks and that are explicitly discussed in the synthesis.

Table 1.
The characteristics of the included studies.
3.2. Individual-Level Determinants
This section focuses on individual-level dietary determinants, including affordability, nutrition knowledge, and personal food preferences, which directly influence dietary behaviors and NCD risk. A consistent association emerged between the consumption of processed, energy-dense foods and increased risk of NCD. In South Africa, frequent intake of takeaway foods, salty snacks, and processed meats was significantly associated with elevated odds of hypertension, diabetes, and cardiac events (adjusted odds ratios [AORs] ranging from 1.42 to 2.45) [43]. In Rwanda, central obesity (prevalence: 48.5%) was strongly linked to weekly meat consumption (OR = 5.3) and alcohol intake (OR = 5.8) [45].
Sugar-sweetened beverage (SSB) consumption exceeded 50% among women in Kenya and Burkina Faso, with higher intake associated with employment status and greater dietary diversity [44].
Micronutrient deficiencies were also prevalent: inadequate intake of protein, iron, and zinc among pregnant women was associated with adverse fetal outcomes [46], while vitamin A deficiency (11.7% prevalence) was linked to systemic inflammation [47]. Dietary determinants such as food insecurity, cultural norms, and limited nutrition knowledge were frequently reported. In contrast, dietary patterns characterized as Western (high in processed foods and sugary beverages) or traditional (rich in legumes and whole grains) were associated with distinct NCD outcomes. Of the 19 studies included in this scoping review, 11 employed only self-reported dietary assessment methods [39,41,42,44,45,46,47,48,49,50,51], 1 relied exclusively on biomarker-based measures [56], and 4 integrated both dietary questionnaires and biomarker analyses [42,45,47,50]. However, the reliance on self-reported tools indicates a methodological gap as well as the use of cross-sectional studies limiting causal interpretation, as discussed in detail in the limitations section. Two studies did not employ dietary assessment or biomarker-based analysis; instead, they focused on anthropometric outcomes and/or socioeconomic and/or reproductive factors as indirect determinants of nutritional status [8,48]. Additionally, one study used a 24-hour dietary recall method (24HR) to assess dietary quality and NCD-related food patterns, focusing on dietary risk indicators rather than direct clinical NCD outcomes (Table 2).

Table 2.
Mapping of dietary factors, assessment methods, and associated NCD outcomes across included studies.
3.3. Socioeconomic and Cultural Influences
