Unveiling TIMPs: A Systematic Review of Their Role as Biomarkers in Atherosclerosis and Coronary Artery Disease
Abstract
1. Introduction
2. Methodology
2.1. Research Question and Search Strategy
2.2. Study Criteria
2.3. Article Selection and Data Extraction
2.4. Risk of Bias Assessment
2.5. Statistical Analysis
3. Results
3.1. Search Results
3.2. Quality Evaluation
3.3. General Characteristics of the Included Studies
3.4. Finding Summary
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Section and Topic | Item # | Checklist Item | Location Where Item Is Reported |
|---|---|---|---|
| Title | 1 | Identify the report as a systematic review. | Title, line 1 |
| Abstract | 2 | See the PRISMA 2020 for Abstracts checklist. | Appendix A and Appendix B |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | Introduction, paragraphs 1–5 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | Introduction, paragraph 5, lines 74–84 |
| Methods | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | Methodology, Section 2.2 |
| Information sources | 6 | Specify all databases, registers, websites, organizations, reference lists, and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | Methodology, Section 2.1 |
| Search strategy | 7 | Present the full search strategies for all databases, registers and websites, including any filters and limits used. | Methodology, Section 2.1 |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. | Methodology, Section 2.3 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details or automation tools used in the process. | Methodology, Section 2.3 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g. for all measures, time points, analyses), and if not, the methods used to decide which results to collect. | Methodology, Section 2.3 |
| 10b | List and define all other variables for which data were sought (e.g. participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | Not applicable | |
| Study risk of bias assessment | 11 | Specify the methods used to assess risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process. | Methodology, Section 2.4 |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g. risk ratio, mean difference) used in the synthesis or presentation of results. | Methodology, Section 2.3, line 6–8 |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g. tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)). | Methodology, Section 2.3, line 1–5 |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions. | Involving article exporting from database into Mendeley web taught by Mrs. Norizam Salamat. | |
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | Not applicable | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | Methodology, Section 2.5 | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g. subgroup analysis, metaregression). | Not applicable | |
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results. | Not applicable | |
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases). | Not applicable |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | Not applicable |
| Results | |||
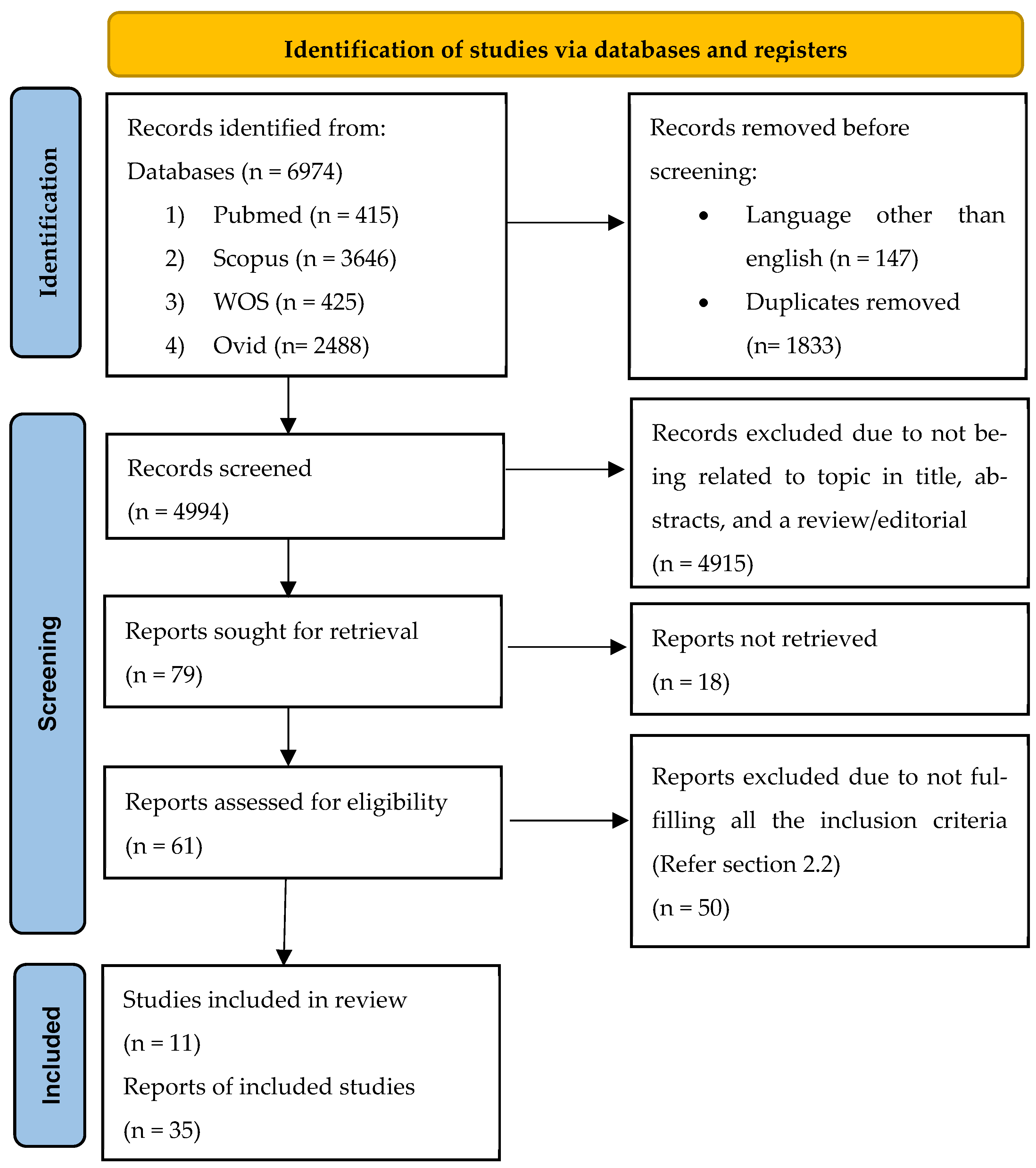
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram (see Figure 1). | Results Section 3.1, see Figure 1 |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded. | Not stated | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | Results, see Table 3 |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | Results Section 3.2, see Table 1 and Table 2 |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g. confidence/credible interval), ideally using structured tables or plots. | Results, see Table 4 column “Findings” and “Conclusion” |
