Unveiling the Unexplored Multifactorial Potential of 5-Aminosalicylic Acid in Diabetic Wound Therapy
Abstract
1. Introduction
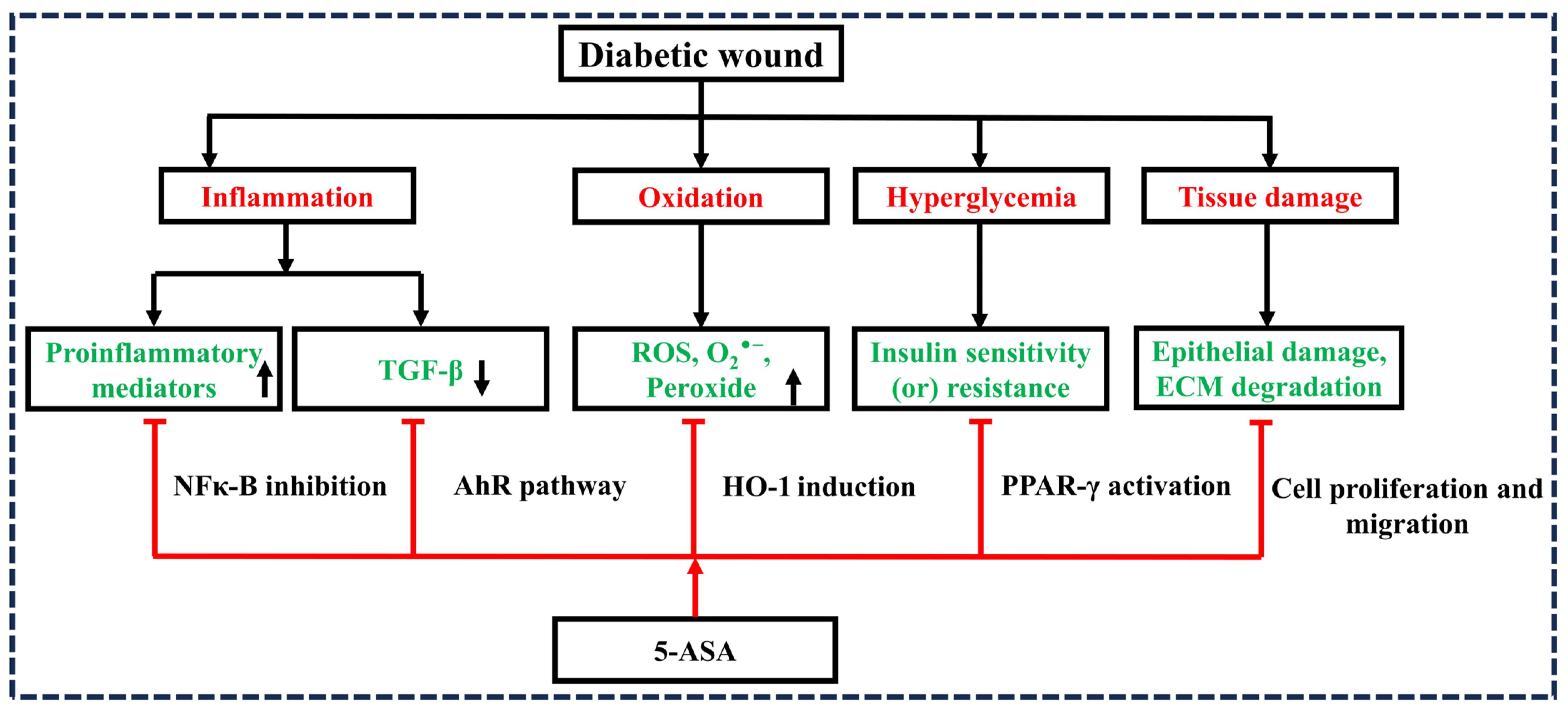
2. Hypothesis
3. Evaluation of the Hypothesis
3.1. 5-ASA and Inflammation
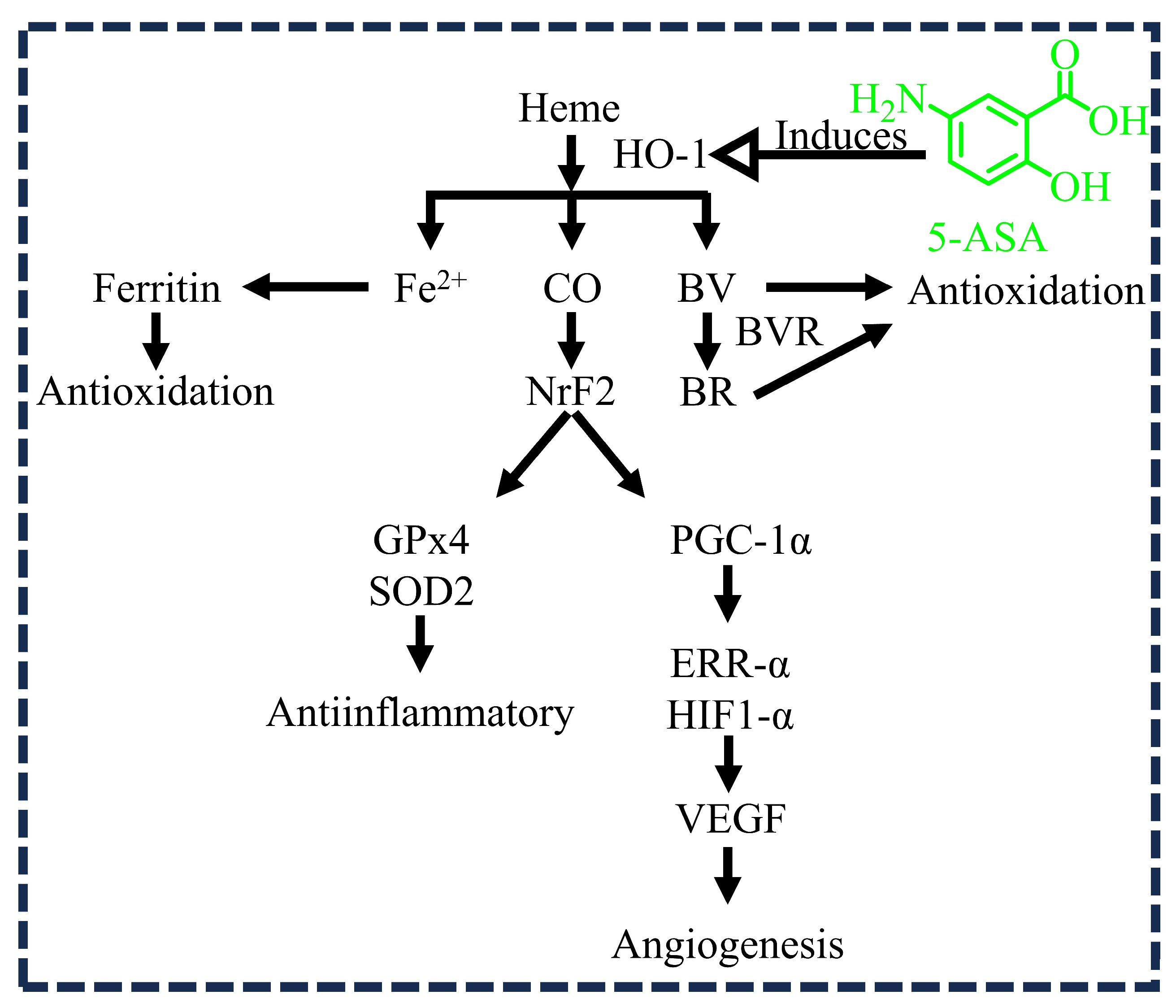
3.2. 5-ASA and Oxidation
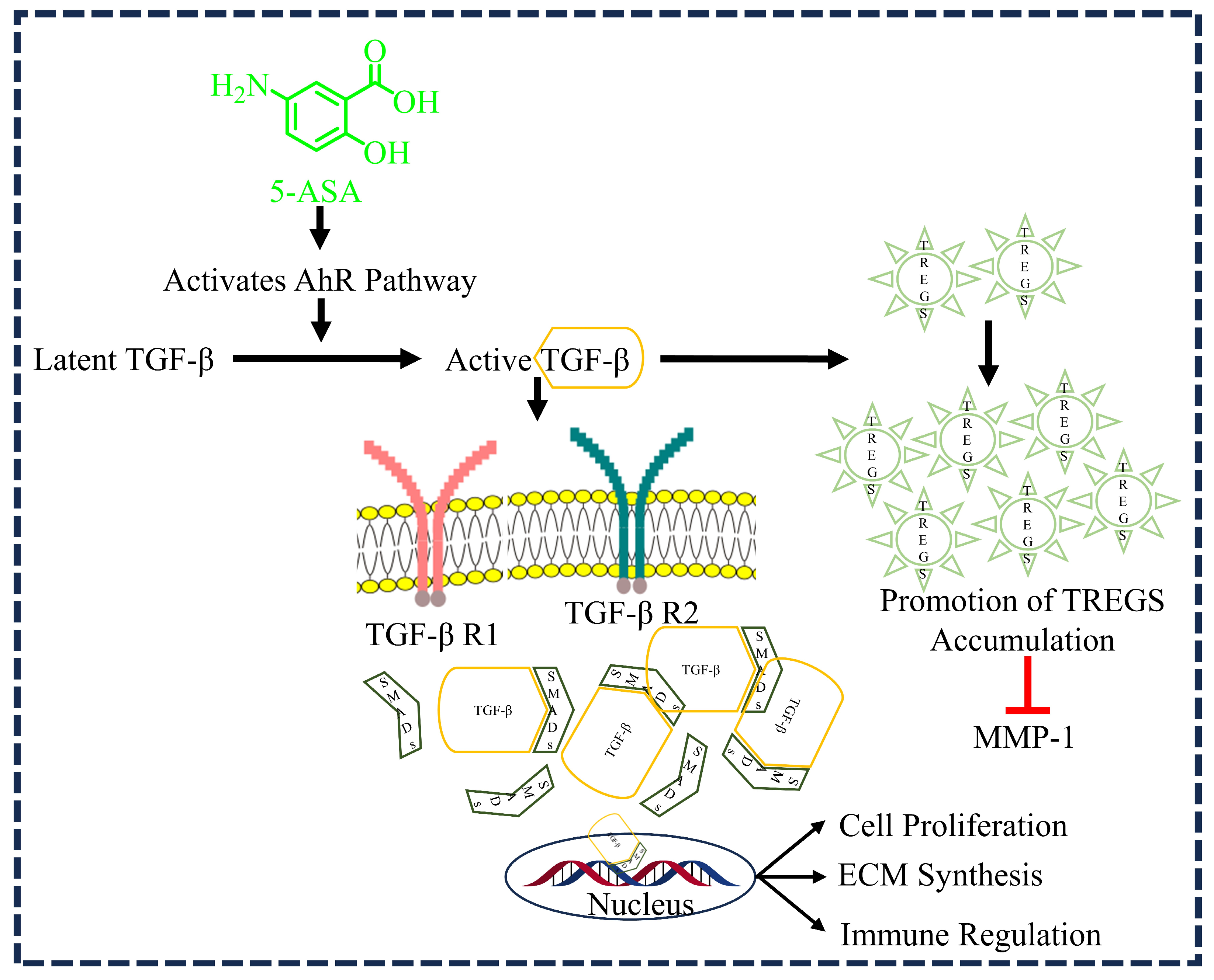
3.3. 5-ASA and TGF-β1 Activation
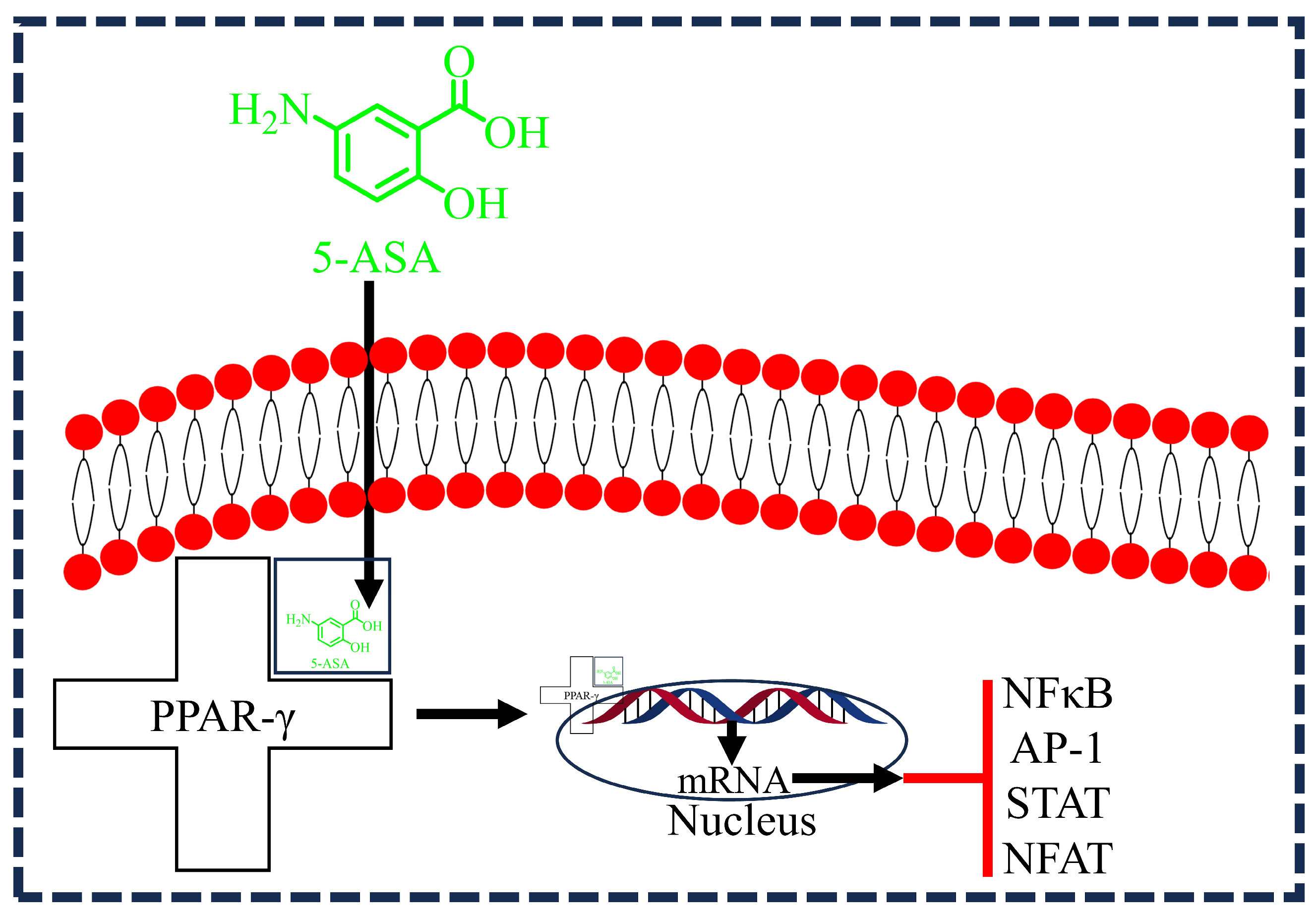
3.4. 5-ASA and PPAR-γ Activation
3.5. 5-ASA and Re-Epithelialization
4. Implications
5. Conclusions
6. Future Directions and Technical Roadmap
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| Abbreviation | Full Form |
| DM | Diabetes mellitus |
| DW | Diabetic wound |
| DFU | Diabetic foot ulcers |
| 5-ASA | 5-aminosalicylic acid |
| PPAR-γ | Peroxisome proliferator-activated receptor gamma |
| TGF-β | Transforming growth factor beta |
| ECM | Extracellular matrix |
| MMP | Matrix metalloproteinase |
| NF-κB | Nuclear factor-kappa B |
| TNF-α | Tumor necrosis factor-alpha |
| HO-1 | Heme oxygenase-1 |
| TREG | Regulatory T cell |
| MPO | Myeloperoxidase |
| CAS | Clinical activity score |
| PC | Phosphatidylcholine |
| ALP | Alkaline phosphatase |
| COMP | Cartilage oligomeric matrix protein |
| MDA | Malondialdehyde |
| SOD | Superoxide dismutase |
References
- Schreml, S.; Szeimies, R.-M.; Prantl, L.; Landthaler, M.; Babilas, P. Wound healing in the 21st century. J. Am. Acad. Dermatol. 2010, 63, 866–881. [Google Scholar] [CrossRef] [PubMed]
- Enoch, S.; Leaper, D.J. Basic science of wound healing. Surgery 2008, 26, 31–37. [Google Scholar]
- Mani, M.P.; Faudzi, A.A.M.; Ramakrishna, S.; Ismail, A.F.; Jaganathan, S.K.; Tucker, N.; Rathanasamy, R. Sustainable electrospun materials with enhanced blood compatibility for wound healing applications—A mini review. Curr. Opin. Biomed. Eng. 2023, 27, 100457. [Google Scholar] [CrossRef]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 1: Normal and chronic wounds: Biology, causes, and approaches to care. Adv. Ski. Wound Care 2012, 25, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Punjataewakupt, A.; Napavichayanun, S.; Aramwit, P. The downside of antimicrobial agents for wound healing. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Sanapalli, B.K.R.; Kannan, E.; Balasubramanian, S.; Natarajan, J.; Baruah, U.K.; Karri, V.V.S.R. Pluronic lecithin organogel of 5-aminosalicylic acid for wound healing. Drug Dev. Ind. Pharm. 2018, 44, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Bello, Y.M.; Falabella, A.F.; Eaglstein, W.H. Tissue-engineered skin: Current status in wound healing. Am. J. Clin. Dermatol. 2001, 2, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.B.; Sporn, M.B.; Assoian, R.K.; Smith, J.M.; Roche, N.S.; Wakefield, L.M.; Heine, U.I.; Liotta, L.A.; Falanga, V.; Kehrl, J.H. Transforming growth factor type beta: Rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc. Natl. Acad. Sci. USA 1986, 83, 4167–4171. [Google Scholar] [CrossRef] [PubMed]