This section focuses on the environmental and sociocultural determinants, such as urbanization, food insecurity, and cultural norms, that shape dietary behaviors at the community and societal levels. Socioeconomic deprivation and cultural norms were found to significantly shape dietary behaviors among women of reproductive age in sub-Saharan Africa. In Zambia, women attributed hypertension not only to the consumption of poor-quality foods, such as chemically grown vegetables, but also to psychosocial stress stemming from caregiving responsibilities [41]. In South Africa, cultural perceptions played a notable role, where body fatness was often valorized as a protective social signal against HIV-related stigma. Additionally, unemployment and low income were consistently associated with a reliance on calorie-dense, nutrient-poor diets [8,47]. Urban residence further compounded nutritional risk, with studies showing a twofold increase in the likelihood of coexisting obesity and micronutrient deficiencies among urban women (adjusted prevalence ratio [aPR] = 2.0) [51].
3.4. Biological Mediators and Health Outcomes
Nutritional inadequacies during pregnancy emerged as a critical concern across several studies. In South Africa, 24.2% of pregnant women were classified as overweight, yet their intake of essential nutrients such as protein and iron remained below the recommended dietary allowances (RDAs) [46]. Adherence to a traditional dietary pattern, characterized by the consumption of whole grains and legumes, was associated with reduced odds of excessive gestational weight gain (odds ratio [OR] = 0.68) [55]. In contrast, a study evaluating the impact of lipid-based nutrient supplementation (LNS) in Ghana found that such interventions did not significantly reduce the risk of hypertension among pregnant women [40].
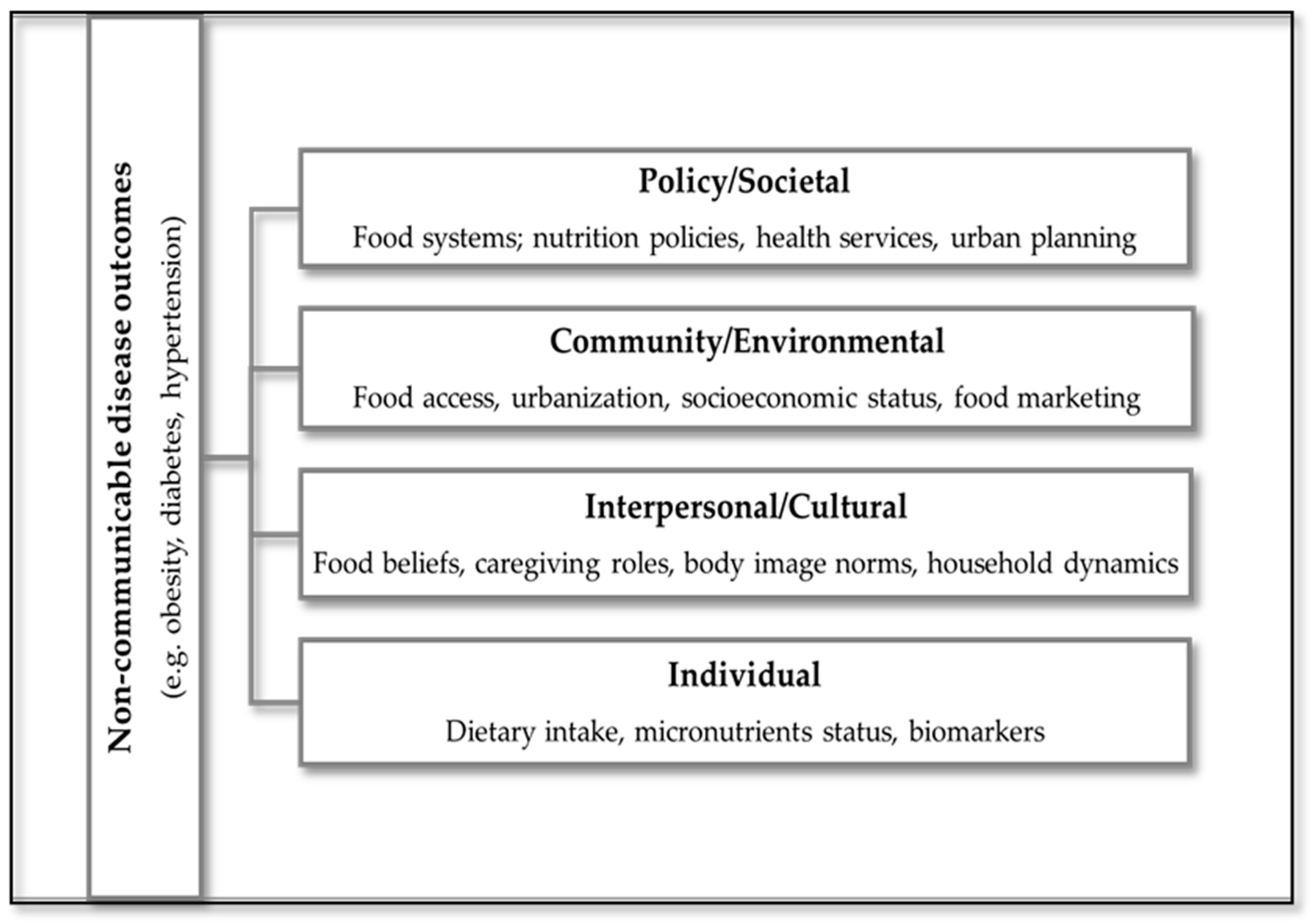
3.5. Conceptual Framework
To visually synthesize the findings of this scoping review, an adapted conceptual framework was developed to illustrate the pathways linking dietary determinants to NCD outcomes among women of reproductive age in SSA (Figure 2). This framework is informed by the socio-ecological model, which has been previously applied to dietary and physical activity behaviors in similar populations [16]. The framework integrates elements of the ecological model, capturing multilevel influences: individual, interpersonal, community, and societal, and incorporates biomedical mediators such as inflammation and micronutrient status. It also reflects cultural and socioeconomic factors that shape dietary behaviors, including urbanization, poverty, food insecurity, and body image norms. This model provides a structured lens through which to understand the complex interplay of determinants and outcomes, and supports the development of context-specific, culturally responsive interventions.
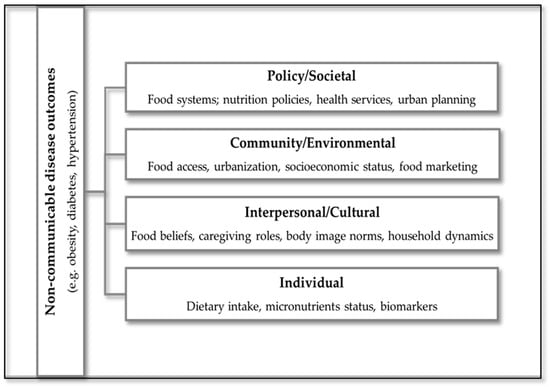
Figure 2.
Conceptual framework illustrating multilevel dietary determinants and their pathways to NCD outcomes among women of reproductive age in SSA.
In this framework, dietary determinants, such as socioeconomic status, food access, and cultural norms, are positioned as upstream influences that shape dietary behaviors. Dietary patterns, including Western and traditional diets, are conceptualized as downstream outcomes of these determinants, which in turn influence NCD risk.
