SARS-CoV-2 Infection during Delivery Causes Histopathological Changes in the Placenta
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Tissue Samples
2.2. Confirmation of the SARS-CoV-2 Infection
2.3. Histochemical Staining
2.4. Histopathological Evaluation
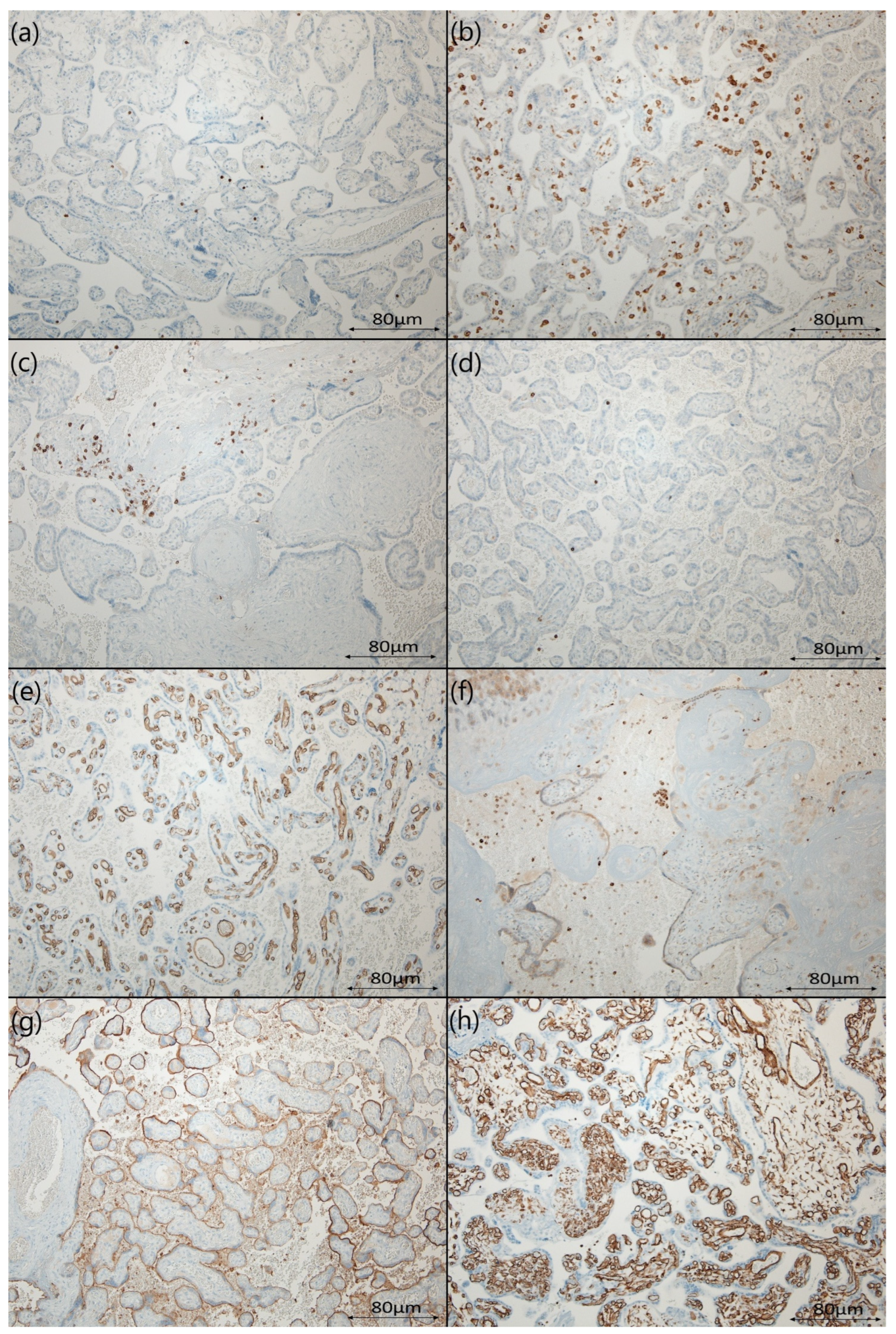
2.5. Immunohistochemical Staining
2.6. IHC Evaluation
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
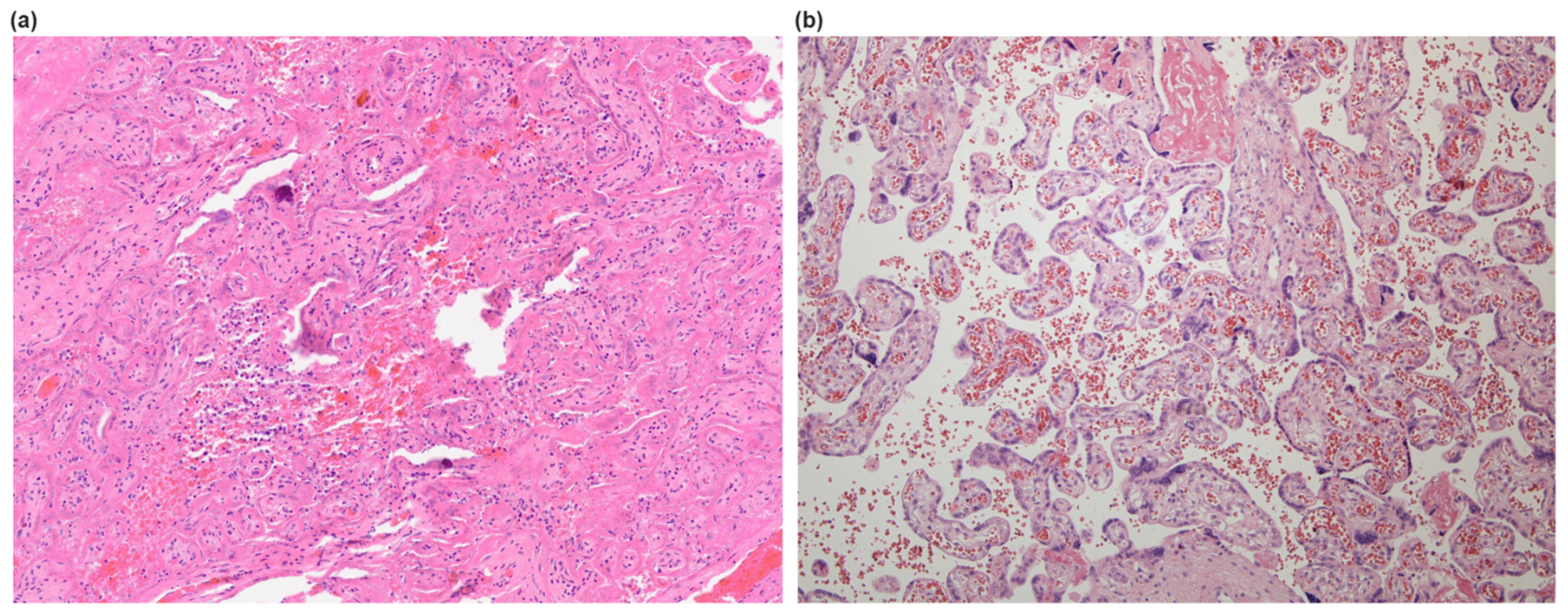
3.2. Histological Findings in Placentas
3.3. The Differences in the Distribution of Assessed Protein Expression
3.4. Correlation between Histopathological Findings and Immunohistochemical Markers
4. Discussion
4.1. Placenta Susceptibility to SARS-CoV-2 Infection
4.2. SARS-CoV-2 Causes Histopathological Changes in Placenta
4.3. SARS-CoV-2-Associated Intervillositis
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, Y.; Wang, G.; Cai, X.-P.; Deng, J.-W.; Zheng, L.; Zhu, H.-H.; Zheng, M.; Yang, B.; Chen, Z. An Overview of COVID-19. J. Zhejiang Univ. Sci. B 2020, 21, 343–360. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Ochani, R.; Asad, A.; Yasmin, F.; Shaikh, S.; Khalid, H.; Batra, S.; Sohail, M.R.; Mahmood, S.F.; Ochani, R.; Hussham Arshad, M.; et al. COVID-19 Pandemic: From Origins to Outcomes. A Comprehensive Review of Viral Pathogenesis, Clinical Manifestations, Diagnostic Evaluation, and Management. Infez. Med. 2021, 29, 20–36. [Google Scholar] [PubMed]
- delli Muti, N.; Finocchi, F.; Tossetta, G.; Salvio, G.; Cutini, M.; Marzioni, D.; Balercia, G. Could SARS-CoV-2 Infection Affect Male Fertility and Sexuality? Apmis 2022, 130, 243–252. [Google Scholar] [CrossRef] [PubMed]
- SeyedAlinaghi, S.; Karimi, A.; Barzegary, A.; Mojdeganlou, H.; Vahedi, F.; Mirghaderi, S.P.; Shobeiri, P.; Ramezani, M.; Yousefi Konjdar, P.; Mirzapour, P.; et al. COVID-19 Mortality in Patients with Immunodeficiency and Its Predictors: A Systematic Review. Eur. J. Med. Res. 2022, 27, 195. [Google Scholar] [CrossRef] [PubMed]
- Lamers, M.M.; Haagmans, B.L. SARS-CoV-2 Pathogenesis. Nat. Rev. Microbiol. 2022, 20, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Majumder, J.; Minko, T. Recent Developments on Therapeutic and Diagnostic Approaches for COVID-19. AAPS J. 2021, 23, 14. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.-L.; Navis, G.J.; Gordijn, S.J.; Bolling, M.C.; Dijkstra, G.; Voors, A.A.; Osterhaus, A.D.; et al. Angiotensin-Converting Enzyme 2 (ACE2), SARS-CoV-2 and the Pathophysiology of Coronavirus Disease 2019 (COVID-19). J. Pathol. 2020, 251, 228–248. [Google Scholar] [CrossRef]
- Beyerstedt, S.; Casaro, E.B.; Rangel, É.B. COVID-19: Angiotensin-Converting Enzyme 2 (ACE2) Expression and Tissue Susceptibility to SARS-CoV-2 Infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 905–919. [Google Scholar] [CrossRef]