- Sanapalli, B.K.R.; Tyagi, R.; Shaik, A.B.; Pelluri, R.; Bhandare, R.R.; Annadurai, S.; Karri, V.V.S.R. L-Glutamic acid loaded collagen chitosan composite scaffold as regenerative medicine for the accelerated healing of diabetic wounds. Arab. J. Chem. 2022, 15, 103841. [Google Scholar] [CrossRef]
- Federation, I.D. IDF Diabetes Atlas, 8th ed.; International Diabetes Federation: Brussels, Belgium, 2017; pp. 905–911. [Google Scholar]
- Burgess, J.L.; Wyant, W.A.; Abdo Abujamra, B.; Kirsner, R.S.; Jozic, I. Diabetic wound-healing science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Berendt, A.R.; Cornia, P.B.; Pile, J.C.; Peters, E.J.; Armstrong, D.G.; Deery, H.G.; Embil, J.M.; Joseph, W.S.; Karchmer, A.W. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin. Infect. Dis. 2012, 54, e132–e173. [Google Scholar] [CrossRef] [PubMed]
- Lavery, L.A.; Davis, K.E.; Berriman, S.J.; Braun, L.; Nichols, A.; Kim, P.J.; Margolis, D.; Peters, E.J.; Attinger, C. WHS guidelines update: Diabetic foot ulcer treatment guidelines. Wound Repair Regen. 2016, 24, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; May, M.J.; Kopp, E.B. NF-κB and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998, 16, 225–260. [Google Scholar] [CrossRef] [PubMed]
- Karri, V.V.S.R.; Kuppusamy, G.; Talluri, S.V.; Yamjala, K.; Mannemala, S.S.; Malayandi, R. Current and emerging therapies in the management of diabetic foot ulcers. Curr. Med. Res. Opin. 2016, 32, 519–542. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Sundaram, C.; Reuter, S.; Aggarwal, B.B. Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochim. Biophys. Acta Gene Regul. Mech. 2010, 1799, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Cavicchi, M.; Gibbs, L.; Whittle, B.J. Heme oxygenase-1 (HO-l) induction in human intestinal epithelial cells by sulphasalazine and 5-aminosalicylic acid: A potential therapeutic mechanism. Gastroenterology 2000, 118, A804. [Google Scholar] [CrossRef]
- Desreumaux, P.; Ghosh, S. mode of action and delivery of 5-aminosalicylic acid–new evidence. Aliment. Pharmacol. Ther. 2006, 24, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Vierziger, K.; Sturm, A.; Wiedenmann, B.; Dignass, A.U. Mesalamine promotes intestinal epithelial wound healing in vitro through a TGF-beta-independent mechanism. Scand. J. Gastroenterol. 2005, 40, 958–964. [Google Scholar] [CrossRef]
- Bertin, B.; Dubuquoy, L.; Colombel, J.-F.; Desreumaux, P. PPAR-gamma in ulcerative colitis: A novel target for intervention. Curr. Drug Targets 2013, 14, 1501–1507. [Google Scholar] [CrossRef]