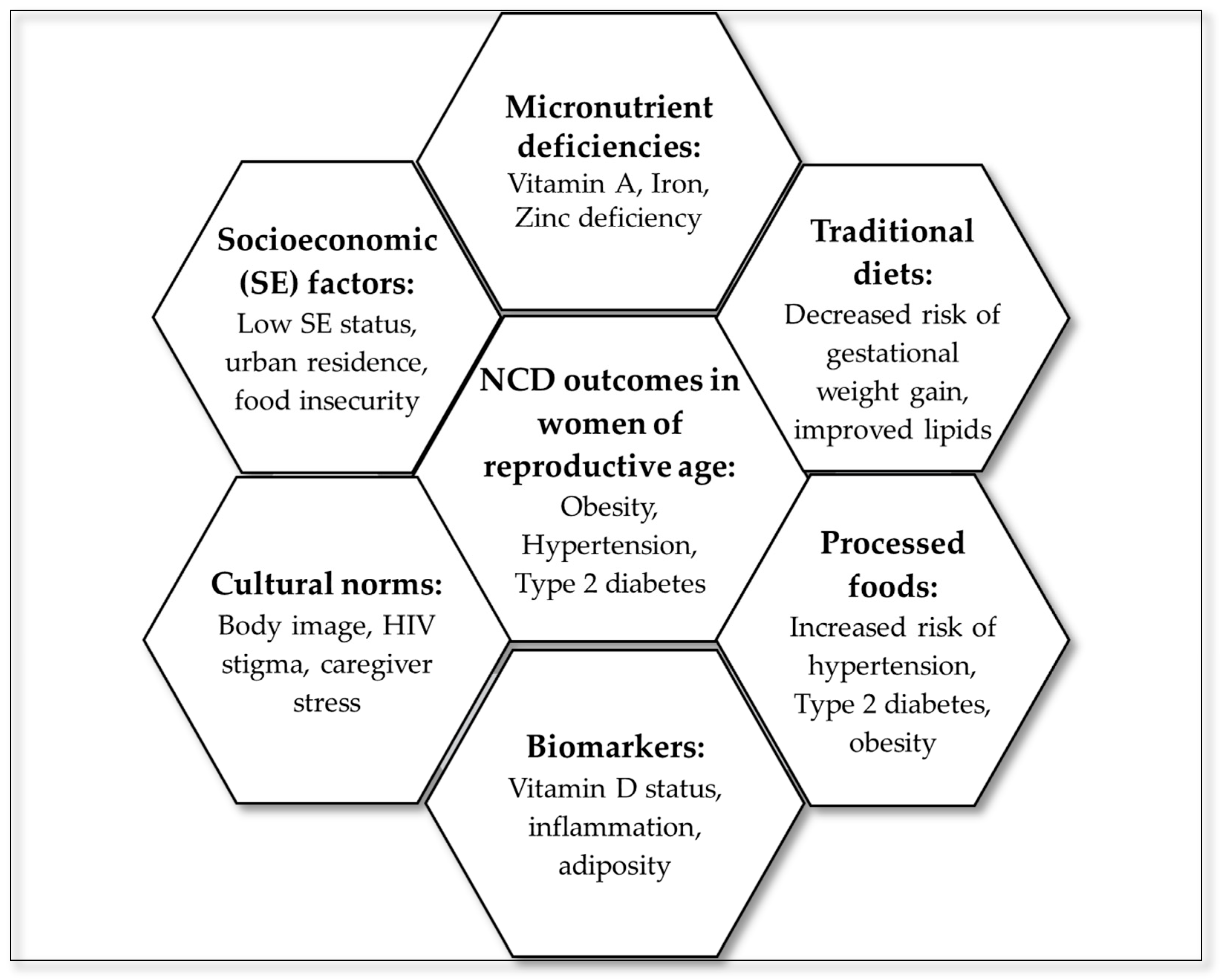
3.6. Summary of Evidence
To visually consolidate the thematic findings of this scoping review, a hexagon radial summary figure was developed (Figure 3), linking dietary domains to NCD outcomes. The central hexagon represents NCD outcomes in women of reproductive age, surrounded by six interlinked domains: processed foods, traditional diets, micronutrient deficiencies, socioeconomic factors, cultural norms, and biomarkers. These domains reflect the multilevel and interconnected nature of dietary determinants and their influence on NCD outcomes.
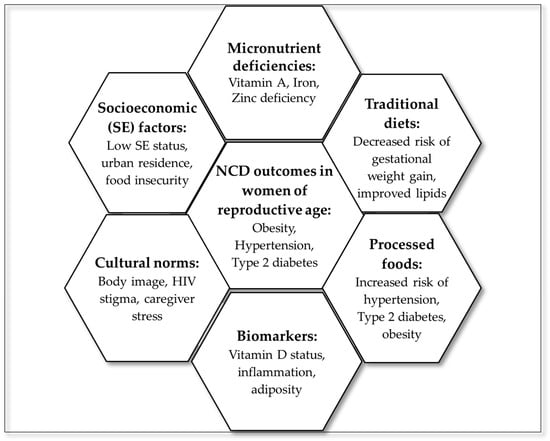
Figure 3.
Summary of evidence from the scoping review.
To highlight geographic disparities in research coverage, an African evidence gap map was developed (Figure 4). This visual illustrates the uneven distribution of studies across SSA, with a notable concentration in South Africa (n = 11) and limited representation from other regions (n = 8).
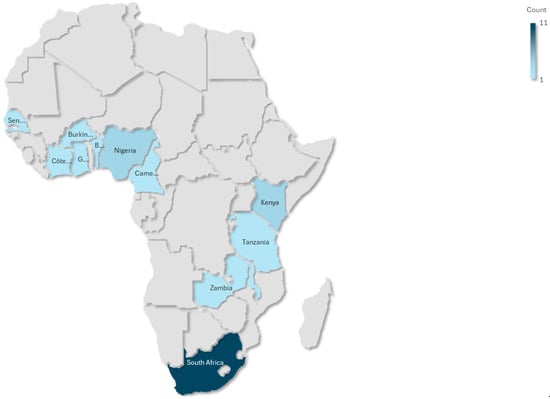
Figure 4.
African evidence map.
4. Discussion
This scoping review synthesizes evidence on dietary determinants contributing to the rising burden of NCDs among women of reproductive age in SSA. The findings showed a complex interplay between poor dietary quality, nutritional inadequacies, and broader socioeconomic and cultural factors. Dietary determinants such as food insecurity, cultural norms, and limited nutrition knowledge influence food choices, while dietary patterns, such as Western (high in processed foods and sugary beverages) or traditional (rich in legumes and whole grains), reflect the habitual combinations of foods consumed. These patterns were associated with distinct NCD outcomes across the studies reviewed. As SSA undergoes a rapid nutrition transition, traditional diets are increasingly replaced by energy-dense, ultra-processed foods, disproportionately affecting women [13,19,24,43,45]. The findings of this review align with a modified ecological framework, which emphasizes the interplay of individual dietary behaviors, interpersonal and cultural norms, community-level food environments, and broader policy influences. This multilevel perspective is essential for designing effective interventions that address both biological and social determinants of NCD risk.
Frequent consumption of processed foods, such as salty snacks, meats, and sugar-sweetened beverages (SSBs), was consistently associated with hypertension, obesity, and type 2 diabetes. These trends mirror global dietary westernization, but are especially concerning in SSA, where health systems are often ill-equipped to manage the dual burden of infectious and chronic diseases. The association between SSB intake and factors like employment and dietary diversity [44] highlights the need for integrated, nutrition-sensitive policies that go beyond economic empowerment to address food environments, marketing, and cultural norms.