| Results of syntheses | 20a | For each synthesis, briefly summarize the characteristics and risk of bias among contributing studies. | Results, see Table 3 column “Subject number and characteristic”. For risk bias, see Table 1 and Table 2 |
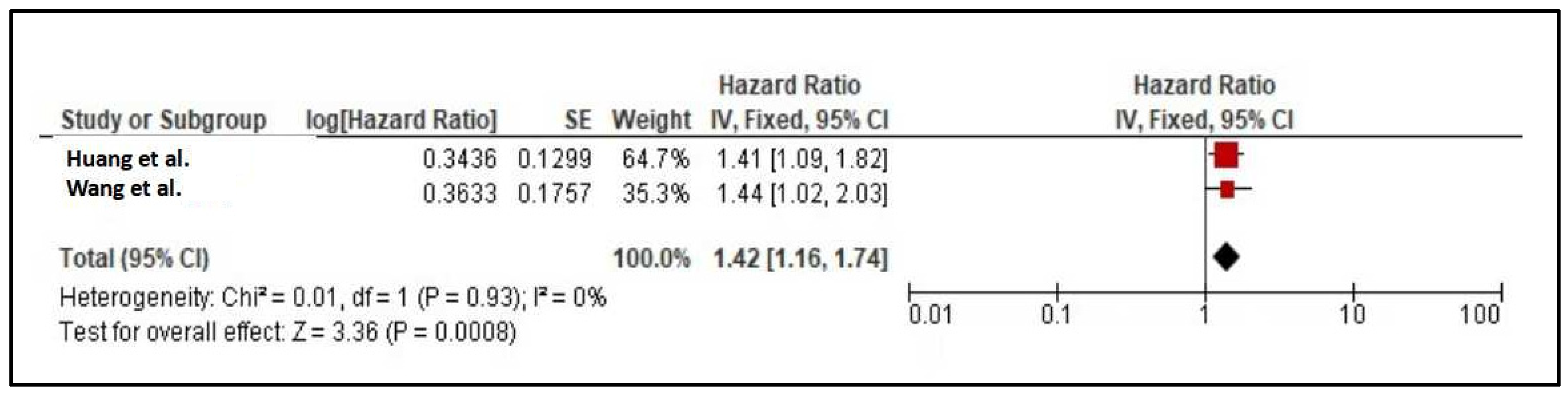
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was done, present for each the summary estimate and its precision (e.g. confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | Results, see Figure 2 and Figure 3 | |
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | Not applicable | |
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | Not applicable | |
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed | Not applicable |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | Not applicable |
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence | Discussion, paragraph 1–10 |
| 23b | Discuss any limitations of the evidence included in the review. | Not applicable | |
| 23c | Discuss any limitations of the review processes used. | Discussion, paragraph 10, line 7–19 | |
| 23d | Discuss implications of the results for practice, policy, and future research. | Conclusions | |
| Other information | |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered. | See “Protocol Registration” |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared | See “Protocol Registration” | |
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | Not applicable | |
| Support | 25 | Describe sources of financial or non-financial support for the review, and the role of the funders or sponsors in the review. | See “Funding” |
| Competing interests | 26 | Declare any competing interests of review authors. | See “Conflict of Interest” |
| Availability of data, code, and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | See “Availability of Data and Materials” |
Appendix B
| Section and Topic | Item # | Checklist Item | Location Where Item Is Reported |
|---|---|---|---|
| Title | 1 | Identify the report as a systematic review. | Abstract, line 8–9 |
| Background | |||
| Objectives | 2 | Provide an explicit statement of the main objective (s) or question (s) the review addresses. | Abstract, line 1–8 |
| Methods | |||
| Eligibility criteria | 3 | Specify the inclusion and exclusion criteria for the review. | Not stated in abstract |
| Information sources | 4 | Specify the information sources (e.g. databases, registers) used to identify studies and the date when each was last searched. | Abstracts, line 10 |
| Risk of bias | 5 | Specify the methods used to assess risk of bias in the included studies. | Abstracts, line 11 |
| Synthesis of results | 6 | Specify the methods used to present and synthesize results. | Abstracts, line 9–12 |
| Results | |||
| Included studies | 7 | Give the total number of included studies and participants and summarize relevant characteristics of studies. | Abstracts, line 13 |
| Synthesis of results | 8 | Present results for main outcomes, preferably indicating the number of included studies and participants for each. If meta-analysis was conducted, reports the summary estimate and confidence/credible interval. If comparing groups, indicate the direction of the effects (i.e. which group is favored). | Abstract, line 13–18 |
| Discussion | |||
| Limitation of evidence | 9 | Provide a brief summary of the limitations of the evidence included in the review (e.g. study risk of bias, inconsistency and imprecision). | Not stated in abstracts |