- Rousseaux, C.; Lefebvre, B.; Dubuquoy, L.; Lefebvre, P.; Romano, O.; Auwerx, J.; Metzger, D.; Wahli, W.; Desvergne, B.; Naccari, G.C. Intestinal antiinflammatory effect of 5-aminosalicylic acid is dependent on peroxisome proliferator–activated receptor-γ. J. Exp. Med. 2005, 201, 1205–1215. [Google Scholar] [CrossRef]
- Oh-Oka, K.; Kojima, Y.; Uchida, K.; Yoda, K.; Ishimaru, K.; Nakajima, S.; Hemmi, J.; Kano, H.; Fujii-Kuriyama, Y.; Katoh, R. Induction of colonic regulatory T cells by mesalamine by activating the aryl hydrocarbon receptor. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 135–151. [Google Scholar] [CrossRef] [PubMed]
- Na, J.; Lee, K.; Na, W.; Shin, J.-Y.; Lee, M.-J.; Yune, T.Y.; Lee, H.K.; Jung, H.-S.; Kim, W.S.; Ju, B.-G. Histone H3K27 demethylase JMJD3 in cooperation with NF-κB regulates keratinocyte wound healing. J. Investig. Dermatol. 2016, 136, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Mirastschijski, U.; Impola, U.; Karsdal, M.A.; Saarialho-Kere, U.; Ågren, M.S. Matrix metalloproteinase inhibitor BB-3103 unlike the serine proteinase inhibitor aprotinin abrogates epidermal healing of human skin wounds ex vivo. J. Investig. Dermatol. 2002, 118, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Wahl, C.; Liptay, S.; Adler, G.; Schmid, R.M. Sulfasalazine: A potent and specific inhibitor of nuclear factor kappa B. J. Clin. Investig. 1998, 101, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Frantz, B.; O’Neill, E.A.; Ghosh, S.; Kopp, E. The effect of sodium salicylate and aspirin on NF-κB. Science 1995, 270, 2017–2019. [Google Scholar] [CrossRef] [PubMed]
- Kopp, E.; Ghosh, S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science 1994, 265, 956–959. [Google Scholar] [CrossRef] [PubMed]
- Bantel, H.; Berg, C.; Vieth, M.; Stolte, M.; Kruis, W.; Schulze-Osthoff, K. Mesalazine inhibits activation of transcription factor NF-κB in inflamed mucosa of patients with ulcerative colitis. Am. J. Gastroenterol. 2000, 95, 3452. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, G.C.; Yan, F.; Polk, D.B. Mesalamine blocks tumor necrosis factor growth inhibition and nuclear factor κB activation in mouse colonocytes. Gastroenterology 1999, 116, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Raju, K.R.S.; Kumar, M.S.; Gupta, S.; Naga, S.T.; Shankar, J.K.; Murthy, V.; Madhunapanthula, S.R.V.; Mulukutla, S.; Ambhore, N.S.; Tummala, S. 5-Aminosalicylic acid attenuates allergen-induced airway inflammation and oxidative stress in asthma. Pulm. Pharmacol. Ther. 2014, 29, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Romero, C.; Sadidi, M.; Feldman, E.L. Mechanisms of disease: The oxidative stress theory of diabetic neuropathy. Rev. Endocr. Metab. Disord. 2008, 9, 301–314. [Google Scholar] [CrossRef]