A key finding of this review is the widespread coexistence of micronutrient deficiencies with overweight and obesity, a double burden of malnutrition increasingly observed in SSA [8,24,57,58]. Deficiencies in vitamin A, iron, and zinc, particularly among pregnant women, have serious implications for maternal and fetal health [59,60], acting not only as nutritional gaps, but also as biological stressors that exacerbate inflammation and disease risk [61,62]. Despite this, most studies relied on self-reported dietary tools such as 24-hour recalls and food frequency questionnaires, with limited use of biomarker-based assessments, highlighting the need for more objective methods to better understand diet–disease relationships. This shows the need for integrating objective biomarker data in future research to better capture physiological responses to dietary exposures and improve causal inference.
In this context, food fortification emerges as a critical and cost-effective strategy to improve the intake of essential micronutrients among women of reproductive age, particularly in regions where the burden of deficiency is highest [24]. Fortification of staple foods like maize meal and wheat flour with iron, folic acid, and vitamin A has demonstrated positive impacts on reducing deficiencies and improving maternal and child health outcomes [29,32]. However, its potential remains underutilized due to inconsistent implementation, limited public awareness, weak regulatory enforcement, and poor access in rural and informal markets. Furthermore, the lack of disaggregated data on micronutrient status among women of reproductive age limits targeted policy responses. We therefore recommend future studies to conduct stratified analyses to better inform targeted interventions. Stratified analyses by age, geographic location, HIV status, and dietary assessment method are essential to uncover subgroup-specific vulnerabilities and guide the development of tailored nutrition and health policies across diverse SSA contexts. Addressing these barriers is essential to fully leverage food fortification as a scalable and sustainable intervention to reduce micronutrient deficiencies and mitigate NCD risk in this vulnerable population [30].
Dietary behaviors are shaped by socioeconomic and cultural contexts. In Zambia, women linked hypertension to both poor-quality food and caregiving stress [41]. In South Africa, cultural norms that valorize body fatness as protection against HIV stigma continue to influence food choices [63,64]. These findings align with the broader literature on food beliefs and social identity [56,65]. Urbanization, while improving access to services, has also increased exposure to obesogenic environments and processed food markets [24,51]. Nutritional inadequacies during pregnancy, such as low protein and iron intake despite high overweight rates, show the mismatch between dietary patterns and physiological needs [46]. Conversely, traditional diets rich in legumes and whole grains were protective against excessive gestational weight gain [55]. In addition to individual and community-level factors, structural interventions targeting the broader food environment are critical. Evidence from SSA and other LMICs suggests that policies such as taxation on sugar-sweetened beverages, front-of-pack labeling, and restrictions on marketing unhealthy foods to children can significantly influence dietary choices and reduce NCD risk [18,66].
The included studies offer several strengths that contribute to the overall value of this review. Many studies employed validated dietary assessment tools and included diverse populations across urban and rural settings. A subset incorporated biomarker-based measures, enhancing the objectivity of nutritional status assessments. Additionally, the geographic spread, though uneven, provides insight into regional dietary patterns and NCD risks among women of reproductive age in SSA.
Further identified key methodological and contextual limitations highlight the need for standardized data collection in future cohort studies. To address these gaps, future studies should consider collecting a standardized minimum dataset that includes dietary diversity scores (DDS), biomarkers such as C-reactive protein (CRP) and HbA1c, and anthropometric indicators to improve data quality and enable cross-study comparisons. To support systematic and comparable research across sub-Saharan Africa (SSA), we propose a minimum data package for NCD-focused nutrition studies. This should include the following: (1) standardized biomarkers such as CRP, HbA1c, and iron indices to assess inflammation and metabolic risk; (2) anthropometric indicators including BMI, waist circumference, and body composition measures; (3) DDS and context-adapted dietary assessment tools; and (4) sociodemographic variables such as age, education, income, and urban/rural residence. Collecting these core data elements will improve comparability, enable stratified analyses, and strengthen the evidence base for targeted interventions. The predominance of South African studies, driven by stronger research infrastructure and national datasets, raises concerns about regional equity in evidence generation, making it difficult to generalize the findings to other African regions. Geographic data gaps may lead to an overreliance on evidence from a limited number of countries or urban settings, potentially resulting in policy blind spots and interventions that fail to address the diverse dietary patterns and health needs of underserved populations. Limited representation from other SSA countries creates gaps in understanding dietary determinants across diverse sociocultural contexts, potentially skewing policy relevance. Future research should prioritize underrepresented regions and populations to ensure inclusive, context-specific interventions. Many studies had small sample sizes, short durations, and relied on cross-sectional designs and self-reported dietary data, which limit causal inference and introduce bias. Future research should also prioritize validating and adapting dietary assessment tools for use in low-literacy and resource-limited settings to ensure accurate data collection and culturally appropriate nutrition interventions. To improve rigor and applicability, longitudinal designs and biomarker-based assessments should be adopted. The quality of evidence, particularly the predominance of cross-sectional designs and reliance on self-reported dietary data, has direct implications for implementation feasibility. These limitations constrain the precision of risk estimates and the ability to design targeted interventions. Strengthening evidence through longitudinal studies and biomarker-informed assessments will enhance the reliability of findings and support the development of scalable, context-specific nutrition policies. To improve rigor and applicability, future cohort studies should incorporate standardized biomarkers, such as CRP, HbA1c, and iron indices, to assess inflammation and metabolic risk. Additionally, dietary assessment tools should be culturally and contextually adapted to ensure accurate data collection across diverse SSA populations. Given the unique biological and sociocultural vulnerabilities of women of reproductive age, interventions must be gender-responsive, integrating reproductive health with nutrition and NCD prevention.