- Iwasaki, M.; Saito, J.; Zhao, H.; Sakamoto, A.; Hirota, K.; Ma, D. Inflammation Triggered by SARS-CoV-2 and ACE2 Augment Drives Multiple Organ Failure of Severe COVID-19: Molecular Mechanisms and Implications. Inflammation 2021, 44, 13–34. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; delli Muti, N.; Balercia, G.; Ciavattini, A.; Giannubilo, S.R.; Marzioni, D. Preeclampsia and Severe Acute Respiratory Syndrome Coronavirus 2 Infection: A Systematic Review. J. Hypertens. 2022, 40, 1629–1638. [Google Scholar] [CrossRef]
- Bhatia, P.; Bhatia, K. Pregnancy and the Lungs. Postgrad. Med. J. 2000, 76, 683–689. [Google Scholar] [CrossRef]
- Wong, Y.P.; Khong, T.Y.; Tan, G.C. The Effects of COVID-19 on Placenta and Pregnancy: What Do We Know So Far? Diagnostics 2021, 11, 94. [Google Scholar] [CrossRef]
- Wastnedge, E.A.N.; Reynolds, R.M.; van Boeckel, S.R.; Stock, S.J.; Denison, F.C.; Maybin, J.A.; Critchley, H.O.D. Pregnancy and COVID-19. Physiol. Rev. 2021, 101, 303–318. [Google Scholar] [CrossRef]
- Vousden, N.; Ramakrishnan, R.; Bunch, K.; Morris, E.; Simpson, N.; Gale, C.; O’Brien, P.; Quigley, M.; Brocklehurst, P.; Kurinczuk, J.J.; et al. Management and Implications of Severe COVID-19 in Pregnancy in the UK: Data from the UK Obstetric Surveillance System National Cohort. Acta Obstet. Gynecol. Scand. 2022, 101, 461–470. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and Pregnancy: The Role of the Immune System at the Implantation Site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Atyeo, C.G.; Shook, L.L.; Brigida, S.; De Guzman, R.M.; Demidkin, S.; Muir, C.; Akinwunmi, B.; Baez, A.M.; Sheehan, M.L.; McSweeney, E.; et al. Maternal Immune Response and Placental Antibody Transfer after COVID-19 Vaccination across Trimester and Platforms. Nat. Commun. 2022, 13, 3571. [Google Scholar]
- Balachandren, N.; Davies, M.C.; Hall, J.A.; Stephenson, J.M.; David, A.L.; Barrett, G.; O’Neill, H.C.; Ploubidis, G.B.; Yasmin, E.; Mavrelos, D. SARS-CoV-2 Infection in the First Trimester and the Risk of Early Miscarriage: A UK Population-Based Prospective Cohort Study of 3041 Pregnancies Conceived during the Pandemic. Hum. Reprod. 2022, 37, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Fallach, N.; Segal, Y.; Agassy, J.; Perez, G.; Peretz, A.; Chodick, G.; Gazit, S.; Patalon, T.; Ben Tov, A.; Goldshtein, I. Pregnancy Outcomes after SARS-CoV-2 Infection by Trimester: A Large, Population-Based Cohort Study. PLoS ONE 2022, 17, e0270893. [Google Scholar] [CrossRef]
- Sessa, R.; Filardo, S.; Masciullo, L.; Di Pietro, M.; Angeloni, A.; Brandolino, G.; Brunelli, R.; D’Alisa, R.; Viscardi, M.F.; Anastasi, E.; et al. SARS-CoV-2 Infection in Pregnancy: Clues and Proof of Adverse Outcomes. Int. J. Environ. Res. Public Health 2023, 20, 2616. [Google Scholar] [CrossRef]
- Golden, T.N.; Simmons, R.A. Maternal and Neonatal Response to COVID-19. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E315–E319. [Google Scholar] [CrossRef]