- White, L.A.; Mitchell, T.I.; Brinckerhoff, C.E. Transforming growth factor β inhibitory element in the rabbit matrix metalloproteinase-1 (collagenase-1) gene functions as a repressor of constitutive transcription. Biochim. Biophys. Acta Gene Struct. Expr. 2000, 1490, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.C.; Kim, H.T.; Park, S.H.; Cha, J.S.; Yufit, T.; Kim, S.J.; Falanga, V. Fibroblasts from chronic wounds show altered TGF-β-signaling and decreased TGF-β Type II Receptor expression. J. Cell. Physiol. 2003, 195, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Pastar, I.; Stojadinovic, O.; Krzyzanowska, A.; Barrientos, S.; Stuelten, C.; Zimmerman, K.; Blumenberg, M.; Brem, H.; Tomic-Canic, M. Attenuation of the transforming growth factor β-signaling pathway in chronic venous ulcers. Mol. Med. 2010, 16, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Michalik, L.; Wahli, W. Involvement of PPAR nuclear receptors in tissue injury and wound repair. J. Clin. Investig. 2006, 116, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, W.; Bi, M.; Wu, J. The molecular mechanisms of action of PPAR-γ agonists in the treatment of corneal alkali burns. Int. J. Mol. Med. 2016, 38, 1003–1011. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shibuya, A.; Wada, K.; Nakajima, A.; Saeki, M.; Katayama, K.; Mayumi, T.; Kadowaki, T.; Niwa, H.; Kamisaki, Y. Nitration of PPARγ inhibits ligand-dependent translocation into the nucleus in a macrophage-like cell line, RAW 264. FEBS Lett. 2002, 525, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Schultz, G.S.; Wysocki, A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009, 17, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Pertuit, D.; Moulari, B.; Betz, T.; Nadaradjane, A.; Neumann, D.; Ismaïli, L.; Refouvelet, B.; Pellequer, Y.; Lamprecht, A. 5-amino salicylic acid bound nanoparticles for the therapy of inflammatory bowel disease. J. Control. Release 2007, 123, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Jhundoo, H.D.; Siefen, T.; Liang, A.; Schmidt, C.; Lokhnauth, J.; Béduneau, A.; Pellequer, Y.; Larsen, C.C.; Lamprecht, A. Anti-inflammatory activity of chitosan and 5-amino salicylic acid combinations in experimental colitis. Pharmaceutics 2020, 12, 1038. [Google Scholar] [CrossRef]
- Jhundoo, H.D.; Siefen, T.; Liang, A.; Schmidt, C.; Lokhnauth, J.; Moulari, B.; Béduneau, A.; Pellequer, Y.; Larsen, C.C.; Lamprecht, A. Anti-inflammatory effects of acacia and guar gum in 5-amino salicylic acid formulations in experimental colitis. Int. J. Pharm. X 2021, 3, 100080. [Google Scholar] [CrossRef]
- Jhundoo, H.D.; Siefen, T.; Liang, A.; Schmidt, C.; Lokhnauth, J.; Moulari, B.; Béduneau, A.; Pellequer, Y.; Larsen, C.C.; Lamprecht, A. Hyaluronic acid increases anti-inflammatory efficacy of rectal 5-amino salicylic acid administration in a Murine Colitis Model. Biomol. Ther. 2021, 29, 536. [Google Scholar] [CrossRef]