| Interpretation | 10 | Provide a general interpretation of the results and important implications. | Abstract, line 19–22 |
| Other | |||
| Funding | 11 | Specify the primary source of funding for the review. | Not stated in abstracts |
| Registration | 12 | Provide the register name and registration number. | Not stated in abstracts |
References
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J. Assessing the role of circulating, genetic, and imaging biomarkers in cardiovascular risk prediction. Circulation 2011, 123, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Hoefer, I.E.; Steffens, S.; Ala-Korpela, M.; Bäck, M.; Badimon, L.; Bochaton-Piallat, M.L.; Boulanger, C.M.; Caligiuri, G.; Dimmeler, S.; Egido, J.; et al. Novel methodologies for biomarker discovery in atherosclerosis. Eur. Heart J. 2015, 36, 2635–2642. [Google Scholar] [CrossRef] [PubMed]
- Adam, C.A.; Șalaru, D.L.; Prisacariu, C.; Marcu, D.T.M.; Sascău, R.A.; Stătescu, C. Novel Biomarkers of Atherosclerotic Vascular Disease-Latest Insights in the Research Field. Int. J. Mol. Sci. 2022, 23, 4998. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.K.; Tse, H.F. Circulating Biomarkers for Cardiovascular Disease Risk Prediction in Patients With Cardiovascular Disease. Front. Cardiovasc. Med. 2021, 8, 713191. [Google Scholar] [CrossRef] [PubMed]
- Wielkoszyński, T.; Zalejska-Fiolka, J.; Strzelczyk, J.K.; Owczarek, A.J.; Cholewka, A.; Furmański, M.; Stanek, A. Oxysterols Increase Inflammation, Lipid Marker Levels and Reflect Accelerated Endothelial Dysfunction in Experimental Animals. Mediat. Inflamm. 2018, 2018, 2784701. [Google Scholar] [CrossRef]
- Zhang, L.; Xiong, L.; Fan, L.; Diao, H.; Tang, M.; Luo, E.; Guo, W.; Yang, X.; Xing, S. Vascular lipidomics analysis reveales increased levels of phosphocholine and lysophosphocholine in atherosclerotic mice. Nutr. Metab. 2023, 20, 1. [Google Scholar] [CrossRef]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Liu, F.; Fan, F.; Chen, N.; Pan, X.; Wei, Z.; Zhang, Y. Exploring the mechanism of atherosclerosis and the intervention of traditional Chinese medicine combined with mesenchymal stem cells based on inflammatory targets. Heliyon 2023, 9, e22005. [Google Scholar] [CrossRef]
- Khuda, F.; Anuar, N.N.M.; Baharin, B.; Nasruddin, N.S. A mini review on the associations of matrix metalloproteinases (MMPs)-1,-8,-13 with periodontal disease. AIMS Mol. Sci. 2021, 8, 13–31. [Google Scholar] [CrossRef]
- Vacek, T.P.; Rehman, S.; Neamtu, D.; Yu, S.; Givimani, S.; Tyagi, S.C. Matrix metalloproteinases in atherosclerosis: Role of nitric oxide, hydrogen sulfide, homocysteine, and polymorphisms. Vasc. Health Risk Manag. 2015, 11, 173–183. [Google Scholar] [CrossRef]
- Vijakumaran, U.; Yazid, M.D.; Hj Idrus, R.B.; Abdul Rahman, M.R.; Sulaiman, N. Molecular Action of Hydroxytyrosol in Attenuation of Intimal Hyperplasia: A Scoping Review. Front. Pharmacol. 2021, 12, 663266. [Google Scholar] [CrossRef]
- Scholtes, V.P.; Johnson, J.L.; Jenkins, N.; Sala-Newby, G.B.; de Vries, J.P.; de Borst, G.J.; de Kleijn, D.P.; Moll, F.L.; Pasterkamp, G.; Newby, A.C. Carotid atherosclerotic plaque matrix metalloproteinase-12-positive macrophage subpopulation predicts adverse outcome after endarterectomy. J. Am. Heart Assoc. 2012, 1, e001040. [Google Scholar] [CrossRef]
- Aminuddin, A.; Cheong, S.S.; Roos, N.A.C.; Ugusman, A. Smoking and Unstable Plaque in Acute Coronary Syndrome: A Systematic Review of The Role of Matrix Metalloproteinases. Int. J. Med. Sci. 2023, 20, 482–492. [Google Scholar] [CrossRef]