Multisectoral collaboration across health, agriculture, education, and urban planning is essential to improve food environments and promote equitable access to nutritious diets. These recommendations align existing policy instruments such as the WHO Package of Essential Noncommunicable Disease Interventions (WHO PEN) with national dietary guidelines, which advocate improved dietary quality, reduced intake of ultra-processed foods, and the integration of nutrition services into primary healthcare. Nutrition policies should focus on reducing ultra-processed food intake and integrating dietary counseling into maternal care. We recommend leveraging the role of community health workers to deliver culturally appropriate nutrition education and counseling, particularly in underserved and resource-limited settings. Urban-specific strategies and biomarker-informed research are vital to address obesity, micronutrient deficiencies, and identify at-risk subgroups. Adolescent women of reproductive age were notably underrepresented in the included studies, and where they were included, age-disaggregated data were often lacking. This limits the ability to assess adolescent-specific dietary patterns, nutritional risks, and NCD vulnerabilities, despite evidence that adolescence is a critical window for establishing lifelong health behaviors and addressing intergenerational health risks. Future research should prioritize adolescent-focused analyses and interventions, incorporating age-specific dietary assessments and biomarker data to inform targeted strategies that address the unique biological, social, and developmental needs of this subgroup.
Additionally, national and regional nutrition strategies should prioritize the scale-up and monitoring of food fortification programs, particularly in underserved areas, to address persistent micronutrient deficiencies and reduce NCD risk among women of reproductive age. Furthermore, the emerging use of digital tools and mobile health platforms to support dietary behavior changes and improve access to nutrition services, especially among younger and urban populations, should be explored.
5. Conclusions
This review highlights the pivotal role of diet in the rising burden of NCDs among women of reproductive age in SSA. Addressing both the quantity and quality of dietary intake is essential to improve individual health outcomes and mitigate intergenerational risks. The evidence highlights the urgent need for context-specific, culturally appropriate, and multisectoral interventions that integrate health, agriculture, education, and urban planning to cultivate supportive food environments and promote sustainable dietary behaviors. Future research should adopt longitudinal cohort designs for causal effects and incorporate biomarker-based assessments to better capture the complex relationships between diet, metabolic health, and disease risk. Specifically, we recommend the inclusion of standardized biomarkers such as CRP, HbA1c, and micronutrient panels to strengthen the evidence base and inform more targeted, context-specific interventions. These approaches will generate actionable insights to inform policies tailored to the diverse needs of women across SSA. Efforts are especially critical in underrepresented regions, where gaps in research and policy implementation persist. Integrating evidence-based dietary strategies, including the scale-up of food fortification programs, into broader health and development agendas can help to reduce NCD prevalence and support long-term well-being among women of reproductive age.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diseases13100313/s1, File S1: PRISMA-ScR checklist; File S2: strategy search.
Author Contributions
Conceptualization, P.M.; writing—original draft preparation, P.M., N.T. and M.D.S.; writing—review and editing, P.M., N.T., M.D.S. and K.D.M.; visualization, P.M., N.T., M.D.S. and K.D.M.; project administration, P.M. and N.T.; funding acquisition, P.M. All authors have read and agreed to the published version of the manuscript.
Funding
The Article Processing Charge will be funded by the Non-Communicable Diseases Research Unit (NCDRU), South African Medical Research Council (SAMRC).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 24HR | 24-hour dietary recall questionnaire |
| aPR | Adjusted prevalence ratio |
| FFQ | Food frequency questionnaire |
| JBI | Joanna Briggs Institute |
| LDL | Low-density lipoprotein cholesterol |
| LNSs | Lipid-based nutrient supplements |
| MEDLINE | Medical Literature Analysis and Retrieval System Online |
| MeSHs | Medical Subject Headings |
| NCDs | Non-communicable diseases |
| PCC | Population–Concept–Context |
| PRISMA-ScR | Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews |
| SSA | Sub-Saharan Africa |
| SSBs | Sugar-sweetened beverages |
| TRE | Time-restricted eating |
References
- Bhuiyan, M.A.; Galdes, N.; Cuschieri, S.; Hu, P. A comparative systematic review of risk factors, prevalence, and challenges contributing to non-communicable diseases in South Asia, Africa, and Caribbeans. J. Health Popul. Nutr. 2024, 43, 140. [Google Scholar] [CrossRef]