- Aguzzi, A.C.; Ortega, A.; Bonferoni, M.C.; Sandri, G.; Cerezo, P.; Salcedo, I.; Sánchez, R.; Viseras, C.; Caramella, C. Assessement of anti-inflammatory properties of microspheres prepared with chitosan and 5-amino salicylic acid over inflamed Caco-2 cells. Carbohydr. Polym. 2011, 85, 638–644. [Google Scholar] [CrossRef]
- Li, K.; Zhu, Y.; Zhang, P.; Alini, M.; Grad, S.; Li, Z. Anti-inflammatory and pro-anabolic effects of 5-aminosalicylic acid on human inflammatory osteoarthritis models. J. Orthop. Transl. 2023, 38, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Qu, T.; Wang, E.; Jin, B.; Li, W.; Liu, R.; Zhao, Z.-b. 5-Aminosalicylic acid inhibits inflammatory responses by suppressing JNK and p38 activity in murine macrophages. Immunopharmacol. Immunotoxicol. 2017, 39, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Xiang, D.; Wang, F.; Mao, J.; Tan, X.; Wang, Y. 5-ASA-loaded SiO2 nanoparticles-a novel drug delivery system targeting therapy on ulcerative colitis in mice. Mol. Med. Rep. 2017, 15, 1117–1122. [Google Scholar] [CrossRef]
- Chiu, C.-T.; Kuo, S.-N.; Hung, S.-W.; Yang, C.-Y. Combined treatment with hyaluronic acid and mesalamine protects rats from inflammatory bowel disease induced by intracolonic administration of trinitrobenzenesulfonic acid. Molecules 2017, 22, 904. [Google Scholar] [CrossRef]
- Ahmad, A.; Ansari, M.M.; Mishra, R.K.; Kumar, A.; Vyawahare, A.; Verma, R.K.; Raza, S.S.; Khan, R. Enteric-coated gelatin nanoparticles mediated oral delivery of 5-aminosalicylic acid alleviates severity of DSS-induced ulcerative colitis. Mater. Sci. Eng. C 2021, 119, 111582. [Google Scholar] [CrossRef]
- Joshi, R.; Kumar, S.; Unnikrishnan, M.; Mukherjee, T. Free radical scavenging reactions of sulfasalazine, 5-aminosalicylic acid and sulfapyridine: Mechanistic aspects and antioxidant activity. Free Radic. Res. 2005, 39, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Couto, D.; Ribeiro, D.; Freitas, M.; Gomes, A.; Lima, J.L.; Fernandes, E. Scavenging of reactive oxygen and nitrogen species by the prodrug sulfasalazine and its metabolites 5-aminosalicylic acid and sulfapyridine. Redox Rep. 2010, 15, 259–267. [Google Scholar] [CrossRef]
- Pereira, S.R.; Almeida, L.M.; Dinis, T.C. Improving the anti-inflammatory activity of 5-aminosalicylic acid by combination with cyanidin-3-glucoside: An in vitro study. J. Funct. Foods 2019, 63, 103586. [Google Scholar] [CrossRef]
- Caltabiano, C.; Máximo, F.R.; Spadari, A.P.P.; da Conceição Miranda, D.D.; Serra, M.M.P.; Ribeiro, M.L.; Martinez, C.A.R. 5-aminosalicylic acid (5-ASA) can reduce levels of oxidative DNA damage in cells of colonic mucosa with and without fecal stream. Dig. Dis. Sci. 2011, 56, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, E.; Almeida, L.M.; Dinis, T.C. Antioxidant activity of 5-aminosalicylic acid against lipid peroxidation in the presence of vitamins C and E. Int. J. Pharm. 1998, 172, 219–228. [Google Scholar] [CrossRef]