- Jackson, H.W.; Defamie, V.; Waterhouse, P.; Khokha, R. TIMPs: Versatile extracellular regulators in cancer. Nat. Rev. Cancer 2017, 17, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Newby, A.C. Matrix metalloproteinase inhibition therapy for vascular diseases. Vasc. Pharmacol. 2012, 56, 232–244. [Google Scholar] [CrossRef]
- Galis, Z.S.; Sukhova, G.K.; Lark, M.W.; Libby, P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J. Clin. Invest. 1994, 94, 2493–2503. [Google Scholar] [CrossRef]
- Chen, H.; Chen, S.; Ye, H.; Guo, X. Protective Effects of Circulating TIMP3 on Coronary Artery Disease and Myocardial Infarction: A Mendelian Randomization Study. J. Cardiovasc. Dev. Dis. 2022, 9, 277. [Google Scholar] [CrossRef]
- Takawale, A.; Zhang, P.; Azad, A.; Wang, W.; Wang, X.; Murray, A.G.; Kassiri, Z. Myocardial overexpression of TIMP3 after myocardial infarction exerts beneficial effects by promoting angiogenesis and suppressing early proteolysis. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H224–H236. [Google Scholar] [CrossRef]
- Moore, L.; Fan, D.; Basu, R.; Kandalam, V.; Kassiri, Z. Tissue inhibitor of metalloproteinases (TIMPs) in heart failure. Heart Fail. Rev. 2012, 17, 693–706. [Google Scholar] [CrossRef]
- Niu, L.; Zhang, L.; Jiao, K.; Li, F.; Ding, Y.; Wang, D.; Wang, M.; Tay, F.; Chen, J. Localization of MMP-2, MMP-9, TIMP-1, and TIMP-2 in human coronal dentine. J. Dent. 2011, 39, 536–542. [Google Scholar] [CrossRef]
- Elgezawi, M.; Haridy, R.; Almas, K.; Abdalla, M.A.; Omar, O.; Abuohashish, H.; Elembaby, A.; Christine Wölfle, U.; Siddiqui, Y.; Kaisarly, D. Matrix Metalloproteinases in Dental and Periodontal Tissues and Their Current Inhibitors: Developmental, Degradational and Pathological Aspects. Int. J. Mol. Sci. 2022, 23, 8929. [Google Scholar] [CrossRef]
- Samah, N.; Ugusman, A.; Hamid, A.A.; Sulaiman, N.; Aminuddin, A. Role of Matrix Metalloproteinase-2 in the Development of Atherosclerosis among Patients with Coronary Artery Disease. Mediat. Inflamm. 2023, 2023, 9715114. [Google Scholar] [CrossRef]
- Fabunmi, R.P.; Sukhova, G.K.; Sugiyama, S.; Libby, P.; Hashimoto, T.; Wen, G.; Lawton, M.T.; Boudreau, N.J.; Bollen, A.W.; Yang, G.-Y.; et al. Expression of tissue inhibitor of metalloproteinases-3 in human atheroma and regulation in lesion-associated cells: A potential protective mechanism in plaque stability. Circ. Res. 1998, 83, 270–278. [Google Scholar] [CrossRef]
- Aoki, T.; Kataoka, H.; Moriwaki, T.; Nozaki, K.; Hashimoto, N. Role of TIMP-1 and TIMP-2 in the progression of cerebral aneurysms. Stroke 2007, 38, 2337–2345. [Google Scholar] [CrossRef]
- Di Gregoli, K.; George, S.J.; Newby, A.C.; Johnson, J.L. Differential effects of tissue inhibitor of metalloproteinase (TIMP)-1 and TIMP-2 on atherosclerosis and monocyte/macrophage invasion. Cardiovasc. Res. 2016, 109, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Howard, E.W.; Bullen, E.C.; Banda, M.J. Preferential inhibition of 72- and 92-kDa gelatinases by tissue inhibitor of metalloproteinases-2. J. Biol. Chem. 1991, 266, 13070–13075. [Google Scholar] [CrossRef]
- Guo, T.; Hao, H.; Zhou, L.; Zhou, F.; Yu, D. Association of SNPs in the TIMP-2 gene and large artery atherosclerotic stroke in southern Chinese Han population. Oncotarget 2018, 9, 4698–4706. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 39. [Google Scholar] [CrossRef]
- Aminuddin, A.; Vijakumaran, U.; Samah, N.; Nor, F.M.; Razali, W.M.H.W.; Mohamad, S.F.; Hamzah, F.A.; Hamid, A.A.; Ugusman, A. INPLASY Protocol 202470070; International Platform of Registered Systematic Review and Meta-Analysis Protocols: Middletown, DE, USA, 2024. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2021. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 1 January 2024).