- Ngaruiya, C.; Bernstein, R.; Leff, R.; Wallace, L.; Agrawal, P.; Selvam, A.; Hersey, D.; Hayward, A. Systematic review on chronic non-communicable disease in disaster settings. BMC Public Health 2022, 22, 1234. [Google Scholar] [CrossRef] [PubMed]
- Modjadji, P. Communicable and non-communicable diseases coexisting in South Africa. Lancet Glob. Health 2021, 9, e889–e890. [Google Scholar] [CrossRef]
- Cuadros, D.F.; Moreno, C.M.; Tomita, A.; Singh, U.; Olivier, S.; Castle, A.; Moosa, Y.; Edwards, J.A.; Kim, H.-Y.; Siedner, M.J.; et al. Geospatial assessment of the convergence of communicable and non-communicable diseases in South Africa. J. Multimorb. Comorbidity 2023, 13, 26335565231204119. [Google Scholar] [CrossRef]
- Mahlangu, K.; Modjadji, P.; Madiba, S. The Nutritional Status of Adult Antiretroviral Therapy Recipients with a Recent HIV Diagnosis; A Cross-Sectional Study in Primary Health Facilities in Gauteng, South Africa. Healthcare 2020, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Modjadji, P.; Mokgalaboni, K.; Nonterah, E.A.; Lebelo, S.L.; Mchiza, Z.J.-R.; Madiba, S.; Kengne, A.P. A Systematic Review on Cardiometabolic Risks and Perinatal Outcomes among Pregnant Women Living with HIV in the Era of Antiretroviral Therapy. Viruses 2023, 15, 1441. [Google Scholar] [CrossRef]
- Bonita, R.; Beaglehole, R. Women and NCDs: Overcoming the neglect. Glob. Health Action 2014, 7, 23742. [Google Scholar] [CrossRef]
- Modjadji, P. Socio-Demographic determinants of overweight and obesity among mothers of primary school children living in a rural health and demographic surveillance system site, South Africa. Open Public Health J. 2020, 13, 518–528. [Google Scholar] [CrossRef]
- Juma, K.; Juma, P.; Shumba, C.; Otieno, P.; Asiki, G. Non-Communicable Diseases and Urbanization in African Cities: A Narrative Review. In Public Health in Developing Countries: Challenges and Opportunities; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Owobi, O.; Okonji, O.; Nzoputam, C.; Ekholuenetale, M. Country-Level Variations in Overweight and Obesity among Reproductive-Aged Women in Sub-Saharan Countries. Women 2022, 2, 313–325. [Google Scholar] [CrossRef]
- Yaya, S.; Ekholuenetale, M.; Bishwajit, G. Differentials in prevalence and correlates of metabolic risk factors of non-communicable diseases among women in sub-Saharan Africa: Evidence from 33 countries. BMC Public Health 2018, 18, 1168. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Ng, S.W. The nutrition transition to a stage of high obesity and noncommunicable disease prevalence dominated by ultra-processed foods is not inevitable. Obes. Rev. 2022, 23, e13366. [Google Scholar] [CrossRef]
- Vorster, H.H.; Kruger, A.; Margetts, B.M. The nutrition transition in Africa: Can it be steered into a more positive direction? Nutrients 2011, 3, 429–441. [Google Scholar] [CrossRef]
- Nnyepi, M.S.; Gwisai, N.; Lekgoa, M.; Seru, T. Evidence of nutrition transition in Southern Africa. Proc. Nutr. Soc. 2015, 74, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Shetty, P. Nutrition Transition and Its Health Outcomes. Indian J. Pediatr. 2013, 80, 21–27. [Google Scholar] [CrossRef]
- Yiga, P.; Seghers, J.; Ogwok, P.; Matthys, C. Determinants of dietary and physical activity behaviours among women of reproductive age in urban sub-Saharan Africa: A systematic review. Br. J. Nutr. 2020, 124, 761–772. [Google Scholar] [CrossRef]
- Delisle, H.; Agueh, V.-D.; Sodjinou, R.; Ntandou-Bouzitou, G.D.; Daboné, C. Dietary Quality and the Nutrition Transition in Sub-Saharan Africa. In Diet Quality: An Evidence-Based Approach; Preedy, V.R., Hunter, L.-A., Patel, V.B., Eds.; Springer: New York, NY, USA, 2013; Volume 2, pp. 263–279. [Google Scholar] [CrossRef]
- Osei-Kwasi, H.; Mohindra, A.; Booth, A.; Laar, A.; Wanjohi, M.; Graham, F.; Pradeilles, R.; Cohen, E.; Holdsworth, M. Factors influencing dietary behaviours in urban food environments in Africa: A systematic mapping review. Public Health Nutr. 2020, 23, 2584–2601. [Google Scholar] [CrossRef]
- Gissing, S.C.; Pradeilles, R.; Osei-Kwasi, H.A.; Cohen, E.; Holdsworth, M. Drivers of dietary behaviours in women living in urban Africa: A systematic mapping review. Public Health Nutr. 2017, 20, 2104–2113. [Google Scholar] [CrossRef] [PubMed]
- Oldewage-Theron, W.H.; Dicks, E.G.; Napier, C. Poverty, household food insecurity and nutrition: Coping strategies in an informal settlement in the Vaal Triangle, South Africa. Public Health 2006, 120, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Labadarios, D.; Steyn, N.P.; Nel, J. How diverse is the diet of adult South Africans? Nutr. J. 2011, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Peñalvo, J.L.; Cudhea, F.; Imamura, F.; Rehm, C.D.; Mozaffarian, D. Association Between Dietary Factors and Mortality From Heart Disease, Stroke, and Type 2 Diabetes in the United States. JAMA 2017, 317, 912–924. [Google Scholar] [CrossRef] [PubMed]
- WHO. Non-Communicable Diseases and Reproductive Health in Sub-Saharan Africa: Bridging the Policy-Implementation Gaps. Reproductive Health, 2020. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 8 July 2025).