- Wei, S.Q.; Bilodeau-Bertrand, M.; Liu, S.; Auger, N. The Impact of COVID-19 on Pregnancy Outcomes: A Systematic Review and Meta-Analysis. CMAJ 2021, 193, E540–E548. [Google Scholar] [CrossRef] [PubMed]
- Di Girolamo, R.; Khalil, A.; Alameddine, S.; D’Angelo, E.; Galliani, C.; Matarrelli, B.; Buca, D.; Liberati, M.; Rizzo, G.; D’Antonio, F. Placental Histopathology after SARS-CoV-2 Infection in Pregnancy: A Systematic Review and Meta-Analysis. Am. J. Obstet. Gynecol. MFM 2021, 3, 100468. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Saadaoui, M.; Al Khodor, S. Infections and Pregnancy: Effects on Maternal and Child Health. Front. Cell. Infect. Microbiol. 2022, 12, 873253. [Google Scholar] [CrossRef] [PubMed]
- Abuova, G.; Ayazbekov, A.; Nurkhasimova, R.; Shaimardenova, G.; Kulbaeva, S.; Nurmagambet, S. Asymptomatic Forms of COVID-19 in Pregnant Women: Long-Term Consequences. Int. J. Infect. Dis. 2022, 116, S46. [Google Scholar] [CrossRef]
- Shanes, E.D.; Mithal, L.B.; Otero, S.; Azad, H.A.; Miller, E.S.; Goldstein, J.A. Placental Pathology in COVID-19. Am. J. Clin. Pathol. 2020, 154, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A.; Avvad-Portari, E.; Babál, P.; Baldewijns, M.; Blomberg, M.; Bouachba, A.; Camacho, J.; Collardeau-Frachon, S.; Colson, A.; Dehaene, I.; et al. Placental Tissue Destruction and Insufficiency From COVID-19 Causes Stillbirth and Neonatal Death From Hypoxic-Ischemic Injury. Arch. Pathol. Lab. Med. 2022, 146, 660–676. [Google Scholar] [CrossRef]
- Yuan, J.; Qian, H.; Cao, S.; Dong, B.; Yan, X.; Luo, S.; Zhou, M.; Zhou, S.; Ning, B.; Zhao, L. Is There Possibility of Vertical Transmission of COVID-19: A Systematic Review. Transl. Pediatr. 2021, 10, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.K.; Mohanta, G.C.; Kumar, V.; Gupta, K. Diagnostic Tools for Rapid Screening and Detection of SARS-CoV-2 Infection. Vaccines 2022, 10, 1200. [Google Scholar] [CrossRef] [PubMed]
- Khong, T.Y.; Mooney, E.E.; Ariel, I.; Balmus, N.C.M.; Boyd, T.K.; Brundler, M.-A.; Derricott, H.; Evans, M.J.; Faye-Petersen, O.M.; Gillan, J.E.; et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch. Pathol. Lab. Med. 2016, 140, 698–713. [Google Scholar] [CrossRef]
- Varghese, F.; Bukhari, A.B.; Malhotra, R.; De, A. IHC Profiler: An Open Source Plugin for the Quantitative Evaluation and Automated Scoring of Immunohistochemistry Images of Human Tissue Samples. PLoS ONE 2014, 9, e96801. [Google Scholar] [CrossRef]
- Knöfler, M.; Haider, S.; Saleh, L.; Pollheimer, J.; Gamage, T.K.J.B.; James, J. Human Placenta and Trophoblast Development: Key Molecular Mechanisms and Model Systems. Cell. Mol. Life Sci. 2019, 76, 3479–3496. [Google Scholar] [CrossRef] [PubMed]