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Huang, J.-T.; Sung, S.-H.; Hsu, C.-P.; Chiang, C.-E.; Yu, W.-C.; Cheng, H.-M.; Huang, C.-H. TIMP-1 in the prognosis of patients who underwent coronary artery bypass surgery: A 12-year follow-up study. Front. Cardiovasc. Med. 2023, 10, 1226449. [Google Scholar] [CrossRef]
- Wang, W.; Song, X.; Chen, Y.; Yuan, F.; Xu, F.; Zhang, M.; Tan, K.; Yang, X.; Yu, X.; Lv, S. The long-term influence of tissue inhibitor of matrix metalloproteinase-1 in patients with mild to moderate coronary artery lesions in a Chinese population: A 7-year follow-up study. Cardiology 2015, 132, 151–158. [Google Scholar] [CrossRef]
- Kormi, I.; Nieminen, M.T.; Havulinna, A.S.; Zeller, T.; Blankenberg, S.; Tervahartiala, T.; Sorsa, T.; Salomaa, V.; Pussinen, P.J. Matrix metalloproteinase-8 and tissue inhibitor of matrix metalloproteinase-1 predict incident cardiovascular disease events and all-cause mortality in a population-based cohort. Eur. J. Prev. Cardiol. 2017, 24, 1136–1144. [Google Scholar] [CrossRef]
- Opstad, T.B.; Seljeflot, I.; Bøhmer, E.; Arnesen, H.; Halvorsen, S. MMP-9 and Its Regulators TIMP-1 and EMMPRIN in Patients with Acute ST-Elevation Myocardial Infarction: A NORDISTEMI Substudy. Cardiology 2018, 139, 17–24. [Google Scholar] [CrossRef]
- Korzeń, D.; Sierka, O.; Dąbek, J. Transcriptional Activity of Metalloproteinase 9 (MMP-9) and Tissue Metalloproteinase 1 (TIMP-1) Genes as a Diagnostic and Prognostic Marker of Heart Failure Due to Ischemic Heart Disease. Biomedicines 2023, 11, 2776. [Google Scholar] [CrossRef]
- Alp, E.; Yilmaz, A.; Tulmac, M.; Dikmen, A.U.; Cengel, A.; Yalcin, R.; Menevse, E.S. Analysis of MMP-7 and TIMP-2 gene polymorphisms in coronary artery disease and myocardial infarction: A Turkish case-control study. Kaohsiung J. Med. Sci. 2017, 33, 78–85. [Google Scholar] [CrossRef]
- Nordeng, J.; Schandiz, H.; Solheim, S.; Åkra, S.; Hoffman, P.; Roald, B.; Bendz, B.; Arnesen, H.; Helseth, R.; Seljeflot, I. TIMP-1 expression in coronary thrombi associate with myocardial injury in ST-elevation myocardial infarction patients. Coron. Artery Dis. 2022, 33, 446–455. [Google Scholar] [CrossRef]
- Ben Braiek, A.; Chahed, H.; Dumont, F.; Abdelhak, F.; Hichem, D.; Gamra, H.; Baudin, B. Identification of biomarker panels as predictors of severity in coronary artery disease. J. Cell. Mol. Med. 2021, 25, 1518–1530. [Google Scholar] [CrossRef]
- Çelebi, G.; Geyik, F.G.; Yılmazbayhan, E.D.; Özsoy, S.; Yıldız, C.; Yıldız, M.; Öksen, D.; Cavlak, M.; Bayrak, A. The Relationship Between The Expression Levels of Tissue Inhibitor of Metalloproteinases-3 (Timp3) and Severity of Atherosclerosis. J. Istanb. Fac. Med. 2021, 84, 472–481. [Google Scholar] [CrossRef]
- Ezhov, M.; Safarova, M.; Afanasieva, O.; Mitroshkin, M.; Matchin, Y.; Pokrovsky, S. Matrix metalloproteinase 9 as a predictor of coronary atherosclerotic plaque instability in stable coronary heart disease patients with elevated lipoprotein (a) levels. Biomolecules 2019, 9, 129. [Google Scholar] [CrossRef]
- Han, Y.; Joo, H.J.; Seo, H.R.; Choi, S.C.; Park, J.H.; Yu, C.W.; Hong, S.J.; Lim, D.S. Serum TIMP-2, NGAL and angiopoietin-2 as biomarkers of coronary artery stenosis. Int. J. Clin. Exp. Med. 2016, 9, 14141–14149. [Google Scholar]
- Halpert, I.; I Sires, U.; Roby, J.D.; Potter-Perigo, S.; Wight, T.N.; Shapiro, S.D.; Welgus, H.G.; A Wickline, S.; Parks, W.C. Matrilysin is expressed by lipid-laden macrophages at sites of potential rupture in atherosclerotic lesions and localizes to areas of versican deposition, a proteoglycan substrate for the enzyme. Proc. Natl. Acad. Sci. USA 1996, 93, 9748–9753. [Google Scholar] [CrossRef] [PubMed]