- Harika, R.; Faber, M.; Samuel, F.; Kimiywe, J.; Mulugeta, A.; Eilander, A. Micronutrient Status and Dietary Intake of Iron, Vitamin A, Iodine, Folate and Zinc in Women of Reproductive Age and Pregnant Women in Ethiopia, Kenya, Nigeria and South Africa: A Systematic Review of Data from 2005 to 2015. Nutrients 2017, 9, 1096. [Google Scholar] [CrossRef] [PubMed]
- Coomson, J.B.; Smith, N.W.; McNabb, W. Impacts of Food Fortification on Micronutrient Intake and Nutritional Status of Women of Reproductive Age in Africa—A Narrative Review. Adv. Nutr. 2025, 16, 100463. [Google Scholar] [CrossRef]
- Leng, G.; Adan, R.A.H.; Belot, M.; Brunstorm, J.M.; de Graaf, K.; Dickson, S.L.; Hare, T.; Maier, S.; Menzies, J.; Preissl, H.; et al. The determinants of food choice. Proc. Nutr. Soc. 2017, 76, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, S.; Byrne, N.M.; Hills, A.P. Cultural influences on dietary choices. Prog. Cardiovasc. Dis. 2025, 90, 22–26. [Google Scholar] [CrossRef]
- Monterrosa, E.C.; Frongillo, E.A.; Drewnowski, A.; de Pee, S.; Vandevijvere, S. Sociocultural Influences on Food Choices and Implications for Sustainable Healthy Diets. Food Nutr. Bull. 2020, 41 (Suppl. S2), 59S–73S. [Google Scholar] [CrossRef]
- Janmohamed, A.; Baker, M.M.; Doledec, D.; Ndiaye, F.; Konan, A.C.L.; Leonce, A.; Kouadio, K.L.; Beye, M.; Danboyi, D.; Jumbe, T.J.; et al. Dietary Quality and Associated Factors among Women of Reproductive Age in Six Sub-Saharan African Countries. Nutrients 2024, 16, 1115. [Google Scholar] [CrossRef]
- Kiran, A.; Wakeel, A.; Mahmood, K.; Mubaraka, R.; Hafsa; Haefele, S.M. Biofortification of Staple Crops to Alleviate Human Malnutrition: Contributions and Potential in Developing Countries. Agronomy 2022, 12, 452. [Google Scholar] [CrossRef]
- Labadarios, D.; Steyn, N.P.; Maunder, E.; MacIntryre, U.; Gericke, G.; Swart, R.; Huskisson, J.; Dannhauser, A.; Vorster, H.H.; Nesmvuni, A.E.; et al. The National Food Consumption Survey (NFCS): South Africa, 1999. Public Health Nutr. 2005, 8, 533–543. [Google Scholar] [CrossRef]
- Modjadji, S.E.P.; Alberts, M.; Mamabolo, R.L. Folate and iron status of South African non-pregnant rural women of childbearing age, before and after fortification of foods. S. Afr. J. Clin. Nutr. 2007, 20, 89–93. [Google Scholar] [CrossRef]
- Thurnham, D.I. Nutrition of Adolescent Girls in Low and Middle Income Countries. Sight Life Mag. Focus Afr. 2013, 27, 26–36. [Google Scholar] [CrossRef]
- Bechoff, A. Fortification Strategies for Nutritional Improvement: Insights from the Journal of Fortification. J. Nutr. Hum. Health 2024, 8, 220. [Google Scholar]
- Peters, M.D.J.; Marnie, C.; Tricco, A.C.; Pollock, D.; Munn, Z.; Alexander, L.; McInerney, P.; Godfrey, C.M.; Khalil, H. Updated methodological guidance for the conduct of scoping reviews. JBI Evid. Synth. 2020, 18, 2119–2126. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef]
- Hoosen, F.; Pico, M.L.; Goedecke, J.H.; Dave, J.A.; Quist, J.S.; Færch, K.; Grunnet, L.G.; de Villiers, A.; Aagaard-Hansen, J.; Mendham, A.E. Development and feasibility testing of a time-restricted eating intervention for women living with overweight/obesity and HIV in a resource-limited setting of South Africa. BMC Public Health 2024, 24, 2768. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.M.; Young, R.R.; Buchanan, A.; Lofgren, I.E.; Okronipa, H.E.T.; Lartey, A.; Ashorn, P.; Adu-Afarwuah, S.; Dewey, K.G.; Oaks, B.M. Maternal Blood Pressure in Relation to Prenatal Lipid-Based Nutrient Supplementation and Adverse Birth Outcomes in a Ghanaian Cohort: A Randomized Controlled Trial and Cohort Analysis. J. Nutr. 2021, 151, 1637–1645. [Google Scholar] [CrossRef]
- Tateyama, Y.; Musumari, P.M.; Techasrivichien, T.; Suguimoto, S.P.; Zulu, R.; Dube, C.; Feldman, M.D.; Ono-Kihara, M.; Kihara, M. Dietary habits, body image, and health service access related to cardiovascular diseases in rural Zambia: A qualitative study. PLoS ONE 2019, 14, e0212739. [Google Scholar] [CrossRef] [PubMed]
- Oldewage-Theron, W.; Egal, A. The Effect of Consumption of Soy Foods on the Blood Lipid Profile of Women: A Pilot Study from Qwa-Qwa. J. Nutr. Sci. Vitaminol. 2013, 59, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Godbharle, S.; Kesa, H.; Jeyakumar, A. Processed food consumption and risk of non-communicable diseases (NCDs) in South Africa: Evidence from Demographic and Health Survey (DHS) VII. J. Nutr. Sci. 2024, 13, e19. [Google Scholar] [CrossRef] [PubMed]
- Semagn, B.E.; Abubakari, A.; Kebede, S.D. Magnitude of sugar-sweetened beverage consumption and associated factors among women aged 15–49 years old in two Sub-Saharan African countries. BMC Women’s Health 2023, 23, 650. [Google Scholar] [CrossRef]
- Kantarama, E.; Uwizeye, D.; Muvunyi, C.M.; Uwineza, A. Prevalence of Central Obesity and its Association with Cardiovascular Risk Factors among Women of Reproductive Age in Rwanda. Afr. J. Biomed. Res. 2023, 26, 37–43. [Google Scholar]