- Kapila, V.; Chaudhry, K. Physiology, Placenta. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Benirschke, K.; Burton, G.J.; Baergen, R.N. Basic Structure of the Villous Trees. In Pathology of the Human Placenta; Benirschke, K., Burton, G.J., Baergen, R.N., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 55–100. ISBN 9783642239410. [Google Scholar]
- Ruan, D.; Ye, Z.-W.; Yuan, S.; Li, Z.; Zhang, W.; Ong, C.P.; Tang, K.; Ka Ki Tam, T.T.; Guo, J.; Xuan, Y.; et al. Human Early Syncytiotrophoblasts Are Highly Susceptible to SARS-CoV-2 Infection. Cell Rep. Med. 2022, 3, 100849. [Google Scholar] [CrossRef] [PubMed]
- Megli, C.J.; Coyne, C.B. Infections at the Maternal-Fetal Interface: An Overview of Pathogenesis and Defence. Nat. Rev. Microbiol. 2022, 20, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Bustamante Helfrich, B.; Chilukuri, N.; He, H.; Cerda, S.R.; Hong, X.; Wang, G.; Pearson, C.; Burd, I.; Wang, X. Maternal Vascular Malperfusion of the Placental Bed Associated with Hypertensive Disorders in the Boston Birth Cohort. Placenta 2017, 52, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Burke, S.D.; Karumanchi, S.A. Imbalances in Circulating Angiogenic Factors in the Pathophysiology of Preeclampsia and Related Disorders. Am. J. Obstet. Gynecol. 2022, 226, S1019–S1034. [Google Scholar] [CrossRef] [PubMed]
- Amraei, R.; Xia, C.; Olejnik, J.; White, M.R.; Napoleon, M.A.; Lotfollahzadeh, S.; Hauser, B.M.; Schmidt, A.G.; Chitalia, V.; Mühlberger, E.; et al. Extracellular Vimentin Is an Attachment Factor That Facilitates SARS-CoV-2 Entry into Human Endothelial Cells. Proc. Natl. Acad. Sci. USA 2022, 119, e2113874119. [Google Scholar] [CrossRef] [PubMed]
- Pilarska, I.; Bizon, M.; Sawicki, W. Influence of COVID-19 Infection on Placental Function. Ginekol. Pol. 2023, 94, 79–83. [Google Scholar] [CrossRef]
- Jaiswal, N.; Puri, M.; Agarwal, K.; Singh, S.; Yadav, R.; Tiwary, N.; Tayal, P.; Vats, B. COVID-19 as an Independent Risk Factor for Subclinical Placental Dysfunction. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 259, 7–11. [Google Scholar] [CrossRef]
- Gychka, S.G.; Brelidze, T.I.; Kuchyn, I.L.; Savchuk, T.V.; Nikolaienko, S.I.; Zhezhera, V.M.; Chermak, I.I.; Suzuki, Y.J. Placental Vascular Remodeling in Pregnant Women with COVID-19. PLoS ONE 2022, 17, e0268591. [Google Scholar] [CrossRef]
- Watkins, J.C.; Torous, V.F.; Roberts, D.J. Defining Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Placentitis. Arch. Pathol. Lab. Med. 2021, 145, 1341–1349. [Google Scholar] [CrossRef]