- Molloy, K.; Thompson, M.; Jones, J.; Schwalbe, E.; Bell, P.; Naylor, A.; Loftus, I. Unstable carotid plaques exhibit raised matrix metalloproteinase-8 activity. Circulation 2004, 110, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Fabris, E.; Berta, B.; Roleder, T.; Hermanides, R.S.; Ijsselmuiden, A.J.; Kauer, F.; Alfonso, F.; von Birgelen, C.; Escaned, J.; Camaro, C.; et al. Thin-Cap Fibroatheroma Rather Than Any Lipid Plaques Increases the Risk of Cardiovascular Events in Diabetic Patients: Insights From the Combine Oct–Ffr Trial. Circ. Cardiovasc. Interv. 2022, 15, e011728. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Cimini, M.; Fedak, P.W.; Altamentova, S.; Fazel, S.; Huang, M.-L.; Weisel, R.D.; Li, R.-K. TIMP-3 deficiency accelerates cardiac remodeling after myocardial infarction. J. Mol. Cell. Cardiol. 2007, 43, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Brew, K.; Nagase, H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim. Biophys. Acta 2010, 1803, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, M.; Tortorella, M.; Nagase, H.; Bre, K. TIMP-3 Is a Potent Inhibitor of Aggrecanase 1 (ADAM-TS4) and Agrrecanase 2 (ADAM-TS5). J. Biol. Chem. 2001, 276, 12501–12504. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Kassiri, Z. Biology of Tissue Inhibitor of Metalloproteinase 3 (TIMP3), and Its Therapeutic Implications in Cardiovascular Pathology. Front. Physiol. 2020, 11, 661. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Qi, X.; Li, Z.; Zhang, W.; Li, C.; Ji, L.; Xu, K.; Zhong, H. TIMP-3 suppresses the proliferation and migration of SMCs from the aortic neck of atherosclerotic AAA in rabbits, via decreased MMP-2 and MMP-9 activity, and reduced TNF-α expression. Mol. Med. Rep. 2018, 18, 2061–2067. [Google Scholar] [CrossRef]
- Stöhr, R.; Cavalera, M.; Menini, S.; Mavilio, M.; Casagrande, V.; Rossi, C.; Urbani, A.; Cardellini, M.; Pugliese, G.; Menghini, R.; et al. Loss of TIMP3 exacerbates atherosclerosis in ApoE null mice. Atherosclerosis 2014, 235, 438–443. [Google Scholar] [CrossRef]
- Barlow, S.C.; Doviak, H.; Jacobs, J.; Freeburg, L.A.; Perreault, P.E.; Zellars, K.N.; Moreau, K.; Villacreses, C.F.; Smith, S.; Khakoo, A.Y.; et al. Intracoronary delivery of recombinant TIMP-3 after myocardial infarction: Effects on myocardial remodeling and function. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H690–H699. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, V.; Menghini, R.; Menini, S.; Marino, A.; Marchetti, V.; Cavalera, M.; Fabrizi, M.; Hribal, M.L.; Pugliese, G.; Gentileschi, P.; et al. Overexpression of Tissue Inhibitor of Metalloproteinase 3 in Macrophages Reduces Atherosclerosis in Low-Density Lipoprotein Receptor Knockout Mice. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.; Salazar, J.; Martínez, M.S.; Palmar, J.; Bautista, J.; Chávez-Castillo, M.; Gómez, A.; Bermúdez, V. Macrophage Heterogeneity and Plasticity: Impact of Macrophage Biomarkers on Atherosclerosis. Scientifica 2015, 2015, 851252. [Google Scholar] [CrossRef]
- Johnson, J.L.; Sala-Newby, G.B.; Ismail, Y.; Aguilera, C.M.; Newby, A.C. Low Tissue Inhibitor of Metalloproteinases 3 and High Matrix Metalloproteinase 14 Levels Defines a Subpopulation of Highly Invasive Foam-Cell Macrophages. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1647–1653. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nanni, S.; Melandri, G.; Hanemaaijer, R.; Cervi, V.; Tomasi, L.; Altimari, A.; Van Lent, N.; Tricoci, P.; Bacchi, L.; Branzi, A. Matrix metalloproteinases in premature coronary atherosclerosis: Influence of inhibitors, inflammation, and genetic polymorphisms. Transl. Res. 2007, 149, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Cavusoglu, E.; Ruwende, C.; Chopra, V.; Yanamadala, S.; Eng, C.; Clark, L.T.; Pinsky, D.J.; Marmur, J.D. Tissue inhibitor of metalloproteinase-1 (TIMP-1) is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction. Am. Heart J. 2006, 151, 1101.e1–1101.e8. [Google Scholar] [CrossRef] [PubMed]