- Motadi, S.A.; Zwidofhelangani, M.; Harriet, M.P.; Phumuzo, M.; Masindi, M.; Mushaphi, L. Assessment of Nutritional Status and Dietary Intake of Pregnant Women in Rural Area of Vhembe District, Limpopo Province. Ecol. Food Nutr. 2020, 59, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.A.; McHiza, Z.J.; Sewpaul, R.; Job, N.; Chola, L.; Sithole, M.; Labadarios, D. The impact of sociodemography, diet, and body size on serum retinol in women 16–35 years of age: SANHANES-1. Ann. N. Y. Acad. Sci. 2017, 1416, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Said-Mohamed, R.; Prioreschi, A.; Nyati, L.H.; van Heerden, A.; Munthali, R.J.; Kahn, K.; Tollman, S.M.; Gómez-Olivé, F.X.; Houle, B.; Dunger, D.B.; et al. Rural-urban variations in age at menarche, adult height, leg-length and abdominal adiposity in black South African women in transitioning South Africa. Ann. Hum. Biol. 2018, 45, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Alaofè, H.; Asaolu, I. Maternal and Child Nutrition Status in Rural Communities of Kalalé District, Benin: The Relationship and Risk Factors. Food Nutr. Bull. 2019, 40, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Mtintsilana, A.; Micklesfield, L.K.; Chorell, E.; Olsson, T.; Shivappa, N.; Hebert, J.R.; Kengne, A.P.; Goedecke, J.H. Adiposity Mediates the Association between the Dietary Inflammatory Index and Markers of Type 2 Diabetes Risk in Middle-Aged Black South African Women. Nutrients 2019, 11, 1246. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, E.C.; Suchdev, P.S.; Narayan, K.M.V.; Cunningham, S.; Weber, M.B.; Tripp, K.; Mapango, C.; Ramakrishnan, U.; Hennink, M.; Williams, A.M. The Co-Occurrence of Overweight and Micronutrient Deficiencies or Anemia among Women of Reproductive Age in Malawi. J. Nutr. 2020, 150, 1554–1565. [Google Scholar] [CrossRef]
- Prioreschi, A.; Wrottesley, S.V.; Norris, S.A. Physical Activity Levels, Food Insecurity and Dietary Behaviours in Women from Soweto, South Africa. J. Community Health 2021, 46, 156–164. [Google Scholar] [CrossRef]
- Soepnel, L.M.; Mabetha, K.; Draper, C.E.; Silubonde, T.M.; Smuts, C.M.; Pettifor, J.M.; Norris, S.A. A Cross-Sectional Study of the Associations between Biomarkers of Vitamin D, Iron Status, and Hemoglobin in South African Women of Reproductive Age: The Healthy Life Trajectories Initiative, South Africa. Curr. Dev. Nutr. 2023, 7, 100072. [Google Scholar] [CrossRef]
- Mosuro, A.; Bodunde, I.; Adeniyi, K. Overweight and obesity are prevalent among female adults in selected areas in Ibadan, Oyo State, Nigeria. Clin. Epidemiol. Glob. Health 2023, 22, 101314. [Google Scholar] [CrossRef]
- Wrottesley, S.V.; Pisa, P.T.; Norris, S.A. The Influence of Maternal Dietary Patterns on Body Mass Index and Gestational Weight Gain in Urban Black South African Women. Nutrients 2017, 9, 732. [Google Scholar] [CrossRef]
- Holdsworth, M.; Delpeuch, F.; Landais, E.; Gartner, A.; Eymard-Duvernay, S.; Maire, B. Knowledge of dietary and behaviour-related determinants of non-communicable disease in urban Senegalese women. Public Health Nutr. 2006, 9, 975–981. [Google Scholar] [CrossRef]
- Stevens, G.A.; Beal, T.; Mbuya, M.N.N.; Luo, H.; Neufeld, L.M. Micronutrient deficiencies among preschool-aged children and women of reproductive age worldwide: A pooled analysis of individual-level data from population-representative surveys. Lancet Glob. Health 2022, 10, e1590–e1599. [Google Scholar] [CrossRef]
- Seifu, B.L.; Mare, K.U.; Legesse, B.T.; Tebeje, T.M. Double burden of malnutrition and associated factors among women of reproductive age in sub-Saharan Africa: A multilevel multinomial logistic regression analysis. BMJ Open 2024, 14, e073447. [Google Scholar] [CrossRef]
- Kiely, M.E.; McCarthy, E.K.; Hennessy, Á. Iron, iodine and vitamin D deficiencies during pregnancy: Epidemiology, risk factors and developmental impacts. Proc. Nutr. Soc. 2021, 80, 290–302. [Google Scholar] [CrossRef]
- Turawa, E.; Awotiwon, O.; Dhansay, M.A.; Cois, A.; Labadarios, D.; Bradshaw, D.; Pillay-van Wyk, V. Prevalence of Anaemia, Iron Deficiency, and Iron Deficiency Anaemia in Women of Reproductive Age and Children under 5 Years of Age in South Africa (1997–2021): A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 12799. [Google Scholar] [CrossRef] [PubMed]
- Miko, E.; Csaszar, A.; Bodis, J.; Kovacs, K. The Maternal-Fetal Gut Microbiota Axis: Physiological Changes, Dietary Influence, and Modulation Possibilities. Life 2022, 12, 424. [Google Scholar] [CrossRef] [PubMed]
- Christian, P.; Stewart, C.P. Maternal Micronutrient Deficiency, Fetal Development, and the Risk of Chronic Disease1. J. Nutr. 2010, 140, 437–445. [Google Scholar] [CrossRef]
- Bosire, E.N.; Cohen, E.; Erzse, A.; Goldstein, S.J.; Hofman, K.J.; Norris, S.A. ‘I’d say I’m fat, I’m not obese’: Obesity normalisation in urban-poor South Africa. Public Health Nutr. 2020, 23, 1515–1526. [Google Scholar] [CrossRef] [PubMed]
- Manafe, M.; Chelule, P.K.; Madiba, S. The Perception of Overweight and Obesity among South African Adults: Implications for Intervention Strategies. Int. J. Environ. Res. Public Health 2022, 19, 12335. [Google Scholar] [CrossRef] [PubMed]
- Draper, C.E.; Davidowitz, K.J.; Goedecke, J.H. Perceptions relating to body size, weight loss and weight-loss interventions in black South African women: A qualitative study. Public Health Nutr. 2016, 19, 548–556. [Google Scholar] [CrossRef] [PubMed]
- WHO. Action Framework for Developing and Implementing Public Food Procurement and Service Policies for a Healthy Diet; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789240018341 (accessed on 8 July 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).