- Managlia, E.; Katzman, R.B.; Brown, J.B.; Barrett, T.A. Antioxidant properties of mesalamine in colitis inhibit phosphoinositide 3-kinase signaling in progenitor cells. Inflamm. Bowel Dis. 2013, 19, 2051–2060. [Google Scholar] [CrossRef] [PubMed]
- Rajeh, N.A.; Al-Dhaheri, N.M. Antioxidant effect of vitamin E and 5-aminosalicylic acid on acrylamide induced kidney injury in rats. Saudi Med. J. 2017, 38, 132. [Google Scholar] [CrossRef]
- Reifen, R.; Nissenkorn, A.; Matas, Z.; Bujanover, Y. 5-ASA and lycopene decrease the oxidative stress and inflammation induced by iron in rats with colitis. J. Gastroenterol. 2004, 39, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Cevallos, S.A.; Lee, J.-Y.; Velazquez, E.M. 5-Aminosalicylic Acid Ameliorates Colitis and Checks Dysbiotic Escherichia coli. mBio 2021, 12, e03227-20. [Google Scholar] [CrossRef] [PubMed]
- Adachi, M.; Kurotani, R.; Morimura, K.; Shah, Y.; Sanford, M.; Madison, B.B.; Gumucio, D.L.; Marin, H.E.; Peters, J.M.; Young, H.A. Peroxisome proliferator activated receptor γ in colonic epithelial cells protects against experimental inflammatory bowel disease. Gut 2006, 55, 1104–1113. [Google Scholar] [CrossRef]
- Mbodji, K.; Charpentier, C.; Guérin, C.; Querec, C.; Bole-Feysot, C.; Aziz, M.; Savoye, G.; Déchelotte, P.; Marion-Letellier, R. Adjunct therapy of n-3 fatty acids to 5-ASA ameliorates inflammatory score and decreases NF-κB in rats with TNBS-induced colitis. J. Nutr. Biochem. 2013, 24, 700–705. [Google Scholar] [CrossRef]
- Jung, E.S.; Jang, H.J.; Hong, E.M.; Lim, H.L.; Lee, S.P.; Kae, S.H.; Lee, J. The protective effect of 5-aminosalicylic acid against non-steroidal anti-inflammatory drug-induced injury through free radical scavenging in small intestinal epithelial cells. Medicina 2020, 56, 515. [Google Scholar] [CrossRef]
- Khare, V.; Krnjic, A.; Frick, A.; Gmainer, C.; Asboth, M.; Jimenez, K.; Lang, M.; Baumgartner, M.; Evstatiev, R.; Gasche, C. Mesalamine and azathioprine modulate junctional complexes and restore epithelial barrier function in intestinal inflammation. Sci. Rep. 2019, 9, 2842. [Google Scholar] [CrossRef]
- Deng, X.; Tolstanova, G.; Khomenko, T.; Chen, L.; Tarnawski, A.; Szabo, S.; Sandor, Z. Mesalamine restores angiogenic balance in experimental ulcerative colitis by reducing expression of endostatin and angiostatin: Novel molecular mechanism for therapeutic action of mesalamine. J. Pharmacol. Exp. Ther. 2009, 331, 1071–1078. [Google Scholar] [CrossRef] [PubMed]

| Drug/ Formulation | Pharmacological Effect | Biomarkers | Type of Study Conducted | References |
|---|---|---|---|---|
| 5-ASA-bound nanoparticles | Inflammation | Clinical activity score (CAS), colon weight/length index, myeloperoxidase (MPO) | Preexistent colitis model in mice | [39] |