- Lubos, E.; Schnabel, R.; Rupprecht, H.J.; Bickel, C.; Messow, C.M.; Prigge, S.; Cambien, F.; Tiret, L.; Münzel, T.; Blankenberg, S. Prognostic value of tissue inhibitor of metalloproteinase-1 for cardiovascular death among patients with cardiovascular disease: Results from the AtheroGene study. Eur. Heart J. 2006, 27, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Masciantonio, M.G.; Lee, C.K.; Arpino, V.; Mehta, S.; Gill, S.E. Chapter Three—The Balance Between Metalloproteinases and TIMPs: Critical Regulator of Microvascular Endothelial Cell Function in Health and Disease. Prog. Mol. Biol. Transl. Sci. 2017, 147, 101–131. [Google Scholar] [PubMed]
- Velagaleti, R.S.; Gona, P.; Sundström, J.; Larson, M.G.; Siwik, D.; Colucci, W.S.; Benjamin, E.J.; Vasan, R.S. Relations of biomarkers of extracellular matrix remodeling to incident cardiovascular events and mortality. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2283–2288. [Google Scholar] [CrossRef] [PubMed]
- De Souza, A.P.; Trevilatto, P.C.; Scarel-Caminaga, R.M.; de Brito, R.B.J.; Barros, S.P.; Line, S.R.P. Analysis of the MMP-9 (C-1562 T) and TIMP-2 (G-418C) gene promoter polymorphisms in patients with chronic periodontitis. J. Clin. Periodontol. 2005, 32, 207–211. [Google Scholar] [CrossRef]
- Hirano, K.; Sakamoto, T.; Uchida, Y.; Morishima, Y.; Masuyama, K.; Ishii, Y.; Nomura, A.; Ohtsuka, M.; Sekizawa, K. Tissue inhibitor of metalloproteinases-2 gene polymorphisms in chronic obstructive pulmonary disease. Eur. Respir. J. 2001, 18, 748–752. [Google Scholar] [CrossRef] [PubMed]
| Author | Year | Type of Study | Selection | Comparability | Outcome | Total Score | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Representatives of the Exposed Cohort | Selection of the Non-Exposed Cohort | Ascertainment of Exposure | Demonstration that Outcome of Interest Was Not Present at Start of Study | Comparability of Cohorts on the Basis of the Design or Analysis | Assessment of Outcome | Was Follow-Up Long Enough for Outcomes to Occur | Adequacy of Follow-Up of Cohorts | ||||
| [33] | 2023 | Cohort | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | 8 |
| [39] | 2022 | Cross-sec | ✵ | ✵ | ✵ | ✵ | ✵ | 5 | |||
| [41] | 2021 | Cross-sec | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | 6 | ||
| [42] | 2019 | Cross-sec | ✵ | ✵ | ✵ | ✵ | ✵ | 5 | |||
| [36] | 2018 | Cohort | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | 7 | |
| [35] | 2017 | Cohort | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | 8 |
| [34] | 2015 | Cohort | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | 8 |
| Author | Year | Type of Study | Selection | Comparability | Exposure | Total Score | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Is the Case Definition Adequate? | Representativeness of the Cases | Selection of Controls | Definition of Controls | Comparability of Cases and Controls on the Basis of the Design or Analysis | Ascertainment of Exposure | Same Method of Ascertainment for Cases and Controls | Non-Response Rate | ||||
| [40] | 2021 | Case–control | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | 8 |
| [43] | 2016 | Case–control | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | 8 |
| [37] | 2023 | Case–control | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | 8 |
| [38] | 2017 | Case–control | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | ✵ | 8 |
| Reference | Type of Study/Trial Design | Subject Number and Characteristics | Sex | Mean Age (Years Old) | Sample Collection/Measurement Analysis | |||
|---|---|---|---|---|---|---|---|---|
| [33] | Prospective cohort- Follow up-12 years |
|
|
|
| |||
| [34] | Prospective study/Cohort-Follow up-1, 3, 6, 12, 24, 36, 48, 60, 72, and 84 months after CAG |
|
|
|
| |||
| [35] | Cohort
|
|
|
|
| |||
| [36] | Prospective/Cohort—follow-up at 3 days and 3 months post-AMI
|
|
| 60 (53, 67-IQR) |
| |||
| [37] | Case–control study |
|
|
|
| |||
| [38] | Case–control study |
|
|
|
| |||
| [39] | Cross-sectional study |
|
|
|
| |||
| [40] | Case–control multi-centric study |
| CAD =
|
|
| |||
| STEMI | NSTEMI | Stable angina | ||||||
| RAASI (%) | 79 | 118 | 35 | |||||
| Insulin (%) | 70 | 104 | 29 | |||||
| Lipid lowering agent (%) | 87 | 133 | 36 | |||||