- Stenton, S.; McPartland, J.; Shukla, R.; Turner, K.; Marton, T.; Hargitai, B.; Bamber, A.; Pryce, J.; Peres, C.L.; Burguess, N.; et al. SARS-CoV-2 Placentitis and Pregnancy Outcome: A Multicentre Experience during the Alpha and Early Delta Waves of Coronavirus Pandemic in England. EClinicalMedicine 2022, 47, 101389. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Morotti, D.; Beigi, B.; Moshfegh, F.; Zafaranloo, N.; Patanè, L. Confirming Vertical Fetal Infection with Coronavirus Disease 2019: Neonatal and Pathology Criteria for Early Onset and Transplacental Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 from Infected Pregnant Mothers. Arch. Pathol. Lab. Med. 2020, 144, 1451–1456. [Google Scholar] [CrossRef] [PubMed]
- Sarno, L.; Locci, M.; Fulgione, C.; Perillo, F.; Dell’Isola, A.; Mantelli, D.; Sibillo, C.; Saccone, G.; Maruotti, G.M.; Terracciano, D.; et al. Characteristics of Placental Histopathology in Women with Uncomplicated Pregnancies Affected by SARS-CoV-2 Infection at the Time of Delivery: A Single-Center Experience. Biomedicines 2022, 10, 3003. [Google Scholar] [CrossRef] [PubMed]
- Shanes, E.D.; Miller, E.S.; Otero, S.; Ebbott, R.; Aggarwal, R.; Willnow, A.S.; Ozer, E.A.; Mithal, L.B.; Goldstein, J.A. Placental Pathology After SARS-CoV-2 Infection in the Pre-Variant of Concern, Alpha / Gamma, Delta, or Omicron Eras. Int. J. Surg. Pathol. 2022, 31, 387–397. [Google Scholar] [CrossRef]
- Khalil, R.A.; Granger, J.P. Vascular Mechanisms of Increased Arterial Pressure in Preeclampsia: Lessons from Animal Models. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R29–R45. [Google Scholar] [CrossRef]
- Stanek, J.; Abdaljaleel, M. CD34 Immunostain Increases the Sensitivity of Placental Diagnosis of Fetal Vascular Malperfusion in Stillbirth. Placenta 2019, 77, 30–38. [Google Scholar] [CrossRef]
- Shimizu, Y. Mechanism Underlying Vascular Remodeling in Relation to Circulating CD34-Positive Cells among Older Japanese Men. Sci. Rep. 2022, 12, 21823. [Google Scholar] [CrossRef]
- Lo, B.C.; Gold, M.J.; Scheer, S.; Hughes, M.R.; Cait, J.; Debruin, E.; Chu, F.S.F.; Walker, D.C.; Soliman, H.; Rossi, F.M.; et al. Loss of Vascular CD34 Results in Increased Sensitivity to Lung Injury. Am. J. Respir. Cell Mol. Biol. 2017, 57, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Kosyreva, A.; Dzhalilova, D.; Lokhonina, A.; Vishnyakova, P.; Fatkhudinov, T. The Role of Macrophages in the Pathogenesis of SARS-CoV-2-Associated Acute Respiratory Distress Syndrome. Front. Immunol. 2021, 12, 682871. [Google Scholar] [CrossRef]
- Brady, C.A.; Williams, C.; Sharps, M.C.; Shelleh, A.; Batra, G.; Heazell, A.E.P.; Crocker, I.P. Chronic Histiocytic Intervillositis: A Breakdown in Immune Tolerance Comparable to Allograft Rejection? Am. J. Reprod. Immunol. 2021, 85, e13373. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Morotti, D. Placental Pathology of COVID-19 with and without Fetal and Neonatal Infection: Trophoblast Necrosis and Chronic Histiocytic Intervillositis as Risk Factors for Transplacental Transmission of SARS-CoV-2. Viruses 2020, 12, 1308. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A.; Baldewijns, M.; Benachi, A.; Bugatti, M.; Collins, R.R.J.; De Luca, D.; Facchetti, F.; Linn, R.L.; Marcelis, L.; Morotti, D.; et al. Chronic Histiocytic Intervillositis With Trophoblast Necrosis Is a Risk Factor Associated With Placental Infection From Coronavirus Disease 2019 (COVID-19) and Intrauterine Maternal-Fetal Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Transmission in Live-Born and Stillborn Infants. Arch. Pathol. Lab. Med. 2021, 145, 517–528. [Google Scholar] [PubMed]
- Ibrahim, E.; Abernathy, E.; Baiulescu, M.; Balarezo, F. 2021 SPP Virtual Spring Meeting. Pediatr. Dev. Pathol. 2021, 24, 269–286. [Google Scholar]
- Kim, C.J.; Romero, R.; Chaemsaithong, P.; Kim, J.-S. Chronic Inflammation of the Placenta: Definition, Classification, Pathogenesis, and Clinical Significance. Am. J. Obstet. Gynecol. 2015, 213, S53–S69. [Google Scholar] [CrossRef]
- Redline, R.W.; Ravishankar, S. Fetal Vascular Malperfusion, an Update. Apmis 2018, 126, 561–569. [Google Scholar] [CrossRef]
- Wallingford, M.C.; Benson, C.; Chavkin, N.W.; Chin, M.T.; Frasch, M.G. Placental Vascular Calcification and Cardiovascular Health: It Is Time to Determine How Much of Maternal and Offspring Health Is Written in Stone. Front. Physiol. 2018, 9, 1044. [Google Scholar] [CrossRef]
- Smith, E.R.; Oakley, E.; Grandner, G.W.; Rukundo, G.; Farooq, F.; Ferguson, K.; Baumann, S.; Adams Waldorf, K.M.; Afshar, Y.; Ahlberg, M.; et al. Clinical Risk Factors of Adverse Outcomes among Women with COVID-19 in the Pregnancy and Postpartum Period: A Sequential, Prospective Meta-Analysis. Am. J. Obstet. Gynecol. 2023, 228, 161–177. [Google Scholar] [CrossRef]
| Antibody | Product ID | Description | Location/Control | Staining |
|---|---|---|---|---|
| CONFIRM anti-CD3 (2GV6) | 05278422001, 790-4341 | Rabbit, primary, monoclonal | Cytoplasm, Membrane/Tonsil | 16 min, 37 °C |
| CONFIRM anti-CD4 (SP35) | 05552737001, 790-4423 | Rabbit, primary, monoclonal | Membrane/Tonsil | 16 min, 37 °C |
| CONFIRM anti-CD8 (SP57) | 05937248001, 790-4460 | Rabbit, primary, monoclonal | Membrane/Tonsil | 16 min, 37 °C |
| CONFIRM anti-CD20 (L26) | 05267099001, 760-2531 | Mouse, primary, monoclonal | Membrane/Tonsil | 8 min, 37 °C |
| CONFIRM anti-CD34 (QBEnd/10) | 05278210001, 790-2927 | Mouse, primary, monoclonal | Membrane/Tonsil | 12 min, 37 °C |
| CONFIRM anti-CD68 (KP-1) | 05278252001, 790-2931 | Mouse, primary, monoclonal | Membrane/Tonsil | 16 min, 37 °C |
| anti-CD138 (B-A38), PAb, Cell Marque | 05269083001, 760-4248 | Mouse, primary, monoclonal | Membrane/Tonsil | 30 min, 37 °C |
| CONFIRM anti-Vimentin (V9) | 05278139001, 790-2917 | Mouse, primary, monoclonal | Cytoplasm/Tonsil | 16 min, 37 °C |
| Variable | COVID-19 Group (n = 18 *) | Control (n = 22) |
|---|---|---|
| Age | 31 (range 24–38) | 31 (range 22–43) |
| Gestational age at delivery | 34 (range 24–38) | 38 (range 31–41) |