| 5-ASA and chitosan combination | Inflammation | Clinical activity score (CAS), colon weight/length index, myeloperoxidase (MPO), alkaline phosphatase (ALP), TNF-α, IL-6, and NF-κB, p65 | Colitis model in male Swiss/CD-1 mice | [40] |
| 5-ASA in combination with acacia and guar gum | Inflammation | Disease activity index, colon weight/length ratio, IL-1β, NF-κB, p65, TNF-α, and IL-6 | Colitis model in male Swiss/CD-1 mice | [41] |
| 5-ASA and hyaluronic acid combination | Inflammation | Clinical activity index, MPO, TNF-α, IL-6, and IL-1β | Colitis model in male Swiss/CD-1 mice | [42] |
| 5-ASA and chitosan microspheres | Inflammation | Cell viability and expression of mRNA levels | Caco-2 cell lines | [43] |
| 5-ASA | Inflammation | IL-6, IL-8, COX-2, nitric oxide, glycosaminoglycan, and anabolic genes (aggrecan (ACAN), alpha-1 chain of type II collagen (COL2A1), proteoglycan 4 (PRG4), cartilage oligomeric matrix protein (COMP)) | In vitro (chondrocyte pellets) and ex vivo (osteochondral explants) human inflammatory osteoarthritis models | [44] |
| 5-ASA | Inflammation | NO, IL-6, induced nitric oxide synthase (iNOS), c-Jun N-terminal kinases (JNKs), p38, and NF-κB | LPS-induced murine macrophages | [45] |
| 5-ASA–silicon oxide nanoparticles | Inflammation | MPO, IL-6, and TNF-α | BALB/c colitis model | [46] |
| 5-ASA in combination with hyaluronic acid | Inflammation | MPO, COX-2, TNF-α, IL-1β, and IL-6 | TNBS-induced colitis rat model | [47] |
| 5-ASA gelatin-coated nanoparticles | Inflammation | TNF-α, IL-1β, COX-2, and iNOS | Dextran sodium sulfate-induced colitis murine model | [48] |
| 5-ASA | Oxidation | Free radicals such as hydroxyl, haloperoxyl, one-electron oxidizing, lipid peroxyl, glutathiyl, superoxide, tryptophany | Nanosecond pulse radiolysis technique coupled with transient spectrophotometry has been used for in situ generation of free radicals and to follow their reaction pathways | [49] |
| 5-ASA | Oxidation | ROS (O2•−, H2O2, 1O2, ROO•, and HOCl) and reactive nitrogen species (•NO and ONOO−) | Chemical scavenging validated test | [50] |
| 5-ASA in combination with cyanidin-3-glucoside (Cy3glc) | Oxidation | ROS and NO | LPS-activated macrophage line | [51] |
| 5-ASA | Oxidation | ROS species for oxidative DNA damage | Diversion colitis model in experimental Wistar rats | [52] |
| 5-ASA and ascorbic acid | Oxidation | Vitamin E consumption, oxygen consumption and formation of conjugated dienes | Lipid peroxidation (thermal decomposition of Azo compounds) in phosphatidylcholine (PC) liposomes as a model | [53] |
| 5-ASA | Oxidation | ROS and catalase | Oxidant-induced cell signaling pathways in HT-29 cells and IECs from mice | [54] |
| 5-ASA and vitamin E | Oxidation | Change in body weight and lactate dehydrogenase activity | Acrylamide-induced kidney injury in Wistar rat model | [55] |