| [41] | Cross-sectional study | (A) Living cases (n = 69) undergone invasive coronary angiography for CAD diagnosis and surgical pre-surgical assessment. Gensini and syntax scores were calculated (cut-off value of 8 for both scores) and divided into the following:
|
|
|
(B) Collection of peri-coronary EATs from those undergone heart valve replacement and CABG: High plaque score (n = 25), low plaque score (n = 9)
| |||
| [42] | Cross-sectional |
|
| 56.1 ± 8.0 |
| |||
| [43] | Case–controlSingle center |
1-vessel disease (n = 22) 2-vessel disease (n = 17) 3-vessel disease (n = 18) |
|
|
| |||
| Author | Methods/Measurement Analysis | Findings | Conclusion |
|---|---|---|---|
| [33] |
|
(B) Model 2, adjusted for age, sex, manifest ACS, eGFR, left ventricular ejection fraction, TC, and triglycerides: 1.41 (1.093–1.819) (C) Model 3, adjusted for age, sex, manifest acute coronary syndrome, estimated glomerular filtration rate, left ventricular ejection fraction, completed revascularization or not: 1.666 (1.296–2.142) | High circulating TIMP-1 is a potential independent predictor of future MACE and mortality among CAD patients |
| [34] |
|
| TIMP-1 is a potential candidate to predict long-term prognosis in patients with mild to moderate coronary artery lesions in a Chinese population |
| [35] |
|
| High-serum TIMP-1 is associated with increased risk for future CVD events and death among health population |
| [36] |
|
| Higher TIMP-1 has association with infarct size, supports TIMP-1 role in cardiac remodeling |
| [37] |
|
| TIMP-1 gene transcriptional activity was significantly lower among CAD and negatively correlated with the severity of heart failure, which make it as useful diagnostic and prognostic markers in clinical practice |
| [38] |
|
| TIMP-2 G-418C polymorphism was not associated with increased or decreased risk of CAD among the Turkish population |
| [39] |
|
| High circulating TIMP-1 plays an independent role in myocardial damage early post-MI |
| [40] |
|
| Plasma TIMP decreased in CAD patients versus control |
| [41] |
| For living cases:
| The reduction in TIMP3 expression is notable in advanced atherosclerotic plaques. Consequently, the elevated TIMP3 expression observed in the normal coronary arterial segments of individuals with CHD suggests that TIMP3 serves a protective function against atherosclerosis development at the molecular level within these arterial regions |
| [42] |
|
| Circulating TIMP-2 was decreased in fibroatheroma type of atherosclerotic lesion |
| [43] |
|
| Increased in TIMP-2 could be a potential biomarker to evaluate CAD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aminuddin, A.; Samah, N.; Vijakumaran, U.; Che Roos, N.A.; Nor, F.M.; Wan Razali, W.M.H.; Mohamad, S.F.; Cong, B.B.; Hamzah, F.A.; Hamid, A.A.; et al. Unveiling TIMPs: A Systematic Review of Their Role as Biomarkers in Atherosclerosis and Coronary Artery Disease. Diseases 2024, 12, 177. https://doi.org/10.3390/diseases12080177
Aminuddin A, Samah N, Vijakumaran U, Che Roos NA, Nor FM, Wan Razali WMH, Mohamad SF, Cong BB, Hamzah FA, Hamid AA, et al. Unveiling TIMPs: A Systematic Review of Their Role as Biomarkers in Atherosclerosis and Coronary Artery Disease. Diseases. 2024; 12(8):177. https://doi.org/10.3390/diseases12080177
Chicago/Turabian StyleAminuddin, Amilia, Nazirah Samah, Ubashini Vijakumaran, Nur Aishah Che Roos, Faridah Mohd Nor, Wan Mohammad Hafiz Wan Razali, Shawal Faizal Mohamad, Beh Boon Cong, Faizal Amri Hamzah, Adila A. Hamid, and et al. 2024. "Unveiling TIMPs: A Systematic Review of Their Role as Biomarkers in Atherosclerosis and Coronary Artery Disease" Diseases 12, no. 8: 177. https://doi.org/10.3390/diseases12080177
APA StyleAminuddin, A., Samah, N., Vijakumaran, U., Che Roos, N. A., Nor, F. M., Wan Razali, W. M. H., Mohamad, S. F., Cong, B. B., Hamzah, F. A., Hamid, A. A., & Ugusman, A. (2024). Unveiling TIMPs: A Systematic Review of Their Role as Biomarkers in Atherosclerosis and Coronary Artery Disease. Diseases, 12(8), 177. https://doi.org/10.3390/diseases12080177