| Gestational age at COVID-19 diagnosis | 34 (range 24–38) | - |
| Mean newborn body mass | 2140 g (range 644–2980 g) | 2395 g (1200–3680 g) |
| Apgar ≥ 7 (1’) | 5/18 (35.71%) | 19/22 (86.36%) |
| Cough | 4/18 (22.22%) | 0/22 (0%) |
| Dyspnea | 9/18 (50%) | 0/22 (0%) |
| Placenta with positive PCR test | 13/15 ** (86.67%) | 0/22 (0%) |
| Placental Lesion | Pathology | Occurrence/ Intensity | COVID-19 Group (n = 23) | Control Group (n = 22) | p-Value |
|---|---|---|---|---|---|
| Infarction | Negative | 18 | 16 | p = 0.43 | |
| Positive | 3 | 5 | |||
| Retroplacental hemorrhage | Negative | 9 | 6 | p = 0.99 | |
| Positive | 0 | 0 | |||
| Maternal vascular malperfusion | Distal villous hypoplasia | Negative | 4 | 10 | p = 0.062 |
| Positive | 16 | 11 | |||
| Accelerated vascular maturation | Negative | 1 | 7 | p = 0.022 More in COVID-19 group | |
| Positive | 19 | 14 | |||
| Decidual arteriopathy | Negative | 0 | 1 | p = 0.36 | |
| Positive | 14 | 12 | |||
| Maternal vascular malperfusion (total) | Negative | 1 | 3 | p = 0.22 | |
| Positive | 20 | 15 | |||
| Venous thrombosis | Negative | 12 | 13 | p = 0.75 | |
| Positive | 9 | 8 | |||
| Arterial thrombosis | Negative | 16 | 18 | p = 0.43 | |
| Positive | 5 | 3 | |||
| Fetal vascular malperfusion | Avascular villi | Negative | 3 | 4 | p = 0.73 |
| Positive | 17 | 17 | |||
| Intramural fibrin depositions | Negative | 13 | 15 | p = 0.84 | |
| Positive | 6 | 6 | |||
| Stroma obliteration | Negative | 3 | 6 | p = 0.35 | |
| Positive | 17 | 15 | |||
| Vascular ectasia | Negative | 7 | 5 | p = 0.44 | |
| Positive | 13 | 16 | |||
| Fetal vascular malperfusion (total) | Negative | 2 | 6 | p = 0.109 | |
| Positive | 16 | 12 | |||
| Delayed vascular maturation | Negative | 13 | 13 | p = 0.67 | |
| Positive | 6 | 8 | |||
| Villitis of unknown etiology | Negative | 20 | 20 | p = 0.99 | |
| Positive | 0 | 0 | |||
| Features | Occurrence/ Intensity | COVID-19 Group (n = 23) | Control Group (n = 22) | p-Value |
|---|---|---|---|---|
| Diffuse fibrin depositions | Sparse | 8 | 13 | p = 0.30 |
| Intermediate | 9 | 8 | ||
| Rich | 6 | 1 | ||
| Positive | 6 | 6 | ||
| Calcifications | No | 4 | 17 | p = 0.0004 More in COVID-19 |
| Yes | 19 | 5 | ||
| Intervillous space collapse | No | 5 | 11 | p = 0.015 More in COVID-19 |
| Yes | 18 | 11 | ||
| Intervillositis (intensity) | Slight | 9 | 20 | p = 0.001 More severe in COVID-19 |
| Moderate | 8 | 2 | ||
| Considerable | 2 | 0 | ||
| Severe | 4 | 0 | ||
| Blood extravasations | Slight | 1 | 17 | p = 0.4 |
| Moderate | 13 | 5 | ||
| Considerable | 6 | 0 | ||
| Severe | 2 | 0 | ||
| Unknown | 1 | 0 | ||
| Trophoblast necrosis | No | 3 | 22 | p < 0.001 More in COVID-19 |
| Yes | 20 | 0 | ||
| Intrauterine infection | Negative | 20 | 21 | p = 0.99 |
| Positive | 0 | 0 |
| Variable | Intensity | COVID Group (n = 23) | Control Group (n = 22) | Intergroup Difference |