| 5-ASA and lycopene | Oxidation and inflammation | Myeloperoxidase (MPO), malondialdehyde (MDA), and superoxide dismutase (SOD) | Colitis model in iodoacetamide rat | [56] |
| 5-ASA | Inflammation and PPAR-γ activation | Oxygen consumption, E. coli growth | Dextran sulfate sodium (DSS)-induced colitis murine model | [57] |
| 5-ASA | Inflammation and PPAR-γ activation | PPAR-γ expression, β-actin, and MPO | 2,4,6-trinitrobenzenesulphonic acid (TNBS)-induced colitis murine model | [21] |
| 5-ASA | Inflammation and PPAR-γ activation | IFN-γ, NF-κB, STAT-1 and -3, SOCS-1 and -3 | 10 Gy γ-irradiation (Co source)-induced colitis rat model | [58] |
| 5-ASA and n-3 ploy unsaturated fatty acids | Inflammation and PPAR-γ activation | NF-κB, COX-2, and leukotriene-B4 | TNBS-induced colitis rat model | [59] |
| 5-ASA | Inflammation, oxidation, and proliferation | ROS, MTT assay, cell apoptosis assay, caspase-3 activity, SOD2 and wound healing assay | Indomethacin-induced injury in IEC-6 cell line of rats | [60] |
| 5-ASA in combination with azathioprine | Inflammation, oxidation, and proliferation | ROS and senescence-associated β-galactosidase activity, cell cycle analysis, BrdU incorporation assay, TNF-α | T-84 cell lines and small intestinal large bowel organoids from C57BL/6J wild-type and IL-10−/− (IL-10 KO) mice | [61] |
| 5-ASA pluronic lecitin organogel | Cell proliferation and migration | MTT assay, cell migration | Full thickness excision wound rat model | [6] |
| 5-ASA | Cell proliferation and migration | MTT assay, migration assay | IEC-6 in vitro wounding model | [19] |
| 5-ASA | Angiogenesis | Expression of VEGF, endostatin, angiostatin, TNF-α, and MMP-2 and -9 | Iodoacetamide-induced ulcerative colitis rat model | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanapalli, B.K.R.; Deshpande, A.; Sanapalli, V.; Sigalapalli, D.K. Unveiling the Unexplored Multifactorial Potential of 5-Aminosalicylic Acid in Diabetic Wound Therapy. Diseases 2024, 12, 172. https://doi.org/10.3390/diseases12080172
Sanapalli BKR, Deshpande A, Sanapalli V, Sigalapalli DK. Unveiling the Unexplored Multifactorial Potential of 5-Aminosalicylic Acid in Diabetic Wound Therapy. Diseases. 2024; 12(8):172. https://doi.org/10.3390/diseases12080172
Chicago/Turabian StyleSanapalli, Bharat Kumar Reddy, Ashwini Deshpande, Vidyasrilekha Sanapalli, and Dilep Kumar Sigalapalli. 2024. "Unveiling the Unexplored Multifactorial Potential of 5-Aminosalicylic Acid in Diabetic Wound Therapy" Diseases 12, no. 8: 172. https://doi.org/10.3390/diseases12080172
APA StyleSanapalli, B. K. R., Deshpande, A., Sanapalli, V., & Sigalapalli, D. K. (2024). Unveiling the Unexplored Multifactorial Potential of 5-Aminosalicylic Acid in Diabetic Wound Therapy. Diseases, 12(8), 172. https://doi.org/10.3390/diseases12080172