|---|---|---|---|---|
| CD3+ | 0 | 1 | 6 | p = 0.07 |
| 1 | 14 | 13 | ||
| 2 | 6 | 0 | ||
| 3 | 2 | 0 | ||
| Unknown * | 0 | 3 | ||
| CD4+ | 1 | 1 | 0 | p = 0.01 Higher in COVID-19 |
| 2 | 10 | 19 | ||
| 3 | 6 | 3 | ||
| 4 | 6 | 0 | ||
| CD8+ | 1 | 17 | 14 | p = 0.33 |
| 2 | 5 | 8 | ||
| Unknown * | 1 | 1 | ||
| CD20+ | 1 | 21 | 18 | p = 0.35 |
| 2 | 2 | 4 | ||
| CD34+ | Low | 18 | 11 | p = 0.048 Lower in COVID-19 |
| High | 5 | 11 | ||
| CD68+ | 1 | 1 | 1 | p = 0.0006 Higher in COVID-19 |
| 2 | 10 | 20 | ||
| 3 | 7 | 0 | ||
| 4 | 4 | 0 | ||
| 5 | 1 | 0 | ||
| Unknown * | 0 | 1 | ||
| CD138+ | 1 | 23 | 18 | p = 0.27 |
| 2 | 0 | 1 | ||
| Unknown * | 0 | 3 | ||
| Vimentin | Low | 13 | 20 | p = 0.009 Higher in COVID-19 |
| High | 10 | 2 |
| Histological Change | Immunohistochemistry | p-Value | Correlation Coefficient (k) |
|---|---|---|---|
| Diffuse fibrin depositions | CD8 | 0.026 | k = 0.49 |
| CD20 | 0.0514 | k = 0.42 | |
| CD68 | 0.014 | k = 0.35 | |
| Calcifications | CD34 | 0.043 | k = −0.48 |
| Intervillositis (intensity) | CD20 | 0.074 | k = 0.39 |
| CD68 | 0.053 | k = 0.516 | |
| Blood extravasations | CD8 | 0.076 | k = 0.36 |
| CD68 | 0.0961 | k = 0.32 | |
| Delayed vascular maturation | CD3 | 0.046 | k = −0.48 |
| Infarction | CD34 | 0.064 | k = 0.46 |
| CD20 | 0.02 | k = 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borowczak, J.; Gąsiorek-Kwiatkowska, A.; Szczerbowski, K.; Maniewski, M.; Zdrenka, M.; Szadurska-Noga, M.; Gostomczyk, K.; Rutkiewicz, P.; Olejnik, K.; Cnota, W.; et al. SARS-CoV-2 Infection during Delivery Causes Histopathological Changes in the Placenta. Diseases 2024, 12, 142. https://doi.org/10.3390/diseases12070142
Borowczak J, Gąsiorek-Kwiatkowska A, Szczerbowski K, Maniewski M, Zdrenka M, Szadurska-Noga M, Gostomczyk K, Rutkiewicz P, Olejnik K, Cnota W, et al. SARS-CoV-2 Infection during Delivery Causes Histopathological Changes in the Placenta. Diseases. 2024; 12(7):142. https://doi.org/10.3390/diseases12070142
Chicago/Turabian StyleBorowczak, Jędrzej, Agnieszka Gąsiorek-Kwiatkowska, Krzysztof Szczerbowski, Mateusz Maniewski, Marek Zdrenka, Marta Szadurska-Noga, Karol Gostomczyk, Paula Rutkiewicz, Katarzyna Olejnik, Wojciech Cnota, and et al. 2024. "SARS-CoV-2 Infection during Delivery Causes Histopathological Changes in the Placenta" Diseases 12, no. 7: 142. https://doi.org/10.3390/diseases12070142
APA StyleBorowczak, J., Gąsiorek-Kwiatkowska, A., Szczerbowski, K., Maniewski, M., Zdrenka, M., Szadurska-Noga, M., Gostomczyk, K., Rutkiewicz, P., Olejnik, K., Cnota, W., Karpów-Greiner, M., Knypiński, W., Sekielska-Domanowska, M., Ludwikowski, G., Dubiel, M., Szylberg, Ł., & Bodnar, M. (2024). SARS-CoV-2 Infection during Delivery Causes Histopathological Changes in the Placenta. Diseases, 12(7), 142. https://doi.org/10.3390/diseases12070142






